Abstract
BACKGROUND:
Sarcoidosis is a multisystem inflammatory disorder characterized by the presence of noncaseating granulomas in involved organs. Prior research has found that sarcoidosis imposes a significant economic burden to U.S. payers. However, the drivers of high health care costs among sarcoidosis patients are unknown.
OBJECTIVE:
To characterize sarcoidosis patients who were among the top 20% of total health care costs.
METHODS:
Patients with a first diagnosis of sarcoidosis between January 1, 1998, and March 31, 2015 (index date) were selected from a deidentified privately insured administrative claims database. Study patients must have at least 12 months of continuous health plan enrollment prior to the index date. High-cost patients were those in the top 20% of total health care costs during the 12 months following the index date (follow-up period), and the remaining patients were classified as lower-cost patients. Patient characteristics, comorbidities, health care resource use, and health care costs in the study period were compared between the high-cost and lower-cost patients. Multiple logistic regression was used to assess the relationship between patient characteristics and being a high-cost sarcoidosis patient.
RESULTS:
A total of 7,173 sarcoidosis patients met the selection criteria. The 20% of patients classified as high-cost patients accounted for approximately 72% of the total health care costs in the 12-month follow-up period. Compared with lower-cost patients, high-cost patients were slightly older (50.6 vs. 49.1 years) and had a higher comorbidity burden at baseline (Charlson Comorbidity Index = 1.8 vs. 0.7). Mean annual total health care cost for high-cost sarcoidosis patients was 10 times that of their lower-cost counterparts ($73,345 vs. $7,073). Mean annual health care cost was $119,878 for patients in the 95th-99th percentile and $375,436 for patients in the top 1% of spend. High-cost patients had greater medical resource use and costs across all places of service (i.e., inpatient, emergency department, outpatient, and other) compared with lower-cost patients. Findings showed that higher total health care cost resulted in a larger proportion of inpatient spend and a smaller proportion of outpatient and pharmacy spend. Adjusting for baseline characteristics, high-cost patients were associated with a number of factors with high ORs: the presence of comorbidities such as deficiency anemia (OR = 1.606; P < 0.001), depression (OR = 1.504; P < 0.001), or cardiac arrhythmia (OR = 1.493; P < 0.001); having an inpatient admission (OR = 9.771; P < 0.001); and use of biologic therapies adalimumab and/or infliximab (OR = 31.821; P < 0.001).
CONCLUSIONS:
This study described the characteristics of high-cost sarcoidosis patients and identified several high-cost indicators using contemporary administrative data. The health care cost distribution for sarcoidosis patients is highly skewed, making it a worthwhile endeavor to focus improvement efforts on patients in the top quintile. The study findings can help population health decision makers identify a subset of patients for targeted interventions aimed at improving quality of care and reducing overall costs.
What is already known about this subject
Sarcoidosis is associated with a significant health and economic burden in the United States—commercial payers incur approximately $19,714 in total annual health care costs per sarcoidosis patient in the first year following diagnosis.
Existing evidence suggests that there is considerable variation in health care spending across sarcoidosis patients; the average annual cost of care for the top 5% of patients has been estimated at $93,201.
Drivers of high health care costs among sarcoidosis patients are unknown.
What this study adds
This retrospective claims database study of commercially insured patients showed that the distribution of total health care cost is highly skewed among sarcoidosis patients; the 20% of patients with the highest costs accounted for approximately 72% of the total health care costs for all patients diagnosed with sarcoidosis.
Mean annual total health care cost for high-cost sarcoidosis patients was 10 times that of their lower-cost counterparts ($73,345 vs. $7,073).
High-cost sarcoidosis patients are complicated patients with higher rates of comorbidities, have increased health care resource use (particularly in the form of inpatient admissions and specialty visits), and likely are treated with biologic therapies (i.e., adalimumab and/or infliximab).
Sarcoidosis is a multisystem inflammatory disorder characterized by the presence of noncaseating granulomas in involved organs.1,2 Sarcoidosis can present in any organ but localization to the lungs is the most common manifestation, affecting more than 90% of sarcoidosis patients; other organs affected by sarcoidosis include the skin, lymph nodes, eyes, and liver.3 Symptoms of sarcoidosis tend to be nonspecific and include cough, fever, fatigue, skin lesions, and joint aches and pain or arthritis.4
The disease course of sarcoidosis varies broadly. The majority of patients experience acute disease, characterized by sudden onset and spontaneous remission. More than half of sarcoidosis patients experience remission within 3 years of disease onset.5 However, up to one third of sarcoidosis patients experience a chronic, progressive disease course, which can lead to clinically significant organ impairment.5 Patients with end-stage sarcoidosis may require lung or cardiac transplantations.6 In more advanced cases, sarcoidosis can cause significant pulmonary morbidity and occasionally be fatal.6,7
Previous research has shown that newly diagnosed sarcoidosis imposes a significant societal economic burden. In a retrospective cohort analysis using privately insured administrative claims data, it was found that commercial payers incurred $19,714 in total annual health care costs per sarcoidosis patient in the first year following diagnosis.8 Based on these findings, it was estimated that sarcoidosis imposes a total direct medical cost burden of approximately $1.3 to $8.7 billion to commercial payers, or approximately $0.3 to $2.3 billion in excess costs over matched controls. A similar retrospective claims database analysis, focusing instead on incident and prevalent sarcoidosis in the United States, reported median annual disease-related medical costs of $18,663.9 The study also found considerable variation in health care spending across sarcoidosis patients; the average annual cost of care for the top 5% of patients was $93,201.
It is unknown the extent to which costs are similarly skewed in newly diagnosed sarcoidosis patients and, importantly, whether there are observable characteristics associated with such high-cost sarcoidosis patients. An improved understanding of these disproportionately costly patients is relevant for physicians, managed care organizations, and other stakeholders who have an interest in addressing the disease and reducing the associated economic burden. To this end, the objective of this study was to characterize sarcoidosis patients who were among the top 20% of total health care costs (known hereafter as high-cost patients).
Methods
Data Source
This study used deidentified administrative claims from OptumHealth Care Solutions (OptumHealth database), a database containing health care utilization records of over 19.1 million beneficiaries (including employees, spouses, dependents, and retirees) with commercial insurance from over 84 large self-insured U.S.-based companies. The database contains information regarding patient age, gender, enrollment history, medical diagnoses, procedures performed, dates and places of service, and payment amounts for the time period spanning January 1, 1998, to March 31, 2015. Prescription drug claims (including fill dates, National Drug Code numbers, and payment amounts) are available for all beneficiaries.
Sample Selection and Baseline Demographics
A retrospective analysis of administrative claims data was conducted to examine the characteristics of high-cost patients diagnosed with sarcoidosis. Patients from the OptumHealth database with at least 1 diagnosis of sarcoidosis were identified by International Classification of Disease, Ninth Revision, Clinical Modification (ICD-9-CM) diagnosis code 135.xx during the study period (January 1, 1998-March 31, 2015). The earliest sarcoidosis diagnosis during the study period was designated as the index date. Patients were required to be aged 18-64 years on the index date and have continuous insurance coverage throughout the 12 months prior to the index date (baseline period) and the 12 months following the index date (follow-up period) to ensure that all relevant drug and medical claims were captured. Patients aged 65 years and older were excluded from the study, as their Medicare eligibility may have limited the ability to observe all relevant drug and medical claims.
High-cost patients were defined as patients with total health care costs in the top quintile (i.e., top 20%) of total health care costs among the study cohort during the 12-month observation period. The remaining 80% of patients were classified as lower-cost patients.
Baseline patient characteristics were examined for all sarcoidosis patients during the 12-month period preceding the index date (i.e., date of earliest sarcoidosis diagnosis). Patient characteristics included demographics such as age, gender, region, and year of the index date.
Comorbidities
Comorbidity burden at baseline was assessed using the Charlson Comorbidity Index (CCI), a composite measure of the patient’s health status based on chronic conditions, such as AIDS, cancer, liver disease, and diabetes.10 Selected comorbidities for which sarcoidosis patients are known to be at a higher risk were evaluated during the 12-month period preceding and 12-month period following the index date. The list of sarcoidosis-related comorbidities was compiled based on published literature8 and clinical input and included a range of conditions such as interstitial lung disease, chronic pulmonary disease, pneumonia, pulmonary aspergillosis, depression, cardiac arrhythmia, deficiency anemia, obesity, hypothyroidism, and pain syndromes. Comorbidities were identified using ICD-9-CM diagnosis codes (see Appendix A, available in online article, for a complete listing of conditions and respective diagnosis codes).
Health Care Resource Utilization and Costs
Health care resource utilization and costs, including medical visits and prescription drug use, were examined during the 12-month follow-up period. Medical visits were categorized by place of service to identify sources of differential utilization. Place of service categories included the following: inpatient, outpatient/physician office, emergency department (ED), and other (e.g., skilled nursing facilities, home health). In addition, utilization of selected medical specialists known to care for sarcoidosis patients (e.g., pulmonologist, ophthalmologist, cardiologist, rheumatologist, nephrologist) was assessed. While the precise manifestation of sarcoidosis can be unknown, these specialist visits may signal the disease manifestation in that patients with pulmonary sarcoidosis may have more visits to pulmonologists and patients with cardiac sarcoidosis may have more visits to cardiologists; as such, these visits may be used as indicators of the manner in which sarcoidosis presents itself. Costs were inflated to 2015 U.S. dollars using the medical care component of the Consumer Price Index.
Statistical Analyses
Categorical variables were summarized using frequencies and percentages; continuous variables were summarized using means and standard deviations. Comparisons between high-cost and lower-cost patients diagnosed with sarcoidosis were conducted using chi-squared tests for categorical variables and Wilcoxon rank-sum tests for continuous variables.
Regression analyses were conducted to examine the relationship between patient demographics, clinical characteristics, and resource utilization and cost during the study period and being a high-cost patient based on total health care costs incurred during the follow-up period. Specifically, we estimated both univariate and multiple logistic regression models in which an indicator for each patient cost cohort (i.e., high-cost = 1, lower-cost = 0) was the dependent variable. Independent variables included age, gender, geographic region, year of index date (categorical grouping), indicator variables for sarcoidosis-related comorbidities occurring during the study period (e.g., bronchiectasis, cataracts, chronic pulmonary disease), indicator variables for evidence of medical visits by place of service and by specialist in the follow-up period, and indicator variables for use of specific classes of prescription drugs in the follow-up period. Interactions between comorbidities and drug use were also considered for model inclusion. All analyses were conducted using SAS software for Windows version 9.4 (SAS Institute, Cary, NC). Results were considered statistically significant at the 0.05 level.
Results
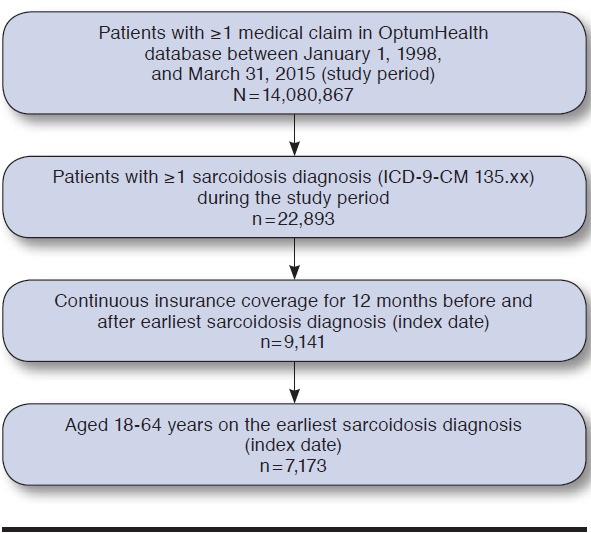
Among the 22,893 patients with evidence of sarcoidosis in the OptumHealth database during the study period, 9,141 patients had continuous coverage for the year before and after their earliest sarcoidosis diagnosis, which was classified as the index date. Of these patients, 7,173 patients were between 18 and 64 years old on their index date and, accordingly, met all sample selection criteria (Appendix B, available in online article). There were 1,434 patients classified as high-cost patients, and the remaining 5,739 patients were classified as lower-cost patients (Figure 1). On average, high-cost patients (i.e., patients in the 80th-100th cost percentile) incurred total health care costs of $73,346 in the 12-month follow-up period, more than 4.5 times that of patients in the fourth cost quintile (i.e., 60th-80th percentile) (average cost = $15,841) and nearly 68 times that of patients in the first cost quintile (i.e., 0-20th percentile) (average cost = $1,082). Total annual health care cost for the sample of newly diagnosed sarcoidosis patients was $146 million; high-cost patients (top 20%) accounted for approximately 72% (over $105 million) of total costs in the 12-month follow-up period (Figure 2).
FIGURE 1.
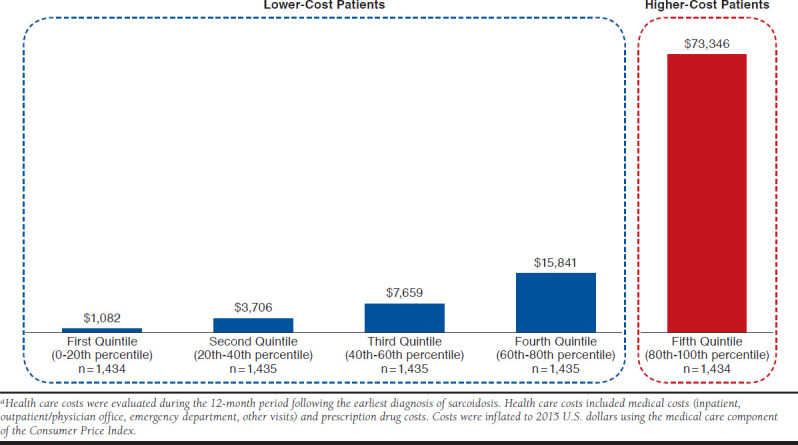
Mean Per-Patient Total Health Care Costs During the Follow-up Perioda
FIGURE 2.
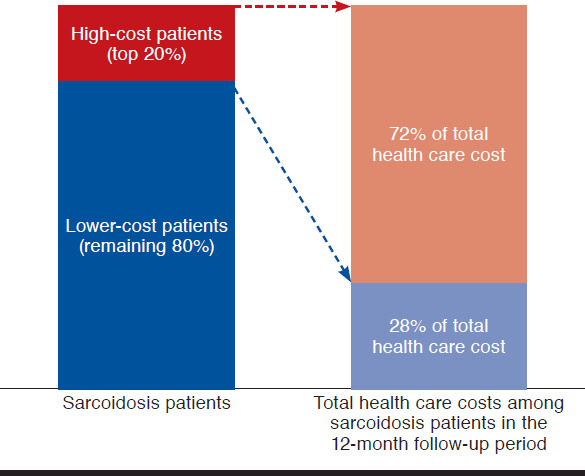
Relative Contributions of High-Cost Patients and Lower-Cost Patients to Total Health Care Costs in the Follow-up Period Among Sarcoidosis Patients
Patient Demographic and Clinical Characteristics
Relative to lower-cost patients, high-cost patients were slightly older (50.6 vs. 49.1) and had more recently diagnosed sarcoidosis (31.5% vs. 26.4% had an index date in 2011-2014; Table 1). In addition, high-cost patients had higher chronic comorbidity burden in the 12-month baseline period, as reflected in the CCI scores (1.8 vs. 0.7).
TABLE 1.
Patient Demographics, Clinical Characteristics, Resource Utilization, and Health Care Costs
| Lower-Cost Patients (n = 5,739) | High-Cost Patients (n = 1,434) | P Valuea | |||
|---|---|---|---|---|---|
| Patient demographicsb | |||||
| Age (year), mean (SD) | 49.1 | (9.8) | 50.6 | (9.1) | < 0.001c |
| Male, % (n) | 42.0 | (2,408) | 39.3 | (564) | 0.071 |
| Region, % (n) | |||||
| Northeast | 24.4 | (1,401) | 24.2 | (347) | 0.866 |
| Midwest | 23.6 | (1,353) | 26.1 | (374) | 0.047c |
| South | 38.1 | (2,188) | 34.0 | (487) | 0.004c |
| West | 9.5 | (543) | 9.5 | (136) | 0.979 |
| Unknown | 4.4 | (254) | 6.3 | (90) | 0.003c |
| Year of index date, % (n) | |||||
| 1999-2004 | 26.3 | (1,508) | 22.2 | (318) | 0.001c |
| 2005-2007 | 23.0 | (1,321) | 22.0 | (316) | 0.428 |
| 2008-2010 | 24.3 | (1,394) | 24.3 | (348) | 0.986 |
| 2011-2014 (Q1 only) | 26.4 | (1,516) | 31.5 | (452) | < 0.001c |
| CCI, mean (SD) | 0.7 | (1.2) | 1.8 | (2.1) | < 0.001c |
| Study period characteristicsd | |||||
| Number of sarcoidosis-related comorbidities,e mean (SD) | 2.7 | (1.9) | 4.4 | (2.4) | < 0.001c |
| Number of sarcoidosis-related comorbidities,e % (n) | |||||
| 0 | 11.4 | (655) | 3.3 | (48) | < 0.001c |
| 1-4 | 72.1 | (4,135) | 50.3 | (722) | < 0.001c |
| 5 or more | 16.5 | (949) | 46.3 | (664) | < 0.001c |
| Sarcoidosis-related comorbidities,e % (n) | |||||
| Bronchiectasis | 1.2 | (66) | 3.1 | (44) | < 0.001c |
| Cardiac arrhythmia | 9.2 | (529) | 28.5 | (408) | < 0.001c |
| Cataracts | 8.5 | (489) | 13.0 | (187) | < 0.001c |
| Chronic pulmonary disease | 26.3 | (1,511) | 42.3 | (606) | < 0.001c |
| Deficiency anemia | 4.7 | (271) | 11.9 | (170) | < 0.001c |
| Depression | 10.6 | (607) | 21.1 | (303) | < 0.001c |
| Diabetes | 14.7 | (845) | 28.7 | (412) | < 0.001c |
| Epilepsy and recurrent seizures | 0.9 | (54) | 2.9 | (41) | < 0.001c |
| Erythema nodosum | 2.2 | (128) | 1.5 | (22) | 0.099 |
| Essential (primary) hypertension | 39.4 | (2,264) | 54.3 | (779) | < 0.001c |
| Hydrocephalus | 0.1 | (8) | 0.7 | (10) | < 0.001c |
| Hypercalcemia | 2.1 | (120) | 3.6 | (52) | < 0.001c |
| Hyperlipidemia | 40.4 | (2,317) | 46.0 | (659) | < 0.001c |
| Hypothyroidism | 13.9 | (795) | 20.2 | (289) | < 0.001c |
| Interstitial lung disease | 27.0 | (1,552) | 41.6 | (597) | < 0.001c |
| Leukocytopenia | 0.6 | (33) | 1.5 | (22) | < 0.001c |
| Malaise and fatigue | 20.9 | (1,201) | 34.5 | (495) | < 0.001c |
| Mild cognitive impairment | 0.2 | (9) | 0.1 | (2) | 1.000 |
| Myalgia and myositis | 9.3 | (532) | 15.6 | (224) | < 0.001c |
| Obesity | 6.2 | (354) | 12.6 | (180) | < 0.001c |
| Osteoporosis | 5.5 | (313) | 7.5 | (108) | 0.003c |
| Pain syndromes | 0.7 | (43) | 2.6 | (38) | < 0.001c |
| Pneumonia | 9.8 | (564) | 20.9 | (299) | < 0.001c |
| Pulmonary aspergillosis | 0.0 | (1) | 0.6 | (9) | < 0.001c |
| Small fiber neuropathy | 0.3 | (15) | 0.6 | (9) | 0.041c |
| Syncope | 3.7 | (211) | 10.4 | (149) | < 0.001c |
| Uveitis | 6.5 | (371) | 5.9 | (85) | 0.456 |
| Vitamin D deficiency | 5.2 | (296) | 7.4 | (106) | 0.001c |
| Follow-up period characteristicsf | |||||
| ≥ 1 medical visit, % (n) | |||||
| Inpatient admission | 14.4 | (824) | 70.8 | (1,015) | < 0.001c |
| Emergency department | 26.6 | (1,528) | 60.0 | (861) | < 0.001c |
| Specialists | |||||
| Cardiologist | 21.7 | (1,246) | 57.7 | (827) | < 0.001c |
| Dermatologist | 21.8 | (1,250) | 22.7 | (325) | 0.470 |
| Gastroenterologist | 13.7 | (786) | 25.2 | (361) | < 0.001c |
| Nephrologist | 2.5 | (146) | 10.0 | (144) | < 0.001c |
| Ophthalmologist | 27.7 | (1,589) | 36.3 | (520) | < 0.001c |
| Orthopedist | 13.5 | (776) | 27.0 | (387) | < 0.001c |
| Pulmonologist | 35.7 | (2,050) | 49.0 | (702) | < 0.001c |
| Radiologist | 70.2 | (4,027) | 91.3 | (1,309) | < 0.001c |
| Rheumatologist | 12.0 | (691) | 19.0 | (273) | < 0.001c |
| ≥ 1 medical or pharmacy claim for drug use, % (n) | |||||
| Adalimumab and/or infliximab | 0.2 | (11) | 3.1 | (44) | < 0.001c |
| ≥ 1 prescription filled, % (n) | |||||
| Antidepressants | 20.5 | (1,177) | 39.9 | (572) | < 0.001c |
| Antimalarial medications | 4.1 | (238) | 6.8 | (97) | < 0.001c |
| Antineoplastic agents | 3.8 | (217) | 12.5 | (179) | < 0.001c |
| Antiasthmatic and bronchodilator agents | 21.6 | (1,238) | 34.0 | (488) | < 0.001c |
| Bisphosphonates | 4.7 | (271) | 7.9 | (113) | < 0.001c |
| Glucocorticosteroids | 29.6 | (1,697) | 51.1 | (733) | < 0.001c |
| Immunosuppressive agents | 1.4 | (80) | 6.0 | (86) | < 0.001c |
| NSAIDs | 25.0 | (1,435) | 34.7 | (498) | < 0.001c |
aP values were calculated using Wilcoxon rank-sum tests for continuous variables and chi-square tests for categorical variables.
bPatient demographics were evaluated at the index date. CCI scores were assessed in the 12-month period preceding the index date.
cP value < 0.05.
dStudy period characteristics were evaluated during the 12-month period preceding and 12-month period following the index date.
eRefer to Appendix A (available in online article) for a list of the sarcoidosis-related comorbidities and the respective ICD-9-CM diagnosis codes.
fFollow-up period characteristics were evaluated during the 12-month period following the index date.
CCI = Charlson Comorbidity Index; ICD-9-CM = International Classification of Diseases, Ninth Revision, Clinical Modification; NSAID = nonsteroidal anti-inflammatory drug; Q1 = quarter 1; SD = standard deviation.
High-cost patients also had several more sarcoidosis-related comorbidities in the 24-month study period than lower-cost patients (4.4 vs. 2.7). Nearly half (46.3%) of high-cost patients had 5 or more of the sarcoidosis-related comorbidities compared to just 16.5% of their lower-cost counterparts. High-cost patients had significantly increased rates of nearly all comorbid conditions evaluated, including primary hypertension (54.3% vs. 39.4%), chronic pulmonary disease (42.3% vs. 26.3%), cardiac arrhythmia (28.5% vs. 9.2%), diabetes (28.7% vs. 14.7%), and depression (21.1% vs. 10.6%). However, lower-cost patients had slightly increased rates of erythema nodosum (2.2% vs. 1.5%), mild cognitive impairment (0.2% vs. 0.1%), and uveitis (6.5% vs. 5.9%) than high-cost patients, though the differences were not statistically significant.
Health Care Resource Utilization
Compared with lower-cost patients, high-cost patients had a greater likelihood of utilizing medical resources across all places of service (Table 1). For example, high-cost patients were more likely to have at least 1 inpatient admission (70.8% vs. 14.4%) and at least 1 ED visit (60.0% vs. 26.6%). They also were significantly more likely to visit specialists including radiologists (91.3% vs. 70.2%), cardiologists (57.7% vs. 21.7%), and pulmonologists (49.0% vs. 35.7%). High-cost patients were also more likely to fill prescriptions for glucocorticosteroids (51.1% vs. 29.6%), nonsteroidal anti-inflammatory drugs (34.7% vs. 25.0%), antineoplastic agents (12.5% vs. 3.8%), and antidepressants (39.9% vs. 20.5%).
Health Care Resource Costs
Among high-cost patients, 41.8% ($30,690) of the $73,346 total average health care costs incurred during the follow-up period was associated with inpatient admissions, 39.1% ($28,676) with outpatient/physician office visits, 2.6% ($1,890) with ED visits, 5.2% ($3,778) with other facility visits (e.g., home health visits, lab visits), and 11.3% ($8,309) with prescription drugs. In comparison, the lower-cost sarcoidosis patients (bottom 80%) incurred only one tenth the total annual health care costs that the high-cost cohort had in the follow-up period ($7,073). In the lower-cost group, inpatient cost accounted for only 8.6% ($606) of the total cost, while outpatient/physician office visits accounted for the majority of the total cost (62.2%; $4,397) followed by prescription drug costs (21.4%; $1,516).
Figure 3 displays the itemization of costs by place of service and further separates the high-cost patients into percentile subgroups. The proportion of costs attributed to inpatient admissions substantially increases across the patient cost quintiles: less than 0.5% in patients in the first quintile of cost (0-20th percentile), 1.2% for patients in the second quintile, 3.7% for patients in the third quintile, 27.5% in the fourth quintile, and 41.8% in the fifth quintile. For patients with total health care costs in the top 1% of the sarcoidosis population, inpatient admissions account for over half of all incurred costs ($201,082 of the $375,436 average total health care costs). Conversely, outpatient services and prescription drugs account for a decreasing proportion as the total health care cost increases.
FIGURE 3.
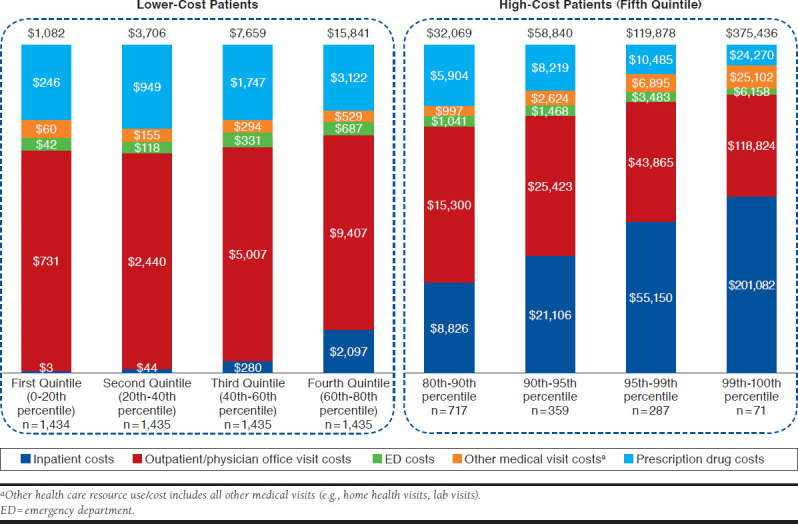
Mean Per-Patient Health Care Costs During the Follow-up Period Among Sarcoidosis Patients
Multiple Regression Results
The multiple logistic regression results, when adjusted for baseline demographic characteristics such as age, gender, region, and year of index date, showed that patients with evidence of sarcoidosis-related comorbidities in the study period were much more likely to have high costs in the follow-up period, and costs increased by the number of comorbidities (Table 2). For example, patients with 5 or more sarcoidosis-related comorbidities were 69.4% (95% confidence interval [CI]: 0.2%-186.6%) more likely to be in the high-cost cohort relative to patients with no sarcoidosis-related comorbid conditions. In particular, sarcoidosis patients with evidence of the following comorbidities were at significantly increased odds of being categorized as high-cost patients: deficiency anemia (odds ratio [OR] = 1.6, 95% CI = 1.2-2.1), depression (OR = 1.5, 95% CI = 1.2-1.9), cardiac arrhythmia (OR = 1.5, 95% CI = 1.2-1.8), and diabetes (OR = 1.4, 95% CI = 1.2-1.7). Patients with pulmonary aspergillosis were 15.0 (95% CI = 1.2-182.9) times more likely to be high-cost patients; the 95% CI for this effect estimate is very wide because the condition was very rare (only 1 patient in the lower-cost cohort and 6 in the high-cost cohort).
TABLE 2.
Multiple Logistic Regression Model to Identify Predictors of High Total Health Care Costs in the Follow-up Period
| OR (95% CI) | P Value | ||
|---|---|---|---|
| Patient demographicsa | |||
| Age (year) | 1.001 | (0.993-1.010) | 0.758 |
| Gender (ref: female) | 1.036 | (0.884-1.214) | 0.661 |
| Year of index date (ref: 1999-2004) | |||
| 2005-2007 | 1.047 | (0.843-1.302) | 0.677 |
| 2008-2010 | 1.111 | (0.891-1.384) | 0.350 |
| 2011-2014 (Q1 only) | 1.283 | (1.034-1.592) | 0.024b |
| Study period characteristicsc | |||
| Number of sarcoidosis-related comorbiditiesd (ref: 0) | |||
| 1-4 | 1.158 | (0.794-1.690) | 0.447 |
| 5 or more | 1.694 | (1.002-2.866) | 0.049b |
| Selected sarcoidosis-related comorbiditiese,f | |||
| Cardiac arrhythmia | 1.493 | (1.221-1.825) | < 0.001b |
| Deficiency anemia | 1.606 | (1.216-2.122) | < 0.001b |
| Depression | 1.504 | (1.222-1.852) | < 0.001b |
| Diabetes | 1.428 | (1.173-1.738) | <0.001b |
| Epilepsy and recurrent seizures | 1.586 | (0.926-2.716) | 0.093 |
| Hydrocephalus | 1.583 | (0.473-5.296) | 0.456 |
| Malaise and fatigue | 1.380 | (1.158-1.645) | < 0.001b |
| Obesity | 1.355 | (1.053-1.742) | 0.018b |
| Pain syndromes | 1.228 | (0.685-2.199) | 0.490 |
| Pulmonary aspergillosis | 14.992 | (1.229-182.917) | 0.034b |
| Follow-up period characteristicsd | |||
| Medical visits (≥1 vs. 0) | |||
| Inpatient admission | 9.771 | (8.376-11.398) | < 0.001b |
| Specialistsg | |||
| Cardiologist | 1.700 | (1.446-1.998) | < 0.001b |
| Gastroenterologist | 1.287 | (1.070-1.546) | 0.007b |
| Nephrologist | 1.739 | (1.256-2.409) | < 0.001b |
| Orthopedist | 1.850 | (1.547-2.213) | < 0.001b |
| Radiologist | 2.090 | (1.670-2.616) | < 0.001b |
| Drug use (medical and pharmacy claims; ≥ 1 vs. 0) | |||
| Adalimumab and/or infliximab | 31.821 | (15.137-66.894) | < 0.001b |
aPatient demographics were evaluated at the index date.
bP value < 0.05.
cStudy period characteristics were evaluated during the 12-month period preceding and 12-month period following the index date.
dFollow-up period characteristics were evaluated during the 12-month period following the index date.
eRefer to Appendix A (available in online article) for a full list of the sarcoidosis-related comorbidities and the respective ICD-9-CM diagnosis codes used to identify the conditions.
fOther sarcoidosis-related comorbidities adjusted for in the regression model, but without statistically significant ORs, included bronchiectasis, cataracts, chronic pulmonary disease, erythema nodosum, essential (primary) hypertension, hypercalcemia, hyperlipidemia, hypothyroidism, interstitial lung disease, leukocytopenia, mild cognitive impairment, myalgia and myositis, osteoporosis, pneumonia, small fiber neuropathy, syncope, uveitis, and vitamin D deficiency.
gSpecialists for whom the OR was greater than 2 in the univariate analysis were included in the model.
CI = confidence interval; ICD-9-CM = International Classification of Diseases, Ninth Revision, Clinical Modification; OR = odds ratio; Q1 = quarter 1; ref = reference.
Sarcoidosis patients with at least 1 inpatient admission during the follow-up period had a nearly 10-fold increase in the odds of being in the high-cost cohort compared with patients without an inpatient admission (OR = 9.8, 95% CI = 8.4-11.4). Patients with visits to the following medical specialists in the follow-up period were also at increased odds: radiologist (OR = 2.1, 95% CI = 1.7-2.6), orthopedist (OR = 1.9, 95% CI = 1.5-2.2), nephrologist (OR = 1.7, 95% CI = 1.3-2.4), and cardiologist (OR = 1.7, 95% CI = 1.4-2.0). In addition, sarcoidosis patients with use of adalimumab and/or infliximab in the follow-up period had 31.8 (95% CI = 15.1-66.9) times the odds of being in the high-cost cohort.
An additional model containing the interactions for the 5 sarcoidosis-related comorbidities with the highest OR estimates in the univariate analysis (i.e., pulmonary aspergillosis, hydrocephalus, cardiac arrhythmia, pain syndromes, and epilepsy and recurrent seizures) was explored. None of the interaction terms were statistically significant and the main effects were largely the same as the model excluding interaction terms (higher-order model not shown).
Discussion
Findings of this study demonstrate that the distribution of total health care cost is highly skewed among sarcoidosis patients. Mean annual health care costs for patients in the top quintile were $73,346 (2015 U.S. dollars), which were 10 times greater than mean annual health care costs for all remaining patients. This skewness is further demonstrated by a mean annual cost of $119,878 and $375,436 in the 95th-99th and 99th-100th percentile groups, respectively. This high cost concentration indicates that it may be worthwhile to invest in quality and outcomes improvement efforts to target this costly patient subgroup. These results are consistent with estimates reported in Baughman et al. (2016), which also suggest significant skewness in costs for sarcoidosis patients.9 Particularly, Baughman et al. (2016) show that patients in the top 5% in terms of costs have costs of $93,201 ($47,097 in sarcoidosis-related care and $46,105 in nondisease costs), which is similar to our findings, although the underlying methodology and data source differ from that employed here. Further, to our knowledge, this is the first study to use such data to characterize high-cost sarcoidosis patients and identify key factors that contribute to costs in this patient population.
Using robust statistical methods, this study found that high-cost sarcoidosis patients were complicated patients with higher rates of comorbidities, had increased health care resource use (particularly in the form of inpatient admissions and specialty visits), and were likely being treated with biologic therapies (i.e., adalimumab and/or infliximab). Additionally, given the ORs for visits to nephrologists and cardiologists, it may be indicative that patients with renal and cardiac sarcoidosis have higher costs compared with patients with other types of sarcoidosis. Using this information, physicians may better be able to identify patients with especially burdensome disease who are at high risk of becoming costly patients. For example, the increased rates of certain physical and mental health conditions highlight the need to consider a patient’s complete medical history to better manage these underlying and concurrent conditions contributing to high health care costs. To further aid care management of patients with sarcoidosis, future research should investigate specific sarcoidosis-related costs and resource utilization.
Limitations
This study has a number of limitations. First, the observational study did not assess the causal relationship between patient characteristics and health care costs among sarcoidosis patients. Moreover, no causal relationship was established between sarcoidosis severity and costs, since data were not available on sarcoidosis disease severity. Because much of the variation in health care costs cannot be explained by observable patient characteristics, further research is necessary to understand which aspect of patient care can affect health care costs. Whether the relationship between costs and associated factors remains comparable beyond the 12-month follow-up period is unknown and has not been addressed in this study. Given that the disease course of sarcoidosis varies significantly across patients, future studies could explore the disease timeline for the patient population, but this identification is not possible with administrative claims data. Finally, the study was based on a population in the United States of continuously enrolled, commercially insured beneficiaries aged 18-64 years old and may not be generalizable to other populations.
Conclusions
This study described characteristics of high-cost sarcoidosis patients and identified several indicators of high costs using contemporary administrative data. The health care cost distribution is highly skewed for sarcoidosis, making it a worthwhile endeavor to focus improvement efforts on patients in the most costly quintile. The findings of this study can help population health decision makers select intervention participants with the aim of improving quality of care and reducing overall costs.
APPENDIX A. Diagnosis Codes Used to Identify Patient Comorbidities
| Sarcoidosis-Related Comorbidities | ICD-9-CM Diagnosis Codes |
|---|---|
| Bronchiectasis | 494.x |
| Cardiac arrhythmia | 426.0, 426.13, 426.7, 426.9, 426.10, 426.12, 427.0-427.4, 427.6-427.9, 785.0, 996.01, 996.04, V45.0, V53.3 |
| Cataracts | 366.x |
| Chronic pulmonary disease | 416.8, 416.9, 490.x-505.x, 506.4, 508.1, 508.8 |
| Deficiency anemia | 280.1-280.9, 281.x |
| Depression | 296.2, 296.3, 296.5, 300.4, 311.x |
| Diabetes | 250.x |
| Epilepsy and recurrent seizures | 345.x |
| Erythema nodosum | 695.2 |
| Essential (primary) hypertension | 401.x |
| Hydrocephalus | 331.3, 331.4, 331.5 |
| Hypercalcemia | 275.42 |
| Hyperlipidemia | 272.0-272.4 |
| Hypothyroidism | 240.9, 243.x, 244.x, 246.1, 246.8 |
| Interstitial lung disease | 508.1, 515.x, 516.3, 518.89, 714.81 |
| Leukocytopenia | 288.50 |
| Malaise and fatigue | 780.7 |
| Mild cognitive impairment | 331.83 |
| Myalgia and myositis | 729.1 |
| Obesity | 278.0, 649.1, 793.91, V85.3, V85.4, V85.54 |
| Osteoporosis | 733.0 |
| Pain syndromes | 337.2, 354.4, 355.71, 338.0, 338.4 |
| Pneumonia | 480-486 |
| Pulmonary aspergillosis | 117.3 |
| Small fiber neuropathy | 356.8 |
| Syncope | 780.2 |
| Uveitis | 364.x |
| Vitamin D deficiency | 268.x |
ICD-9-CM = International Classification of Diseases, Ninth Revision, Clinical Modification.
APPENDIX B. Selection of Sarcoidosis Patients for Study Inclusion
REFERENCES
- 1.Hunninghake GW, Costabel U, Ando M, et al. ATS/ERS/WASOG statement on sarcoidosis. American Thoracic Society/European Respiratory Society/World Association of Sarcoidosis and other Granulomatous Disorders. Sarcoidosis Vasc Diffuse Lung Dis. 1999;16(2):149-73. [PubMed] [Google Scholar]
- 2.Baughman RP, Drent M, Kavuru M, et al. Infliximab therapy in patients with chronic sarcoidosis and pulmonary involvement. Am J Respir Crit Care Med. 2006;174(7):795-802. [DOI] [PubMed] [Google Scholar]
- 3.Baughman RP, Lower EE, Gibson K.. Pulmonary manifestations of sarcoidosis. Presse Med. 2012;41(6 Pt 2):e289-302. [DOI] [PubMed] [Google Scholar]
- 4.Judson MA, Thompson BW, Rabin DL, et al. The diagnostic pathway to sarcoidosis. Chest. 2003;123(2):406-12. [DOI] [PubMed] [Google Scholar]
- 5.Iannuzzi MC, Rybicki BA, Teirstein AS.. Sarcoidosis. N Engl J Med. 2007;357(21):2153-65. [DOI] [PubMed] [Google Scholar]
- 6.Wu JJ, Schiff KR.. Sarcoidosis. Am Fam Physician. 2004;70(2):312-22. [PubMed] [Google Scholar]
- 7.Shorr AF, Helman DL, Davies DB, Nathan SD.. Pulmonary hypertension in advanced sarcoidosis: epidemiology and clinical characteristics. Eur Respir J. 2005;25(5):783-88. [DOI] [PubMed] [Google Scholar]
- 8.Rice JB, White A, Lopez A, et al. Economic burden of sarcoidosis in a commercially-insured population in the United States. J Med Econ. 2017;20(10):1048-55. [DOI] [PubMed] [Google Scholar]
- 9.Baughman RP, Field S, Costabel U, et al. Sarcoidosis in America. Analysis based on health care use. Ann Am Thorac Soc. 2016;13(8):1244-52. [DOI] [PubMed] [Google Scholar]
- 10.Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130-9. [DOI] [PubMed] [Google Scholar]


