Abstract
BACKGROUND:
Previous studies demonstrated substantial economic and comorbidity burden associated with psoriasis (PsO) before biologics were available. Biologics have changed PsO treatment paradigms and potentially improved patient outcomes. There is a need to reassess the economic and comorbidity burden of PsO in the biologic era.
OBJECTIVE:
To compare the prevalence of comorbidities, health care resource utilization, and costs between moderate-to-severe PsO patients and demographically matched controls.
METHODS:
Adults aged 18-64 years with at least 2 PsO diagnoses (ICD-9-CM code 696.1) were identified in the OptumHealth Reporting and Insights claims database from January 2007 to March 2012. Moderate-to-severe PsO patients were identified as those receiving ≥ 1 systemic therapy or phototherapy during the 12-month study period following the index date (randomly selected date after the first PsO diagnosis). Controls were free of PsO and psoriatic arthritis (PsA) and were matched 1:1 with PsO patients on age, gender, and geographic region. All patients had at least 12 months of continuous enrollment after the index date. Selected comorbidities, medication use, all-cause health care utilization, and costs were compared between PsO patients and controls. Multivariate regression models were performed to examine the association between PsO and selected comorbidities, medication use, and health care costs and utilization, adjusting for demographics, index year, insurance type, and other comorbidities. Odds ratios (ORs) were reported for any medication use, hospitalization, emergency room visit, and outpatient visit, and incidence rate ratios (IRRs) were reported for the number of medications filled. Adjusted cost differences between PsO patient and controls were also estimated.
RESULTS:
A total of 5,492 matched pairs of moderate-to-severe PsO patients and controls were selected, with a mean age of 47.6 years and 55.5% of patients being male. PsO patients were significantly more likely to have most of the comorbidities examined, with the top 3 most common in both groups being hyperlipidemia (33.3% vs. 27.3%), hypertension (32.8% vs. 23.5%), and diabetes (15.8% vs. 9.7%). Compared with controls, PsO patients were more likely to have any medication filled (OR = 27.5) and had more distinct number of prescription medications (IRR = 2.1; both P < 0.01). PsO patients were more likely to have any inpatient admission (OR = 1.3), emergency room visit (OR = 1.3), and outpatient visit (OR = 29.3; all P < 0.01). PsO patients also incurred significantly higher total, pharmacy, and medical costs (adjusted annual costs differences: $18,960, $13,990, and $3,895 per patient, respectively; all P < 0.01) than controls.
CONCLUSIONS:
Compared with PsO- and PsA-free controls, moderate-to-severe PsO patients were more likely to have selected comorbidities and higher health care utilization and costs.
What is already known about this subject
Psoriasis (PsO) is associated with considerable comorbidity and medical expenditures, with most PsO-related economic burden being attributed to moderate-to-severe patients.
The recent introduction of biologics has significantly changed PsO treatment paradigms and is expected to improve patient outcomes.
What this study adds
This study used recent data from a large administrative claims database to assess the comorbidity and economic burden associated with moderate-to-severe PsO.
In the biologic era, moderate-to-severe PsO patients experienced higher disease burden and incurred significantly higher health care utilization and costs compared with controls.
Psoriasis (PsO) is the most common immune-mediated disease in the United States, affecting approximately 7.5 million (2%) individuals.1,2 Its primary skin manifestations include scaling and erythematous plaques, which often lead to inflammation and pain and impaired quality of life.3,4 The pathophysiology of PsO involves an abnormal immune response characterized by increased activity of T cells, antigen presenting cells, Th-1 cytokines, and B lymphocytes that cause inflammation.5-7
PsO affects multiple systems in addition to the skin.8,9 Because of the immune dysregulation and ensuing inflammation, PsO is associated with an increased risk of various comorbidities. Psoriatic arthritis (PsA) is a common comorbidity affecting 6%-42% of PsO patients.10-12 PsA has clinical presentations of pain and swelling of the joints, which can progress to joint damage and long-term disability.12 PsO is also associated with a higher prevalence of metabolic syndrome (e.g., obesity, hypertension, hyperlipidemia, and diabetes); cardiovascular diseases (e.g., myocardial infarction and stroke); other autoimmune diseases (e.g., inflammatory bowel diseases such as Crohn’s disease and ulcerative colitis); and nonmelanoma skin cancer and lymphoma.3,8,13-16 Because patients often feel stigmatized, they may develop mental health conditions, such as depressive symptoms and psychiatric disorders.6 The burden of comorbidities increases with disease severity among PsO patients.17
Due to its chronic nature and comorbidities, PsO incurs substantial economic burden to society.18 In 2006, the total U.S. direct costs associated with PsO were estimated to be approximately $5 billion.19 All cost studies in the prebiologic era demonstrated increased economic burden among PsO patients in the United States and Canada,20-23 and all suggested that most PsO-related economic burden was attributed to moderate-to-severe patients.
Treatments for PsO vary across disease severity. For decades, immunosuppressants were the only pharmacological treatments for moderate-to-severe PsO. The introduction of biologics in recent years has significantly changed PsO treatment paradigms. Biologics are cost-effective in improving clinical outcomes, quality of life, and work productivity, compared with conventional therapies.24,25 The pathogenic targets of biologics can include proinflammatory cytokines that play an imperative role in the pathogenesis of PsA and Crohn’s disease; cardiometabolic pathways (insulin resistance, lipid metabolism, and general body weight homeostasis); and atherogenesis.7 Therefore, effective treatment of PsO with biologics may reduce medical resource utilization and costs over time. One recent study revealed significant reductions in all hospital resource use and costs in the year following initiation of biologics among patients with moderate-to-severe PsO.26
Although the economic burden of PsO was examined during the prebiologics time,20-22,27,28 a study is needed to determine whether there remains a high economic and comorbidity burden among PsO patients since the introduction of biologics, particularly for moderate-to-severe PsO patients. Our study assessed the comorbidity burden, health care utilization, and costs associated with moderate-to-severe PsO patients compared with a demographically matched cohort without PsO or PsA.
Methods
Data Source
This is a retrospective study using data from the OptumHealth Reporting and Insights claims database from January 1, 2007, to March 31, 2012, which represents approximately 15.5 million privately insured individuals from 69 companies in a wide range of industries and occupations across the United States. Medical and pharmacy claims, as well as eligibility data are available for all beneficiaries for services provided. Enrollment/eligibility records for each patient contain demographic information, including age, gender, and census region. Medical claims comprise information on inpatient and outpatient care, including the service date, type and place of service, provider type, International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) diagnosis codes, and the Current Procedural Terminology (CPT) code. Pharmacy claims capture the National Drug Code (NDC) number, dispensing date, quantity of drug, and number of days supplied. Payment information is available at the claims level.
Sample Selection
Patients with at least 2 diagnoses of PsO (ICD-9-CM code 696.1) on different dates from January 1, 2007, to March 31, 2012, were selected into the PsO cohort. The index date was randomly selected among potential calendar dates after the first PsO diagnosis that met the following criteria: (a) patients were continuously enrolled for 12 months (study period) after a potential index date, and (b) patients were aged 18-64 years as of a potential index date. Patient enrollment periods covered by health maintenance organizations (HMOs) were excluded due to incomplete cost information in the database. Moderate-to-severe PsO patients were further identified as those who received at least 1 nontopical systemic therapy (see Appendix A for Generic Product Identifier [GPI] codes, available in online article) or phototherapy (see Appendix B for CPT codes, available in online article) during the study period.
Patients free of PsO (ICD-9-CM code 696.1) and PsA (ICD-9-CM code 696.0) throughout the entire claims history (i.e., January 1, 1999-March 31, 2012) were included as control candidates and matched 1:1 with moderate-to-severe PsO patients based on age, gender, and geographic region as of the index date using the exact matching technique. Specifically, for each moderate-to-severe PsO patient, control candidates were assigned the same index date and had to have the same age, gender, and geographic region as of the index date. Control candidates were further required to be continuously enrolled for 12 months following the index date (excluding enrollment periods covered by HMOs). One control patient was selected among all control candidates meeting these criteria. If a patient was selected as a control for a PsO patient, the patient was removed from the control candidates for the next PsO patients.
Patient Characteristics and Outcomes
Patient characteristics as of the index date included demographics (age, gender, and geographic region); insurance type; and calendar year of index date. Two types of comorbidities, selected and common comorbidities, and other comorbid medical conditions were identified based on the ICD-9-CM codes in either the primary or secondary diagnosis code during the 12-month study period. Selected and common comorbidities (see Appendix C, available in online article) included PsA, hyperlipidemia, hypertension, diabetes, obesity, coronary heart disease, cerebrovascular disease (stroke), peripheral vascular disease, depression, anxiety, rheumatoid arthritis, Crohn’s disease or ulcerative colitis, multiple sclerosis, skin cancer, lymphoma, other autoimmune disorders, and other malignancies. Other comorbid medical conditions (see Appendix D, available in online article) included all components of the Charlson Comorbidity Index (CCI) except for those included in the selected and common comorbidities already mentioned. A modified CCI was calculated based on these comorbidities.29
Medication use was measured by any medication filled and number of medications filled. All-cause and selected medications of interest, such as antidepressants, antidiabetic drugs, and cardiovascular drugs/antihypertensives (identified based on GPI codes; Appendix E, available in online article), were analyzed separately. All-cause health care utilization outcomes were categorized by inpatient visit (any visit, number of visits, and length of inpatient stay); emergency room (ER) visit (any visit and number of visits); and outpatient visit (any visit and number of visits). Claims for inpatient, ER, and outpatient services were identified from the database based on the place of service, type of service, and the CPT codes. All-cause health care costs and PsO-related costs (PsO treatment costs and PsO-related medical costs, calculated for moderate-to-severe PsO patients only) were calculated as reimbursed amounts from payers and were adjusted to 2012 U.S. dollars using the medical care component of the Consumer Price Index.30 Total health care costs and each cost component (i.e., pharmacy costs and medical costs including inpatient, ER, outpatient, and other costs) were examined.
Statistical Analyses
Descriptive statistics were provided for all patient characteristics and outcomes. Continuous variables were summarized using means and standard deviations (SDs); categorical variables were described using frequency counts and percentages. Unadjusted values were compared between the matched PsO patients and controls using Wilcoxon signed-rank tests for continuous variables and McNemar’s tests for binary variables.
Multivariate regression models were performed to assess the association between PsO and comorbidities, medication use, medical resource utilization, and health care costs, adjusting for demographics, index year, insurance type, other comorbid medical conditions, and modified CCI measured during the 12-month study period. Specifically, conditional logistic regressions were used to compare binary variables, including occurrence of comorbidities, any medication fill, and any medical service utilization. Odds ratios (ORs) and 95% confidence intervals (CIs) were estimated. Negative binomial regressions were used to compare count variables, including number of distinctive medications filled and the number of medical service visits. Generalized estimating equation (GEE) was used to account for correlation between matched pairs. Incidence rate ratios (IRRs) and 95% CIs were estimated. Two-part models with GEE were used to determine the adjusted cost differences between the matched cohorts. The first part was a logistic regression modeling the probability of having a nonzero cost, and the second part was a generalized linear regression estimating cost values with a gamma distribution and log link.31 The bootstrap resampling method was used to estimate the adjusted mean cost differences between cohorts and associated P values.32 All data analyses were conducted using SAS version 9.2 (SAS Institute, Carey, NC).
Results
A total of 48,843 patients had at least 2 ICD-9-CM diagnoses of PsO on different dates between January 1, 2007, and March 31, 2012 (Figure 1). The final sample comprised 5,492 moderate-to-severe PsO patients who met all inclusion and exclusion criteria and included 5,492 pairs of moderate-to-severe PsO patients and their matched controls based on age, gender, and geographic region. Per study design, the PsO patients and controls had similar patient demographics with a mean age of 47.6 years and 44.5% being female (Table 1). More than 30% of the sample lived in the South, followed by the Midwest and Northeast regions. Approximately two thirds of the matched pairs had insurance coverage from preferred provider organizations. Compared with matched controls, PsO patients had higher modified CCI scores (0.26 vs. 0.13, P < 0.001) and increased burden in all other comorbid medical conditions assessed (all P < 0.01; Table 2).
FIGURE 1.
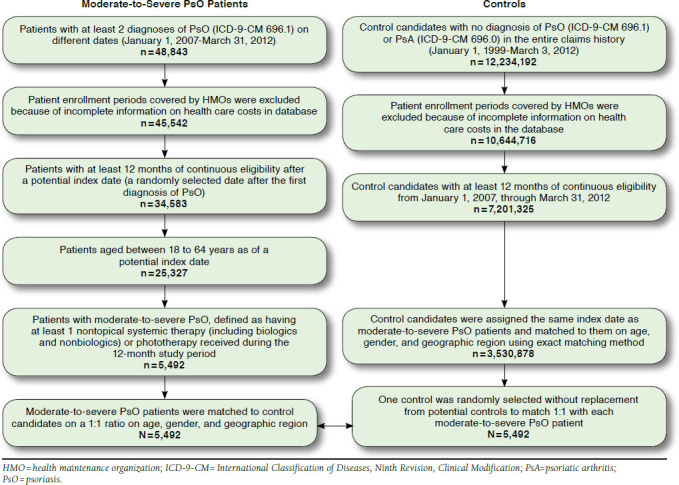
Selection of Study Cohorts
TABLE 1.
Patient Characteristics in Moderate-to-Severe PsO Patients and Matched Controls
| Demographic Characteristicsa | Moderate-to-Severe PsO Patients | Controls | P Valueb | ||
|---|---|---|---|---|---|
| N = 5,492 [A] | N = 5,492 [B] | [A] vs. [B] | |||
| Years of age, mean (SD) | 47.62 | (1.65) | 47.62 | (1.65) | - |
| Age group, n (%) | |||||
| 18-29 | 489 | (8.9) | 489 | (8.9) | - |
| 30-39 | 850 | (15.5) | 850 | (15.5) | - |
| 40-49 | 1,390 | (25.3) | 1,390 | (25.3) | - |
| 50-59 | 1,822 | (33.2) | 1,822 | (33.2) | - |
| 60-64 | 941 | (17.1) | 941 | (17.1) | - |
| Gender, n (%) | |||||
| Male | 3,048 | (55.5) | 3,048 | (55.5) | - |
| Female | 2,444 | (44.5) | 2,444 | (44.5) | - |
| Geographic region, n (%) | |||||
| Northeast | 1,126 | (20.5) | 1,126 | (20.5) | - |
| Midwest | 1,308 | (23.8) | 1,308 | (23.8) | - |
| South | 1,741 | (31.7) | 1,741 | (31.7) | - |
| West | 742 | (13.5) | 742 | (13.5) | - |
| Unknown | 575 | (10.5) | 575 | (10.5) | - |
| Index year, n (%) | |||||
| 2007 | 773 | (14.1) | 773 | (14.1) | - |
| 2008 | 939 | (17.1) | 939 | (17.1) | - |
| 2009 | 1,207 | (22.0) | 1,207 | (22.0) | - |
| 2010 | 1,745 | (31.8) | 1,745 | (31.8) | - |
| 2011 | 828 | (15.1) | 828 | (15.1) | - |
| Health insurance type, n (%) | |||||
| Preferred provider organization | 3,627 | (66.0) | 3,630 | (66.1) | 0.950 |
| Point of service | 862 | (15.7) | 834 | (15.2) | 0.458 |
| Indemnity | 811 | (14.8) | 885 | (16.1) | 0.034 |
| Other | 192 | (3.5) | 143 | (2.6) | 0.006 |
aDemographics were measured as of the index date.
bUnivariate comparison was made using Wilcoxon signed rank tests for continuous variables and McNemar’s tests for categorical variables (exact binomial distribution was used if number of discordant pairs was ≤ 25).
PsO = psoriasis; SD = standard deviation.
TABLE 2.
Prevalence of Other Comorbid Medical Conditions in Moderate-to-Severe PsO Patients and Matched Controls
| Moderate-to-Severe PsO Patients | Controls | P Valuea | |||
|---|---|---|---|---|---|
| N = 5,492 [A] | N = 5,492 [B] | [A] vs. [B] | |||
| Modified Charlson Comorbidity Index,b mean (SD) | 0.26 | (0.91) | 0.13 | (0.52) | < 0.001 |
| Other comorbid medical conditions,c n (%) | |||||
| Dementia | 23 | (0.4) | 12 | (0.2) | 0.063 |
| Lung diseased | 505 | (9.2) | 340 | (6.2) | < 0.001 |
| Liver disease | 170 | (3.1) | 59 | (1.1) | < 0.001 |
| Renal disease | 172 | (3.1) | 85 | (1.5) | < 0.001 |
| Peptic ulcer disease | 32 | (0.6) | 19 | (0.3) | 0.069 |
| Rheumatologic diseasee | 38 | (0.7) | 8 | (0.1) | < 0.001 |
| Hemiplegia | 34 | (0.6) | 15 | (0.3) | 0.007 |
| AIDS | 36 | (0.7) | 10 | (0.2) | < 0.001 |
aMcNemar’s tests were used for the comparison of other comorbidities (exact binomial distribution was used if number of discordant pairs was ≤ 25).
bExcluding common comorbidities in PsO using the methodology described by Romano et al. (1993).29
cOther comorbid medical conditions were measured during the 12-month study period.
dIncluding cor pulmonale, pulmonary heart disease, chronic obstructive pulmonary disease, and asthma.
eExcluding rheumatoid arthritis, systemic lupus erythematosus, systemic sclerosis, and Sjögren’s syndrome.
AIDS = acquired immune deficiency syndrome; PsO = psoriasis; SD = standard deviation.
The prevalence of all selected and common comorbidities was higher, and the risk of having almost all these comorbidities assessed was elevated among PsO patients during the 12-month study period, compared with controls (Table 3). PsA was present among 22.1% of PsO patients. Excluding PsA, the most common comorbidities in PsO patients were related to metabolic syndrome, including hyperlipidemia (33.3% vs. 27.3%, adjusted OR [aOR] = 1.3), hypertension (32.8% vs. 23.5%, aOR = 1.6), and diabetes (15.8% vs. 9.7%, aOR = 1.7). Obesity, another condition related to metabolic syndrome, was observed in 4.8% of PsO patients, compared with 2.3% of controls (aOR = 1.9). Depression and anxiety affected 9.1% and 6.3% PsO patients, respectively (aOR = 1.7 and 1.4). PsO patients were also more likely to have rheumatic arthritis (aOR = 9.9), Crohn’s disease/ulcerative colitis (aOR = 3.4), and other autoimmune diseases (aOR = 2.7), compared with controls. Additionally, PsO patients had an elevated risk of coronary heart disease (aOR = 1.5), cerebrovascular disease (aOR = 1.7), peripheral vascular disease (aOR = 1.6), skin cancer (aOR = 1.9) including nonmelanoma skin cancer (aOR = 2.1), and lymphoma (aOR = 22.7).
TABLE 3.
Selected and Common Comorbidities in Moderate-to-Severe PsO Patients and Matched Controls
| Moderate-to-Severe PsO Patients | Controls | P Valuea | OR (95% CI)b | P Valueb | ||||
|---|---|---|---|---|---|---|---|---|
| N = 5,492 [A] | N = 5,492 [B] | [A] vs. [B] | ~[A]/[B] | [A] vs. [B] | ||||
| Selected and common comorbidities,c n (%) | ||||||||
| Hyperlipidemia | 1,831 | (33.3) | 1,499 | (27.3) | < 0.001 | 1.3 | (1.2-1.4) | < 0.001 |
| Hypertension | 1,803 | (32.8) | 1,292 | (23.5) | < 0.001 | 1.6 | (1.4-1.7) | < 0.001 |
| Psoriatic arthritis | 1,212 | (22.1) | 0 | (0.0) | < 0.001 | NA | NA | |
| Diabetes | 867 | (15.8) | 532 | (9.7) | < 0.001 | 1.7 | (1.5-1.9) | < 0.001 |
| Depression | 497 | (9.1) | 299 | (5.4) | < 0.001 | 1.7 | (1.4-2.0) | < 0.001 |
| Coronary heart disease | 406 | (7.4) | 241 | (4.4) | < 0.001 | 1.5 | (1.2-1.8) | < 0.001 |
| Acute myocardial infarction | 41 | (0.7) | 27 | (0.5) | 0.085 | 1.3 | (0.7-2.5) | 0.474 |
| Rheumatoid arthritisd | 349 | (6.4) | 47 | (0.9) | < 0.001 | 9.9 | (6.7-14.7) | < 0.001 |
| Anxiety | 345 | (6.3) | 231 | (4.2) | < 0.001 | 1.4 | (1.2-1.7) | < 0.001 |
| Obesity | 265 | (4.8) | 124 | (2.3) | < 0.001 | 1.9 | (1.5-2.4) | < 0.001 |
| Other autoimmune disorderse | 237 | (4.3) | 80 | (1.5) | < 0.001 | 2.7 | (2.0-3.7) | < 0.001 |
| Cerebrovascular disease (stroke) | 201 | (3.7) | 100 | (1.8) | < 0.001 | 1.7 | (1.3-2.3) | < 0.001 |
| Occlusion and stenosis of precerebral arteries | 93 | (1.7) | 48 | (0.9) | < 0.001 | 1.9 | (1.2-3.0) | 0.005 |
| Skin cancer | 177 | (3.2) | 95 | (1.7) | < 0.001 | 1.9 | (1.4-2.5) | < 0.001 |
| Nonmelanoma | 165 | (3.0) | 81 | (1.5) | < 0.001 | 2.1 | (1.5-2.8) | < 0.001 |
| Peripheral vascular disease | 175 | (3.2) | 95 | (1.7) | < 0.001 | 1.6 | (1.2-2.2) | 0.002 |
| Other malignanciesf | 170 | (3.1) | 124 | (2.3) | 0.004 | 1.0 | (0.8-1.4) | 0.860 |
| Crohn’s disease or ulcerative colitisd | 135 | (2.5) | 41 | (0.7) | < 0.001 | 3.4 | (2.2-5.2) | < 0.001 |
| Multiple sclerosisd | 36 | (0.7) | 21 | (0.4) | 0.047 | 1.3 | (0.5-3.0) | 0.576 |
| Lymphoma | 32 | (0.6) | 2 | (0.0) | < 0.001 | 22.7 | (3.1-169.2) | 0.002 |
aUnivariate comparison was made using McNemar’s tests (exact binomial distribution was used if number of discordant pairs was ≤ 25).
bORs and P values were estimated using conditional logistic regression, controlling for insurance type, individual other comorbid medical conditions, and modified Charlson Comorbidity Index scores calculated excluding selected and common comorbidities in PsO. ORs greater than 1 indicate increased risk for PsO patients compared with controls.
cSelected and common comorbidities were measured during the 12-month study period.
dClassified as autoimmune diseases.
eOther autoimmune disorders included alopecia areata, celiac disease, systemic sclerosis, Sjögren’s syndrome, vitiligo, chronic urticaria, systemic lupus erythematosus, Addison’s disease, giant cell arteritis, pulmonary fibrosis, and chronic glomerulonephritis.
fOther malignancies included cancers of lung, pharynx, liver, pancreas, breast, vulva, penis, bladder, and kidney.
CI = confidence interval; NA = not applicable; OR = odds ratio; PsO = psoriasis.
During the 12-month study period, 3,582 (65.2%) of the moderate-to-severe PsO patients used biologics; 1,502 (27.3%) used nonbiologic systemic therapies; and 906 (16.5%) used phototherapies (Table 4). Patients who used any combination of these systemic PsO therapies were counted in more than 1 group. In addition, PsO patients were more likely to have any medication filled (98.8% vs. 76.6%, aOR = 27.5) and have selected medications of interest filled (57.8% vs. 42.8%, aOR = 1.9), including antidepressants (25.0% vs. 13.9%, aOR = 2.1), antidiabetic drugs (11.3% vs. 6.8%, aOR = 1.7), and cardiovascular drugs/antihypertensives (47.9% vs. 36.1%, aOR = 1.7; all P < 0.001). On average, PsO patients filled more distinct prescription medications (10.96 vs. 5.16, adjusted IRR [aIRR] = 2.1) and selected medications of interest (1.43 vs. 1.02, aIRR = 1.5), including antidepressants (0.48 vs. 0.26, aIRR = 1.8), antidiabetic medications (0.28 vs. 0.17, aIRR = 1.6), and cardiovascular medications/antihypertensives (2.19 vs. 1.45, aIRR=1.4; all P < 0.001). In addition, PsO patients were more likely to incur all-cause medical services, including any inpatient admissions (9.3% vs. 6.4%, aOR = 1.3), ER visits (21.4% vs. 16.4%, aOR = 1.3), and outpatient visits (99.0% vs. 84.5%, aOR = 29.3; all P < 0.001). On average, PsO patients had more inpatient admissions (0.15 vs. 0.09, aIRR = 1.3; P = 0.001), ER visits (0.40 vs. 0.27, aIRR = 1.3; P < 0.001), and outpatient visits (20.46 vs. 8.41, aIRR = 2.4; P < 0.001). PsO patients also spent more hospitalization days (1.03 vs. 0.55, aIRR = 1.9; P < 0.001) than controls.
TABLE 4.
Medication Use and Medical Service Utilization in Moderate-to-Severe PsO Patients and Matched Controls
| Moderate-to-Severe PsO Patients | Controls | P Valueb | OR, IRR (95% CI)c | P Valuec | ||||
|---|---|---|---|---|---|---|---|---|
| N = 5,492 [A] | N = 5,492 [B] | [A] vs. [B] | ~[A]/[B] | [A] vs. [B] | ||||
| Medication Usea | ||||||||
| All-cause medication | ||||||||
| Any medication filled, n (%) | 5,428 | (98.8) | 4,205 | (76.6) | < 0.001 | 27.5 | (20.2-37.4) | < 0.001 |
| Number of distinct medications filled, mean (SD) | 10.96 | (8.85) | 5.16 | (5.99) | < 0.001 | 2.1 | (2.0-2.2) | < 0.001 |
| Medications of interest (antidepressants, antidiabetic drugs, and cardiovascular drugs) | ||||||||
| Any prescription filled, n (%) | 3,176 | (57.8) | 2,351 | (42.8) | < 0.001 | 1.9 | (1.8-2.1) | < 0.001 |
| Number of distinct medications filled, mean (SD) | 1.43 | (2.22) | 1.02 | (1.88) | < 0.001 | 1.5 | (1.4-1.6) | < 0.001 |
| Antidepressants | ||||||||
| Any prescription filled, n (%) | 1,372 | (25.0) | 762 | (13.9) | < 0.001 | 2.1 | (1.9-2.3) | < 0.001 |
| Number of distinct medications filled, mean (SD) | 0.48 | (1.04) | 0.26 | (0.79) | < 0.001 | 1.8 | (1.7-2.0) | < 0.001 |
| Antidiabetic drugs | ||||||||
| Any prescription filled, n (%) | 623 | (11.3) | 373 | (6.8) | < 0.001 | 1.7 | (1.4-1.9) | < 0.001 |
| Number of distinct medications filled, mean (SD) | 0.28 | (0.93) | 0.17 | (0.72) | < 0.001 | 1.6 | (1.4-1.9) | < 0.001 |
| Cardiovascular drugs | ||||||||
| Any prescription filled, n (%) | 2,630 | (47.9) | 1,980 | (36.1) | < 0.001 | 1.7 | (1.6-1.9) | < 0.001 |
| Number of distinct medications filled, mean (SD) | 2.19 | (3.11) | 1.45 | (2.47) | < 0.001 | 1.4 | (1.3-1.5) | < 0.001 |
| PsO treatment | ||||||||
| Any biologic therapy, n (%) | 3,582 | (65.2) | — | — | — | — | — | — |
| Any nonbiologic systemic therapy, n (%) | 1,502 | (27.3) | — | — | — | — | — | — |
| Any phototherapy, n (%) | 906 | (16.5) | — | — | — | — | — | — |
| All-Cause Medical Service Utilizationa | ||||||||
| Inpatient admissions | ||||||||
| Any admission, n (%) | 513 | (9.3) | 352 | (6.4) | < 0.001 | 1.3 | (1.1-1.5) | 0.001 |
| Number of admissions, mean (SD) | 0.15 | (0.60) | 0.09 | (0.42) | < 0.001 | 1.3 | (1.1-1.5) | 0.001 |
| Total length (days) of stay, mean (SD) | 1.03 | (6.49) | 0.55 | (4.39) | < 0.001 | 1.9 | (1.5-2.5) | < 0.001 |
| Emergency room visits | ||||||||
| Any visit, n (%) | 1,177 | (21.4) | 902 | (16.4) | < 0.001 | 1.3 | (1.2-1.4) | < 0.001 |
| Number of visits, mean (SD) | 0.40 | (1.21) | 0.27 | (0.91) | < 0.001 | 1.3 | (1.2-1.5) | < 0.001 |
| Outpatient visits | ||||||||
| Any visit, n (%) | 5,437 | (99.0) | 4,642 | (84.5) | < 0.001 | 29.3 | (19.1-44.7) | < 0.001 |
| Number of visits, mean (SD) | 20.46 | (20.09) | 8.41 | (11.06) | < 0.001 | 2.4 | (2.3-2.5) | < 0.001 |
aMedication use and medical service utilization were measured during the 12-month study period.
bUnivariate comparison was made using McNemar’s tests for at least 1 medication filled and Wilcoxon signed rank tests for number of distinct medications filled and number of visits or total length of stay.
cORs and P values were calculated for any medication filled and any visit from conditional logistic regression. IRRs and P values were calculated for number of distinct medications filled, number of visits, and total length of stay from a negative binomial model with generalized estimating equation. All models controlled for age, gender, geographic region, index year, insurance type, individual other comorbid medical conditions, and modified CCI scores calculated excluding selected and common comorbidities in PsO. ORs greater than 1 or IRRs greater than 1 indicate an increased risk or incidence rate for PsO patients compared with controls.
CCI = Charlson Comorbidity Index; CI = confidence interval; IRR = incidence rate ratio; OR = odds ratio; PsO = psoriasis; SD = standard deviation.
Compared with controls, PsO patients incurred higher annual total health care costs per patient ($22,713 vs. $4,993, adjusted cost difference [ACD] = $18,960; P < 0.001), driven by significantly higher costs in all-cause medications ($14,698 vs. $1,101, ACD = $13,990; P < 0.001) and all-cause medical services ($8,015 vs. $3,892, ACD = $3,895; P = 0.002; Figure 2). Compared with matched controls, all components of all-cause medical service costs were higher in PsO patients, including inpatient cost ($2,609 vs. $1,137, ACD = $1,056), ER ($277 vs. $145, ACD = $104), outpatient cost ($4,694 vs. $2,421, ACD = $2,151), and other medical costs ($435 vs. $189, ACD = $194; all P < 0.001). During the 12-month study period, the moderate-to-severe PsO patients incurred an average of $13,731 PsO-related costs, a majority of which were attributed to treatment costs for systemic PsO therapies ($12,958) that included biologics, nonbiologics, and phototherapies (Table 5).
FIGURE 2.
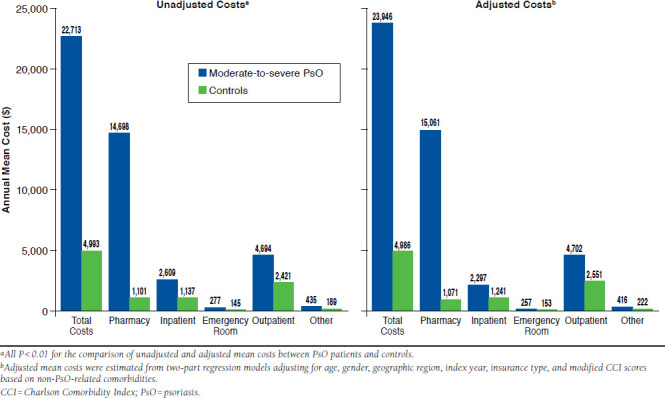
All-Cause Health Care Costs in Moderate-to-Severe PsO Patients and Matched Controls During 12-Month Study Period: Univariate and Multivariate Analyses
TABLE 5.
PsO-Related Costs in Moderate-to-Severe PsO Patientsa
| Moderate-to-Severe PsO Patients | ||
|---|---|---|
| N = 5,492 | ||
| Total PsO-related costs, mean (SD) $ | 13,731 | (11,878) |
| Treatment costs | 12,958 | (11,632) |
| Biologic therapy | 12,084 | (12,090) |
| Nonbiologics systemic therapy | 464 | (1,778) |
| Phototherapy | 409 | (1,321) |
| Medical costsb | 773 | (2,863) |
aMeasured during the 12-month study period.
bPsO-related medical costs were calculated based on medical claims associated with a diagnosis for PsO.
PsO = psoriasis; SD = standard deviation.
Discussion
Using a recent and large U.S. administrative claims database, this study assessed the comorbidity and economic burden of moderate-to-severe PsO in the biologic era. The findings demonstrated that moderate-to-severe PsO patients are associated with significantly higher prevalence of comorbidities; greater health care resource utilization, including medication use and medical service utilization; and higher health care costs, compared with controls without PsO or PsA.
Our findings were consistent with earlier PsO economic burden studies, including those before biologics were available.20-22 Using large claims databases, previous studies found that the annual incremental total cost for the overall PsO population ranged between $900 ($2,006 using 1998-2005 data) and $1,976 ($2,007 using 2003 data),21,22 while the annual incremental total health care costs for moderate-to-severe PsO patients were $7,084 (medical services: $3,167; medications: $3,917) higher than controls without PsO or PsA.22 Using a more recent database, our incremental total costs were over 2.6 times that estimated by Yu et al. (2009), with over 70% of the cost difference attributed to pharmacy.22 Similar to estimates from Yu et al., our moderate-to-severe PsO patients were more likely to have inpatient admissions, ER visits, and outpatient visits than controls without PsO or PsA.
This study clearly showed an elevated risk for selected and common comorbidities among moderate-to-severe PsO patients, particularly for cardiometabolic diseases. The leading comorbidities in our moderate-to-severe sample were hyperlipidemia (33.3%) and hypertension (32.8%), which were slightly higher than the prevalence among the overall PsO population reported in a previous study (27% for hyperlipidemia and 25% for hypertension).9 Furthermore, Neimann et al. (2006) estimated a 62% higher adjusted odds for diabetes mellitus and 79% higher adjusted odds for obesity in patients with severe PsO, which is comparable to the 70% and 90% found in our study.15 Another analysis using 2 large U.S. health plan databases during 2001-2002 reported a roughly 20% higher odds for coronary heart disease and 13%-18% higher odds for cerebrovascular disease in the overall PsO population, compared with 50% and 70% in our moderate-to-severe PsO sample.33
Besides cardiovascular and metabolic syndrome, moderate-to-severe PsO patients face higher risks for other comorbidities. In our moderate-to-severe PsO sample, 22% were comorbid with PsA, which is comparable to the data in a 2001 U.S. survey with PsA reported in 18% moderate PsO patients and 56% in severe PsO patients.34 Our study found increased risks of depression (aOR = 1.7) and autoimmune disorders such as rheumatoid arthritis (aOR = 9.9) and Crohn’s disease/ulcerative colitis (aOR = 3.4) among moderate-to-severe PsO patients. Our findings were consistent with those reported in a German case-control study (aOR = 1.49 for depression),6 and a U.S. retrospective cohort study (aOR = 3.6 for rheumatoid arthritis, aOR = 1.5 for ulcerative colitis, and aOR = 1.8 for Crohn’s disease).35 Similarly, a higher risk for nonmelanoma skin cancer (aOR = 1.9) and lymphoma (aOR = 22.7) was found in our study, consistent with the findings in a U.S. national survey (relative risk = 1.7-2.2) and a large United Kingdom cohort study (adjust hazard ratio = 1.59 for lymphoma).36,37 The risks of comorbidities in our moderate-to-severe sample are generally higher than those reported for the overall PsO sample in the literature. Despite different study design and populations, the increasing trend could be explained by the positive relationship between severity of PsO and likelihood of these conditions.
This study revealed that the economic and comorbidity burden of moderate-to-severe PsO remains high even with the availability of biologic treatments. Biologics may work on both symptoms commonly observed among PsO patients and other comorbidities by modifying the immune-inflammatory pathway. 7 Future studies comparing the health care utilization and costs between patients on biologics versus those on nonbiologic systemic therapies are warranted to further understand the economic impact of biologics.
The high economic burden identified in the current study demonstrates that unmet needs remain in this patient population. Treatment plans should reflect the severity of PsO and the need of care for comorbidities that include PsA, metaboliccardiovascular conditions, psychiatric disorders, autoimmune conditions, skin cancer, and lymphoma. More patient-centered care coordination through multispecialty PsO teams (e.g., dermatology, rheumatology, primary care, and endocrinology) is a priority area in research and practice to improve care for PsO.38
Limitations
This study has several limitations. First, it is a retrospective observational study that could be affected by unobserved differences between comparison cohorts. Second, classification bias may exist. Since comorbidities were identified using administrative ICD-9-CM codes, medical conditions might not be accurately captured in the data because of undercoding, upcoding, or miscoding. Another classification bias may arise from the identification of moderate-to-severe PsO patients using treatment claims. Due to lack of clinical measures of PsO severity (e.g., the Psoriasis Area and Severity Index) in the claims datasets, we used systemic treatments (pharmacologic or phototherapy) to define the moderate-to-severe PsO cohort. By definition, PsO patients would incur these treatments that did not apply to the control patients. However, treatment as a proxy of disease severity is commonly used in commercial claims database analyses for PsO studies.22,27 It is rare that moderate-to-severe PsO patients would not receive any of these treatments. Therefore, we do not expect substantial bias generated using this approach. Third, this study is from a payer perspective and may understate direct costs because patient out-of-pocket costs were not considered. Fourth, our study assessed only the direct health care costs associated with PsO. The costs associated with decrements in quality of life, lost productivity, and work absenteeism were not included in our analysis. Finally, generalization of the study findings to populations beyond the commercially insured patients should be done cautiously, since the Medicare and Medicaid populations may have different characteristics.
Conclusions
Using updated data, this study indicates that moderate-to-severe PsO patients were more likely to have selected comorbidities and higher all-cause health care utilization and costs than PsO- and PsA-free controls. The incremental comorbidity and economic burdens associated with moderate-to-severe PsO highlight the need for more effective treatments.
APPENDIX A. GPI Codes for PsO-Related Nontopical Systemic Medications
| Drug Class | GPI Codes | Description |
|---|---|---|
| Biologics | ||
| Etanercept | 6629003000* | Etanercept SC Inj 50 mg/mL (66290030002020) Etanercept SC Inj 25 mg/0.5 mL (66290030002025) Etanercept for SC Inj Kit 25 mg (66290030006420) |
| Infliximab | 5250504000* | Infliximab for IV Inj 100 mg (52505040002120) |
| Adalimumab | 6627001500* | Adalimumab Inj 40 mg/0.8 mL (50 mg/mL) (66270015002020) Adalimumab Inj Kit 20 mg/0.4 mL (662700150064) Adalimumab Inj Kit 40 mg/0.8 mL (50 mg/mL) (66270015006420) |
| Ustekinumab | 9025058500* | Ustekinumab Inj 45 mg/0.5 mL (90250585002020) Ustekinumab Inj 90 mg/mL (90250585002025) |
| Alefacept | 9025051500* | Alefacept for IV Inj 7.5 mg (90250515002120) Alefacept for IM Inj 15 mg (90250515002130) |
| Efalizumab | 9025052700* | Efalizumab for Subcutaneous Inj Kit 125 mg (150 mg) (902505270064 0) |
| Nonbiologics | ||
| Acitretin (oral retinoids) | 90250510* | Acitretin Cap 10 mg (90250510000110) Acitretin Cap 17.5 mg (90250510000115) Acitretin Cap 22.5 mg (90250510000120) Acitretin Cap 25 mg (90250510000125) |
| Isotretinoin capsules (oral retinoids) | 900500130001* | Isotretinoin Cap 5 mg (90050013000105) Isotretinoin Cap 10 mg (90050013000110) Isotretinoin Cap 20 mg (90050013000120) Isotretinoin Cap 30 mg (90050013000130) Isotretinoin Cap 40 mg (90050013000140) |
| Cyclosporine | 9940202000* | Cyclosporine Cap 25 mg (99402020000110) Cyclosporine Cap 50 mg (99402020000130) Cyclosporine Cap 100 mg (99402020000140) Cyclosporine IV Soln 50 mg/mL (99402020002005) Cyclosporine IV Soln 100 mg/mL (99402020002010) |
| Methotrexate | 2130005000* | Methotrexate Tab 2.5 mg (21300050000305) Methotrexate Tab 10 mg (21300050000320) Methotrexate Powder (21300050002900) |
| Methoxsalen | 9025056000* | Methoxsalen Cap 10 mg (90250560000110) |
| Other medications commonly used off-label for moderate-to-severe PsO | 2170003000* | Hydroxyurea Hydroxyurea Cap 500 mg (21700030000105) Hydroxyurea Tab 1000 mg (21700030000330) |
| 2130006000* | Thioguanine Thioguanine Tab 40 mg (21300060000305) |
|
| 5250006000* | Sulfasalazine Sulfasalazine Cap 1 mg (52500060000110) Sulfasalazine Cap 10 mg (52500060000120) Sulfasalazine Cap 100 mg (52500060000150) Sulfasalazine Cap CR 100 mg (52500060000240) Sulfasalazine Tab 500 mg (52500060000310) Sulfasalazine Tab Delayed Release 500 mg (52500060000610) Sulfasalazine Susp 250 mg/5mL (52500060001810) Sulfasalazine Powder (52500060002900) Sulfasalazine Enema 3 GM (52500060005120) Sulfasalazine Supp 500 mg (52500060005220) Sulfasalazine Cap Desensitization Kit (52500060006450) |
|
| 9940408000* | Tacrolimus Tacrolimus Cap 0.5 mg (99404080000105) Tacrolimus Cap 1 mg (99404080000110) Tacrolimus Cap 5 mg (99404080000120) Tacrolimus Inj 5 mg/mL (99404080002010) Tacrolimus Cap SR 24HR 0.5 mg (99404080007005) Tacrolimus Cap SR 24HR 1 mg (99404080007010) Tacrolimus Cap SR 24HR 3 mg (99404080007015) Tacrolimus Cap SR 24HR 5 mg (99404080007020) |
|
| 9940303010* | Mycophenolate Mofetil Mycophenolate Mofetil Cap 250 mg (99403030100120) Mycophenolate Mofetil Cap 500 mg (99403030100330) Mycophenolate Mofetil for Oral Susp 200 mg/mL (99403030101920) |
|
| 6628005000* | Leflunomide Leflunomide Tab 10 mg (66280050000310) Leflunomide Tab 20 mg (66280050000320) Leflunomide Tab 100 mg (66280050000340) |
|
| 9940601000* | Azathioprine Azathioprine Tab 25 mg (99406010000303) Azathioprine Tab 50 mg (99406010000305) Azathioprine Tab 75 mg (99406010000315) Azathioprine Tab 100 mg (99406010000325) Azathioprine Powder (99406010002900) |
Cap = capsule; GPI = Generic Product Identifier; IM = intramuscular; Inj = injection; IV = intravenous; mg = milligram; mL = milliliter; PsO = psoriasis; SC = subcutaneous; susp = suspension; tab = tablet.
APPENDIX B. CPT Codes for PsO-Related Phototherapies
| CPT Codes | Description |
|---|---|
| 96900 | Actinotherapy (ultraviolet light [UVL]) |
| 96910 | Photochemotherapy; tar and ultraviolet B (Goeckerman treatment) or petrolatum and ultraviolet B |
| 96912 | Photochemotherapy; psoralens and ultraviolet A (PUVA) |
| 96913 | Photochemotherapy (Goeckerman and/or PUVA) for severe photoresponsive dermatoses requiring at least 4 to 8 hours of care under direct supervision of the physician (includes application of medication and dressings) |
| 96920 | Laser treatment for inflammatory skin disease (PsO); total area less than 250 sq cm |
| 96921 | Laser treatment for inflammatory skin disease (PsO); 250 sq cm to 500 sq cm |
| 96922 | Laser treatment for inflammatory skin disease (PsO); over 500 sq cm |
CPT = Current Procedural Terminology; PsO = psoriasis; sq cm = square centimeters.
APPENDIX C. ICD-9-CM Codes for Selected and Common Comorbidities
| Comorbidity | ICD-9-CM Codes |
|---|---|
| Diabetes | 250 |
| Anxiety | 300.0 |
| Depression | 296.2, 296.3, 298.0, 300.4, 309.1, 311 |
| Hypertension | 401-404 |
| Hyperlipidemia | 272.0-272.4 |
| Coronary heart disease | 410-414 |
| Cerebrovascular disease (stroke) | 430-438 |
| Peripheral vascular disease | 440, 441, 443, 447.1, 557.1, 557.9, V43.4 |
| Obesity | 278.0 |
| Rheumatoid arthritis | 714 |
| Crohn’s disease or ulcerative colitis | 555-556 |
| Multiple sclerosis | 340 |
| Other autoimmune disorders | 704.01 (alopecia areata), 579.0 (celiac disease), 710.1 (systemic sclerosis), 710.2 (Sjögren’s syndrome), 709.01, 374.53 (vitiligo), 708 (chronic urticaria), 710.0 (systemic lupus erythematosus), 255.4 (Addison’s disease), 446.5 (giant cell arteritis), 515, 516.31 (pulmonary fibrosis), 582 (chronic glomerulonephritis) |
| Skin cancer | 172 (melanoma skin cancer), 173 (nonmelanoma skin cancer) |
| Lymphoma | 200.0-200.7, 202.1, 202.2, 202.7 (non-Hodgkin’s lymphoma), 201.0-201.9 (Hodgkin’s lymphoma) |
| Other malignancies | 162, 146, 155, 157, 174, 184.4, 187, 156, 189 (cancers of lung, pharynx, liver, pancreas, breast, vulva, penis, bladder, and kidney) |
| Psoriatic arthritis | 696.0 |
ICD-9-CM = International Classification of Diseases, Ninth Revision, Clinical Modification.
APPENDIX D. ICD-9-CM Codes for Other Comorbid Medical Conditions
| Comorbidity | ICD-9-CM Codes |
|---|---|
| Dementia | 290, 331.0, 331.1, 331.2 |
| Chronic pulmonary disease | 415.0, 416.8, 416.9, 491-494, 496 |
| Liver disease | 571.2, 571.5, 571.6, 571.8, 571.9, 572.2, 572.3, 572.4, 456.0, 456.1, 456.2 |
| Renal disease | 585, 586, V420, V451, V56 |
| Rheumatologic disease other than rheumatoid arthritis, systemic lupus erythematosus, systemic sclerosis, and Sjögren’s syndrome | 710.3, 710.4, 710.5, 710.8, and 710.9; excluding 714.0, 710.0, 710.1, 710.2 |
| Peptic ulcer disease | 531-534 |
| Hemiplegia | 342, 344 |
| AIDS | 042, 043, 044 |
AIDS = acquired immune deficiency syndrome; ICD-9-CM = International Classification of Diseases, Ninth Revision, Clinical Modification.
APPENDIX E. GPI Codes for Selected Medications
| Drug Class | GPI Codes | Description |
|---|---|---|
| Antidepressants | 58* | Alpha-2 Receptor Antagonists (Tetracyclics) (5803*) |
| Monoamine Oxidase Inhibitors (MAOIs) (5810*) | ||
| Modified Cyclics (5812*) | ||
| Selective Serotonin Reuptake Inhibitors (SSRIs) (5816*) | ||
| Serotonin-Norepinephrine Reuptake Inhibitors (SNRIs) (5818*) | ||
| Tricyclic Agents (5820*) | ||
| Antidepressants-Misc. (5830*) | ||
| Antidepressant Combinations (5899*) | ||
| Modified Cyclics-Nutritional Supplement Combinations (589980) | ||
| SSRIs-Nutritional Supplement Combinations (589985*) | ||
| Tricyclic Agents-Nutritional Supplement Combinations (589987) | ||
| Antidepressant Misc-Nutritional Supplement Combinations (589990) | ||
| Antidiabetics | 27* | Insulin (2710*) |
| Antidiabetic-Amylin Analogs (2715*) | ||
| Incretin Mimetic Agents (GLP-1 Receptor Agonists) (2717*) | ||
| Sulfonylureas (2720*) | ||
| Antidiabetic-Amino Acid Derivatives (2723*) | ||
| Biguanides (2725*) | ||
| Meglitinide Analogues (2728*) | ||
| Diabetic Other (2730*) | ||
| Progesterone Receptor Antagonists (273040*) | ||
| Diabetic Other-Combinations (273099) | ||
| Aldose Reductase Inhibitors (2740*) | ||
| Alpha-Glucosidase Inhibitors (2750*) | ||
| Dipeptidyl Peptidase-4 (DPP-4) Inhibitors (2755*) | ||
| Dopamine Receptor Agonists-Antidiabetic (2757*) | ||
| Insulin Sensitizing Agents (2760*) | ||
| Antidiabetic Combinations (2799*) | ||
| Dipeptidyl Peptidase-4 Inhibitor-Biguanide Combinations (279925) | ||
| DPP-4 Inhibitor-HMG CoA Reductase Inhibitor Comb (279930) | ||
| Meglitinide-Biguanide Combinations (279950) | ||
| Sulfonylurea-Biguanide Combinations(279970) | ||
| Sulfonylurea-Thiazolidinedione Combinations (279978) | ||
| Thiazolidinedione-Biguanide Combinations (279980) | ||
| Biguanide-Nutritional Supplement Combinations (279990) | ||
| Cardiovascular/antihypertensive agents | 31*-40* | Cardiotonics (31*) |
| Antianginal Agents (32*) | ||
| Beta Blockers (33*) | ||
| Calcium Channel Blockers (34*) | ||
| Antiarrhythmics (35*) | ||
| Antihypertensives (36*) | ||
| Diuretics (37*) | ||
| Vasopressors (38*) | ||
| Antihyperlipidemics (39*) | ||
| Cardiovascular Agents-Misc. (40*) |
GPI = Generic Product Identifier.
References
- 1. National Psoriasis Foundation. . Statistics. Available at: https://www.psoriasis.org/cure_known_statistics. Accessed September 6, 2015.
- 2. Stern RS, Nijsten T, Feldman SR, Margolis DJ, Rolstad T.. Psoriasis is common, carries a substantial burden even when not extensive, and is associated with widespread treatment dissatisfaction. J Investig Dermatol Symp Proc. 2004; 9(2): 136-39. [DOI] [PubMed] [Google Scholar]
- 3. Gelfand JM, Feldman SR, Stern RS, Thomas J, Rolstad T, Margolis DJ.. Determinants of quality of life in patients with psoriasis: a study from the U.S. population. J Am Acad Dermatol. 2004; 51(5): 704-08. [DOI] [PubMed] [Google Scholar]
- 4. Rapp SR, Feldman SR, Exum ML, Fleischer AB Jr, Reboussin DM.. Psoriasis causes as much disability as other major medical diseases. J Am Acad Dermatol. 41(3 Pt 1):401-07. [DOI] [PubMed] [Google Scholar]
- 5. Gelfand JM, Neimann AL, Shin DB, Wang X, Margolis DJ, Troxel AB.. Risk of myocardial infarction in patients with psoriasis. JAMA. 2006; 296(14): 1735-41. [DOI] [PubMed] [Google Scholar]
- 6. Schmitt J, Ford DE.. Psoriasis is independently associated with psychiatric morbidity and adverse cardiovascular risk factors, but not with cardiovascular events in a population-based sample. J Eur Acad Dermatol Venereol. 2010; 24(8): 885-92. [DOI] [PubMed] [Google Scholar]
- 7. Vena GA, Vestita M, Cassano N.. Can early treatment with biologicals modify the natural history of comorbidities? Dermatol Ther. 2010; 23(2): 181-93. [DOI] [PubMed] [Google Scholar]
- 8. Gottlieb AB, Dann F.. Comorbidities in patients with psoriasis. Am J Med. 2009; 122(12): 1150. [DOI] [PubMed] [Google Scholar]
- 9. Kimball AB, Guerin A, Tsaneva M, et al. . Economic burden of comorbidities in patients with psoriasis is substantial. J Eur Acad Dermatol Venereol. 2011; 25(2): 157-63. [DOI] [PubMed] [Google Scholar]
- 10. Zachariae H. Prevalence of joint disease in patients with psoriasis: implications for therapy. Am J Clin Dermatol. 2003; 4(7): 441-47. [DOI] [PubMed] [Google Scholar]
- 11. Myers WA, Gottlieb AB, Mease P.. Psoriasis and psoriatic arthritis: clinical features and disease mechanisms. Clin Dermatol. 2006; 24(5): 438-47. [DOI] [PubMed] [Google Scholar]
- 12. Gladman DD, Antoni C, Mease P, Clegg DO, Nash P.. Psoriatic arthritis: epidemiology, clinical features, course, and outcome. Ann Rheum Dis. 2005; 64(Suppl 2): ii14-ii17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Armstrong AW, Harskamp CT, Armstrong EJ.. The association between psoriasis and obesity: a systematic review and meta-analysis of observational studies. Nutr Diabetes. 2012;3(2):26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Love TJ, Qureshi AA, Karlson EW, Gelfand JM, Choi HK.. Prevalence of the metabolic syndrome in psoriasis: results from the National Health and Nutrition Examination Survey, 2003-2006. Arch Dermatol. 2011; 147(4): 419-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Neimann AL, Shin DB, Wang X, Margolis DJ, Troxel AB, Gelfand JM.. Prevalence of cardiovascular risk factors in patients with psoriasis. J Am Acad Dermatol. 2006; 55(5): 829-35. [DOI] [PubMed] [Google Scholar]
- 16. Gelfand JM, Dommasch ED, Shin DB, et al. . The risk of stroke in patients with psoriasis. J Invest Dermatol. 2009; 129(10): 2411-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yeung H, Takeshita J, Mehta NN, et al. . Psoriasis severity and the prevalence of major medical comorbidity: a population-based study. JAMA Dermatol. 2013; 149(10): 1173-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bickers DR, Lim HW, Margolis D, et al. . The burden of skin diseases: 2004 a joint project of the American Academy of Dermatology Association and the Society for Investigative Dermatology. J Am Acad Dermatol. 2006; 55(3): 490-500. [DOI] [PubMed] [Google Scholar]
- 19. Gunnarsson C, Chen J, Rizzo JA, Ladapo JA, Naim A, Lofland JH.. The direct healthcare insurer and out-of-pocket expenditures of psoriasis: evidence from a United States national survey. J Dermatolog Treat. 2012; 23(4): 240-54. [DOI] [PubMed] [Google Scholar]
- 20. Feldman SR, Evans C, Russell MW.. Systemic treatment for moderate to severe psoriasis: estimates of failure rates and direct medical costs in a north-eastern US managed care plan. J Dermatolog Treat. 2005; 16(1): 37-42. [DOI] [PubMed] [Google Scholar]
- 21. Fowler JF, Duh MS, Rovba L, et al. . The impact of psoriasis on health care costs and patient work loss. J Am Acad Dermatol. 2008; 59(5): 772-80. [DOI] [PubMed] [Google Scholar]
- 22. Yu AP, Tang J, Xie J, et al. . Economic burden of psoriasis compared to the general population and stratified by disease severity. Curr Med Res Opin. 2009; 25(10): 2429-38. [DOI] [PubMed] [Google Scholar]
- 23. Levy AR, Davie AM, Brazier NC, et al. . Economic burden of moderate to severe plaque psoriasis in Canada. Int J Dermatol. 2012; 51(12): 1432-40. [DOI] [PubMed] [Google Scholar]
- 24. Ahn CS, Gustafson CJ, Sandoval LF, Davis SA, Feldman SR.. Cost effectiveness of biologic therapies for plaque psoriasis. Am J Clin Dermatol. 2013; 14(4): 315-26. [DOI] [PubMed] [Google Scholar]
- 25. Anis AH, Bansback N, Sizto S, Gupta SR, Willian MK, Feldman SR.. Economic evaluation of biologic therapies for the treatment of moderate to severe psoriasis in the United States. J Dermatolog Treat. 2011; 22(2): 65-74. [DOI] [PubMed] [Google Scholar]
- 26. Fonia A, Jackson K, Lereun C, Grant DM, Barker JN, Smith CH.. A retrospective cohort study of the impact of biologic therapy initiation on medical resource use and costs in patients with moderate to severe psoriasis. Br J Dermatol. 2010; 163(4): 807-16. [DOI] [PubMed] [Google Scholar]
- 27. Crown WH, Bresnahan BW, Orsini LS, Kennedy S, Leonardi C.. The burden of illness associated with psoriasis: cost of treatment with systemic therapy and phototherapy in the U.S. Curr Med Res Opin. 2004; 20(12): 1929-36. [DOI] [PubMed] [Google Scholar]
- 28. Javitz HS, Ward MM, Farber E, Nail L, Vallow SG.. The direct cost of care for psoriasis and psoriatic arthritis in the United States. J Am Acad Dermatol. 2002; 46(6): 850-60. [DOI] [PubMed] [Google Scholar]
- 29. Romano PS, Roos LL, Jollis JG.. Adapting a clinical comorbidity index for use with ICD-9-CM administrative data: differing perspectives. J Clin Epidemiol. 1993; 46(10): 1075-79. [DOI] [PubMed] [Google Scholar]
- 30. U.S. Bureau of Labor Statistics. . Consumer Price Index databases. Available at: http://www.bls.gov/cpi/data.htm. Accessed September 6, 2015.
- 31. Mullahy J. Specification and testing of some modified count data models. J Econometrics. 1986; 33(3): 341-65. [Google Scholar]
- 32. Poi BP. From the help desk: Some bootstrapping techniques. Stata J. 2004; 4(3): 312-28. [Google Scholar]
- 33. Kimball AB, Robinson JD, Wu Y, et al. . Cardiovascular disease and risk factors among psoriasis patients in two U.S. healthcare databases, 2001-2002. Dermatology. 2008; 217(1): 27-37. [DOI] [PubMed] [Google Scholar]
- 34. Gelfand JM, Gladman DD, Mease PJ, et al. . Epidemiology of psoriatic arthritis in the population of the United States. J Am Acad Dermatol. 2005; 53(4): 573. [DOI] [PubMed] [Google Scholar]
- 35. Wu JJ, Nguyen TU, Poon K-YT, Herrinton LJ.. The association of psoriasis with autoimmune diseases. J Am Acad Dermatol. 2012; 67(5): 924-30. [DOI] [PubMed] [Google Scholar]
- 36. Stern RS, Scotto J, Fears TR.. Psoriasis and susceptibility to nonmelanoma skin cancer. J Am Acad Dermatol. 1985; 12(1 Pt 1): 67-73. [DOI] [PubMed] [Google Scholar]
- 37. Gelfand JM, Shin DB, Neimann AL, Xingmei W, Margolis DJ, Troxel AB.. The risk of lymphoma in patients with psoriasis. J Invest Dermatol. 2006; 126(10): 2194-201. [DOI] [PubMed] [Google Scholar]
- 38. Velez NF, Wei-Passanese EX, Husni ME, Mody EA, Qureshi AA.. Management of psoriasis and psoriatic arthritis in a combined dermatology and rheumatology clinic. Arch Dermatol Res. 2012; 304(1): 7-13. [DOI] [PubMed] [Google Scholar]


