Hereditary angioedema (HAE) is a rare autosomal dominant genetic disorder characterized by recurrent and unpredictable episodes of tissue swelling (angioedema).1 HAE is caused by mutations in the SERPING1 gene that encodes for the C1 inhibitor (C1-INH), a protease inhibitor involved in limiting bradykinin production. Low levels of C1-INH (HAE type 1) or dysfunctional C1-INH (HAE type 2) lead to the buildup of bradykinin, resulting in capillary leakage and ultimately tissue swelling.2,3 There are also other less common forms of HAE in which C1-INH levels and function are normal.
HAE affects approximately 1 in 50,000 individuals, with males and females equally affected.4 The mean age of onset is 10 years, although attacks can begin at any age.5 Attacks are usually characterized by mild to severe tissue swelling at 1 or more sites in the body (typically the face, hands, feet, airways, and intestinal tract). The frequency and duration of HAE attacks are highly variable.6 On average, HAE attacks can occur every 1-2 weeks.1 If untreated, swelling is self-limited and usually resolves spontaneously in 2-5 days; however, laryngeal edema poses the risk of death due to asphyxiation.6,7 When treatments are administered, death is rare.
HAE attacks are typically treated with on-demand administration of C1-INH replacement products, kallikrein inhibitors, or bradykinin receptor antagonists. Some patients with frequent attacks receive routine (long-term) prophylaxis to prevent or reduce the frequency and severity of attacks. Human plasma-derived C1-INH concentrate products (Cinryze or Haegarda) have been approved by the U.S. Food and Drug Administration for long-term prophylaxis, and lanadelumab-flyo (Takzyhro), a monoclonal antibody that inhibits plasma kallikrein, was approved on August 23, 2018, as another alternative.
The Institute for Clinical and Economic Review (ICER) conducted a review of Cinryze, Haegarda, and Takzyhro as prophylactic therapy for HAE types 1 and 2 (HAE 1/2). In this article, we present a summary of a systematic literature review of the clinical effectiveness of the drugs, a cost-effectiveness analysis, and a policy discussion with key stakeholders regarding the overall value of these therapies held at a public meeting of the California Technology Assessment Forum on October 25, 2018. The detailed report is available on the ICER website at https://icer-review.org/material/angioedema-final-report/.8
Summary of Findings
Clinical Effectiveness
We evaluated the literature supporting the clinical effectiveness and safety of Cinryze, Haegarda, and Takhyzro for long-term prophylaxis in patients with HAE 1/2 compared with on-demand treatment only. Due to differences in trial entry criteria (particularly age and baseline attack rates) and study design, as well as small study populations, we did not perform a quantitative indirect comparison of the 3 drugs through network meta-analysis. As such, our review evaluated the comparative findings from the clinical trials of each agent. Complete details on ICER’s systematic literature search and protocol, including search strategy and inclusion and exclusion criteria, are included in the final evidence report, which is available on ICER’s website.8
Cinryze.
The pivotal trial for Cinryze was a crossover randomized controlled trial (RCT) of 22 HAE 1/2 patients aged 6 years and older, who had a history of 2 or more attacks per month. Patients were randomized to receive either 1,000 IU of Cinryze or placebo intravenously (every 3-4 days) for two 12-week treatment periods.9 Patients in both arms of the trials received on-demand treatment for attacks as needed. The primary outcome was patient-reported HAE attacks. Compared with the placebo arm, prophylactic treatment with Cinryze significantly reduced the frequency of HAE attacks (estimated mean attack rate per month: 4.24 vs. 2.09; P < 0.001).9 In addition, Cinryze was also shown to significantly reduce the severity and duration of HAE attacks and use of rescue medication when compared with placebo.9 Furthermore, Cinryze appeared to improve health-related quality of life based on increased SF-36 scores in the treatment group, although statistical significance was not reported.9 Another randomized crossover study, conducted in an exclusively pediatric population (N = 12), also found that long-term prophylaxis with Cinryze significantly reduced the mean HAE arrack rate by 71%-85% when compared with placebo.10 In addition, data from an open label extension study conducted over 2.6 years (N = 146) showed a sustained reduction in the monthly rate of HAE attacks.11
Haegarda.
For Haegarda, only 1 trial met our inclusion criteria.12 The trial was a crossover RCT of 90 patients aged 12 years and older, who had a history of 2 or more HAE attacks per month requiring immediate medical attention. Patients were randomized to either Haegarda (40 IU/kg or 60 IU/kg) or placebo and followed over two 16-week treatment periods. The primary outcome was HAE attack, confirmed by the investigators. Prophylactic treatment with Haegarda at both dosages significantly reduced the frequency of HAE attacks when compared with placebo, with greater improvement shown in the 60 IU/kg group (0.5 per month vs. 4.0 per month; P < 0.001; 84% mean reduction in attacks). More patients on Haegarda prophylaxis were attack-free over the duration of the study compared with those on placebo (38%-40% vs. 9%). Haegarda also showed effectiveness in other secondary outcomes including severity and duration of HAE attacks and use of rescue medication.12 Exploratory analyses examining the effect of Haegarda prophylaxis on quality of life observed no meaningful difference on the European Quality of Life-5 Dimensions Questionnaire (EQ-5D) compared with placebo.13 However, prophylaxis with Haegarda appeared to result in clinically meaningful improvement on work presenteeism and productivity.13
Takhyzro.
Data to inform our assessment of Takhyzro were drawn from a conference presentation of a 26-week, parallelarm RCT of 125 patients aged 12 years and older, who had a history of 1 or more HAE attacks per month.14 Participants were treated with placebo or 1 of 3 dosing regimens of Takhyzro (150 mg every 4 weeks, 300 mg every 4 weeks, or 300 mg every 2 weeks). The primary outcome was investigator-confirmed HAE attack.14 Prophylactic treatment with Takhyzro significantly decreased the frequency of HAE attacks when compared with placebo (0.26, 0.53, and 0.48 attacks per month vs. 1.97 attacks per month; all P < 0.001), a 73%-87% reduction in the frequency of HAE attacks.14 In addition, more patients on Takhyzro were attack-free over the duration of the study compared with placebo (39%-44% vs. 2%). Secondary outcomes, including severity of attacks and use of rescue medication, were also in favor of Takhyzro. In addition, prophylaxis with Takhyzro resulted in clinically meaningful improvement on the angioedema quality of life questionnaire (AE-QoL).14
Harms of C1-INHs and Lanadelumab-flyo.
Side effects observed during randomized controlled trials of the C1-INH products and lanadelumab-flyo were mild to moderate and included mild infections, headaches, hypersensitivity, dizziness, and injection site reactions for the subcutaneous drugs.9,12,14 Serious adverse events and adverse events leading to trial discontinuation were rare and generally similar between active treatment and placebo. Long-term safety data related to prophylaxis use were identified only for Cinryze, which was the first drug approved for long-term prophylaxis, and it was found to be well tolerated when used over 2.6 years.11
Limitations of the Clinical Evidence
As is common with treatments for rare diseases, the evidence base for our review is limited by small study populations and a lack of long-term safety and efficacy data. While there are fewer concerns about the safety profile of the C1-INH products, given the long experience with their use for on-demand treatment, data on the safety profile of lanadelumab-flyo are extremely limited at this time. We found very limited data on patient-reported outcomes. Quality of life measures were infrequently and inconsistently measured across the trials, and none of the trials used a quality of life measure specific to “hereditary angioedema.”
Long-Term Cost-Effectiveness
We also evaluated the cost-effectiveness of prophylaxis with the C1-INH products and lanadelumab-flyo, using a Markov model to compare these interventions to no prophylaxis in patients with HAE 1/2. The model was developed with monthly cycles over a lifetime horizon. The baseline characteristics of the population in the model reflected the weighted average across the 3 pivotal clinical trials for the interventions, with a mean age of 39.6 years, 68.4% female, mean weights of 88.8 kg (male) and 76.4 kg (female), and a baseline attack rate of 3.39 attacks per month.
For each intervention, the model estimated the number of acute attacks, the probability of death given the number of attacks in each cycle, patient survival, time spent attack-free, quality-adjusted survival, and health care costs. Indirect productivity costs such as missed work or school for acute attacks were also accounted for in the model. The effect of prophylaxis treatment on attack rates was derived from the pivotal trials previously described. Differences in survival, quality-adjusted survival, and costs between each prophylactic therapy and no prophylaxis were used to calculate incremental cost-effectiveness ratios.
The base-case analysis used a U.S. health care system perspective with a 3% discount rate for costs and health outcomes. All patients in the model were assumed to have received on-demand treatment for moderate and severe acute attacks in the analysis. We used net prices for prophylactic and on-demand drug costs. Uncertainty in the model was assessed through sensitivity analyses of key model inputs. Full details on ICER’s cost-effectiveness analysis and model are included in the final evidence report on the ICER website.8
Results from our model showed that all 3 drugs had incremental cost-effectiveness ratios above the commonly accepted willingness-to-pay threshold of $150,000 per quality-adjusted life-year (Table 1). However, the results of the models were very sensitive to baseline attack rates, prophylactic and on-demand drug costs, and treatment effect estimates. For example, these drugs will achieve cost-effectiveness at a threshold of $150,000 per quality-adjusted life-year for a monthly baseline attack rate of 3.43 for Haegarda, 3.78 for Takhzyro, and 5.85 for Cinryze.
TABLE 1.
Health Care Sector Perspective Results for Cinryze, Haegarda, and Takhzyro Cost-Effectiveness
| Intervention | Discounted Cost and QALYs | Versus No Prophylaxis | |||
|---|---|---|---|---|---|
| Prophylaxis Drug Costs $ | Acute Treatment Costs, $ | Total Costs: U.S. Health System Perspective $ | QALYs | ICER (per QALY) $ | |
| No prophylaxis | 0 | 9,953,000 | 9,953,000 | 17.47 | - |
| Cinryze | 9,469,000 | 4,927,000 | 14,396,000 | 18.21 | 5,954,000 |
| Haegarda | 8,897,000 | 1,446,000 | 10,343,000 | 18.65 | 328,000 |
| Takhzyro | 9,970,000 | 1,304,000 | 11,274,000 | 18.66 | 1,108,000 |
ICER = incremental cost-effectiveness ratio; QALY = quality-adjusted life-year.
For Takhzyro, the product label suggests that patients who remain attack-free for 6 months on the approved dose of 300 mg every 2 weeks may consider decreasing to an every 4-week dosing schedule. We modeled this reduced dosing frequency in a scenario analysis among all attack-free patients taking Takhzyro and found that if approximately 75% of all eligible patients switch to the less frequent dosing, Takhzyro would be cost-effective at the $150,000 willingness-to-pay threshold.
Limitations of the Cost-Effectiveness Model
We were limited to the measures of effectiveness captured in the clinical trials. The limitations of these trials have already been described. In addition, because U.S.-specific data on utilities and HAE mortality were not available, we used estimates from European studies.
Policy Discussion
The California Technology Assessment Forum (CTAF) is one of the independent appraisal committees convened by ICER to engage in the public deliberation of the evidence on clinical and cost-effectiveness of health care interventions. CTAF is composed of medical evidence experts (e.g., practicing clinicians and methodologists) and leaders in patient engagement and advocacy. Their deliberation includes input from clinical experts and patient representatives specific to the condition under review, as well as formal comment from manufacturers and the public. A policy roundtable concludes each meeting during which representatives from insurers and manufacturers join with clinical experts and patient representatives to discuss how best to apply the findings of the evidence to clinical practice, insurance coverage, and pricing negotiations.
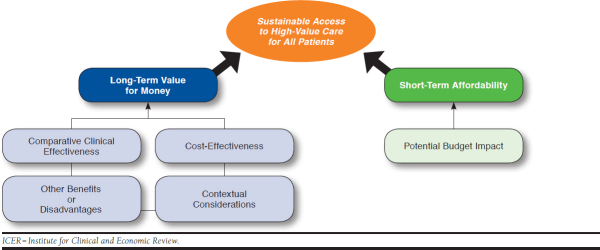
The structure through which ICER evidence reports present information for the CTAF is presented in Figure 1. This “value assessment framework” represents the conceptual framework through which considerations of different elements of value are integrated in judgments on long-term value for money and short-term affordability.15,16
FIGURE 1.
The ICER Value Assessment Framework
The ICER report on Cinryze, Haegarda, and Takhzyro was the subject of a CTAF meeting in October 2018. Following discussion, the CTAF panel members voted 14-1 that the evidence was adequate to demonstrate that the net health benefits of long-term prophylaxis with C1-INHs for HAE 1/2 are superior to on-demand therapy only. CTAF also voted 14-1 that the evidence was not adequate to distinguish between the net health benefits provided by the 2 C1-INHs (Cinryze and Haegarda) for long-term prophylactic therapy for HAE 1/2. For Takhzyro, the CTAF panel voted 11-4 that the evidence at the time of the public meeting was not yet adequate to demonstrate that its net health benefit when used for long-term prophylaxis is superior to on-demand therapy only.
The CTAF panel also voted on “other potential benefits” and “contextual considerations” of these treatments as part of a process intended to signal to policymakers whether there are important considerations when making judgments about long-term value for money that are not adequately captured in the analyses of clinical effectiveness and cost-effectiveness. The results of these votes are shown in Tables 2 and 3 and serve to highlight several factors that the CTAF panel felt were particularly important for judgments of value.
TABLE 2.
Other Benefits or Disadvantages
| Does treating HAE type 1 or 2 with long-term prophylactic therapy offer 1 or more of the following potential “other benefits” versus on-demand treatment? | |
|---|---|
| Potential Benefit | Panel Votesa |
| a. Haegarda offers reduced complexity that will significantly improve patient outcomes. | 15 |
| b. Takhzyro offers reduced complexity that will significantly improve patient outcomes. | 13 |
| c. This intervention will reduce important health disparities across racial, ethnic, gender, socioeconomic, or regional categories. | 0 |
| d. This intervention will significantly reduce caregiver or broader family burden. | 13 |
| e. Takhzyro offers a novel mechanism of action or approach that will allow successful treatment of many patients for whom other available treatments have failed. | 7 |
| f. This intervention will have a significant impact on improving patients’/caregivers’ ability to return to work or school and/or their overall productivity. | 13 |
| g. This intervention will have a significant positive impact outside the family, including on schools and/or communities. | 4 |
| h. This intervention will have a significant impact on the entire “infrastructure” of care, including effects on screening for affected patients, on the sensitization of clinicians, and on the dissemination of understanding about the condition, that may revolutionize how patients are cared for in many ways that extend beyond the treatment itself. | 2 |
| i. There are other important benefits or disadvantages that should have an important role in judgments of the value of this intervention. | 2 |
aFifteen CTAF panelists voted.
CTAF = California Technology Assessment Forum; HAE = hereditary angioedema.
TABLE 3.
Contextual Considerations
| Are any of the following contextual considerations important in assessing the long-term value for money of long-term prophylactic therapy for HAE type 1 or 2? | |
|---|---|
| Contextual Consideration | Panel Votesa |
| a. This intervention is intended for the care of individuals with a condition of particularly high severity in terms of impact on length of life and/or quality of life. | 11 |
| b. This intervention is intended for the care of individuals with a condition that represents a particularly high lifetime burden of illness | 10 |
| c. This intervention is the first to offer any improvement for patients with this condition. | 0 |
| d. Compared to on-demand treatment only, there is significant uncertainty about the long-term risk of serious side effects of using C1-INHs. | 5 |
| e. Compared to on-demand treatment only, there is significant uncertainty about the long-term risk of serious side effects of using lanadelumab. | 14 |
| f. Compared to on-demand treatment only, there is significant uncertainty about the magnitude or durability of the long-term benefits of using C1-INHs. | 9 |
| g. Compared to on-demand treatment only, there is significant uncertainty about the e magnitude or durability of the long-term benefits of using lanadelumab. | 15 |
| h. There are additional contextual considerations that should have an important role in judgments of the value of this intervention. | 0 |
aFifteen CTAF panelists voted.
C1-INH = C1 inhibiter; CTAF = California Technology Assessment Forum; HAE = hereditary angioedema.
The culminating vote of the CTAF panel, intended to reflect the members’ integration of the elements of the value assessment framework, was on the “long-term value for money” of Cinryze, Haegarda, and Takhzyro. Voting categories for long-term value for money are “low,” “intermediate,” or “high.” The 15 CTAF panel members voted that, for patients with HAE 1/2, the long-term value for money of prophylaxis compared with on-demand therapy alone for Cinryze was 14 (low) and 1 (intermediate); for Haegarda the long-term value votes were 7 (low), 7 (intermediate), and 1 (high); and for Takhzyro the votes were 13 (low), and 2 (intermediate).
The policy roundtable discussion explored how best to translate the evidence and broader perspectives discussed into clinical practice and into pricing and insurance coverage policies. The full set of policy recommendations can be found in the final evidence report; however, the key policy recommendations are as follows:
Payers seeking to negotiate better prices may consider giving all market share to the 2 treatments administered subcutaneously, Haegarda and Takhzyro, due to the simpler administration of these therapies compared with intravenous drugs.
Prior authorization criteria should be based on clinical evidence with input from clinical experts and patient groups. Suggestions for elements of coverage criteria include confirmation of HAE through lab tests or physician attestation, determination of the appropriateness of long-term prophylaxis based on the frequency and severity of attacks, and use of a patient’s weight to more precisely manage dosing of weight-based treatments. Specific coverage criteria options are described in greater detail in the final evidence report.
Given that the cost-effectiveness of Takhzyro can be vastly improved by switching the dosing for attack-free patients from every 2 weeks to every 4 weeks, payers should work with clinicians to encourage trial periods of the less frequent dosing if patients are attack-free after 6 months of therapy.
Conclusions
For patients with HAE 1/2, prophylaxis with Cinryze, Haegarda, and Takhzyro showed significant clinical benefits by reducing the number and severity of HAE attacks, without significant adverse events when compared with no long-term prophylaxis. However, the data were not sufficient to distinguish any drug as clinically superior to the other. In the absence of long-term safety data for lanadelumab-flyo, which targets a different pathway than the C1-INH products, we are less certain about its overall net health benefit. In the base case and at current pricing, all 3 drugs were judged to represent low or intermediate long-term value for money. However, these findings were highly influenced by the baseline attack rate, with improved cost-effectiveness for a population with a higher mean attack rate per month and by the cost of treating patients with on-demand therapy, with higher on-demand treatment costs leading to improved cost-effectiveness for prophylaxis. Further efforts are needed to help align the price of these treatments with their demonstrated benefit in order to ensure sustainable access to high-value care for all patients.
Acknowledgments
The authors thank David Rind for his contributions to the ICER’s HAE CTAF report.
References
- 1.Zuraw BL. Clinical practice. Hereditary angioedema. New Engl J Med. 2008;359(10):1027-36. [DOI] [PubMed] [Google Scholar]
- 2.Germenis AE, Speletas M. Genetics of hereditary angioedema revisited. Clin Rev Allergy Immunol. 2016;51(2):170-82. [DOI] [PubMed] [Google Scholar]
- 3.Henry Li H, Riedl M, Kashkin J. Update on the use of C1-esterase inhibitor replacement therapy in the acute and prophylactic treatment of hereditary angioedema. Clin Rev Allergy Immunol. June 16, 2018. [Epub ahead of print]. Available at: https://link.springer.com/article/10.1007%2Fs12016-018-8684-1. Accessed January 15, 2019. [DOI] [PubMed]
- 4.Lumry WR. Overview of epidemiology, pathophysiology, and disease progression in hereditary angioedema. Am J Manag Care. 2013;19(7 Suppl):s103-10. [PubMed] [Google Scholar]
- 5.Bygum A. Hereditary angio-oedema in Denmark: a nationwide survey. Br J Dermatol. 2009;161(5):1153-58. [DOI] [PubMed] [Google Scholar]
- 6.Bork K, Meng G, Staubach P, Hardt J. Hereditary angioedema: new findings concerning symptoms, affected organs, and course. Am J Med. 2006;119(3):267-74. [DOI] [PubMed] [Google Scholar]
- 7.Cicardi M, Bork K, Caballero T, et al. Evidence-based recommendations for the therapeutic management of angioedema owing to hereditary C1 inhibitor deficiency: consensus report of an international working group. Allergy. 2012;67(2):147-57. [DOI] [PubMed] [Google Scholar]
- 8.Lin GA, Lubinga S, Agboola F, Carlson J, et al. Prophylaxis for hereditary angioedema with lanadelumab and C1 inhibitors: effectiveness and value. Final evidence report. Institute for Clinical and Economic Review. November 15, 2018. Available at: https://icer-review.org/material/angioedema-final-report/. Accessed January 15, 2019.
- 9.Zuraw BL, Busse PJ, White M, et al. Nanofiltered C1 inhibitor concentrate for treatment of hereditary angioedema. N Engl J Med. 2010;363(6):513-22. [DOI] [PubMed] [Google Scholar]
- 10.Aygören-Pürsün E, Soteres D, Moldovan D, et al. Preventing hereditary angioedema attacks in children using Cinryze: interim efficacy and safety phase 3 findings. Int Arch Allergy Immunol. 2017;173(2):114-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zuraw BL, Kalfus I. Safety and efficacy of prophylactic nanofiltered C1-inhibitor in hereditary angioedema. Am J Med. 2012;125(9):938.e1-7. [DOI] [PubMed] [Google Scholar]
- 12.Longhurst H, Cicardi M, Craig T, et al. Prevention of hereditary angioedema attacks with a subcutaneous C1 inhibitor. N Engl J Med. 2017;376(12):1131-40. [DOI] [PubMed] [Google Scholar]
- 13.Lumry WR, Miller DP, Newcomer S, Fitts D, Dayno J. Quality of life in patients with hereditary angioedema receiving therapy for routine prevention of attacks. Allergy Asthma Proc. 2014;35(5):371-76. [DOI] [PubMed] [Google Scholar]
- 14.Banerji A, Riedl M, Bernstein J, et al. Lanadelumab for prevention of attacks in hereditary angioedema: results from the phase 3 help study. Ann Allergy Asthma Immunol. 2017;119(5):S5. [Google Scholar]
- 15.Pearson SD. The ICER value framework: integrating cost effectiveness and affordability in the assessment of health care value. Value Health. 2018;21(3):258-65. [DOI] [PubMed] [Google Scholar]
- 16.Institute for Clinical and Economic Review . ICER value assessment framework. Available at: https://icer-review.org/methodology/icers-methods/icer-value-assessment-framework/. Accessed January 15, 2019.