Abstract
BACKGROUND:
Widespread use of statins has improved hypercholesterolemia management, yet a significant proportion of patients remain at risk for cardiovascular (CV) events. Analyses of treatment patterns reveal inadequate intensity and duration of statin therapy among patients with hypercholesterolemia, and little is known about real-world statin use, specifically in subgroups of patients at high risk for CV events.
OBJECTIVE:
To examine patterns of statin use and outcomes among patients with high-risk features who newly initiated statin monotherapy.
METHODS:
Adult patients (aged > 18 years) at high CV risk who received > 1 prescription for statin monotherapy and who had not received lipid-modifying therapy during the previous 12 months were identified from the Truven MarketScan Commercial and Medicare Supplemental databases (from January 2007 to June 2013). Patients with atherosclerotic cardiovascular disease (ASCVD) or diabetes were hierarchically classified into 5 mutually exclusive CV risk categories (listed here in order from highest to lowest risk): (1) recent CV event (subcategorized by hospitalization for acute coronary syndrome [ACS] or other non-ACS CV event within 90 days of index); (2) coronary heart disease (CHD); (3) history of ischemic stroke; (4) peripheral artery disease (PAD); and (5) diabetes. Outcomes of interest included changes in therapy, proportion of days covered (PDC), time to discontinuation, and proportion of patients with ASCVD-related inpatient visit during the follow-up period. Statin therapy was subdivided into high-intensity treatment (atorvastatin 40 mg or 80 mg, rosuvastatin 20 mg or 40 mg, or simvastatin 80 mg) or moderate- to low-intensity treatment (all other statins and statin dosing regimens). Follow-up data were obtained from the index date (statin initiation) until the end of continuous enrollment.
RESULTS:
A total of 541,221 patients were included in the analysis. The majority of patients were stratified in the diabetes cohort (61.1%), followed in frequency by recent ACS event (15.8%), recent non-ACS CV event (9.9%), PAD (4.7%), CHD (4.4%), and history of ischemic stroke (4.1%). Only 15.0% of the population initiated therapy with a high-intensity statin, and 22.5% of these high-intensity statin initiators switched to a moderate- to low-intensity regimen during the follow-up period. Median time to statin discontinuation was approximately 15 months. Duration of treatment was longer among those who were treated with a high-intensity versus a moderate- to low-intensity statin regimen (21 and 15 months, respectively). The PDC was highest in the recent ACS hospitalization cohort (66.4%) and lowest in the diabetes cohort (55.5%). The PDC was significantly greater among patients who initiated treatment with a high-intensity statin regimen than with a moderate- to low-intensity statin regimen (62.1% vs. 57.5%, respectively; P< 0.001). At 1 year, Kaplan-Meier estimates of the cumulative rates for ASCVD-related hospitalizations ranged from 3.5% (diabetes) to 21.8% (recent ACS hospitalization).
CONCLUSIONS:
Patients at high risk for CV events are suboptimally dosed with statins, have high rates of discontinuation, and have low rates of adherence. Despite the use of statin therapy, ASCVD-related inpatient visit rates were high, particularly among those patients at highest risk because of a recent ACS hospitalization. Future interventions are required to ensure that high-risk patients are effectively managed to reduce subsequent morbidity and mortality.
What is already known about this subject
Patients with established cardiovascular (CV) disease or CV risk factors are at high risk for CV events. For these patients, optimal secondary preventive care, including lipid-modifying therapy, can improve outcomes.
Available evidence suggests that patients at high CV risk because of the presence of atherosclerotic cardiovascular disease (ASCVD) are not receiving sufficient lipid-modifying therapy to mitigate disease-associated risk.
The shift toward high-intensity statin therapy recommended in the 2013 American College of Cardiology/American Heart Association guidelines may be challenging, given the large proportion of moderate- to low-intensity statin use.
What this study adds
High-intensity statin therapy is infrequently prescribed to patients at high risk for CV events, regardless of the underlying risk factor(s) (e.g., recent acute coronary syndrome [ACS] hospitalization, diagnosed coronary heart disease, and diabetes).
Adherence to statin therapy is suboptimal, with high discontinuation rates among all high CV-risk patient subgroups.
Despite the use of statin therapy, patients with recent ACS have a high 1-year risk of ASCVD-related rehospitalization.
Since their introduction in the late 1980s, 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase inhibitors (statins) have become the cornerstone of hypercholesterolemia treatment. Although recommendations in guidelines for cholesterol management differ, major guidelines endorse statin therapy to reduce cardiovascular (CV) risk for patients with elevated cholesterol levels for whom lifestyle change alone is insufficient.1-4 In 2013, the American College of Cardiology and the American Heart Association (ACC/AHA) guideline on cholesterol management shifted away from specific low-density lipoprotein cholesterol (LDL-C) levels to overall atherosclerotic CV disease (ASCVD) risk reduction.3 This guideline recommends the use of high-intensity statins in patients at highest risk (e.g., ASCVD) for CV events.
Approximately 35.9 million Americans, or 15% of the population aged 18 years and older, have either ASCVD or diabetes mellitus.5 The downstream consequences of uncontrolled hypercholesterolemia, including ASCVD events such as those related to coronary heart disease (CHD), ischemic stroke, and peripheral artery disease (PAD), are a significant burden for patients and for the health care system. CHD alone accounted for approximately 1 of every 6 deaths in the United States in 2010,6 and it is projected to result in direct and indirect costs of nearly $220 billion in 2030.7 Despite these known risks, statin therapy is widely underutilized across patient populations and clinical conditions.
Patients at high risk for CV events, especially those with ASCVD, stand to gain the most from statins.3 This effect has been demonstrated in several randomized, placebocontrolled clinical studies.8-10 Furthermore, studies indicate that intensive dosing reduced CV events more than standard doses of statins.11,12 Nonetheless, research from real-world studies indicates that statin use is suboptimal in populations with high CV risk.13-17 Available evidence also suggests that clinicians frequently opt for a less aggressive statin regimen, even when treatment goals are not achieved.18-22 As with the primary prevention population, adherence to therapy is a significant challenge to maintaining adequate risk reduction in patients with established CV disease (CVD).17,23,24
To further understand statin use among subgroups of patients with ASCVD, we conducted a large-scale, comprehensive evaluation of statin intensity, modification, adherence, and associated outcomes in a treatment-naive cohort of patients at high risk for CV events.
Methods
Study Population and Data Source
This retrospective study identified patients from the Truven MarketScan Commercial and Medicare Supplemental databases from January 2007 through June 2013. These databases contain medical, pharmacy, and enrollment data from large self-insured employers representing approximately 30 million lives. Major data contributors include employers and health plans that cover employees and their dependents through a variety of offerings, including fee-for-service, fully capitated, and partially capitated health plans. The Truven MarketScan Medicare Supplemental database encompasses 2.9 million covered lives of individuals aged ≥ 65 years who had Medicare coverage plus employer-paid commercial plans. The datasets fully integrate pharmacy and medical claims and provide longitudinal information on patient treatment history.
Adult patients (≥ 18 years) who newly initiated statin mono-therapy (index date) and had ≥ 1 medical claim for 1 of the following ASCVD conditions or diabetes during the pre-index period were included in the analysis: acute coronary syndrome (ACS), history of myocardial infarction (MI), stable or unstable angina, coronary or other arterial revascularization, ischemic stroke, PAD, or diabetes. Patients were required to have continuous enrollment for 12 months pre-index.
Patient Subgroups
Patients were hierarchically classified into the highest mutually exclusive CV risk category.25 Stratification was performed such that a patient was assigned a CV risk category based on the presence or absence of the foremost risk factor using the following organizational scheme: (a) recent CV event (subcategorized by hospitalization for ACS or other non-ACS CV event); (b) CHD; (c) history of ischemic stroke; (d) PAD; and (e) diabetes. CVD/CV risk factors were identified by International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) diagnostic codes26 and procedure codes in the Current Procedural Terminology, 4th edition (CPT-4), Healthcare Common Procedure Coding System Level II (HCPCSII), or revenue code format. A complete list of included ICD-9-CM diagnostic codes and CPT and HCPCSII procedure codes is provided in the Appendix (available in online article).
The following definitions were applied to patient classification:
-
Recent CV event
Recent ACS hospitalization: ≥ 1 inpatient stay for acute MI or unstable angina within 90 days pre-index
Recent non-ACS CV event: ≥ 1 inpatient stay for revascu-larization or ischemic stroke within 90 days pre-index
CHD: > 1 inpatient or ≥ 2 outpatient visits for stable angina or a prior MI (≥ 90 days) during the 12 months pre-index
History of ischemic stroke: ischemic stroke that occurred between 90 days and 12 months pre-index
PAD: ≥ 1 inpatient or ≥ 2 outpatient visits for PAD or carotid artery disease during the 12 months pre-index
Diabetes: ≥ 1 inpatient or ≥ 2 outpatient visits for diabetes management during the 12 months pre-index
According to this stratification algorithm, a patient with diabetes and CHD would be classified within the CHD CV risk category. The existence of CV risk factors was determined by their presence in medical claims records within 12 months before statin initiation, with the exception of recent CV events, which were evaluated 90 days pre-index.
Study Definitions and Measures
Statin monotherapy was defined as a prescription for a drug within the HMG-CoA reductase inhibitor drug class (i.e., atorvastatin, fluvastatin, lovastatin, pitavastatin, pravastatin, rosuvastatin, and simvastatin) without any concomitant lipid-modifying therapies at baseline. Statin therapy was subdivided into high-intensity (atorvastatin 40 mg or 80 mg, rosuvastatin 20 mg or 40 mg, or simvastatin 80 mg) or moderate- to low-intensity (all other statins and statin-dosing regimens) treatment.
A variable follow-up period was used, with patients tracked from the index date until the end of continuous enrollment. Follow-up measures included statin discontinuation, changes to the statin therapy regimen, proportion of days covered (PDC) by a statin, and the occurrence of ASCVD-related hospitalization. A patient was considered to have discontinued statin therapy if he or she failed to receive any statin treatment for 90 consecutive days. Modifications of the statin monotherapy regimen included switching to another statin class member, adding concomitant lipid-modifying therapy or prescription of a fixed-dose combination (e.g., ezetimibe-simvastatin), and upward or downward adjustments to the intensity of statin therapy. PDC for statin therapy was calculated as the total number of covered days with statin supply divided by the total number of days from index until the end of continuous enrollment. The proportion of patients experiencing an ASCVD-related hospitalization was determined based on the presence of an ICD-9-CM code indicative of ≥ 1 ASCVD condition of interest (i.e., ASCVD conditions used to identify high CV risk during patient selection, such as MI, unstable angina, and ischemic stroke).
Statistical Analyses
Descriptive statistics were calculated for baseline patient demographic and clinical characteristics and for statin treatment utilization patterns. Analyses of postbaseline treatment patterns (e.g., discontinuation, switching, changes in statin intensity, and PDC) included all patients with > 120 days of continuous enrollment post-index. The requirement of > 120 days of continuous enrollment was selected to allow patients sufficient time on the index statin before discontinuing their statin. A minimum of >30 days supplied was assumed, and discontinuation was defined as the absence of any statin use for > 90 days.
All baseline and outcome measures were stratified by CV risk category (i.e., recent CV event, CHD, history of ischemic stroke, PAD, and diabetes). Kaplan-Meier survival analyses were conducted for time to statin discontinuation and ASCVDrelated hospitalizations during the follow-up period, which was defined as index to the end of continuous enrollment. Two-sided log-rank tests comparing across all cohorts were used to determine statistical significance. A Cox proportional hazards regression model was used to estimate the hazard ratio for the relationship between high-risk cohort and discontinuation while adjusting for age, gender, and comorbidity index. The regression analysis on discontinuation was run on a subset of patients with > 120 days of continuous enrollment post-index. Kaplan-Meier survival analysis and Cox regression models were conducted with Statistical Analysis System (SAS) software package, version 9.3 (SAS Institute, Cary, NC). All other analyses of descriptive statistics were conducted using R, version 3.1.2 (The R Foundation, Vienna, Austria).
Results
A total of 541,221 patients met the eligibility criteria and were included in the baseline assessments (Figure 1). The proportion of patients per CV risk category were as follows: 15.8% recent ACS event, 9.9% recent non-ACS CV event, 4.4% CHD, 4.1% history of ischemic stroke, 4.7% PAD, and 61.1% diabetes. Age distribution was generally comparable across cohorts, with the exception of the diabetes cohort, which had a younger mean age and more patients in the 18-44 years category (Table 1). The mean Charlson comorbidity score for the overall population was 1.9 and varied by cohort (intercohort range = 1.7 to 2.6). Hypertension and diabetes were prevalent across all CV risk categories. The mean observation period following the index event (i.e., statin initiation) was 20.4 months (median = 16.6 months; range = < 1 to 65.9 months).
FIGURE 1.
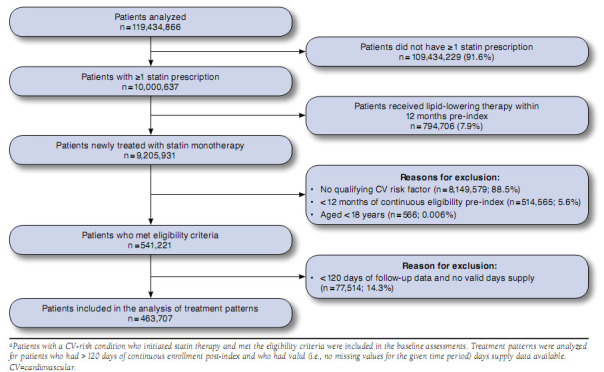
Patient Selection Flowcharta
TABLE 1.
Demographics and Baseline Characteristics of the Study Population (N = 541,221)
| Parameter | Recent CV Event (n = 139,222) | CHD (n = 23,621) | Ischemic Stroke (n = 22,431) | PAD (n = 25,357) | Diabetes (n = 330,590) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| ACS Eventa (n = 85,272) | Non-ACS Eventb (n = 53,950) | ||||||||||
| Mean age [SD], years | 60.5 [13.1] | 63.9 [13.7] | 60.3 [12.2] | 66.0 [12.8] | 64.9 [12.8] | 54.7 [11.4] | |||||
| Age group, years, % (n) | |||||||||||
| 18-44 | 8.9 (7,589) | 6.6 (3,561) | 7.8 (1,842) | 4.0 (897) | 4.2 (1,065) | 17.5 (57,853) | |||||
| 45-64 | 61.8 (52,698) | 52.2 (28,162) | 64.6 (15,259) | 48.2 (10,812) | 52.2 (13,236) | 69.1 (228,438) | |||||
| > 65 | 29.3 (24,985) | 41.3 (22,281) | 27.6 (6,519) | 47.7 (10,700) | 43.6 (11,056) | 13.4 (44,299) | |||||
| Female, % (n) | 32.8 (27,969) | 44.1 (23,792) | 42.3 (9,992) | 51.7 (11,597) | 46.8 (11,867) | 49.7 (164,303) | |||||
| Region, % (n) | |||||||||||
| Northeast | 13.7 (11,682) | 13.8 (7,445) | 12.2 (2,882) | 14.3 (3,208) | 16.9 (4,285) | 10.7 (35,373) | |||||
| South | 39.0 (33,256) | 38.6 (20,825) | 48.8 (11,527) | 39.0 (8,748) | 37.1 (9,407) | 46.6 (154,055) | |||||
| Midwest | 31.8 (27,116) | 31.9 (17,210) | 27.6 (6,519) | 32.2 (7,223) | 31.5 (7,987) | 28.0 (92,565) | |||||
| West | 15.5 (13,217) | 15.7 (8,470) | 11.5 (2,716) | 14.5 (3,252) | 14.5 (3,677) | 14.7 (48,597) | |||||
| Hypertension, % (n) | 52.2 (44,512) | 62.6 (33,773) | 62.5 (14,763) | 60.6 (13,593) | 58.4 (14,808) | 45.3 (149,757) | |||||
| Cancer, % (n) | 6.6 (5,628) | 8.1 (4,370) | 7.5 (1,772) | 9.0 (2,019) | 9.1 (2,307) | 4.6 (15,207) | |||||
| Diabetes, % (n) | 21.1 (17,992) | 23.0 (12,409) | 29.9 (7,063) | 28.1 (6,303) | 34.1 (8,647) | 100.0 (330,590) | |||||
| Mean Charlson comorbidity score [SD] | 2.0 [1.8] | 2.3 [1.8] | 1.8 [1.9] | 2.6 [1.9] | 2.5 [1.9] | 1.7 [1.2] | |||||
| Mean follow-up duration [SD], months | 20.2 [16.3] | 19.5 [16.0] | 19.9 [15.8] | 19.5 [15.7] | 19.3 [15.5] | 20.8 [16.2] | |||||
aACS event category includes patients with > 1 inpatient hospital stay for acute myocardial infarction or unstable angina within 90 days pre-index.
bNon-ACS CV event category includes patients with > 1 inpatient hospital stay for revascularization or ischemic stroke within 90 days pre-index.
ACS = acute coronary syndrome; CHD = coronary heart disease; CV = cardiovascular; PAD = peripheral artery disease; SD = standard deviation.
At the time of index, 15.0% of the overall population was started on high-intensity statin therapy (Figure 2). High-intensity therapy was most frequent among patients with a recent ACS hospitalization (31.4%) and least frequent among patients with diabetes (10.4%). Post-index treatment patterns were analyzed for 463,707 patients who had > 120 days of continuous post-index enrollment and available valid (i.e., no missing values for the given time period) days supply data (Figure 1). During the course of follow-up, 15% to 20% of patients per cohort switched from their index statins to monotherapy with another statin (Figure 3A). Patients who had experienced a recent ACS event changed their statin regimens numerically more frequently than patients in other CV risk categories. Add-on or combination therapy was used by 3% to 4% of patients per cohort. Less than 3% of patients switched from a statin to a nonstatin agent for lipid lowering.
FIGURE 2.
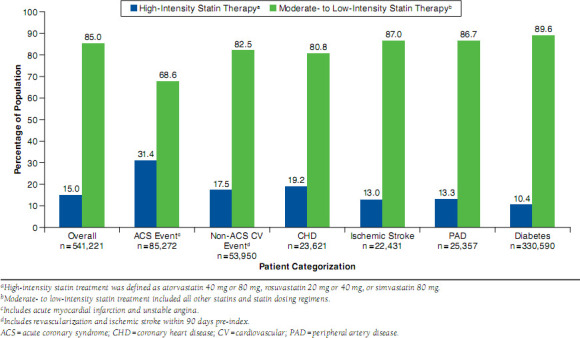
Intensity of Statin Therapy at Index
FIGURE 3.
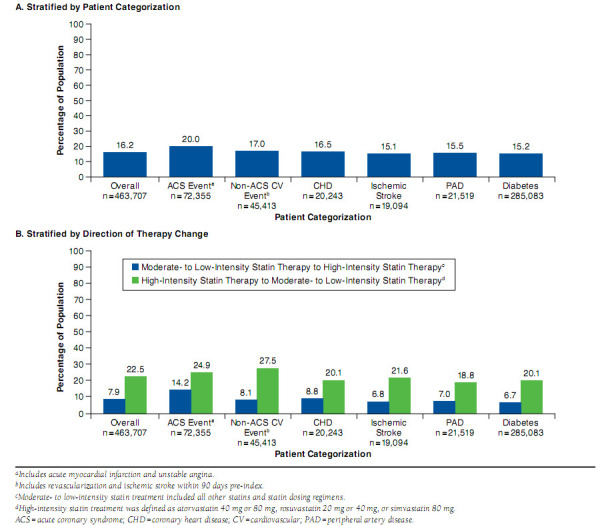
Changes in Statin Therapy Intensity
More than 20% of patients initially treated with a high-intensity regimen switched to moderate- to low-intensity therapy (Figure 3B). Reduction in treatment intensity was most frequently observed among patients who had experienced a recent ACS event. Statin intensity was increased (through changing drug or dosage) in 6.7% to 14.2% of patients during follow-up. Overall and by cohort, patients with high-intensity therapy at index were more likely to switch statin monotherapy than patients who initiated treatment with a moderate- to low-intensity regimen.
During the course of follow-up, 53.0% of patients discontinued statin therapy. Kaplan-Meier curves showed a median time to statin discontinuation of approximately 15 months (Figure 4A). Time to discontinuation was significantly higher in the recent ACS event cohort (median = 27 months) compared with the other CV risk groups (median = 13 to 18 months; log-rank P < 0.001 comparing across all cohorts). Patients receiving a high-intensity statin regimen had a significantly longer median time to discontinuation (Figure 4B) and had lower rates of discontinuation (47.9% vs. 53.9%) than patients who started on moderate- to low-intensity treatment (log-rank P < 0.001 comparing across all cohorts). Variables significantly associated with statin discontinuation on regression analysis included younger age (18-44 years), female sex, and a Charlson comor-bidity score > 2 (P < 0.001 for all). Recent ACS event and history of stroke risk categories were associated with lower hazard ratio for discontinuation (P < 0.001).
FIGURE 4.
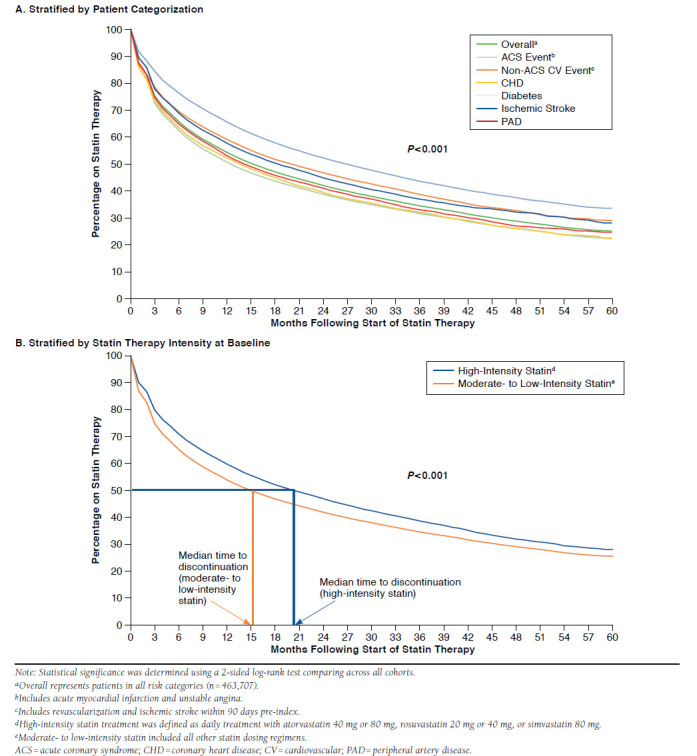
Kaplan-Meier Analysis of Time to Statin Discontinuation
The PDC on statin therapy was 58.2% for all cohorts, ranging from 55.5% (diabetes) to 66.4% (recent ACS hospitalization; Figure 5). Patients who initiated treatment with a high-intensity statin regimen had a significantly greater PDC than patients who initiated treatment with a moderate- to low-intensity regimen (62.1% vs. 57.5%, respectively; P < 0.001).
FIGURE 5.
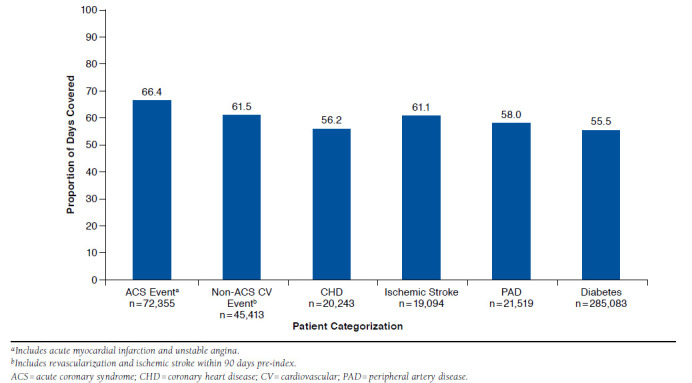
Proportion of Days Covered for Statin Therapy (of Any intensity)
Hospitalizations related to ASCVD occurred in 12.0% of patients during the follow-up period (i.e., index to end of continuous enrollment). These hospitalizations were significantly higher among patients with a recent ACS hospitalization or PAD and lowest for the diabetes cohort (Figure 6; log-rank P < 0.001 comparing across all cohorts; comparisons were not made between specific cohorts).
FIGURE 6.
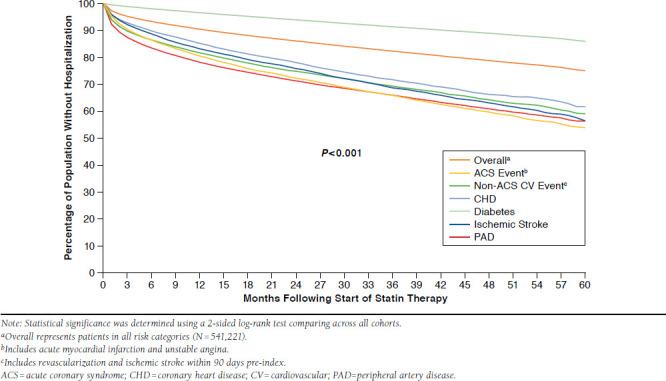
ASCVD-Related Hospitalizations During Follow-up
Discussion
This retrospective claims database analysis demonstrated that real-world patients at high risk for CV events are not optimally receiving and maintaining high-intensity statin therapy. Most patients in all CV risk categories initiated treatment with a moderate- to low-intensity statin regimen. Although increases in statin intensity were infrequent, more than one fifth of patients who initiated on a high-intensity statin regimen switched to a moderate- to low-intensity regimen during follow-up. Discontinuation was a common occurrence, regardless of baseline therapy, and the PDC for statin therapy was low.
Widespread use of suboptimal-intensity therapy has been routinely reported in high-risk populations.18-22 In the current study, 15% of patients initiated treatment with high-intensity statin therapy. These patients were likely to change dosage or switch statin therapy type (possibly to achieve a better response), and approximately half discontinued treatment within 20 months. Previously published data from a large cohort of patients (N = 11,473) with ASCVD or diabetes who had not reached the LDL-C goal of < 70 mg/dL showed that most patients (76%) were initially treated with moderate-intensity statin therapy, and < 2% of patients received a high-intensity statin regimen.20 After reevaluation of LDL-C levels posttreatment, only 13% of patients were prescribed a higher-intensity regimen either through switching or uptitrating statin therapy or via the addition of another lipid-lowering agent. Notably, 47% of patients not at the LDL-C goal discontinued therapy within 12 months of beginning treatment without first attempting a change in their lipid-lowering regimens. A modestly higher rate of statin intensification (25.3%) was reported in a recent retrospective observational study that evaluated patients with CHD or CHD risk equivalents (N = 656,807), yet the majority of patients (70.2%) continued with their initial regimen or downtitrated statin therapy during the follow-up period.18 Interestingly, as we had observed in our high-risk patient cohort, add-on therapy was infrequently used as an adjunct to lipid-lowering with a statin.
Analysis of data from the Prospective Registry Evaluating Myocardial Infarction: Events and Recovery (PREMIER) and Translational Research Investigating Underlying disparities in acute Myocardial infarction Patients' Health status (TRIUMPH) registries reinforces the suboptimal utilization of target statin intensity in high-risk patients, specifically those who had experienced an acute MI (n = 6,748).21 At hospital discharge, one third of patients were prescribed statin therapy at the goal level. Intensification of treatment after hospital discharge was rare, with fewer patients (26%) receiving their target statin doses at the 12-month follow-up time point than at the time of hospital discharge.
The generally low rate of adherence observed in our high-risk cohort is compatible with previous observations for statins.17,24 Nonadherence to therapy is an ongoing issue in the management of hypercholesterolemia.27,28 The underlying reasons for statin nonadherence range from concerns about adverse effects to perceived lack of therapeutic benefit.29,30 Several treatment-related factors that decrease the likelihood of statin adherence, including higher copays and higher drug doses, have also been identified.31-34 Higher rates of statin discontinuation have also been reported among younger patients and women—findings that are consistent with our observations.35 Routine monitoring is suggested as a means to promote adherence. Although treating to goal is no longer the emphasis in U.S. lipid management guidelines, it is recommended that practitioners monitor lipid levels within 4 to 12 weeks of initiating treatment to evaluate therapeutic response and patient adherence.3 It is also advised that adherence to therapy be reinforced at routine follow-up visits every 3 to 12 months.
Statin treatment patterns and adherence to therapy in our study differed among cohorts. Patients with diabetes and no other ASCVD diagnosis were among the least aggressively managed groups in terms of intensity of statin therapy and PDC. Less intensive therapy for this patient subgroup is consistent with the ACC/AHA guideline, which gives a Level I recommendation for moderate-intensity statin therapy in adults aged 40-75 years who have diabetes.3 High-intensity therapy would be appropriate for those patients who also have a 7.5% or greater estimated risk for ASCVD. Whether more patients in the diabetes CV risk category should have received high-intensity therapy is not possible to ascertain from our analysis because of the lack of CV risk scoring. The observed differences in adherence based on cohort designation align with previous data demonstrating that patients at very high CV risk (i.e., those with ACS or established CVD plus either diabetes or metabolic syndrome) are more likely to be adherent to statin therapy.17 In our assessment, patients with a recent hospitalization for ACS had the greatest statin therapy PDC (66.4%) and the longest duration of statin treatment (median = 27 months).
Patients with a recent hospitalization for ACS were also more likely than patients in other CV risk categories to initiate treatment with a high-intensity statin regimen. We observed a significant difference in PDC between statin intensity groups, with a greater PDC (62.1%) for high-intensity treatment compared with patients who received a moderate- to low-intensity regimen (57.5%; P < 0.001). Data from previous evaluations of prescription/administrative data have reported that higher statin dose/intensity was associated with lower adherence rates.31,32 However, previous studies have not specifically assessed patients who were newly initiating statin therapy and who had high CV risk conditions. In the recent study by Virani et al. (2014),32 the difference in PDC between high-intensity and moderate- to low-intensity statin regimens in patients with established CVD (i.e., CHD, PAD, or ischemic stroke) who were receiving ongoing statin therapy was statistically significant (86% and 87%, respectively; P < 0.0001) but not considered to be clinically relevant. Importantly, patients with diabetes and no other ASCVD diagnosis—a group that made up a substantial proportion of our study population and had both the lowest PDC rates and the lowest high-intensity therapy rates—were not included in the analysis.
The rate of ASCVD-related hospitalizations in the current analysis underscores the high-risk status of these patients and the lack of adequate risk mitigation with current management strategies. Twelve percent of patients experienced an
ASCVD-related hospitalization during a median follow-up duration of 20.4 months. These values varied substantially by CV risk cohort, ranging from 5% among patients in the diabetes CV risk category to 23% for patients who had experienced a recent CV event. Some possible factors affecting the hospitalization rate were the prevalence of statin nonadherence and the high rate of statin discontinuation. Both of these factors have been shown to adversely affect CV outcomes.23,24,36,37 Accumulating evidence suggests that patients with established CV or cerebrovascular disease who discontinue chronic statin therapy are at increased mortality risk compared with those who maintain therapy and, in some cases, patients who had never been treated with a statin.24,36,37
Limitations
Several limitations inherent to the nature of the data source affect the conclusions that can be drawn from this assessment. The claims databases did not capture information on the reasons for initial therapy choice or for modifying or discontinuing therapy. Clinical considerations such as contraindications or conditions that increase the risk for treatment-related adverse effects may have led to the selection of less intensive therapy. For patients on statin treatment, discontinuation or switching to a nonstatin lipid-modifying therapy, the latter of which occurred in 2%-3% of patients per cohort in our study, could have been the result of apparent statin intolerance. Clinicians may have chosen not to intensify statin therapy during the follow-up period for patients who were tolerating and adhering to therapy because of the minimal additional reduction in LDL-C that is observed with statin dose escalation and/or the increased likelihood for side effects and intolerance at higher doses.38,39 Experiencing a treatment-emergent side effect increases the probability that a patient will discontinue therapy altogether. In the Understanding Statin Use in America and Gaps in Education (USAGE) survey, side effects, particularly muscle-related side effects, were the predominant reason cited by former statin users for discontinuing therapy.40,41 Emerging concerns regarding an increased risk for new-onset diabetes42-44 or acute kidney injury45,46 with high-intensity statin therapy may also have factored into the decision to limit treatment to a moderate- to low-intensity regimen.
An additional consideration regarding study interpretation is the method by which statin discontinuation was detected. Discontinuation was identified based on a 90-day gap in statin treatment; use of any other statins was not assessed after this 90-day gap. Therefore, we were unable to distinguish between “temporary” and “permanent” treatment discontinuation, potentially overestimating our discontinuation rates. The metric used to gauge adherence is also subject to limitations that may underestimate (e.g., missing prescriptions filled outside the insured setting) or overestimate (e.g., assuming a prescription filled is a prescription consumed) adherence, and it does not distinguish physician-mandated changes from patient choice.
Finally, a number of factors could influence the decision to switch between statins, including the availability of generics, regulatory approval of new agents, or changes in recommended use for a particular agent. For example, in June 2011 (during the data collection window), the U.S. Food and Drug Administration advised physicians to limit the use of simv-astatin 80 mg (a high-intensity regimen) because of the risk of muscle injury.47 The proportion of patients switching from high-intensity therapy was potentially affected by this guidance.
Conclusions
These data indicate a lack of intensive statin-based therapy among patients at high risk for CV events. The continued use of moderate- to low-intensity therapy, failure to intensify treatment, poor adherence, and high rates of discontinuation result in a large number of patients who are inadequately protected from recurrent CV events. The current ACC/AHA lipid management guidelines recommend high-intensity statin treatment for men and women who are aged 75 years or younger and who have clinical ASCVD (e.g., CHD, stroke, or PAD).3 Similarly, the National Lipid Association advocates the use of moderate- to high-intensity statins for the first-line treatment of hyperlip-idemia and consideration of moderate- to high-intensity statin therapy for patients with ASCVD or type 2 diabetes, regardless of baseline atherogenic cholesterol levels.48 Given the results of this study, health care system-based interventions and new therapeutic paradigms are necessary to optimize patient care, manage risk, and enhance outcomes.
Acknowledgments
Medical writing support for this manuscript was provided by Crystal Murcia, PhD, of MicroMass Communications, Cary, North Carolina, with funding from Regeneron Pharmaceuticals, Tarrytown, New York, and Sanofi US, Bridgewater, New Jersey.
Appendix. Diagnostic and Procedure Codes
| Brief Code Description | ICD-9-CM, CPT, or HCPCSII Code | Description |
|---|---|---|
| Abdominal aortic aneurysm | 35081-35103 | Open repair of abdominal aortic aneurysm |
| Abdominal aortic aneurysm | 441.3x, 441.3 | Abdominal aortic aneurysm |
| Abdominal aortic aneurysm | 441.4x, 441.4 | Abdominal aortic aneurysm |
| Abdominal aortic aneurysm | 34800-34805 | Endovascular repair of infrarenal abdominal aortic aneurysm |
| Acute MI | 410.xx | Acute myocardial infarction |
| Carotid artery disease | 00.63 | Percutaneous insertion of carotid artery stent(s) |
| Carotid artery disease | 38.13, 38.18 | Endarterectomy of upper limb vessels/lower limb arteries |
| Carotid artery disease | 433.1x | Occlusion and stenosis of carotid artery without mention of cerebral infarction |
| Carotid artery disease | 35301 | Endarterectomy |
| Carotid artery disease | 37215-37216 | Stenting of carotid artery |
| Coronary revascularization procedures | G0290 | Transcatheter placement of a drug eluting intracoronary stent(s) |
| Coronary revascularization procedures | G0291 | Transcatheter placement of a drug eluting intracoronary stent(s) |
| Coronary revascularization procedures | S2205-S2209 | Minimally invasive direct coronary artery bypass surgery |
| Coronary revascularization procedures | 00.66 | Percutaneous transluminal coronary angioplasty [PTCA] or coronary atherectomy |
| Coronary revascularization procedures | 36.0x | Removal of coronary artery obstruction and insertion of stent(s) |
| Coronary revascularization procedures | 36.1x | Bypass anastomosis for heart revascularization |
| Coronary revascularization procedures | 36.2x | Heart revascularization by arterial implant |
| Coronary revascularization procedures | 36.3x | Other heart revascularization |
| Coronary revascularization procedures | 92980 | Transcatheter placement of an intracoronary stent(s) |
| Coronary revascularization procedures | 92981 | Transcatheter placement of an intracoronary stent(s) |
| Coronary revascularization procedures | 92982 | Percutaneous transluminal coronary balloon angioplasty |
| Coronary revascularization procedures | 92984 | Percutaneous transluminal coronary balloon angioplasty |
| Coronary revascularization procedures | 92995 | Percutaneous transluminal coronary atherectomy |
| Coronary revascularization procedures | 92996 | Percutaneous transluminal coronary atherectomy |
| Coronary revascularization procedures | 33510-33536 (except 33530) | Coronary artery bypass |
| Diabetes with complication | 250.1x | Diabetes with ketoacidosis |
| Diabetes with complication | 250.1 | Diabetes with ketoacidosis, type II or unspecified type, not stated as uncontrolled |
| Diabetes with complication | 250.11 | Diabetes with ketoacidosis, type I [juvenile type], not stated as uncontrolled |
| Diabetes with complication | 250.12 | Diabetes with ketoacidosis, type II or unspecified type, uncontrolled |
| Diabetes with complication | 250.13 | Diabetes with ketoacidosis, type I [juvenile type], uncontrolled |
| Diabetes with complication | 250.2x | Diabetes with hyperosmolarity |
| Diabetes with complication | 250.20 | Diabetes with hyperosmolarity, type II or unspecified type, not stated as uncontrolled |
| Diabetes with complication | 250.21 | Diabetes with hyperosmolarity, type I [juvenile type], not stated as uncontrolled |
| Diabetes with complication | 250.22 | Diabetes with hyperosmolarity, type II or unspecified type, uncontrolled |
| Diabetes with complication | 250.23 | Diabetes with hyperosmolarity, type I [juvenile type], uncontrolled |
| Diabetes with complication | 250.3x | Diabetes with other coma |
| Diabetes with complication | 250.30 | Diabetes with other coma, type II or unspecified type, not stated as uncontrolled |
| Diabetes with complication | 250.31 | Diabetes with other coma, type I [juvenile type], not stated as uncontrolled |
| Diabetes with complication | 250.32 | Diabetes with other coma, type II or unspecified type, uncontrolled |
| Diabetes with complication | 250.33 | Diabetes with other coma, type I [juvenile type], uncontrolled |
| Diabetes with complication | 250.4x | Diabetes with renal manifestations |
| Diabetes with complication | 250.40 | Diabetes with renal manifestations, type II or unspecified type, not stated as uncontrolled |
| Diabetes with complication | 250.41 | Diabetes with renal manifestations, type I [juvenile type], not stated as uncontrolled |
| Diabetes with complication | 250.42 | Diabetes with renal manifestations, type II or unspecified type, uncontrolled |
| Diabetes with complication | 250.43 | Diabetes with renal manifestations, type I [juvenile type], uncontrolled |
| Diabetes with complication | 250.5x | Diabetes with ophthalmic manifestations |
| Diabetes with complication | 250.50 | Diabetes with ophthalmic manifestations, type II or unspecified type, not stated as uncontrolled |
| Diabetes with complication | 250.51 | Diabetes with ophthalmic manifestations, type I [juvenile type], not stated as uncontrolled |
| Diabetes with complication | 250.52 | Diabetes with ophthalmic manifestations, type II or unspecified type, uncontrolled |
| Diabetes with complication | 250.53 | Diabetes with ophthalmic manifestations, type I [juvenile type], uncontrolled |
| Diabetes with complication | 250.6x | Diabetes with neurological manifestations |
| Diabetes with complication | 250.60 | Diabetes with neurological manifestations, type II or unspecified type, not stated as uncontrolled |
| Diabetes with complication | 250.61 | Diabetes with neurological manifestations, type I [juvenile type], not stated as uncontrolled |
| Diabetes with complication | 250.62 | Diabetes with neurological manifestations, type II or unspecified type, uncontrolled |
| Diabetes with complication | 250.63 | Diabetes with neurological manifestations, type I [juvenile type], uncontrolled |
| Diabetes with complication | 250 7x | |
| Diabetes with complication | 250.70 | Diabetes with peripheral circulatory disorders, type II or unspecified type, not stated as uncontrolled |
| Diabetes with complication | 250.71 | Diabetes with peripheral circulatory disorders, type I [juvenile type], not stated as uncontrolled |
| Diabetes with complication | 250.72 | Diabetes with peripheral circulatory disorders, type II or unspecified type, uncontrolled |
| Diabetes with complication | 250.73 | Diabetes with peripheral circulatory disorders, type I [juvenile type], uncontrolled |
| Diabetes with complication | 250.8x | Diabetes with other specified manifestations |
| Diabetes with complication | 250.80 | Diabetes with other specified manifestations, type II or unspecified type, not stated as uncontrolled |
| Diabetes with complication | 250.81 | Diabetes with other specified manifestations, type I [juvenile type], not stated as uncontrolled |
| Diabetes with complication | 250.82 | Diabetes with other specified manifestations, type II or unspecified type, uncontrolled |
| Diabetes with complication | 250.83 | Diabetes with other specified manifestations, type I [juvenile type], uncontrolled |
| Diabetes with complication | 250.9x | Diabetes with unspecified complication |
| Diabetes with complication | 250.90 | Diabetes with unspecified complication, type II or unspecified type, not stated as uncontrolled |
| Diabetes with complication | 250.91 | Diabetes with unspecified complication, type I [juvenile type], not stated as uncontrolled |
| Diabetes with complication | 250.92 | Diabetes with unspecified complication, type II or unspecified type, uncontrolled |
| Diabetes with complication | 250.93 | Diabetes with unspecified complication, type I [juvenile type], uncontrolled |
| Diabetes without complication | 250.0x | Diabetes mellitus without mention of complication |
| Diabetes without complication | 250.00 | Diabetes mellitus without mention of complication, type II or unspecified type, not stated as uncontrolled |
| Diabetes without complication | 250.01 | Diabetes mellitus without mention of complication, type I [juvenile type], not stated as uncontrolled |
| Diabetes without complication | 250.02 | Diabetes mellitus without mention of complication, type II or unspecified type, uncontrolled |
| Diabetes without complication | 250.03 | Diabetes mellitus without mention of complication, type I [juvenile type], uncontrolled |
| Ischemic stroke | 433.xx, 433.01, 433.11, 433.21, 433.31, 433.81, 433.91, 434.01, 434.11, 434.91 |
Occlusion and stenosis of precerebral arteries |
| Old MI | 412.xx, 412 | Old myocardial infarction |
| Other chronic ischemic heart disease | 414.xx | Other forms of chronic ischemic heart disease |
| PAD | 37220-37235 | Endovascular revascularization, lower extremities |
| PAD | 00.55 | Insertion of drug-eluting peripheral vessel stent(s) |
| PAD | 00.61 | Percutaneous angioplasty or atherectomy of precerebral (extracranial) vessel(s) |
| PAD | 00.64 | Percutaneous insertion of other precerebral (extracranial) artery stent(s) |
| PAD | 39.5x | Angioplasty or atherectomy of other non-coronary vessel(s) |
| PAD | 39.72 | Endovascular repair or occlusion of head and neck vessels |
| PAD | 39.74 | Endovascular removal of obstruction from head and neck vessel(s) |
| PAD | 39.9x | Insertion of non-drug-eluting peripheral vessel stent(s) |
| PAD | 443.8x | Other specified peripheral vascular diseases |
| PAD | 443.9x | Peripheral vascular disease, unspecified |
| PAD | 444.2x | Embolism and thrombosis of arteries of the extremities |
| PAD | 445.0x | Atheroembolism of extremities |
| PAD | 440.xx, 440.2x, | Atherosclerosis (Excludes following which are coded separately: basilar, carotid, cerebral, coro- |
| 440.3x, 440.4 | nary, mesenteric, precerebral, pulmonary, and vertebral) | |
| PAD | 35450-35459 | Transluminal angioplasty: open (excluding venous) |
| PAD | 35470-35475 | Transluminal angioplasty: percutaneous (excluding venous) |
| PAD | 35480-35485 | Transluminal atherectomy: cutdown |
| PAD | 35490-35495 | Transluminal atherectomy: percutaneous |
| PAD | 35501-35571 | Arterial bypass using vein grafts |
| PAD | 35583-35587 | Lower extremity revascularization: in-situ vein bypass |
| PAD | 35601-35671 | Arterial bypass with synthetic grafts |
| PAD | 37205-37208 | Insertion of intravascular stent |
| PAD | 93668 | Rehabilitation services: peripheral arterial disease |
| Stable angina | 413.xx, 413.x | Angina pectoris |
| Transient ischemic attack | v12.54 | Transient ischemic attack (TIA), and cerebral infarction without residual deficits |
| Transient ischemic attack | 435.0x | Basilar artery syndrome (Transient cerebral ischemia) |
| Transient ischemic attack | 435.1x | Vertebral artery syndrome (Transient cerebral ischemia) |
| Transient ischemic attack | 435.8x | Other specified transient cerebral ischemias (Transient cerebral ischemia) |
| Transient ischemic attack | 435.9x | Unspecified transient cerebral ischemia (Transient cerebral ischemia) |
| Unstable angina | 411.81 | Acute coronary occlusion without myocardial infarction |
| Unstable angina | 411.1x, 411.1 | Intermediate coronary syndrome |
CPT = Current Procedural Terminology; HCPCSII = Healthcare Common Procedure Coding System Level II; ICD-9-CM = International Classification of Diseases, Ninth Revision, Clinical Modification; MI = myocardial infarction; PAD = peripheral artery disease.
References
- 1.Anderson TJ, Gregoire J, Hegele RA, et al. 2012 update of the Canadian Cardiovascular Society guidelines for the diagnosis and treatment of dys-lipidemia for the prevention of cardiovascular disease in the adult. Can J Cardiol. 2013;29(2):151-67. [DOI] [PubMed] [Google Scholar]
- 2.European Association for Cardiovascular Prevention & Rehabilitation, Reiner Z, Catapano AL, et al. ESC/EAS Guidelines for the management of dyslipidaemias: the Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS). Eur Heart J. 2011;32(14):1769-818. [DOI] [PubMed] [Google Scholar]
- 3.Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(25 Pt B):2889-934. [DOI] [PubMed] [Google Scholar]
- 4.Teramoto T, Sasaki J, Ishibashi S, et al. Comprehensive risk management for the prevention of cardiovascular disease: executive summary of the Japan Atherosclerosis Society (JAS) guidelines for the diagnosis and prevention of atherosclerotic cardiovascular diseases in Japan—2012. J Atheroscler Thromb. 2013;20(7):603-15. [DOI] [PubMed] [Google Scholar]
- 5.Gorcyca KM, Khan I, Wadhera R, et al. Prevalence of atherosclerotic cardiovascular disease and diabetes in the United States. Poster presented at: 2015 National Lipid Association Scientific Sessions; June 11-14, 2015; Chicago, IL. Available at: https://www.lipid.org/util/eposters/PDF/118.pdf. Accessed March 21, 2016. [Google Scholar]
- 6.Go AS, Mozaffarian D, Roger VL, et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2014 update: a report from the American Heart Association. Circulation. 2014;129(3):e28-e292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heidenreich PA, Trogdon JG, Khavjou OA, et al. Forecasting the future of cardiovascular disease in the United States: a policy statement from the American Heart Association. Circulation. 2011;123(8):933-44. [DOI] [PubMed] [Google Scholar]
- 8.Long-term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group. Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. N Engl J Med. 1998;339(19):1349-57. [DOI] [PubMed] [Google Scholar]
- 9.Scandinavian Simvastatin Survival Study Group. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet. 1994;344(8934):1383-89. [PubMed] [Google Scholar]
- 10.Sacks FM, Pfeffer MA, Moye LA, et al. Cholesterol and Recurrent Events Trial Investigators. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. N Engl J Med. 1996;335(14):1001-09. [DOI] [PubMed] [Google Scholar]
- 11.Cannon CP, Braunwald E, McCabe CH, et al. Pravastatin or Atorvastatin Evaluation and Infection Therapy-Thrombolysis in Myocardial Infarction 22 Investigators. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med. 2004;350(15):1495-504. [DOI] [PubMed] [Google Scholar]
- 12.LaRosa JC, Grundy SM, Waters DD, et al. Treating to New Targets (TNT) Investigators. Intensive lipid lowering with atorvastatin in patients with stable coronary disease. N Engl J Med. 2005;352(14):1425-35. [DOI] [PubMed] [Google Scholar]
- 13.Fu AZ, Zhang Q, Davies MJ, Pentakota SR, Radican L, Seck T.. Underutilization of statins in patients with type 2 diabetes in US clinical practice: a retrospective cohort study. Curr Med Res Opin. 2011;27(5):1035-40. [DOI] [PubMed] [Google Scholar]
- 14.Gamboa CM, Safford MM, Levitan EB, et al. Statin underuse and low prevalence of LDL-C control among U.S. adults at high risk of coronary heart disease. Am J Med Sci. 2014;348(2):108-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoeks SE, Scholte op Reimer WJ, van Gestel YR, et al. Medication underuse during long-term follow-up in patients with peripheral arterial disease. Circ Cardiovasc Qual Outcomes. 2009;2(4):338-43. [DOI] [PubMed] [Google Scholar]
- 16.Subherwal S, Patel MR, Kober L, et al. Missed opportunities: despite improvement in use of cardioprotective medications among patients with lower-extremity peripheral artery disease, underuse remains. Circulation. 2012;126(11):1345-54. [DOI] [PubMed] [Google Scholar]
- 17.Kern DM, Balu S, Tunceli O, Anzalone D.. Statin treatment patterns and clinical profile of patients with risk factors for coronary heart disease defined by National Cholesterol Education Program Adult Treatment Panel III. Curr Med Res Opin. 2014;30(12):2443-51. [DOI] [PubMed] [Google Scholar]
- 18.Toth PP, Foody JM, Tomassini JE, et al. Therapeutic practice patterns related to statin potency and ezetimibe/simvastatin combination therapies in lowering LDL-C in patients with high-risk cardiovascular disease. J Clin Lipidol. 2014;8(1):107-16. [DOI] [PubMed] [Google Scholar]
- 19.Melloni C, Shah BR, Ou FS, et al. Lipid-lowering intensification and low-density lipoprotein cholesterol achievement from hospital admission to 1-year follow-up after an acute coronary syndrome event: results from the Medications ApplIed aNd SusTAINed Over Time (MAINTAIN) registry. Am Heart J. 2010;160(6):1121-29. [DOI] [PubMed] [Google Scholar]
- 20.Simpson RJ Jr, Tunceli K, Ramey DR, et al. Treatment pattern changes in high-risk patients newly initiated on statin monotherapy in a managed care setting. J Clin Lipidol. 2013;7(5):399-407. [DOI] [PubMed] [Google Scholar]
- 21.Arnold SV, Spertus JA, Masoudi FA, et al. Beyond medication prescription as performance measures: optimal secondary prevention medication dosing after acute myocardial infarction. J Am Coll Cardiol. 2013;62(19):1791-801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arnold SV, Kosiborod M, Tang F, et al. Patterns of statin initiation, intensification, and maximization among patients hospitalized with an acute myocardial infarction. Circulation. 2014;129(12):1303-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Slejko JF, Ho M, Anderson HD, Nair KV, Sullivan PW, Campbell JD.. Adherence to statins in primary prevention: yearly adherence changes and outcomes. J Manag Care Pharm. 2014;20(1):51-57. Available at: http://www.jmcp.org/doi/abs/10.18553/jmcp.2014.20.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Phan K, Gomez YH, Elbaz L, Daskalopoulou SS.. Statin treatment non-adherence and discontinuation: clinical implications and potential solutions. Curr Pharm Des. 2014;20(40):6314-24. [DOI] [PubMed] [Google Scholar]
- 25.Steen DL, Khan I, Song X, et al. Cardiovascular event rates in a high-risk managed care population in the United States. J Am Coll Cardiol. 2015;65(10):A1647. [Abstract] [Google Scholar]
- 26.Centers for Disease Control and Prevention/National Center for Health Statistics. Classifications of disease, functioning, and disability. Updated June 19, 2013. Available at: http://www.cdc.gov/nchs/icd.htm. Accessed March 21, 2016. [Google Scholar]
- 27.Yeaw J, Benner JS, Walt JG, Sian S, Smith DB.. Comparing adherence and persistence across 6 chronic medication classes. J Manag Care Pharm. 2009;15(9):728-40. Available at: http://www.amcp.org/data/jmcp/728-740.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zullig LL, Melnyk SD, Stechuchak KM, et al. The Cardiovascular Intervention Improvement Telemedicine Study (CITIES): rationale for a tailored behavioral and educational pharmacist-administered intervention for achieving cardiovascular disease risk reduction. Telemed J E Health. 2014;20(2):135-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fung V, Sinclair F, Wang H, Dailey D, Hsu J, Shaber R.. Patients' perspectives on nonadherence to statin therapy: a focus-group study. Perm J. 2010;14(1):4-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McNaughton RJ, Shucksmith J.. Reasons for (non)compliance with intervention following identification of “high-risk” status in the NHS Health Check programme. J Public Health (Oxf). 2015;37(2):218-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pedan A, Varasteh L, Schneeweiss S.. Analysis of factors associated with statin adherence in a hierarchical model considering physician, pharmacy, patient, and prescription characteristics. J Manag Care Pharm. 2007;13(6):487-96. Available at: http://www.amcp.org/data/jmcp/pages%20487-96.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Virani SS, Woodard LD, Akeroyd JM, Ramsey DJ, Ballantyne CM, Petersen LA.. Is high-intensity statin therapy associated with lower statin adherence compared with low- to moderate-intensity statin therapy? Implications of the 2013 American College of Cardiology/American Heart Association Cholesterol Management Guidelines. Clin Cardiol. 2014;37(11):653-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Watanabe JH, Kazerooni R, Bounthavong M.. Association of copayment with likelihood and level of adherence in new users of statins: a retrospective cohort study. J Manag Care Pharm. 2014;20(1):43-50. Available at: http://www.jmcp.org/doi/abs/10.18553/jmcp.2014.20.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen S-Y, Shah S-N, Lee YC, Boulanger L, Mardekian J, Kuznik A.. Moving branded statins to lowest copay tier improves patient adherence. J Manag Care Pharm. 2014;20(1):34-42. Available at: http://www.jmcp.org/doi/abs/10.18553/jmcp.2014.20.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Caspard H, Chan AK, Walker AM.. Compliance with a statin treatment in a usual-care setting: retrospective database analysis over 3 years after treatment initiation in health maintenance organization enrollees with dys-lipidemia. Clin Ther. 2005;27(10):1639-46. [DOI] [PubMed] [Google Scholar]
- 36.Tziomalos K, Athyros VG, Mikhailidis DP.. Statin discontinuation: an underestimated risk? Curr Med Res Opin. 2008;24(11):3059-62. [DOI] [PubMed] [Google Scholar]
- 37.Gomez Sandoval YH, Braganza MV, Daskalopoulou SS.. Statin discontinuation in high-risk patients: a systematic review of the evidence. Curr Pharm Des. 2011;17(33):3669-89. [DOI] [PubMed] [Google Scholar]
- 38.Mancini GB, Baker S, Bergeron J, et al. Diagnosis, prevention, and management of statin adverse effects and intolerance: proceedings of a Canadian Working Group Consensus Conference. Can J Cardiol. 2011;27(5):635-62. [DOI] [PubMed] [Google Scholar]
- 39.Robinson JG. Management of familial hypercholesterolemia: a review of the recommendations from the National Lipid Association Expert Panel on Familial Hypercholesterolemia. J Manag Care Pharm. 2013;19(2):139-49. Available at: http://www.jmcp.org/doi/abs/10.18553/jmcp.2013.19.2.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cohen JD, Brinton EA, Ito MK, Jacobson TA.. Understanding Statin Use in America and Gaps in Patient Education (USAGE): an internet-based survey of 10,138 current and former statin users. J Clin Lipidol. 2012;6(3):208-15. [DOI] [PubMed] [Google Scholar]
- 41.Wei MY, Ito MK, Cohen JD, Brinton EA, Jacobson TA.. Predictors of statin adherence, switching, and discontinuation in the USAGE survey: understanding the use of statins in America and gaps in patient education. J Clin Lipidol. 2013;7(5):472-83. [DOI] [PubMed] [Google Scholar]
- 42.Carter AA, Gomes T, Camacho X, Juurlink DN, Shah BR, Mamdani MM.. Risk of incident diabetes among patients treated with statins: population based study. BMJ. 2013;346:f2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dormuth CR, Filion KB, Paterson JM, et al. Higher potency statins and the risk of new diabetes: multicentre, observational study of administrative databases. BMJ. 2014;348:g3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Preiss D, Seshasai SR, Welsh P, et al. Risk of incident diabetes with intensive-dose compared with moderate-dose statin therapy: a meta-analysis. JAMA. 2011;305(24):2556-64. [DOI] [PubMed] [Google Scholar]
- 45.Dormuth CR, Hemmelgarn BR, Paterson JM, et al. Use of high potency statins and rates of admission for acute kidney injury: multicenter, retrospective observational analysis of administrative databases. BMJ. 2013;346:f880. [DOI] [PubMed] [Google Scholar]
- 46.Corrao G, Soranna D, Casula M, Merlino L, Porcellini MG, Catapano AL.. High-potency statins increase the risk of acute kidney injury: evidence from a large population-based study. Atherosclerosis. 2014;234(1):224-29. [DOI] [PubMed] [Google Scholar]
- 47.U.S. Food and Drug Administration. Limit use of 80 mg simvastatin. Consumer Health Information. June 8, 2011. Available at: http://www.fda.gov/ForConsumers/ConsumerUpdates/ucm257884.htm. Accessed March 21, 2016. [Google Scholar]
- 48.Jacobson TA, Ito MK, Maki KC, et al. National lipid association recommendations for patient-centered management of dyslipidemia: part 1—full report. J Clin Lipidol. 2015;9(2):129-69. [DOI] [PubMed] [Google Scholar]


