Abstract
BACKGROUND:
Opioid-induced constipation (OIC), a common side effect of opioid treatment for chronic pain, affects patient health-related quality of life (HRQL) and may prompt some patients to lower the dose or alter adherence to their opioid medication, compromising pain relief. Although health care providers (HCPs) are aware of the potential for OIC, patients may not inform their HCPs of their OIC symptoms, and HCPs may not initiate conversation regarding OIC if their patients’ pain is controlled. Patients often try to address OIC symptoms on their own by using natural approaches or over-the-counter options. When OIC is discussed in an office visit, HCPs typically recommend conventional laxatives to relieve symptoms, but the efficacy of this approach is unproven and often suboptimal. In many areas of medicine, HCP perceptions of the impact of adverse effects of treatment on a patient’s HRQL do not align with the patient’s experience.
OBJECTIVES:
To (a) describe HCP-reported understanding of his or her patients’ experiences with OIC and (b) evaluate the level of agreement or discordance in perception between patients and their HCPs of OIC’s impact on clinical outcomes.
METHODS:
This was a prospective, longitudinal, observational cohort study conducted in the United States, Canada, Germany, and the United Kingdom (NCT01928953) in patients aged 18 to 85 years who had been receiving daily opioid therapy for ≥ 4 weeks for chronic noncancer pain with presence of OIC in the past 2 weeks. Data were collected from retrospective chart reviews, HCP questionnaires, and web-based patient surveys. Eligible patients enrolled online and completed the Patient Assessment of Constipation-Symptoms, the Work Productivity and Activity Impairment Questionnaire-Specific Health Problem, the EuroQOL 5 Dimensions, and the Global Assessment of Treatment Benefit, Satisfaction, and Willingness to Continue standardized questionnaires. The patient-reported component included 1 baseline survey and 8 follow-up surveys over 24 weeks. HCPs completed a web-based survey at baseline and at week 24 to assess their perceptions of OIC burden, treatment patterns, laxative use, and overall treatment satisfaction. The correspondence of patient- and HCP-reported data was evaluated for all similar outcomes from these 2 databases.
RESULTS:
Patients (N = 489) reported a mean (SD) number of bowel movements (BMs) per week and spontaneous BMs per week of 3.7 (2.9) and 1.4 (2.3), respectively, at baseline. Most (87%) reported chronic pain of ≥ 2 years duration; 65% had used opioids for ≥ 2 years; and the mean pain score at baseline was 6.3, consistent with a moderate-to-severe pain population. Most (97%) patients at baseline reported any gastrointestinal-related symptom of at least moderate intensity, with 82% reporting the same intensity of any symptom at week 24. Of the 405 patients who had seen their HCPs in the past month, 63% reported that they had spoken to their HCPs about constipation, and 62% reported that their HCPs had asked them if they had constipation in the past month. The proportion of agreement between HCPs and their patients on the presence of constipation at baseline was 61%. Similar average pain ratings between HCPs and patients (5.9 vs. 6.0) at week 24 suggested clear communication regarding the level of pain experienced by the patient; however, OIC symptoms, laxative use and effectiveness, and impact of OIC on pain management and HRQL were not fully appreciated by HCPs.
CONCLUSIONS:
The importance and severity of OIC are perceived differently by patients and their HCPs, a discordance that complicates pain management and demonstrates a need for greater communication. These disparate perceptions indicate a need for clinical education and coordination of care by HCPs to improve understanding and proactively manage OIC in patients with chronic noncancer pain.
What is already known about this subject
In the United States, patient surveys and medical record reviews suggest that 17%-67% of patients experience opioid-induced constipation (OIC) as an adverse event of their opioid pain medication.
In the majority of these patients, the symptoms of OIC affect activities of daily living and health-related quality of life (HRQL) and prompt patients to decrease the dose or temporarily stop using their opioid medication to relieve their constipation.
Discordance between the perceptions of health care providers (HCPs) and patients is found in many areas of medicine and may compromise patient care and disease management.
What this study adds
This study investigated the perceptions of patients and HCPs about the burden of OIC with regard to symptoms, adequacy of pain management, use and effectiveness of laxative therapy, and activities of daily living in patients receiving opioids for chronic noncancer pain with the presence of OIC.
Similar average pain ratings between HCPs and patients (5.9 vs. 6.0) at week 24 suggested clear communication regarding the level of pain experienced by the patient.
However, OIC symptoms, laxative use and effectiveness, and the negative effects of OIC on pain management and HRQL were not fully appreciated by HCPs.
Effective communication between patients and their health care providers (HCPs) is recognized as essential for optimal patient outcomes, but it is often difficult to achieve in today’s time-constrained health care environment.1-3 Discordance between the perceptions of HCPs and patients is found in many areas of medicine and may compromise patient care and disease management. For example, in surveys carried out to determine the impact of gastroesophageal reflux disease (GERD) on health-related quality of life (HRQL), a disconnect was noted between patients and HCPs regarding treatment of GERD, with HCPs underestimating the use of over-the-counter therapies by their patients with GERD.4 Similarly, results obtained on HRQL questionnaires completed by HCPs and their patients with melanoma who were receiving adjuvant PEGylated interferon-α2b therapy showed that HCPs significantly underestimated the HRQL impairments that their patients were experiencing.5
Opioids are commonly used to treat chronic noncancer pain.6 Data from U.S. patient surveys and medical record reviews suggest that 17%-67% of patients experience constipation as an adverse effect of their opioid pain medication.7-9 The absence of general agreement on the magnitude of the problem is an indication that patients may have different perceptions of when opioid-induced constipation (OIC) becomes an issue for them. This lack of agreement further underscores the importance of better understanding patient-physician communication. Patients with OIC may experience abdominal discomfort, nausea, gas, decreased appetite, reflux, bloating, straining, incomplete evacuation, and hard bowel movements (BMs).10,11 In the majority (≥ 80%) of patients, these symptoms affect activities of daily living and HRQL,12 with individuals receiving opioids who developed OIC reporting significantly worse HRQL than those who did not develop OIC.13
Nonpharmacologic interventions, such as increasing fluid and dietary intake of fiber, are common first-line approaches for treating OIC but are rarely sufficient to manage the condition.11 Laxatives are often used to treat OIC, but they have limited efficacy.14,15 Laxatives exist in a variety of formulations, including surfactants that soften the stool, osmotic agents that retain water in the gastrointestinal (GI) tract, and stimulants that promote motor activity and the secretion of fluids in the GI tract.15,16 Bulk-forming laxatives may not be effective for the treatment of OIC because the receptors stimulated by the increased bulk are blocked by opioids.15 Safety and low cost render over-the-counter laxatives and natural remedies appropriate first choices for pharmacotherapy; however, they have no ability to block opioid actions at GI opioid receptors.15
The symptoms of OIC may prompt patients to decrease the dose or stop using their opioid medication to relieve their constipation.12,14 In the international, Internet-based Patient Reports of Opioid-Related Bothersome Effects (PROBE 1) survey, 33% of patients reported that they had skipped, decreased, or ceased use of opioids to facilitate having a BM.12 Thus, the inability to achieve a balance between pain relief and development of constipation impairs HRQL and compromises effective pain management.17 A better understanding of OIC or more active HCP participation in monitoring and managing OIC may help with this balance.
The objectives of this study were (a) to describe HCP-reported understanding of his or her patients’ experiences with OIC, including the symptoms experienced, adequacy of treatment, and adequacy of pain management among patients receiving chronic opioid therapy for chronic noncancer pain; and (b) to evaluate the level of agreement or discordance in perception between patients and their HCPs of OIC’s impact on clinical outcomes.
Methods
Study Design
This was a prospective, longitudinal, observational cohort study conducted in the United States, Canada, Germany, and the United Kingdom (NCT01928953). A detailed description of the methods of this study has been published.17 Briefly, patients with usual-care visits to primary care, pain, or other specialty clinics (neurology, orthopedics, rheumatology) were recruited. Data were collected from web-based patient surveys, retrospective chart reviews, and HCP questionnaires. Both patients and their respective HCPs completed surveys, and the responses on the HCP survey were based on HCP knowledge of his or her patients who completed the patient survey. The protocol and informed consent forms were reviewed and approved by institutional review boards and ethics committees to meet country- and site-specific requirements.
Patients
Patients aged 18 to 85 years who had been receiving daily opioid therapy for ≥ 4 weeks to treat chronic noncancer pain with presence of OIC in the 2 weeks before screening were eligible for enrollment. Patients were receiving a minimum total daily dose of 30 mg oral morphine or equianalgesic amounts of 1 or more other opioid therapies and were expected to continue on opioid therapy for ≥ 6 months. Presence of OIC was based on patient-reported symptoms. Patients were required to report laxative use if the number of BMs in the past 2 weeks was ≥ 3 per week, whereas those with < 3 BMs per week in the past 2 weeks were eligible regardless of reported laxative use. Patients who did not report laxative use were required to report at least 1 symptom of OIC (e.g., straining during BM, hard/lumpy stools, or sensation of incomplete emptying) in the past 2 weeks. Patients who were unable or who refused to provide informed consent, who were unable to use or were without access to a computer with Internet connectivity to complete the web-based survey, or who had a history of chronic constipation were excluded.
Assessments
Eligible patients enrolled online and completed web-based surveys to provide data regarding sociodemographic and clinical characteristics, treatment patterns, BMs, constipation history, laxative use, and patient-reported outcome measures, including the Patient Assessment of Constipation-Symptoms (PACSYM),18 with 5 additional symptom items to assess nausea, vomiting, flatulence, GERD, and headache/migraine. Other standardized questionnaires in the patient survey included the Work Productivity and Activity Impairment Questionnaire-Specific Health Problem,19 the EuroQOL 5 Dimensions,20 and the Global Assessment of Treatment Benefit, Satisfaction, and Willingness to Continue.21 The prospective, patient-reported component contained 1 baseline survey and 8 follow-up surveys over 24 weeks. Patients were nominally remunerated for their time to complete each survey. Retrospective chart reviews assessed patient medical history, pharmacotherapy (including opioid and laxative prescriptions), and health care resource utilization at 2 points in time—at baseline, which included the 12 months leading up to the baseline visit, and at the end of the 6-month prospective study period (week 24 visit), covering a total period of 18 months (Figure 1). HCPs completed an Internet-based questionnaire to assess their perception of OIC burden, symptoms, and treatment patterns for OIC, laxative use, and overall OIC treatment satisfaction of their patients at baseline and at week 24 for each patient who completed a baseline web-based survey.
FIGURE 1.
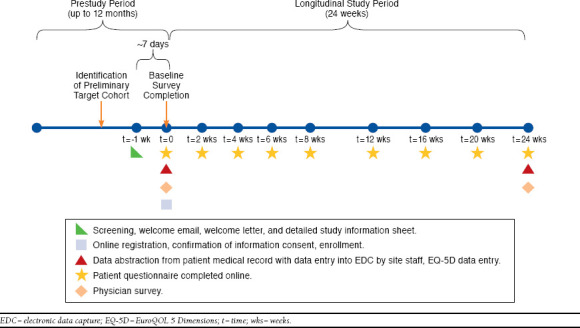
Study Design
Data Analysis
All analyses were performed with SAS software package 9.4 (SAS Institute, Cary, NC). Owing to the extensive nature of the objectives and number of analyses, descriptive statistics were applied. Summary measures such as means and standard deviations (SDs) for continuous variables and counts, frequencies, and percentages for categorical variables were reported; no statistical significance tests were applied. The correspondence of patient- and HCP-reported data was evaluated for all similar outcomes from these 2 databases, including OIC symptoms and treatment use. All data were analyzed without imputation for missing responses.
Results
Survey Metrics
Of the 617 patients initially recruited, 500 (81%) were enrolled and completed the patient baseline survey. Of those, 489 (98%) met criteria confirming the presence of OIC. Chart reviews were completed for 486 of 489 (99%) eligible patients at baseline and for 477 (98%) patients at week 24. HCP baseline and week 24 surveys were completed for 464 (95%) and 457 (94%) patients, respectively.
Patient Demographic and Clinical Characteristics and Baseline Constipation History
Demographic and clinical characteristics of the overall patient population are shown in Table 1. Most patients were female (62%) and white (85%), with a mean age of 53 years. More than half of the patients (62%) rated their overall health as fair or poor. Twenty-seven percent of patients were working full- or part-time, and 34% were unable to work because of disability. The most frequently reported pain conditions were back pain (77%) and joint pain (52%). The mean (SD) duration of chronic pain and the mean (SD) duration of chronic opioid use was 9.8 (8.9) years and 6.4 (6.3) years, respectively. Most (87%) patients reported chronic pain of ≥ 2 years in duration, and 65% had used opioids for ≥ 2 years. The mean pain score at baseline was 6.3, both for pain in the last 24 hours and for pain in the last 7 days, consistent with a moderate-to-severe pain population.
TABLE 1.
Demographic and Clinical Characteristics of the Overall Patient Population
| Patients (N = 489) | ||
|---|---|---|
| Male, % (n) | 37.8 | (185) |
| Age, years, mean [SD] | 52.6 | [11.6] |
| Race, % (n) | ||
| White | 84.9 | (415) |
| Black | 4.9 | (24) |
| Asian | 4.5 | (22) |
| Hispanic/Latino/Latin American | 1.8 | (9) |
| Other | 2.9 | (14) |
| Prefer not to state | 0.8 | (4) |
| Missing | 0.2 | (1) |
| Marital status, % (n) | ||
| Single, never married | 14.7 | (72) |
| Married | 52.4 | (256) |
| Living together, not married | 5.3 | (26) |
| Common-law partnership | 2.2 | (11) |
| Divorced | 12.9 | (63) |
| Separated | 1.4 | (7) |
| Widowed | 4.5 | (22) |
| Prefer not to answer | 5.3 | (26) |
| Missing | 1.2 | (6) |
| Type of chronic pain, % (n)a | ||
| Back pain | 76.7 | (375) |
| Joint pain | 51.5 | (252) |
| Fibromyalgia | 14.5 | (71) |
| Headache or migraine | 16.0 | (78) |
| Osteoarthritis | 19.4 | (95) |
| Rheumatoid arthritis | 8.4 | (41) |
| Neuralgia | 23.5 | (115) |
| Pain syndrome | 33.1 | (162) |
| Other | 15.5 | (76) |
| Employment status, % (n) | ||
| Employed, full-time | 19.0 | (93) |
| Employed, part-time | 8.0 | (39) |
| Studying full- or part-time | 1.2 | (6) |
| Both employed and studying | 0.6 | (3) |
| Unable to work because of disability or handicap | 34.2 | (167) |
| Unemployed | 4.5 | (22) |
| Not currently working (e.g., full-time homemaker) | 3.7 | (18) |
| Retired | 21.1 | (103) |
| Other | 1.4 | (7) |
| Prefer not to answer | 6.1 | (30) |
| Missing | 0.2 | (1) |
| Body mass index, kg/m2, mean [SD] | 29.7 | [7.3] |
| Health rating, % (n) | ||
| Excellent | 1.4 | (7) |
| Very good | 6.7 | (33) |
| Good | 29.7 | (145) |
| Fair | 42.3 | (207) |
| Poor | 19.2 | (94) |
| Missing | 0.6 | (3) |
| Duration of chronic pain, years, mean [SD] | 9.8 | [8.9] |
| < 2 years, % (n) | 13.3 | (65) |
| ≥ 2 years, % (n) | 86.7 | (424) |
| Duration of opioid medication, years, mean [SD] | 6.4 | [6.3] |
| < 2 years, % (n) | 23.9 | (117) |
| ≥ 2 years, % (n) | 65.4 | (320) |
| Unknown, % (n)b | 10.6 | (52) |
| Pain severity rating | ||
| Average pain in last 24 hours, mean [SD] | 6.3 | [1.8] |
| Average pain in last 7 days, mean [SD] | 6.3 | [1.7] |
aPatients could report more than 1 type of chronic pain.
bIncludes participants who reported “I don’t know” for duration of opioid medication; patients reported duration of pain of ≥ 2 years (n = 49) and < 2 years (n = 3). The mean (SD) number of years of chronic pain duration for this group was 12.6 (10.1).
SD = standard deviation.
The mean (SD) number of BMs per week was 3.7 (2.9), and the mean (SD) number of spontaneous BMs per week was 1.4 (2.3) (Table 2). On the basis of PAC-SYM and additional symptom assessments, 97% of patients at baseline reported any symptom of at least moderate intensity that remained high throughout follow-up, with 82% reporting the same intensity of any symptom at week 24. Laxative use was sufficient (defined as use of ≥ 1 laxative ≥ 4 times over the past 2 weeks) in 48%, insufficient in 25%, and absent in 27% of patients. Of the 405 patients who had seen their HCPs in the past month, 63% reported that they had spoken to their HCPs about constipation, and 62% reported that their HCPs had asked them if they had constipation in the past month. Results did not vary appreciably across countries (data not shown), although fewer patients in the United Kingdom discussed their OIC with their HCPs (46%) as opposed to patients in other countries (United States, 64%; Canada, 52%; Germany, 76%). Of the 149 participants who did not speak to their HCPs about constipation in the past month, more than half (58%) had not done so because they had spoken to their HCPs about constipation in the past, whereas 13% had not done so because of concerns that their pain medication would be changed or the dose would be reduced. In addition, 8% of patients reported that they adjusted their opioid pain medication to have a BM, with the majority experiencing a worsening of pain as a result. Of these patients, 49% temporarily interrupted and 43% reduced the use of their pain medication.
TABLE 2.
Patient-Reported Constipation History and Opioid Use Management at Baseline
| Overall (N = 489) | ||
|---|---|---|
| Over the past 2 weeks, how many BMs were spontaneous? | N = 488 | |
| Mean [SD] | 1.4 | [2.3] |
| Ideally, how often would you like to have BMs? % (n) | ||
| More than once a day | 12.7 | (62) |
| Once a day | 69.9 | (342) |
| Every other day | 10.4 | (51) |
| 2-3 times per week | 5.9 | (29) |
| Once per week | 1.0 | (5) |
| If you saw your HCP in the last month, did you speak about constipation? | N = 405 | |
| Yes, % (n) | 63.2 | (256) |
| If you saw your HCP in the last month, did your HCP ask if you were having constipation in the last month? | N = 405 | |
| Yes, % (n) | 61.8 | (251) |
| If you did not speak to your HCP about constipation, why not? % (n) | N = 149 | |
| Constipation not a problem | 4.0 | (6) |
| Embarrassed | 9.4 | (14) |
| Ran out of time | 4.7 | (7) |
| Concerned about need to change/reduce pain medication | 13.4 | (20) |
| Discussed with doctor in the past | 58.4 | (87) |
| Other | 10.1 | (15) |
| Since starting opioid medication, have you been experiencing constipation or worsening of current constipation? % (n) | ||
| Yes | 93.7 | (458) |
| Missing | 0.2 | (1) |
| How long after did you begin experiencing constipation? % (n) | N = 458 | |
| 1 day after beginning | 4.4 | (20) |
| Within first week | 34.7 | (159) |
| 2-4 weeks | 26.0 | (119) |
| 5-8 weeks | 6.1 | (28) |
| > 8 weeks | 5.2 | (24) |
| Don’t remember | 23.6 | (108) |
| In past 7 days, did you change how you used your opioid medication(s) so that you could have BMs? % yes (n)a | ||
| If yes, how change,b n (%) | 7.6 | (37) |
| No longer take my pain medication | 2.7 | (1) |
| Reduced how much of my pain medication I use | 43.2 | (16) |
| Temporarily interrupted the use of pain medication | 48.6 | (18) |
| Switched to a different pain medication | 10.8 | (4) |
| Other | 2.7 | (1) |
a8% of responders.
bParticipants were able to report more than 1 response.
BM = bowel movement; HCP = health care provider; SD = standard deviation.
HCP Awareness of Patients’ OIC and Impact of OIC on Patients’ Lives: Baseline Results
Overall, 77% of HCPs reported that they discussed OIC symptoms and concerns with patients; however, only 65% were actually aware that the patients met criteria for OIC and only 58% of HCPs reported that patients complained of having too few BMs per week (Table 3). A subset of HCPs responded to additional follow-up questions about the specific number of BMs patients experienced per week and what number of BMs per week a patient considered too few. Of those responding, HCPs reported that their patients experienced 2.5 BMs per week on average, which is 1 BM per week fewer, on average, than what patients actually reported. Additionally, 17% of HCPs were unaware of the number of BMs a patient experienced per week, and 39% were unaware of how many BMs were considered too few, consistent with the reported lack of awareness by HCPs that patients met criteria for OIC. Of those who did provide a response, HCPs reported that 3.5 BMs per week on average were considered too few for patients, which is still under the desired 1 BM or more per day that 83% of patients reported would be ideal. Almost half (48%) of HCPs responded that treatments to relieve OIC were moderately to completely adequate. Forty percent of HCPs reported that they believed that OIC did not have an effect on patient quality of life with regard to activities of daily living.
TABLE 3.
HCP-Reported Awareness of Patients’ Bowel Symptoms and OIC at Baseline
| Overall (N = 464) a | ||
|---|---|---|
| Knows patient is experiencing OIC, % (n) | 65.1 | (302) |
| Patient discussed OIC symptoms/concerns, % (n) | ||
| Yes | 76.9 | (357) |
| No | 22.8 | (106) |
| Missing | 0.2 | (1) |
| Patient’s pain level | N = 404 | |
| Mean [SD] | 6.3 | [1.8] |
| Patient complained about having too few BMs per week, % (n) | 58.4 | (271) |
| Typical number of BMs this patient has per week | N = 193 | |
| Mean [SD] | 2.5 | [1.4] |
| Don’t know, % (n) | 16.8 | (78) |
| Number of BMs per week this patient considers to be too few | N = 90 | |
| Mean [SD] | 3.5 | [2.1] |
| Don’t know, % (n) | 39.0 | (181) |
| How does OIC affect this patient’s quality of life? % (n) | ||
| Life can be carried on as usual | 39.7 | (184) |
| Quality of life is restricted | 34.1 | (158) |
| Lifestyle is highly affected | 6.5 | (30) |
| Don’t know | 19.2 | (89) |
| Missing | 0.6 | (3) |
aNumber of HCPs responding to the survey.
BM = bowel movement; HCP = health care provider; OIC = opioid-induced constipation; SD = standard deviation.
Concordance Between Patient- and HCP-Reported OIC and Pain Symptoms at Baseline
The proportion of agreement between HCPs and patients on the presence of constipation at baseline was 61%. The most commonly reported symptoms rated as moderate to severe in intensity by patients were straining/squeezing to pass a BM (83%), BMs too hard (75%), flatulence (69%), incomplete BM (69%), bloating in abdomen (69%), painful BM (67%), and abdominal discomfort (64%); however, the proportion of HCPs who had observed or discussed each OIC symptom reported by the patient as being of moderate or greater severity ranged from 1% to 41% (Figure 2).
FIGURE 2.
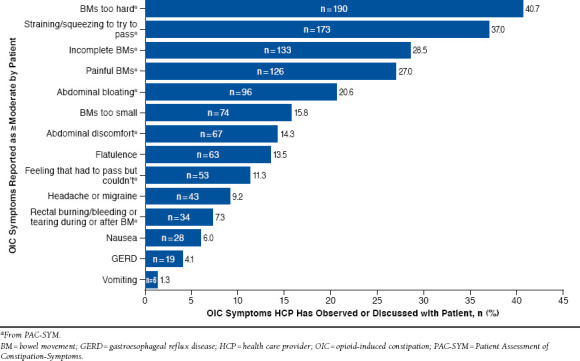
Agreement of Patient- and HCP-Reported OIC Symptoms at Baseline: Overall
In contrast with OIC symptomology, HCPs were well aware of patients’ pain severity, with both patients and HCPs reporting an average pain rating of 6.3. Nevertheless, HCPs overestimated how well patients managed their pain and OIC concurrently. Although 49% of patients reported that OIC interfered moderately or completely with the ability of their opioid medication to adequately control their pain, a majority (73%) of HCPs reported that their patients’ pain was mostly or adequately managed (Figure 3). This pattern continued at week 24, with 46 participants (45%) reporting moderate to complete interference of OIC with their pain management, and 73% of HCPs reporting their patients’ pain was mostly or adequately managed. The proportion of agreement between HCPs and patients on the current use of laxatives was 55%, and the proportion of agreement on the level of benefit and satisfaction from laxatives varied between patients and HCPs. Among the 305 patients who reported little to no benefit of laxative treatment at baseline, 53% of their HCPs reported moderate-to-complete satisfaction with laxative treatment at baseline. A similar pattern was present at week 24, with 60% of HCPs overestimating treatment benefit. For satisfaction, 99 patients (51%) reported they were a little to very dissatisfied with laxative treatment at baseline, with 54% of HCPs overestimating the baseline level of satisfaction of their patients as moderate-to-complete satisfaction. A similar pattern was observed at week 24, when 62% of HCPs overestimated their patients’ satisfaction.
FIGURE 3.
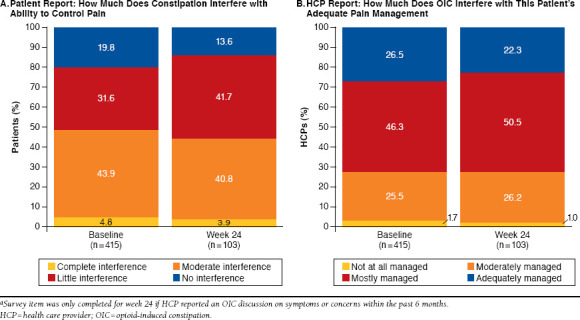
Discordance Between Patient and HCP Reports of OIC Interference with Adequate Pain Managementa
Results at Week 24 Follow-up
Overall, 72% (n = 330) of HCPs reported that they had a follow-up visit or other consultation with their patients since the baseline visit. The patient-reported mean pain score was similar to that estimated by HCPs (5.9 vs. 6.0) at week 24 for pain in the last 24 hours and for pain in the last 7 days. Of the 330 HCPs who had a follow-up visit, 38% (n = 125) reported having discussed OIC symptoms and concerns with patients. Of these, HCPs responded that 75% of patients had taken laxatives to manage their OIC since the baseline survey and that about half (52%) had achieved moderate-to-complete satisfaction with laxative treatment. Almost half (46%) of the HCPs responded that treatments to relieve OIC were moderately to completely adequate. About half (54%) of HCPs expressed that OIC had little to no interference with the patients’ reports of HRQL. Of those visits in which the patients discussed OIC symptoms/concerns (n = 125), the most common treatment recommendations were to decrease the frequency of pain medication (39% of HCPs, n = 49) or to take an opioid holiday (47% of HCPs, n = 59).
Discussion
In this study, HCP-reported understanding of the patient experience with OIC and pain management when receiving chronic opioid therapy for chronic noncancer pain was assessed. Analyses of consistency between patient and HCP responses on web-based surveys were interpreted as evidence of limited or incomplete HCP awareness of the patient disease burden of OIC. Identical average pain ratings by HCPs and patients at baseline and similar average pain ratings by HCPs and patients at week 24 indicated that there appears to be clear communication between HCPs and patients regarding the level of pain experienced by the patients; however, OIC symptoms (at baseline), laxative use, satisfaction and benefit, and the impact of OIC on the impairment of pain management are not fully appreciated by HCPs. A potential factor playing into this disparity is a lack of communication about OIC between HCPs and patients that may contribute to a lack of awareness of the impact of OIC by HCPs and ultimately patient dissatisfaction. However, identification of reasons for this disconnect would be a topic for further health promotion research.
Although most HCPs reported that they discussed OIC symptoms and pain severity with their patients, many were unaware of their patients’ OIC symptom severity, level of laxative use, and limited benefits with laxative use, which increased somewhat during the 6 months of the study. Despite inclusion in a study of OIC, there appears to be very little advancement in the conversation between patients and HCPs and very little improvement in HCP insight into the disease burden of their patients. Thus, patients’ ability to concurrently manage their pain and OIC is commonly underestimated by HCPs.
The reasons for a lack of discussion of OIC symptoms may be related to both HCPs and patients. The HCPs may run out of time or believe the patients should initiate the conversation, and/or the patients may not mention OIC symptoms for fear of losing the opioid medication. There may be a lack of appreciation of the relationship between OIC and opioid use, a belief that OIC is a condition that should be self-managed and not shared with a physician, or embarrassment.
Patients in this survey experienced chronic pain for an average of 9 years and used opioids for an average of 6 years. The gap in communication may have widened after being in this state for so long. A more proactive approach to prophylaxis, in which patients with chronic pain are targeted to have a conversation with their HCPs about constipation at the initiation of opioid therapy, may be a way to close this gap. It is also possible that the availability of better prescription therapies for OIC may also bring patients closer to their HCPs.
A previously published analysis of baseline data from this study showed that OIC had a negative impact on patient activities of daily living, work productivity, overall HRQL, and, for some patients, ability to manage pain.17 Other studies have also shown that OIC causes patients to compromise their pain management to avoid constipation.13 In the current analysis, OIC interfered with patients’ pain management more than HCPs realized.
Discordance in patient-physician communication is not restricted to patients with chronic pain and OIC. For example, patient overreporting of symptom severity and underrating of HRQL compared with physician assessments has been observed in surveys of patients with cardiovascular disease, psoriatic arthritis, rheumatic diseases, and systemic sclerosis.22-25 In a longitudinal study in cancer patients, those with severe constipation scores were more likely than patients with mild or moderate constipation scores to initiate a discussion of their symptoms with a physician at first consultation.26 Studies in other indications point to a failure of physicians to initiate a conversation about disease symptoms and quality of life, race/ethnicity-related discordance between patients and physicians, and the presence of comorbid conditions24 (e.g., physical inactivity, depression, fatigue, GI symptoms) as possible predictors of discordance between patient-physician perceptions.23,25-28 However, further research is needed to better understand the reasons for the discordance between patient-physician perceptions in patients with OIC.
We hypothesized that differences may exist between patient and HCP perceptions of the burden of OIC on the symptom experience, the adequacy of pain management, the use and effectiveness of laxative therapy, and the day-to-day activities of patients receiving opioids for chronic noncancer pain.29
To improve patient care, the HCP treatment team may bridge this gap by including an assessment of OIC as part of routine appointments with patients receiving chronic opioid medication. Increasingly, patients are requested to complete brief questionnaires about their pain severity and other symptoms as part of the check-in process for routine-care visits. Inclusion of a single question to assess the presence of OIC may prompt physicians to follow up with patients about this issue. Clear treatment guidelines should be developed to establish best practices in the treatment of OIC when identified. A stepwise approach, with over-the-counter osmotic agents, surfactants, and/or stimulants as first-line therapy and prescription treatment options for those who do not respond to these therapies, should be considered. Clinical education and coordination of care by additional HCPs, including nursing staff, may add to the critical need to appreciate and proactively address the burden of disease. Development of a consensus definition of OIC and perhaps a validated assessment tool (i.e., patient-reported outcome) in OIC and empirically based treatment guidelines are areas of further research that could close the gap. Chronic pain management and treatment side effects, including OIC, present complex challenges for patients and their providers.
Limitations
This study was subject to limitations, given that it involved patient self-reports and may have been influenced by cultural factors, differences in clinical practice, and, in some cases, small sample size.
Conclusions
The importance and severity of OIC are perceived differently by patients and their HCPs, a discordance that complicates pain management and demonstrates a need for greater communication. These disparate perceptions indicate a need for clinical education and coordination of care by HCPs to improve understanding and proactively manage OIC in patients with chronic noncancer pain.
Acknowledgments
Medical writing services were provided by Stephanie Leinbach, PhD, and Erica Wehner, RPh, CMPP, Complete Healthcare Communications, Chadds Ford, Pennsylvania, with funding from AstraZeneca Pharmaceuticals. The authors would like to acknowledge the work of the following employees of AstraZeneca who contributed to the study design and implementation: Fred King, Soheil Chavoshi, and Ron Dirienzi. The authors would also like to acknowledge the work of the following Evidera employees: Jun Chen, Chris Sexton, and Christine Thompson, who contributed to the data analysis and interpretation. Lastly, Colleen Valenzuela and Jersino Jean-Mary at United BioSource Corporation contributed to the study implementation and data collection.
References
- 1. ACOG Committee Opinion No. 587: Effective patient-physician communication. Obstet Gynecol. 2014;123(2 Pt 1):389-93. Available at: http://journals.lww.com/greenjournal/pages/articleviewer.aspx?year=2014&issue=02000&article=00036&type=abstract. Accessed December 14, 2015. [DOI] [PubMed] [Google Scholar]
- 2. Travaline JM, Ruchinskas R, D’Alonzo GE Jr.. Patient-physician communication: why and how. J Am Osteopath Assoc. 2005;105(1):13-18. Available at: http://jaoa.org/article.aspx?articleid=2093086. Accessed December 14, 2015. [PubMed] [Google Scholar]
- 3. Zolnierek KB, DiMatteo MR. Physician communication and patient adherence to treatment: a meta-analysis. Med Care. 2009;47(8):826-34. Available at: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2728700/. Accessed December 14, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Liker HR, Ducrotté P, Malfertheiner P. Unmet medical needs among patients with gastroesophageal reflux disease: a foundation for improving management in primary care. Dig Dis. 2009;27(1):62-67. [DOI] [PubMed] [Google Scholar]
- 5. Loquai C, Schmidtmann I, Beutel M, et al. Quality of life in melanoma patients during adjuvant treatment with pegylated interferon-α2b: patients’ and doctors’ views. Eur J Dermatol. 2011;21(6):976-84. [DOI] [PubMed] [Google Scholar]
- 6. Chou R, Fanciullo GJ, Fine PG, et al. Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. J Pain.2009;10(2):113-30. Available at: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4043401/pdf/nihms-578614.pdf. Accessed December 14, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brown RT, Zuelsdorff M, Fleming M. Adverse effects and cognitive function among primary care patients taking opioids for chronic nonmalignant pain. J Opioid Manag. 2006;2(3):137-46. [DOI] [PubMed] [Google Scholar]
- 8. Mahowald ML, Singh JA, Majeski P. Opioid use by patients in an orthopedics spine clinic. Arthritis Rheum. 2005;52(1):312-21. Available at: http://onlinelibrary.wiley.com/doi/10.1002/art.20784/pdf. Accessed December 14, 2015. [DOI] [PubMed] [Google Scholar]
- 9. Cook SF, Lanza L, Zhou X, et al. Gastrointestinal side effects in chronic opioid users: results from a population-based survey. Aliment Pharmacol Ther. 2008;27(12):1224-32. Available at: http://onlinelibrary.wiley.com/doi/10.1111/j.1365-2036.2008.03689.x/pdf. Accessed December 14, 2015. [DOI] [PubMed] [Google Scholar]
- 10. Camilleri M, Drossman DA, Becker G, Webster LR, Davies AN, Mawe GM. Emerging treatments in neurogastroenterology: a multidisciplinary working group consensus statement on opioid-induced constipation. Neurogastroenterol Motil. 2014;26(10):1386-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pappagallo M. Incidence, prevalence, and management of opioid bowel dysfunction. Am J Surg. 2001;182(5A Suppl):11S-18S. [DOI] [PubMed] [Google Scholar]
- 12. Bell TJ, Panchal SJ, Miaskowski C, et al. The prevalence, severity, and impact of opioid-induced bowel dysfunction: results of a US and European Patient Survey (PROBE 1). Pain Med. 2009;10(1):35-42. [DOI] [PubMed] [Google Scholar]
- 13. Bell T, Annunziata K, Leslie JB. Opioid-induced constipation negatively impacts pain management, productivity, and health-related quality of life: findings from the National Health and Wellness Survey. J Opioid Manag. 2009;5(3):137-44. [DOI] [PubMed] [Google Scholar]
- 14. Holzer P, Ahmedzai SH, Niederle N, et al. Opioid-induced bowel dysfunction in cancer-related pain: causes, consequences, and a novel approach for its management. J Opioid Manag. 2009;5(3):145-51. [DOI] [PubMed] [Google Scholar]
- 15. Kumar L, Barker C, Emmanuel A. Opioid-induced constipation: pathophysiology, clinical consequences, and management. Gastroenterol Res Pract. 2014;2014:141737. Available at: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4027019/pdf/GRP2014-141737.pdf. Accessed December 14, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Twycross R, Sykes N, Mihalyo M, Wilcock A. Stimulant laxatives and opioid-induced constipation. J Pain Symptom Manage. 2012;43(2):306-13. [DOI] [PubMed] [Google Scholar]
- 17. Coyne KS, LoCasale RJ, Datto CJ, Sexton CC, Yeomans K, Tack J. Opioid-induced constipation in patients with chronic noncancer pain in the USA, Canada, Germany, and the UK: descriptive analysis of baseline patient-reported outcomes and retrospective chart review. Clinicoecon Outcomes Res. 2014;6:269-81. Available at: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4041290/pdf/ceor-6-269.pdf. Accessed December 14, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Frank L, Kleinman L, Farup C, Taylor L, Miner P Jr.. Psychometric validation of a constipation symptom assessment questionnaire. Scand J Gastroenterol. 1999;34(9):870-77. [DOI] [PubMed] [Google Scholar]
- 19. Reilly MC, Zbrozek AS, Dukes EM. The validity and reproducibility of a work productivity and activity impairment instrument. Pharmacoeconomics. 1993;4(5):353-65. [DOI] [PubMed] [Google Scholar]
- 20. Rabin R, Oemar M, Oppe M. EQ-5D-3L user guide: basic information on how to use the EQ-5D-3L instrument. Rotterdam: EuroQol Group; 2011. [Google Scholar]
- 21. Pleil AM, Coyne KS, Reese PR, et al. The validation of patient-rated global assessments of treatment benefit, satisfaction, and willingness to continue—the BSW. Value Health.2005;8(Suppl 1):S25-34. [DOI] [PubMed] [Google Scholar]
- 22. Eder L, Thavaneswaran A, Chandran V, Cook R, Gladman DD. Factors explaining the discrepancy between physician and patient global assessment of joint and skin disease activity in psoriatic arthritis patients. Arthritis Care Res (Hoboken). 2015;67(2):264-72. [DOI] [PubMed] [Google Scholar]
- 23. Hudson M, Impens A, Baron M, et al. Discordance between patient and physician assessments of disease severity in systemic sclerosis. J Rheumatol. 2010;37(11):2307-12. [DOI] [PubMed] [Google Scholar]
- 24. von Eisenhart Rothe A, Bielitzer M, Meinertz T, Limbourg T, Ladwig KH, Goette A. Predictors of discordance between physicians’ and patients’ appraisals of health-related quality of life in atrial fibrillation patients: findings from the Angiotensin II Antagonist in Paroxysmal Atrial Fibrillation Trial. Am Heart J. 2013;166(3):589-96. [DOI] [PubMed] [Google Scholar]
- 25. Castrejón I, Yazici Y, Samuels J, Luta G, Pincus T. Discordance of global estimates by patients and their physicians in usual care of many rheumatic diseases: association with 5 scores on a Multidimensional Health Assessment Questionnaire (MDHAQ) that are not found on the Health Assessment Questionnaire (HAQ). Arthritis Care Res (Hoboken).2014;66(6):934-42. [DOI] [PubMed] [Google Scholar]
- 26. Takeuchi EE, Keding A, Awad N, et al. Impact of patient-reported outcomes in oncology: a longitudinal analysis of patient-physician communication. J Clin Oncol. 2011;29(21):2910-17. Available at: http://jco.ascopubs.org/content/29/21/2910.full.pdf+html. Accessed December 14, 2015. [DOI] [PubMed] [Google Scholar]
- 27. Ghosh S, Mitchell R. Impact of inflammatory bowel disease on quality of life: Results of the European Federation of Crohn’s and Ulcerative Colitis Associations (EFCCA) patient survey. J Crohns Colitis. 2007;1(1):10-20. Available at: http://ecco-jcc.oxfordjournals.org/content/eccojc/1/1/10.full.pdf. Accessed December 14, 2015. [DOI] [PubMed] [Google Scholar]
- 28. August KJ, Nguyen H, Ngo-Metzger Q, Sorkin DH. Language concordance and patient-physician communication regarding mental health needs. J Am Geriatr Soc. 2011;59(12):2356-62. [DOI] [PubMed] [Google Scholar]
- 29. Crawley JA, Horowicz-Mehler N, Hawryluk EA, King F. Impact of opioid-induced constipation: a multinational cross-sectional survey of patients and health care providers [Abstract PGI21]. Value Health.2013;16(3):A215. Available at: http://www.valueinhealthjournal.com/article/S1098-3015(13)01162-5/pdf. Accessed December 14, 2015. [Google Scholar]


