Abstract
BACKGROUND:
Osteoporosis-related fractures are a considerable economic burden on the U.S. health care system. Since 2008, the Centers for Medicare & Medicaid Services have adopted a Medicare Part C Five-Star Quality Rating measure to ensure that a woman’s previously unaddressed osteoporosis is managed appropriately after a fracture. Despite the effort to improve this gap in care, the 2013 CMS plan ratings fact sheet reported an average star rating of 1.4 stars for the osteoporosis measure, the lowest score for any measure across all health plans.
OBJECTIVE:
To evaluate the impact of conducting a pharmacist-led, telephone outreach program to members or their providers to improve osteoporosis management in elderly women after experiencing fractures.
METHODS:
This was a prospective, randomized study to evaluate the effectiveness of 3 different intervention strategies within a nationwide managed care population. Women aged 66 years and older who experienced a new bone fracture between January 1, 2012-August 31, 2012, were identified through medical claims. Women who were treated with an osteoporosis medication or received a bone mineral density (BMD) test within a year of their fractures were excluded. Study patients were randomized into 3 intervention cohorts: (1) baseline intervention consisting of member educational mailing and provider educational mail or fax notification; (2) baseline intervention plus a live outbound intervention call to members by a pharmacist; and (3) baseline intervention plus a pharmacist call to members’ providers to recommend starting osteoporosis therapy and/or a bone mineral density (BMD) test. An intent-to-treat and per protocol analyses were employed, and appropriate osteoporosis management (initiation of osteoporosis therapy and/or BMD testing) 120 days after the baseline intervention and 180 days after a fracture were measured.
RESULTS:
The study identified 6,591 members who were equally randomized into 3 cohorts. The baseline demographics in each cohort were similar. Results of the intent-to-treat analysis showed more members in cohort 3 receiving appropriate osteoporosis management (13.0%) compared with those in cohort 2 (10.3%, P < 0.005) or compared with those in cohort 1 (9.1%, P < 0.001). No difference was detected between those receiving additional member calls (cohort 2) and those receiving only the baseline intervention (cohort 1). Similar results were observed utilizing the 180 days after fracture time frame.
CONCLUSIONS:
The effectiveness of a pharmacist-led telephone intervention directed at providers or members was examined in this randomized study. Pharmacist calls to members did not improve osteoporosis management over member and provider mail and fax notifications. Greater impact was demonstrated by performing a pharmacist call intervention with providers rather than with members.
What is already known about this subject
Less than 25% of previously untreated women over aged 66 years in the United States receive appropriate screening and treatment for osteoporosis within 6 months of a primary bone fracture.
Current strategies (primarily the sending of written education materials to patients or providers) are unlikely to significantly improve screening and treatment rates.
Pharmacists contribute to the health care team by educating patients on drug therapies and also by contacting prescribers to close gaps in patient medical care.
What this study adds
Eligible study patients whose prescribers were contacted by a pharmacist by telephone were more likely to receive osteoporosis management than those that received written materials only (15.3% vs. 12.3%) within 6 months of a primary bone fracture.
Pharmacist calls to providers were more effective than calls directly to patients in improving osteoporosis care (15.3% vs. 12.1%) within 6 months of a primary bone fracture.
A pharmacist-led telephone intervention directed at patients did not improve osteoporosis care any better than written educational notifications.
Despite nearly a decade after the release of the Surgeon General’s 2004 report on bone health, osteoporosis remains a significant yet treatable illness that afflicts nearly 1 in 6 women over the age of 50 in the United States.1,2 Osteoporosis-related fractures resulted in nearly half a million hospital admissions in 2005 with the combined costs to the U.S. health care system estimated at $17 billion and growing.
Osteoporosis is difficult to detect and is often only discovered when a patient suffers a bone fracture. Current guidelines recommend bone mineral density (BMD) testing in all women aged 65 years and older and initiating pharmacologic treatment in those women aged 50 years and older with hip or vertebral fractures.3,4 Since 2007, the National Committee on Quality Assurance (NCQA) has employed a Healthcare Effectiveness Data and Information Set (HEDIS) quality measure, Osteoporosis Management in Women who had a Fracture (OMW), that compares OMW across health plans. The measure determines the rate at which previously untreated older women receive appropriate osteoporosis management (either a BMD test or osteoporosis pharmacotherapy) within 6 months of a fracture. Results from the 2013 NCQA’s state of health care quality yearly report indicate that little progress had been made within the last 5 years to improve the OMW rate.5
Since 2008, the Centers for Medicare & Medicaid Services (CMS) have adopted the OMW measure as part of their Five Star Quality Rating System for Medicare Advantage Plans. In 2012, high performance was rewarded with bonus payments totaling $3.1 billion distributed to all participants.6 Despite these efforts, the 2013 CMS plan ratings fact sheet shows that across all plans and out of 55 individual part C and D measures, the OMW measure received the lowest average star rating (1.4 stars), even though the cut point for achieving 2 stars was only 24%.7
In the past decade, numerous interventions have been employed to improve osteoporosis management in postfracture patients.8 As part of a multidisciplinary health care team, pharmacists may be uniquely positioned to intervene with gaps in osteoporosis management.9 In a series of randomized controlled trials by Solomon et al. (2007), educational visits to physician offices by a team of pharmacists demonstrated an increase (14% vs. 10%) in BMD testing and initiation of osteoporosis medications in postfracture patients compared with performing no additional interventions.10,11 In another study, dedicated pharmacist-run fracture liaison services resulted in 65% of patients receiving appropriate care within 6 months of their fractures compared with a similar service run by nurses (46%).12
To further discern the impact of pharmacists on improving rates of osteoporosis management, we examined the comparative effectiveness of adding an intensive pharmacist-led telephone intervention to a standard mail and fax-based notification program. From the perspective of a large, nationwide managed care organization, having telephone conversations with eligible members and providers would cost less to operate than face-to-face conversations.
Methods
Study Design
We compared 3 interventions simultaneously in a nationwide, prospective, multicycle, randomized controlled trial. National medical and pharmacy claims databases were used to identify women aged 66 years and older with fractures and no previous osteoporosis treatment. Patients were identified every 2 months (4 cycles) and randomized into 3 cohorts between January 1, 2012, and August 31, 2012. All interventions were completed within 4-5 months after a patient was identified as having a fracture. All aspects of the trial were approved prior to implementation by the Ethical & Independent West Coast Review Board.
Study Population
Study patients were health plan members of a large, nationwide Medicare Advantage insurance plan and OptumRx, a pharmacy benefits management company serving commercial and Medicare clients across the United States. All bone fractures and BMD tests were identified through International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) diagnosis and procedure codes, Current Procedural Terminology (CPT) codes, or Healthcare Common Procedure Coding System (HCPCS) codes as specified in the HEDIS 2012 OMW measure.13 A data warehouse that stores all medical encounters involving these codes was queried for fracture claims. The fracture index date (FID) for each patient was defined as the earliest date of service in which a fracture diagnosis was identified during the identification period. Patients with a previous fracture claim between July 1, 2011, and the FID were excluded. In patients with multiple fractures, only the earliest fracture was included in the study. Patients were excluded if they received a BMD test or prescription claims for medications that treat osteoporosis as specified in the HEDIS 2012 OMW measure within 1 year prior to the FID through the end of the identification period for each cycle.14 Patients were also excluded due to invalid or unknown mailing addresses and phone numbers and if their providers were unidentifiable, had invalid contact information, or requested not to be contacted by the health plan but not excluded due to returned mail. Patients who were in institutional care settings have a higher level of care and oversight by pharmacy and physician staff and were excluded from the study. Patients not using OptumRx as a pharmacy benefits management company, defined as having no pharmacy claims during the identification period or 4 months prior to the identification period, were also excluded.
Interventions
Study patients in all 3 cohorts received the baseline mailing and faxing intervention. The baseline intervention consisted of written materials sent to every patient and corresponding provider in the study. The patient mailing materials consisted of an educational brochure explaining the importance of diagnosing and treating osteoporosis. All primary providers were faxed or mailed (if no fax number was available) an initial written notification informing them of the identified patient and suggesting appropriate osteoporosis management based on national guidelines and quality measures. The baseline intervention was sent 1-2 months after the identification period for each cycle. The patients and their providers in cohort 1 received no further intervention. Outbound phone calls began 2 weeks after the baseline intervention was sent and were completed over 2 to 4 weeks for each cycle. Patients randomized to cohort 2 received an additional live unscheduled call from a pharmacist who delivered a scripted message highlighting the benefits of osteoporosis therapy and BMD testing in postfracture patients. Their providers received no further intervention. Providers of patients randomized to cohort 3 also received a live unscheduled call from a pharmacist who delivered a scripted message regarding guidelines from the North American Menopause Society on postfracture osteoporosis treatment and BMD testing and its application to the identified patient. In this cohort, a second follow-up courtesy fax was sent to the contacted provider after the completion of a successful call. The format of the second fax was different than the first notification and summarized the conversation that occurred between the provider and pharmacist. The patients in cohort 3 received no further intervention.
Providers were identified based on prescription drug claim patterns. The primary targeted provider was the most common prescriber of medications for the patient during the identification period. If contact with the first provider failed, contact with a second provider was attempted if available. The second provider was the second most common prescriber of medications during the identification period. In cases where multiple secondary providers prescribed the same number of medications, the prescriber of the most recently dispensed medication was selected.
A group of 5 licensed pharmacists conducted the outbound calls after receiving 2 hours of osteoporosis management training as their sole responsibility. The same group of pharmacists performed patient and provider calls. Patient and provider calls were randomly assigned to each pharmacist. A call intervention was determined to be successful if the entire message was relayed to the intended recipient. During the outbound phone call, pharmacists relayed a scripted message, answered questions, and addressed patient and provider concerns. Calls averaged 4 and 5 minutes for patients and providers, respectively. Up to 3 call attempts at different times and on different days were conducted between the hours of 8:00am to 5:00pm, in the patients’ time zones. The outbound call interventions occurred over the span of 2 to 4 weeks, approximately 2 to 5 months after the patients’ fracture dates.
Study Outcomes
The primary outcome measured resolution within 120 days after the launch date of the baseline interventions. This more conservative, shorter time frame provided a better assessment of cause and effect, since measurement occurred after the interventions began. The secondary outcome followed HEDIS specifications, which calls for resolution measurement within 180 days after the FID for each patient. Appropriate osteoporosis management was the primary composite resolution measured and was defined, according to the 2012 HEDIS OMW measure, as either one or some combination of the following: a medical encounter with ICD-9-CM, CPT, or HCPCS codes indicating a BMD test or a claim representing an osteoporosis medication via a J-code or prescription medication claim during the measurement period, with the exception of alendronate 40 milligrams (mg) and risedronate 30 mg, since those specific dosages are reserved for Paget’s disease. The disposition of each call intervention (success, declined, or wrong number) and the individual resolution event types that made up the composite resolution (BMD test or osteoporosis medication) were also measured and reported.
Statistical Analysis
Intent-to-treat and per protocol analyses were completed. The intent-to-treat population included all patients initially randomized to each cohort. The per protocol population consisted of patients continuously eligible with the plan during the measurement period and who underwent the entire telephone intervention successfully. Patients were excluded from the per protocol analysis if during the call it was discovered that the patient was already receiving appropriate osteoporosis management or did not meet some other eligibility criteria.
Statistical analyses were performed using SAS version 9 (SAS Institute, Inc., Carey, NC). All statistical tests were 2-sided. Assuming a 3% absolute difference in primary composite resolution rates between cohorts, a sample size of 1,059 intervened patients per cohort (3,177 total patients) was estimated to provide a statistical power of 80% with a 2-sided significance level of 0.05. Based on past experiences, a 50% attrition of the number of patients initially identified for the program was expected because of invalid contact information, death, inability to complete the entire intervention successfully, and health plan eligibility. In order to obtain the necessary number of patients to conduct the per protocol analysis, 2,118 identified patients per cohort (6,354 patients) were needed to account for the 50% attrition. Resolution is a binary outcome that was compared using the chi-square test of proportions for independent samples.
Results
Nationwide, 6,591 eligible women were enrolled into the study (Figure 1). Table 1 describes baseline demographics among study cohorts. No differences were noted between the cohorts for any of the variables reported. The average age of study patients was 80 years, and about 1 in 4 patients qualified for some form of Medicare low-income subsidy.
FIGURE 1.
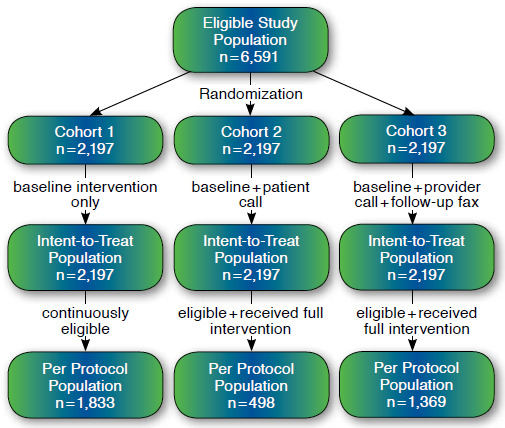
Study Flow Diagram
TABLE 1.
Study Population Demographics
| Cohort 1 | Cohort 2 | Cohort 3 | |
|---|---|---|---|
| Randomized members, n | 2,197 | 2,197 | 2,197 |
| Age, years, average (range) | 79.8 (66-105) | 80 (66-104) | 80 (66-105) |
| Medicare low-income subsidy eligible,a n (%) | 547 (24.89) | 549 (24.99) | 570 (25.94) |
| Female, % | 100.00 | 100.00 | 100.00 |
| Number of unique providers targeted for intervention, n | 2,101 | 2,111 | 2,076 |
| Number of randomized members continuously enrolled during study period, n | 1,833 | 1,832 | 1,841 |
a Individuals with income less than 150% of the federal poverty level based on criteria set by the health plan and the Centers for Medicare & Medicaid Services Prescription Drug Benefit Manual.19
Call outcomes for all patients randomized into cohort 2 and 3 are reported in Figure 2. The average outbound patient call lasted 4 minutes, and outbound provider calls lasted 5 minutes. The success rate in delivering the phone message to providers was almost 3 times greater than for patients. Although at least 3 separate attempts were made to reach all patients in cohort 2 by phone, the targeted patient was reached by phone only half the time. Only 25% of patients listened to the full message, while 14% declined the message. A failed message was defined as a call that went directly to voice mail or if the patient or provider was not available to talk. Due to privacy considerations, no messages were left on a patient’s voice mail. For provider calls, a message was left on a provider’s voice mail only if the front office staff confirmed the patient as the provider’s patient. Providers or their confirmed voice mail were reached over 78% of the time, and the message was successfully delivered 72% of the time. The primary reason a successful message was not delivered to a provider was due to targeting the incorrect provider. For 12% of study patients, the primary and secondary provider (if available) either did not know the patient or could not address the patient’s osteoporosis management and thus were classified as “incorrect provider.” Patients were classified as “criteria not met” if during either the patient or provider outbound call, it was discovered that the patient had already received a BMD test or osteoporosis therapy or had some contraindication to receiving appropriate osteoporosis management.
FIGURE 2.
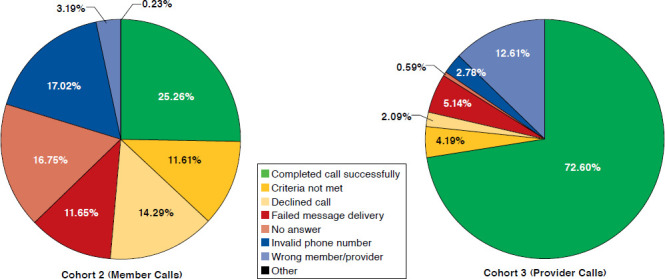
Call Outcomes of the Intent-to-Treat Population
The results of the intent-to-treat analysis (Table 2) at 120 days after the baseline intervention showed that significantly more patients randomized into cohort 3 (13%) received appropriate osteoporosis management than patients in cohort 1 (9.1%, P <0.001) or cohort 2 (10.3%, P <0.005). No significant increase in osteoporosis management was demonstrated for members in cohort 2 when compared with cohort 1; their P values are not reported. Given the longer follow-up period of the 180-day secondary measurement period, resolution rates across all cohorts increased and the relative differences decreased, but cohort 3 patients (15.3%) continued to receive more appropriate osteoporosis management than patients in cohort 1 (12.3%, P <0.005) or cohort 2 (12.1%, P <0.005). A secondary analysis of the component outcomes, using either measurement time frame, determined that the BMD test rate was statistically greater in cohort 3 patients than in any other cohort. Although rates for initiating osteoporosis medications were greater in cohort 3 patients than in any other cohort, results measured at 180 days after FID were not statistically significant.
TABLE 2.
Intent-to-Treat Analysis
| Cohort 1 Baseline Intervention | Cohort 2 Baseline+ Patient Call | Cohort 3 Baseline+ Provider Call | P Value Cohort 3 vs. Cohort 1 | P Value Cohort 3 vs. Cohort 2 | |
|---|---|---|---|---|---|
| Intent-to-treat population, n | 2,197 | 2,197 | 2,197 | — | — |
| Underwent BMD test 120 days after intervention, n (%) | 117 (5.3) | 124 (5.6) | 179 (8.1) | <0.001 | 0.001 |
| Initiated osteoporosis medication 120 days after intervention, n (%) | 116 (5.3) | 126 (5.7) | 153 (7.0) | 0.019 | 0.095 |
| Either outcome 120 days after intervention, n (%) | 201 (9.1) | 226 (10.3) | 286 (13.0) | <0.001 | <0.005 |
| Underwent BMD test 180 days after FID, n (%) | 187 (8.5) | 175 (8.0) | 238 (10.8) | 0.009 | 0.001 |
| Initiated osteoporosis medication 180 days after FID, n (%) | 127 (5.8) | 128 (5.8) | 150 (6.8) | 0.153 | 0.173 |
| Either outcome 180 days after FID, n (%) | 271 (12.3) | 266 (12.1) | 336 (15.3) | <0.005 | <0.005 |
BMD = bone mineral density; FID = fracture index date.
The results of the per protocol analysis (Table 3) at 120 days after baseline intervention show that more patients in cohort 3 (15.2%), who successfully completed the intervention and stayed eligible with the health plan, received appropriate osteoporosis management than cohort 1 (10.3%, P <0.001). The number of patients remaining in the per protocol population for cohort 2 failed to meet sample size to detect a statistically significant difference in resolution rate when compared with any other cohort. Although their outcomes are still reported, a significance test comparing cohort 2 outcomes to other cohorts was not conducted. The longer secondary 180-day measurement period continued to show a significant, albeit smaller, difference in resolution rate for the composite outcome between patients in cohort 3 (16.5%) and cohort 1 (13.4%, P = 0.012).
TABLE 3.
Per Protocol Analysis
| Cohort 1 Baseline Intervention | Cohort 2 Baseline +Patient Call | Cohort 3 Baseline +Provider Call | P Value Cohort 3 vs. Cohort 1 | |
|---|---|---|---|---|
| Per protocol population, n | 1,833 | 498 | 1,369 | — |
| Underwent BMD test 120 days after intervention, n (%) | 112 (6.1) | 36 (7.2) | 126 (9.2) | 0.001 |
| Initiated osteoporosis medication 120 days after intervention, n (%) | 107 (5.8) | 26 (5.2) | 113 (8.3) | <0.008 |
| Either outcome 120 days after intervention, n (%) | 189 (10.3) | 55 (11.0) | 208 (15.2) | <0.001 |
| Underwent BMD test 180 days after FID, n (%) | 171 (9.3) | 41 (8.2) | 156 (11.4) | 0.148 |
| Initiated osteoporosis medication 180 days after FID, n (%) | 115 (6.3) | 25 (5.0) | 102 (7.5) | 0.059 |
| Either outcome 180 days after FID, n (%) | 245 (13.4) | 58 (11.6) | 226 (16.5) | 0.012 |
BMD = bone mineral density; FID = fracture index date.
Discussion
Applying the additional provider intervention resulted in 42% more appropriate osteoporosis management in cohort 3 (13.0%) when compared with traditional methods (9.1%), despite pharmacists having no pre-existing personal relationships with patients or providers and only being successfully implemented on 72% of the population. Additionally, those who actually completed the provider call intervention successfully, and were included in the per protocol analysis, received appropriate osteoporosis management more often (15.2%) compared with traditional methods (10.3%), which further supports the causal relationship. Not only is the additional provider intervention effective, but it is also timely, since more appropriate osteoporosis management was achieved when measuring outcomes either 120 days after the initiation of the intervention or 180 days after the fracture, which reflects the time frame utilized by the HEDIS quality measure.
Pharmacist-initiated outbound calls to patients resulted in no measurable improvement in osteoporosis management over traditional methods. We were unable to determine if greater osteoporosis management would have been observed if message delivery success rates were on par with the provider intervention. Nonetheless, results of the intent-to-treat analysis are a reflection of real-world conditions, where effectiveness is determined in part by how realistically the intervention can be fully applied to the entire study population. A recent study reported a similarly low 49.6% patient contact rate by mail-order pharmacists as they conducted a telephone-based patient intervention to improve diabetes management for 4,022 patients.15
The additional telephone intervention to providers resulted in significantly more appropriate osteoporosis management than the additional patient intervention. Attributing this finding at least in part to the different contact rates cannot be ruled out. The higher success rate in contacting providers can in part be attributed to providers having front office staff who were always available to answer phone calls during normal business hours. Results from a recent focus group of patients who had sustained fractures outlines a theory that explains why some patients receive appropriate osteoporosis management and not others.16 Patients alone must complete a series of steps and encounter many barriers in order to receive appropriate treatment. The study pointed out a subgroup of patients who more directly received appropriate care by simply trusting and following their physicians’ recommendations. This places the impetus on physicians to correctly diagnose, provide appropriate care, and guide their patients through the entire process. Our results fit into this framework of viewing how patients receive appropriate osteoporosis care following a fracture. Barriers to initiating osteoporosis treatment were not any better overcome by additional call attempts to the patient than mailed educational materials. Instead, having pharmacists call to notify and inform providers about the need for treatment and subsequently having providers coordinate patient care was a more successful strategy.
We did not determine the impact of the 3% absolute increase in osteoporosis management by provider intervention compared with traditional methods at 180 days post-FID (15.3% vs. 12.3%) on individual plan star ratings nor perform a thorough cost-effectiveness analysis. Although study outcomes may not exactly replicate CMS star rating methodology, the 2014 OMW measure 2-star cut point of 16%, with only half the plans exceeding the average score of 1.9 stars, is noticeably similar to the higher success rates achieved by the additional provider call intervention in this study.7 Although unable to obtain the higher resolution rates often observed by on-site fracture liaison services, the telephone intervention is possibly more scalable and easier to operate. Costs per intervention were primarily attributed to the approximately 5 minutes that pharmacists spent on each intervention, and although for every 1,000 calls conducted only 30 additional members would receive osteoporosis management, the Surgeon General reports that a lifetime cost of $81,000 could be saved for any single subsequent hip fracture avoided.1
Limitations
Our study exhibits some key limitations. Due to exclusion criteria, primarily for incomplete contact information or other data elements necessary for the study, the population selected for the study is not the entire population eligible for the HEDIS measure. However, no further exclusions occurred after randomization for the intent-to-treat analysis, and outcomes appear comparable to national rates. The intensity and contact rate of the intervention varied for each cohort and may have created an unfair comparison between groups. The provider intervention was designed to maximize the opportunity for contact, including the ability to leave a message, contact a second provider, and send a follow-up fax. These options were not available for the patient intervention because of health information privacy considerations. This may have contributed to the difference in contact rate. A control group receiving no intervention was also not employed, since it would be inappropriate to withhold some form of intervention from any group. There is the possibility of some cross-contamination of the intervention among study cohorts, since patients—and not providers—were randomized. In a large nationwide study such as this, some providers contacted for patients in cohort 3 may also have treated patients in the other cohorts.
Patient age, mobility, socioeconomic factors, and comorbidities, as well as recent warnings associating bisphosphonates with atypical fractures, may have hindered therapy initiation.17 We suspect some osteoporosis medications and BMD testing may have been paid out of pocket by patients, resulting in the absence of claims for reimbursement. Also, it may not be inappropriate for providers to utilize a BMD test obtained just outside the HEDIS-specified 1-year exclusion window prior to fracture. Initiating treatment outside the HEDIS-specified 6-month time frame may also be appropriate. It is also possible that the population HEDIS chose to measure for receiving appropriate treatment may be relatively more challenging and resistant to receiving treatment than expected. Patients more open to preventative management may have already initiated appropriate prophylactic therapy, leaving behind those less likely to initiate osteoporosis therapy thereby constituting a large proportion of the population not receiving appropriate management. We did notice that the average age of study patients (80 years) was noticeably higher than the starting age required for inclusion in the study and HEDIS measure (66 years). This suggests that a very elderly (>80 years) subpopulation constituted a significant portion of the HEDIS measure denominator and was resistant to appropriate care, thus requiring more personal contact and more intensive interventions to overcome resistance to receiving appropriate care. We did not stratify outcomes by age but suspect that the resistance of this elderly population was due to provider reluctance to initiate treatment for very elderly patients, who often have complex comorbid diseases for which clinical practice guideline recommendations may not apply.18 Additional studies are needed to establish the benefits of osteoporosis medications in the very elderly and to determine even more cost-effective methods to further improve the management of osteoporosis in this vulnerable population, such as utilizing pharmacy technicians, retail pharmacists, or nurses to conduct interventions.
Conclusions
This study provided pharmacists with dedicated time and analytical resources to engage patients or providers by telephone in order to improve appropriate osteoporosis management in a large nationwide population of elderly women who had recently experienced a fracture. We measured the effect of these additional personal contact interventions compared with traditional methods of sending written educational materials. The additional outbound call to providers demonstrated a clear improvement in care. However, additional outbound calls to patients appeared to not reach enough patients or be an intensive enough intervention to initiate a change. Given the comparable effort in conducting either intervention, we believe the provider intervention is the favored approach to enhance existing clinical campaigns based solely on written materials to improve osteoporosis care nationwide.
References
- 1. Office of the Surgeon General. . Bone Health and Osteoporosis: A Report of the Surgeon General. Rockville, MD: Office of the Surgeon General; 2004. Available at: http://www.ncbi.nlm.nih.gov/books/NBK45513/. Accessed July 23, 2015. [PubMed] [Google Scholar]
- 2. Looker AC, Borrud LG, Dawson-Hughes B, Shepherd JA, Wright NC.. Osteoporosis or low bone mass at the femur neck or lumbar spine in older adults: United States, 2005-2008. NCHS Data Brief, No 93. Hyattsville, MD: National Center for Health Statistics. April 2012. Available at: http://stacks.cdc.gov/view/cdc/12136. Accessed July 23, 2015. [PubMed] [Google Scholar]
- 3. The National Osteoporosis Foundation. . Clinician’s guide to prevention and treatment of osteoporosis 2014. National Osteoporosis Foundation, Washington, DC. Available at: http://www.nof.org/hcp/clinicians-guide. Accessed July 23, 2015. [Google Scholar]
- 4. Management of osteoporosis in postmenopausal women: 2010 position statement of The North American Menopause Society. Menopause. 2010; 17(1): 25-54. [DOI] [PubMed] [Google Scholar]
- 5. National Committee on Quality Assurance. . The state of health care quality. 2014. Available at: http://www.ncqa.org/ReportCards/HealthPlans/StateofHealthCareQuality.aspx. Accessed July 23, 2015.
- 6. Jacobson G, Neuman T, Damico A, Huang J.. Medicare Advantage Plan star ratings and bonus payments in 2012. Kaiser Family Foundation Data Brief. November 2011. Available at: https://kaiserfamilyfoundation.files.wordpress.com/2013/01/8257.pdf. Accessed July 23, 2015.
- 7. Centers for Medicare & Medicaid Services. . Part C and D performance data. 2013 Part C and D ratings data. Available at: http://www.cms.gov/Medicare/Prescription-Drug-Coverage/PrescriptionDrugCovGenIn/PerformanceData.html. Accessed July 23, 2015.
- 8. Sale JE, Beaton D, Posen J, Elliot-Gibson V, Bogoch E.. Systematic review on interventions to improve osteoporosis investigation and treatment in fragility fracture patients. Osteoporos Int. 2011; 22(7): 2067-82. [DOI] [PubMed] [Google Scholar]
- 9. Elias MN, Burden AM, Cadarette SM.. The impact of pharmacist interventions on osteoporosis management: a systematic review. Osteoporos Int. 2011; 22(10): 2587-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Solomon DH, Katz JN, Finkelstein JS, et al. . Osteoporosis improvement: a large-scale randomized controlled trial of patient and primary care physician education. J Bone Miner Res. 2007; 22(11): 1808-15. [DOI] [PubMed] [Google Scholar]
- 11. Solomon DH, Polinski JM, Stedman M, et al. . Improving care of patients at-risk for osteoporosis: a randomized controlled trial. J Gen Intern Med. 2007; 22(3): 362-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Heilmann RM, Friesleben CR, Billups SJ.. Impact of a pharmacist-directed intervention in postmenopausal women after fracture. Am J Health Syst Pharm. 2012; 69(6): 504-09. [DOI] [PubMed] [Google Scholar]
- 13. National Committee on Quality Assurance. . HEDIS 2012, volume 2: technical specifications for health plans. Available at: http://www.ncqa.org/HEDISQualityMeasurement/HEDISMeasures/HEDIS2012.aspx. Accessed July 23, 2015.
- 14. National Committee for Quality Assurance. . HEDIS 2012. Table OMW-C: FDA-approved osteoporosis therapies. Available at: http://www.ncqa.org/Portals/0/HEDISQM/HEDIS2012/NDC/Table%20OMW-C.doc. Accessed July 23, 2015.
- 15. Brennan TA, Dollear TJ, Hu M, et al. . An integrated pharmacy-based program improved medication prescription and adherence rates in diabetes patients. Health Aff (Millwood). 2012; 31(1): 120-29. [DOI] [PubMed] [Google Scholar]
- 16. Beaton DE, Sujic R, McIlroy Beaton K, Sale J, Elliot-Gibson V, Bogoch ER.. Patient perceptions of the path to osteoporosis care following a fragility fracture. Qual Health Res. 2012; 22(12): 1647-58. [DOI] [PubMed] [Google Scholar]
- 17. Shane E, Burr D, Abrahamsen B, et al. . Atypical subtrochanteric and diaphyseal femoral fractures: second report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2014; 29(1): 1-23. [DOI] [PubMed] [Google Scholar]
- 18. Boyd CM, Darer J, Boult C, Fried LP, Boult L, Wu AW.. Clinical practice guidelines and quality of care for older patients with multiple comorbid diseases: implications for pay for performance. JAMA. 2005; 294(6): 716-24. [DOI] [PubMed] [Google Scholar]
- 19. Centers for Medicare & Medicaid Services. . Prescription drug benefit manual. Available at: http://www.cms.gov/Medicare/Prescription-Drug-Coverage/PrescriptionDrugCovContra/PartDManuals.html. Accessed July 23, 2015.


