The Centers for Disease Control and Prevention estimates that 20.4 million Americans aged ≥ 18 years currently have asthma and that an additional 6.1 million children have asthma.1,2 There are approximately 14.2 million office visits, 1.8 million emergency department visits, and 440,000 hospitalizations due to asthma each year in the United States.2 The societal costs are estimated to be $82 billion, which includes $50 billion in direct medical costs, $29 billion from asthma-related mortality, and $3 billion from missed work and school.2 Severe asthma comprises a small but important subset of all individuals with asthma. Those with severe asthma represent fewer than 5%-10% of all individuals with asthma but account for approximately 50% of all costs. In addition to being treated with inhaled corticosteroids and long-acting beta agonist therapy, these patients are often treated with oral corticosteroids.3
Asthma has been divided into different phenotypes with some overlap. Approximately half of all patients with asthma have “allergic” asthma, which is associated with allergic rhinitis, atopy, and elevated immunoglobin E (IgE) levels. Another group has “eosinophilic” asthma, with elevated eosinophil levels in blood and airways. Both of these phenotypes are linked to type 2 inflammation with increases in T helper 2 cells.4 These cells secrete interleukin-4 (IL-4), IL-5, and IL-13, which increase the proliferation, survival, and recruitment of eosinophils and increase IgE levels.5,6
There are 5 biologic therapies approved by the U.S. Food and Drug Administration (FDA) that affect the pathways involved in the allergic or eosinophilic phenotypes of asthma. These drugs, along with their mechanisms of action and their FDA indications for asthma, are summarized in Table 1. Omalizumab is a monoclonal antibody to IgE, which is indicated for the treatment of patients with moderate to severe asthma with the allergic phenotype. Mepolizumab, reslizumab, and benralizumab target the IL-5 pathway either with monoclonal antibodies to IL-5 itself (mepolizumab and reslizumab) or to the IL-5 receptor (benralizumab). Dupilumab is a monoclonal antibody to the IL-4 receptor alpha, which modulates the IL-4 and IL-13 pathways.
TABLE 1.
Biologic Therapies for Asthma with Type 2 Inflammation
| Drug | FDA Indication | Dosing | Mechanism |
|---|---|---|---|
| Omalizumab (Xolair, Genentech) | Aged ≥ 6 years with moderate to severe persistent asthma who test positive for year-round allergens14 | 75-375 mg SC Q 2-4 weeks | Anti-IgE |
| Mepolizumab (Nucala, GlaxoSmithKline) | Aged ≥ 12 years with severe asthma and eosinophilic phenotype15 | 100 mg SC Q 4 weeks | Anti-IL-5 |
| Reslizumab (Cinqair, Teva) | Aged ≥ 18 years with severe asthma and eosinophilic phenotype16 | 3 mg/kg IV Q 4 weeks | Anti-IL-5 |
| Benralizumab (Fasenra, AstraZeneca) | Aged ≥ 12 years with severe asthma and eosinophilic phenotype17 | 30 mg SC Q 4 weeks × 3, then Q 8 weeks | Anti-IL-5Ra |
| Dupilumab (Dupixent, Sanofi/Regeneron) | Aged ≥ 12 years with moderate to severe asthma with eosinophilic phenotype or with oral corticosteroid-dependent asthma18 | 200 mg SC Q 2 weeks 300 mg SC Q 2 weeks | Anti-IL-4Ra |
FDA = U.S. Food and Drug Administration; IgE = immunoglobin E; IL-4Ra = interleukin-4 receptor alpha; IL-5 = interleukin-5; IL-5Ra = interleukin-5 receptor alpha; IV = intravenous; Q = every; SC = subcutaneous.
There are important differences in the indications for each of these drugs, such as age, severity of asthma, and asthma phenotype. These differences make direct comparisons challenging. In addition, dupilumab is the only drug approved for self-administration; the other 4 drugs must be administered by a health care professional.
The Institute for Clinical and Economic Review (ICER) conducted a review of omalizumab, mepolizumab, reslizumab, benralizumab, and dupilumab as add-on therapy for patients with uncontrolled moderate to severe asthma to coincide with the expected FDA approval of dupilumab. In this article, we summarize the systematic literature review of the clinical effectiveness of the drugs, the cost-effectiveness analysis, and the policy discussion with key stakeholders regarding the overall value of these therapies held at a public meeting of the Midwest Comparative Effectiveness Public Advisory Council (Midwest CEPAC) on November 29, 2018. The detailed report is available on the ICER website at https://icer-review.org/material/asthma-final-evidence-report/.
Summary of Findings
Clinical Effectiveness
We compared the evidence on the clinical effectiveness of the 5 biologics added to standard of care (inhaled corticosteroids plus at least 1 additional controller therapy) versus standard of care alone. The primary measures of clinical benefit were reductions in asthma exacerbations and improvements in quality of life. The primary harms were severe adverse events and adverse events leading to discontinuation of therapy.
There are no head-to-head randomized or observational trials of the 5 biologics. We used summary estimates from Cochrane meta-analyses for each of the drugs,7,8 in addition to the estimates for dupilumab from its pivotal trials.9,10 The evidence showed that all 5 drugs reduced the annual exacerbation rate by approximately 50%, with broadly overlapping confidence intervals. Similarly, the effect on quality of life for all of the drugs, as assessed by the Asthma Quality of Life Questionnaire and by the Asthma Control Questionnaire, was similar, with modest but statistically significant improvements. All 5 of the drugs are more effective in patients with higher baseline eosinophil counts.
These drugs are well tolerated. The risk for serious adverse events was lower in the active drug group than the placebo group for all 5 drugs, and there were no differences in withdrawals due to adverse events. Minor injection site reactions occurred in approximately twice as many patients treated with a biologic compared with placebo.
Limitations of the Clinical Evidence
Across the study populations for these drugs, differences in the mix of asthma phenotypes, age ranges, baseline eosinophil levels, and asthma severity make it impossible to perform a quantitative indirect comparison, and even qualitative comparison of relative clinical benefits is highly uncertain. In addition, the length of follow-up in some of the randomized trials was only 24 weeks, and no trial was longer than 15 months, so there is no evidence on the long-term safety and effectiveness of these drugs, all of which could potentially be used by patients for decades.
Long-Term Cost-Effectiveness
We estimated the cost-effectiveness of each of the 5 drugs compared with standard of care using a Markov model that included 3 primary health states: an asthma nonexacerbation state (i.e., day-to-day asthma symptoms); an asthma exacerbation state (including an oral corticosteroid burst, asthma-related emergency department visit, or asthma-related hospitalization); and death (including asthma-related mortality and other cause mortality). Full details on ICER’s cost-effectiveness analysis and model are available on ICER’s website at https://icer-review.org/material/asthma-final-evidence-report/.
The reductions in annual exacerbation rates resulting in outpatient steroid bursts, emergency department visits, and hospitalizations for each drug versus standard of care were derived from meta-analyses of the randomized trials for each drug and were specific to the individual drug. For the nonexacerbation health state, we assumed higher utilities for the biologics versus standard of care due to improvements in day-to-day symptoms. In order to eliminate differences across baseline characteristics, such as age, which may affect lifetime costs and outcomes, we averaged baseline characteristics to estimate the same model cohort’s baseline age, gender, weight, proportion of chronic oral steroid users, and standard of care annualized exacerbation rates.
The annual net price supplied by the manufacturers for each of the 5 drugs was approximately $30,000. The incremental cost-effectiveness ratios for the 5 drugs were similar, ranging from $325,000 to $391,000 per quality-adjusted life-year (QALY; Table 2). The cost-effectiveness findings were robust to one-way and probabilistic sensitivity analyses across all biologic agents. In probabilistic sensitivity analyses, no biologic achieved a greater than zero likelihood of meeting the $150,000 per QALY or lower threshold.
TABLE 2.
Health Care Sector Cost-Effectiveness Results for the Biologics
| Annual Price, $a | Cost per QALY, $ | |
|---|---|---|
| Omalizumab | 28,900 | 325,000 |
| Mepolizumab | 29,500 | 344,000 |
| Reslizumab | 28,900 | 391,000 |
| Benralizumab | 27,800 | 371,000 |
| Dupilumab | 31,000 | 351,000 |
aAverage annual price of each treatment, net of discounts and rebates, as reported to ICER by each manufacturer.
ICER = Institute for Clinical and Economic Review; QALY = quality-adjusted life-year.
Limitations of the Cost-Effectiveness Model
Important assumptions in the model were required given that long-term evidence on outcomes for biologic treatment responders, as well as discontinuation rates, was not available. Significant uncertainty also remains about the quality of life of patients on biologic treatment in the nonexacerbation health state. Another limitation of the model is that differences in mortality related to treatment were not observed in the trials or other available evidence; therefore, effect on mortality could be modeled only as a function of reduced asthma-related hospitalizations and emergency department visits.
Policy Discussion
The Midwest CEPAC is 1 of 3 independent appraisal committees convened by ICER to engage in the public deliberation of the evidence on clinical and cost-effectiveness of health care interventions. The Midwest CEPAC is composed of medical evidence experts, including practicing clinicians, methodologists, and leaders in patient engagement and advocacy. Their deliberation includes input from clinical experts and patient representatives specific to the condition under review, as well as formal comment from manufacturers and the public. A policy roundtable concludes each meeting during which representatives from insurers and manufacturers join with clinical experts and patient representatives to discuss how best to apply the findings of the evidence to clinical practice, insurance coverage, and pricing negotiations.
The structure through which ICER evidence reports present information to the Midwest CEPAC is presented in Figure 1. This value assessment framework represents the conceptual framework through which considerations of different elements of value are integrated in judgments on long-term value for money and short-term affordability.11,12
FIGURE 1.
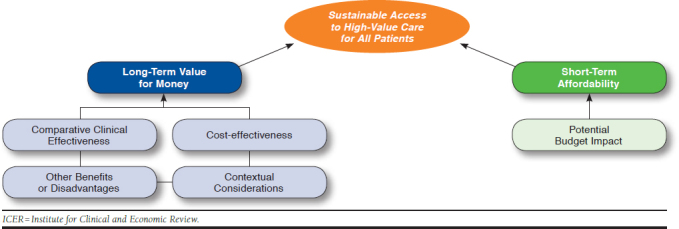
The ICER Value Framework
The ICER report on biologics for asthma was the subject of a Midwest CEPAC meeting on November 29, 2018. Because dupilumab was the only drug receiving new regulatory approval for asthma, the CEPAC first voted 12-3 that the evidence was adequate to demonstrate its superiority to the standard of care. Next, the CEPAC voted with near unanimity that there was not adequate evidence to distinguish among mepolizumab, reslizumab, and benralizumab (14-1), and the panel also voted that the evidence was not adequate to distinguish between dupilumab or omalizumab and these 3 treatments (both votes 15-0).
The CEPAC panel also voted on “other potential benefits” and “contextual considerations” related to dupilumab that may not be fully captured in the clinical or economic evidence but are important for policymakers to consider when making judgments about long-term value for money (Table 3 and Table 4).
TABLE 3.
Other Benefits or Disadvantages
| In the treatment of patients aged ≥ 12 years with moderate to severe asthma, does dupilumab offer 1 or more of the following potential other benefits or disadvantages compared with standard of care without biologic treatment? | |
|---|---|
| Potential Benefit | Panel Votesa |
| Dupilumab offers reduced complexity that will significantly improve patient outcomes | 3 |
| Dupilumab will reduce important health disparities across racial, ethnic, gender, socioeconomic, or regional categories | 0 |
| Dupilumab will significantly reduce caregiver or broader family burden | 6 |
| Dupilumab offers a novel mechanism of action or approach that will allow successful treatment of many patients who have failed other treatments | 8 |
| Dupilumab will have a significant impact on improving patients’ ability to return to work and/or their overall productivity | 7 |
| There are other important benefits or disadvantages that should have an important role in judgements of the value of this intervention | 3 |
aFifteen panelists voted.
TABLE 4.
Contextual Considerations
| Are any of the following contextual considerations important in assessing the long-term value for money of dupilumab versus standard of care without biologics? | |
|---|---|
| Contextual Consideration | Panel Votesa |
| Dupilumab is intended for the care of individuals with a condition of particularly high severity in terms of impact on length of life and/or quality of life | 11 |
| Dupilumab is intended for the care of individuals with a condition that represents a particularly high lifetime burden of illness. | 12 |
| Dupilumab is the first to offer any improvement for patients with this condition | 0 |
| There is significant uncertainty about the long-term risk of serious side effects of this intervention. | 8 |
| There is significant uncertainty about the magnitude or durability of the long-term benefits of this intervention. | 11 |
| There are additional contextual considerations that should have an important role in judgements of the value of this intervention | 3 |
aFifteen panelists voted.
As described in ICER’s recent update to its value assessment framework, questions on “long-term value for money” are subject to a value vote only when incremental cost-effectiveness ratios for the interventions of interest are between $50,000 and $175,000 per QALY in the primary “base case” analysis.13 As shown in Table 2, the estimates for all 5 biologics exceed the higher end of the range, thus, all interventions were deemed “low value” without a vote of the panel.
The policy roundtable discussion explored how best to translate the evidence and broader perspectives discussed into clinical practice and into pricing and insurance coverage policies. The full set of policy recommendations can be found in the final evidence report on the ICER website at https://icer-review.org/material/asthma-final-evidence-report/. Several key policy recommendations are described below:
Given that manufacturers have not priced biologics for asthma at a value-based level, payers should offer preferential formulary status in return for lower prices. For many patients, the evidence is not adequate to determine which drug would be superior as a first option; therefore, it is reasonable for payers to consider step therapy as a mechanism to achieve lower costs without harming patients.
In addition to step therapy, payers should develop prior authorization criteria to ensure that prescriptions are covered only for appropriate patients and that use of these expensive medications is prudent.
Payers should not deny ongoing coverage of biologic therapy if patients are able to reduce the intensity of their inhaled corticosteroids or other long-acting controller medications during treatment with the biologic.
Plan sponsors should work with payers to develop benefit design and negotiation platforms that can provide a clear pathway for all asthma biologics that are priced fairly to be covered with minimum prior authorization controls. In addition, fair pricing as established in comparison to external, independent assessment should be matched with low out-of-pocket requirements for patients.
Conclusions
Adequate evidence demonstrates that all 5 current biologic therapies for asthma related to type 2 inflammation reduce asthma exacerbations and modestly improve quality of life. The therapies also appear to be relatively safe. Omalizumab and mepolizumab have been followed longer than the other drugs in extension studies of the pivotal trials, so there is less uncertainty about long-term effectiveness and safety for these 2 drugs. Nonetheless, the evidence is insufficient to distinguish the overall clinical benefits and safety among these therapies. Despite their incremental clinical benefits when added to standard care for asthma, at current average net prices in the U.S. commercial market, all 5 therapies have high incremental cost-effectiveness ratios, representing low long-term value for money for their labeled indications. Further efforts are needed to help align the prices of these treatments with their demonstrated benefits in order to ensure sustainable access to high-value care for all patients.
ACKNOWLEDGMENTS
The authors thank Laura Cianciolo and Madeline O’Grady for their contributions to ICER’s Midwest CEPAC report on biologic therapies for asthma.
REFERENCES
- 1.Centers for Disease Control and Prevention, National Center for Health Statistics . Faststats: Asthma. 2017. Available at: https://www.cdc.gov/nchs/fastats/asthma.htm. Accessed April 16, 2019.
- 2.Centers for Disease Control and Prevention . Asthma facts: CDC’s National asthma control program grantees. July 2013. Available at: https://www.cdc.gov/asthma/pdfs/asthma_facts_program_grantees.pdf. Accessed April 16, 2019.
- 3.Expert Panel Report 3 (EPR-3): Guidelines for the Diagnosis and Management of Asthma-Summary Report 2007 . J Allergy Clin Immunol. 2007;120(5 Suppl):S94-138. [DOI] [PubMed] [Google Scholar]
- 4.Schatz M, Rosenwasser L. The allergic asthma phenotype. J Allergy Clin Immunol Pract. 2014;2(6):645-48. [DOI] [PubMed] [Google Scholar]
- 5.Fahy JV. Type 2 inflammation in asthma—present in most, absent in many. Nat Rev Immunol. 2015;15(1):57-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moore WC, Meyers DA, Wenzel SE, et al. Identification of asthma phenotypes using cluster analysis in the Severe Asthma Research Program. Am J Respir Crit Care Med. 2010;181(4):315-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Normansell R, Walker S, Milan SJ, Walters EH, Nair P. Omalizumab for asthma in adults and children. Cochrane Database Syst Rev. 2014;(1):CD003559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Farne HA, Wilson A, Powell C, Bax L, Milan SJ. Anti-IL5 therapies for asthma. Cochrane Database Syst Rev. 2017;(9):CD010834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Castro M, Corren J, Pavord ID, et al. Dupilumab efficacy and safety in moderate-to-severe uncontrolled asthma. New Engl J Med. 2018;378(26):2486-96. [DOI] [PubMed] [Google Scholar]
- 10.Rabe KF, Nair P, Brusselle G, et al. Efficacy and safety of dupilumab in glucocorticoid-dependent severe asthma. New Engl J Med. 2018;378(26):2475-85. [DOI] [PubMed] [Google Scholar]
- 11.Pearson SD. The ICER value framework: integrating cost effectiveness and affordability in the assessment of health care value. Value Health. 2018;21(3):258-65. [DOI] [PubMed] [Google Scholar]
- 12.Institute for Clinical and Economic Review . ICER value assessment framework. Available at: https://icer-review.org/methodology/icers-methods/icer-value-assessment-framework/. Accessed April 16, 2019.
- 13.Institute for Clinical and Economic Review . Overview of the ICER value assessment framework and update for 2017-2019. Available at: http://icer-review.org/wp-content/uploads/2017/06/ICER-value-assessment-framework-Updated-050818.pdf. Accessed April 16, 2019.
- 14.XOLAIR (omalizumab) for subcutaneous use . Genentech. 2007. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2007/103976s5102lbl.pdf. Accessed April 16, 2019.
- 15.NUCALA (mepolizumab) for injection, for subcutaneous use. GlaxoSmithKline . Revised November 2015. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/125526Orig1s000Lbl.pdf. Accessed April 16, 2019.
- 16.CINQAIR (reslizumab) injection, for intravenous use . Teva Pharmaceutical Industries. Revised March 2016. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/761033lbl.pdf. Accessed April 16, 2019.
- 17.FASENRA (benralizumab) injection, for subcutaneous use . AstraZeneca. 2017. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/761070s000lbl.pdf. Accessed April 16, 2019.
- 18.DUPIXENT (dupilumab) injection, for subcutaneous use . Regeneron Pharmaceuticals. Revised October 2018. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/761055s007lbl.pdf. Accessed April 16, 2019.


