Abstract
BACKGROUND:
In the United States, more than 50% of patients with type 2 diabetes mellitus (T2DM) have hemoglobin A1c (A1c) levels that fail to achieve the recommended target of < 7.0%. Of these, 30%-45% have an A1c > 9.0%, the threshold for poorly controlled T2DM per National Committee for Quality Assurance (NCQA) measures. Treatment inertia is a known challenge. However, recent treatment intensification patterns and outcomes after treatment fails 2 classes of oral antidiabetic agents (OADs) are not well understood.
OBJECTIVE:
To characterize treatment intensification patterns and glycemic control outcomes in patients with A1c ≥ 7.0% on 2 OADs.
METHODS:
A retrospective cohort study was conducted in patients with T2DM from a regional health plan claims dataset augmented with A1c results between January 1, 2010, and March 31, 2017. Patients were identified with an A1c ≥ 7.0% (baseline), while on 2 OADs, and whose treatment was intensified with basal/biphasic insulin (insulin), glucagon-like peptide-1 receptor antagonist (GLP-1RA), or a third OAD within 365 days after the baseline A1c ≥ 7.0%. Patients had at least 1 A1c value 60-365 days (follow-up period) after treatment intensification. The proportion of patients with an A1c < 7.0% and < 9.0% at follow-up were identified by therapeutic intensification strategy. Odds ratios for achieving A1c < 7.0% and < 9.0% were calculated.
RESULTS:
1,226 patients were included in the analysis, and 33.5% of the patients had a baseline A1c ≥ 9.0%. 24% of patients received insulin; 16% received GLP-1RA; and 60% received a third OAD for the treatment intensification. Overall, 26.0% achieved A1c < 7.0% and 76.1% of patients achieved < 9.0%, with a median follow-up of 119 days. The proportion of patients intensified with insulin who had an A1c ≥ 9.0% at follow-up was 34.6% versus 53.2% at baseline (P < 0.01). The corresponding percentages for those intensified with a GLP-1RA and OAD were 21.6% versus 27.1% (P = 0.24) and 20.1% versus 27.3% (P < 0.01). After controlling for baseline characteristics, the odds ratio (95% CI) of achieving A1c < 7.0% and < 9.0% was 2.05 (1.45-2.90) for GLP-1RA and 0.92 (0.61-1.40) for OAD. The association between goal attainment and GLP-1RA versus OAD intensification was influenced by the time to the A1c follow-up and baseline A1c.
CONCLUSIONS:
Treatment intensification was associated with improved glycemic control in patients after therapy failed 2 OADs. Patients with higher A1c at baseline were likely to initiate insulin, which was associated with a greater drop in A1c. GLP-1RA was associated with a higher likelihood of achieving NCQA-suggested glycemic control compared with a third OAD. However, the association varied by the follow-up period. These findings are important to health plans seeking to improve patient outcomes as reflected in high performance on NCQA diabetes quality measures by promoting effective and timely treatment intensification.
What is already known about this subject
Switching to or addition of an antidiabetic agent in a third class leads to better outcomes across varying levels of hemoglobin A1c (A1c) goals in patients who already failed to achieve glycemic control on metformin alone or in combination with an oral antidiabetic agent (OAD).
According to the National Committee for Quality Assurance (NCQA) Healthcare Effectiveness Data and Information Set (HEDIS) measures, the proportion of adult patients meeting A1c goals of < 7.0% to < 9.0% indicates the quality of comprehensive diabetes care.
What this study adds
This study identified the pattern of treatment intensification in patients whose A1c was insufficiently controlled on OADs in 2 classes: Patients continued to OAD in a third class more often than insulin or glucagon-like peptide-1 receptor antagonist (GLP-1RA).
The proportion of patients meeting A1c goals of < 7.0% to < 9.0% increased after the third treatment intensification, which was consistently observed across the 3 intensification groups: insulin, GLP-1RA, and third OAD.
The odds of achieving A1c < 7.0%, after adjusting for baseline A1c and other potential confounders, were significantly higher in the GLP-1RA group relative to the third OAD group.
Type 2 diabetes mellitus (T2DM) is a chronic condition that requires long-term management and self-care. In 2012, the medical cost of treating a patient with diabetes averaged $13,700 annually, which was 2.3 times the expenditure for patients without diabetes.1 Given that the prevalence of diabetes in the United States has reached 9.4%, with more than 90% of these patients diagnosed with T2DM, the economic burden of diabetes in 2012 was $245 billion including medical costs and lost productivity.2 Inadequate glycemic control contributes to a substantial increase in the risk of vascular complications and potentially leads to blindness or premature death.3-5 Consequently, T2DM patients who have insufficiently controlled hyperglycemia are expected to use more medical resources.6
The National Committee for Quality Assurance (NCQA) has compiled a comprehensive diabetes care quality measurement set using Healthcare Effectiveness Data and Information Set (HEDIS) measures and has promoted high-quality care for patients through the Diabetes Recognition Program.7 Current comprehensive diabetes care measures assess 3 levels of hemoglobin A1c (A1c) as indicators of population-level diabetes control and diabetes quality of care. The measures include the proportion of adult patients who are candidates for aggressive control as A1c < 7.0%, proportion with A1c < 8.0% as a level appropriate for most patients to be below, and proportion with A1c > 9.0% as poorly controlled glycemia.7
The NCQA diabetes measures have been used to evaluate the performance of clinical practice and patient education in multiple studies.8-10 Patients with A1c < 7.0% were expected to live with a manageable risk of complications.11 However, it is estimated that around 50% of patients with diabetes in the United States have A1c levels that failed to achieve sustained glycemic control of < 7.0%, and about 15% of patients had A1c ≥ 9.0%, an indicator of poorly controlled hyperglycemia.12
Existing studies have demonstrated that shorter clinical inertia in patients whose A1c is not sufficiently controlled is potentially associated with a lower follow-up A1c value. In patients with newly diagnosed T2DM whose treatment failed metformin (MET) monotherapy, time to A1c goal attainment was shorter among patients who received early treatment intensification regardless of the A1c goal.13 Fu et al. (2016, 2017) also analyzed change in A1c associated with treatment intensification among T2DM patients with A1c > 8.0% and demonstrated a positive association between treatment intensification and A1c reduction.14,15 Practice guidelines recommend treatment intensification, including switching to or addition of an antidiabetic agent in patients with insufficient glycemic control, based on the patient’s cardiovascular risk and the drug’s ability to lower A1c.16,17
Previous studies included patients on MET monotherapy or MET combined with another oral antidiabetic agent (OAD). Therefore, the identified pattern of treatment intensification and the outcomes have limited generalizability to patients receiving other OAD combinations.13-15 Levin et al. (2014) described treatment patterns in patients whose glycemia was insufficiently controlled on 2 OADs.18 However, the Levin study included treatment intensification between January 2000 and March 2009; therefore, recent patterns of antidiabetic agent use, specifically for glucagon-like peptide-1 receptor antagonist (GLP-1RA) after failure on 2 OADs, have not been studied.18
Given the limitations of previous studies, there is a need for a description of recent trends in treatment intensification after treatment fails 2 OADs. Further, assessment of the NCQA-HEDIS criteria to determine the A1c outcome for an observational study will facilitate better interpretation of quality measures in clinical practice, particularly within a managed care setting. The objectives of this study were to (a) characterize treatment intensification patterns and time to treatment intensification in patients with T2DM whose diabetes was insufficiently controlled per A1c > 7.0% on 2 OADs; (b) assess diabetes control associated with treatment intensification per NCQA-HEDIS measures; and (c) compare glycemic control between 3 treatment intensification strategies—adding basal/biphasic insulin (insulin), GLP-1RA, or a third OAD.
Methods
Study Design and Analytic Cohort
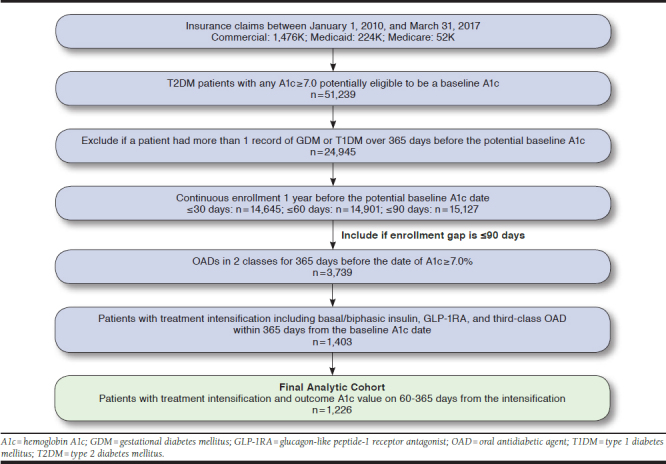
This was a retrospective observational study using deidentified medical and pharmacy claims and A1c test results from SelectHealth, a regional managed care organization, between January 1, 2010, and March 31, 2017. SelectHealth operates in the Intermountain Region of the United States and collects medical and pharmacy claims for approximately 800,000 patients. The enrollees are predominantly from commercial lines but include some Medicaid and Medicare Advantage beneficiaries. Therefore, the population of this study is younger than a typical T2DM population. SelectHealth also captures A1c values for patients with T2DM, promoted by a diabetes incentive program for providers. This research was deemed exempt from the University of Utah’s institutional review board (IRB) review (January 27, 2017) and was approved by the Intermountain Healthcare IRB expedited review (May 25, 2017).
The analytic cohort consisted of patients who had a record of A1c ≥ 7.0% (i.e., baseline A1c) after receiving prescriptions for 2 different OAD classes, including fixed-dose combinations. The baseline period was up to 365 days before the baseline A1c date, during which demographic information and clinical characteristics were captured. Charlson Comorbidity Index (CCI) and Diabetes Complication Severity Index (DCSI) scores were calculated using a published algorithm.19,20 To confirm insufficient response to therapy, the second OAD agent was required to have been dispensed at least 60 days before the baseline A1c date. Included patients had a diagnosis of T2DM (International Classification of Diseases, Ninth/Tenth Revision, Clinical Modification codes 250.x0 or 250.x2; E11x) on at least 2 different days during the 365-day baseline period; those with a diagnosis for type 1 diabetes or gestational diabetes were excluded. Also excluded were patients who received insulin or other injectable antidiabetic agents, including GLP-1RA or pramlintide, during the baseline period. Patients who filled pramlintide, an injectable amylin analogue, on the date of treatment intensification were also excluded. Pramlintide is specifically indicated for T2DM without optimal glycemic control despite an insulin therapy and therefore would not qualify as a treatment intensification option in our analysis. Included patients were required to have continuous enrollment, defined as constant coverage or a gap of no more than 90 days over the baseline period (Figure 1). The influence of patients having a coverage gap of 30+ or 60+ days was assessed as a sensitivity analysis. However, these shorter, allowed gaps had minimal effect on cohort size (~3% difference; see Appendix A, available in online article).
FIGURE 1.
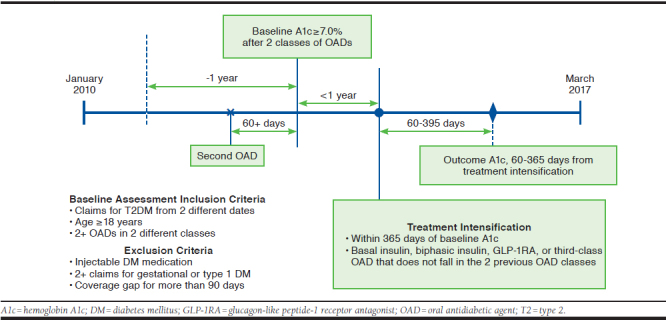
Analyses Timeline
Treatment Intensification and Outcomes
Treatment intensification was defined as a prescription for the first antidiabetic agent that differed from the 2 previous OAD classes and was classified by the type of antidiabetic agent—insulin, GLP-1RA, or a third OAD including thiazolidinedione (TZD), sulfonylurea (SU), alpha-glucosidase inhibitor, dipeptidyl peptidase 4 inhibitor (DPP-4), or sodium-glucose cotransporter inhibitor (SGLT2). Patients had to have a treatment intensification on or within 365 days from the baseline A1c date. Time (in days) from baseline A1c date to the treatment intensification date was also captured.
The primary outcome for the treatment intensification was glycemic control based on the first A1c measure and was captured between 60 and 365 days after treatment intensification (Figure 1). Glycemic control was defined as A1c < 7.0% and < 9.0%, the lowest and highest A1c criteria from the
NCQA-HEDIS performance measures for diabetes care. The change in A1c from baseline to follow-up period was evaluated as a secondary outcome.
Statistical Analysis
All baseline and outcomes variables were descriptively analyzed by treatment intensification group. Continuous variables including age, morbidity index scores, A1c measures, number of days to treatment intensification, and the number of days to follow-up A1c were summarized using mean and standard deviation. Frequencies and percentages were reported for categorical variables that included gender, age over 65 years, geographic location (by state), type of insurance, baseline A1c > 9.0%, and grouped comorbidity index scores.
Statistical significance was tested for insulin versus noninsulin, GLP-1RA versus third OAD intensification, and across the 3 groups using either Student’s t-test or analysis of variance (ANOVA) for continuous variables and a chi-square test for categorical variables. When the expected number of patients in a cell of a 2-by-2 table was less than 5, the Fisher’s exact test was used for the categorical variable.
The odds of achieving A1c < 7.0% and < 9.0% with GLP-1RA intensification compared with intensification with a third OAD was assessed using a logistic regression model. Iterations of the model included baseline A1c < 9.0% versus ≥ 9.0% as a covariate, as well as with the cohort stratified by A1c < 9.0% versus ≥ 9.0%, in order to account for the influence of baseline glycemic control on treatment outcomes. Once adjusted for the baseline A1c measure, the model predictability based on Akaike Information Criteria (AIC) values and likelihood ratio test results did not improve with the inclusion of any additional covariates. To alleviate concerns of residual confounding, the odds ratio (OR) was further adjusted for age, gender, baseline treatment group, and CCI score in a multivariate regression model. Covariate selection between the CCI and DCSI scores was based on the AIC and Bayesian Information Criterion, where the lower score represents the better model fit.
Glycemic control could be influenced by the length of follow-up from the intensification to the outcome assessment, and length of follow-up type could differ by the class of agent prescribed to intensify therapy. To address this potential bias, the analytic cohort was further stratified by the time to follow-up A1c measurement from 60 to 120 days from the intensification and > 120 days after intensification. The 120-day cut-off was determined based on the median follow-up (i.e., 119 days) from the overall cohort. Therefore, the analysis maintained a similar number of patients between the 2 subcohorts and was identically powered for the 2 subcohorts.
Results
Cohort Extraction and Baseline Characteristics by Treatment Intensification
The analytic cohort included 1,226 patients who met the baseline inclusion criteria and had a record of treatment intensification and an outcome A1c measure. Of the cohort, 54.6% (n = 669) received MET + SU during the 1-year baseline period, and 28.3% (n = 347) received MET + DPP-4. Baseline treatment with MET + TZD and SU + DPP-4 accounted for 7.7% (n = 95) and 4.7% (n = 58) of the cohort, respectively (Table 1).
TABLE 1.
Baseline Characteristics by the Type of Treatment Intensification
| Insulina n = 295 | GLP-1RAb n = 199 | OADc n = 732 | P Value | |||
|---|---|---|---|---|---|---|
| 3 Arms | Insulin vs. Other | GLP-1RA vs. OAD | ||||
| Age, mean (SD) | 53.93 (11.22) | 52.24 (10.34) | 55.47 (9.98) | 0.22 | 0.25 | < 0.01 |
| < 65 (%) | 86.8 | 92.0 | 85.7 | 0.07 | 0.98 | 0.03 |
| ≥ 65 (%) | 13.2 | 8.0 | 14.3 | |||
| Male, gender, % | 55.3 | 51.8 | 62.7 | 0.07 | 0.37 | 0.04 |
| Geographic region | 0.36 | 0.78 | 0.15 | |||
| Utah (%) | 95.3 | 95.0 | 95.1 | |||
| Idaho (%) | 3.4 | 2.0 | 3.7 | |||
| Other (%) | 1.0 | 3.0 | 1.4 | |||
| Insurance line | 0.06 | 0.21 | 0.04 | |||
| Commercial (%) | 86.4 | 94.5 | 88.3 | |||
| Medicare (%) | 10.2 | 4.5 | 9.6 | |||
| Medicaid (%) | 3.4 | 1.0 | 2.2 | |||
| Baseline A1c (%) | ||||||
| Mean (SD) | 9.55 (1.97) | 8.42 (1.27) | 8.49 (1.39) | < 0.01 | < 0.01 | 0.48 |
| A1c ≥ 7.0% to < 9.0% | 46.8 | 72.9 | 72.7 | < 0.01 | < 0.01 | 1.00 |
| A1c ≥ 9.0% | 53.2 | 27.1 | 27.3 | |||
| CCId score, mean (SD) | 1.90 (1.31) | 1.68 (1.21) | 1.66 (1.10) | < 0.01 | 0.01 | 0.76 |
| 1 (%) | 54.9 | 61.8 | 63.0 | 0.11 | 0.02 | 0.83 |
| 2 (%) | 22.0 | 23.6 | 21.2 | |||
| 3 (%) | 10.8 | 6.5 | 7.9 | |||
| ≥ 4 (%) | 12.2 | 8.0 | 7.9 | |||
| DCSId score, mean (SD) | 0.79 (1.29) | 0.60 (1.04) | 0.59 (1.02) | 0.01 | 0.02 | 0.89 |
| 0 (%) | 62.0 | 64.3 | 65.3 | 0.08 | 0.01 | 0.98 |
| 1 (%) | 16.6 | 21.1 | 19.8 | |||
| 2 (%) | 10.2 | 9.0 | 9.0 | |||
| ≥ 3 (%) | 11.2 | 5.5 | 5.9 | |||
| Baseline treatment | < 0.01 | < 0.01 | 0.32 | |||
| MET +SU (%) | 65.4 | 54.8 | 50.1 | |||
| MET + DPP-4 (%) | 22.7 | 26.1 | 31.1 | |||
| MET + TZD (%) | 4.1 | 8.5 | 9.0 | |||
| SU + DPP-4 (%) | 5.1 | 6.5 | 4.1 | |||
| Other (%) | 2.7 | 4.0 | 5.6 | |||
aInsulin refers to treatment intensification with basal or biphasic insulin.
bGLP-1RA refers to treatment intensification with GLP-1RA without basal or biphasic insulin.
cOAD refers to treatment intensification with a third OAD without injectable antidiabetic agent.
dIndividual comorbid conditions are presented in Appendix B (available in online article).
A1c = hemoglobin A1c; CCI = Charlson Comorbidity Index; DCSI = Diabetes Complication Severity Index; DPP-4 = dipeptidyl peptidase 4 inhibitor; GLP-1RA = glucagon-like peptide-1 receptor antagonist; MET = metformin; OAD = oral antidiabetic agent; SD = standard deviation; SGLT2 = sodium-glucose cotransporter inhibitor; SU = sulfonylurea; TZD = thiazolidinedione.
Treatment intensification with insulin was observed in 295 (24.1%) patients, while 199 (16.2%) patients were prescribed a GLP-1RA, and 732 (59.7%) were prescribed a third OAD to intensify therapy (Table 1). GLP-1RA recipients were slightly younger with a mean age of 52.2 ± 10.3 years versus those receiving insulin (53.9 ± 11.2 years) or a third OAD (55.5 ± 10.0 years). The CCI and DCSI scores were significantly higher in the insulin group (CCI: 1.90 ± 1.31, P < 0.01; DCSI: 0.79 ± 1.29, P = 0.01) versus others.
There was no statistically significant difference in the comorbidity scores between the GLP-1RA (CCI: 1.68 ± 1.21; DCSI: 0.60 ± 1.04) and the third OAD (CCI: 1.66 ± 1.10; DCSI: 0.59 ± 1.02) groups. The number of days from treatment intensification to the follow-up A1c value was similar for the insulin, GLP-1RA, and OAD groups, with follow-up periods of 141.3, 138.5, and 145.0 days, respectively (Table 2).
TABLE 2.
A1c Outcomes, Number of Days to Treatment Intensification from Baseline A1c, and Number of Days to Follow-Up A1c from Treatment Intensification by Type of Treatment Intensification
| Insulin n = 295 | GLP-1RA n = 199 | OAD only n = 732 | P Value | |||
|---|---|---|---|---|---|---|
| 3 Arms | Insulin vs. Other | GLP-1RA vs. OAD | ||||
| A1c at baseline (mean, SD) | 9.56 (1.97) | 8.42 (1.27) | 8.49 (1.39) | < 0.01 | <0.01 | 0.48 |
| A1c at follow-up (60-365 days after treatment intensification; mean, SD) | 8.54 (1.89) | 7.80 (1.71) | 7.96 (1.54) | < 0.01 | < 0.01 | 0.20 |
| Change in A1c (mean, SD) | -1.02 (2.08) | -0.62 (1.59) | -0.52 (1.60) | < 0.01 | < 0.01 | 0.45 |
| Baseline A1c < 9.0% (%) | 46.8 | 72.9 | 72.7 | < 0.01 | < 0.01 | 1.00 |
| Follow-up A1c < 7.0% (%) | 18.6 | 38.7 | 25.5 | <0.01 | < 0.01 | < 0.01 |
| Follow-up < 9.0% (%) | 65.4 | 78.4 | 79.9 | < 0.01 | < 0.01 | 0.73 |
| Days from baseline to treatment intensification (mean, SD) | 111.6 (112.2) | 121.9 (114.0) | 102.8 (108.4) | 0.13 | 0.52 | 0.03 |
| Days from treatment intensification to follow-up (mean, SD) | 141.3 (69.7) | 138.5 (71.0) | 145.0 (70.5) | 0.35 | 0.63 | 0.25 |
A1c = hemoglobin A1c; GLP-1RA = glucagon-like peptide-1 receptor antagonist; OAD = oral antidiabetic agent; SD = standard deviation.
More than half of the patients whose therapy was intensified with insulin had baseline A1c ≥ 9.0%, whereas the corresponding proportions of patients in the GLP-1RA and third OAD groups with baseline A1c ≥ 9.0% was less than 30% (Table 1). This accounts for the predominantly higher mean baseline A1c in patients receiving insulin (9.55%) versus the GLP-1RA (8.42%) or third OAD (8.49%) intensification groups (P < 0.01). The number of days to treatment intensification was not statistically different (P = 0.13, ANOVA) when the analysis was performed across the insulin (111.6 days), GLP-1RA (121.9 days), and third OAD groups (102.8 days; Table 2). However, the comparison limited to the 2 noninsulin groups showed that patients who received GLP-1RA had significantly longer time to receive the intensification than third OAD group (P = 0.03; Table 2).
Outcomes After Treatment Intensification
In general, glycemic control in the study cohort improved from baseline to follow-up with 26.0% of the cohort achieving A1c < 7.0% at follow-up (60-365 days from the treatment intensification). The proportion of patients with A1c between 7.0% and 7.9% decreased from 40.4% to 31.6% from baseline to follow-up. Similarly, the proportion of patients with A1c between 8.0% and 8.9% dropped from 26.1% to 18.5%, and the proportion with A1c ≥ 9.0% changed from 33.5% to 23.8% during the same time period (Figure 2).
FIGURE 2.
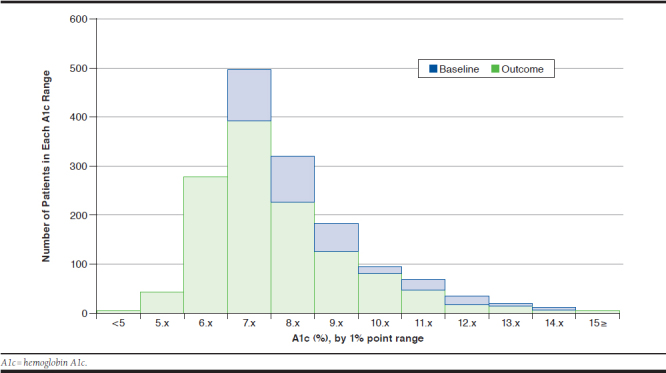
Distribution of Baseline and Follow-Up A1c
Patients whose treatment was intensified with insulin experienced a significantly larger A1c reduction (-1.02%) than those receiving a GLP-1RA (-0.62%) or third OAD (-0.52%; P < 0.01). However, the percentage of patients achieving A1c < 7.0% and < 9.0% at follow-up was significantly lower in patients with insulin intensification (18.6% and 65.4%, respectively) compared with the GLP-1RA (38.7% and 78.4%, respectively) and third OAD groups (25.5% and 79.9%), respectively. This finding reflects the higher average baseline A1c in the insulin intensification group.
There was no statistically significant difference in the follow-up A1c levels between GLP-1RA (7.80%) and third OAD (7.96%) patients or in A1c change from baseline. On the other hand, a significantly larger proportion of patients in the GLP-1RA group achieved A1c < 7.0% compared with the patients in the third OAD group (38.7% vs. 25.5%, P < 0.01; Table 2). GLP-1RA patients were also significantly more likely to achieve A1c < 7.0% at follow-up than third OAD patients, specifically when the analysis was limited to those with a baseline A1c < 9.0% (OR = 2.29, 95% CI = 1.56-3.37; Table 3).
TABLE 3.
Odds of Achieving A1c < 7.0 and < 9.0 for GLP-1RA Versus Third OADs per Multivariable Logistic Regression Analyses
| Outcome | By Baseline A1c | Time to Outcome A1c | ||
|---|---|---|---|---|
| 60-365 Days, n=1,226 OR (95% CI) | 60-120 Days, n=620 OR (95% CI) | 121-365 Days, n=606 OR (95% CI) | ||
| A1c < 9.0% | Alla | 2.05 (1.45-2.90) | 2.46 (1.11-5.47) | 0.48 (0.28-0.83) |
| Allb | 2.28 (1.55-3.36) | 2.63 (0.89-7.76) | 0.67 (0.32-1.42) | |
| Baseline A1c ≥ 9.0% | 1.24 (0.53-2.87) | 2.62 (0.88-7.75) | 0.66 (0.31-1.41) | |
| Baseline A1c < 9.0% | 2.29 (1.56-3.37) | 2.75 (0.78-9.69) | 0.28 (0.11-0.68) | |
| A1c < 7.0% | Alla | 2.05 (1.45-2.90) | 2.03 (1.27-3.23) | 1.90 (1.13-3.18) |
| Allb | 2.28 (1.55-3.36) | 2.10 (1.26-3.50) | 2.20 (1.23-3.94) | |
| Baseline A1c ≥ 9.0% | 1.24 (1.24-2.87) | 2.07 (1.24-3.45) | 2.22 (1.24-3.98) | |
| Baseline A1c < 9.0% | 2.29 (1.56-3.37) | 1.87 (0.55-6.39) | 1.01 (0.30-3.45) | |
Note: All ORs were adjusted for age (< 65 vs. ≥ 65 years), gender, baseline treatment group, and Charlson Comorbidity Index score (1, 2, 3, or ≥ 4).
aModel also adjusted for baseline A1c ≥ 9.0% versus < 9.0%.
bModel also adjusted for baseline A1c ≥ 9.0% versus < 9.0% and an interaction term of baseline A1c and the intensification group.
A1c = hemoglobin A1c; CI = confidence interval; GLP-1RA = glucagon-like peptide-1 receptor antagonist; OAD = oral antidiabetic agent; OR = odds ratio.
The effect of insulin versus noninsulin maintained when the analysis was stratified by the time to follow-up (data not provided). In patients having the outcome A1c within 120 days, the insulin arm achieved a significantly larger A1c reduction (-1.24%) than those receiving a GLP-1RA (-0.95%) or third OAD (-0.69%; P < 0.01). The A1c reduction after 120 days from the insulin intensification (-0.78%) was significantly greater (P = 0.02) than the reduction after the GLP-1RA (-0.24%) or third OAD (-0.36%).
The outcome after the intensification with GLP-1RA versus OAD was influenced by the time to follow-up. The OR of achieving A1c < 9.0% or 7.0% consistently favored GLP-1RA for the 120-day follow-up, with the point estimates between 1.87 and 2.75. In patients having the outcome after 120 days OAD, the ORs of GLP-1RA versus OAD for the outcome A1c < 9.0% were negative and statistically significant, specifically in patients with a baseline A1c < 9.0% (OR = 0.28, 95% CI = 0.11-0.68; Table 3). On the other hand, in patients with a baseline A1c ≥ 9.0%, GLP-1RA was associated with better odds of the outcome A1c < 7.0% than OADs after 120 days (OR = 2.22, 95% CI = 1.24-3.98; Table 3).
Discussion
This study identified that intensification of diabetes therapy improved glycemic control outcomes in patients with suboptimally controlled glycemia (A1c ≥ 7.0%) on 2 OADs. The proportion of patients with A1c < 7.0% increased at follow-up, while those with A1c between 7.0% and 9.0% or over 9.0% declined. Patients whose treatment was intensified with insulin had a greater mean A1c reduction than those receiving a GLP-1RA or a third OAD. However, a smaller proportion of patients in the insulin group achieved A1c < 7.0% and < 9.0% at follow-up, presumably because of higher baseline A1c levels. In general, our results are consistent with previous studies that showed a controlled A1c outcome after treatment intensification.14,15,18 Specifically, the results are comparable in that patients who received insulin for the treatment intensification achieved a substantial drop in A1c.18
The study findings also indicate that patients and providers turn to oral agents more often than either insulin or a GLP-1RA. The proportion of patients in our study who continued to use OADs (60%) was similar to a previous study.18 The choice to use insulin was presumably determined by the baseline A1c, with a difference in baseline A1c between insulin versus noninsulin groups exceeding 1% point. Before treatment intensification, more than half of the patients in the insulin intensification group were classified as “poorly controlled” at baseline, based on the NCQA comprehensive diabetes care recommendation.7 At follow-up, this proportion was decreased to 34.6% in patients receiving insulin. The insulin group also experienced a clinically substantial reduction in A1c (over 1% point), reflecting insulin’s potency when dosed appropriately. However, regression and stratified analyses suggested that confounding by baseline A1c was considerable and that we were unable to fully control for baseline diabetes severity. Thus, our results comparing intensification with insulin versus GLP-1RA or OADs in our target population may be biased. Nonetheless, the significant and substantial A1c reduction from baseline to follow-up with insulin therapy sufficiently demonstrates the relative benefit of insulin intensification in patients whose glycemia is poorly or not optimally controlled with a regimen of 2 OADs.
GLP-1RA, a relatively newer strategy compared with insulin or OADs in general, accounted for 16% of the treatment intensifications. Patients whose treatment was intensified with a GLP-1RA tended to be younger and female with commercial coverage than patients whose treatment was intensified with a third OAD. When it comes to the other clinical characteristics, however, there was no significant difference in the baseline A1c or comorbidity profiles between the GLP-1RA and third OAD cohorts.
Among the noninsulin patients, A1c reduction with intensification did not differ between GLP-1RA and a third OAD nor the odds of achieving A1c < 9.0%. However, the odds of achieving A1c < 7.0% favored GLP-1RA relative to a third OAD, particularly in patients with a baseline A1c between 7.0% and 9.0%. The association between intensification with GLP-1RA versus an OAD was further influenced by the time to follow-up A1c. For example, GLP-1RA was associated with higher odds of having outcome A1c < 7.0, as well as A1c < 9.0 within 120 days from the intensification across the different levels of A1c at baseline. The lower A1c with the use of GLP-1RA did not replicate in patients with baseline A1c ≥ 9.0% and whose follow-up A1c occurred more than 120 days after intensification.
Multiple prospective studies have identified more favorable glycemic control outcomes with GLP-1RA versus OADs, including MET, SU, and TZD. However, only a limited number of studies demonstrated the superiority of GLP-1RA over OADs in a real-world setting.21 Our study generally supports the use of GLP-1RA as third-line therapy after failure on 2 OADs based on overall treatment goals and patient characteristics. However, our mixed findings from the subgroup analysis demonstrated that the benefits of GLP-1RA may be greatest in patients with higher baseline A1c and whose glycemic control is monitored per treatment guidelines, which suggest that A1c should be measured every 90 days in patients with inadequate glycemic control and after treatment changes.22
An important finding of this study is that even with intensification, there was an average delay of 3.5 months between baseline A1c > 7.0% and treatment intensification. Practice recommendations encourage timely intensification when treatment fails to reduce the risk of poor outcomes, thus, managed care organizations have an opportunity to help improve patient care and outcomes by encouraging timely intensification.16 Successful interventions will need to address barriers to treatment intensification. While this study was not designed to assess these barriers, this topic is worthy of further investigation.15
In recognizing the value of optimizing diabetes care, over 90% of U.S. health plans have adopted the NCQA-HEDIS measures, including comprehensive diabetes care measures.23 In addition to recommended processes of care for diabetes (e.g., annual eye and foot exams and A1c testing), the comprehensive diabetes care set includes A1c outcomes as a measure of diabetes care performance for health plans overall, as well as for providers. As a further incentive to improve diabetes outcomes and reduce costs, public and private payers are basing a component of provider reimbursement on diabetes quality measures performance, such as foot and eye screenings on aregular basis.24 Further, the Centers for Medicare & Medicaid Services (CMS) assesses A1c < 9.0% as a performance measure in the Quality Payment Program for accountable care organizations and group practices, as well as in the Medicare Star Quality Rating System.24-26 Thus, high performance on diabetes outcomes-based quality measures is an important goal for health plans and is considered when prioritizing patient interventions, such as targeting patients with poorly controlled diabetes (A1c ≥ 9.0%) for education and treatment intensification.
While there is solid trial-based evidence supporting the use of diabetes agents and informing place in therapy for a given patient, outcomes are not regularly reported in a way that can shed light on whether interventions and treatment strategies will have a positive influence on performance to diabetes care measures. This study has elucidated the outcomes of treatment intensification in patients whose A1c levels failed to attain optimal control after therapy with 2 OADs from a quality measures perspective. Thus, the information from this study will give health plans and providers information on the value of treatment intensification in terms of improving patient outcomes and improving diabetes care performance in the eyes of employers and CMS as the ultimate health care purchasers and payer.
Limitations
Any interpretation of this study is accompanied by study limitations. First, we used health plan claims data that are augmented by A1c laboratory records. We did not have access to additional clinical data or information on patient behaviors that could influence treatment selection and patient outcomes (e.g., weight/body mass index, blood pressure, and adherence to diet and exercise recommendations). This limitation is partially addressed by a robust linkage of claims and medical records data.
Second, we were unable to completely control for differences in baseline A1c between insulin and the other treatment intensification groups. Future retrospective studies may consider matching techniques to more effectively isolate the effect of treatment on outcomes, but such techniques may introduce selection bias and reduce generalizability.
Third, treatment intensification was determined by a claim for a new class of diabetes medication, but we did not determine if the newly prescribed class represented an add-on or a switch in therapy. We also did not identify dose escalations as an alternate form of treatment intensification. In addition, we did not assess medication compliance, which could affect treatment selection and outcomes and possibly be a determinant of time to intensification.
Fourth, we limited follow-up assessment to the first A1c measure 60-365 days after treatment intensification versus using all available follow-up A1c data. Our future research will consider the longitudinal changes in A1c after the treatment intensification to assess time to goal and sustainability of goal attainment by treatment intensification strategy.
Finally, this study was subject to general limitations in the use of claims data, including the potential for miscoding, unmeasured confounders, and situations when medications are not reimbursed through the health plan, such as medication samples and cash purchases of low-cost generics.
Conclusions
This study provided insight into current treatment intensification patterns for patients with T2DM whose regimen is intensified after failing to attain glycemic control while on 2 OADs. We identified that treatment intensification is associated with improved glycemic control, as expected. Further, improved glycemic control translates to better performance relative to diabetes quality measures in this study population. Health plans should encourage appropriate treatment intensification in patients with poorly controlled diabetes while on multiple oral agents. Future research to provide data on glycemic control sustainability and barriers to treatment intensification will help to optimize health plan efforts to reduce therapeutic inertia.
APPENDIX A. Cohort Extraction Flowchart
APPENDIX B. Comorbidities Based on Diagnoses Coding Used in Calculating Charlson Comorbidity Index and Diabetes Complication Severity Index Scores
| Insulina n = 295, % | GLP-1RAb n = 199, % | OADc n = 732, % | P Value | |||
|---|---|---|---|---|---|---|
| 3 Arms | Insulin vs. Other | GLP-1RA vs. OAD | ||||
| Comorbidities in CCI 20 | ||||||
| History of myocardial infarction | 1.7 | 2.0 | 1.8 | 0.95 | 1.00 | 0.77 |
| Congestive heart failure | 5.1 | 2.5 | 3.8 | 0.34 | 0.30 | 0.50 |
| Peripheral vascular disorder | 2.0 | 1.5 | 2.0 | 0.92 | 1.00 | 0.78 |
| Cerebrovascular disorder | 2.4 | 1.5 | 1.4 | 0.51 | 0.29 | 0.75 |
| Dementia | 0.0 | 0.0 | 0.5 | 0.49 | 0.58 | 0.58 |
| Pulmonary disorder | 12.9 | 13.6 | 11.1 | 0.52 | 0.61 | 0.39 |
| Rheumatic | 1.4 | 1.0 | 1.1 | 0.93 | 0.75 | 1.00 |
| Peptic ulcer | 1.0 | 0.0 | 0.8 | 0.45 | 0.45 | 0.35 |
| Hepatic disorder, mild | 6.8 | 7.0 | 7.4 | 0.95 | 0.88 | 1.00 |
| Diabetes with complication | 17.6 | 16.1 | 14.3 | 0.39 | 0.25 | 0.61 |
| Para/hemiplegia | 0.3 | 0.0 | 0.1 | 0.64 | 0.42 | 1.00 |
| Renal disorder | 9.5 | 5.0 | 5.7 | 0.05 | 0.02 | 0.83 |
| Cancer/malignancy | 8.5 | 5.0 | 3.8 | 0.01 | 0.00 | 0.58 |
| Hepatic disorder, moderate to severe | 0.3 | 0.5 | 0.1 | 0.35 | 0.56 | 0.38 |
| Metastatic cancer | 0.3 | 0.5 | 0.4 | 1.00 | 1.00 | 1.00 |
| HIV/AIDS | 0.0 | 0.0 | 0.0 | N/A | N/A | N/A |
| Comorbidities in DCSId,19 | ||||||
| Ophthalmic complication (any) | 6.1 | 5.0 | 4.8 | 0.67 | 0.47 | 1.00 |
| Ophthalmic complication (severe) | 1.0 | 0.5 | 1.2 | 0.73 | 0.57 | 0.57 |
| Nephropathy (any) | 14.2 | 8.5 | 9.8 | 0.06 | 0.03 | 0.68 |
| Nephropathy (severe) | 7.5 | 5.5 | 4.5 | 0.12 | 0.07 | 0.35 |
| Neuropathy | 19.7 | 18.6 | 16.0 | 0.31 | 0.24 | 0.44 |
| Cerebrovascular disease (any) | 1.7 | 1.5 | 1.2 | 0.74 | 0.57 | 0.73 |
| Cerebrovascular disease (severe) | 1.4 | 1.5 | 1.1 | 0.78 | 0.46 | 0.77 |
| Cardiovascular disease (any) | 10.2 | 8.5 | 11.5 | 0.47 | 0.84 | 0.30 |
| Cardiovascular disease (severe) | 4.7 | 3.5 | 4.6 | 0.77 | 0.81 | 0.50 |
| Peripheral vascular disease (any) | 5.1 | 3.0 | 2.3 | 0.07 | 0.04 | 0.61 |
| Peripheral vascular disease (severe) | 4.4 | 3.0 | 1.4 | 0.03 | 0.03 | 0.13 |
| Metabolic disease (any) | 1.4 | 0.5 | 0.5 | 0.40 | 0.23 | 1.00 |
| Metabolic disease (severe) | 1.0 | 0.5 | 0.3 | 0.45 | 0.22 | 0.70 |
aInsulin refers to treatment intensification with basal or biphasic insulin.
bGLP-1RA refers to treatment intensification with GLP-1RA without basal or biphasic insulin.
cOAD refers to treatment intensification with a third OAD without injectable antidiabetic agent.
dComplication (any), the number of patients having a record of the condition over the baseline that adds any point to the DSCI calculation; complication (severe), the number of patients having a record of the condition that needs to add 2 points to the DSCI calculation.
CCI = Charlson Comorbidity Index; DCSI = Diabetes Complication Severity Index; GLP-1RA = glucagon-like peptide-1 receptor antagonist; N/A = not applicable; OAD = oral antidiabetic agent.
REFERENCES
- 1.American Diabetes Association . Economic costs of diabetes in the U.S. in 2012. Diabetes Care. 2013;36(4):1033-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention . National diabetes statistics report, 2017: estimates of diabetes and its burden in the United States. 2017. Available at: https://www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf. Accessed January 8, 2019.
- 3.Fowler MJ. Microvascular and macrovascular complications of diabetes. Clin Diabetes. 2008;26(2):77-82. [Google Scholar]
- 4.Monami M, Adalsteinsson JE, Desideri CM, Ragghianti B, Dicembrini I, Mannucci E. Fasting and post-prandial glucose and diabetic complication: a meta-analysis. Nutr Metab Cardiovasc Dis. 2013;23(7):591-98. [DOI] [PubMed] [Google Scholar]
- 5.Stratton IM, Adler AI, Neil HA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321(7258):405-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shi L, Ye X, Lu M, et al. Clinical and economic benefits associated with the achievement of both HbA(1c) and LDL cholesterol goals in veterans with type 2 diabetes. Diabetes Care. 2013;36(10):3297-304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.National Committee for Quality Assurance . Comprehensive diabetes care. October 31, 2017. Available at: http://www.ncqa.org/report-cards/health-plans/state-of-health-care-quality/2017-table-of-contents/diabetes-care. Accessed January 8, 2019.
- 8.Dickerson LM, Ables AZ, Everett CJ, et al. Measuring diabetes care in the national interdisciplinary primary care practice-based research network (NIPC-PBRN). Pharmacotherapy. 2011;31(1):23-30. [DOI] [PubMed] [Google Scholar]
- 9.Planas LG, Crosby KM, Farmer KC, Harrison DL. Evaluation of a diabetes management program using selected HEDIS measures. J Am Pharm Assoc (2003). 2012;52(6):e130-38. [DOI] [PubMed] [Google Scholar]
- 10.Ragucci KR, Fermo JD, Wessell AM, Chumney EC. Effectiveness of pharmacist-administered diabetes mellitus education and management services. Pharmacotherapy. 2005;25(12):1809-16. [DOI] [PubMed] [Google Scholar]
- 11.Cavero-Redondo I, Peleteiro B, Álvarez-Bueno C, Rodriguez-Artalejo F, Martínez-Vizcaíno V. Glycated haemoglobin A1c as a risk factor of cardiovascular outcomes and all-cause mortality in diabetic and non-diabetic populations: a systematic review and meta-analysis. BMJ Open. 2017;7(7):e015949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ali MK, Bullard KM, Saaddine JB, Cowie CC, Imperatore G, Gregg EW. Achievement of goals in U.S. diabetes care, 1999-2010. N Engl J Med. 2013;368(17):1613-24. [DOI] [PubMed] [Google Scholar]
- 13.Pantalone KM, Wells BJ, Chagin KM, et al. Intensification of diabetes therapy and time until A1C goal attainment among patients with newly diagnosed type 2 diabetes who fail metformin monotherapy within a large integrated health system. Diabetes Care. 2016;39(9):1527-34. [DOI] [PubMed] [Google Scholar]
- 14.Fu AZ, Sheehan JJ. Treatment intensification for patients with type 2 diabetes and poor glycaemic control. Diabetes Obes Metab. 2016;18(9):892-98. [DOI] [PubMed] [Google Scholar]
- 15.Fu AZ, Sheehan JJ. Change in HbA1c associated with treatment intensification among patients with type 2 diabetes and poor glycemic control. Curr Med Res Opin. 2017;33(5):853-58. [DOI] [PubMed] [Google Scholar]
- 16.Garber AJ, Abrahamson MJ, Barzilay JI, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm-2018 executive summary. Endocr Pract. 2018;24(1):91-120. [DOI] [PubMed] [Google Scholar]
- 17.American Diabetes Association . Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes-2018. Diabetes Care. 2018;41(Suppl 1):S73-S85. [DOI] [PubMed] [Google Scholar]
- 18.Levin PA, Wei W, Zhou S, Xie L, Baser O. Outcomes and treatment patterns of adding a third agent to 2 OADs in patients with type 2 diabetes. J Manag Care Pharm. 2014;20(5):501-12. Available at: https://www.jmcp.org/doi/10.18553/jmcp.2014.20.5.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Glasheen WP, Renda A, Dong Y. Diabetes Complications Severity Index (DCSI)-update and ICD-10 translation. J Diabetes Complications. 2017;31(6):1007-13. [DOI] [PubMed] [Google Scholar]
- 20.Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130-39. [DOI] [PubMed] [Google Scholar]
- 21.Levin PA, Nguyen H, Wittbrodt ET, Kim SC. Glucagon-like peptide-1 receptor agonists: a systematic review of comparative effectiveness research. Diabetes Metab Syndr Obes. 2017;10:123-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.American Diabetes Association . Glycemic targets: standards of medical care in diabetes-2018. Diabetes Care. 2018;41(Suppl 1):S55-64. [DOI] [PubMed] [Google Scholar]
- 23.National Committee for Quality Assurance . HEDIS and performance measurement. February 20, 2018. Available at: http://www.ncqa.org/hedisquality-measurement. Accessed January 8, 2019.
- 24.American Diabetes Association . Standard of medical care in diabetes-2016. Diabetes Care. 2016;39(Suppl 1):S4-S5.26696680 [Google Scholar]
- 25.Centers for Medicare & Medicaid Services . Medicare Shared Savings Program quality measure benchmarks for the 2015 reporting year. 2015. Available at: https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/sharedsavingsprogram/Downloads/MSSP-QM-Benchmarks-2015.pdf. Accessed January 8, 2019.
- 26.Center for Medicare & Medicaid Services . Medicare 2018 Part C & D Star Ratings Technical Notes. Draft. September 6, 2017. Available at: https://www.cms.gov/Medicare/Prescription-Drug-Coverage/PrescriptionDrugCovGenIn/Downloads/2018-Star-Ratings-Technical-Notes-2017_09_06.pdf. Accessed January 24, 2019.


