Abstract
BACKGROUND:
Given the availability of a number of alternative biologic treatment options and other novel disease-modifying antirheumatic drugs (DMARDs) for the treatment of patients with rheumatoid arthritis (RA), clinicians are faced with an increasingly challenging choice regarding optimal treatment. Biologics are usually combined with traditional DMARDs, primarily methotrexate (MTX), but some biologics and tofacitinib (together referred to in this article as novel DMARDs) have been shown to be efficacious as monotherapy as well. In real-world practice, approximately one-third of RA patients receiving biologics are on monotherapy, primarily because of intolerance of, or noncompliance with, MTX. Limited data, however, are available analyzing the effectiveness of monotherapy compared with combination therapy across novel DMARDs.
OBJECTIVE:
To compare American College of Rheumatology (ACR) responses to approved novel DMARDs used as monotherapy or as combination therapy with methotrexate (MTX) at 24 weeks in RA patients who have shown inadequate response to conventional DMARDs (DMARD-IR).
METHODS:
Through a systematic review of the literature, we identified randomized controlled trials that assessed approved novel DMARDs used as monotherapy or as combination therapy with MTX in DMARD-IR RA patients. Twenty-eight RCTs were identified that evaluated abatacept, anakinra, adalimumab, certolizumab pegol, etanercept, golimumab, infliximab, tocilizumab, or tofacitinib. ACR responses at 24 weeks were extracted and combined by means of Bayesian network meta-analyses.
RESULTS:
With the exception of anakinra plus MTX, which was less efficacious, most novel DMARDs, when used in combination with MTX, demonstrated comparable ACR responses. When novel DMARDs were used as monotherapies, greater ACR20/50/70 responses were observed with tocilizumab than with anti-tumor necrosis factor agents (aTNF) or tofacitinib. Furthermore, ACR20/50/70 responses with tocilizumab plus MTX were similar to those with tocilizumab monotherapy (odds ratio [OR] for the indirect comparison = 1.08, 95% credible interval [CrI] = 0.40-2.84; OR = 1.24, CrI = 0.44-3.61; OR = 0.95, CrI = 0.33-2.72, respectively), whereas greater responses were observed with aTNF plus MTX than with aTNF monotherapy (OR = 2.41, CrI = 0.51-11.61; OR = 2.85, CrI = 0.51-17.67; OR = 1.28, CrI = 0.21-8.42, respectively). Relative efficacy estimates for the indirect comparison of tofacitinib plus MTX with tofacitinib monotherapy were very uncertain.
CONCLUSIONS:
Results suggest that in combination with MTX most of the available novel DMARDs have similar levels of efficacy in DMARD-IR patients. As monotherapy, however, tocilizumab displayed higher ACR responses than aTNF or tofacitinib. ACR responses with tocilizumab plus MTX were similar to those with tocilizumab as monotherapy, whereas aTNF in combination with MTX demonstrated greater ACR responses than aTNF as monotherapy.
What is already known about this subject
Biologics and tofacitinib—referred to as novel disease-modifying antirheumatic drugs (DMARDs)— are usually combined with traditional DMARDs, primarily methotrexate (MTX). In realworld practice, however, approximately one-third of rheumatoid arthritis (RA) patients receiving biologics are on monotherapy mainly because of intolerance of, or noncompliance with, MTX.
In the past few years, several network meta-analyses of novel DMARDs for RA have been published. Most network meta-analyses have shown that in combination therapy, the efficacy of most novel DMARDs is comparable; this is confirmed by the current analysis. Comparisons of the efficacy of novel DMARDs as monotherapy and comparisons of monotherapy with combination therapy, however, are rare and none include all currently approved treatments.
What this study adds
Contrary to several earlier published network meta-analyses, this study did not group patients with inadequate response to conventional DMARDs (DMARD-IR) with DMARD-naive or biologic-experienced patients. Additionally, monotherapy and combination therapies were considered different regimens, and all currently approved novel DMARDs were included in the analysis.
Agents in combination with MTX and agents as monotherapy were evaluated simultaneously as part of a single network and could, therefore, also be indirectly compared (monotherapy vs. combination therapy).
Patients with rheumatoid arthritis (RA), a chronic inflammatory joint disorder, experience alternating episodes of joint stiffness, swelling, and pain. Without treatment, most patients become severely disabled over time. The primary goal of treating patients with RA is to maximize long-term health-related quality of life through control of symptoms, prevention of structural damage, normalization of function, and social participation. Amelioration of inflammation is the most important way to achieve these goals.1-3
According to American College of Rheumatology (ACR) and European League Against Rheumatism recommendations for the management of RA, treatment should begin with the use (alone or in combination) of traditional (nonbiologic) disease-modifying antirheumatic drugs (DMARDs), most commonly methotrexate (MTX).4,5 Patients who are intolerant of, or show an inadequate response (IR) to, traditional DMARDs (DMARD-IR) are often treated with a biologic agent. Five classes of biologic agents are approved to treat patients with RA: (1) tumor necrosis factor blockers, otherwise known as anti-tumor necrosis factor agents (aTNF; etanercept, infliximab, adalimumab, certolizumab pegol, and golimumab); (2) interleukin-1 receptor antagonists (anakinra); (3) a selective T-cell costimulatory modulator (abatacept); (4) a monoclonal antibody that inhibits B cells (rituximab); and (5) a monoclonal antibody that inhibits the interleukin-6 receptor (tocilizumab). Recently, data for tofacitinib, an oral Janus kinase inhibitor, have also become available. For DMARD-IR patients, biologics are usually combined with traditional DMARDs, primarily MTX, but some biologics and tofacitinib (together referred to in this article as novel DMARDs) have been shown to be efficacious as monotherapy as well.6-8 In real-world practice, approximately one-third of RA patients receiving biologics are on monotherapy mainly because of intolerance of, or noncompliance with, MTX.9-11 Side effects (primarily gastrointestinal symptoms, respiratory symptoms, and hepatotoxicity) are the main reason (> 75%) for MTX withdrawal.12
Given the availability of a number of alternative biologic treatment options and other novel DMARDs, clinicians are faced with an increasingly challenging choice regarding optimal treatment. No all-encompassing, head-to-head randomized controlled trial (RCT) has been conducted to evaluate all the different novel DMARDs to help inform medical decision making. Rather, the available evidence base consists of many placebo-controlled trials and only a limited number of head-to-head RCTs comparing not more than 2 interventions each. In general, when the available RCTs of interest do not compare the same interventions but each instead compares only a subset of the interventions of interest, it is possible to develop a network of RCTs in which all trials have at least 1 intervention in common. The results of these trials in such an evidence network can be synthesized by means of a network meta-analysis.
Network meta-analysis is a generalization of standard pairwise meta-analysis and includes multiple pairwise comparisons across a range of interventions.13,14 In addition to obtaining pooled results of multiple studies comparing the same interventions, network meta-analysis provides estimates of relative treatment effects of interventions not studied in a head-to-head fashion. In the past few years, several network meta-analyses of biologic treatments for RA have been published.15 In terms of methodology, some published network meta-analyses focus only on combination therapy (i.e., a biologic with MTX), whereas others include monotherapy and combination therapy and either ignore the effect of MTX or explicitly acknowledge the effect of MTX in a meta-regression model. None of the latter analyses, however, include all currently approved biologic agents.
The recent review by Thorlund et al. (2013) provides an overview of recently published network meta-analyses of biologic treatments in RA and discusses why findings vary.15 The authors recommended that DMARD-naïve and DMARD-experienced patients not be grouped together in an analysis.15 Similarly, patients who have previously experienced treatment failure with a biologic should not be pooled with those who are biologic-naïve. Furthermore, the authors questioned whether the concomitant use of DMARDs and MTX does, in fact, yield a modification of the relative treatment effect. Accordingly, a robust approach would consider monotherapy and combination therapy as different regimens but would still investigate their comparative effectiveness in 1 network meta-analysis to allow comparisons of monotherapy and combination therapy.15
The objective of the present study was to compare the efficacy of available novel DMARDs as monotherapy and as combination therapy in the treatment of biologic DMARD-naïve and DMARD-IR RA patients based on evidence from RCTs identified by means of a systematic literature review, providing prescribers and payers an additional piece of evidence when comparing the efficacy of RA treatment options. Indeed, given the devastating nature of RA, these patients should be administered the best treatment option first.
Methods
Identification and Selection of Studies and Data Extraction
A predefined search strategy of the MEDLINE, Embase, and Cochrane databases used terms related to RA, novel DMARDs, and RCTs to allow for a search of studies published between January 1990 and April 2013 (see Appendix A for search strategy terms). Titles and abstracts were screened to ascertain whether studies met predefined selection criteria. Studies that either met the criteria or had unclear criteria were further screened using the full text report.
The following criteria were used when considering published studies for inclusion:
Study design: RCTs.
Population of interest: biologic DMARD-naïve and DMARD-IR RA patients. Although some studies had patients from non-Western countries, populations that were exclusively non-Western were not considered to be comparable; therefore, studies conducted in these populations were excluded.
Interventions: tocilizumab (subcutaneous [SC] or intravenous [IV]), TNF blockers, abatacept (SC or IV), anakinra and tofacitinib in their usual dose, alone and in combination with conventional DMARDs. Rituximab was not considered because its label is restricted to TNF-IR patients.
Comparators: placebo or 1 of the regimens described under interventions. Comparison of different dosages of the same intervention only and comparison of the same interventions with different background treatments were excluded.
Outcomes/end points: American College of Rheumatology (ACR) response criteria.16,17
For each identified study that met the selection criteria, details were extracted on study design, study population characteristics, interventions, and number of patients with a 20% improvement in ACR criteria (ACR20 response), ACR50 response, and ACR70 response, all assessed at 24 weeks follow-up. The ACR criteria require a percentage improvement (for example, ACR20 requires 20%) in both tender and swollen joint counts and that same percentage improvement in 3 of the following 5 parameters: physician global assessment of disease, patient global assessment of disease, patient assessment of pain, C-reactive protein (or erythrocyte sedimentation rate), and degree of disability according to the Health Assessment Questionnaire–Disability Index.16,17
Network Meta-Analysis
To synthesize the results of the included studies, Bayesian network meta-analysis models were used.13,14,18,19 For the analysis, we grouped the different aTNFs. Additionally, because head-to-head comparisons of SC and IV administration of individual therapies did not show any meaningful difference, these modes of delivery were grouped for each of these therapies.20,21 Within a Bayesian framework, analysis involves data, a likelihood distribution, a model with parameters, and prior distributions for these parameters.22 A logistic regression model with a binomial likelihood relates the data from the individual studies to basic parameters reflecting the (pooled) treatment effect of each intervention compared with placebo. Based on these basic parameters, the relative efficacy was calculated between each of the interventions as monotherapy and as combination therapy.
Both fixed and random effects models were considered and were compared regarding the goodness-of-fit to the data, calculated as the posterior mean residual deviance. The deviance information criterion (DIC) provides a measure of model fit that penalizes model complexity.23 In general, a more complex model will result in a better fit to the data, demonstrating a smaller residual deviance. The model with the lowest DIC is the model providing the “best” fit to the data adjusted for the number of parameters. The random effects model resulted in the lowest DIC for ACR20 and ACR50 and was considered appropriate for the synthesis of the available evidence. Regarding ACR70, the DICs of the fixed and random effects models were similar. Despite the lack of strong evidence against the fixed effects model on statistical grounds, we preferred using the random effects model for ACR70 to be consistent with the ACR20 and ACR50 analyses. In addition, the random effects model can be considered more plausible because it assumes that the studies included in the analysis are clinically and methodologically diverse, and it addresses associated between-study heterogeneity in treatment effects. This is especially relevant for the current analysis because we compare different drug classes and pool different aTNFs.
To avoid the influence of prior distributions required for Bayesian analyses on the results, noninformative prior distributions were used. Prior distributions of the treatment effects (i.e., the log-odds ratio of ACR response) were normal distributions with a mean of 0 and a variance of 10,000. A uniform distribution with range of 0 to 2 was used for the prior distribution of heterogeneity needed for the random effects analyses. WinBUGS statistical software was used for the analyses.24 Results of the network meta-analysis provided us with posterior distributions of relative treatment effects of each treatment compared with another in terms of odds ratio (OR). To transform the OR into an expected ACR response, the OR of each regimen compared with placebo was combined with the average estimate of the odds of response with placebo across studies. Posterior distribution of OR and the expected response were summarized with the median to represent the most likely estimate and the 2.5th and 97.5th percentiles reflecting each 95% credible interval (95% CrI). Unlike 95% confidence intervals obtained with frequentist analysis that show that with repeated analyses the calculated confidence interval would contain the true estimate 95% of the time, the 95% CrI can be interpreted in terms of probability. The 95% CrI reflects with 95% probability that the true OR or the expected response would fall between the boundaries of the 95% CrI. Given the posterior distributions of relative treatment effects corresponding to the different comparisons obtained, we were also able to calculate the probability that a certain intervention was more efficacious than a competitor intervention.
Results
Study Identification
The literature search resulted in 2,635 potentially relevant citations; abstract review excluded 2,515 (95%; Figure 1). Of the remaining 120 retrieved full-text publications, 74 (3%) were excluded through the full-text review, leaving 46 publications plus 2 additional publications identified through bibliography searching, corresponding to 35 different RCTs that met the selection criteria.
FIGURE 1.
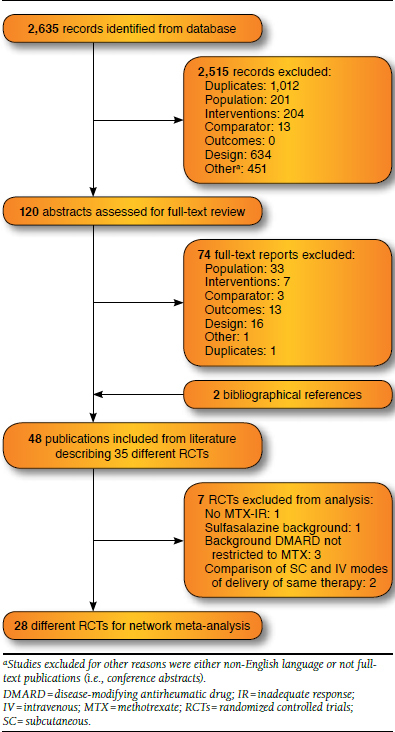
Flow Diagram of Study Identification and Selection
Evidence Base
All studies were double-blind, parallel RCTs. Most of the trials (28/35) were explicitly reported as having been conducted at multiple centers and included patients predominantly from Europe and North America. Some of these studies were also reported to include patients from South America (7 studies) and Asia (3 studies).
Most studies included adult patients with diagnoses of RA based on the 1987 revised ACR classification criteria, with active disease in spite of previous treatment with traditional DMARDs, including MTX. The TEMPO trial included patients who were DMARD-IR but disqualified patients for whom MTX treatment failed.25 Definitions of active disease varied around the minimum levels of erythrocyte sedimentation rate (ESR; 10 millimeters per hour [mm/h], 28 mm/h) and C-reactive protein (CRP; 2 milligrams per deciliter [mg/dL], 1 mg/dL, 1.5 mg/dL, 7 milligrams per milliliter [mg/mL]) and around the minimum number of required tender (6-12) and swollen (6-10) joints. Not all studies reported whether RA disease duration and DMARD treatment duration were eligibility criteria.
In almost all RCTs evaluating the efficacy of biologics in combination with a traditional DMARD (29 studies), MTX was the background treatment of choice (25/29 studies). The exception was the study by Combe et al. (2006, 2009) in which sulfasalazine was used.26,27 Three studies allowed multiple DMARDs as background therapy.
To achieve a group of studies sufficiently comparable to allow for indirect comparison of ACR responses at 24 weeks, 7 of the 35 identified studies were excluded (Klareskog et al., 2004 [TEMPO, MTX failures excluded],25 Combe et al. [sulfasalazine background treatment],26,27 3 studies that allowed background DMARD therapy with multiple DMARDs,28-30 and 2 studies that compared IV and SC modes of delivery of the same treatment20,21). Given that modes of delivery of each therapy were grouped for the purpose of analysis, these studies could not contribute to the network.
Table 1 provides information on the characteristics of the patients included in the 28 RCTs.7,31-54 Mean age in the study arms ranged from 42 to 57 years. Female patients were predominant; the participation of women in the study arms ranged from 66% to 90%. Average disease duration ranged from 1.7 to 13 years. Rheumatoid factor positivity ranged from 67% to 100%; ESR ranged from 24.4 to 56.1 mm/h; average swollen and tender joint counts (66/68 count) ranged from 11.3 to 25 and from 15.9 to 35.5, respectively. In Figure 2, the network of the 28 RCTs is presented such that each line between nodes reflects the available direct comparisons The right side of the network, which concerns biologics in combination with MTX, shows multiple connections between the different interventions. The left side of the network, which concerns interventions as monotherapy, consists of 1 path with a limited number of studies for each edge. This means that any comparison between interventions on the left side of the network and interventions on the right side will be uncertain.
TABLE 1.
Study and Patient Baseline Characteristics Grouped by Intervention
| Study | Interventions | Patients (n) | Mean Age (years) | Female (%) | Mean Disease Duration (years) | SJC | TJC | ESR (mm/h) | CRP (mg/L) | RF + (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| Kremer 200337 | ABT 10 mg/kg Q4W + MTX | 115 | 56 | 75 | 10 | 21.3 | 30.8 | NR | 29 | 90 |
| Placebo + MTX | 119 | 55 | 66 | 9 | 21.8 | 29.2 | NR | 32 | 90 | |
| Kremer 200638 | ABT 10 mg/kg Q4W + MTX | 433 | 52 | 78 | 9 | 21.4 | 31 | NR | 33 | 82 |
| Placebo + MTX | 219 | 50 | 82 | 9 | 22.1 | 32.3 | NR | 28 | 79 | |
| Kay 200833 | GLB 50 mg Q4W + MTX | 35 | 57M | 86 | 8 | NR | NR | NR | 21M | NR |
| Placebo + MTX | 35 | 52M | 74 | 6 | NR | NR | NR | 20M | NR | |
| Keystone 200934 (GO-FORWARD) | GLB 50 mg Q4W + MTX | 89 | 52M | 81 | 4.5M | 13M | 26M | NR | 10M | 81 |
| Placebo + MTX | 133 | 52M | 82 | 6.5M | 12M | 21M | NR | 8M | 81 | |
| Maini 199939 (ATTRACT) | IFX 3 mg/kg Q8W + MTX | 86 | 54 | 81 | 10 | 22 | 32 | 49 | 39 | 84 |
| Placebo + MTX | 88 | 51 | 80 | 11 | 21 | 31 | 49 | 40 | 77 | |
| Westhovens 200646 (START) | IFX 3 mg/kg Q8W + MTX | 360 | 53 | 80 | 8 | 15 | 22 | NR | 16 | 83 |
| Placebo + MTX | 363 | 42 | 83 | 8 | 15 | 22 | NR | 12 | 81 | |
| Schiff 200840 (ATTEST) | ABT 10 mg/kg Q4W + MTX | 156 | 49 | 83 | 8 | 21.3 | 31.6 | 49.4 | 31 | 87 |
| IFX 3 mg/kg Q8W + MTX | 165 | 49 | 87 | 8 | 20.1 | 30.3 | 47 | 27 | 77 | |
| Placebo + MTX | 110 | 49 | 82 | 7 | 20.3 | 31.7 | 47.8 | 33 | 85 | |
| Cohen 200455 | ANA 100 mg QD + MTX | 250 | 56 | 79 | 11 | 20.1 | 26.8 | 41.5 | 27 | 76 |
| Placebo + MTX | 251 | 57 | 75 | 10 | 20 | 24.5 | 42.9 | 26 | 78 | |
| Keystone 200435 | ADA 40 mg Q2W + MTX | 207 | 56 | 76 | 11 | 19.3 | 27.3 | NR | 18 | 82 |
| Placebo + MTX | 200 | 56 | 73 | 11 | 19 | 28.1 | NR | 18 | 90 | |
| Weinblatt 200345 (ARMADA) | ADA 40 mg Q2W + MTX | 67 | 57 | 75 | 12 | 17.3 | 28 | NR | 21 | NR |
| Placebo + MTX | 62 | 56 | 82 | 11 | 16.9 | 28.7 | NR | 31 | NR | |
| van Vollenhoven 201148 (AUGUST-2) | ADA 40 mg Q2W + MTX | 79 | 53 | 81 | 8.8 | 16.2 | 27.8 | 41.7 | 16.6 | 81 |
| Placebo + MTX | 76 | 54 | 84 | 8.4 | 16.4 | 24.3 | 39.3 | 16.5 | 83 | |
| van de Putte 20048 | ADA 40 mg Q2W | 113 | 53 | 80 | 11 | 20.5 | 33.7 | 55.8 | 52.6 | 80 |
| Placebo | 110 | 54 | 77 | 12 | 19.8 | 35.5 | 56.1 | 57 | 82 | |
| Weinblatt 201349 (AMPLE) | ABT 125 mg QW SC + MTX | 318 | 51.4 | 81.4 | 1.9 | 15.8 | 25.4 | NR | 16 | 75.5 |
| ADA 40 mg Q2W + MTX | 328 | 51 | 82.3 | 1.7 | 15.9 | 26.3 | NR | 15 | 77.4 | |
| Keystone 200836 (RAPID 1) | CTZ 200 mg Q2W + MTX | 393 | 51 | 82 | 6 | 9.9M | 12.4M | 43.5M | 16M | 80 |
| Placebo + MTX | 199 | 52 | 84 | 6 | 9.7M | 13M | 45M | 16M | 83 | |
| Smolen 200941 (RAPID 2) | CTZ 200 mg Q2W + MTX | 246 | 52 | 84 | 6 | 20.5 | 30.1 | 43.7 | 14.2 | 78 |
| Placebo + MTX | 127 | 52 | 84 | 6 | 21.9 | 30.4 | 40.8 | 13.5 | 78 | |
| Choy 201250 | CTZ 400 mg Q4W + MTX | 126 | 53 | 72.2 | 9.4 | 22.8 | 29 | 24.4 | 11.9 | 73.8 |
| Placebo + MTX | 121 | 55.6 | 66.1 | 9.9 | 22.2 | 31 | 25.9 | 13.1 | 78.5 | |
| Fleischmann 200931 (FAST4WARD) | CTZ 400 mg Q4W | 111 | 53 | 78 | 9 | 21.2 | 29.6 | 30.9 | 11.6 | 100 |
| Placebo | 109 | 55 | 89 | 10 | 19.9 | 28.3 | 35.6 | 11.3 | 100 | |
| Weinblatt 199944 | ETN 25 mg BW + MTX | 59 | 48 | 90 | 13 | 20 | 28 | 25 | 22 | 84 |
| Placebo + MTX | 30 | 53 | 73 | 13 | 17 | 28 | 36 | 26 | 90 | |
| Moreland 19997 | ETN 25 mg BW | 78 | 53 | 74 | 11 | 25 | 33 | 35 | 47 | 79 |
| Placebo | 80 | 51 | 76 | 12 | 25 | 35 | 39 | 41 | 79 | |
| Kremer 201251 | TOF 5 mg BID + MTX | 71 | 52 | 80 | 9.0 | 14.1 | 21.5 | NR | 18 | 82.8 |
| Placebo | 69 | 53 | 81 | 9.2 | 15.7 | 21.6 | NR | 19 | 83 | |
| van der Heijde 201343 | TOF 5 mg BID + MTX | 321 | 53.7 | 83.8 | 8.9 | 14.1 | 24.1 | 50.1 | 15.5 | 75.2 |
| Placebo + MTX | NR | NR | NR | NR | NR | NR | NR | NR | NR | |
| van Vollenhoven 201252 | TOF 5 mg BID + MTX | 204 | 53 | 85 | 7.6 | 16.7 | 28.5 | 48.6 | 15 | 66.8 |
| ADA + MTX | 204 | 52.5 | 79 | 8.1 | 16.4 | 26.7 | 48.5 | 18 | 68.2 | |
| Placebo + MTX | 108 | NR | NR | NR | NR | NR | NR | NR | NR | |
| Fleischmann 201228 | TOF 5 mg BID | 49 | 54 | 87.8 | 8.1 | 17.4 | 27.1 | 47.4 | 24.5 | 77.5 |
| Placebo | 59 | 53 | 88.1 | 10.8 | 16.9 | 25.9 | 46.2 | 23.5 | 74.5 | |
| Fleischmann 201253 (ORAL SOLO) | TOF 5 mg BID | 243 | 52.2 | 85.2 | 8.0 | 16.3 | 29.4 | 53.1 | 22.9 | |
| Placebo | 122 | 49.7 | 86.1 | 7.7 | 17.3 | 28.9 | 50.9 | 17.8 | 68 | |
| Smolen 200842 (OPTION) | TCZ 8 mg/kg Q4W + MTX | 205 | 51 | NR | 8 | 19.5 | 31.9 | 51.2 | 26 | 83 |
| Placebo + MTX | 204 | 51 | NR | 8 | 20.7 | 32.8 | 49.7 | 24 | 71 | |
| Kremer 201147 (LITHE) | TCZ 8 mg/kg Q4W + MTX | 398 | 53.4 | 82 | 9.3 | 17.3 | 29.3 | 46.4 | 23 | NR |
| TCZ 4 mg/kg Q4W + MTX | ||||||||||
| Placebo + MTX | 393 | 51.3 | 83 | 9 | 16.6 | 27.9 | 46.5 | 22 | NR | |
| Dougados 201354 (ACT-RAY) | TCZ 8 mg/kg | 277 | 53 | 81.9 | 8.2 | 14.4 | 25.8 | 39.9 | NR | NR |
| Q4W + MTX | ||||||||||
| TCZ 8 mg/kg Q4W | 276 | 53.6 | 78.6 | 8.3 | 15.3 | 26.6 | 39.6 | NR | NR | |
| Gabay 201332 (ADACTA) | TCZ 8 mg/kg | 163 | 54.4 | 79 | 7.3 | 11.3 | 15.9 | 50.5 | 26 | NR |
| ADA 40 mg | 162 | 53.3 | 82 | 6.3 | 12.4 | 16.5 | 45.5 | 25 | NR |
ABT = abatacept; ADA = adalimumab; ANA = anakinra; BID = twice a day; BW = body weight; CRP = C-reactive protein; CTZ = certolizumab; ESR = erythrocyte sedimenation rate; ETN = etanercept; GLB = golimumab; IFX = infliximab; M = median; mg = milligram; mg/kg = milligram per kilogram; mg/L = milligram per liter; mm/h = millimeters per hour; MTX = methotrexate; NR = not reported; QD = every day; QW = every week; Q2W = every 2 weeks; Q4W = every 4 weeks; Q8W = every 8 weeks; RF = rheumatoid factor; SC = subcutaneous; SJC = swollen joint count; TCZ = tocilizumab; TJC = tender joint count; TOF = tofacitinib.
FIGURE 2.
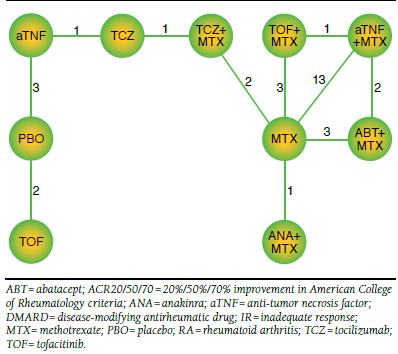
Network of RCTs Evaluating Agents for DMARD-IR RA Patients in Terms of ACR20/50/70 Response at 24 Weeks
Network meta-analysis allows a treatment effect of an intervention compared with another intervention in the same network to be obtained. Despite some variation in patient characteristics across studies (e.g., duration of disease), no differences beyond what can be expected due to chance were observed across the different types of comparisons, indicating the feasibility of the network meta-analysis. ACR responses at 24 weeks by study are provided in Table 2.7,8,31-55
TABLE 2.
Proportions of ACR20/50/70 Responders at 24 Weeks with Treatments Reported in Individual Studies Used for Network Meta-Analysis
| Placebo | Placebo + MTX | ADA | CTZ | ETN | TOF | TCZ | ABT + MTX | ANA + MTX | GLB + MTX | IFX + MTX | ADA + MTX | CTZ + MTX | ETN + MTX | TOF + MTX | TCZ + MTX | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| van de Putte 20048 | 0.19 | 0.46 | ||||||||||||||
| 0.08 | 0.22 | |||||||||||||||
| 0.02 | 0.12 | |||||||||||||||
| Fleischmann 200931 | 0.09 | 0.46 | ||||||||||||||
| 0.04 | 0.23 | |||||||||||||||
| 0.00 | 0.06 | |||||||||||||||
| Moreland 19997 | 0.13 | 0.59 | ||||||||||||||
| 0.05 | 0.40 | |||||||||||||||
| 0.10 | 0.15 | |||||||||||||||
| Fleischmann 201228 | 0.25 | 0.51 | ||||||||||||||
| 0.10 | 0.35 | |||||||||||||||
| 0.07 | 0.20 | |||||||||||||||
| Fleischmann 201253 | 0.27 | 0.70 | ||||||||||||||
| Gabay 201332 | 0.49 | 0.65 | ||||||||||||||
| 0.28 | 0.47 | |||||||||||||||
| 0.18 | 0.33 | |||||||||||||||
| Kremer 200337 | 0.35 | 0.60 | ||||||||||||||
| 0.12 | 0.37 | |||||||||||||||
| 0.02 | 0.17 | |||||||||||||||
| Kremer 200638 | 0.40 | 0.68 | ||||||||||||||
| 0.18 | 0.38 | |||||||||||||||
| 0.07 | 0.20 | |||||||||||||||
| Schiff 200840 | 0.42 | 0.67 | 0.59 | |||||||||||||
| 0.20 | 0.40 | 0.37 | ||||||||||||||
| 0.09 | 0.21 | 0.24 | ||||||||||||||
| Cohen 200455 | 0.22 | 0.38 | ||||||||||||||
| 0.08 | 0.17 | |||||||||||||||
| 0.02 | 0.06 | |||||||||||||||
| Kay 200833 | 0.37 | 0.60 | ||||||||||||||
| 0.06 | 0.37 | |||||||||||||||
| 0.00 | 0.09 | |||||||||||||||
| Keystone 200934 | 0.28 | 0.60 | ||||||||||||||
| 0.14 | 0.37 | |||||||||||||||
| 0.05 | 0.20 | |||||||||||||||
| Maini 199939 | 0.20 | 0.52 | ||||||||||||||
| 0.05 | 0.27 | |||||||||||||||
| 0.00 | 0.08 | |||||||||||||||
| Westhovens 200646 | 0.24 | 0.55 | ||||||||||||||
| 0.09 | 0.31 | |||||||||||||||
| 0.04 | 0.13 | |||||||||||||||
| Keystone 200435 | 0.30 | 0.63 | ||||||||||||||
| 0.10 | 0.39 | |||||||||||||||
| 0.03 | 0.21 | |||||||||||||||
| Weinblatt 200345 | 0.15 | 0.67 | ||||||||||||||
| 0.08 | 0.55 | |||||||||||||||
| 0.05 | 0.27 | |||||||||||||||
| van Vollenhoven 201148 | 0.46 | 0.71 | ||||||||||||||
| 0.15 | 0.38 | |||||||||||||||
| 0.05 | 0.18 | |||||||||||||||
| Weinblatt 201349 | 0.66 | 0.65 | ||||||||||||||
| 0.46 | 0.43 | |||||||||||||||
| 0.24 | 0.22 | |||||||||||||||
| Keystone 200836 | 0.14 | 0.59 | ||||||||||||||
| 0.08 | 0.37 | |||||||||||||||
| 0.03 | 0.21 | |||||||||||||||
| Smolen 200941 | 0.09 | 0.57 | ||||||||||||||
| 0.03 | 0.33 | |||||||||||||||
| 0.01 | 0.16 | |||||||||||||||
| Choy 201250 | 0.23 | 0.46 | ||||||||||||||
| 0.06 | 0.18 | |||||||||||||||
| 0.02 | 0.00 | |||||||||||||||
| Weinblatt 199944 | 0.27 | 0.71 | ||||||||||||||
| 0.03 | 0.39 | |||||||||||||||
| 0.00 | 0.15 | |||||||||||||||
| Kremer 201251 | 0.34 | 0.48 | ||||||||||||||
| 0.23 | 0.34 | |||||||||||||||
| 0.07 | 0.20 | |||||||||||||||
| van der Heijde 201343 | 0.25 | 0.52 | ||||||||||||||
| 0.09 | 0.32 | |||||||||||||||
| 0.02 | 0.16 | |||||||||||||||
| van Vollenhoven 201252 | 0.28 | 0.47 | 0.52 | |||||||||||||
| 0.28 | 0.37 | |||||||||||||||
| 0.09 | 0.20 | |||||||||||||||
| Smolen 200842 | 0.26 | 0.59 | ||||||||||||||
| 0.11 | 0.44 | |||||||||||||||
| 0.02 | 0.22 | |||||||||||||||
| Kremer 201147 | 0.27 | 0.57 | ||||||||||||||
| 0.10 | 0.32 | |||||||||||||||
| 0.02 | 0.12 | |||||||||||||||
| Dougados 201354 | 0.70 | 0.72 | ||||||||||||||
| 0.40 | 0.46 | |||||||||||||||
| 0.25 | 0.25 |
ABT = abatacept; ACR20/50/70 = 20%/50%/70% improvement in American College of Rheumatology criteria; ADA = adalimumab; ANA = anakinra; CTZ = certolizumab; ETN = etanercept; GLB = golimumab; IFX = infliximab; MTX = methotrexate; TCZ = tocilizumab; TOF = tofacitinib.
Monotherapy
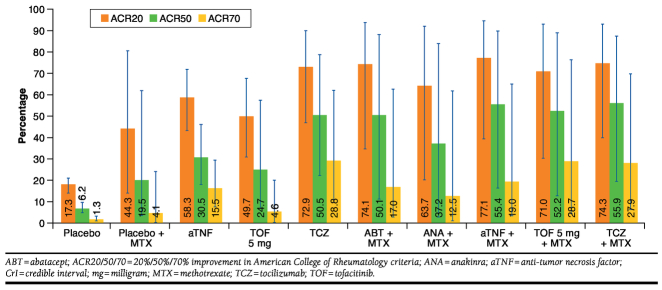
Table 3 presents the results of the network meta-analysis. Each cell presents the OR of response at 24 weeks with the intervention (in the rows) relative to a comparator (in the column). Both aTNF and tocilizumab as monotherapy showed greater ACR20 response than placebo, with ORs of 6.67 and 12.89, respectively (see Table 3, under ACR20, where the tocilizumab row and the aTNF row intersect with the placebo column). Consistent with these estimates, the OR of tocilizumab relative to aTNF monotherapy obtained with the network meta-analysis equals 1.94 (95% CrI = 0.71-5.36; Table 3). Although this difference was not thought to be statistically significant because the 95% CrI includes the 1, this estimate still corresponds to a 91% probability that tocilizumab as monotherapy would result in a greater ACR20 response than aTNF as monotherapy. For ACR50 and ACR70 responses, similar findings were observed with ORs of 6.65 and 14.00 for aTNF relative to placebo and ORs of 15.51 and 31.19 for tocilizumab relative to placebo (Appendices B and C). The ORs of tocilizumab relative to aTNF were 2.34 and 2.22 for ACR50 and ACR70, respectively. Although this is not statistically relevant at a 95% level, it still indicates a greater than 93% chance that tocilizumab results in greater responses than aTNF. Tofacitinib showed greater ACR response than placebo (OR = 4.72/4.98/3.76 for ACR20/50/70, respectively). Figure 3 shows the expected ACR20/50/70 responses for each treatment, calculated by combining the ORs of each intervention compared with placebo obtained with the network meta-analysis and the average placebo response across all trials. This is a more tangible way of illustrating the findings, representing the most likely ACR responses at 24 weeks for all treatment options in the network as if they had been compared in a huge head-to-head study. However, we would like to stress that this figure cannot be used to identify “significant” differences between interventions because the whiskers concern both the uncertainty about the relative efficacy measures as presented in Table 3 and Appendices B and C and the uncertainty about the overall placebo response. The whiskers indicate the uncertainty in the expected ACR20/50/70 responses. To compare the efficacy of the different interventions, the ORs, along with the uncertainty measures presented in Table 3 and Appendices B and C, must be used.
TABLE 3.
Treatment Effects for All Contrasts in Terms of OR of ACR20 Response Along with 95% CrI and Probability That Treatment Is Better Than Comparator
| Intervention | Comparator | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Placebo | Placebo + MTX | aTNF | TOF 5 mg | TCZ | ABT + MTX | ANA + MTX | aTNF + MTX | TOF 5 mg + MTX | TCZ + MTX | ||
| Placebo | OR (95% CrI) |
1 (1, 1) |
0.26 (0.05, 1.34) |
0.15 (0.08, 0.29) |
0.21 (0.10, 0.47) |
0.08 (0.02, 0.25) |
0.07 (0.01, 0.40) |
0.12 (0.02, 0.82) |
0.06 (0.01, 0.33) |
0.09 (0.01, 0.48) |
0.07 (0.01, 0.32) |
| P(better) | NA | 0.05 | < 0.01 | < 0.01 | < 0.01 | < 0.01 | 0.02 | < 0.01 | < 0.01 | < 0.01 | |
| Placebo + MTX | OR (95% CrI) |
3.80 (0.75, 20.53) |
1.00 (1, 1) |
0.57 (0.13, 2.68) |
0.80 (0.14, 5.40) |
0.30 (0.09, 0.97) |
0.28 (0.17, 0.46) |
0.46 (0.17, 1.24) |
0.24 (0.18, 0.32) |
0.33 (0.18, 0.59) |
0.27 (0.14, 0.55) |
| P(better) | 0.95 | NA | 0.23 | 0.40 | 0.02 | < 0.01 | 0.06 | < 0.01 | < 0.01 | < 0.01 | |
| aTNF | OR (95% CrI) |
6.67 (3.48, 13.08) |
1.75 (0.37, 7.97) |
1.00 (1, 1) |
1.41 (0.51, 4.08) |
0.52 (0.19, 1.41) |
0.48 (0.10, 2.41) |
0.79 (0.12, 5.02) |
0.41 (0.09, 1.94) |
0.57 (0.11, 2.94) |
0.48 (0.12, 1.88) |
| P(better) | > 0.99 | 0.77 | NA | 0.75 | 0.09 | 0.18 | 0.4 | 0.13 | 0.24 | 0.14 | |
| TOF 5 mg | OR (95% CrI) |
4.72 (2.11, 10.33) |
1.24 (0.19, 7.34) |
0.71 (0.24, 1.96) |
1.00 (1, 1) |
0.37 (0.08, 1.48) |
0.34 (0.05, 2.22) |
0.56 (0.07, 4.45) |
0.29 (0.04, 1.77) |
0.41 (0.06, 2.68) |
0.34 (0.06, 1.78) |
| P(better) | > 0.99 | 0.60 | 0.25 | NA | 0.08 | 0.13 | 0.29 | 0.09 | 0.17 | 0.10 | |
| TCZ | OR (95% CrI) |
12.89 (3.96, 44.39) |
3.38 (1.03, 11.17) |
1.94 (0.71, 5.36) |
2.74 (0.68, 12.11) |
1.00 (1, 1) |
0.94 (0.26, 3.43) |
1.55 (0.33, 7.54) |
0.80 (0.23, 2.74) |
1.10 (0.30, 4.22) |
0.92 (0.35, 2.48) |
| P(better) | > 0.99 | 0.98 | 0.91 | 0.92 | NA | 0.46 | 0.72 | 0.35 | 0.56 | 0.43 | |
| ABT + MTX | OR (95% CrI) |
13.72 (2.49, 79.12) |
3.60 (2.18, 5.96) |
2.06 (0.41, 10.45) |
2.91 (0.45, 20.52) |
1.06 (0.29, 3.86) |
1.00 (1, 1) |
1.64 (0.54, 5.04) |
0.85 (0.51, 1.43) |
1.17 (0.56, 2.55) |
0.99 (0.42, 2.32) |
| P(better) | > 0.99 | > 0.99 | 0.82 | 0.87 | 0.54 | NA | 0.82 | 0.26 | 0.67 | 0.49 | |
| ANA + MTX | OR (95% CrI) |
8.37 (1.22, 59.84) |
2.19 (0.80, 5.98) |
1.26 (0.20, 8.02) |
1.77 (0.22, 15.29) |
0.64 (0.13, 3.06) |
0.61 (0.20, 1.87) |
1.00 (1, 1) |
0.52 (0.18, 1.49) |
0.71 (0.22, 2.29) |
0.60 (0.18, 2.10) |
| P(better) | 0.98 | 0.94 | 0.6 | 0.71 | 0.28 | 0.18 | NA | 0.10 | 0.27 | 0.19 | |
| aTNF + MTX | OR (95% CrI) |
16.06 (3.07, 88.89) |
4.22 (3.17, 5.69) |
2.41 (0.51, 11.61) |
3.40 (0.56, 23.22) | 1.25 (0.37, 4.27) |
1.17 (0.70, 1.98) |
1.93 (0.67, 5.51) |
1.00 (1, 1) |
1.37 (0.74, 2.59) |
1.16 (0.55, 2.51) |
| P(better) | > 0.99 | > 0.99 | 0.87 | 0.91 | 0.65 | 0.74 | 0.90 | NA | 0.86 | 0.66 | |
| TOF 5 mg + MTX | OR (95% CrI) |
11.66 (2.07, 68.81) |
3.08 (1.70, 5.46) |
1.75 (0.34, 9.07) |
2.46 (0.37, 17.67) |
0.91 (0.24, 3.38) |
0.86 (0.39, 1.80) |
1.40 (0.44, 4.45) |
0.73 (0.39, 1.34) |
1.00 (1, 1) |
0.84 (0.34, 2.09) |
| P(better) | > 0.99 | > 0.99 | 0.76 | 0.83 | 0.44 | 0.33 | 0.73 | 0.14 | NA | 0.34 | |
| TCZ + MTX | estimate (95% CrI) |
13.86 (3.11, 66.90) |
3.65 (1.81, 7.36) |
2.09 (0.53, 8.35) |
2.93 (0.56, 17.34) |
1.08 (0.40, 2.84) |
1.01 (0.43, 2.41) |
1.66 (0.48, 5.66) |
0.86 (0.40, 1.83) |
1.18 (0.48, 2.97) |
1.00 (1, 1) |
| P(better) | > 0.99 | > 0.99 | 0.86 | 0.90 | 0.57 | 0.51 | 0.81 | 0.34 | 0.66 | NA | |
ABT = abatacept; ACR20 = 20% improvement in American College of Rheumatology criteria; ANA = anakinra; aTNF = anti-tumor necrosis factor; CrI = credible interval; mg = milligram; MTX = methotrexate; NA = not applicable; OR = odds ratio; P(better) = probability that treatment (in row) is showing greater response than comparator (in column); TCZ = tocilizumab; TOF = tofacitinib.
FIGURE 3.
Probability of ACR20/50/70 Response with 95% CrI for Different Classes of Biologic Treatment with and Without MTX
Combination Therapy with Methotrexate
In combination therapy with MTX, all classes of novel DMARDs demonstrated greater ACR20/50/70 responses than MTX alone in this DMARD-IR population (Table 3 and Appendices B and C). ACR20/50/70 responses with aTNF, abatacept, tocilizumab, and tofacitinib were comparable. Response with anakinra was lower than with other novel DMARDs.
Comparison Between Monotherapy and Combination Treatment with Methotrexate
There is an 87% probability that aTNF in combination with MTX results in greater ACR20 response than aTNF monotherapy (OR = 2.41, 95% CrI = 0.51-11.61). For ACR50, the probability of a higher response in combination therapy than in monotherapy was 90% (OR = 2.85, 95% CrI = 0.51-17.67). For ACR70, the probability was 63% that aTNF in combination with MTX would result in greater response than aTNF monotherapy (OR = 1.28, 95% CrI = 0.21-8.42). For tocilizumab, however, ACR20/50/70 responses with MTX were similar to ACR20/50/70 responses without MTX at 24 weeks (ORs = 1.08/1.24/0.95). Greater ACR20/50/70 responses were observed for tofacitinib in combination with MTX than for tofacitinib monotherapy, but the estimates of relative efficacy were uncertain because of the long path in the network (Figure 2) required for this indirect comparison.
Discussion
The objective of this study was to compare the efficacy of different classes of novel DMARD treatments with or without MTX in biologic DMARD-naïve and DMARD-IR RA patients based on available RCT evidence. Agents in combination with MTX and agents as monotherapy were evaluated simultaneously as part of 1 network of RCTs by means of a network meta-analysis and could therefore be indirectly compared. The results of the present analysis are in line with previously conducted network meta-analyses, although many of these are limited to combination therapy or do not include all treatments. The results also align well with those of a recent independent analysis initiated by the National Institute for Health and Care Excellence that compared RA biologics (including agents as monotherapy and agents in combination with MTX in 1 network, as in our study)56 and with another recently published network meta-analysis57 that compared patient-reported outcomes between agents as monotherapy and agents in combination with MTX using a similar approach.
The results of this analysis do suggest that an aTNF as monotherapy is likely to be less effective than an aTNF in combination with MTX. This finding is corroborated by observational studies that demonstrate greater aTNF retention rates in combination with MTX than in monotherapy.10,58-60 Tocilizumab monotherapy resulted in an ACR20/50/70 response similar to that of tocilizumab in combination with MTX. The findings of this network meta-analysis may have important clinical implications for patients who cannot tolerate or are not compliant with MTX. Indeed, in patients who require monotherapy, the analysis may suggest that tocilizumab results in a greater likelihood of good clinical response than aTNF or tofacitinib and may represent a better treatment option. However, additional randomized comparisons of aTNF or tofacitinib in combination with MTX compared with each respective monotherapy are required to validate this finding.
Limitations
The evidence of efficacy for all interventions was obtained from RCTs identified by means of a systematic literature review. We do not believe there were any unpublished primary trials that should have been consulted for this study. We came to this conclusion by comparing the identified trials with information from ClinicalTrials.gov. In general, funnel plots can help provide information regarding the presence of publication bias. However, the number of studies for each direct comparison is too small to generate meaningful funnel plots.
It is important to realize that the value of randomization holds within trials but not across trials. As such, it is possible that there are differences in study and patient characteristics across studies that are modifiers of the treatment effects. If the distribution of treatment effect modifiers is imbalanced across the different types of direct comparisons (i.e., the different edges), the indirect comparison obtained with the network meta-analysis will be biased. The longer the path concerning an indirect comparison of interest, the more we rely on the transitivity assumption and the more we trust that there are no systematic differences in treatment effect modifiers along all the edges of the path.
The studies do not show clear differences in demographics and patient characteristics regarding swollen and tender joint counts, ESR, and CRP levels, making these factors unlikely to have been sources of bias. Disease duration showed variation across studies, but we did not observe systematic differences in the distribution of disease duration across different types of direct comparisons. As such, disease duration could not have been a cause of heterogeneity (variation in true treatment effect across studies compared) but was unlikely to bias the indirect comparisons. Of course, there is always the risk of unmeasured differences in patient characteristics or other differences in contextual factors between studies that result in unmeasured confounding bias in indirect comparisons.
In this context, it is relevant to mention that placebo responses were lower in the certolizumab trials than in the other aTNF trials.36,41 Adjustment with a meta-regression model for differences in placebo response across the aTNF trials of certolizumab and other aTNFs resulted in similar ORs (analysis not shown), suggesting that the greater ORs observed in the certolizumab trials might have been attributed to the low placebo response in the trials and not to different efficacy. This finding supports our approach to consider the different aTNFs as 1 class. With the results of different aTNF trials pooled as 1 class, the heterogeneity in ORs across the different aTNF trials was acknowledged by a random effects approach. We did not adjust for differences in placebo response in the network meta-analysis because it did not result in biased indirect comparisons between the classes. That said, the responses for aTNF as a class in Figure 3 may be marginally overestimated compared with the presented placebo and MTX responses.
The indirect comparison of aTNF with and without MTX spans multiple connection nodes in the network (depicted in Figure 2). Not only does this require a greater assumption of transitivity, it also results in relative treatment effect estimates that are not very precise (i.e., relatively wide 95% CrIs). Tofacitinib monotherapy and tofacitinib in combination therapy with MTX are also linked through a long path that includes pooled aTNFs. This may artificially skew the comparison between these treatments, and caution must be taken when interpreting the results.
Another limitation of the current analysis is the sole focus on ACR responses. Although ACR response is a composite measure that captures improvement in tender and swollen joint counts, patient and physician global assessment of disease, pain, CRP, and disability, the analysis does not provide information about how the efficacy of biologics with and without MTX compares for these different components of ACR response. It also does not make a distinction in a response obtained from a patient with a reduction in all components and a response based on improvement in only 3 of the 5 components. Furthermore, because this study did not address differences in risk from adverse events, only the benefit, rather than the risk/benefit, can be compared. Unfortunately, such a risk/benefit analysis of relatively short-term RCT data would not provide a valid picture associated with long-term use.
Although the MTX doses used in the studies are not necessarily reflective of doses used in clinical practice, they decreased the variability between studies, which is a strength of the analysis and underscores the importance of comparing agents in combination with MTX and as monotherapy simultaneously as part of 1 network, showing the magnitude of the effect of concomitant MTX use for each treatment option.
It has often been suggested that the efficacy of tocilizumab, as measured by composite end points such as ACR and Disease Activity Score response, should be interpreted with caution because of its strong effect on CRP, a component of these end points. In the more recent tocilizumab trials, however (including ACT-RAY and ADACTA, which provide the monotherapy data for the network meta-analysis), these composite end points are exclusively calculated using ESR values, not CRP, thus avoiding this potential CRP bias. Post hoc analysis of ADACTA using the Clinical Disease Activity Index (CDAI)—a parameter that does not include CRP or ESR—to compare disease activity and remission rates confirms the significant efficacy difference between the 2 treatment arms, as shown by the primary end point.61
Conclusions
Based on a network meta-analysis involving indirect comparisons of RCT findings, we found that aTNF, abatacept, tocilizumab, and tofacitinib in combination with MTX had comparable ACR responses in DMARD-IR patient populations. Anakinra in combination with MTX was less efficacious than the other novel DMARDs in combination with MTX. In monotherapy, tocilizumab may be associated with a higher ACR response than observed with aTNF or tofacitinib. ACR responses with tocilizumab in combination with MTX were similar to those of tocilizumab monotherapy, whereas aTNF in combination with MTX demonstrated greater ACR responses than aTNF monotherapy. These findings suggest tocilizumab is a valuable treatment option for patients who cannot tolerate MTX or are not compliant with an MTX regimen. This conclusion is increasingly being reflected in clinical and payer guidelines in many countries, with some of these guidelines recommending, or even mandating, its use as a first-line biologic in patients who cannot take MTX.
APPENDIX A. Search Strategy Terms
| #1 rheumatoid arthritis |
| #2 antirheumatic agent |
| #3 (#1 AND #2) |
| #4 adalimumab OR Humira |
| #5 infliximab OR Remicade |
| #7 golimumab OR Simponi OR CNTO 148 |
| #8 certolizumab OR Cimzia OR CDP870 |
| #9 tocilizumab OR Actemra OR RoActemra |
| #10 rituximab OR Rituxan OR Mabthera |
| #11 abatacept OR Orencia |
| #12 anakinra OR Kineret |
| #13 (tofacitinib or tasocitinib or CP-690550).ti,ab. |
| #14 tumor necrosis factor OR tumor necrosis factor inhibitor OR tumor necrosis factor blocker OR tumor necrosis factor receptor OR anti-tumor necrosis factor OR TNF OR anti-TNF |
| #15 biologic OR biological |
| #16 (#4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15) |
| #17 randomized controlled trial OR randomized-controlled-trial OR controlled-clinical-trial OR randomized OR clinical trial OR random OR RCT OR random allocation OR double-blind method OR single-blind method OR placebo |
| #18 (#3 AND #16 AND #17) |
| #19 Limit #18 to Humans, Adults, and the years 1990-2011 |
APPENDIX B. Treatment Effects for All Contrasts in Terms of OR of ACR50 Response Along with 95% CrI and Probability That Treatment Is Better Than Comparator
| Intervention | Comparator | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Placebo | Placebo + MTX | aTNF | TOF 5mg | TCZ | ABT + MTX | ANA + MTX | aTNF + MTX | TOF 5mg + MTX | TCZ + MTX | ||
| Placebo | OR (95% CrI) |
1.00 (1,1) |
0.27 (0.04, 1.80) |
0.15 (0.06, 0.33) |
0.20 (0.05, 0.83) |
0.06 (0.02, 0.24) |
0.07 (0.01, 0.47) |
0.11 (0.01, 1.01) |
0.05 (0.01, 0.35) |
0.06 (0.01, 0.46) |
0.05 (0.01, 0.29) |
| P(better) | NA | 0.08 | < 0.01 | 0.01 | < 0.01 | 0.01 | 0.03 | < 0.01 | < 0.01 | < 0.01 | |
| Placebo + MTX | OR (95% CrI) |
3.66 (0.56, 26.40) |
1.00 (1, 1) |
0.56 (0.10, 3.17) |
0.74 (0.07, 8.55) |
0.24 (0.06, 0.88) |
0.24 (0.14, 0.41) |
0.41 (0.13, 1.25) |
0.19 (0.13, 0.27) |
0.22 (0.11, 0.44) |
0.19 (0.09, 0.41) |
| P(better) | 0.92 | NA | 0.23 | 0.39 | 0.02 | < 0.01 | 0.05 | < 0.01 | < 0.01 | < 0.01 | |
| aTNF | OR (95% CrI) |
6.65 (3.05, 15.43) |
1.80 (0.32, 10.07) |
1.00 (1, 1) |
1.34 (0.25, 7.07) |
0.43 (0.14, 1.26) |
0.43 (0.07, 2.62) |
0.74 (0.09, 5.64) |
0.35 (0.06, 1.95) |
0.40 (0.06, 2.59) |
0.34 (0.07, 1.54) |
| P(better) | > 0.99 | 0.77 | NA | 0.64 | 0.05 | 0.16 | 0.37 | 0.10 | 0.15 | 0.07 | |
| TOF 5mg | OR (95% CrI) |
4.98 (1.21, 21.62) |
1.36 (0.12, 14.42) |
0.75 (0.14, 3.95) |
1.00 (1, 1) |
0.32 (0.04, 2.31) |
0.33 (0.03, 3.75) |
0.55 (0.04, 7.56) |
0.26 (0.02, 2.89) |
0.30 (0.02, 3.63) |
0.26 (0.03, 2.47) |
| P(better) | 0.99 | 0.61 | 0.36 | NA | 0.12 | 0.17 | 0.32 | 0.13 | 0.16 | 0.11 | |
| TCZ | OR (95% CrI) |
15.51 (4.12, 62.04) |
4.20 (1.14, 15.56) |
2.34 (0.80, 7.05) |
3.13 (0.43, 23.22) |
1.00 (1, 1) |
1.01 (0.24, 4.14) |
1.72 (0.30, 9.58) |
0.82 (0.20, 3.06) |
0.93 (0.22, 4.12) |
0.81 (0.28, 2.25) |
| P(better) | > 0.99 | 0.98 | 0.95 | 0.88 | NA | 0.51 | 0.75 | 0.37 | 0.46 | 0.32 | |
| ABT + MTX | OR (95% CrI) |
15.25 (2.13, 122.40) |
4.15 (2.43, 7.19) |
2.30 (0.38, 14.49) |
3.04 (0.27, 38.72) |
0.99 (0.24, 4.12) |
1.00 (1, 1) |
1.71 (0.48, 5.92) |
0.80 (0.45, 1.38) |
0.92 (0.41, 2.16) |
0.79 (0.31, 2.05) |
| P(better) | 0.99 | > 0.99 | 0.84 | 0.83 | 0.49 | NA | 0.81 | 0.20 | 0.42 | 0.30 | |
| ANA + MTX | OR (95% CrI) |
8.95 (0.99, 87.80) |
2.44 (0.80, 7.81) |
1.35 (0.18, 10.59) |
1.80 (0.13, 26.84) |
0.58 (0.10, 3.35) |
0.59 (0.17, 2.09) |
1.00 (1, 1) |
0.47 (0.14, 1.53) |
0.54 (0.15, 2.12) |
0.47 (0.12, 1.85) |
| P(better) | 0.97 | 0.95 | 0.63 | 0.68 | 0.25 | 0.19 | NA | 0.09 | 0.17 | 0.12 | |
| aTNF + MTX | OR (95% CrI) |
18.83 (2.85, 148.01) |
5.16 (3.74, 7.53) |
2.85 (0.51, 17.67) |
3.79 (0.35, 47.14) |
1.22 (0.33, 4.94) |
1.24 (0.73, 2.23) |
2.11 (0.65, 7.05) |
1.00 (1, 1) |
1.15 (0.59, 2.42) |
0.98 (0.44, 2.36) |
| P(better) | > 0.99 | > 0.99 | 0.90 | 0.87 | 0.63 | 0.80 | 0.91 | NA | 0.66 | 0.48 | |
| TOF 5mg + MTX | OR (95% CrI) |
16.47 (2.19, 129.20) |
4.49 (2.25, 8.70) |
2.51 (0.39, 15.67) |
3.30 (0.28, 42.02) |
1.07 (0.24, 4.60) |
1.08 (0.46, 2.46) |
1.84 (0.47, 6.79) |
0.87 (0.41, 1.68) |
1.00 (1, 1) |
0.86 (0.30, 2.33) |
| P(better) | > 0.99 | > 0.99 | 0.85 | 0.84 | 0.54 | 0.58 | 0.83 | 0.34 | NA | 0.38 | |
| TCZ + MTX | OR (95% CrI) |
19.23 (3.45, 114.60) |
5.22 (2.45, 11.39) |
2.90 (0.65, 13.75) |
3.86 (0.41, 38.66) |
1.24 (0.44, 3.61) |
1.26 (0.49, 3.24) |
2.14 (0.54, 8.43) |
1.02 (0.42, 2.29) |
1.16 (0.43, 3.33) |
1.00 (1, 1) |
| P(better) | > 0.99 | > 0.99 | 0.93 | 0.89 | 0.68 | 0.70 | 0.88 | 0.52 | 0.62 | NA | |
ABT = abatacept; ACR50 = 50% improvement in American College of Rheumatology criteria; ANA = anakinra; aTNF = anti-tumor necrosis factor; CrI = credible interval; mg = milligram; MTX = methotrexate; NA = not applicable; OR = odds ratio; P(better) = probability that treatment (in row) is showing greater response than comparator (in column); TCZ = tocilizumab; TOF = tofacitinib.
APPENDIX C. Treatment Effects for All Contrasts in Terms of OR of ACR70 Response Along with 95% CrI and Probability That Treatment Is Better Than Comparator
| Intervention | Comparator | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Placebo | Placebo + MTX | aTNF | TOF 5mg | TCZ | ABT + MTX | ANA + MTX | aTNF + MTX | TOF 5mg + MTX | TCZ + MTX | ||
| Placebo | OR (95% CrI) |
1.00 (1, 1) |
0.31 (0.03, 2.66) |
0.07 (0.01, 0.24) |
0.27 (0.05, 1.22) |
0.03 (0.00, 0.16) |
0.06 (0.00, 0.58) |
0.09 (0.01, 1.17) |
0.06 (0.00, 0.47) |
0.03 (0.00, 0.34) |
0.03 (0.00, 0.23) |
| P(better) | NA | 0.11 | < 0.01 | 0.04 | < 0.01 | 0.01 | 0.03 | 0.01 | < 0.01 | < 0.01 | |
| Placebo + MTX | OR (95% CrI) |
3.26 (0.38, 39.62) |
1.00 (1, 1) |
0.23 (0.04, 1.38) |
0.88 (0.06, 15.52) |
0.11 (0.02, 0.42) |
0.21 (0.11, 0.37) |
0.30 (0.07, 1.22) |
0.18 (0.11, 0.26) |
0.11 (0.05, 0.24) |
0.11 (0.04, 0.27) |
| P(better) | 0.89 | NA | 0.05 | 0.46 | < 0.01 | < 0.01 | 0.04 | 0.04 | < 0.01 | < 0.01 | |
| aTNF | OR (95% CrI) |
14.00 (4.23, 75.38) |
4.26 (0.73, 26.83) |
1.00 (1, 1) |
3.79 (0.50, 35.41) |
0.45 (0.15, 1.39) |
0.89 (0.13, 5.90) |
1.29 (0.13, 13.11) |
0.78 (0.12, 4.82) |
0.45 (0.07, 3.57) |
0.47 (0.10, 2.29) |
| P(better) | > 0.99 | 0.95 | NA | 0.90 | 0.06 | 0.44 | 0.60 | 0.37 | 0.18 | 0.13 | |
| TOF 5 mg | OR (95% CrI) |
3.76 (0.82, 20.18) |
1.14 (0.06, 17.29) |
0.26 (0.03, 2.01) |
1.00 (1, 1) |
0.12 (0.01, 1.19) |
0.24 (0.01, 3.70) |
0.34 (0.01, 7.36) |
0.21 (0.01, 3.08) |
0.12 (0.01, 2.15) |
0.12 (0.01, 1.61) |
| P(better) | 0.96 | 0.54 | 0.10 | NA | 0.03 | 0.13 | 0.23 | 0.11 | 0.06 | 0.05 | |
| TCZ | OR (95% CrI) |
31.19 (6.23, 242.41) |
9.42 (2.40, 40.26) |
2.22 (0.72, 6.82) |
8.36 (0.84, 103.30) |
1.00 (1,1) |
1.97 (0.43, 9.08) |
2.85 (0.40, 21.09) |
1.72 (0.40, 7.25) |
1.00 (0.22, 5.62) |
1.05 (0.37, 3.07) |
| P(better) | > 0.99 | > 0.99 | 0.94 | 0.97 | NA | 0.85 | 0.87 | 0.80 | 0.50 | 0.55 | |
| ABT + MTX | OR (95% CrI) |
15.69 (1.71, 208.60) |
4.80 (2.71, 9.34) |
1.12 (0.17, 7.50) |
4.19 (0.27, 83.54) |
0.51 (0.11, 2.31) |
1.00 (1, 1) |
1.46 (0.30, 6.86) |
0.88 (0.47, 1.59) |
0.51 (0.21, 1.47) |
0.54 (0.18, 1.60) |
| P(better) | 0.99 | > 0.99 | 0.56 | 0.87 | 0.15 | NA | 0.70 | 0.30 | 0.09 | 0.11 | |
| ANA + MTX | OR (95% CrI) |
11.17 (0.85,192.01) |
3.30 (0.82, 14.83) |
0.78 (0.08, 7.42) |
2.96 (0.14, 74.77) |
0.35 (0.05, 2.52) |
0.69 (0.15, 3.34) |
1.00 (1, 1) |
0.60 (0.13, 2.72) |
0.36 (0.07, 1.99) |
0.37 (0.07, 2.07) |
| P(better) | 0.97 | 0.96 | 0.40 | 0.77 | 0.13 | 0.30 | NA | 0.23 | 0.10 | 0.11 | |
| aTNF + MTX | OR (95% CrI) |
17.84 (2.13, 237.71) |
5.50 (3.80, 8.85) |
1.28 (0.21, 8.42) |
4.85 (0.32, 95.11) |
0.58 (0.14, 2.51) |
1.14 (0.63, 2.13) |
1.66 (0.37, 7.44) |
1.00 (1, 1) |
0.59 (0.28, 1.47) |
0.61 (0.23, 1.69) |
| P(better) | 0.99 | > 0.99 | 0.63 | 0.89 | 0.20 | 0.70 | 0.77 | NA | 0.11 | 0.15 | |
| TOF 5mg + MTX | OR (95% CrI) |
30.84 (2.95, 403.71) |
9.30 (4.10, 20.36) |
2.21 (0.28, 14.55) |
8.32 (0.47, 154.70) |
1.00 (0.18, 4.62) |
1.95 (0.68, 4.86) |
2.81 (0.50, 13.39) |
1.70 (0.68, 3.61) |
1.00 (1, 1) |
1.04 (0.29, 3.36) |
| P(better) | > 0.99 | > 0.99 | 0.82 | 0.94 | 0.50 | 0.91 | 0.90 | 0.89 | NA | 0.53 | |
| TCZ + MTX | OR (95% CrI) |
29.53 (4.35, 295.43) |
8.95 (3.72, 23.36) |
2.12 (0.44, 9.84) |
8.00 (0.62,122.50) |
0.95 (0.33, 2.72) |
1.86 (0.63, 5.61) |
2.72 (0.48, 15.04) |
1.63 (0.59, 4.40) |
0.96 (0.30, 3.50) |
1.00 (1, 1) |
| P(better) | > 0.99 | > 0.99 | 0.87 | 0.95 | 0.45 | 0.89 | 0.89 | 0.85 | 0.47 | NA | |
ABT = abatacept; ACR70 = 70% improvement in American College of Rheumatology criteria; ANA = anakinra; aTNF = anti-tumor necrosis factor; CrI = credible interval; mg = milligram; MTX = methotrexate; NA = not applicable; OR = odds ratio; P(better) = probability that treatment (in row) is showing greater response than comparator (in column); TCZ = tocilizumab; TOF = tofacitinib.
REFERENCES
- 1.Saag KG, Teng GG, Patkar NM, et al. American College of Rheumatology 2008 recommendations for the use of nonbiologic and biologic disease-modifying antirheumatic drugs in rheumatoid arthritis. Arthritis Rheum. 2008;59(6):762-84. [DOI] [PubMed] [Google Scholar]
- 2.Luqmani R, Hennell S, Estrach C, et al. British Society for Rheumatology and British Health Professionals in Rheumatology guideline for the management of rheumatoid arthritis (after the first 2 years). Rheumatology (Oxford). 2009;48(4):436-39. [DOI] [PubMed] [Google Scholar]
- 3.Smolen JS, Aletaha D, Bijlsma JW, et al. Treating rheumatoid arthritis to target: recommendations of an international task force. Ann Rheum Dis. 2010;69(4):631-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Singh JA, Furst DE, Bharat A, et al. 2012 update of the 2008 American College of Rheumatology recommendations for the use of disease-modifying antirheumatic drugs and biologic agents in the treatment of rheumatoid arthritis. Arthritis Care Res (Hoboken). 2012;64(5):625-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smolen JS, Landewé R, Breedveld FC, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2013 update. Ann Rheum Dis. 2014;73(3):492-509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mathias SD, Colwell HH, Miller DP, Moreland LW, Buatti M, Wanke L. Health-related quality of life and functional status of patients with rheumatoid arthritis randomly assigned to receive etanercept or placebo. Clin Ther. 2000;22(1):128-39. [DOI] [PubMed] [Google Scholar]
- 7.Moreland LW, Schiff MH, Baumgartner SW, et al. Etanercept therapy in rheumatoid arthritis: a randomized, controlled trial. Ann Intern Med. 1999;130(6):478-86. [DOI] [PubMed] [Google Scholar]
- 8.van de Putte LB, Atkins C, Malaise M, et al. Efficacy and safety of adalimumab as monotherapy in patients with rheumatoid arthritis for whom previous disease modifying antirheumatic drug treatment has failed. Ann Rheum Dis. 2004;63(5):508-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Listing J, Strangfeld A, Rau R, et al. Clinical and functional remission: even though biologics are superior to conventional DMARDs overall success rates remain low—results from RABBIT, the German biologics register. Arthritis Res Ther. 2006;8(3):R66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heiberg MS, Koldingsnes W, Mikkelsen K, et al. The comparative one-year performance of anti-tumor necrosis factor alpha drugs in patients with rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis: results from a longitudinal, observational, multicenter study. Arthritis Rheum. 2008;59(2):234-40. [DOI] [PubMed] [Google Scholar]
- 11.Soliman MM, Ashcroft DM, Watson KD, et al. Impact of concomitant use of DMARDs on the persistence with anti-TNF therapies in patients with rheumatoid arthritis: results from the British Society for Rheumatology Biologics Register. Ann Rheum Dis. 2011;70(4):583-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nikiphorou E, Negoescu A, Fitzpatrick JD, et al. Indispensable or intolerable? Methotrexate in patients with rheumatoid and psoriatic arthritis: a retrospective review of discontinuation rates from a large UK cohort. Clin Rheumatol. 2014;33(5):609-14. [DOI] [PubMed] [Google Scholar]
- 13.Jansen JP, Fleurence R, Devine B, et al. Interpreting indirect treatment comparisons and network meta-analysis for health-care decision making: report of the ISPOR Task Force on Indirect Treatment Comparisons Good Research Practices: part 1. Value Health. 2011;14(4):417-28. [DOI] [PubMed] [Google Scholar]
- 14.Lu G, Ades AE. Combination of direct and indirect evidence in mixed treatment comparisons. Stat Med. 2004;23(20):3105-24. [DOI] [PubMed] [Google Scholar]
- 15.Thorlund K, Druyts E, Aviña-Zubieta JA, Wu P, Mills EJ. Why the findings of published multiple treatment comparison meta-analyses of biologic treatments for rheumatoid arthritis are different: an overview of recurrent methodological shortcomings. Ann Rheum Dis. 2013;72(9):1524-35. [DOI] [PubMed] [Google Scholar]
- 16.Felson DT, Anderson JJ, Boers M, et al. The American College of Rheumatology preliminary core set of disease activity measures for rheumatoid arthritis clinical trials: the Committee on Outcome Measures in Rheumatoid Arthritis Clinical Trials. Arthritis Rheum. 1993;36(6):729-40. [DOI] [PubMed] [Google Scholar]
- 17.Fries JF, Spitz PW, Young DY. The dimensions of health outcomes: the health assessment questionnaire, disability and pain scales. J Rheumatol. 1982;9(5):789-93. [PubMed] [Google Scholar]
- 18.Caldwell DM, Ades AE, Higgins JP. Simultaneous comparison of multiple treatments: combining direct and indirect evidence. BMJ. 2005;331(7521):897-900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoaglin DC, Hawkins N, Jansen JP, et al. Conducting indirect-treatment-comparison and network-meta-analysis studies: report of the ISPOR Task Force on Indirect Treatment Comparisons Good Research Practices: part 2. Value Health. 2011;14(4):429-37. [DOI] [PubMed] [Google Scholar]
- 20.Burmester GR, Rubbert-Roth A, Cantagrel AG, et al. A randomised, double-blind, parallel-group study of the safety and efficacy of subcutaneous tocilizumab versus intravenous tocilizumab in combination with traditional disease-modifying antirheumatic drugs in patients with moderate to severe rheumatoid arthritis (SUMMACTA study). Ann Rheum Dis. 2014;73(1):69-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Genovese MC, Covarrubias A, Leon G, et al. Subcutaneous abatacept versus intravenous abatacept: a phase IIIb noninferiority study in patients with an inadequate response to methotrexate. Arthritis Rheum. 2011;63(10): 2854-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sutton AJ, Abrams KR. Bayesian methods in meta-analysis and evidence synthesis. Stat Methods Med Res. 2001;10(4):277-303. [DOI] [PubMed] [Google Scholar]
- 23.Spiegelhalter DJ, Best NG Carlin BP, van der Linde A. Bayesian measures of model complexity and fit. J R Stat Soc. 2002;64(4):583-639. [Google Scholar]
- 24.Spiegelhalter D, Thomas A, Best N, Lunn D. WinBUGS User Manual. Version 1.4. Cambridge, UK: MRC Biostatistics Unit; 2003. [Google Scholar]
- 25.Klareskog L, van der Heijde D, de Jager JP, et al. Therapeutic effect of the combination of etanercept and methotrexate compared with each treatment alone in patients with rheumatoid arthritis: double-blind randomised controlled trial. Lancet. 2004;363(9410):675-81. [DOI] [PubMed] [Google Scholar]
- 26.Combe B, Codreanu C, Fiocco U, et al. Etanercept and sulfasalazine, alone and combined, in patients with active rheumatoid arthritis despite receiving sulfasalazine: a double-blind comparison. Ann Rheum Dis. 2006;65(10):1357-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Combe B, Codreanu C, Fiocco U, et al. Efficacy, safety and patient-reported outcomes of combination etanercept and sulfasalazine versus etanercept alone in patients with rheumatoid arthritis: a double-blind randomised 2-year study. Ann Rheum Dis. 2009;68(7):1146-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fleischmann R, Cutolo M, Genovese MC, et al. Phase IIb dose-ranging study of the oral JAK inhibitor tofacitinib (CP-690,550) or adalimumab monotherapy versus placebo in patients with active rheumatoid arthritis with an inadequate response to disease-modifying antirheumatic drugs. Arthritis Rheum. 2012;64(3):617-29. [DOI] [PubMed] [Google Scholar]
- 29.Weinblatt ME, Fleischmann R, Huizinga TW, et al. Efficacy and safety of certolizumab pegol in a broad population of patients with active rheumatoid arthritis: results from the REALISTIC phase IIIb study. Rheumatology. 2012;51(12):2204-14. [DOI] [PubMed] [Google Scholar]
- 30.Yazici Y, Curtis JR, Ince A, et al. Efficacy of tocilizumab in patients with moderate to severe active rheumatoid arthritis and a previous inadequate response to disease-modifying antirheumatic drugs: the ROSE study. Ann Rheum Dis. 2012;71(2):198-205. [DOI] [PubMed] [Google Scholar]
- 31.Fleischmann R, Vencovsky J, van Vollenhoven RF, et al. Efficacy and safety of certolizumab pegol monotherapy every 4 weeks in patients with rheumatoid arthritis failing previous disease-modifying antirheumatic therapy: the FAST4WARD study. Ann Rheum Dis. 2009;68(6):805-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gabay C, Emery P, van Vollenhoven R, et al. Tocilizumab monotherapy versus adalimumab monotherapy for treatment of rheumatoid arthritis (ADACTA): a randomised, double-blind, controlled phase 4 trial. Lancet. 2013;381(9877):1541-50. [DOI] [PubMed] [Google Scholar]
- 33.Kay J, Matteson E, Dasgupta B, et al. Golimumab in patients with active rheumatoid arthritis despite treatment with methotrexate: a randomized, double-blind, placebo-controlled, dose-ranging study. Arthritis Rheum. 2008;58(4):964-75. [DOI] [PubMed] [Google Scholar]
- 34.Keystone EC, Genovese MC, Klareskog L, et al. Golimumab, a human antibody to tumour necrosis factor α given by monthly subcutaneous injections, in active rheumatoid arthritis despite methotrexate therapy: the GO-FORWARD Study. Ann Rheum Dis. 2009;68(6):789-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Keystone EC, Kavanaugh AF, Sharp JT, et al. Radiographic, clinical, and functional outcomes of treatment with adalimumab a human anti-tumor necrosis factor monoclonal antibody in patients with active rheumatoid arthritis receiving concomitant methotrexate therapy: a randomized, place-bo-controlled, 52-week trial. Arthritis Rheum. 2004;50(5):1400-11. [DOI] [PubMed] [Google Scholar]
- 36.Keystone E, Heijde D, Mason D Jr, et al. Certolizumab pegol plus methotrexate is significantly more effective than placebo plus methotrexate in active rheumatoid arthritis: findings of a fifty-two-week, phase III, multicenter, randomized, double-blind, placebo-controlled, parallel-group study. Arthritis Rheum. 2008;58(11):3319-29. [DOI] [PubMed] [Google Scholar]
- 37.Kremer JM, Westhovens R, Leon M, et al. Treatment of rheumatoid arthritis by selective inhibition of T-cell activation with fusion protein CTLA4Ig. N Engl J Med. 2003;349(20):1907-15. [DOI] [PubMed] [Google Scholar]
- 38.Kremer JM, Genant HK, Moreland LW, et al. Effects of abatacept in patients with methotrexate-resistant active rheumatoid arthritis: a randomized trial. Ann Intern Med. 2006;144(12):865-76. [DOI] [PubMed] [Google Scholar]
- 39.Maini R, St Clair EW, Breedveld F, et al. Infliximab chimeric antitumour necrosis factor alpha monoclonal antibody versus placebo in rheumatoid arthritis patients receiving concomitant methotrexate: a randomised phase III trial ATTRACT Study Group. Lancet. 1999;354(9194):1932-39. [DOI] [PubMed] [Google Scholar]
- 40.Schiff M, Keiserman M, Codding C, et al. Efficacy and safety of abatacept or infliximab vs placebo in ATTEST: a phase III, multi-centre, randomised, double-blind, placebo-controlled study in patients with rheumatoid arthritis and an inadequate response to methotrexate. Ann Rheum Dis. 2008;67(8):1096-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Smolen J, Landewé RB, Mease P, et al. Efficacy and safety of certolizumab pegol plus methotrexate in active rheumatoid arthritis: the RAPID 2 study. A randomised controlled trial. Ann Rheum Dis. 2009;68(6):797-804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smolen JS, Beaulieu A, Rubbert-Roth A, et al. Effect of interleukin-6 receptor inhibition with tocilizumab in patients with rheumatoid arthritis (OPTION study): a double-blind, placebo-controlled, randomised trial. Lancet. 2008;371(9617):987-97. [DOI] [PubMed] [Google Scholar]
- 43.van der Heijde D, Tanaka Y, Fleischmann R, et al. Tofacitinib (CP-690,550) in patients with rheumatoid arthritis receiving methotrexate: twelve-month data from a twenty-four-month phase III randomized radiographic study. Arthritis Rheum. 2013;65(3):559-70. [DOI] [PubMed] [Google Scholar]
- 44.Weinblatt ME, Kremer JM, Bankhurst AD, et al. A trial of etanercept, a recombinant tumor necrosis factor receptor:Fc fusion protein, in patients with rheumatoid arthritis receiving methotrexate. N Engl J Med. 1999;340(4):253-59. [DOI] [PubMed] [Google Scholar]
- 45.Weinblatt ME, Keystone EC, Furst DE, et al. Adalimumab, a fully human anti-tumor necrosis factor alpha monoclonal antibody, for the treatment of rheumatoid arthritis in patients taking concomitant methotrexate: the ARMADA trial. Arthritis Rheum. 2003;48(1):35-45. [DOI] [PubMed] [Google Scholar]
- 46.Westhovens R, Yocum D, Han J, et al. The safety of infliximab, combined with background treatments, among patients with rheumatoid arthritis and various comorbidities: a large, randomized, placebo-controlled trial. Arthritis Rheum. 2006;54(4):1075-86. [DOI] [PubMed] [Google Scholar]
- 47.Kremer JM, Blanco R, Brzosko M, et al. Tocilizumab inhibits structural joint damage in rheumatoid arthritis patients with inadequate responses to methotrexate: results from the double-blind treatment phase of a randomized placebo-controlled trial of tocilizumab safety and prevention of structural joint damage at one year. Arthritis Rheum. 2011;63(3):609-21. [DOI] [PubMed] [Google Scholar]
- 48.van Vollenhoven RF, Kinnman N, Vincent E, Wax S, Bathon J. Atacicept in patients with rheumatoid arthritis and an inadequate response to methotrexate: results of a phase II, randomized, placebo-controlled trial. Arthritis Rheum. 2011;63(7):1782-92. [DOI] [PubMed] [Google Scholar]
- 49.Weinblatt ME, Schiff M, Valente R, et al. Head-to-head comparison of subcutaneous abatacept versus adalimumab for rheumatoid arthritis: findings of a phase IIIb, multinational, prospective, randomized study. Arthritis Rheum. 2013;65(1):28-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Choy E, McKenna F, Vencovsky J, et al. Certolizumab pegol plus MTX administered every 4 weeks is effective in patients with RA who are partial responders to MTX. Rheumatology (Oxford). 2012;51(7):1226-34. [DOI] [PubMed] [Google Scholar]
- 51.Kremer JM, Cohen S, Wilkinson BE, et al. A phase IIb dose-ranging study of the oral JAK inhibitor tofacitinib (CP-690,550) versus placebo in combination with background methotrexate in patients with active rheumatoid arthritis and an inadequate response to methotrexate alone. Arthritis Rheum. 2012;64(4):970-81. [DOI] [PubMed] [Google Scholar]
- 52.van Vollenhoven RF, Fleischmann R, Cohen S, et al. Tofacitinib or adalimumab versus placebo in rheumatoid arthritis. N Engl J Med. 2012;367(6):508-19. [DOI] [PubMed] [Google Scholar]
- 53.Fleischmann R, Kremer J, Cush J, et al. Placebo-controlled trial of tofacitinib monotherapy in rheumatoid arthritis. N Engl J Med. 2012;367(6):495-507. [DOI] [PubMed] [Google Scholar]
- 54.Dougados M, Kissel K, Sheeran T, et al. Adding tocilizumab or switching to tocilizumab monotherapy in methotrexate inadequate responders: 24-week symptomatic and structural results of a 2-year randomised controlled strategy trial in rheumatoid arthritis (ACT-RAY). Ann Rheum Dis. 2013;72(1):43-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cohen SB, Moreland LW, Cush JJ, et al. A multicentre, double blind, randomised, placebo controlled trial of anakinra Kineret, a recombinant interleukin 1 receptor antagonist, in patients with rheumatoid arthritis treated with background methotrexate. Ann Rheum Dis. 2004;63(9):1062-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stevenson MD, Archer R, Tosh J, et al. Adalimumab, etanercept, infliximab, certolizumab pegol, golimumab, tocilizumab and abatacept for the treatment of rheumatoid arthritis not previously treated with disease-modifying anti-rheumatic drugs and after the failure of conventional disease-modifying anti-rheumatic drugs only: systematic review and economic evaluation. Health Technol Assess. August 12, 2013. Available at: https://www.nice.org.uk/guidance/gid-tag313/documents/rheumatoid-arthritis-adalim-umab-etanercept-infliximab-certolizumab-pegol-golimumab-abatacept-and-tocilizumab-review-assessment-report2. Accessed March 29, 2015.
- 57.Jansen JP, Buckley F, Dejonckheere F, Ogale S. Comparative efficacy of biologics as monotherapy and in combination with methotrexate on patient-reported outcomes (PROs) in rheumatoid arthritis patients with an inadequate response to conventional DMARDs—a systematic review and network meta-analysis. Health Qual Life Outcomes. 2014;12:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Finckh A, Dehler S, Gabay C; SCQM doctors. The effectiveness of leflunomide as a co-therapy of tumour necrosis factor inhibitors in rheumatoid arthritis: a population-based study. Ann Rheum Dis. 2009;68(1):33-39. [DOI] [PubMed] [Google Scholar]
- 59.Kristensen LE, Saxne T, Nilsson JA, Geborek P. Impact of concomitant DMARD therapy on adherence to treatment with etanercept and infliximab in rheumatoid arthritis: results from a six-year observational study in southern Sweden. Arthritis Res Ther. 2006;8(6):R174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ostergaard M, Unkerskov J, Linde L, et al. Low remission rates but long drug survival in rheumatoid arthritis patients treated with infliximab or etanercept: results from the nationwide Danish DANBIO database. Scand J Rheumatol. 2007;36(2):151-54. [DOI] [PubMed] [Google Scholar]
- 61.Gabay C, Emery P, van Vollenhoven R, et al. Tocilizumab monotherapy is superior to adalimumab monotherapy in reducing disease activity in patients with rheumatoid arthritis: 24-week data from the phase 4 ADACTA trial. Ann Rheum Dis. 2013;71(Suppl 3):152. Available at: http://ard.bmj.com/content/71/Suppl_3/152.1.abstract?sid=62a8bace-6070-45e5-bb86-2861428a31ab. Accessed April 8, 2015. [Google Scholar]