Abstract
BACKGROUND:
Medication nonadherence is a major public health problem. Identification of patients who are likely to be and not be adherent can guide targeted interventions and improve the design of comparative-effectiveness studies.
OBJECTIVE:
To evaluate multiple measures of patient previous medication adherence in light of predicting future statin adherence in a large U.S. administrative claims database.
METHODS:
We identified a cohort of patients newly initiating statins and measured their previous adherence to other chronic preventive medications during a 365-day baseline period, using metrics such as proportion of days covered (PDC), lack of second fills, and number of dispensations. We measured adherence to statins during the year after initiation, defining high adherence as PDC ≥ 80%. We built logistic regression models from different combinations of baseline variables and previous adherence measures to predict high adherence in a random 50% sample and tested their discrimination using concordance statistics (c-statistics) in the other 50%. We also assessed the association between previous adherence and subsequent statin high adherence by fitting a modified Poisson model from all relevant covariates plus previous mean PDC categorized as < 25%, 25%-79%, and ≥ 80%.
RESULTS:
Among 89,490 statin initiators identified, a prediction model including only demographic variables had a c-statistic of 0.578 (95% CI = 0.573-0.584). A model combining information on patient comorbidities, health care services utilization, and medication use resulted in a c-statistic of 0.665 (95% CI = 0.659-0.670). Models with each of the previous medication adherence measures as the only explanatory variable yielded c-statistics ranging between 0.533 (95% CI = 0.529-0.537) for lack of second fill and 0.666 (95% CI = 0.661-0.671) for maximum PDC. Adding mean PDC to the combined model yielded a c-statistic of 0.695 (95% CI = 0.690-0.700). Given a sensitivity of 75%, the predictor improved the specificity from 47.7% to 53.6%. Patients with previous mean PDC < 25% were half as likely to show high adherence to statins compared with those with previous mean PDC ≥ 80% (risk ratio = 0.49, 95% CI = 0.46-0.50).
CONCLUSIONS:
Including measures of previous medication adherence yields better prediction of future statin adherence than usual baseline clinical measures that are typically used in claims-based studies.
What is already known about this subject
Various effective medication adherence interventions exist; however, it is critical to identify patients who are at risk of nonadherence to maximize the efficiency of the interventions.
Many attempts to predict medication adherence using routinely collected electronic health care data have had limited predictive performance.
What this study adds
Measures of previous adherence to chronic medications were relatively strong predictors of future adherence to newly initiated statins compared with other claims-based measures.
Addition of previous adherence measures to usual claims-based adherence predictors modestly improved the performance of the models for predicting future adherence.
Medication nonadherence is a major public health problem.1 On average, up to 50% of patients do not adhere to their prescribed therapies.2,3 Less than half of patients persist with cardiovascular drugs for a year following a heart attack,4 despite compelling evidence of the clinical benefits of these life-saving treatments.5 Poor adherence has substantial clinical and economic consequences.6 In the United States, suboptimal adherence accounts for 33%-69% of medication-related hospital admissions and $100 billion of potentially avoidable health spending each year.1
Various effective medication adherence interventions exist.7-9 Even small improvements in adherence to evidence-based treatment at the population level can improve clinical outcomes for patients.10,11 A key challenge in maximizing the benefit and value of certain interventions is identifying populations of patients who are expected to have low adherence. Although technologic advances have enabled real-time adherence monitoring, identification of patients who are likely to adhere or not adhere to treatment at the time of treatment initiation would enable early intervention at the first encounter.
Many attempts have been made to predict medication adherence using routinely collected health care data.12,13 Although previously developed medication adherence algorithms have had limited predictive performance, some evidence suggests that using measures of previous adherence to other chronically used medications may be a strong predictor of future adherence to newly initiated medication.14-17 However, it is not known how best to measure and use previous medication adherence to predict adherence to a newly initiated drug.
By focusing on statins, one of the most frequently prescribed drug classes in the United States, we sought to systematically evaluate different metrics and chronic medication classes for measuring previous adherence and to assess the added value of these measures of previous adherence to adherence prediction using administrative claims data.
Methods
Data Source
We used data from Optum Clinformatics Data Mart, which includes medical and pharmacy claims data (e.g., diagnoses, procedures, and medication dispensations); demographic data; and plan information for patients with commercial insurance plans across the United States administered by a large national insurer. The database has been used extensively for observational research and is part of the U.S. Food and Drug Administration’s Sentinel System.18 The data undergo quality checks by OptumInsight and Sentinel. The use of the data was approved by the institutional review board of Brigham and Women's Hospital, Boston, Massachusetts.
Study Cohort
We identified patients aged 18 years or older who initiated a statin between July 1, 2010, and December 31, 2011. Initiation of a statin was defined as a new statin dispensation following a 365-day baseline period in which patients were required to have continuous enrollment in the health plan and no statin dispensation. The day of the first statin dispensation was defined as the cohort entry date. For patients with multiple eligible cohort entries, only the first was included.
The primary analysis focused on patients who were continuously enrolled in the plan for the 365 days following statin initiation and who had at least 1 dispensation for a medication used for measuring previous adherence in the 365-day baseline period (Figure 1).
FIGURE 1.
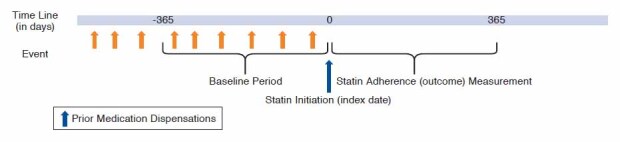
Study Design
Study Outcomes
The outcome of interest was adherence to statins in the 365 days following the first dispensation. We measured adherence using proportion of days covered (PDC), defined as the total number of days covered by the dispensed medication supplies in the 365-day follow-up divided by 365.19-21 We chose PDC as the measure for adherence as it is 1 of the most frequently used adherence measure in administrative claims data.
Baseline Patient Characteristics
Demographic variables included age and sex. We used claims with services dates occurring during each patient’s 365-day baseline period before cohort entry to define an extensive list of variables as potential predictors. These included comorbidities, medication use, and health service utilization measures (e.g., number of physician visits, hospitalized days, or use of colonoscopy, mammography, and vaccinations; Appendix A, available in online article, contains the list of covariates).
We also assessed measures of patient medication burden by counting the number of dispensations of all drugs and the number of unique drug types dispensed during the baseline period; evidence for medication refill synchronization, which indicates patients filling multiple prescriptions on the same day; and the number of concurrently used medications on the cohort entry date.22
Finally, we assessed proxies of patient medication cost burden, including plan benefit type, total copayment for all drugs during baseline period, and index statin copayment. These variables formed the set of basic potential predictors.
Medications for Previous Adherence Measurement
We measured patient adherence to the following types of medications using dispensations in the 365-day baseline period: angiotensin-converting enzyme (ACE) inhibitors or angiotensin II receptor blockers (ARBs), renin inhibitors, beta blockers, calcium channel blockers, thiazide diuretics, loop diuretics, potassium-sparing diuretics, other antihypertensives, oral anticoagulants, digoxin, antianginal agents, selective serotonin reuptake inhibitors (SSRIs), conventional antipsychotics, atypical antipsychotics, other lipid-lowering agents, antidiabetics, osteoporosis drugs, thyroid hormone, nonbiologic disease-modifying antirheumatic drugs, antiparkinson agents, anti-convulsants, and antiglaucoma agents. We aimed to include a broad range of medications to balance including as many patients as possible in the analysis cohort while focusing on drugs that are intended to treat chronic conditions.
Previous Adherence Measurement
We assessed previous adherence using 7 measures of prescription coverage, drug discontinuation, and dispensation counts identified from the literature (Table 1). Measurement was performed over the 365-day baseline period before statin initiation. Measurement for each drug began at the first observed dispensation of the target medication in the baseline period and ended on the statin initiation date. Prescriptions filled before the start of the baseline period, but with days supply that elapsed at the start of the baseline period, were not considered. Medications that were first dispensed in the 90 days immediately preceding statin initiation were excluded to ensure at least a 90-day assessment period for each drug. Switching between medications within the same pharmacologic class was allowed. For patients who used multiple medications for previous adherence measurement, we summarized across drugs by taking the mean, median, maximum, and minimum for each adherence metric.
TABLE 1.
Definitions of Previous Adherence Measures
| Measurement Type | Measurement Name | Definition |
|---|---|---|
| Measures based on prescription coverage days | Continuous measure of medication acquisition (CMA) |
Numerator: total days supply dispensed during denominator Denominator: time between first dispensation in baseline period and the end of baseline period (date of statin initiation) |
| Continuous measure of medication gaps (CMG) |
Numerator: total gap days (number of days without medication days supply) Denominator: time between first dispensation in baseline period and end of baseline period |
|
| Proportion of days covered (PDC) |
Numerator: total days supply capped at maximum of number of days in denominators Denominator: time between first dispensation in baseline period and the end of baseline period |
|
| Measures based on discontinuation | Discontinuation(dichotomous;DD) | Presence (yes = 1 or no = 0) of 1 or more periods of at least 30 days without medication supply following the days supply of a dispensation in baseline period |
| Time to discontinuation(TTD) | Days between date of first dispensation and discontinuation or the end of baseline period | |
| Measures based on dispensation counts | Refill counts (counts) | Number of dispensations in baseline period beyond first fill for each drug |
| Lack of second fills (dichotomous) | Not having a second dispensation of a drug in baseline period when the first dispensation occurred more than 30 days + days supply before the statin initiation |
Statistical Analysis
We tabulated the characteristics of the cohort based on its demographics, previous medication use, comorbidities, health services utilizations, medication burden, and financial burden.
We first divided the cohort into a training cohort and a testing cohort in a 1:1 ratio. We selected predictors of future statin PDCs in the training cohort from among the list of covariates other than measures of previous adherence using the least absolute shrinkage and selection operator (lasso). We fit lasso logistic regression models with a binary outcome of good adherence defined as PDC ≥ 80%, with a shrinkage parameter lambda chosen to minimize the models’ Bayesian information criterion. Variables with nonzero beta coefficients were selected as predictors of the statin adherence for subsequent models. Appendix B contains the full list of variables selected via the lasso procedure (available in online article).
We then developed multivariable logistic regression models predicting PDC ≥ 80% in the training cohort by including various groups of the lasso-selected variables. For example, we built a model including only demographic characteristics and another model including only comorbidities. Next, we built a model that included all variables selected by lasso from the set of basic predictors. We also built separate univariable logistic regression models in which each of the previous medication measures was included as the only explanatory variable. We then developed models that included all of the variables selected by lasso from the basic set of predictors plus the best-performing measures of previous adherence.
Finally, we developed a separate model using only data available at the pharmacy at the time of prescription dispensation (i.e., demographics, baseline medication use, index statin type, medication burden, and previous medication adherence) to examine the performance of adherence prediction if medical data are not available.
Each model created in the training cohort was applied to the testing cohort to predict each patient’s probability of having a 1-year PDC ≥ 80% for the newly initiated statin. The predicted probabilities were used to calculate c-statistics to compare discrimination across models. We assessed the calibration of the models using calibration plots.
To assess the strength of association between previous adherence and subsequent statin adherence, we fit a modified Poisson regression model using sandwich variance estimators,23 with a binary dependent variable of high adherence (PDC ≥ 80%). We categorized patients into 3 previous adherence groups based on their mean PDC during the baseline period: high previous adherence (mean PDC ≥ 80%), moderate previous adherence (25% ≤ mean PDC < 80%), and low previous adherence (mean PDC < 25%). We compared the probability of having high adherence to statins across previous adherence groups, adjusting for all previously selected predictors via lasso regression.
Expanded Cohort and Subgroup Analyses
To assess the performance of the prediction models in a typical patient population, we conducted an expanded cohort analysis that added patients excluded from the primary study cohort because they had no dispensation of drugs used for previous adherence measurement in the baseline period. Because measures of previous adherence could not be calculated for these patients, we expected that model performance would be lower but that the analysis population would better reflect real-world patient cohorts. In this cohort, we applied the model that included all lasso-selected baseline predictors + mean PDC, since this was 1 of the best performing models in the primary analysis and required only 1 measure of previous adherence. We replaced the missing previous adherence values with the median observed previous adherence measurement from patients in the training cohort. We estimated c-statistics to assess the performance of the model in this expanded cohort.
To examine the performance of individual drug classes for previous adherence measurement, we conducted 4 subgroup analyses in which we restricted the training cohort to patients who used specific classes of medications in the baseline period: (1) antihypertensives, (2) antidiabetics, (3) lipid-lowering agents other than statins, and (4) SSRIs. These medication classes were selected because they are commonly used and are intended to be used chronically.
We then assessed the performance of each of the 4 models in the following 3 cohorts: (1) those in the primary analysis cohort who had nonmissing previous adherence values for the drug class of interest; (2) the entire primary cohort (i.e., those with any previous adherence measurement, not necessarily based on the drug class of interest); and (3) the expanded cohort described earlier in this article.
As a separate sensitivity analysis, we developed and assessed the performance of the adherence prediction models in a cohort including the patients originally excluded for having less than 365 days of follow-up after statin initiation. Statin adherence during follow-up was measured by PDC, accounting for variable length of follow-up. Patients were followed until they were censored or until 365 days, whichever came first, and PDC was calculated using a denominator defined as the length of the varying follow-up period and a numerator defined as the number of denominator days on which drugs were available during that period. We repeated the modeling process as described for the primary cohort in the 50% randomly selected training cohort and assessed the discrimination of the model in the other 50%.
Results
Patient Characteristics and Adherence to Statins
We identified 243,051 statin initiators in the database between July 1, 2010, and December 31, 2011; 165,620 had complete follow-up during the 365 days after cohort entry. The primary analysis cohort comprised 89,490 patients (54% of all identified statin initiators) who had at least 1 dispensation for a medication used for previous adherence measurement. The average age of patients in the analysis cohort was 54.6 years (standard deviation [SD] 10.2), and 54.5% were female. The most common comorbidities were hypertension (56.1%), diabetes (26.0%), depression (14.6%), and cancer (13.4%). Frequently dispensed medications during the baseline period included ACE inhibitors (34.7%), beta blockers (25.3%), antidiabetics (25.2%), nonsteroidal anti-inflammatory drugs (22.8%), SSRIs (21.6%), and calcium channel blockers (20.7%). Mean medication synchronization metric was 0.26 (SD 0.21), indicating that many of the patients did not consolidate their medication dispensations. The median PDC for statins was 57.5%, and the 25th and 75th percentiles were 24.7% and 89.6%, respectively; 11.8% of patients had PDCs less than 10%, approximately reflecting the proportion of patients who filled only a single statin prescription.
Adherence Prediction Models
Using the basic set of predictors selected by lasso models including medication burden as the only explanatory variables yielded the highest c-statistic in the testing cohort (0.614, 95% confidence interval [CI] = 0.609-0.619; Table 2), followed by demographics (0.578, 95% CI = 0.573-0.584) and proxies of drug cost burden (0.575, 95% CI = 0.570-0.581). The model including only comorbidities had the lowest c-statistic (0.540, 95% CI = 0.535-0.545). Combining all of these baseline data components yielded a c-statistic of 0.665 (95% CI = 0.659-0.670).
TABLE 2.
C-statistics from Models Predicting High Statin Adherence (PDC ≥ 80%) Including Different Sets of Patient Baseline Information in the Testing Cohort
| Variables Included in Each Model | C-statistics (95% CI) |
|---|---|
| Demographics (age, sex) | 0.578(0.573-0.584) |
| Health services use (number of physician visits, colonoscopy, mammography, vaccinations, and hospitalized days) | 0.549(0.543-0.554) |
| Comorbidities (PVD, liver disease, renal disease, recent MI, previous stroke, ischemic heart disease, HTN, DM, depression, cancer) | 0.540(0.535-0.545) |
| Baseline medication use | 0.545(0.539-0.551) |
| Index statin information (high-/low-intensity dose, dispensed days supply) | 0.543(0.538-0.547) |
| Medication burden (number of any drug dispensations, of unique drugs, and of concurrent medication dispensed) | 0.614(0.609-0.619) |
| Plan benefit type and financial burden (total copayment during baseline, copay of the index statin) | 0.575(0.570-0.581) |
| All components combined | 0.665(0.659-0.670) |
c-statistics = concordance statistics; CI = confidence interval; DM = diabetes mellitus; HTN = hypertension; MI = myocardial infarction; PDC = proportion of days covered; PVD = peripheral vascular diseases.
Among models including only previous adherence measures, the continuous coverage measures achieved the highest c-statistics (range = 0.614-0.666; Table 3). Among these, PDC yielded the highest c-statistic for prediction of PDC ≥ 80% during follow-up (c-statistic for the model with max PDC = 0.666, 95% CI = 0.661-0.671). Minimum PDC or minimum continuous measure of medication acquisition (CMA) yielded a slightly lower c-statistic as compared with mean, median, and maximum of the 2, which were all similar. Count measures including the refill counts and lack of second fill achieved the poorest discrimination (c-statistics = 0.533-0.575).
TABLE 3.
C-statistics from Models Predicting High Statin Adherence (PDC ≥ 80%) Including Combinations of Measures of Previous Adherence and Patient Baseline Information in the Testing Cohort
| Variables Included in Each Model | C-statistics (95% CI) |
|---|---|
| Univariable models | |
| Refill count measures | |
| Any “no second fill” | 0.533 (0.529-0.537) |
| Median refills | 0.574 (0.569-0.580) |
| Mean refills | 0.575 (0.569-0.580) |
| Discontinuation measures | |
| Any discontinuation | 0.587 (0.583-0.592) |
| Mean TTD | 0.625 (0.620-0.630) |
| Coverage measures | |
| Min CMA | 0.614 (0.608-0.619) |
| Max CMA | 0.640 (0.635-0.646) |
| Median CMA | 0.639 (0.634-0.644) |
| Mean CMA | 0.640 (0.635-0.646) |
| Min CMG | 0.661 (0.656-0.666) |
| Max CMG | 0.629 (0.623-0.634) |
| Median CMG | 0.659 (0.654-0.665) |
| Mean CMG | 0.657 (0.651-0.662) |
| Min PDC | 0.633 (0.628-0.639) |
| Max PDC | 0.666 (0.661-0.671) |
| Median PDC | 0.665 (0.659-0.670) |
| Mean PDC | 0.663 (0.658-0.668) |
| Clinical variables + previous adherence measuresa | |
| a. All baseline information + median CMA | 0.684 (0.679-0.689) |
| b. All baseline information + mean CMA | 0.685 (0.680-0.690) |
| c. All baseline information + median PDC | 0.694 (0.689-0.699) |
| d. All baseline information + mean PDC | 0.695 (0.690-0.700) |
| e. All baseline information + mean PDC + mean refills | 0.696 (0.691-0.701) |
| f. All baseline information + mean PDC + DD | 0.695 (0.690-0.700) |
| g. All baseline information + mean PDC + DD + no second fill + mean TTD + mean CMA | 0.696 (0.691-0.701) |
| Pharmacy-based data components | |
| h. Demographics + medication use + index statin information + medication burden + mean PDC | 0.687 (0.682-0.692) |
a “All baseline information” includes all variables listed in Table 2: patient demographics, health services use, comorbidities, medication use, index statin information, medication burden, and financial burden.
c-statistics = concordance statistics; CI = confidence interval; CMA = continuous measure of medication acquisition; CMG = continuous measure of medication gaps; DD = discontinuation (dichotomous); max = maximum; min = minimum; PDC = proportion of days covered; TTD = time to discontinuation.
Combining a previous adherence measure with the baseline information increased c-statistics for predicting good adherence (PDC ≥ 80%; Table 3, models a through d), with addition of mean PDC achieving the highest c-statistic of 0.695 (95% CI = 0.690-0.700) and good calibration confirmed in the calibration plot (plot not shown). Adding combinations of different previous adherence measures to the basic set of predictors selected by lasso did not result in substantive improvement over the model with a single previous adherence measure (Table 3, models e through g). For a fixed sensitivity of 75%, the specificity of the model improved from 47.7% for the baseline variable–only model to 53.6% for the baseline + mean PDC model. This translates into an improvement in positive predictive value (PPV) from 43.2% to 46.1%. Exclusion of variables defined using medical data yielded c-statistics of 0.687, 95% CI = 0.682-0.692 (Table 3, model h).
In Appendix B, we present the beta coefficients and odds ratio point estimates for the variables and the intercept from the model, which included all basic predictors + mean PDC. In addition to previous adherence, male gender (odds ratio [OR] = 1.37, 95% CI = 1.31-1.44); history of mammography exams (OR = 1.21, 95% CI = 1.14-1.28); past medical events, including myocardial infarction and stroke; past or current use of oral anticoagulants and nonstatin lipid-lowering drugs; and the use of high-dose statins were strong predictors of good adherence. Information for the model without medical data is also shown in the same table.
A strong association was observed between previous adherence and future adherence after adjusting for all of the predefined clinical variables, despite the modest incremental improvement in predictive accuracy. For patients with mean previous PDC of < 25%, and for those with mean previous PDC 25%-79%, the likelihood of having high adherence to newly initiated statins was 51% lower (risk ratio [RR] = 0.49, 95% CI = 0.46-0.50), and 36% lower (RR = 0.64, 95% CI = 0.62-0.65), respectively, as compared with those with mean previous PDC of 80% and above.
Subgroup Analysis and Expansion of Target Patient Population
When we applied the prediction model with baseline variables + mean PDC from the primary analysis to the expanded cohort that included patients with no previous medication adherence measurement, the c-statistic was slightly lower (0.676, 95% CI = 0.672-0.679).
Subgroup analyses restricting to specific classes of medications for previous adherence measurement substantially reduced the number of patients included in the cohort (Table 4) but led to higher c-statistics of up to 0.720 (range = 0.709-0.731). The c-statistics were reduced when the models were applied to the full primary analysis cohort and to the expanded cohort, in which most patients lacked information on previous adherence to these specific medications (c-statistics = 0.671, 95% CI = 0.666-0.676 and 0.663, 95% CI = 0.659-0.667, respectively, for antidiabetics, and 0.669, 95% CI = 0.664-0.674 and 0.661, 95% CI = 0.657-0.665, respectively, for nonstatin lipid-lowering drugs). The model that included previous adherence only to SSRIs had similar c-statistics in all 3 testing cohorts (range = 0.661- 0.669).
TABLE 4.
C-statistics from Models Predicting High Statin Adherence (PDC ≥80%) Based on Previous Adherence Measured Using Only Specific Medication Classes, Applied to Various Testing Cohorts
| Medication Group | Drugs/Classes Included | Among Patients with Previous Adherence Measure for the Specific Medication Group | Among All Patients in the Primary Analysis Cohorta | Among Patients in the Expanded Cohort Including Patients with No Previous Use of Medications for Adherence Measurementa | |||
|---|---|---|---|---|---|---|---|
| n | C-statisticsb (95% CI) | n | C-statisticsb (95% CI) | n | C-statisticsb (95% CI) | ||
| All medications | See Appendix B for full list | - | - | 44,745 | 0.695 (0.690-0.700) | 82,810 | 0.676 (0.672-0.679) |
| Antihypertensives | ACEI/ARBs, diuretics, DHP-CCB | 27,912 | 0.692 (0.686-0.698) | 44,745 | 0.682 (0.677-0.687) | 82,810 | 0.669 (0.665-0.673) |
| Antidiabetics | Metformin, sulfonylurea | 8,952 | 0.720 (0.709-0.731) | 44,745 | 0.671 (0.666-0.676) | 82,810 | 0.663 (0.659-0.667) |
| Lipid-lowering drugs other than statins | Fibrates, bile acid sequestrants, ezetimibe | 2,196 | 0.717 (0.695-0.739) | 44,745 | 0.669 (0.663-0.674) | 82,810 | 0.661 (0.657-0.665) |
| SSRIs | SSRIs | 9,140 | 0.665 (0.653-0.676) | 44,745 | 0.669 (0.664-0.674) | 82,810 | 0.661 (0.657-0.665) |
aPatients without previous adherence measure for the specific medication group were given a median adherence value from the training cohort.
bAll models included baseline information variables from table 2 + mean PDC.
ACEI = angiotensin-converting enzyme inhibitors, ARBs = angiotensin II receptor blockers; c-statistics = concordance statistics; CI = confidence interval; DHP-CCB = dihydropyridine calcium channel blockers; PDC = proportion of days covered, SSRI = selective serotonin reuptake inhibitors.
In the sensitivity analysis in which we included patients with less than 365 days of follow-up, the pattern of results was similar to that from the primary analysis (full data not shown), although the c-statistics were consistently slightly lower for the models with previous adherence measures. The model including the basic set of predictors selected by lasso and mean PDC yielded a c-statistic of 0.680 (95% CI = 0.676-0.685).
Discussion
Our large, systematic evaluation found that previous adherence to chronically used medications was associated with future adherence to newly initiated statin treatment. Addition of measures of previous adherence to prediction models led to models capable of identifying whether patients would have high adherence to the newly started statins with improved accuracy. Building on previous work,14,17 we found that previous adherence as a predictor of future adherence was best measured using the PDC. Adding multiple previous adherence measures to the prediction model did not lead to substantive improvement in discriminatory ability over a model with mean PDC as a single previous adherence measure.
Few studies have examined previous medication adherence as a predictor of future adherence. Solomon et al. (2011) described the strength of association between previous nonadherence to medications for chronic medical conditions, defined as having less than 2 prescriptions of the drugs despite having diagnoses for the conditions and very-low future adherence to osteoporosis medication in women.16 Muntner et al. (2014) reported a strong association between past adherence to anti-hypertensive medication and the risk of statin discontinuation and low adherence among patients discharged from hospitals for acute myocardial infarction or coronary revascularization.14 Neither study specifically assessed the extent to which measures of previous adherence improve the performance of models to predict future medication adherence. Our study is the first study to systematically compare the predictive performance of a large number of previous adherence metrics across a large set of drugs used to measure previous adherence.
Curtis et al. (2009) previously evaluated improvements in c-statistics for models predicting bisphosphonate adherence in administrative claims data using adherence to other medications. They found that the use of adherence to previous and concurrently used chronic medications such as statins, ACE inhibitors/ARBs, and SSRIs improved the c-statistics of the prediction model up to 0.70 from the base model’s 0.62.15 An obvious and important difference between this study and ours is the inclusion of adherence measured for medications dispensed both before and concurrent to the bisphosphonates. This is also an important difference between our study and that by Franklin et al.24 where patient adherence to newly initiated statins for the first 3 months was used to predict future adherence, achieving a model with c-statistics as high as 0.83. Estimating patient adherence before the start of a new medication is useful to determine whether patients are likely to be nonadherent at the time of the initial dispensation because it would enable pharmacists to intervene at a critical time for patients initiating therapy. At the same time, it is important to point out that these prediction methods can be used to achieve improved prediction and intervention in combination and need not be used separately.
We observed good discrimination when we used previous adherence measures from only antidiabetics or only lipid-lowering agents other than statins. It is possible that previous adherence behaviors with these drugs are better indicators of future statin adherence. However, because only a small subset of patients used these drugs, models based on only these drugs performed less well when applied to the expanded cohort of all statin initiators as compared with models in which all previous adherence drugs were considered. Future work should focus on identifying the optimal set of previous adherence drugs to predict adherence to statins as well as to other drugs of interest.
The c-statistics of the prediction model without medical data were slightly reduced compared with the full model but still had good discrimination, which is consistent with the findings from previous studies.17,24 We built this model with the idea that adherence prediction could happen at the point of a pharmacy visit when only prescription data may be available. For example, the pharmacy or pharmacy benefits manager could identify patients at risk of nonadherence in real time at the point of initial prescription dispensation and use the pharmacy encounter to provide adherence improvement interventions.25,26
Limitations
Several important limitations of this study should be acknowledged. First, we measured statin adherence in claims data using PDC to define high adherence. Although this has been shown to correlate well with other adherence measures, including drug presence measured by serum levels, and is a widely used measure of adherence,20,27 dispensation patterns may not exactly correspond to patient medication-taking behavior.
Second, we selected a lookback period of 365 days for the assessment of baseline covariates and of previous adherence. Requiring longer lookback periods may lead to more complete capture of chronic conditions but would reduce the number of eligible patients, which would potentially limit the generalizability of the prediction models. Future work should examine the effect of different lookback periods on adherence prediction.
Third, we focused the statin adherence outcome on the first year following statin initiation, whereas some patients continued to use the drugs for much longer. However, because monthly PDC for chronic cardiovascular medications tends to stabilize after the first 10 months of treatment,28 we believe our results should be largely generalizable to adherence behavior in the longer term.
Fourth, patients needed to have baseline use of the selected chronic medications to estimate their previous medication adherence, which was available for 54% of all identified statin initiators. By definition, measures of previous adherence were not applicable to those patients with no use of drugs used to assess previous adherence.
Fifth, the generalizability of our findings may in part be compromised by the introduction of the Centers for Medicare & Medicaid Services Medicare Health Plan Quality and Performance Ratings program, which includes medication adherence programs and which may have led to heightened attention to adherence by the health care providers and insurers or by an introduction of new treatment guidelines and generics that occurred since the study period set in 2010-2011.
Sixth, because our study relied on a claims database as the data source, we were unable to capture primary nonadherence as an outcome. Finally, the study was conducted using a U.S. claims database consisting mostly of commercial insurance beneficiaries, and we evaluated adherence among patients initiating a single chronic medication. We chose statins as our target medication because of their widespread and chronic use for asymptomatic conditions, which are features common to other cardiovascular medications. Future studies should evaluate the generalizability of the study’s results to older patients and those with different insurance providers and to other medication classes.
Conclusions
Previous adherence to chronic medications was a strong predictor of future adherence to newly initiated statins and was a stronger determinant than demographic variables, clinical variables, and other medication-based measures. When predicting medication adherence in administrative claims data, whether for targeted adherence improvement interventions or to better design comparative effectiveness research studies, models should include measures of previous medication adherence, such as mean PDC.
APPENDIX A. Distribution of Baseline Characteristics Among Patients in the Training and Testing Cohorts for the Primary Analysis
| Characteristics | Training Cohort | Testing Cohort |
|---|---|---|
| Patients, n | 44,745 | 44,745 |
| Age, mean (SD) | 54.5 (10.2) | 54.6 (10.2) |
| Female, n (%) | 24,289 (54.3) | 24,424 (54.6) |
| Regions, n (%) | ||
| Midwest | 10,422 (23.3) | 10,471 (23.4) |
| Northeast | 3,431 (7.7) | 3,566 (8.0) |
| South | 25,054 (56.0) | 25,013 (55.9) |
| West | 5,838 (13.0) | 5,695 (12.7) |
| Use of preventive service, n (%) | ||
| Fecal blood tests | 4,166 (9.3) | 4,178 (9.3) |
| Colonoscopy | 3,984 (8.9) | 3,904 (8.7) |
| Mammography | 8,585 (19.2) | 8,540 (19.1) |
| Number of hospitalizations, mean (SD) | 0.17 (0.6) | 0.17 (0.6) |
| Days in hospital, mean (SD) | 0.88 (4.6) | 0.90 (4.5) |
| Comorbidities, n (%) | ||
| PVD | 1,529 (3.4) | 1,502 (3.4) |
| Liver disease | 1,938 (4.3) | 1,957 (4.4) |
| Renal disease | 2,399 (5.4) | 2,434 (5.4) |
| Recent MI | 588 (1.3) | 593 (1.3) |
| Prior MI | 754 (1.7) | 770 (1.7) |
| Recent stroke | 289 (0.7) | 313 (0.7) |
| Prior stroke | 391 (0.9) | 418 (0.9) |
| Ischemic heart disease | 5,191 (11.6) | 5,207 (11.6) |
| Transient ischemic attack | 158 (0.4) | 132 (0.3) |
| Hypertension | 25,051 (56.0) | 25,156 (56.2) |
| Diabetes | 11,653 (26.0) | 11,579 (25.9) |
| Depression | 6,517 (14.6) | 6,544 (14.6) |
| Cancer | 5,978 (13.4) | 5,882 (13.2) |
| Combined comorbidity score, mean (SD) | 0.16 (1.5) | 0.17 (1.5) |
| Baseline medications, n (%) | ||
| ACEI | 15,548 (34.8) | 15,480 (34.6) |
| ARB | 8,580 (19.2) | 8,576 (19.2) |
| Beta blockers | 11,182 (25.0) | 11,445 (25.6) |
| Calcium channel blockers | 9,253 (20.7) | 9,266 (20.7) |
| Thiazides | 14,850 (33.2) | 14,861 (33.2) |
| Baseline medications, n (%) | ||
| Oral anticoagulants | 1,484 (3.3) | 1,537 (3.4) |
| Antiplatelets | 1,708 (3.8) | 1,713 (3.8) |
| Antidiabetics | 11,286 (25.2) | 11,229 (25.1) |
| NSAIDs | 10,200 (22.8) | 10,105 (22.6) |
| SSRIs | 9,652 (21.6) | 9,698 (21.7) |
| Other lipid-lowering agents | 2,169 (4.9) | 2,223 (5.0) |
| Index statin, n (%) | ||
| Atorvastatin | 7,466 (16.7) | 7,601 (17.0) |
| Fluvastatin | 47 (0.1) | 57 (0.1) |
| Lovastatin | 2,208 (4.9) | 2,174 (4.9) |
| Pitavastatin | 501 (1.1) | 545 (1.2) |
| Pravastatin | 8,257 (18.5) | 8,127 (18.2) |
| Rosuvastatin | 6,440 (14.4) | 6,271 (14.0) |
| Simvastatin | 19,826 (44.3) | 19,970 (44.6) |
| High-intensity dose | 4,090 (9.1) | 3,931 (8.9) |
| Refill synchronization measure | 0.26 (0.2) | 0.26 (0.2) |
| Medication burden, mean (SD) | ||
| Number of drug dispensations | 26.4 (21.9) | 26.5 (21.9) |
| Number of unique drugs dispensed | 7.6 (5.2) | 7.6 (5.2) |
| Number of drugs dispensed with days supply overlapping index | 2.4 (2.3) | 2.4 (2.3) |
| Plan benefit type, n (%) | ||
| POS | 30,753 (68.7) | 30,747 (68.7) |
| EPO | 7,118 (15.9) | 7,028 (15.7) |
| HMO | 3,706 (8.3) | 3,733 (8.3) |
| IND | 1,753 (3.9) | 1,856 (4.2) |
| PPO | 1,357 (3.1) | 1,336 (3.0) |
| Other | 58 (0.1) | 45 (0.1) |
| Financial burden | ||
| Total deductibles in U.S. dollars, mean (SD) | 557.8 (547.6) | 559.6 (560.6) |
| Copay of index statin in U.S. dollars, mean (SD) | 17.0 (19.5) | 16.9 (19.3) |
ACEI = angiotensin-converting enzyme inhibitors, ARBs = angiotensin II receptor blockers; EPO = exclusive provider program; HMO = health maintenance organization; IND = individual health plan; MI = myocardial infarction; NSAIDs = nonsteroidal anti-inflammatory drugs; POS = point-of-service; PPO = preferred provider organization; PVD = peripheral vascular disease; SD = standard deviation; SSRIs = selective serotonin reuptake inhibitors.
APPENDIX B. Coefficients and Corresponding Odds Ratios for High Adherence for the Variables in the Full Model with All Baseline Information and Mean PDC and the Model Without Medical Information Built Among the Primary Analysis Cohort
| Data Type | Variable Name | Full Model | Model Without Medical Information | ||
|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | ||
| Demographics | Age | 1.015 | 1.012-1.017 | 1.017 | 1.015-1.020 |
| Female gender | 0.730 | 0.695-0.767 | 0.765 | 0.733-0.799 | |
| Health services use | Physician visits | 1.005 | 1.003-1.007 | ||
| Hospitalizations | 1.066 | 1.017-1.118 | |||
| Colonoscopy | 1.074 | 0.999-1.155 | |||
| Mammography | 1.206 | 1.137-1.280 | |||
| Vaccinations | 1.104 | 1.062-1.147 | |||
| Comorbidities | PVD | 0.885 | 0.787-0.996 | ||
| Prior liver disease | 0.869 | 0.783-0.964 | |||
| Renal dysfunction | 0.911 | 0.826-1.005 | |||
| Recent MI | 2.322 | 1.921-2.806 | |||
| Previous stroke | 1.833 | 1.471-2.285 | |||
| Ischemic heart disease | 1.163 | 1.081-1.252 | |||
| Diabetes | 0.905 | 0.835-0.980 | |||
| Cancer | 1.063 | 1,000-1.131 | |||
| Drug use | ACEIs | 0.997 | 0.950-1.047 | 0.982 | 0.936-1.031 |
| Antidiabetics | 0.922 | 0.848-1.003 | 0.834 | 0.790-0.881 | |
| Antiparkinson agents | 0.820 | 0.692-0.971 | 0.816 | 0.690-0.966 | |
| Antiplatelets | 0.886 | 0.791-0.993 | 0.957 | 0.859-1.066 | |
| ARBs | 0.869 | 0.819-0.923 | 0.885 | 0.834-0.938 | |
| Calcium channel blockers | 0.852 | 0.806-0.900 | 0.849 | 0.804-0.897 | |
| Digoxin | 0.724 | 0.569-0.922 | 0.742 | 0.583-0.945 | |
| Loop diuretics | 1.040 | 0.939-1.152 | 1.043 | 0.943-1.153 | |
| NSAIDs | 0.944 | 0.895-0.995 | 0.924 | 0.876-0.973 | |
| Oral anticoagulants | 1.304 | 1.157-1.471 | 1.415 | 1.259-1.591 | |
| SSRIs | 1.000 | 0.947-1.057 | 0.997 | 0.944-1.053 | |
| TCAs | 1.203 | 1.063-1.362 | 1.168 | 1.032-1.321 | |
| Thiazide diuretics | 0.987 | 0.938-1.039 | 0.976 | 0.927-1.027 | |
| Nonstatin lipid-lowering drugs | 1.490 | 1.354-1.641 | 1.486 | 1.351-1.634 | |
| Number of any drug | 1.008 | 1.005-1.010 | 1.010 | 1.008-1.012 | |
| Prescription burden | Number of unique drug type | 0.949 | 0.941-0.957 | 0.962 | 0.955-0.969 |
| Number of concurrently prescribed drugs as the index statin | 1.089 | 1.075-1.103 | 1.086 | 1.072-1.100 | |
| Number of drugs among those listed above | 0.999 | 0.996-1.002 | 0.998 | 0.995-1.001 | |
| Refill synchronization | 0.865 | 0.777-0.963 | 0.825 | 0.742-0.918 | |
| Sum of copayment for drugs | 1.000 | 1.000-1.000 | |||
| Benefit plan type | EPO vs. PPO | 0.722 | 0.635-0.820 | 0.723 | 0.636-0.821 |
| HMO vs. PPO | 0.896 | 0.783-1.026 | 0.900 | 0.787-1.030 | |
| IND vs. PPO | 1.072 | 0.917-1.253 | 1.052 | 0.901-1.228 | |
| Other vs. PPO | 1.155 | 0.662-2.012 | 1.184 | 0.680-2.064 | |
| POS vs. PPO | 0.906 | 0.805-1.020 | 0.904 | 0.804-1.017 | |
| Index statin information | Copay amount | 0.996 | 0.994-0.997 | 0.996 | 0.995-0.997 |
| Days supply | 1.007 | 1.006-1.008 | 1.006 | 1.005-1.007 | |
| High-dose statin (no vs. yes) | 1.289 | 1.192-1.394 | 1.219 | 1.129-1.317 | |
| Previous medication adherence | Mean PDC | 5.985 | 5.410-6.621 | 6.081 | 5.500-6.722 |
ACEI = angiotensin-converting enzyme inhibitors, ARBs = angiotensin II receptor blockers; CI = confidence interval; EPO = exclusive provider organization; HMO = health maintenance organization; IND = individual health plan; MI = myocardial infarction; NSAIDS = nonsteroidal anti-inflammatory drugs; OR = odds ratio; PDC = proportion of days covered; POS = point-of-service; PPO = preferred provider organization; PVD = peripheral vascular disease; SSRIs = selective serotonin reuptake inhibitors; TCAs = tricyclic antidepressants.
REFERENCES
- 1.Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353(5):487-97. [DOI] [PubMed] [Google Scholar]
- 2.Naderi SH, Bestwick JP, Wald DS. Adherence to drugs that prevent cardiovascular disease: meta-analysis on 376,162 patients. Am J Med. 2012;125(9):882-87. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization. Adherence to long-term therapies: evidence for action. 2003. Available at: http://www.who.int/chp/knowledge/publications/adherence_report/en/. Accessed August 6, 2018.
- 4.Yusuf S, Reddy S, Ounpuu S AS. Global burden of cardiovascular diseases part I: general considerations, the epidemiologic transition, risk factors, and impact of urbanization. Circulation. 2001;104(22):2746-53. [DOI] [PubMed] [Google Scholar]
- 5.Antman EM, Hand M, Armstrong PW, et al. 2007 focused update of the ACC/AHA 2004 guidelines for the management of patients with ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2008;51(2):210-47. [DOI] [PubMed] [Google Scholar]
- 6.Desai NR, Choudhry NK. Impediments to adherence to post myocardial infarction medications. Curr Cardiol Rep. 2013;15(1):332. [DOI] [PubMed] [Google Scholar]
- 7.Misono AS, Cutrona SL, Choudhry NK, et al. Healthcare information technology interventions to improve cardiovascular and diabetes medication adherence. Am J Manag Care. 2010;16(12 Suppl HIT):SP82-92. [PubMed] [Google Scholar]
- 8.Cutrona S, Choudhry N, Fischer M, et al. Targeting cardiovascular medication adherence interventions. J Am Pharm Assoc. 2012;52(3):381-97. [DOI] [PubMed] [Google Scholar]
- 9.Viswanathan M, Golin C, Jones C, et al. Interventions to improve adherence to self-administered medications for chronic diseases in the United States. Ann Intern Med. 2012;157(11):785-95. [DOI] [PubMed] [Google Scholar]
- 10.Gagne JJ, Choudhry NK, Kesselheim AS, et al. Comparative effectiveness of generic and brand-name statins on patient outcomes: a cohort study. Ann Intern Med. 2014;161(6):400-07. [DOI] [PubMed] [Google Scholar]
- 11.Choudhry NK, Avorn J, Glynn RJ, et al. Full coverage for preventive medications after myocardial infarction. N Engl J Med. 2011;365(22):2088-97. [DOI] [PubMed] [Google Scholar]
- 12.Steiner JF, Ho PM, Beaty BL, et al. Sociodemographic and clinical characteristics are not clinically useful predictors of refill adherence in patients with hypertension. Circ Cardiovasc Qual Outcomes. 2009;2(5):451-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chan DC, Shrank WH, Cutler D, Choudhry NK. Patient, physician, and payment predictors of statin adherence. Med Care. 2010;48(3):196-202. [DOI] [PubMed] [Google Scholar]
- 14.Muntner P, Yun H, Sharma P, et al. Ability of low antihypertensive medication adherence to predict statin discontinuation and low statin adherence in patients initiating treatment after a coronary event. Am J Cardiol. 2014;114(6):826-31. [DOI] [PubMed] [Google Scholar]
- 15.Curtis JR, Xi J, Westfall AO, et al. Improving the prediction of medication compliance: the example of bisphosphonates for osteoporosis. Med Care. 2009;47(3):334-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Solomon DH, Brookhart MA, Tsao P, et al. Predictors of very low adherence with medications for osteoporosis: towards development of a clinical prediction rule. Osteoporos Int. 2011;22(6):1737-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krumme AA, Franklin J, Isaman D, et al. Predicting 1-year statin adherence among prevalent users: a retrospective cohort study. J Manag Care Spec Pharm. 2017;23(4):494-502. Available at: https://www.jmcp.org/doi/10.18553/jmcp.2017.23.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sentinel. Data partners. Available at: https://www.sentinelinitiative.org/sentinel/data/data-partners. Accessed August 16, 2018.
- 19.Ho PM, Bryson CL, Rumsfeld JS. Medication adherence: its importance in cardiovascular outcomes. Circulation. 2009;119(23):3028-35. [DOI] [PubMed] [Google Scholar]
- 20.Steiner JF, Prochazka A. The assessment of refill compliance using pharmacy records: methods, validity, and applications. J Clin Epidemiol. 1997;50(1):105-16. [DOI] [PubMed] [Google Scholar]
- 21.Steiner JF, Koepsell TD, Fihn SD, Inui TS. A. general method of compliance assessment using centralized pharmacy records. Description and validation. Med Care. 1988;26(8):814-23. [DOI] [PubMed] [Google Scholar]
- 22.Choudhry NK, Fischer MA, Avorn J, et al. The implications of therapeutic complexity on adherence to cardiovascular medications. Arch Intern Med. 2011;171(9):814-22. [DOI] [PubMed] [Google Scholar]
- 23.Zou G. A modified Poisson regression approach to prospective studies with binary data. 2004;159(7):702-06. [DOI] [PubMed] [Google Scholar]
- 24.Franklin JM, Shrank WH, Lii J, et al. Observing versus predicting: initial patterns of filling predict long-term adherence more accurately than high-dimensional modeling techniques. Health Serv Res. 2016;51(1):220-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eussen SR, van der Elst ME, Klungel OH, et al. A pharmaceutical care program to improve adherence to statin therapy: a randomized controlled trial. Ann Pharmacother. 2010;44(8):1905-13. [DOI] [PubMed] [Google Scholar]
- 26.Evans CD, Eurich DT, Remillard AJ, Shevchuk YM, Blackburn D. First-fill medication discontinuations and nonadherence to antihypertensive therapy: an observational study. Am J Hypertens. 2009;25(2):195-203. [DOI] [PubMed] [Google Scholar]
- 27.Lau H, de Boer A, Beuning K, Porsius A. Validation of pharmacy records in drug exposure assessment. J Clin Epidemiol. 1997;50(5):619-25. [DOI] [PubMed] [Google Scholar]
- 28.Setoguchi S, Shrank WH, Liu J, et al. Angiotensin receptor blockers and angiotensin-converting enzyme inhibitors: challenges in comparative effectiveness using Medicare data. Clin Pharmacol Ther. 2011;89(5):674-82. [DOI] [PMC free article] [PubMed] [Google Scholar]


