Abstract
BACKGROUND:
Chronic hepatitis C (CHC) is associated with substantial morbidity and mortality, with the future burden of disease predicted to significantly increase. The recent addition of 2 direct-acting antiviral (DAA) protease inhibitors, telaprevir and boceprevir, to peginterferon alfa (PEG) and ribavirin (RBV) therapy has been shown to significantly improve sustained virologic response rates and thus has become standard of care. While the efficacy and safety of DAAs has been assessed in the clinical trial setting, less is known about real-world use of these new therapies.
OBJECTIVES:
To (a) evaluate the treatment patterns, health care utilization, and costs of CHC patients receiving DAA-based therapies in the United States using a retrospective analysis of a large administrative claims database and (b) evaluate factors associated with therapy noncompletion using multivariable analyses.
METHODS:
Adult patients with ≥ 1 claim for CHC and a prescription filled for boceprevir or telaprevir were selected from a de-identified U.S.-based claims database. The date of the first fill for a DAA after May 13, 2011 (date of first DAA availability) was defined as the index date, and patients were categorized into either the telaprevir or boceprevir cohort. Patients were required to have continuous eligibility and no claims for hepatitis B during the 6 months before (baseline) and 12 months following (study period) the index date. Baseline characteristics and study period treatment patterns, health care utilization, and costs were described. Factors associated with therapy noncompletion were examined using multivariable logistic regression, and adjusted health care costs were compared between the DAA cohorts using multivariable analyses.
RESULTS:
A total of 871 telaprevir and 284 boceprevir patients were identified. DAA patients were aged 54 years on average and more often were male (60%, n = 688). Approximately 25% (n = 216) of telaprevir and 18% (n = 52) of boceprevir patients had cirrhosis, and 9% (n = 82) of telaprevir and 7% (n = 20) of boceprevir patients had decompensated cirrhosis at baseline. Less than 1% (n = 9) of patients were HIV co-infected. Approximately 54% (n = 470) of telaprevir and 74% (n = 210) of boceprevir patients did not complete the minimum duration of therapy as per the prescribing information (telaprevir: 12 weeks of triple + 12 weeks of dual; boceprevir: 3 weeks of lead-in + 24 weeks of triple). In multivariable analyses, females (vs. males) and patients taking boceprevir (vs. telaprevir) were more likely to not complete therapy (P = 0.011). CHC patients experienced high medical and drug-related resource utilization. Telaprevir patients had numerically higher study period unadjusted medical (boceprevir: $16,927; telaprevir: $19,519) and drug costs (boceprevir: $59,953; telaprevir: $76,497) than boceprevir patients; however, after adjusting for baseline characteristics, only drug costs remained significantly different (P < 0.001).
CONCLUSIONS:
These results indicate that a large proportion of CHC patients receiving telaprevir or boceprevir did not complete minimum duration of therapy as per the prescribing information. CHC patients on a DAA regimen also experienced high resource utilization and high medical and drug costs.
What is already known about this subject
Chronic hepatitis C (CHC) is associated with considerable morbidity and mortality, which results in high medical expenditures. The addition of direct-acting antiviral (DAA) protease inhibitors (i.e., telaprevir and boceprevir) to peginterferon alfa and ribavirin therapy significantly improves the efficacy of treatment for CHC in the clinical trial setting.
However, previous real-world studies conducted among hepatitis C patients initiating DAA therapy found rates of early treatment discontinuation that ranged from 28%-52% for patients receiving telaprevir-based therapy and 30%-55% for patients receiving boceprevir-based therapy.
What this study adds
This study used a large administrative claims database to assess the treatment patterns, health care utilization, and costs of CHC patients treated with DAA-based therapy in a real-world setting.
CHC patients receiving DAAs incurred substantial health care utilization and costs, with patients receiving telaprevir-based therapy having significantly higher adjusted total drug costs than patients receiving boceprevir-based therapy.
These CHC patients also experienced high discontinuation rates of DAA-based therapy, with boceprevir patients less likely to complete minimum recommended therapy than telaprevir patients.
Chronic hepatitis C (CHC) develops in approximately 75%-85% of people infected with the hepatitis C virus (HCV), a single-stranded ribonucleic acid (RNA) virus categorized into 6 major genotypes, of which genotype 1 is predominant in the United States (70%).1,2 The prevalence of CHC in the United States is approximately 1% (2.7 million people),3 and among those infected with the HCV virus who do develop CHC, chronic liver disease is a major source of morbidity and mortality, which results in high medical expenditures.4,5
Prior to 2011, standard therapy for CHC was combination therapy consisting of peginterferon alfa (PEG) and ribavirin (RBV). In May 2011, 2 direct-acting antiviral agents (DAA), telaprevir and boceprevir, were approved by the U.S. Food and Drug Administration (FDA) for treatment of HCV genotype 1 in combination with PEG and RBV (triple therapy). Both are inhibitors for NS3/4A protease, an enzyme required for self-cleavage during viral replication. FDA prescribing information for telaprevir recommends administration of triple therapy for 12 weeks followed by 12-36 weeks of dual therapy with PEG and RBV, with duration of dual therapy depending on viral response and prior response status.6 FDA prescribing information for boceprevir recommends a 4-week therapy lead-in period with PEG and RBV followed by 24-44 weeks of triple therapy (boceprevir, PEG, and RBV). The duration of triple therapy is based on cirrhosis status, prior treatment experience, and viral response. An additional 12 weeks of dual therapy (PEG and RBV) may also be administered depending on viral response.7 Clinical trials demonstrated that the addition of either boceprevir or telaprevir to PEG and RBV significantly increases sustained virologic response (SVR) rates (defined as undetectable HCV RNA level, which probably equates to a cure) in both treatment naïve and previously treated populations compared with PEG and RBV.8-11 In 1 study, DAA-based regimens were associated with more adverse events, including anemia and rash, compared with PEG and RBV alone.12
Patient population and treatment-level differences between clinical trial and real-world settings may result in different rates of response and safety outcomes. For example, adherence to therapy may be lower in the real-world setting. SVR rates have been shown to be significantly related to adherence rates in boceprevir trials.13 Existing real-world data on the efficacy, safety, and adherence to DAA regimens in the United States are limited. Previous studies have examined the use of DAAs in specific subpopulations, such as the Veterans Administration (VA) health care system in the United States14-17 or in small scale health center-based studies.18,19 However, few of them investigated the treatment patterns or health care utilization and cost outcomes. This study seeks to contribute to the growing literature on real-world use of DAA therapies by describing the treatment patterns (e.g., initiation, duration, and noncompletion of a minimum duration of therapy as per the prescribing information); health care resource utilization; and health care costs of a commercially insured U.S. population of CHC patients treated with boceprevir or telaprevir. It also presents adjusted costs analyses to compare the cost burden experienced by boceprevir and telaprevir patients, controlling for demographic factors and other patient characteristics.
Methods
Data Source
This study used data from Truven Health MarketScan Commercial Claims and Encounters and Medicare Supplemental and Coordination of Benefits Databases (January 1, 2006-September 30, 2012). These databases contain deidentified information on health insurance claims of employees of large, self-insured corporations and their dependents, along with a few commercial health plans, and of Medicare-eligible persons who are also covered by self-insured employers.
Study Population
Patients with at least 1 diagnosis code for CHC (International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM] codes 070.44, 070.54, 070.70, 070.71) and a prescription for boceprevir or telaprevir after May 13, 2011 (date of first DAA availability) were selected. The first prescription for a DAA was defined as the index therapy. For patients who received telaprevir, the date of the first prescription was defined as the index date; for patients who received boceprevir, the date 4 weeks prior to boceprevir initiation was defined as the index date, since boceprevir requires a 4-week lead-in treatment period with PEG and RBV. Patients were required (a) to be at least aged 18 years as of the index date, (b) to be continuously enrolled in a health plan for at least 6 months before (baseline) and 12 months following (study period) the index date, and (c) to have no diagnosis for hepatitis B during the baseline or study periods.
Patient Characteristics
Patient demographics and disease characteristics were summarized over a 6-month baseline period prior to the index date; treatment pattern outcomes, resource utilization, and costs were analyzed over a 12-month study period. All results were stratified by cohort (telaprevir or boceprevir). Patient demographic and baseline characteristics included patient’s age; gender; region of residence; insurance type; Charlson Comorbidity Index (CCI)20; comorbidities of interest (liver-related complications, including cirrhosis, decompensating cirrhosis, liver cancer, liver transplant, other sequelae of chronic liver disease, unspecified disorder of the liver); and previous CHC treatment (any time in the patient’s claims history). Refer to Appendices A and B (available in online article) for codes used to identify CHC therapies and conditions.
Treatment Patterns
Treatment pattern outcomes included therapy segment initiations (i.e., lead-in, triple, and dual therapy); therapy durations overall and by segment; and therapy noncompletion rates. Lead-in therapy initiation (for boceprevir only) was defined as the first day of concurrent treatment with PEG and RBV during the 4 weeks prior to boceprevir initiation. Triple therapy initiation was defined as the first day of concurrent treatment with the index drug, PEG, and RBV. Dual therapy initiation was defined as the first day of concurrent treatment with PEG and RBV following the last day of continuous treatment with the index drug after triple therapy initiation. The duration of each therapy was defined as the length of concurrent and continuous treatment with all drugs required by FDA prescribing information in that segment. Continuous treatment allowed for gaps of 45 days in medication supply for any of the therapies, following a treatment definition used in previous HCV studies.21,22 Therapy noncompletion rates were defined for each drug based on minimum recommended therapy according to prescribing information (Figure 1). For telaprevir patients, this was defined as not completing at least 12 weeks of triple therapy followed by at least 12 weeks of dual therapy; for boceprevir patients, this was defined as not completing at least 3 weeks of lead-in treatment followed by at least 24 weeks of triple therapy. Additionally, factors related to therapy noncompletion were evaluated by multivariable logistic regression, including treatment cohort (telaprevir or boceprevir), gender, prior CHC treatment, age, and CCI. The factors investigated were defined a priori, and no model selection or model building was planned or conducted. A predetermined significance level of 0.05 (two-tailed) was used for the logistic regression.
FIGURE 1.
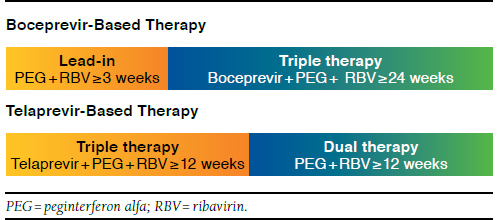
Minimum Duration of Recommended Therapy As Per Prescribing Information
Resource Utilization and Costs
Resource utilization outcomes included rates and frequencies of inpatient (IP), emergency room (ER), and outpatient (OP) care, as well as number of prescriptions for CHC-related treatment and other drugs (i.e., non-CHC related). IP and OP claims were used as provided in the databases. Claims for ER services were identified from IP and OP claims based on the place of service. All cost values were measured as the amount paid by third-party payers and were inflated to 2012 U.S. dollars using the medical care component of the Consumer Price Index.23 CHC treatment costs included costs for all pharmacy claims as well as medical claims identified via Healthcare Common Procedure Coding System codes for CHC treatment (Appendix A, available in online article). Unadjusted health care costs included total medical costs, IP costs, ER costs, OP costs, and drug costs, including CHC treatment costs and other drug costs. Adjusted health care costs were estimated by a regression model for total medical, IP, ER, OP, and drug costs. Cost adjustment models controlled for cohort (boceprevir or telaprevir), baseline costs (of the same type), gender, prior CHC treatment, age, and CCI. All covariates adjusted for in the regression models were defined a priori, and no model selection or model building was planned or conducted. One-part generalized linear regression models with a gamma distribution and log link were used for the comparison of total medical costs, OP costs, and drug costs. Two-part models were used for IP and ER costs, since zero costs were observed in more than 5% of the patients. The first part of the model used logistic regression to model the probability of incurring any health care costs, and the second part used a generalized linear regression with a gamma distribution and log link to model health care costs among those with nonzero costs. Standard errors and P values were obtained using a bootstrap-based approach (1,000 replications). The Kolmogorov-Smirnov test for the equality of distributions was used to test the null hypothesis that the adjusted costs for telaprevir and boceprevir arise from identical distributions.
Results
Patient Characteristics
A total of 871 telaprevir patients and 284 boceprevir patients who satisfied eligibility criteria were identified in the claims database (Figure 2). The average age was 54.1 (standard deviation [SD] = 7.6) years for the telaprevir cohort and 53.3 (SD = 8.9) years for the boceprevir cohort, and males composed 63.4% (n = 522) and 58.5% (n = 166) of telaprevir and boceprevir cohorts, respectively. Approximately 61.6% (n = 537) of telaprevir patients and 48.6% (n = 138) of boceprevir patients were CHC treatment naïve on the index date (i.e., received no CHC treatment at any time in their claims history prior to the index date; Table 1).
FIGURE 2.
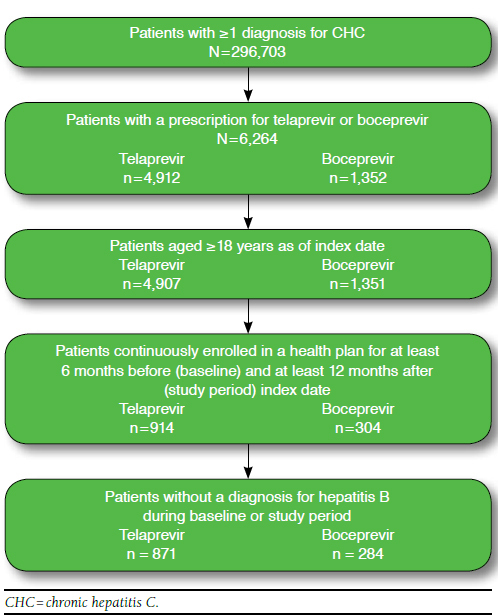
Flowchart of Sample Selection
TABLE 1.
Baseline Characteristics and Conditions a
| Telaprevir Patients (n = 871) | Boceprevir Patients (n = 284) | |
|---|---|---|
| Demographics | ||
| Age (years), mean ± SD | 54.1 ± 7.6 | 53.3 ± 8.9 |
| Male, n (%) | 522 (63.4) | 166 (58.5) |
| Charlson Comorbidity Index, mean ± SD | 0.93 ± 1.3 | 0.73 ± 1.1 |
| Select comorbidities, n (%) | ||
| HIV/AIDS | 9 (1.0) | 0 (0.0) |
| Acute renal failure | 3 (0.3) | 2 (0.7) |
| Cerebrovascular disease | 7 (0.8) | 6 (2.1) |
| Congestive heart failure | 5 (0.6) | 3 (1.1) |
| Depression | 76 (8.7) | 19 (6.7) |
| Diabetes | 119 (13.7) | 34 (12.0) |
| Diabetes with chronic complications | 15 (1.7) | 5 (1.8) |
| Hypertension | 244 (28.0) | 66 (23.2) |
| Myocardial infarction | 3 (0.3) | 1 (0.4) |
| Peripheral vascular disease | 4 (0.5) | 3 (1.1) |
| Renal disease | 9 (1.0) | 2 (0.7) |
| Previous CHC treatment (any history),b n (%) | 334 (38.4) | 146 (51.4) |
| Liver-related complications, n (%) | ||
| Any liver-related complication | 268 (30.8) | 67 (23.6) |
| Cirrhosis | 216 (24.8) | 52 (18.3) |
| Decompensating cirrhosis | 82 (9.4) | 20 (7.0) |
| Liver cancer | 15 (1.7) | 3 (1.1) |
| Liver transplant | 12 (1.4) | 1 (0.4) |
| Other sequelae of chronic liver disease | 5 (0.6) | 1 (0.4) |
| Unspecified disorder of the liver | 28 (3.2) | 9 (3.2) |
a The baseline period was defined as 6 months prior to the index date.
b Previous treatment for CHC was based on a patient’s entire claims history prior to the index date.
CHC = chronic hepatitis C; HIV/AIDS = human immunodeficiency virus/acquired immunodeficiency syndrome; SD = standard deviation.
During the 6-month baseline period, telaprevir patients had an average CCI of 0.93, and boceprevir patients had an average CCI of 0.73. The most common comorbidities were hypertension, diabetes, and depression. Less than 1% of patients were co-infected with human immunodeficiency virus, and approximately 1%-2% of patients in both cohorts had renal disease. Liver-related complications were observed in 30.8% of telaprevir and 23.6% of boceprevir patients; 24.8% and 18.3% of telaprevir and boceprevir patients, respectively, had cirrhosis, which was the most common liver-related complication observed in the study population (Table 1).
Treatment Patterns
During the study period, approximately 97.0% of telaprevir patients and 97.9% of boceprevir patients initiated triple therapy consisting of the DAA (telaprevir or boceprevir), PEG, and RBV, and 74.7% of telaprevir patients and 42.6% of boceprevir patients also initiated dual therapy with PEG and RBV. The mean overall therapy duration for those who initiated triple therapy was 25.6 weeks for telaprevir patients (triple + dual therapy segments) and 28.6 for boceprevir patients (lead-in + triple + dual therapy segments), while duration of index therapy was 11.9 weeks for telaprevir patients and 24.9 weeks for boceprevir patients. Approximately 54% of telaprevir patients and 74% of boceprevir patients did not complete minimum recommended therapy according to prescribing information (Table 2).
TABLE 2.
Study Period Treatment Patterns
| Treatment Pattern Outcomesa | Telaprevir Patients (n = 871) | Boceprevir Patients (n = 284) |
|---|---|---|
| Therapy segment initiation, n (%) | ||
| Lead-in therapy (PEG + RBV) | — | 185 (65.1) |
| Triple therapy (index drug + PEG + RBV) | 845 (97.0) | 278 (97.9) |
| Dual therapy (PEG + RBV) | 651 (74.7) | 121 (42.6) |
| Therapy duration (weeks),b mean ± SD | ||
| Index drug | 11.9 ± 4.4 | 24.9 ± 13.3 |
| Overall (lead-in+triple+dual) | 25.6 ± 14.9 | 28.6 ± 13.6 |
| Lead-in therapy | — | 3.3 ± 0.9 |
| Triple therapy | 11.6 ± 4.2 | 23.9 ± 12.8 |
| Dual therapy | 18.2 ± 12.0 | 5.8 ± 6.0 |
| Therapy noncompletion,c n (%) | 470 (54.0) | 210 (73.9) |
a Guidelines differ for each DAA; as such, certain outcomes of interest vary by treatment.
b All therapy duration outcomes are calculated only among those patients with nonzero duration for that segment.
c Noncompletion of the DAA-based regimen was defined as not completing at least 12 weeks of triple therapy followed by at least 12 weeks of dual therapy for telaprevir and not completing at least 3 weeks of lead-in treatment followed by at least 24 weeks of triple therapy for boceprevir.
DAA = direct-acting antiviral; PEG = peginterferon alfa; RBV = ribavirin; SD = standard deviation.
Logistic regression models of noncompletion of the DAA-based regimens found that telaprevir patients were more likely to complete minimum recommended therapy than boceprevir patients (noncompletion odds ratio [OR] = 0.42, 95% confidence interval [CI] = 0.31-0.56, P < 0.001) and that women were less likely to complete minimum recommended therapy than men (noncompletion OR = 1.38, 95% CI = 1.08-1.78, P < 0.001; Table 3).
TABLE 3.
Risk Factors for Therapy Noncompletion a
| Risk Factor | Odds Ratio | 95% CI | P Valueb |
|---|---|---|---|
| Unadjusted | |||
| Cohort (reference = boceprevir) | 0.41 | [0.31-0.56] | < 0.001 |
| Adjusted | |||
| Cohort (reference = boceprevir) | 0.42 | [0.31-0.56] | < 0.001 |
| Gender (reference = male) | 1.38 | [1.08-1.78] | 0.011 |
| Age | 1.00 | [0.99-1.02] | 0.809 |
| Any prior CHC treatment (reference = no prior treatment) | 0.93 | [0.72-1.18] | 0.541 |
| Charlson Comorbidity Index | 0.97 | [0.88-1.06] | 0.479 |
a Noncompletion of the DAA-based regimen was based on completion of a minimum duration of recommended therapy as per the prescribing information and was defined as not completing at least 12 weeks of triple therapy followed by at least 12 weeks of dual therapy for telaprevir and not completing at least 3 weeks of lead-in treatment followed by at least 24 weeks of triple therapy for boceprevir.
b P values were generated from multivariable logistic regression models, including treatment cohort (telaprevir or boceprevir), gender, prior CHC treatment, age, and Charlson Comorbidity Index.
CHC = chronic hepatitis C; CI = confidence interval; DAA = direct-acting antiviral.
Resource Utilization and Costs
Almost all patients had at least 1 outpatient visit (telaprevir: 99.4%, boceprevir: 97.2%) during the 6-month baseline period; telaprevir and boceprevir patients averaged 10.6 and 9.5 baseline outpatient visits, respectively (Table 4). Mean total baseline medical costs were $7,107 for telaprevir and $5,562 for boceprevir, with outpatient costs comprising over three-fourths of baseline medical costs in both cohorts. IP costs in both cohorts made up approximately 15% of baseline medical costs. Total baseline drug costs were $2,834 in the telaprevir cohort and $3,151 in the boceprevir cohort (Table 4).
TABLE 4.
Health Care Resource Utilization During Baseline and Study Periods a
| Telaprevir Patients (n = 871) | Boceprevir Patients (n = 284) | |
|---|---|---|
| Baseline period (6 months) | ||
| At least 1 visit, n (%) | ||
| IP visits | 38 (4.4) | 15 (5.3) |
| ER visits | 105 (12.1) | 44 (15.5) |
| OP visits | 866 (99.4) | 276 (97.2) |
| Number of visits, mean ± SD | ||
| IP visits | 0.1 ± 0.3 | 0.1 ± 0.3 |
| ER visits | 0.2 ± 0.5 | 0.2 ± 0.5 |
| OP visits | 10.6 ±9.3 | 9.5 ± 7.0 |
| Drug prescriptions for CHC treatment | ||
| At least 1 drug prescription, n (%) | 154 (17.7) | 93 (32.8) |
| Number of drug prescriptions, mean ± SD | 0.5 ± 1.5 | 1.2 ± 2.6 |
| Study period (12 months) | ||
| At least 1 visit, n (%) | ||
| IP visits | 151 (17.3) | 47 (16.5) |
| ER visits | 242 (27.8) | 86 (30.3) |
| OP visits | 871 (100.0) | 282 (99.3) |
| Number of visits, mean ± SD | ||
| IP visits | 0.3 ± 0.9 | 0.2 ± 0.7 |
| ER visits | 0.5 ± 1.2 | 0.5 ± 1.3 |
| OP visits | 29.1 ± 18.6 | 29.9 ± 16.9 |
| Drug prescriptions for CHC treatment | ||
| At least 1 drug prescription, n (%) | 871 (100.0) | 284 (100.0) |
| Number of drug prescriptions, mean ± SD | 15.2 ±8.0 | 18.9 ± 9.5 |
a The baseline period was defined as 6 months before the index date, and the study period was defined as 12 months after the index date.
CHC = chronic hepatitis C; ER = emergency room; IP = inpatient; OP = outpatient; SD = standard deviation.
During the 12-month study period, telaprevir and boceprevir patients had on average 29.1 and 29.9 OP visits, respectively, almost tripling the numbers during the baseline period, which can be attributable to the fact that DAA-based therapies require constant monitoring, including laboratory testing and physician visits. Approximately 17.3% of telaprevir patients and 16.5% of boceprevir patients had an IP visit, and 27.8% of telaprevir patients and 30.3% of boceprevir patients had an ER visit. On average, telaprevir patients received 15.2 prescriptions for CHC treatment, and boceprevir patients received 18.9 prescriptions for CHC treatment (Table 4). Mean total study period medical costs were $19,519 for telaprevir and $16,927 for boceprevir, with OP costs comprising slightly more than half and IP costs consisting of over 40% of study period medical costs in both cohorts. Study period drug costs were $76,497 in the telaprevir cohort and $59,953 in the boceprevir cohort, and CHC treatment costs accounted for approximately 91% of study period drug costs in the telaprevir cohort and 85% in the boceprevir cohort (Table 5).
TABLE 5.
Unadjusted and Adjusted Health Care Costs During Baseline and Study Periods a
| Telaprevir Patients (n = 871) | Boceprevir Patients (n = 284) | P Valuec | |
|---|---|---|---|
| Unadjusted Health Care Costs ($) | |||
| Baseline period (6 months) costs, mean ± SD | |||
| Medical costs | 7,107 ± 36,348 | 5,562 ± 9,242 | |
| IP costs | 1,123 ± 12,811 | 972 ± 4,750 | |
| ER costs | 556 ± 12,395 | 161 ± 547 | |
| OP costs | 5,427 ± 25,938 | 4,429 ± 7,251 | |
| Drug costs | 2,834 ± 17,352 | 3,151 ± 5,245 | |
| Drug costs for CHC treatment | 809 ± 2,678 | 1,931 ± 4,145 | |
| Other drug costs | 2,024 ± 16,979 | 1,220 ± 3,340 | |
| Study period (12 months) costs, mean ± SD | |||
| Medical costs | 19,519 ± 57,986 | 16,927 ± 40,915 | |
| IP costs | 7,975 ± 42,729 | 7,607 ± 34,589 | |
| ER costs | 430 ± 1,742 | 462 ± 1,144 | |
| OP costs | 11,114 ± 35,661 | 8,858 ± 12,187 | |
| Drug costs | 76,497 ± 37,341 | 59,953 ± 41,665 | |
| Drug costs for CHC treatment | 69,426 ± 21,210 | 51,014 ± 24,657 | |
| Other drug costs | 7,071 ± 31,287 | 8,939 ± 29,831 | |
| Adjusted Health Care Costsb ($) | |||
| Study period (12 months) costs, mean (SE) | |||
| Medical costs | 18,148 (1,593) | 18,301 (2,181) | 0.966 |
| IP costs | 7,507 (1,363) | 9,476 (2,915) | 0.475 |
| ER costs | 421 (46) | 486 (76) | 0.491 |
| OP costs | 9,848 (721) | 9,663 (769) | 0.881 |
| Drug costs | 76,560 (1,180) | 59,678 (2,254) | < 0.001 |
a The baseline period was defined as 6 months before the index date, and the study period was defined as 12 months after the index date.
b All models included the following covariates: cohort (telaprevir or boceprevir), gender, age, prior CHC treatment, CCI, and the log of baseline cost of the same type. One-part generalized linear regression models with a gamma distribution and log link were used for the comparison of total medical costs, OP costs, and drug costs. Two-part models were used for IP and ER costs, since zero costs were observed in more than 5% of the patients.
c P values were obtained using a bootstrap-based approach (1,000 replications). The Kolmogorov-Smirnov test for the equality of distributions was used to test the null hypothesis that the adjusted costs for telaprevir and boceprevir arise from identical distributions.
CCI = Charlson Comorbidity Index; CHC = chronic hepatitis C; ER = emergency room; IP = inpatient; OP = outpatient; SD = standard deviation; SE = standard error.
After adjusting for cohort (telaprevir or boceprevir), gender, age, prior CHC treatment, CCI, and the log of baseline costs of the same type (because a log link function was used for the dependent cost variable), no significant differences between telaprevir and boceprevir patients were found for total medical, IP, ER, and OP costs. However, in multivariable models of total drug costs controlling for the same variables, telaprevir patients were found to have statistically significantly higher total annual drug costs than boceprevir patients (P < 0.001; Table 5).
Discussion
This study adds to existing literature on CHC treatment by describing the patient characteristics, treatment patterns, health care resource utilization, health care costs, and factors associated with therapy noncompletion of a cohort of CHC patients from a large, commercially insured population treated with the DAA agents telaprevir and boceprevir. The study found that 54% of telaprevir patients and 74% of boceprevir patients did not complete minimum duration of therapy as per the FDA prescribing information. Telaprevir patients were more likely to complete minimum recommended therapy than boceprevir patients, and women were less likely to do so than men. Additionally, patients taking telaprevir or boceprevir incurred high medical and drug costs following the initiation of a DAA-based regimen, with telaprevir patients experiencing significantly higher adjusted total drug costs.
Directly comparing the rates of therapy noncompletion found in this study with discontinuation rates in the literature is challenging given differing definitions of discontinuation, differing lengths of follow-up, and the lack of SVR data in this study to determine the recommended regimen for any given patient. However, this study finds rates similar to a 2 center-based study of U.S. CHC patients and a study of CHC treatment in France.18,24 In a chart review study conducted among HCV patients in the United States, Bichoupan et al. (2014) found that 52% of telaprevir patients discontinued therapy early, which is comparable to the approximately 54% of telaprevir patients who did not complete therapy in our study.18 Similarly, a French multicenter prospective cohort reported early discontinuation rates of 47% among telaprevir patients; however, 42% of boceprevir patients discontinued therapy early, a finding that is lower than the current study.24 This difference is potentially due to the prospective cohort study nature that requires monitoring and assessment of patients throughout the study and clearly defined treatment protocol for lead-in therapy as part of boceprevir treatment in the French study.24
Several other real-world studies of telaprevir and boceprevir used the U.S. Veteran’s Affairs (VA) electronic medical record database and found rates of therapy discontinuation generally lower than those observed in this study.14-17 Backus et al. (2014) and Belperio et al. (2013) defined duration of treatment from the start of boceprevir or telaprevir to the last day of treatment covered by PEG and found that approximately one-third of telaprevir and boceprevir patients discontinued therapy before week 24.14,15 In another VA database analysis, Ioannou et al. (2014) defined duration of treatment from the first fill of PEG/RBV to the last day of treatment covered by PEG/RBV and found that approximately 41%-45% of telaprevir patients did not complete at least 24 weeks of therapy, and 40%-55% of boceprevir patients did not complete at least 28 weeks of therapy.17 Finally, Majid et al. (2014) found that 49% of boceprevir patients discontinued therapy prior to 24 weeks.16
The higher rates of therapy noncompletion found in the current study are likely attributable to a stricter definition of therapy completion and differences in the study populations. For example, this study required that patients take all 3 drugs for the minimum required duration of triple therapy and that patients take PEG and RBV for the minimum recommended duration of lead-in or dual therapy, whereas other definitions did not require that individual segments of a regimen meet guidelines, such as simultaneous possession of all required drugs. Furthermore, studies using data from the VA analyzed patient populations that consisted mostly of males. However, this study found that female patients were at an increased risk for therapy noncompletion, and the higher percentage of females in this study relative to studies of the VA population may partially explain the higher discontinuation rate found in this study.14-17
Taken holistically, the results from the current study support evidence in the literature that completion rates of DAA therapy regimens in the real-world are lower than in clinical trials, which report 66%-73% completion rates in telaprevir patients10,11 and 60%-65% completion rates in boceprevir patients.8,9 The higher completion rates found in clinical trials likely reflect the carefully selected patient populations and additional resources used to encourage patient compliance with medications.
In addition to high therapy noncompletion rates, the currently available treatment is limited by several contraindications that may preclude the treatment of CHC patients. In research conducted in parallel to the current study, we found that approximately 60% of untreated CHC patients had a contraindicated condition for DAA-based regiments including PEG and RBV, and the most common contraindications included arterial hypertension (32%), hepatic decompensation (22%), major system impairment (19%), and psychiatric depression (11%).25 Thus, new therapies that are less burdensome with fewer contraindications have the potential to greatly benefit this patient population.
This study also demonstrates that CHC patients used substantial health care resources during the 12-month period following initiation of a DAA-based regimen. Patients frequently used outpatient services, with both cohorts having approximately 29 outpatient visits during the 12-month study period. Additionally, approximately one-third of patients visited the ER and one-sixth of patients had a hospitalization during this same time period. Further, telaprevir and boceprevir patients incurred approximately $19,500 and $17,000 in medical costs, respectively, during the study period. Outpatient costs composed the largest component of medical costs in both cohorts. Inpatient costs in the study period also composed a substantial component of costs (> 40%) and increased by more than a factor of 7 compared with the 6-month baseline period. This suggests that inpatient costs during the 12-month study period increased for reasons other than the longer duration of the study period compared with the baseline.
Finally, drug costs during the study period were approximately $76,500 for telaprevir and $60,000 for boceprevir; these drug costs were nearly 4 times the magnitude of total medical costs, demonstrating the substantial costs of telaprevir- and boceprevir-based regimens. Telaprevir patients experienced higher adjusted drug costs despite undergoing shorter overall (25.6 weeks vs. 28.6 weeks) and index (11.9 weeks vs. 24.9 weeks) therapy durations. Despite its higher costs, more patients were treated with telaprevir than boceprevir in our study (75% and 25%, respectively), a trend potentially suggesting that patients and providers in a commercial population may prefer a shorter duration of therapy. None of the real-world studies discussed previously conducted analyses of costs; however, the results found here are consistent with the observation that the wholesale acquisition cost (WAC) for boceprevir was typically less expensive than telaprevir (boceprevir: $26,410 for 24 weeks, $35,213 for 32 weeks; telaprevir: $49,200 for 12 weeks [prices reflect WAC in 2011]).26
Limitations
The present study is subject to several limitations. First, the study assumed that possession of medication translates into actual use of medication; however, this cannot be confirmed using claims data. Second, the MarketScan dataset only contains data from employer-sponsored plans or Medicare Supplemental plans, and these patients may differ from other patient populations. So, our results may not be generalizable to patients who are uninsured or not covered by commercial insurance, such as those in the VA system or Medicaid. Third, since medical conditions and medical services were identified based on administrative codes in the claims database, such information could be underestimated. For example, a patient may have been treated as part of a clinical study, which would not be captured in the insurance claims. Finally, the MarketScan dataset does not contain RNA test results. Therefore, this study could not assess adherence to therapy guidelines beyond adherence to minimum therapy, since therapy guidelines are dependent upon these values. As such, a future study for patients with RNA test values is warranted to refine the findings of the present study.
Conclusions
This study finds high therapy noncompletion rates and high medical and drug costs among a cohort of commercially insured patients receiving DAA treatment for CHC. Furthermore, the study finds that women are less likely than men to complete minimum recommended durations of therapy based on FDA prescribing information. Additionally, telaprevir patients completed shorter durations of therapy and were more likely to complete the minimum recommended duration of therapy despite telaprevir being more expensive than boceprevir. The results of this study highlight the need for new CHC treatment regimens that facilitate improved adherence.
APPENDIX A. CPT-4/HCPCS and GPI Codes to Identify CHC Therapy
| Drug | CPT-4/HCPCS Code | GPI Code |
|---|---|---|
| Interferon only | J9212, J9213, J9214 | 12-35-30-40-10, 21-70-00-60-10, 21-70-00-60-20 |
| PEG only | S0145, S0146, S0148 | 12-35-30-60-05, 12-35-30-60-10 |
| RBV only | — | 12-35-30-70-00 |
| Interferon and RBV | — | 12-99-50-02-60 |
| PEG and RBV | — | 12-99-50-02-67, 12-99-50-02-70 |
| Boceprevir only | — | 12-35-30-15-00 |
| Telaprevir only | — | 12-35-30-85-00 |
| Boceprevir, PEG, and RBV | — | 12-99-60-03-20 |
CHC = chronic hepatitis C; CPT-4 = Current Procedural Terminology, 4th edition; HCPCS = Healthcare Common Procedure Coding System; GPI = Generic Product Identifier; PEG = peginterferon alfa; RBV = ribavirin.
APPENDIX B. Codes to Identify Conditions
| Condition | ICD-9-CM Diagnosis, ICD-9-CM Procedure, or CPT-4 Codes (ICD-9-CM Diagnosis Codes Unless Otherwise Specified) |
|---|---|
| Liver-Related Complications | |
| Cirrhosis | 571.1, 571.2, 571.5, 571.6 |
| Decompensated cirrhosis | 070.44, 070.71, 348.3, 456.0, 456.1, 456.2, 572.2, 572.3, 572.4, 782.4, 789.5 |
| Liver cancer | 155 |
| Liver transplant | V42.7, V49.83; ICD-9-CM Procedure: 50.5x; CPT-4: 47135, 47136 |
| Other sequelae of chronic liver disease | 572.8 |
| Unspecified disorder of the liver | 573.9 |
| Comorbidities | |
| Charlson Comorbidity Index a | |
| HIV/AIDS | 042-044 |
| Cerebrovascular disease | 430-438 |
| Chronic pulmonary disease | 490-496, 500-505, 506.4 |
| Congestive heart failure | 428-428.9 |
| Dementia | 290-290.9 |
| Diabetes | 250.0-250.3, 250.7 |
| Diabetes with chronic complications | 250.4-250.6 |
| Hemiplegia or paraplegia | 342-342.9, 344.1 |
| Malignancy | 140-172.9, 174-195.8, 200-208.9 |
| Metastatic solid tumor | 196-199.1 |
| Mild liver disease | 571.2, 571.4, 571.5, 571.6 |
| Moderate or severe liver disease | 456-456.21, 572.2-572.8 |
| Myocardial infarction | 410-410.9, 412 |
| Peptic ulcer disease | 531-534.9 |
| Peripheral vascular disease | 441, 443.9, 785.4, V43.4; ICD-9-CM Procedure: 38.48 |
| Renal disease | 582, 583.0-583.7, 585, 586, 588 |
| Rheumatologic disease | 710.0, 710.1, 710.4, 714.0-714.2, 714.81, 725 |
| Additional comorbidities | |
| Acute renal failure | 584.5-584.9 |
| Depression | 296.2, 296.3, 309.0, 309.1, 311 |
| Hypertension | 401-405 |
a Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases.20
CPT-4 = Current Procedural Terminology, 4th edition; HIV/AIDS = human immunodeficiency virus/acquired immunodeficiency syndrome; ICD-9-CM = International Classification of Diseases, Ninth Revision, Clinical Modification.
Funding Statement
Funding for this study was provided by Bristol-Myers Squibb (BMS) to Analysis Group. Le, Kalsekar, and Yuan are employees of BMS. Macaulay, Sorg, Wei, and Wu are employees of Analysis Group, and received support from BMS for this study. Behrer was employed by Analysis Group at the time of this study.
REFERENCES
- 1.Centers for Disease Control and Prevention. Hepatitis C FAQs for the public. Available at: http://www.cdc.gov/hepatitis/c/cfaq.htm. Accessed February 23, 2015.
- 2.Manos MM, Shvachko VA, Murphy RC, Arduino JM, Shire NJ.. Distribution of hepatitis C virus genotypes in a diverse U.S. integrated health care population. J Med Virol. 2012;84(11):1744-50. [DOI] [PubMed] [Google Scholar]
- 3.Denniston MM, Jiles RB, Drobeniuc J, et al. . Chronic hepatitis C virus infection in the United States, National Health and Nutrition Examination Survey 2003 to 2010. Ann Intern Med. 2014;160(5):293-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wong JB, McQuillan GM, McHutchison JG, Poynard T.. Estimating future hepatitis C morbidity, mortality, and costs in the United States. Am J Public Health. 2000;90(10):1562-69. Available at: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1446368/pdf/11029989.pdf. Accessed February 23, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McAdam-Marx C, McGarry LJ, Hane CA, Biskupiak J, Deniz B, Brixner DI.. All-cause and incremental per patient per year cost associated with chronic hepatitis C virus and associated liver complications in the United States: a managed care perspective. J Manag Care Pharm. 2011;17(7):531-46. Available at: http://www.amcp.org/WorkArea/DownloadAsset.aspx?id=10710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.INCIVEK (telaprevir) tablets, for oral use. Vertex Pharmaceuticals. Revised October 2013. Available at: http://www.accessdata.fda.gov/drugsatfda_docs/label/2013/201917s012lbl.pdf. Accessed February 23, 2015.
- 7.VICTRELIS (boceprevir) capsules for oral use. Merck Sharp & Dohme Corp. Revised February 2014. Available at: http://www.accessdata.fda.gov/drugsatfda_docs/label/2014/202258s014lbl.pdf. Accessed February 23, 2015.
- 8.Poordad F, McCone J Jr, Bacon BR, et al. . Boceprevir for untreated chronic HCV genotype 1 infection. N Engl J Med. 2011;364(13):1195-206. Available at: http://www.nejm.org/doi/pdf/10.1056/NEJMoa1010494. Accessed February 23, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bacon BR, Gordon SC, Lawitz E, et al. . Boceprevir for previously treated chronic HCV genotype 1 infection. N Engl J Med. 2011;364(13):1207-17. Available at: http://www.nejm.org/doi/pdf/10.1056/NEJMoa1009482. Accessed February 23, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jacobson IM, McHutchison JG, Dusheiko G, et al. . Telaprevir for previously untreated chronic hepatitis C virus infection. N Engl J Med. 2011;364(25):2405-16. Available at: http://www.nejm.org/doi/pdf/10.1056/NEJMoa1012912. Accessed February 23, 2015. [DOI] [PubMed] [Google Scholar]
- 11.Zeuzem S, Andreone P, Pol S, et al. . Telaprevir for retreatment of HCV infection. N Engl J Med. 2011;364(25):2417-28. Available at: http://www.nejm.org/doi/pdf/10.1056/NEJMoa1013086. Accessed February 23, 2015. [DOI] [PubMed] [Google Scholar]
- 12.Ghany MG, Nelson DR, Strader DB, Thomas DL, Leonard SB.. An update on treatment of genotype 1 chronic hepatitis C virus infection: 2011 practice guideline by the American Association for the Study of Liver Diseases. Hepatology. 2011;54(4):1433-44. Available at: http://onlinelibrary.wiley.com/doi/10.1002/hep.24641/pdf. Accessed February 23, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gordon SC, Yoshida EM, Lawitz EJ, et al. . Adherence to assigned dosing regimen and sustained virological response among chronic hepatitis C genotype 1 patients treated with boceprevir plus peginterferon alfa-2b/ribavirin. Aliment Pharmacol Ther. 2013:38(1):16-27. [DOI] [PubMed] [Google Scholar]
- 14.Backus LI, Belperio PS, Shahoumian TA, Cheung R, Mole LA.. Comparative effectiveness of the hepatitis C virus protease inhibitors boceprevir and telaprevir in a large U.S. cohort. Aliment Pharmacol Ther. 2014;39(1):93-103. Available at: http://onlinelibrary.wiley.com/doi/10.1111/apt.12546/pdf. Accessed February 23, 2015. [DOI] [PubMed] [Google Scholar]
- 15.Belperio PS, Hwang EW, Thomas IC, et al. . Early virologic responses and hematologic safety of direct-acting antiviral therapies in veterans with chronic hepatitis C. Clin Gastroenterol Hepatol. 2013;11(8):1021-27. [DOI] [PubMed] [Google Scholar]
- 16.Majid A, McAninch J, Morgan DJ, et al. . Predictors of early treatment discontinuation in a cohort of patients treated with boceprevir-based therapy for hepatitis C infection. J Viral Hepat. 2014;21(8):585-89. [DOI] [PubMed] [Google Scholar]
- 17.Ioannou GN, Beste LA, Green PK.. Similar effectiveness of boceprevir and telaprevir treatment regimens for hepatitis C virus infection on the basis of a nationwide study of veterans. Clin Gastroenterol Hepatol. 2014;12(8):1371-80. [DOI] [PubMed] [Google Scholar]
- 18.Bichoupan K, Schwartz JM, Martel-Laferriere V, et al. . Effect of fibrosis on adverse events in patients with hepatitis C treated with telaprevir. Aliment Pharmacol Ther. 2014;39(2):209-16. Available at: http://onlinelibrary.wiley.com/doi/10.1111/apt.12560/pdf. Accessed February 23, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maasoumy B, Port K, Markova AA, et al. . Eligibility and safety of triple therapy for hepatitis C: lessons learned from the first experience in a real world setting. PLoS One. 2013;8(2):e55285. Available at: http://www.plosone.org/article/fetchObject.action?uri=info%3Adoi%2F10.1371%2Fjournal.pone.0055285&representation=PDF. Accessed February 23, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deyo RA, Cherkin DC, Ciol MA.. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613-19. [DOI] [PubMed] [Google Scholar]
- 21.Kramer JR, Kanwal F, Richardson P, Mei M, El-Serag HB.. Gaps in the achievement of effectiveness of HCV treatment in national VA practice. J Hepatol. 2012;56(2):320-25. [DOI] [PubMed] [Google Scholar]
- 22.Backus LI, Boothroyd DB, Phillips BR, Mole LA.. Predictors of response of US veterans to treatment for the hepatitis C virus. Hepatology. 2007;46(1):37-47. Available at: http://onlinelibrary.wiley.com/doi/10.1002/hep.21662/pdf. Accessed February 23, 2015. [DOI] [PubMed] [Google Scholar]
- 23.U.S. Department of Labor, Bureau of Labor Statistics. Consumer Price Index. CPI databases. July 10, 2013. Available at: http://www.bls.gov/cpi/data.htm. Accessed February 23, 2015.
- 24.Fontaine H, Hezode C, Dorival C, et al. . SVR12 rates and safety of triple therapy including telaprevir or boceprevir in 221 cirrhotic non responders treated in the French Early Access Program (ANRS CO20-CUPIC). J Hepatol. 2013;58:S27. [Abstract]. [Google Scholar]
- 25.Le TK, Kalsekar A, Yuan Y, et al. . Contraindications for HCV therapy in US patients with untreated chronic hepatitis C. Poster presented at: ISPOR 19th Annual International Conference; May 31-June 4, 2014; Montreal, QC, Canada. [PGI3]. Available at: http://www.analysisgroup.com/uploadedFiles/News_and_Events/Events/AnalysisGroup_ISPOR2014_Guide.pdf. Accessed February 23, 2015. [Google Scholar]
- 26.Tungol A, Rademacher K, Schafer JA.. Formulary management of the protease inhibitors boceprevir and telaprevir for chronic hepatitis C virus. J Manag Care Pharm. 2011;17(9):685-94. Available at: http://www.amcp.org/WorkArea/DownloadAsset.aspx?id=13668. [DOI] [PMC free article] [PubMed] [Google Scholar]


