Abstract
BACKGROUND:
Suboptimal treatment of exacerbations is a major concern in management of chronic obstructive pulmonary disease (COPD). The Pharmacotherapy Management of COPD Exacerbation (PCE) Healthcare Effectiveness Data and Information Set (HEDIS) measure is a quality measure included by the National Committee for Quality Assurance that focuses on appropriate use of steroids and bronchodilators during an acute COPD exacerbation. There is limited evidence evaluating predictors of this quality measure, as well as its association with hospital readmission and cost outcomes.
OBJECTIVES:
To (a) describe characteristics of patients hospitalized for COPD, (b) evaluate factors associated with appropriate receipt of pharmacotherapy upon discharge, and (c) evaluate factors associated with the rate of readmission.
METHODS:
In this retrospective, observational, event-based study of COPD-related hospital and ED visits, events were identified between 2007 and 2013 from a Central Texas health plan using administrative claims data. The index date was defined as the date of admission. Subjects were included if they were aged ≥ 40 years and had a medical claim with a primary diagnosis for COPD or a pharmacy claim for a COPD maintenance medication during the 1-year pre-index period. Study groups were identified based on the receipt of PCE within the time frame specified by HEDIS: (a) a systemic corticosteroid within 14 days of discharge (PCE-C) or (b) a bronchodilator within 30 days of discharge (PCE-D). Bivariate analyses of potential factors associated with the receipt of PCE were performed using t-tests for continuous data and chi-square tests for categorical data. Generalized estimating equations, including significant predictors from the bivariate analyses, were used to determine factors associated with receipt of PCE-C and/or PCE-D, as well association with COPD-related and all-cause readmission within 6 months of discharge.
RESULTS:
Of 375 identified index admissions, 254 (68%) patients received PCE-C; 299 (80%) received PCE-D; and 229 (61%) received both. Patients were more likely to receive PCE with an index inpatient visit as compared with an ED visit (PCE-C: RR = 2.25, 95% CI = 1.21-4.17, P = 0.010; PCE-D: RR = 1.90, 95% CI = 1.01-3.58, P = 0.048). Those with previous use of rescue medication were also more likely to receive PCE (PCE-C: RR = 1.88, 95% CI = 1.12-3.17, P = 0.018; PCE-D: RR = 2.11, 95% CI = 1.16-3.83, P = 0.014). Patients with greater adherence (proportion of days covered [PDC] ≥ 75%) to COPD maintenance medication before admission (RR = 8.67, 95% CI = 1.60-46.78, P = 0.012) were also more likely to receive PCE-D. Older patients were more likely to have a COPD-related readmission (RR = 1.07, 95% CI = 1.01-1.13, P = 0.028), while use of maintenance medication before admission was associated with lower risk of an all-cause readmission (RR = 0.49, 95% CI = 0.30-0.79, P = 0.004). In addition, patients with higher medical and pharmacy costs before the index event were more likely to have all-cause readmission (RR = 1.01, 95% CI = 1.00-1.02, P = 0.013). Receipt of PCE was not shown to be a significant predictor of all-cause or COPD-related readmission.
CONCLUSIONS:
The use of bronchodilators and systemic corticosteroids after a COPD-related inpatient or ED visit may be related to the severity of the index COPD exacerbation or patients' previous pattern of bronchodilator use. However, the use of maintenance medication before the index event was associated with a significant reduction in all-cause readmission, so proper treatment of the underlying disease may be an effective strategy in reducing readmission.
What is already known about this subject
In patients with chronic obstructive pulmonary disease (COPD), exacerbations resulting in hospital or emergency department admissions are a significant driver of clinical and economic burden.
Despite established guidelines and quality measures that use evidence-based treatments to improve disease management, COPD patients remain undertreated with maintenance medications.
What this study adds
COPD patients with a previous history of rescue medication use, those who are more adherent to maintenance medications, and those who had their exacerbation managed in an inpatient setting were more likely to receive inhaled bronchodilators and systemic corticosteroids after their COPD exacerbation.
Patients on maintenance medication before admission were associated with lower risk of an all-cause readmission, while patients with higher health care costs before admission were more likely to have an all-cause readmission. Older patients were more likely to have a COPD-related readmission.
Chronic obstructive pulmonary disease (COPD) is a progressive illness affecting approximately 6.3% of the U.S. adult population.1 The condition is characterized by persistent airflow limitation, resulting in the cardinal symptoms of cough, sputum production, and exertional dyspnea, with periodic exacerbations.2 Exacerbations of COPD are acute episodes of worsening respiratory symptoms that can result in hospitalizations, a change in medication, temporary decline in lung function, or a fatal event.3-5 After an exacerbation, a patient's symptoms and lung function can take several weeks to recover, and quality of life may decline drastically.4,6
Aside from its humanistic burden, COPD is also associated with significant economic burden, with an estimated total cost of $36 billion annually in the United States, $32.1 billion of which is attributed to direct medical costs.7 A large study of commercially insured COPD patients found that total medical and pharmacy costs ranged from about $2,000 to $40,000 per patient per year among those admitted to the emergency department (ED) or inpatient hospital.8 Not surprisingly, most of COPD costs have been shown to be a result of direct medical costs, with severe exacerbations being the biggest cost driver.7-9 Patients with severe exacerbations have been shown to incur over $43,000 in total health care costs annually.10 To reduce the disease burden and improve patient care, the Global Initiative for Chronic Obstructive Lung Disease developed guidelines for management of COPD.
Despite recommendations from established guidelines, suboptimal treatment of exacerbations remains a major concern in COPD management. Studies have shown that 36% to 71% of patients with COPD are undertreated with maintenance medications as recommended by established guidelines.11-13 To assess the use of evidence-based treatments for this common and costly consequence of COPD, the National Committee for Quality Assurance (NCQA) includes a quality measure that focuses on proper use of systemic corticosteroids and bronchodilators following an acute exacerbation.
The NCQA is a private, nonprofit organization dedicated to improving the quality of health care through performance measurement. Using Healthcare Effectiveness Data and Information Set (HEDIS) measures, the NCQA compares the performance of health plans based on ability to meet specified quality criteria.6 The Centers for Medicare & Medicaid Services (CMS) have also recently added COPD as a condition in their Hospital Readmissions Reduction Program, which penalizes hospitals with excessive readmissions for COPD.14 The role of quality measurement is becoming more important in an evolving health care environment, where reimbursement is tied to quality performance rather than volume. This is evident in the recent goal set by the U.S. Department of Health & Human Services to tie 90% of all traditional Medicare payments to value or quality by 2018.15
Of particular interest is the Pharmacotherapy Management of COPD Exacerbation (PCE) measure, which focuses on appropriate use of systemic corticosteroids and bronchodilators after an acute exacerbation. This measure examines the percentage of patients aged 40 years and older who receive a systemic corticosteroid within 14 days or a bronchodilator within 30 days after a COPD-related inpatient admission or ED visit. Understanding factors associated with receiving appropriate therapy for a COPD exacerbation, as well as how receipt of that treatment is associated with outcomes, can assist with population-based management of this chronic respiratory disease.
The objectives of this study were to (a) describe the characteristics of patients admitted to the ED or hospital for COPD exacerbation, (b) evaluate patient demographics and baseline use associated with receipt of appropriate pharmacotherapy after a COPD hospitalization or ED visit as defined by HEDIS, and (c) examine the factors that affect the rate of all-cause and COPD-related 6-month readmission.
Methods
Study Design
This retrospective, observational event-based study examined data between January 1, 2006, and June 30, 2014, which was referred to as the study period. Hospital or ED events for a COPD exacerbation during the enrollment period of January 1, 2007, to December 31, 2013, were identified. The date of admission to the hospital or ED was considered the index date. Baseline characteristics in the 1-year time frame before the index date were obtained. Study groups were identified based on the receipt of PCE within the time frame specified by HEDIS: a systemic corticosteroid within 14 days of discharge (PCE-C) and/or a bronchodilator within 30 days of discharge (PCE-D). The Appendix (available in online article) shows the time line and variables of interest. This study was approved by the study institution's institutional review board before initiation.
Data Source
Data were extracted from an integrated delivery network in Central Texas that includes a network of hospitals, clinics, pharmacies, and a health plan with over 250,000 covered lives. Pharmacy and medical claims were linked to patient enrollment data containing demographic information. Pharmacy claims contained details from all prescriptions dispensed, including drug name, date and quantity dispensed, days supplied, and plan- and patient-paid amounts. Medical claims provided detailed information on inpatient and outpatient services, including date and place of service, payments, procedure codes, and up to 5 International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) diagnosis codes.
Sample Selection
Inpatient and ED admissions between January 1, 2007, and December 31, 2013, with a primary diagnosis of COPD (ICD-9-CM diagnosis codes 491.xx, 492.xx, 493.2x, and 496.xx) were identified. For the primary analysis, patients were included who were aged 40 years or older at time of admission with at least 1 pre-index medical claim with a primary or secondary diagnosis code consistent with COPD, as previously defined, or at least 1 pre-index pharmacy claim for a COPD maintenance medication (long-acting muscarinic agonist [LAMA], long-acting beta-antagonist [LABA], and/or inhaled corticosteroid [ICS]) and continuous enrollment in the health plan for 1 year before the index date and 30 days after the discharge date. The age of 40 years or older was used to be consistent with HEDIS specifications. Patients with enrollment at least 180 days after discharge were included in the secondary analysis, which evaluated 6-month readmission. Because of sample size, 6-month readmission was evaluated rather than 30-day readmission to allow for capture of more events. Inpatient or ED visits were not included as index events, if they resulted in readmission to an acute or nonacute care facility for any diagnosis within 14 days of the date of discharge. ED visits resulting in an inpatient admission were counted as a single inpatient admission. Patient-level data were analyzed for each year; however, this sample included multiple admissions for the same patient, if they had hospital events in more than 1 year.
Baseline Characteristics and Outcome Measures
The following pre-index variables during the 1 year before index admissions were summarized and compared between those who received PCE-D or PCE-C and those who did not: demographics (age and gender); Charlson Comorbidity Index (CCI)16; number of rescue (i.e., albuterol- or ipratropium-containing products) and maintenance (i.e., LAMA, LABA, ICS, or any combination thereof) COPD medications; higher adherence to COPD maintenance medication (measured by proportion of days covered [PDC]); number of COPD exacerbations; all-cause and COPD-related health care resource utilization (inpatient, outpatient, and pharmacy); and associated costs. The primary outcomes were proportion of COPD hospitalization and/or ED visits that received PCE-C or PCE-D and factors associated with receiving PCE based on the baseline characteristics measured. Secondary outcomes were all-cause and COPD-related hospital readmissions for those enrolled 6 months after discharge and factors (including receipt of PCE) associated with readmission.
Data Analysis
Descriptive statistics (means and proportions) were used to characterize the study sample based on pre-index variables. Patient characteristics of those who did and did not receive PCE-C or PCE-D were compared using independent sample t-tests for continuous data and chi-square tests for nominal data. Generalized estimating equations (GEE) in which receipt of PCE-C or PCE-D was the dependent variable were performed. Predictor variables were age, gender, CCI, type of admission (inpatient or ED visit), length of stay, admission year and quarter, adherence, whether or not the patient was on a maintenance or rescue medication, pre-index medical and pharmacy costs, and number of COPD exacerbations in the year before index admission. Costs were analyzed using log transformation. A COPD exacerbation was defined as either (a) an inpatient hospital stay or ED visit with a primary diagnosis for COPD or (b) a claim for systemic corticosteroids within 7 days following an outpatient claim for COPD. Among those with 6-month enrollment after discharge, GEEs were used to assess factors associated with readmission within 6 months. These factors included the pre-index and index variables previously listed, as well as receipt of PCE. All statistical tests were conducted using SAS version 9.4 (SAS Institute, Cary, NC), testing a hypothesis at a significance level of 0.05.
Results
Receipt of Pharmacotherapy Management as Defined by HEDIS
A total of 375 index admissions encompassing 330 patients were identified (Figure 1), with 254 (68%) events receiving PCE-C, 299 (80%) receiving PCE-D, and 229 (61%) receiving both. Table 1 compares characteristics of index admissions per patient per year for those who received PCE with those who did not.
FIGURE 1.
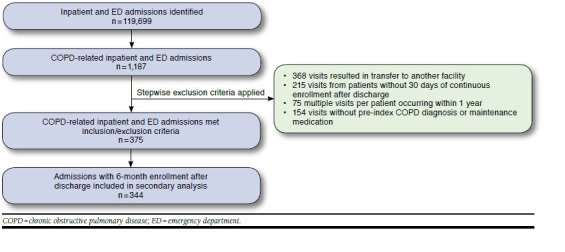
Sample Selection
TABLE 1.
Characteristics of Index Admissions with or Without Receipt of PCE-C or PCE-D
| Visit Variables, N = 375 Admissions | Received Systemic Corticosteroid Within 14 Days of Discharge (PCE-C) | Received Bronchodilator Within 30 Days of Discharge (PCE-D) | ||||
|---|---|---|---|---|---|---|
|
Yes
n = 254 (68%) |
No
n = 121 (32%) |
P Value |
Yes
n = 299 (80%) |
No
n = 76 (20%) |
P Value | |
| Pre-Index Variables | ||||||
| Age | 60 (7) | 58 (8) | 0.312 | 60 (7) | 57 (8) | 0.012 |
| Gender | ||||||
| Female | 168 (70%) [66%] | 72 (30%) [60%] | 0.211 | 192 (80%) [64%] | 48 (20%) [63%] | 0.864 |
| Male | 86 (64%) [34%] | 49 (36%) [40%] | 107 (79%) [36%] | 28 (21%) [37%] | ||
| Charlson Comorbidity Index | 1.68 (1.31) | 1.82 (1.63) | 0.403 | 1.69 (1.34) | 1.84 (1.7) | 0.474 |
| On a rescue medication | ||||||
| Yes | 224 (73%) [88%] | 84 (27%) [69%] | < 0.001 | 262 (85%) [88%] | 46 (15%) [61%] | < 0.001 |
| No | 30 (45%) [12%] | 37 (55%) [31%] | 37 (55%) [12%] | 30 (45%) [39%] | ||
| Number of rescue medicationsa | 6.19 (7.27) | 4.17 (6.82) | 0.011 | 6.57 (7.61) | 1.46 (2.38) | < 0.001 |
| On a maintenance medication | ||||||
| Yes | 164 (73%) [65%] | 60 (27%) [50%] | 0.005 | 195 (87%) [65%] | 29 (13%) [38%] | < 0.001 |
| No | 90 (60%) [35%] | 61 (40%) [50%] | 104 (69%) [35%] | 47 (31%) [62%] | ||
| Number of maintenance medicationsa | 4.59 (6.17) | 3.01 (4.92) | 0.008 | 4.88 (6.21) | 0.93 (1.99) | < 0.001 |
| Proportion of days covered | 0.29 (0.34) | 0.2 (0.3) | 0.012 | 0.31 (0.34) | 0.07 (0.15) | < 0.001 |
| PDC ≥ 75% | 40 (77%) [16%] | 12 (23%) [10%] | 0.017 | 51 (98%) [17%] | 1 (2%) [1%] | < 0.001 |
| PDC < 75% | 124 (72%) [49%] | 48 (28%) [40%] | 144 (84%) [48%] | 28 (16%) [37%] | ||
| No maintenance drug | 90 (60%) [35%] | 61 (40%) [50%] | 104 (69%) [35%] | 47 (31%) [62%] | ||
| Total medical + pharmacy costs, $ | 18,718 (23,056) | 25,838 (39,890) | 0.280 | 20,180 (29,378) | 24,304 (30,846) | 0.070 |
| COPD-related, $ | 3,492 (5,078) | 3,373 (6,422) | 0.135 | 3,713 (5,104) | 2,434 (6,941) | 0.858 |
| Total medical costs, $ | 15,126 (22,383) | 22,971 (39,231) | 0.042 | 16,624 (28,847) | 21,723 (29,874) | 0.173 |
| Inpatient costs, $ | 7,665 (16,199) | 14,026 (32,812) | 0.045 | 9,228 (23,580) | 11,645 (20,929) | 0.415 |
| COPD-related, $ | 1,178 (3,391) | 1,908 (5,827) | 0.202 | 1,329 (3,533) | 1,746 (6,629) | 0.598 |
| Outpatient costs, $ | 5,330 (7,805) | 6,748 (9,666) | 0.161 | 5,272 (7,657) | 7,818 (10,908) | 0.058 |
| COPD-related, $ | 398 (1,246) | 291 (617) | 0.268 | 401 (1,195) | 215 (392) | 0.025 |
| Total pharmacy costs, $ | 3,591 (3,629) | 2,865 (3,306) | 0.063 | 3,554 (3,438) | 2,579 (3,840) | 0.105 |
| COPD-related, $ | 1,474 (1,886) | 941 (1,361) | 0.002 | 1,569 (1,856) | 251 (416) | < 0.001 |
| Total inpatient visits | 1.46 (2.1) | 2.00 (2.83) | 0.062 | 1.5 (2.12) | 2.16 (3.15) | 0.087 |
| COPD-related | 0.32 (0.75) | 0.36 (0.9) | 0.700 | 0.32 (0.8) | 0.36 (0.81) | 0.764 |
| Total outpatient visits | 9.82 (7.96) | 9.17 (7.81) | 0.461 | 9.47 (7.79) | 10.16 (8.37) | 0.500 |
| COPD-related | 1.35 (2.32) | 1.2 (2.29) | 0.542 | 1.36 (2.43) | 1.07 (1.73) | 0.221 |
| COPD exacerbationsb | 0.80 (1.64) | 0.45 (1.03) | 0.010 | 0.73 (1.58) | 0.50 (0.96) | 0.110 |
| Event (Index) Variables | ||||||
| Length of stay, days | 1.92 (2.35) | 1.56 (3.54) | 0.312 | 1.90 (2.88) | 1.45 (2.37) | 0.161 |
| Cost of hospitalization/ED, $ | 4,491 (4,346) | 4,503 (10,160) | 0.990 | 4,511 (6,962) | 4,430 (6,022) | 0.926 |
| Location | ||||||
| ED | 131 (63%) [52%] | 78 (37%) [64%] | 0.019 | 160 (77%) [54%] | 49 (23%) [64%] | 0.086 |
| Inpatient hospital | 123 (74%) [48%] | 43 (26%) [36%] | 139 (84%) [46%] | 27 (16%) [36%] | ||
Note: Expressed as mean (SD) for continuous variables or count (Row %) [Column %] for categorical variables.
a Number of medications are expressed as mean inhalers per patient per year and include patients who have no medications.
b COPD exacerbations were defined as an inpatient hospitalization or an ED visit with a primary diagnosis for COPD or a claim for oral or parenteral corticosteroids within 7 days following an outpatient claim for COPD, expressed as per patient per year.
COPD = chronic obstructive pulmonary disease; ED = emergency department; PCE = pharmacotherapy management of COPD exacerbation; PDC = proportion of days covered; SD = standard deviation.
Univariate Analysis.
Patient demographics were similar between those who received PCE-C and those who did not (mean age: 60 years vs. 58 years, P = 0.312; 66% vs. 60% female, P = 0.211). Length of stay (mean: 1.92 days vs. 1.56 days, P = 0.312) and cost of index hospitalization per patient (mean: $4,491 vs. $4,503, P = 0.990) were also similar between the 2 groups. Those with receipt of PCE-C had more rescue inhalers (mean: 6.19 vs. 4.17, P = 0.011) and more maintenance inhalers (mean: 4.59 vs. 3.01, P = 0.008) dispensed in the year before the index event. The mean PDC of previous maintenance medication was greater in patients who received PCE-C (mean: 0.29 vs. 0.20, P = 0.012). Total all-cause medical costs before the index event were lower in those who received PCE-C (mean: $15,126 vs. $22,971, P = 0.042), whereas previous COPD-related pharmacy costs were higher in those who received PCE-C (mean: $1,474 vs. $941, P = 0.002).
Patients who received PCE-D were younger (Table 1; mean age: 60 years vs. 57 years, P > 0.012) with a similar gender distribution (64% vs. 63% female, P = 0.864) compared with those who did not receive PCE-D. Those with receipt of PCE-D had more rescue medications (mean: 6.57 vs. 1.46, P > 0.001) and maintenance medications (mean: 4.88 vs. 0.93, P > 0.001) before the index exacerbation. Mean PDC of previous maintenance medication was greater in patients who received PCE-D (mean: 0.31 vs. 0.07, P > 0.001), while COPD-related outpatient costs before the index event were higher in those who received PCE-D (mean: $401 vs. $215, P = 0.025).
Multivariate Analysis.
After controlling for pre-index and index variables, patients with an index inpatient event as compared with an ED visit were more than twice as likely to receive PCE-C (Table 2; relative risk [RR] = 2.25, 95% confidence interval [CI] = 1.21-4.17, P = 0.010). Those with previous use of rescue medication were also more likely to receive PCE-C (RR = 1.88, 95% CI = 1.12-3.17, P = 0.018). Additionally, lower pharmacy and medical costs before admission were associated with receipt of PCE-C (RR = 0.99, 95% CI = 0.98-0.99, P = 0.029).
TABLE 2.
Adjusted Generalized Estimating Equations Predicting Receipt of PCE (N = 375)
| Parameter | PCE-C | PCE-D | ||||
|---|---|---|---|---|---|---|
| RR | 95% CI | P Value | RR | 95% CI | P Value | |
| Pre-index variables | ||||||
| Female vs. male | 1.14 | 0.71-1.83 | 0.575 | 0.93 | 0.52-1.69 | 0.823 |
| Age | 1.02 | 0.98-1.06 | 0.423 | 1.04 | 0.99-1.09 | 0.086 |
| Charlson Comorbidity Index | 0.98 | 0.80-1.19 | 0.818 | 0.87 | 0.70-1.08 | 0.208 |
| Adherent vs. nonadherent | 1.09 | 0.50-2.38 | 0.824 | 8.67 | 1.60-46.78 | 0.012 |
| On maintenance medication vs. not | 1.49 | 0.91-2.44 | 0.112 | 1.54 | 0.88-2.72 | 0.131 |
| On rescue medication vs. not | 1.88 | 1.12-3.17 | 0.018 | 2.11 | 1.16-3.83 | 0.014 |
| Pre-index costsa | 0.99 | 0.98-0.99 | 0.029 | 0.99 | 0.98-1.00 | 0.288 |
| Number of exacerbations | 1.14 | 0.95-1.36 | 0.173 | 0.94 | 0.80-1.12 | 0.507 |
| Index (event) variables | ||||||
| Inpatient vs. emergency room | 2.25 | 1.21-4.17 | 0.010 | 1.90 | 1.01-3.58 | 0.048 |
| Length of stay | 0.95 | 0.85-1.06 | 0.331 | 1.02 | 1.01-1.03 | 0.799 |
| Admission year | 1.11 | 0.98-1.26 | 0.107 | 1.10 | 0.95-1.27 | 0.210 |
| Admission quarter | 1.04 | 0.85-1.27 | 0.700 | 0.82 | 0.65-1.04 | 0.097 |
a Costs multiplied by $1,000.
CI = confidence interval; PCE-C = pharmacotherapy management of COPD exacerbation with systemic corticosteroid; PCE-D = pharmacotherapy management of COPD exacerbation with bronchodilator; RR = relative risk.
Similar to PCE-C, patients with an inpatient index event were more likely to receive PCE-D, compared with those with an ED index event (Table 2; RR = 1.90, 95% CI = 1.01-3.58, P = 0.048). Those with previous use of rescue medication (RR = 2.11, 95% CI = 1.16-3.83, P = 0.014) and with greater adherence (PDC ≥ 75%) to pre-index COPD maintenance medication (RR = 8.67, 95% CI = 1.60-46.78, P = 0.012) were also more likely to receive PCE-D.
Readmissions Among Those with 6-Month Postdischarge Follow-up
Univariate Analysis.
A total of 344 admissions with 6 months of continuous enrollment after discharge were identified (Table 3). Of these, 60 (17%) admissions had a subsequent COPD-related readmission, and 155 (45%) had an all-cause readmission within 6 months after discharge. The index events that resulted in PCE had fewer all-cause readmissions (PCE-C: 0.77 vs. 0.84, P = 0.578; PCE-D: 0.74 vs. 1.01, P = 0.098) and more COPD-related readmissions (PCE-C: 0.22 vs. 0.19, P = 0.592; PCE-D: 0.23 vs. 0.13, P = 0.055) compared with index events that did not result in PCE, although these differences were not statistically significant.
TABLE 3.
Postdischarge Characteristics in Patients with 6-Month Follow-up by Receipt of PCE
| Visit Variables, N = 344 Admissions | Received Systemic Corticosteroid Within 14 Days of Discharge (PCE-C) | Received Systemic Bronchodilator Within 30 Days of Discharge (PCE-D) | ||||
|---|---|---|---|---|---|---|
|
Yes
n = 230 (67%) |
No
n = 114 (33%) |
P Value |
Yes
n = 274 (80%) |
No
n = 70 (20%) |
P Value | |
| Readmissions | ||||||
| All-cause | 0.77 (1.38) | 0.84 (1.13) | 0.576 | 0.74 (1.25) | 1.01 (1.47) | 0.098 |
| COPD-related | 0.22 (0.59) | 0.19 (0.55) | 0.592 | 0.23 (0.62) | 0.13 (0.34) | 0.055 |
| Readmission costs, $ | ||||||
| All-cause | 5,161 (22,825) | 4,114 (8,590) | 0.522 | 611 (1,840) | 382 (1,574) | 0.343 |
| COPD-related | 602 (1,863) | 488 (1,629) | 0.566 | 5,126 (21,296) | 3,631 (8,537) | 0.320 |
| ≥ 1 all-cause readmission | ||||||
| Yes | 101 (65%) [44%] | 54 (35%) [47%] | 0.544 | 118 (76%) [43%] | 37 (24%) [53%] | 0.142 |
| No | 129 (68%) [56%] | 60 (32%) [53%] | 156 (83%) [57%] | 33 (17%) [47%] | ||
| ≥ 1 COPD-related readmission | ||||||
| Yes | 42 (70%) [18%] | 18 (30%) [16%] | 0.570 | 50 (83%) [18%] | 10 (17%) [14%] | 0.436 |
| No | 188 (66%) [82%] | 96 (34%) [84%] | 224 (79%) [82%] | 60 (21%) [86%] | ||
Note: Expressed as mean (SD) for continuous variables or count (Row %) [Column %] for categorical variables.
COPD = chronic obstructive pulmonary disease; PCE = pharmacotherapy management of COPD exacerbation; SD = standard deviation.
Multivariate Analysis.
After controlling for pre-index and index variables, receipt of PCE was not shown to be a significant predictor of all-cause or COPD-related readmission. Older patients were more likely to have a COPD-related readmission (Table 4; RR = 1.07, 95% CI = 1.01-1.13, P = 0.028), while patients with higher all-cause medical and pharmacy costs before admission were more likely to have an all-cause readmission (RR = 1.01, 95% CI = 1.00-1.02, P = 0.013). However, patients using maintenance medication before their index event were 51% less likely to have an all-cause readmission (RR = 0.49, 95% CI = 0.30-0.79, P = 0.004).
TABLE 4.
Adjusted Generalized Estimating Equations Predicting 6-Month Readmission (N = 344)
| Parameter | COPD-Related Readmissions | All-Cause Readmissions | ||||
|---|---|---|---|---|---|---|
| RR | 95% CI | P Value | RR | 95% CI | P Value | |
| Pre-index variables | ||||||
| Received PCE-C vs. not | 1.12 | 0.56-2.22 | 0.756 | 1.06 | 0.64-1.75 | 0.827 |
| Received PCE-D vs. not | 1.16 | 0.53-2.53 | 0.711 | 0.62 | 0.34-1.16 | 0.137 |
| Female vs. male | 0.64 | 0.34-1.20 | 0.162 | 1.00 | 0.61-1.63 | 0.999 |
| Age | 1.07 | 1.01-1.13 | 0.028 | 1.03 | 1.00-1.07 | 0.082 |
| Charlson Comorbidity Index | 1.03 | 0.82-1.30 | 0.770 | 1.15 | 0.94-1.40 | 0.169 |
| Adherent vs. nonadherent | 1.08 | 0.46-2.54 | 0.869 | 1.10 | 0.54-2.26 | 0.798 |
| On maintenance medication vs. not | 0.78 | 0.43-1.43 | 0.430 | 0.49 | 0.30-0.79 | 0.004 |
| On rescue medication vs. not | 1.45 | 0.71-2.94 | 0.303 | 1.68 | 0.95-2.96 | 0.072 |
| Pre-index costsa | 1.00 | 0.99-1.01 | 0.672 | 1.01 | 1.00-1.02 | 0.013 |
| Number of exacerbations | 1.13 | 0.90-1.43 | 0.290 | 1.05 | 0.85-1.30 | 0.629 |
| Index (event) variables | ||||||
| Inpatient vs. ED | 0.80 | 0.39-1.65 | 0.542 | 0.77 | 0.43-1.37 | 0.368 |
| Length of stay | 0.99 | 0.90-1.10 | 0.851 | 1.04 | 0.95-1.14 | 0.392 |
| Admission year | 0.95 | 0.81-1.12 | 0.567 | 1.00 | 0.88-1.14 | 0.980 |
| Admission quarter | 1.09 | 0.85-1.38 | 0.507 | 0.90 | 0.73-1.10 | 0.292 |
a Costs multiplied by $1,000.
CI = confidence interval; COPD = chronic obstructive pulmonary disease; ED = emergency department; PCE-C = pharmacotherapy management of COPD exacerbation with systemic corticosteroid; PCE-D = pharmacotherapy management of COPD exacerbation with bronchodilator; RR = relative risk.
Discussion
To our knowledge, this study is the first to determine potential factors associated with the HEDIS quality measure assessing use of systemic corticosteroids and bronchodilators, and explore whether receiving this quality care is associated with hospital readmissions. The rates of PCE-C (68%) and PCE-D (80%) receipt found in this study were slightly higher than the actual average rates of 62% and 74% reported to the NCQA by the Midwest health plan over the study period. This difference is likely because of differences in methodology; for example, the events reported by NCQA are measured over an 11-month intake period (January 1 to November 30 of the measurement year), whereas our study included events throughout the entire 12-month period.
We found that the receipt of bronchodilators and systemic corticosteroids after a COPD-related inpatient or ED visit were more likely to occur in patients with a previous history of rescue medication use, patients who are more adherent to their maintenance medication, and patients with inpatient versus ED visits. As such, the likelihood of treatment may be related to patients' previous use of bronchodilators or greater severity of the index COPD exacerbation. In contrast, another study found that younger patients with less severe COPD are more likely to receive maintenance treatment earlier after an exacerbation; however, this study was conducted in maintenance treatment-naïve patients.17
Our study found that older patients were more likely to have a COPD-related readmission, and those who were previously on a maintenance medication had a lower risk of having an all-cause readmission. This observation may be supportive of the PCE-D measure, considering that use of maintenance medication before the index event was more likely to occur in the PCE-D group. Although a direct comparison cannot be made because of differences in study design, another study focusing on timing of maintenance treatment and outcomes found that delayed treatment of a COPD exacerbation resulted in a 43% higher risk of a future COPD-related hospitalization or ED visit compared with those who received early treatment, with every 30-day delay associated with a 9% risk increase.17 Treatment delay was also shown to increase COPD-related costs.17 Other studies have also shown that older age, longer length of stay during index event, comorbid conditions, and number of previous inpatient hospitalizations were significant predictors of readmission.18,19
Although long-term therapies such as LABA, LAMA, and ICS have been shown to reduce the risk and frequency of exacerbations, there is a need for evidence to demonstrate whether current quality measures are associated with improved patient outcomes.20-22 There are also several concerns with the PCE measure that have been raised by decision makers. For example, in response to the proposed 2015 CMS star ratings, Part C and D sponsors commented that certain circumstances should be considered for exclusion in the measure, such as patients with contraindications to the treatment, patients with extensive length-of-stay hospitalizations, and patients who may have been treated during admission.23 Based on these concerns and available data, a potential solution could be to change the specifications of the measure to include the previously mentioned exclusion criteria or to assess timing of maintenance therapy, since early treatment has been previously shown to reduce COPD-related hospitalization and costs.17
Limitations
This study is not without limitations. Retrospective analyses using claims data and electronic medical records may not include complete patient information and rationale behind the therapy prescribed by physicians. In addition, the misclassification of COPD may have occurred, since not all patients have documented spirometry results. There are several ongoing COPD initiatives within this study's health plan that may have had an impact on care and could potentially bias results for some patients; however, these initiatives could have potentially affected all patients with COPD and not just those with PCE.
Adherence was measured using PDC, which is calculated using claims data that provides information on medications filled and the days supplied. This calculation indicates that patients picked up their medication from their pharmacies, not that they inhaled the medication as directed, but it is a good proxy of patients' medication-taking behavior. The small sample size and lack of COPD severity measurements (e.g., spirometry data) are also limitations. Finally, the patient population is predominantly in rural Central Texas and may differ from the rest of Texas and the United States, thereby potentially limiting the generalizability of this study.
Conclusions
The severity of an index exacerbation or previous pattern of bronchodilator use may be related to the appropriate use of bronchodilators and systemic corticosteroids after a COPD-related inpatient or ED visit. The use of maintenance medication before the index event was associated with a significant reduction in all-cause readmission; therefore, proper treatment of the underlying disease may be an effective strategy in preventing readmission.
APPENDIX. Study Design to Evaluate Pharmacotherapy Management of COPD Exacerbation
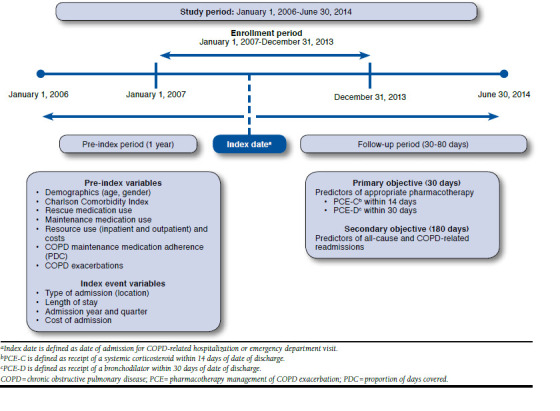
References
- 1.Kosacz NM. Chronic obstructive pulmonary disease among adults— United States, 2011. MMWR Morb Mortal Wkly Rep. 2012;61(46):938-43. [PubMed] [Google Scholar]
- 2.Reilly JJ, Silverman EK, Shapiro SD.. Chronic obstructive pulmonary disease. In: Kasper D, Fauci A, Hauser S, Longo D, Jameson JL, Loscalzo J, eds. Harrison's Principles of Internal Medicine. 19th ed. New York: McGraw-Hill Education; 2015. [Google Scholar]
- 3.Global Initiative for Chronic Obstructive Lung Disease. Global Strategy for the diagnosis, management and prevention of COPD. 2016. Available at: http://goldcopd.org/global-strategy-diagnosis-management-prevention-copd-2016/. Accessed September 6, 2016. [Google Scholar]
- 4.Anzueto A. Impact of exacerbations on COPD. Eur Respir Rev. 2010;19(116):113-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.American Lung Association. Trends in COPD (chronic bronchitis and emphysema): morbidity and mortality. 2013. Available at: http://www.lung.org/assets/documents/research/copd-trend-report.pdf. Accessed September 6, 2016. [Google Scholar]
- 6.National Committee for Quality Assurance. Continuous improvement and expansion of quality measurement. The state of healthcare quality 2011. Available at: http://www.ncqa.org/portals/0/sohc-web1.pdf. Accessed August 24, 2016. [Google Scholar]
- 7.Ford ES, Murphy LB, Khavjou O, Giles WH, Holt JB, Croft JB.. Total and state-specific medical and absenteeism costs of COPD among adults aged ≥ 18 years in the United States for 2010 and projections through 2020. Chest. 2015;147(1):31-45. [DOI] [PubMed] [Google Scholar]
- 8.Dalal AA, Christensen L, Liu F, Riedel AA.. Direct costs of chronic obstructive pulmonary disease among managed care patients. Int J Chron Obstruct Pulmon Dis. 2010;5:341-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dalal AA, Liu F, Riedel AA.. Cost trends among commercially insured and Medicare Advantage-insured patients with chronic obstructive pulmonary disease: 2006 through 2009. Int J Chron Obstruct Pulmon Dis. 2011;6:533-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pasquale MK, Sun SX, Song F, Hartnett HJ, Stemkowski SA.. Impact of exacerbations on health care cost and resource utilization in chronic obstructive pulmonary disease patients with chronic bronchitis from a predominantly Medicare population. Int J Chron Obstruct Pulmon Dis. 2012;7:757-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diette GB, Dalal AA, D'Souza AO, Lunacsek OE, Nagar SP.. Treatment patterns of chronic obstructive pulmonary disease in employed adults in the United States. Int J Chron Obstruct Pulmon Dis. 2015;10:415-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lindenauer PK, Pekow P, Gao S, Crawford AS, Gutierrez B, Benjamin EM.. Quality of care for patients hospitalized for acute exacerbations of chronic obstructive pulmonary disease. Ann Intern Med. 2006;144(12):894-903. [DOI] [PubMed] [Google Scholar]
- 13.Make B, Dutro MP, Paulose-Ram R, Marton JP, Mapel DW.. Undertreatment of COPD: a retrospective analysis of US managed care and Medicare patients. Int J Chron Obstruct Pulmon Dis. 2012;7:1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Centers for Medicare & Medicaid Services. Readmissions Reduction Program (HRRP). 2016. Available at: https://www.cms.gov/medicare/medicare-fee-for-service-payment/acuteinpatientpps/readmissions-reductionprogram.html. Accessed August 24, 2016. [Google Scholar]
- 15.U.S. Department of Health and Human Services. Press release. Better, smarter, healthier: in historic announcement, HHS sets clear goals and timeline for shifting Medicare reimbursements from volume to value. January 26, 2015. Available at: http://www.hhs.gov/about/news/2015/01/26/better-smarter-healthier-in-historic-announcement-hhs-sets-clear-goals-and-timeline-for-shifting-medicare-reimbursements-from-volume-to-value.html. Accessed August 24, 2016. [Google Scholar]
- 16.Charlson ME, Pompei P, Ales KL, MacKenzie CR.. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-83. [DOI] [PubMed] [Google Scholar]
- 17.Dalal AA, Shah MB, D'Souza AO, Dhamane AD, Crater GD.. Outcomes associated with timing of maintenance treatment for COPD exacerbation. Am J Manag Care. 2012;18(9):e338-45. [PubMed] [Google Scholar]
- 18.Baker CL, Zou KH, Su J.. Risk assessment of readmissions following an initial COPD-related hospitalization. Int J Chron Obstruct Pulmon Dis. 2013;8:551-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Candrilli SD, Dhamane AD, Meyers JL, Kaila S.. Factors associated with inpatient readmission among managed care enrollees with COPD. Hosp Pract (1995). 2015;43(4):199-207. [DOI] [PubMed] [Google Scholar]
- 20.Decramer M, Celli B, Kesten S, Lystig T, Mehra S, Tashkin DP.. Effect of tiotropium on outcomes in patients with moderate chronic obstructive pulmonary disease (UPLIFT): a prespecified subgroup analysis of a randomised controlled trial. Lancet. 2009;374(9696):1171-78. [DOI] [PubMed] [Google Scholar]
- 21.Niewoehner DE, Rice K, Cote C, et al.. Prevention of exacerbations of chronic obstructive pulmonary disease with tiotropium, a once-daily inhaled anticholinergic bronchodilator: a randomized trial. Ann Intern Med. 2005;143(5):317-26. [DOI] [PubMed] [Google Scholar]
- 22.Calverley PM, Anderson JA, Celli B, et al.. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med. 2007;356(8):775-89. [DOI] [PubMed] [Google Scholar]
- 23.Centers for Medicare & Medicaid Services. Summary of comments to the star ratings request for comments. 2014. Available at: https://www.cms.gov/medicare/prescription-drug-coverage/prescriptiondrugcovgenin/downloads/2014starratingsrequestforcomments112213-.pdf. Accessed August 24, 2016. [Google Scholar]


