Abstract
BACKGROUND: Treatment adherence and persistence are crucial to achieve glycemic control in patients with type 2 diabetes (T2D). Early response to a new therapy may lead to improved treatment adherence and associated outcomes.
OBJECTIVE:
To assess the effect of early response to glucagon-like peptide-1 receptor agonist (GLP-1 RA) therapy, as indicated by reduced hemoglobin A1c (A1c) and body weight, on long-term adherence and persistence.
METHODS:
Adults aged ≥ 18 years with T2D initiated with GLP-1 RA therapy after January 1, 2010, were identified from the IBM Explorys Therapeutic Dataset. Patients were required to have health care utilization ≥ 6 months before and ≥ 18 months after the index prescription. Changes in A1c and body weight from baseline through 6 months were assessed for all patients; early response was defined by > 1% reduction in A1c and > 3% reduction in body weight within 3-6 months. Adherence (assessed as the proportion of days covered [PDC] ≥ 80%) and nonpersistence/discontinuation (indicated by a gap in therapy ≥ 60 days) over 18 months were evaluated among early responders versus nonresponders. Multivariable logistic regression was used to assess the effect of early response to GLP-1 RA therapy on adherence and discontinuation over 18 months.
RESULTS:
Among 8,329 identified patients, 33.3% and 31.2% experienced early response as indicated by reductions in A1c > 1% point and in body weight > 3% from baseline, respectively. Significantly higher proportions (P < 0.001) of early responders in both reduced A1c and body weight were adherent over 18 months compared with patients without an early response (A1c: 45.0% vs. 37.1%; body weight: 43.3% vs. 38.0%). Significantly lower proportions (P < 0.001) of early responders discontinued over 18 months compared with patients without an early response (A1c: 61.4% vs. 67.9%; body weight: 61.9% vs. 67.5%). After controlling for baseline demographic and clinical characteristics including baseline weight, baseline A1c, oral antidiabetes drug use, insulin use, and the presence of comorbidity of diabetes, patients were more likely to be adherent over 18 months if they had reductions in A1c > 1% (OR = 1.59, 95% CI = 1.36-1.85) or body weight reduction > 3% (OR = 1.18, 95% CI = 1.02-1.36) at 3-6 months compared with those without an early response. Similarly, the early responders had significantly lower likelihood of discontinuation compared with those without early response (A1c > 1%; OR = 0.62, 95% CI = 0.53-0.72; body weight > 3%; OR = 0.81, 95% CI = 0.70-0.94).
CONCLUSIONS:
Early response to GLP-1 RA therapy was associated with significantly increased adherence and reduced likelihood of discontinuation.
What is already known about this subject
Medication adherence is crucial to achieve and sustain glycemic control in type 2 diabetes patients.
Early glycemic controls and treatment intensification with glucagon-like peptide-1 receptor agonists (GLP-1 RAs) result in long-term clinical and economic benefits.
What this study adds
Early response to GLP-1 RA therapy is associated with increased adherence and reduced likelihood of discontinuation versus no early response in terms of hemoglobin A1c or weight reductions.
This study presents a novel analysis of how patient outcomes affect adherence and persistence.
Type 2 diabetes (T2D) is a chronic, progressive, metabolic condition governed by a decline in beta-cell function, insufficient insulin secretion, and worsening of insulin resistance.1 T2D accounts for approximately 95% of all diabetes cases in the United States.2
Treatment approach to achieve glycemic control for eliminating symptoms and preventing diabetes-related complications in T2D patients is based on hemoglobin A1c (A1c) levels.1 Clinical practice guidelines recommend lifestyle modifications coupled with dietary changes as the first-line therapy. When lifestyle changes fail to achieve adequate glycemic control, prescribers usually initiate patients on metformin followed by other antihyperglycemic agents, such as sulfonylureas, thiazolidinediones, alpha-glucosidase inhibitors, dipeptidyl peptidase-4 (DPP-4) inhibitors, sodium glucose cotransporter-2 inhibitors, meglitinides, basal insulin, and glucagon-like peptide-1 receptor agonists (GLP-1 RAs).1
Pharmacologic treatments of T2D have progressed considerably over the past several years. Currently, there are 12 classes of drugs approved in the United States for T2D, 3 of which were introduced in the past decade.1,3 However, based on a U.S. National Survey during 2011-2014, only 63.8% of patients achieved their individualized A1c targets for glycemic control despite treatment.3
Medication adherence is crucial to achieving and sustaining glycemic control in T2D patients.5-9 Suboptimal adherence and persistence remain a major barrier for optimal treatment response in patients with T2D. A recent study based on administrative claims data, which assessed patient adherence to various antidiabetic drug classes using a medication possession ratio of greater than 80%, reported that over a 1-year period only 45.1% of the T2D patients were adherent to treatment.10
The factors influencing medication adherence in diabetes are multidimensional and include age,10 safety and tolerability, dosing,11 regimen complexity, and cost.10,12,13 In addition, for GLP-1 RAs, a study by Wilke et al. (2016) concluded that previous experience with antidiabetic and concomitant medications influence treatment adherence.14 In chronic conditions such as T2D, patient-perceived control, self-monitoring, and perceived treatment efficacy have an effect on treatment adherence and outcomes.15-17 Patient empowerment, along with motivation, was also reported to improve medication adherence and glycemic control in T2D.17,18 In addition, early glycemic control, occurring within the first 6 months of treatment in T2D patients, has been shown to positively influence the long-term treatment benefits for up to at least 5 years.19 Even early treatment intensification with GLP-1 RAs in T2D patients showed improvement in clinical and economic outcomes.20 However, the direct effect of early treatment efficacy of GLP-1 RAs on medication adherence and the extent of it have not been well documented.
This retrospective analysis sought to assess the effect of early response to GLP-1 RA therapy, as indicated by reduced A1c and body weight, on long-term adherence and persistence among patients with T2D.
Methods
Data Source
This retrospective database study used patient-level deidentified U.S. electronic medical record (EMR) data from the IBM Watson Health Explorys Universe Dataset (IBM, Armonk, NY) for the period from July 1, 2009, to January 1, 2017. Explorys contains data for approximately 55 million patients (≈ 15% of U.S. population) derived from integrated data networks across 23 large health systems comprising approximately 360 hospitals and 330,000 providers. The data provide a full longitudinal view of a patient’s medical history across the care continuum, including diagnoses, procedures, and medications.
Sensitivity analysis of medication adherence and persistence was conducted using the IBM MarketScan Explorys Linked Claims–EMR Dataset, which includes the clinical detail available in the IBM Explorys Therapeutic Dataset along with the detailed pharmacy utilization data found in the IBM MarketScan Research Database, which are administrative claims databases that contain patient-level, deidentified claims from the IBM MarketScan Commercial and Medicare Supplemental Databases, allowing for a more detailed analysis of drug utilization.
All database records are statistically deidentified and certified to be fully compliant with U.S. patient confidentiality requirements set forth in the Health Insurance Portability and Accountability Act of 1996. Because this study used only deidentified patient records and did not involve the collection, use, or transmittal of individually identifiable data, institutional review board approval to conduct this study was not necessary.
Study Design and Patient Selection
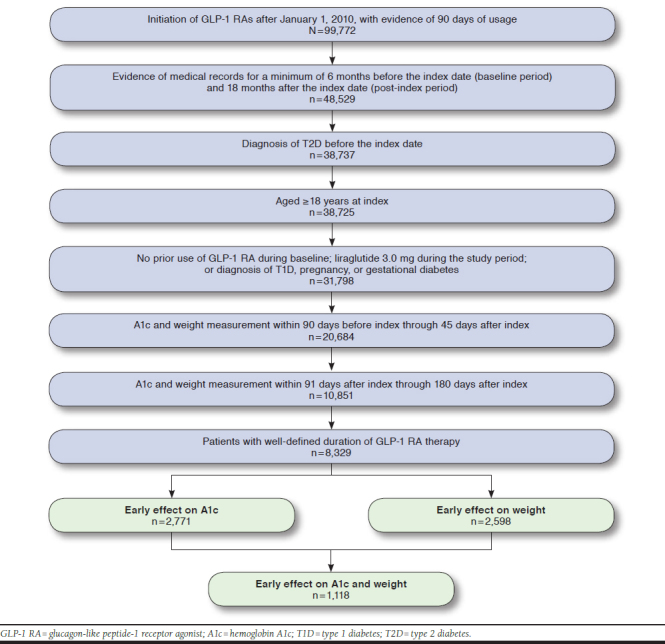
Adults with T2D initiating GLP-1 RA therapy on or after January 1, 2010, through January 1, 2017, were included. Patients with evidence of health care utilization at least 6 months before index (baseline period) and 18 months afterward were selected for the study. Patients with use of GLP-1 RA therapy during the baseline period were excluded. Also, patients with a diagnosis of type 1 diabetes, pregnancy, or gestational diabetes during the study period, identified by the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) and International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM) diagnosis codes, as well as those receiving liraglutide 3.0 mg per day (approved for weight management) at any point during the study period, were excluded.
Patients were required to have a valid recorded A1c (range of actual A1c values in sample: 4.3%-20.3%) and valid recorded body weight (actual values ranged 51 kg-214 kg) between the 2 periods: 90 days pre-index to 45 days post-index (index measurement) and 91-180 days post-index (3- to 6-month measurement). Relevant codes can be found in Appendix A (available in online article). Early response to GLP-1 RA therapy was determined based on changes from the index to the 3- to 6-month measurement period in A1c and body weight. Using the National Institute for Health and Care Excellence guidelines for successful response to GLP-1 RA therapy, early responders were defined by a reduction in A1c > 1% and a reduction in body weight > 3%.21 Same criteria were used for the sensitivity analysis, with diagnosis and drug utilization data drawn from claims data in the IBM MarketScan Research Database and A1c and weight data from Explorys.
Baseline Characteristics
Patient demographic characteristics, such as age; gender; race/ethnicity (Caucasian, African American, Hispanic, other, and unknown); payer type (Medicaid, Medicare, private payer, and other, including self-pay); index year; and index GLP-1 RA were reported. Potential index GLP-1 RAs included albiglutide, dulaglutide, exenatide twice daily, exenatide once weekly, and liraglutide. Baseline A1c and body weight were captured. The Deyo Charlson Comorbidity Index score, an aggregate measure of comorbidity using select diagnoses associated with chronic disease, was calculated for the 6-month baseline period.22 Comorbidities and diabetes-related complications of interest were captured during the baseline period and included cardiovascular disease, hypertension, diabetic nephropathy, diabetic neuropathy, and diabetic retinopathy. Finally, oral antidiabetic drug (OAD) and insulin use during baseline and follow-up were captured.
Outcomes
For each GLP-1 RA treatment period, the starting date of therapy was extracted from EMR records and the end date was calculated using 1 of the following 3 methods listed in order of preference: (1) the recorded end date, if available in the records; (2) the start date plus the number of recorded refills multiplied by 30 days per refill; and (3) the start date plus 365 days. Patients with a calculated end date that fell outside the study period (after January 1, 2017), along with patients with only 1 record of GLP-1 RA treatment and an ascribed end date of exactly 365 days after the start date, were considered ill-defined and excluded from the study. Lines of continuous therapy were constructed by appending consecutive treatment periods as long as the gap between the stop date of the previous period and the start date of the subsequent period was less than 60 days.
Adherence and persistence with GLP-1 RA therapy at 18 months were assessed based on the lines of therapy as previously described. Persistence was defined as the indexing line of therapy lasting for at least 12 or 18 months, respectively. A quasi-measure of proportion of days covered (quasi-PDC) was defined by days covered by medication lines as previously defined, without allowances for gap periods, and divided by the follow-up periods (365 and 545 days). Patients were considered adherent if the quasi-PDC was > 0.80.
Sensitivity analyses assessed adherence and discontinuation using the IBM MarketScan Explorys Linked Claims–EMR Dataset, which contains detailed pharmacy claims data, including dispensed days of supply, allowing for consideration of patient fill behavior and potential drug stockpiling in the determination of adherence and persistence. By having exact fill dates and fill volumes, periods of medication persistence could be calculated more precisely. For example, a patient with an index fill, 9 refills, and no recorded end date in his/her EMR records would be considered nonpersistent at 12 months using the quasi-PDC method described. However, if the same patient had records in the linked dataset suggesting that the patient was consistently a few days late in filling the prescription, then the patient would be considered persistent over the same 12-month period for the sensitivity analysis.
Analysis
All study measures were summarized using descriptive statistics. Bivariate comparisons were made between response cohorts. Chi-square tests were used to evaluate the statistical significance of differences for dichotomous or categorical variables, and 2-sided t-tests or analysis of variance were used for comparison of continuous variables. Multivariable logistic regression was used to assess the effect of early response to GLP-1 RA therapy, as measured by change in A1c and separately change in body weight, on adherence and persistence over 18 months. Models also included interaction terms for early response via A1c and body weight. Models were adjusted for age, gender, primary payer, index year, baseline weight and A1c, OAD and insulin use during baseline and follow-up, and the following conditions identified during the baseline period: cardiovascular disease, hypertension, diabetic nephropathy, diabetic neuropathy, and diabetic retinopathy. The nominal level of significance for all statistical tests was set at 0.05.
Results
A total of 8,329 T2D patients initiating GLP-1 RA therapy on or after January 1, 2010, met the patient selection criteria. One third (33.3%) of patients experienced early response for A1c (i.e., reduction > 1%); one third (31.2%) experienced early response in body weight (reduction > 3%); and 13.4% of patients who initiated GLP-1 RA experienced early response as indicated by both measures from baseline through 6 months of follow-up (Appendix B, available in online article). Table 1 displays the demographic and clinical characteristics of the study cohorts. The mean (standard deviation [SD]) age of the study population was 57 (10.8) years, and one half (54%) of patients were female. Across all patients, the mean change in A1c was –0.71%, and the mean change in weight was –1.65%.
TABLE 1.
Baseline Demographic and Clinical Characteristics by Response Cohort
| Response: A1c | Response: Body Weight | Response: A1c and Weight | |||||||
|---|---|---|---|---|---|---|---|---|---|
| No Early Effect | Dropped > 1% | No Early Effect | Dropped > 3% | No Early Effect | Early Effect | ||||
| n = 5,558 | n = 2,771 | n = 5,731 | n = 2,598 | n = 7,211 | n = 1,118 | ||||
| Age, mean (SD) | 57.7 (10.8) | 56.3 (10.8) | a | 57.0 (10.9) | 57.7 (10.7) | b | 57.2 (10.9) | 57.5 (10.3) | |
| Female, % | 55.8 | 51.3 | a | 52.9 | 57.4 | a | 54.0 | 56.2 | |
| Race, % | b | ||||||||
| African American | 12.2 | 14.7 | 13.6 | 11.8 | 13.1 | 12.3 | |||
| Caucasian | 78.9 | 76.7 | 77.1 | 80.6 | 77.9 | 80.2 | |||
| Hispanic/Latino | 0.6 | 0.4 | 0.6 | 0.3 | 0.6 | 0.1 | |||
| Other | 8.3 | 8.2 | 8.8 | 7.3 | 8.4 | 7.4 | |||
| Payer, % | a | b | |||||||
| Medicaid | 5.1 | 6.2 | 5.3 | 5.9 | 5.4 | 6.1 | |||
| Medicare | 32.4 | 28.2 | 30.1 | 32.9 | 30.9 | 31.8 | |||
| Private | 56.9 | 60.9 | 58.9 | 56.8 | 58.2 | 58.2 | |||
| Other | 5.6 | 4.7 | 5.7 | 4.3 | 5.5 | 3.8 | |||
| Index GLP-1 RA, % | a | a | b | ||||||
| Albiglutide | 1.5 | 1.5 | 1.7 | 1.1 | 1.6 | 1.3 | |||
| Dulaglutide | 1.6 | 2.0 | 1.9 | 1.3 | 1.7 | 1.7 | |||
| Exenatide | 17.4 | 13.3 | 16.6 | 14.7 | 16.5 | 12.9 | |||
| Exenatide QW | 17.7 | 18.0 | 18.4 | 16.4 | 17.9 | 17.3 | |||
| Liraglutide | 61.8 | 65.2 | 61.3 | 66.4 | 62.3 | 66.9 | |||
| Baseline A1c, mean (SD) | 7.8 (1.4) | 9.6 (1.7) | a | 8.4 (1.8) | 8.3 (1.6) | b | 8.3 (1.7) | 9.2 (1.6) | a |
| Change in A1c, mean (SD) | 0.1 (1.0) | -2.4 (1.4) | a | -0.6 (1.6) | -1.0 (1.7) | a | -0.5 (1.5) | -2.3 (1.3) | a |
| Baseline weight (kg), mean (SD) | 108.7 (24.6) | 109.6 (24.3) | 109.2 (24.5) | 108.6 (24.5) | 109.1 (24.6) | 108.8 (23.8) | |||
| Change in weight (%) | -1.4 (3.6) | -2.2 (4.3) | a | 0.2 (2.6) | -5.8 (2.7) | a | -1.0 (3.5) | -6.0 (2.9) | a |
| Charlson Comorbidity Index score, mean (SD) | 1.49 (1.58) | 1.46 (1.49) | 1.48 (1.55) | 1.49 (1.55) | 1.47 (1.55) | 1.54 (1.53) | |||
| Retinopathy, % | 4.4 | 4.0 | 4.0 | 4.7 | 4.3 | 3.9 | |||
| Nephropathy, % | 17.0 | 15.4 | 16.1 | 17.2 | 16.4 | 16.8 | |||
| Cerebrovascular disease, % | 7.4 | 5.9 | b | 6.5 | 7.7 | 6.9 | 6.7 | ||
aP < 0.001.
bP < 0.05.
A1c = hemoglobin A1c; GLP-1 RA = glucagon-like peptide-1 receptor agonist; QW = once weekly; SD = standard deviation.
Patients with reduced A1c > 1% were younger than those who did not experience such a reduction in A1c (56.3 [10.8] vs. 57.7 [10.8]; P < 0.001). In contrast, those with reduced body weight > 3% were older than those who did not experience the same reduction (57.7 [10.7] vs. 57.0 [10.9]; P < 0.006). Most patients initiated GLP-1 RA therapy with liraglutide (61%-67%). Patients not showing early response as indicated by > 1% reduction in A1c had a lower mean A1c at baseline (7.8% [1.4] vs. 9.6% [1.7]) compared with patients who did experience a significant reduction in A1c (P < 0.001). Patients not showing early response as indicated by > 3% reduction in body weight tended to have higher body weight at baseline, but the difference was not statistically significant. Table 2 summarizes the antidiabetic therapy use of the study cohorts. Of the early responders, 18% had no use of any other OADs during baseline compared with 23% for those without early response (P < 0.001).
TABLE 2.
Antidiabetic Therapy During the Baseline and Follow-Up Periods by Response Cohort
| Response: A1c | Response: Body Weight | Response: A1c and Weight | |||||||
|---|---|---|---|---|---|---|---|---|---|
| No Early Effect | Dropped > 1% | No Early Effect | Dropped > 3% | No Early Effect | Early Effect | ||||
| n = 5,558 | n = 2,771 | n = 5,731 | n = 2,598 | n = 7,211 | n = 1,118 | ||||
| Therapy during baseline period, % | |||||||||
| Number of OADs | a | a | a | ||||||
| 0 | 23.3 | 18.1 | 23.2 | 18.1 | 22.4 | 16.6 | |||
| 1-2 | 67.9 | 73.1 | 68.5 | 72.2 | 68.8 | 75.1 | |||
| 3+ | 8.8 | 8.8 | 8.4 | 9.7 | 8.9 | 8.2 | |||
| Insulin and OAD use | b | ||||||||
| Insulin only | 6.0 | 6.5 | 6.3 | 5.9 | 6.3 | 5.6 | |||
| Insulin + 1-2 OADs | 20.4 | 22.8 | 21.8 | 19.9 | 21.4 | 19.9 | |||
| Insulin + 3 + OADs | 2.3 | 2.2 | 2.2 | 2.5 | 2.3 | 2.1 | |||
| Therapy during follow-up period, % | |||||||||
| Number of OADs | a | b | |||||||
| 0 | 13.2 | 9.7 | 12.0 | 12.0 | 12.3 | 10.6 | |||
| 1-2 | 72.2 | 75.2 | 72.7 | 74.3 | 72.7 | 76.6 | |||
| 3+ | 14.6 | 15.1 | 15.3 | 13.7 | 15.0 | 12.9 | |||
| Insulin and OAD use | a | a | |||||||
| Insulin only | 6.2 | 5.5 | 6.2 | 5.5 | 6.1 | 5.1 | |||
| Insulin + 1-2 OADs | 33.4 | 32.4 | 35.0 | 28.8 | 34.1 | 26.4 | |||
| Insulin + 3 + OADs | 6.3 | 6.1 | 6.4 | 5.7 | 6.4 | 4.7 | |||
aP < 0.001.
bP < 0.05.
A1C = hemoglobin A1c; OAD = oral antidiabetic drugs.
The rates of adherence and discontinuation by a change in A1c, body weight, and a combined measure of change in A1c and body weight were also assessed. Significantly higher proportions of early responders (P < 0.001) were adherent over 18 months compared with patients without early response (A1c: 45.0% vs. 37.1%; body weight: 43.3% vs. 38.0%; A1c and body weight: 46.4% vs. 38.6%). Likewise, significantly lower proportions of early responders (P < 0.001) discontinued over 18 months compared with other patients (A1c: 61.4% vs. 67.9%; body weight: 61.9% vs. 67.5%; A1c and body weight: 60.0% vs. 66.7%; Table 3).
TABLE 3.
GLP-1 RA Adherence and Discontinuation over 18 Months by Response Cohort
| Response: A1c | Response: Body Weight | Response: A1c and Weight | |||||||
|---|---|---|---|---|---|---|---|---|---|
| No Early Effect | Dropped > 1% | No Early Effect | Dropped > 3% | No Early Effect | Early Effect | ||||
| n = 5,558 | n = 2,771 | n = 5,731 | n = 2,598 | n = 7,211 | n = 1,118 | ||||
| Adherent (PDC ≥ 0.80), % | 37.1 | 45.0 | a | 38.0 | 43.3 | a | 38.6 | 46.4 | a |
| PDC, mean(SD) | 0.61 (0.32) | 0.67 (0.31) | a | 0.62 (0.32) | 0.66 (0.32) | a | 0.63 (0.32) | 0.68 (0.31) | a |
| Discontinued, % | 67.9 | 61.4 | a | 67.5 | 61.9 | a | 66.7 | 60.0 | a |
| Days on medication, mean (SD) | 343.8 (176.5) | 378.5 (170.8) | a | 348.8 (175.6) | 369.8 (174.0) | a | 351.1 (175.7) | 382.8 (170.3) | a |
aP < 0.001.
A1c = hemoglobin A1c; GLP-1 RA=glucagon-like peptide-1 receptor agonist; PDC=proportion of days covered; SD = standard deviation.
After controlling for differences in demographic and clinical characteristics, previous and concomitant diabetes treatment, and baseline A1c and body weight, the odds of adherence and discontinuation over 18 months were predicted using logistic regression models. The odds of adherence over 18 months were significantly higher for patients with early response, in terms of reduced A1c > 1% (odds ratio [OR] = 1.59, 95% confidence interval [CI] = 1.36-1.85) and reduced body weight > 3% (OR = 1.18, 95% CI = 1.02-1.36) compared with those without early response. Early responders by both measures (reduced A1c > 1% and reduced body weight > 3%) were significantly more likely to be adherent over 18 months (OR = 1.64, 95% CI = 1.52-1.76; Figure 1A).
Figure 1.
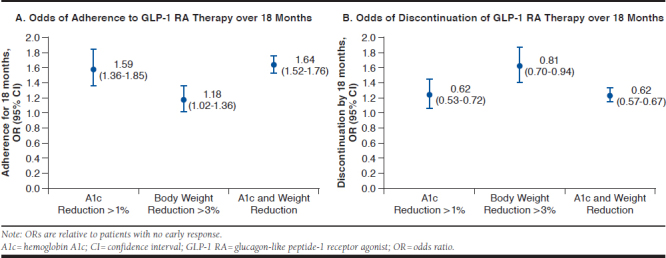
Odds of Adherence to and Discontinuation of GLP-1 RA Therapy over 18 Months
Similarly, the odds of discontinuation over 18 months were significantly lower for early responders having reductions in A1c > 1% (OR = 0.62, 95% CI = 0.53-0.72) and in body weight > 3% (OR = 0.81, 95% CI = 0.70-0.94) compared with those without early response. Patients with both reduced A1c > 1% and body weight reduction > 3% were significantly less likely to discontinue over 18 months (OR = 0.62, 95% CI = 0.57-0.67; Figure 1B).
Sensitivity Analysis
For the sensitivity analysis, 492 T2D patients initiating GLP-1 RA therapy on or after January 1, 2010, who had data availability in IBM MarketScan Explorys Linked Claims-EMR Database were identified. Of these patients, 43.0% experienced early response by a reduction in A1c > 1%; 37.6% experienced early response by a reduction in body weight > 3%; and 18.1% experienced early response as indicated by both measures, from baseline through 3-6 months of follow-up. Higher proportions of early responders with both reduced A1c and body weight were adherent over 18 months compared with other patients (A1c: 37.7% vs. 32.4%; body weight: 41.9% vs. 30.7%; A1c and body weight: 42.7% vs. 32.9%). Lower proportions of early responders discontinued over 18 months compared with other patients (A1c: 54.2% vs. 59.1%; body weight: 49.7% vs. 61.3%; A1c and body weight: 50.6% vs. 58.4%). Multivariable regression analyses conducted within this linked database show the effect of early response measures to be in the same direction as in the primary analyses but were not statistically significant. Patients with reduced A1c > 1% and reduced body weight > 3% were more likely to be adherent (OR = 1.53 and OR = 1.59, respectively) and less likely to discontinue therapy at 18 months (OR = 0.55 and OR = 0.55, respectively). Patients with both indicators of early response had a similar likelihood of adherence and discontinuation (OR = 1.70 and OR = 0.57, respectively, though all P > 0.05).
Discussion
This real-world study assessed the effect of early response to GLP-1 RA treatment on medication adherence and discontinuation, as defined by nonpersistence, over a period of 18 months in patients with T2D. Within this study population, one third of the patients showed an early response to treatment with GLP-1 RA depicted by A1c reduction > 1% and reduction in body weight > 3% within 3-6 months after treatment initiation. The findings of this study suggest that early response was associated with a higher likelihood of medication adherence and lower likelihood of discontinuation over a period of up to 18 months compared with those patients who did not achieve changes of more than 1% reduction in A1c and > 3% reduction in body weight within 3-6 months. The majority of patients in this U.S. study initiated GLP-1 RA treatment with liraglutide, similar to a previous report by another real-world, claim-based multinational European study.23
GLP-1 RAs are recommended as third-line therapy in the treatment sequelae for T2D.2 Currently, 7 GLP-1 RAs, most recently including semaglutide, are approved in the United States.24,25 Evidence from phase 3 clinical trials has shown that GLP-1 RAs are superior to other antidiabetic drugs in A1c and weight reduction with low hypoglycemic risk.26 There is a growing body of literature reporting the benefits of GLP-1 RAs in real-world settings. A recent retrospective claims-based study showed that 6 months after treatment initiation with GLP-1 RAs, mean A1c was reduced by 0.9%, with a larger effect among patients with no previous GLP-1 RA use (1.03%); those who were adherent (1.14%); and those who were persistent (1.12%).27 Consistent with these outcomes, within 3-6 months 30% of our patient population who initiated on GLP-1 RAs, showed reductions in A1c > 1%. In addition, patients showing early response by > 1% reductions in A1c levels had higher baseline A1c levels. Singhal et al. (2015) conducted an analysis using EMR-based data and reported greater A1c reductions among GLP-1 RA patients with poor baseline glycemic control (baseline A1c ≥ 9%).28
Previous studies also have suggested that early treatment with GLP-1 RAs compared with other antidiabetic agents is associated with improved glycemic control and weight loss, which may then affect improved adherence.29 Additionally, use of some GLP-1 RAs (liraglutide and semaglutide) in subjects with high risk of cardiovascular events was associated with cardiovascular benefits in terms of reduced risk of cardiovascular events and cardiovascular and all-cause mortality risk.30-32 The implications of a potential cardiovascular benefit are substantial given that approximately one half (55%-60%) of patients initiating GLP-1 RAs in this study had cardiovascular disease at baseline. Our analysis found that a significantly higher proportion of patients with baseline cardiovascular disease showed an early response in terms of both reductions in A1c and weight.
Compared with other antidiabetics, such as DPP-4 inhibitors and sulfonylureas, GLP-1 RA use has been associated with better weight loss within a year, which is also associated with improved medication adherence.4 A 5-year survey-based study assessing medication-taking behavior, suggested that medications resulting in weight loss by at least 1% within a year might motivate for better medication adherence.33
In addition, a recent study using an administrative claims database to compare adherence and discontinuation within 6 months reported that 38%-54% of patients initiating on GLP-1 RAs were adherent (PDC ≥ 0.80).34 Consistent with these findings, one third of the patients in this study showed > 3% reduction in body weight within 3-6 months after treatment initiation, and those showing this early response had significantly better adherence over 18 months (PDC: 0.66 vs. 0.62). The use of additional therapy with insulin alone or in combination with other OADs was significantly lower for the early responders.
Limitations
Several limitations that are related to database research should be considered when interpreting these study results. First, patient medical and prescription history in the primary analysis was limited to EMRs during the reporting months in this study. EMR data, including diagnoses, prescriptions, and procedures, are only available when the patient is seen by a provider who contributes to the EMR system; any services conducted by providers external to contributing EMR systems were not captured.
Second, misclassification error is possible when relying on diagnosis coding from EMRs, where the extent of missing or inaccurate codes is unknown. Third, because EMR drug records reflect the perspective of the health care provider, data may not reflect patient behavior.
Fourth, the Explorys Universe Database is a convenience sample of contributing data sources in the United States, and findings may not be fully generalizable to other U.S. or international patient populations. Fifth, due to the need for sufficiently flexible testing windows to capture the variability of real-world patient experiences, it is possible that only 45 days might have passed between the 2 testing intervals and a patient may have been taking the index treatment for up to 45 days before the initial test. In addition, multivariate analyses of outcomes provided adjustment for potentially confounding covariates; however, there is always the potential for unmeasured and residual confounders, as well as measurable differences in patient characteristics.
Sixth, some factors that may influence patient adherence and could not be controlled due to lack of information or a high degree of variability include prescribing changes in other antidiabetes medications, the number of follow-up visits with providers, time since initial T2D diagnosis, nondiabetic comorbidities, and medication cost to the patient.
Finally, EMR databases are also limited in their contents as it relates to prescription records, and our methods of estimating the duration of therapy were necessarily conservative. It is likely that these methods underestimated patient adherence and persistence; however, there is no evidence that this bias would affect one cohort over another. To address this concern, adherence and persistence were also estimated in a sensitivity analysis using pharmacy claims data, which provided a more precise record of prescription fills. However, with both record types, it is not possible to ensure that medication has been taken as prescribed.
Despite these limitations, the present study has some important strengths. This large-scale study included patients with T2D enrolled from diverse health care centers and health plans across the United States. To our knowledge, this is the first study that has focused on the early response of T2D patients to GLP-1 RAs within 3-6 months in real-world settings. The sensitivity analysis conducted using a linked database of claims and EMR data elements confirmed the study findings, albeit on the smaller subset of linked patients.
Conclusions
Findings from this study demonstrated that early response to GLP-1 RA therapy is associated with increased adherence and reduced likelihood of discontinuation over 18 months. Although low sample sizes precluded drug-specific analysis, further analysis is warranted on the effect of early response to specific therapies.
ACKNOWLEDGMENTS
The authors thank Machaon Bonafede, PhD, MPH (IBM Watson Health) for his support in managing this study. They also thank Jessamine Winer-Jones, PhD, and Shaswati Khan, PhD (IBM Watson Health), for their support in writing the manuscript.
APPENDIX A. Code List
| Clinical Codes | ||
|---|---|---|
| Condition | ICD-9-CM | ICD-10-CM |
| Type 2 diabetes | 25000, 25002, 25020, 25022, 25010, 25012, 25060, 25062, 25050, 25052, 25030, 25032, 25080, 25082, 25070, 25072, 25040, 25042, 25090, 25092 | E1144, E1143, E1136, E1122, E11620, E1141, E1121, E11610, E1140, E1152, E1151, E1142, E11621, E1165, E1101, E1100, E11641, E11649, E11321, E11329, E11331, E11339, E1159, E11618, E1129, E1149, E1139, E11638, E11628, E11622, E1169, E11630, E11351, E11359, E11341, E11349, E118, E11311, E11319, E119 |
| Type 1 diabetes | 25001, 25003, 25021, 25023, 25011, 25013, 25061, 25063, 25051, 25053, 25031, 25033, 25081, 25083, 25071, 25073, 25041, 25043, 25091, 25093 | E1044, E1043, E1036, E1022, E10620, E1041, E1021, E10610, E1040, E1052, E1051, E1042, E10621, E1065, E10641, E10649, E1011, E1010, E10321, E10329, E10331, E10339, E1059, E10618, E1029, E1049, E1039, E10638, E10628, E10622, E1069, E10630, E10351, E10359, E10341, E10349, E108, E10311, E10319, E109 |
| Test Codes | ||
| Measurement | LOINC | |
| BMI | 39156-5 | |
| A1c | 17855-8, 17856-6, 41995-2, 4548-4, 4549-2, 55454-3, 59261-8, 62388-4, 71875-9 | |
| Height | 8308-9 | |
| Weight | 29463-7, 3141-9, 58229-6, 79348-9, 8350-1, 8351-9 | |
A1c = hemoglobin A1c; BMI = body mass index; ICD-9-CM = International Classification of Diseases, Ninth Revision, Clinical Modification; ICD-10-CM = International Classification of Diseases, Tenth Revision, Clinical Modification; LOINC = Logical Observation Identifiers Names and Codes.
APPENDIX B. Patient Selection
REFERENCES
- 1.American Diabetes Association. Standards of medical care in diabetes—2018. Diabetes Care. 2018;41(1):S1-S159.29222369 [Google Scholar]
- 2.Centers for Disease Control and Prevention. National diabetes statistics report, 2017. Estimates of diabetes and its burden in the United States. 2017. Available at: https://www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf. Accessed February 23, 2019.
- 3.White JR Jr. A brief history of the development of diabetes medications. Diabetes Spectr. 2014;27(2):82-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carls G, Huynh J, Tuttle E, Yee J, Edelman SV. Achievement of glycated hemoglobin goals in the U.S. remains unchanged through 2014. Diabetes Ther. 2017;8(4):863-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buysman EK, Anderson A, Bacchus S, Ingham M. Retrospective study on the impact of adherence in achieving glycemic goals in type 2 diabetes mellitus patients receiving canagliflozin. Adv Ther. 2017;34(4):937-53. [DOI] [PubMed] [Google Scholar]
- 6.Egede LE, Gebregziabher M, Hunt KJ, et al. Regional, geographic, and racial/ethnic variation in glycemic control in a national sample of veterans with diabetes. Diabetes Care. 2011;34(4):938-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McAdam-Marx C, Bellows BK, Unni S, et al. Determinants of glycaemic control in a practice setting: the role of weight loss and treatment adherence (the DELTA Study). Int J Clin Pract. 2014;68(11):1309-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pladevall M, Williams LK, Potts LA, Divine G, Xi H, Lafata JE. Clinical outcomes and adherence to medications measured by claims data in patients with diabetes. Diabetes Care. 2004;27(12):2800-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rhee MK, Slocum W, Ziemer DC, et al. Patient adherence improves glycemic control. Diabetes Educ. 2005;31(2):240-50. [DOI] [PubMed] [Google Scholar]
- 10.Curkendall SM, Thomas N, Bell KF, Juneau PL, Weiss AJ. Predictors of medication adherence in patients with type 2 diabetes mellitus. Curr Med Res Opin. 2013;29(10):1275-86. [DOI] [PubMed] [Google Scholar]
- 11.Nguyen H, Dufour R, Caldwell-Tarr A. Glucagon-like peptide-1 receptor agonist (GLP-1RA) therapy adherence for patients with type 2 diabetes in a Medicare population. Adv Ther. 2017;34(3):658-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garcia-Perez LE, Alvarez M, Dilla T, Gil-Guillen V, Orozco-Beltran D. Adherence to therapies in patients with type 2 diabetes. Diabetes Ther. 2013;4(2):175-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tamblyn R, Eguale T, Huang A, Winslade N, Doran P. The incidence and determinants of primary nonadherence with prescribed medication in primary care: a cohort study. Ann Intern Med. 2014;160(7):441-50. [DOI] [PubMed] [Google Scholar]
- 14.Wilke T, Mueller S, Groth A, et al. Non-persistence and non-adherence of patients with type 2 diabetes mellitus in therapy with GLP-1 receptor agonists: a retrospective analysis. Diabetes Ther. 2016;7(1):105-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Broadbent E, Donkin L, Stroh JC. Illness and treatment perceptions are associated with adherence to medications, diet, and exercise in diabetic patients. Diabetes Care. 2011;34(2):338-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kassahun A, Gashe F, Mulisa E, Rike WA. Nonadherence and factors affecting adherence of diabetic patients to anti-diabetic medication in Assela General Hospital, Oromia Region, Ethiopia. J Pharm Bioallied Sci. 2016;8(2):124-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.von Arx LB, Gydesen H, Skovlund S. Treatment beliefs, health behaviors and their association with treatment outcome in type 2 diabetes. BMJ Open Diabetes Res Care. 2016;4(1):e000166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reach G. Obedience and motivation as mechanisms for adherence to medication: a study in obese type 2 diabetic patients. Patient Prefer Adherence. 2011;5:523-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Svensson E, Baggesen LM, Johnsen SP, et al. Early glycemic control and magnitude of HbA1c reduction predict cardiovascular events and mortality: population-based cohort study of 24,752 metformin initiators. Diabetes Care. 2017;40(6):800-06. [DOI] [PubMed] [Google Scholar]
- 20.Tong L, Pan C, Wang H, Bertolini M, Lew E, Meneghini LF. Impact of delaying treatment intensification with a glucagon-like peptide-1 receptor agonist in patients with type 2 diabetes uncontrolled on basal insulin: a longitudinal study of a US administrative claims database. Diabetes Obes Metab. 2018;20(4):831-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.National Institute for Health and Care Excellence. Type 2 diabetes in adults: management. NICE Guideline 28 . 2015. Available at: https://www.nice.org.uk/guidance/ng28. Accessed February 23, 2019. [PubMed]
- 22.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613-19. [DOI] [PubMed] [Google Scholar]
- 23.Divino V, DeKoven M, Khan FA, Boye KS, Sapin H, Norrbacka K. GLP-1 RA treatment patterns among type 2 diabetes patients in five European countries. Diabetes Ther. 2017;8(1):115-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Novo Nordisk. Novo Nordisk receives FDA approval of Ozempic (semaglutide) injection for the treatment of adults with type 2 diabetes [news release] . Available at: http://press.novonordisk-us.com/2017-12-5-Novo-Nordisk-Receives-FDA-Approval-of-OZEMPIC-R-semaglutide-Injection-For-the-Treatment-of-Adults-with-Type-2-Diabetes. Accessed February 23, 2019.
- 25.Clements JN. New GLP-1 agonist approved by FDA: semaglutide. March 27, 2018. Available at: https://www.diabeteseducator.org/news/aade-blog/aade-blog-details/jennifer-n.-clements-pharmd-bcps-cde-bcacp/2018/03/27/new-glp-1-agonist-approved-by-FDA. Accessed February 23, 2019.
- 26.Tran KL, Park YI, Pandya S, et al. Overview of glucagon-like peptide-1 receptor agonists for the treatment of patients with type 2 diabetes. Am Health Drug Benefits. 2017;10(4):178-88. [PMC free article] [PubMed] [Google Scholar]
- 27.Mody R, Grabner M, Yu M, et al. Real-world effectiveness, adherence and persistence among patients with type 2 diabetes mellitus initiating dulaglutide treatment. Curr Med Res Opin. 2018;34(6):995-1003. [DOI] [PubMed] [Google Scholar]
- 28.Singhal M, Nguyen H, Schauerhamer M, Unni S, Cobden D, McAdam-Marx C. Effect of daily or weekly GLP-1 receptor agonists on glycemic control in insulin-naive patients with poorly controlled type 2 diabetes: a real-world study [abstract]. Value Health. 2015;18(3):A54. [Google Scholar]
- 29.Ross SA, Ballantine J. Early use of glucagon-like peptide-1 receptor agonists (GLP-1 RAs) in type 2 diabetes. Curr Med Res Opin. 2013;29(12):1617-26. [DOI] [PubMed] [Google Scholar]
- 30.Bethel MA, Patel RA, Merrill P, et al. Cardiovascular outcomes with glucagon-like peptide-1 receptor agonists in patients with type 2 diabetes: a meta-analysis. Lancet Diabetes Endocrinol. 2018;6(2):105-13. [DOI] [PubMed] [Google Scholar]
- 31.Marso SP, Bain SC, Consoli A, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375(19):1834-44. [DOI] [PubMed] [Google Scholar]
- 32.Marso SP, Daniels GH, Brown-Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375(4):311-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grandy S, Fox KM, Hardy E, Group SS. Association of weight loss and medication adherence among adults with type 2 diabetes mellitus: SHIELD (Study to Help Improve Early evaluation and management of risk factors Leading to Diabetes). Curr Ther Res Clin Exp. 2013;75:77-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alatorre C, Fernandez Lando L, Yu M, et al. Treatment patterns in patients with type 2 diabetes mellitus treated with glucagon-like peptide-1 receptor agonists: higher adherence and persistence with dulaglutide compared with once-weekly exenatide and liraglutide. Diabetes Obes Metab. 2017;19(7):953-61. [DOI] [PMC free article] [PubMed] [Google Scholar]


