Abstract
BACKGROUND:
Renin-angiotensin system (RAS) antagonists are recommended for people with diabetes and hypertension or with elevated urinary albumin excretion. RAS antagonists are beneficial for some, yet clinically inappropriate for others. The percentage of patients for whom RASs are clinically inappropriate has not been compared across health plans.
OBJECTIVES:
To (a) identify reasons why RAS therapy was not recommended and (b) compare exception percentages between health plans.
METHODS:
This retrospective, cross-sectional analysis included Medicare Part D beneficiaries with diabetes, enrolled in health plans (n = 96) participating in a university-based medication therapy management (MTM) program between January 1 and December 31, 2013. The MTM program evaluated patient eligibility for RAS therapy via (1) a clinically derived software system assessing demographics and medication history, and (2) telepharmacist-delivered medication reviews. The MTM program database calculated the number of patients with diabetes and percentage of RAS therapy exceptions.
RESULTS:
An average of 55% of patients with diabetes qualified for MTM (range: 19%-88%). Of the 218,589 eligible, 94,359 had 1 or more reasons contraindicating RAS therapy (exception). For an average of 29% of patients, it was inappropriate to recommend the addition of an RAS antagonist; the overall exception rate ranged from 3% to 83%, suggesting a wide variation of exception rates for all health plans.
CONCLUSIONS:
A substantial difference existed across health plans where RAS therapy was considered clinically inappropriate to recommend for patients with diabetes. Future research must examine variations in therapy exceptions to understand the effect of encouraging broad-population RAS antagonist use.
What is already known about this subject
In 2013, medication therapy management sponsors began offering Medicare beneficiaries with diagnoses of diabetes and high blood pressure certain types of hypertensive medications (i.e., renin-angiotensin system [RAS] antagonists such as angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers).
Although RAS antagonists are recommended as a treatment option for diabetes and hypertension patients, certain side effects (e.g., cough) and contraindications (e.g., unsuitable for pregnant patients or those with poor kidney function) are associated with their use.
RAS antagonists are recommended and beneficial for some patients yet clinically inappropriate for others.
What this study adds
This study evaluated RAS antagonist recommendations for Medicare beneficiaries with diabetes across health plans to better understand the effect of encouraging its broad population use in managed care settings.
This study found variation across health plans, which suggests the need for future evaluation involving risk adjustment for quality measures to account for differences in patients’ conditions and characteristics across health plans.
The Centers for Medicare & Medicaid Services (CMS) uses star ratings to compare Medicare Part D plan performance related to providing better care, creating healthier communities, and spending efficiently.1 Star ratings comprise several domains, with multiple measures within each domain assigned the same or different weights, depending on the emphasis placed on the respective measure. To meet Medicare Star Rating System thresholds, health plans are incentivized to ensure that beneficiaries’ therapy complies with current recommendations.2 Plans earning an overall 5-star rating may display CMS’s gold star icon on their marketing mate-rials,3 while lower performers (rating of 2.5 or lower) receive a low-performing icon.2
In 2012, Medicare Advantage plans became eligible for bonus payments based on their star rating performance.4 Some Part D star measures focus on beneficiaries’ medications (e.g., adherence for hypertension), whereby plans are assessed according to the number of beneficiaries with a proportion of days covered (PDC) at or above the 80% threshold for the same or other medication in the respective therapeutic class (indicating high medication adherence). Thus, plans are incentivized to help beneficiaries improve medication adherence. Medicare Part D plans must include medication therapy management (MTM) programs to ensure that members’ medications are effectively improving health outcomes; beneficiaries must meet certain criteria, established by CMS, to receive MTM services.5
To address these medication issues, health plans collaborate with pharmacists and MTM sponsors who offer medication review and reconciliation. Additionally, patient counseling is provided to assist beneficiaries in achieving optimal medication use; help health plans improve star rating performance; and reduce medication costs.6 MTM programs have pharmacists and other health care providers who address medication-related problems, concerns, and questions regarding medication use and its associated costs, and other relevant problems. After the initial pharmacist-beneficiary interaction, enrollees receive a copy of their medication list and personal action plan to share with their physicians and other health care providers.5
In 2013, patients with diabetes mellitus (DM) and high blood pressure were targeted to ensure that they were offered certain types of hypertensive medications (i.e., renin-angiotensin system [RAS] antagonists, including an angiotensin-converting enzyme inhibitor [ACEI], angiotensin II receptor blocker [ARB], and direct renin inhibitors).7 The hypertension treatment guidelines for 2013 included recommendations for adding an ACEI or ARB for hypertensive patients with DM8; however, certain side effects are associated with their use (e.g., cough),9-11 as well as contraindications (e.g., patients who are pregnant or have poor kidney function).12 While these medications may be inappropriate for some, they can still benefit from MTM services to help identify viable substitutes.
Beneficiaries with DM who have any of these contraindications are not candidates for ACEI or ARB therapy (denoted as “exceptions” in this study). However, a gap exists regarding the description of these exceptions and overall rates among health plans. The purpose of this study was to determine the frequency that exceptions occur overall and, in particular, by health plan. The objectives of this study were to (a) identify reasons why ACEI or ARB therapy was not recommended for certain patients and (b) compare percentage of exceptions to ACEI or ARB therapy recommendations across health plans contracted with the University of Arizona Medication Management Center (UAMMC) to provide MTM services.
Methods
Study Design
This retrospective, cross-sectional study involved Medicare beneficiaries with DM at increased risk for cardiovascular disease affiliated with 1 of the UAMMC’s contracted health plans; the UAMMC provides MTM services to beneficiaries throughout the United States. UAMMC pharmacists performed telephonic consultations with health plan beneficiaries who qualified for MTM services through their Medicare or private insurance plans (non-Medicare). During consultations, pharmacists provided comprehensive medication reviews (CMRs) and targeted medication reviews (TMRs) to ensure that patients were on appropriate medication regimens related to Medicare Part D quality measure specifications. The study period was from January 1, 2013, through December 31, 2013. The University of Arizona’s Institutional Review Board approved the study protocol.
Data Sources
This study used 3 data sources. The first source was the data file, including beneficiary-level data on patients with the exceptions, identified through a 2-phase process. This process was used to determine the appropriateness of recommending an ACEI or ARB for a particular patient via (1) a clinically derived software system and (2) pharmacist-initiated, telephonic medication review with the patient. This multistep assessment process is outlined here and in Figure 1.
FIGURE 1.
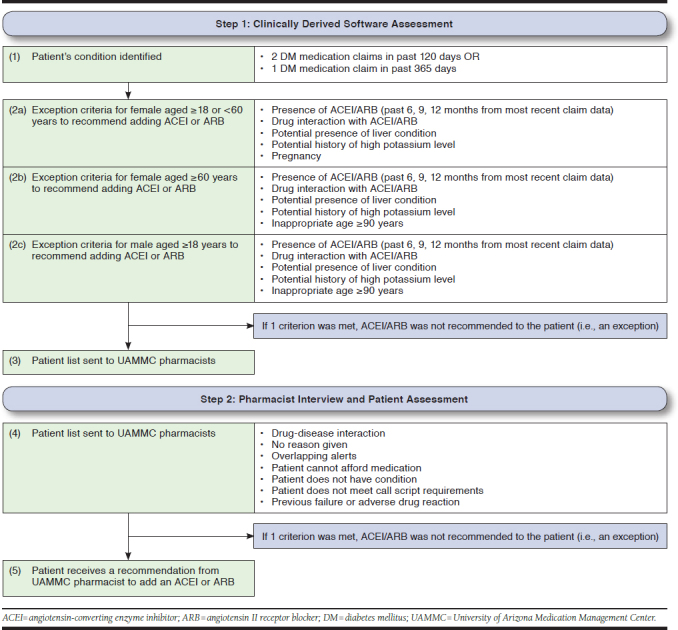
Assessment Process for Selecting Patients Ineligible to Receive an ACEI or ARB Therapy Recommendation
Step 1: Clinically Derived Software System Assessment.
The clinically derived software system assessed patients’ demographics and medication history to identify the condition (DM) using patient age, gender, medications listed in the health plan’s prescription claims database, and/or International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) diagnosis codes (if provided). Subsequently, the software assessed patients with DM (condition). Next, patients were grouped accordingly: (1) females aged ≥ 18 and < 60 years; (2) females aged ≥ 60 years; and (3) males aged ≥ 18 years. Based on the call script, females aged less than 60 years were identified and prompted about pregnancy status or plans to become pregnant (e.g., pregnancy exception). For every step in assessing each patient group, a series of exception criteria were compared with the patient records to determine eligibility to receive a recommendation for adding an ACEI or ARB to the therapy regimen. Finally, a list of eligible patients identified to receive an ACEI or ARB recommendation to add to their therapy regimen was sent to UAMMC pharmacists. Additionally, each time an eligible health plan submitted a new prescription claim to the UAMMC for a particular patient, the software system initiated an assessment to determine if the recommendation was still appropriate.
Step 2: Pharmacist Interview and Patient Assessment.
Following the software system assessment, UAMMC pharmacists interviewed and further assessed patient eligibility to recommend an ACEI or ARB during telephone-administered medication reviews, using comprehensive call scripts to guide assessment of patient eligibility for therapy recommendations. During the consultation, the UAMMC pharmacist confirmed that the patient was diabetic (condition) and subsequently asked him or her a series of questions to determine eligibility to receive a recommendation for an ACEI or ARB. During this telephone interview and consultation, the UAMMC pharmacist may have concluded, for various reasons, that ACEI or ARB therapy was inappropriate for the particular patient. Further, if patients had any of these circumstances they were considered ineligible for ACEI or ARB therapy: (1) experienced problems with hypotension13; (2) had a medical history of angioedema14; (3) may have had a previous therapy failure (e.g., adverse drug reaction); (4) were unable to afford the medication; (5) were pregnant or planning to become pregnant if female; and/or (6) had another reason identified by the pharmacist.
Additionally, researchers used a 2013 annual report as a second data source to obtain the number of patients with DM as the numerator (for DM rates) and the total number of patients for each plan as the denominator for exception rate computation. The dataset was not accessible to researchers to conduct the analysis at the beneficiary level; however, health plan-level analysis was conducted. The third data source was the prescription fills history file for an RAS antagonist (i.e., ACEIs, ARBs, and direct renin inhibitors).
Inclusion and Exclusion Criteria
Overall, 112 health plans were evaluated for the study. Eligible plans met these inclusion criteria: (1) considered a noncommercial Medicare health plan; (2) had at least 1 exception to recommending an ACEI or ARB; and (3) had outcomes reported in the UAMMC database through the end of 2013. Of these, 96 health plans were eligible for the study.
Patients participating in 1 of the 96 health plans contracted with the UAMMC were included in the analysis if they met these clinical criteria: (1) a Medicare Part D beneficiary with DM with hypertension, any degree of proteinuria (i.e., history of kidney disease), or known cardiovascular disease (e.g., history of chest pain, heart attack, or heart surgeries); (2) at least 1 exception to receiving a recommendation to add an ACEI or ARB; and (3) no prescription claims for an RAS antagonist (i.e., ACEIs, ARBs, and direct renin inhibitors) during 2013 using the prescription claim data file.
All patients were assessed to determine if adding hypertension therapy was clinically appropriate. Patients for whom it was inappropriate to recommend addition of an ACEI or ARB medication (i.e., who had an exception to adding these medications) were included in the study.
Outcomes Definitions
The study outcomes included (1) number of patients with DM who had an exception in each health plan; (2) reasons why addition of ACEI or ARB therapy was deemed inappropriate for respective patients; and (3) exception rates for each health plan. Patient eligibility to receive the therapy recommendation was conducted using methods previously described (i.e., clinically derived software and pharmacist consultation). Patients for whom these therapies were deemed unacceptable or inappropriate were classified as “exceptions” and included in this study.
Data Analysis
Types of exceptions identified in UAMMC’s software system or via the pharmacist’s review were reported using count and percentage to describe the proportion. Overall percentage of patients with DM in each health plan was calculated. Of the patients enrolled in each health plan, the percentage for whom it was an exception to add ACEI or ARB therapy also was calculated. Health plans were stratified into quartiles for reporting purposes based on the number of Medicare beneficiaries. Health plans in quartile 1 were those with the smallest numbers of eligible Medicare beneficiaries relative to the other 3 groups. Quartile 4 contained health plans with the largest numbers of Medicare beneficiaries relative to the other 3 groups. The number of patients with DM was calculated from the number of beneficiaries with an MTM diabetic alert, identified in the clinically derived software system (Appendix A and Appendix B, available in online article).
Results
From the 96 health plans, 218,589 patients with diabetes were identified during 2013. Of these, 189,267 patients were identified either in UAMMC’s software system or via the pharmacist’s review as having an exception to receive an ACEI or ARB (Figure 2). Finally, 94,908 patients who received at least 1 ACEI or ARB or direct renin inhibitor prescription during the 2013 calendar year were excluded from the analysis; the remaining 94,359 patients met the inclusion criteria (as exceptions) and were included in the study. Table 1 shows the characteristics of the eligible patients who had exceptions to an ACEI or ARB recommendation.
FIGURE 2.
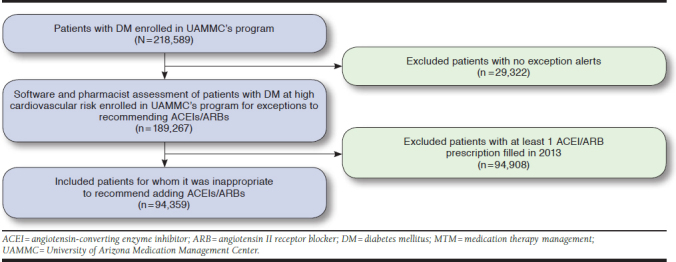
Flowchart Illustrating Selection of Patients for Study Inclusion
TABLE 1.
Characteristics of Patients with DM with an Exception for ACEI or ARB Therapy in 96 Eligible Health Plans During the 2013 Calendar Year
| Characteristics | All Patients (N = 94,359) |
|---|---|
| Age, years (SD) | 73 (10) |
| Females, n (%) | 51,302 (53) |
| Geographic regiona | |
| Midwest, n (%) | 39,600 (42) |
| Northeast, n (%) | 12,563 (13) |
| South, n (%) | 22,901 (24) |
| West, n (%) | 19,281 (20) |
aSome beneficiaries had missing data.
DM = diabetes mellitus; SD = standard deviation.
A total of 101,183 exception reasons were identified by the clinically derived software system (n = 98,206) and the pharmacist (n = 2,977). Individual patients could have multiple reasons for exceptions to therapy (n = 6,445 [7%]). The most common software-derived reason was (1) presence of ACEI/ARB (89%), followed by (2) inappropriate age ≥ 90 years (3%); (3) drug interaction (3%) with ACEI/ARBs, detected based on whether the patient was taking a medication that interacted with an ACEI/ARB or was taking medications that inferred conditions would make an ACEI or ARB recommendation inappropriate; (4) potential liver condition (2%) detected based on whether the patient was taking medication that infers he or she has liver disease; (5) potential history of high potassium levels (2%; i.e., sodium polystyrene sulfate, indicating the patient has had previous problems with high potassium levels, is a known adverse drug event [ADE] of ACEI/ARBs); or (6) pregnancy (0.2%; Table 2).
TABLE 2.
Reasons for Exceptions Derived from Clinical Software System and Pharmacist Review to Recommend Adding ACEI or ARB Therapy for Patients with DM in Participating 96 Health Plans During the 2013 Calendar Year
| Exception Reasons (N = 101,183) | n (%) |
| Clinical software exception reasons (n = 98,206)a | |
| Past presence of ACEI/ARB | 87,746 (89) |
| Inappropriate age (≥ 90 years) | 3,349 (3) |
| Drug/condition interaction with ACEI/ARB | 3,144 (3) |
| Potential liver condition | 1,932 (2) |
| Potential history of high potassium levels | 1,808 (2) |
| Pregnancy | 227 (0.2) |
| Pharmacist exception reasons (n =2,977)a | |
| Patient does not meet call script requirementsb | 767 (26) |
| Previous failure or adverse drug reactionc | 698 (23) |
| Patient states he/she does not have DM | 307 (10) |
| Drug-disease interactionsd | 188 (6) |
| Overlapping alerts | 20 (0.7) |
| Patient cannot afford medication | 1 (0.03) |
| Other | 878 (29) |
aException reasons exceed the number of patients (n = 94,359); individual patients could have multiple reasons for exceptions to ACEI/ARB therapy.
bExamples of not meeting call script requirements included patients without high blood pressure, kidney problems, or heart problems or a history of heart attack, stroke, or heart surgeries.
cExamples of previous failure or adverse drug reaction included patients who previously took ACEI/ARB and had angioedema or low blood pressure.
dAn example of a drug-disease interaction included patient-reported hyperkalemia.
ACEI = angiotensin-converting enzyme inhibitor; ARB = angiontensin II receptor blocker; DM = diabetes mellitus.
Reasons why the pharmacist deemed it inappropriate to recommend an ACEI/ARB included (1) patient did not meet call script requirements (e.g., those without high blood pressure); (2) previous failure or ADE (e.g., patient previously took ACEI/ARB and had angioedema or low blood pressure); (3) patient cited not having DM or DM with conditions(e.g., hypertension) during the pharmacist-patient call although the clinically derived software system indicated he or she did; (4) drug-disease interactions (e.g., patient-reported hyperkalemia); (5) overlapping alerts; (6) patient could not afford medication; or (7) other (e.g., patient refused recommendation and claimed his or her physician provided excellent care).
Overlapping alerts indicated the patient was already flagged in the software system to receive the recommendation to add an ACEI or ARB for another condition (e.g., heart failure) or that a second recommendation was not made to prevent sending the prescribers a similar therapy recommendation for the same patient, potentially resulting in duplicate ACEI or ARB prescribing. A review of pharmacists’ consultation summaries highlighted that “other” was selected most frequently (29%) for reasons such as patient refused recommendation; therapy recommendation was on hold pending other doctor appointments for outstanding medical conditions; or patient was already taking another hypertensive agent and did not want to add an ACEI or ARB. The next most common reason via the pharmacist’s review was that the patient did not meet call script requirements (26%). This reason indicated that the patient had no risk factors or conditions to necessitate adding an ACEI or ARB at that time. Examples of not meeting call script requirements included patients with no history of high blood pressure, kidney problems, or heart problems or patients with a history of heart attack, stroke, or heart surgeries.
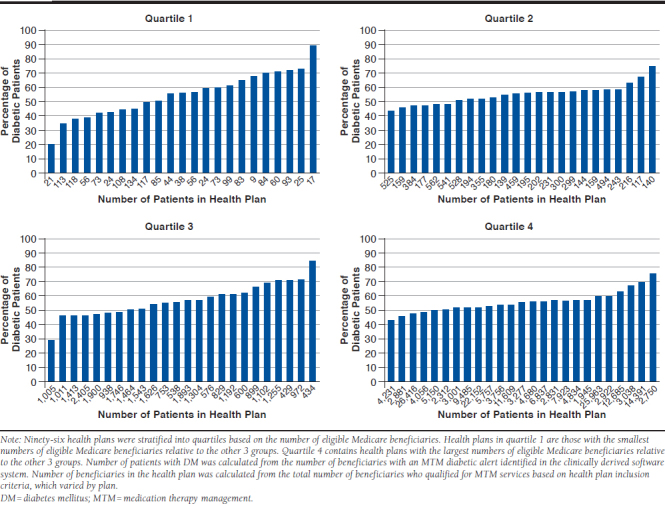
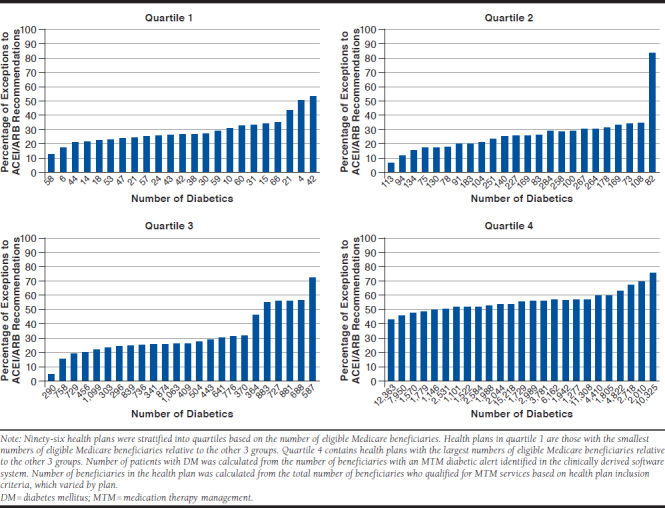
Among the 96 health plans in this study, an average of 55% (range: 19%-88%) of patients with DM qualified for MTM. When health plans were stratified into quartiles based on the number of eligible Medicare beneficiaries, the percentage of patients varied numerically by plan size: quartile 1 (smaller health plans): 19%-88%; quartile 2: 43%-74%; quartile 3: 29%-84%; and quartile 4 (larger health plans): 42%-74%. For an average of 29% of patients with DM, it was inappropriate to recommend adding an ACEI or ARB; however, the overall recommendation exception rate for all health plans ranged from 3%-83%. Additionally, when health plans were stratified based on number of beneficiaries with an MTM diabetic alert (i.e., identified in the clinically derived software system), patients ineligible to receive a recommendation to add an ACEI or ARB varied within the quartiles (quartile 1: 12%-52%; quartile 2: 5%-83%; quartile 3: 4%-72%; and quartile 4: 3%-60%).
Discussion
This analysis showed variations in the percentage of patients with DM for whom adding an ACEI or ARB was an exception across the 96 participating health plans. However, the proportion of DM patients also differed across health plans, possibly indicating case-mix inequity. The published literature describing variation in exception to recommend ACEI or ARB therapy among health plans is quite limited. However, many different factors may explain the variation observed in this study.
A retrospective study by Dharmarajan et al. (2014) studied 137,497 Medicare beneficiaries and found variation in patients’ characteristics across pharmacies in Mississippi. The researchers hypothesized that the variation could have influenced pharmacy quality measures.15 Another study by Trivedi et al. (2006) provided evidence that enrollees’ socioeconomic characteristics had an influence on quality Healthcare Effectiveness Data and Information Set (HEDIS) measures16; the distribution of overall performance and racial disparity varied widely across health plans (35%-70%).16 Case-mix adjustment or risk adjustment therefore may help account for differences in factors, such as demographics or comorbidities.17
Dharmarajan et. al performed a risk adjustment to account for patient characteristics such as age, race, and average number of prescriptions. As a result, risk-adjusted scores produced more reliable indicators of pharmacy quality performance score.15 Thus, the authors emphasized the importance of evaluating the effect of performance thresholds and medication utilization on health plan performance, subsequent bonus reimbursement, and incorporation of case-mix adjustment into value-based models for patients’ medication use.15 In contrast, Zaslavsky and Epstein (2005) reported that adjusting for patient characteristics had minimal effect on health plan performance.18
In the Medicare Part D population, Young et al. (2014) found that star ratings in the adherence domain were affected by adjusting performance scores for enrollees’ socioeconomic characteristics, leading them to recommend risk adjusting the adherence score.19 As these previous studies indicate, many factors, including demographic characteristics (e.g., age and gender) and socioeconomics (e.g., income and education), may influence performance scores. Eisenberg and Butterfield (2015) stated that many factors preceding health care service may affect health outcomes. For example, considerable evidence suggests that various sociodemographic factors influence health care outcomes and thus influence performance measures.20 In the interim, disparities in health care may correlate with sociodemographic status, suggesting the potential effect on performance measurement scores.20
Under the 2016 Medicare Star Rating System, CMS adopted 5 pharmacy quality measures developed by the Pharmacy Quality Alliance (PQA), a nonprofit organization developing medication use measures across various sectors, such as safety and appropriateness. Additionally, CMS is collaborating with PQA to account for the disproportionate numbers of patients with low socioeconomic status participating in the Medicare health plans. To this end, the first analysis of CMS data reporting on disparities in performance due to low income subsidy, dual eligibility, and/or disability showed that these factors had an effect on health plan performance.21 As a result of adjusting for these disparities, PQA and CMS reported modest movement in performance ranking and star ratings.22,23 Although the ACEIs or ARBs quality measure was retired in 2015-2016 due to various reasons (e.g., the first-line antihypertensive drug recommended by the 8th Joint National Committee can include other medication in addition to ACEIs and ARBs),24 the recommendation is to account for the exceptions for clinical appropriateness for current and future quality measures. It is important to better understand how these omissions may have an effect on health plans’ performance on star quality measures.
Limitations
This study has several limitations. First, the UAMMC uses a broad algorithm that targets beneficiaries with DM and hypertension (per Medicare Star Rating System measure recommendation) for adding ACEIs or ARBs. The system also targets patients with other risk factors including kidney disease and cardiovascular events, as suggested by the American Diabetes Association. Therefore, it was impossible to determine whether patients identified as having a DM diagnosis (e.g., flagged to receive a pharmacist intervention) also had hypertension or whether other DM-related risk factors prompted the alert for pharmacist consultation. Despite this limitation, the high proportion of “past presence of ACEI/ARB” (89%) among the exceptions suggests that the majority of the sample may have had DM and hypertension. If this is the case, it is feasible that these results are generalizable to the targeted population for this quality measure.
Second, different interpretations of the results are possible, so it is challenging to make any strong conclusions. For example, a high rate of “past presence of ACEI/ARB” exceptions could actually reflect a health plan whose members are already taking ACEIs or ARBs. The challenge is due to the uncertainty of internal validity. Internal validity is a commonly voiced concern when conducting real-world data studies.25 The dataset used for this study was not purposely designed or organized with the aim of supporting research. Based on the definition of an exception as “past presence of ACEI/ARB,” there were 2 scenarios: (1) those members who were already taking an ACEI or ARB and (2) those with high risk of failing the medication regimen for other reasons such as allergy, nonadherence, or stopping the medication altogether. Unfortunately, the exception did not record the date of the patient’s last ACEI/ARB fill, and as such, we could not differentiate between these 2 groups. However, excluding the exception would have erroneously omitted the latter group, jeopardizing the interval validity and thus not reflecting the reality of the situation. Therefore, the advantage of keeping the exception outweighed the advantage of excluding it. The investigators were well aware of this limitation. Future research is needed to redefine the exception more precisely to differentiate the cases, based on current versus previous ACEI or ARB use, to facilitate inclusion of the latter beneficiaries (as exceptions) to receive the pharmacist intervention.
Finally, the beneficiary-level data for the entire population were not accessible to the investigators.
Conclusions
This preliminary study suggests that substantial variation may exist in ACEI or ARB appropriateness for Medicare beneficiaries with DM across health plans. This finding provides initial evidence to support the need for plan-level risk adjustment when conducting large database analyses. Further research is needed to understand plan-level differences based on individual patient characteristics and to examine variations in therapy recommendation exceptions to determine the effect of incentives and programs promoting ACEI or ARB use across broad populations.
APPENDIX A. Percentage of Patients with DM Enrolled in Participating Health Plans During the 2013 Calendar Year
APPENDIX B. Percentage of Exceptions to Recommend Adding ACEIs or ARBs for Eligible Patients with DM in Participating Health Plans During the 2013 Calendar Year
REFERENCES
- 1.Centers for Medicare & Medicaid Services . Better care, smarter spending, healthier people: improving our health care delivery system. September 29, 2015. Available at: https://www.cms.gov/newsroom/fact-sheets/better-care-smarter-spending-healthier-people-improving-our-health-care-delivery-system-0. Accessed January 31, 2019.
- 2.Centers for Medicare & Medicaid Services . Part C and D performance data. 2015. Available at: https://www.cms.gov/medicare/prescription-drug-coverage/prescriptiondrugcovgenin/performancedata.html. Accessed January 31, 2019.
- 3.Centers for Medicare & Medicaid Services . Medicare marketing guidelines. June 10, 2016. Available at: https://www.cms.gov/Medicare/Health-Plans/ManagedCareMarketing/Downloads/2017MedicareMarketingGuidelines2.pdf. Accessed January 31, 2019.
- 4.Jacobson G, Neuman T, Damico A, Huang J. Medicare Advantage plan star ratings and bonus payments in 2012. Data brief. The Henry J. Kaiser Family Foundation. November 2011. Available at: https://kaiserfamilyfoundation. files.wordpress.com/2013/01/8257.pdf. Accessed January 31, 2019.
- 5.Centers for Medicare & Medicaid Services . 2013 Medicare Part D medication therapy management (MTM) programs. Fact sheet. September 12, 2013. Available at: https://www.cms.gov/medicare/prescription-drug-coverage/prescriptiondrugcovcontra/downloads/cy2013-mtm-fact-sheet.pdf. Accessed January 31, 2019.
- 6.Leslie RS, Tirado B, Patel BV, Rein PJ. Evaluation of an integrated adherence program aimed to increase Medicare Part D star rating measures. J Manag Care Spec Pharm. 2014;20(12):1193-203. Available at: https://www. jmcp.org/doi/10.18553/jmcp.2014.20.12.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.American Diabetes Association . Standards of medical care in diabetes—2013. Diabetes Care. 2013;36(Suppl 1):S11-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Centers for Medicare & Medicaid Services . Medicare health & drug plan quality and performance ratings 2013 Part C & D technical notes. 2013. Available at: https://www.cms.gov/medicare/prescription-drug-coverage/prescriptiondrugcovgenin/performance-data.html. Accessed February 8, 2019.
- 9.Glenn C, Taylor JL.. JNC 8 hypertension guideline algorithm. 2014. Available at: http://www.nmhs.net/documents/27JNC8HTNGuidelinesBook Booklet.pdf. Accessed January 31, 2019.
- 10.Aronow WS. Treating hypertension in older adults: safety considerations. Drug Saf. 2009;32(2):111-18. [DOI] [PubMed] [Google Scholar]
- 11.Grossman E, Messerli FH. Long-term safety of antihypertensive therapy. Prog Cardiovasc Dis. 2006;49(1):16-25. [DOI] [PubMed] [Google Scholar]
- 12.James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311(5):507-20. [DOI] [PubMed] [Google Scholar]
- 13.Ma TK, Kam KK, Yan BP, Lam YY. Renin-angiotensin-aldosterone system blockade for cardiovascular diseases: current status. Br J Pharmacol. 2010;160(6):1273-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaplan AP, Greaves MW. Angioedema. J Am Acad Dermatol. 2005;53(3): 373-88. [DOI] [PubMed] [Google Scholar]
- 15.Dharmarajan S, Bentley JP, Banahan Iii BF, West-Strum DS. Measuring pharmacy performance in the area of medication adherence: addressing the issue of risk adjustment. J Manag Care Spec Pharm. 2014;20(10):1057-68. Available at: https://www.jmcp.org/doi/10.18553/jmcp.2014.20.10.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Trivedi AN, Zaslavsky AM, Schneider EC, Ayanian JZ. Relationship between quality of care and racial disparities in Medicare health plans. JAMA. 2006;296(16):1998-2004. [DOI] [PubMed] [Google Scholar]
- 17.Calsbeek H, Markhorst J, Voerman GE, Braspenning JC. Case-mix adjustment for diabetes indicators: a systematic review. Am J Manag Care. 2016;22(2):e45-e52. [PubMed] [Google Scholar]
- 18.Zaslavsky AM, Epstein AM. How patients’ sociodemographic characteristics affect comparisons of competing health plans in California on HEDIS quality measures. Int J Qual Health Care. 2005;17(1):67-74. [DOI] [PubMed] [Google Scholar]
- 19.Young GJ, Rickles NM, Chou CH, Raver E. Socioeconomic characteristics of enrollees appear to influence performance scores for medicare part D contractors. Health Aff (Millwood). 2014;33(1):140-46. [DOI] [PubMed] [Google Scholar]
- 20.Eisenberg W, Butterfield KA. What roles do patient characteristics play in value-based performance? Am J Pharm Benefits. 2015;7(4):165-67. [Google Scholar]
- 21.Centers for Medicare & Medicaid Services . Examining the potential effects of socioeconomics factors on star ratings. September 8, 2015. Available at: https://www.cms.gov/Medicare/Prescription-Drug-Coverage/PrescriptionDrugCovGenIn/Downloads/Research-on-the-Impact-of-Socioeconomic-Status-on-Star-Ratingsv1-09082015.pdf. Accessed January 31, 2019.
- 22.Centers for Medicare & Medicaid Services . Advance Notice of Methodological Changes for Calendar Year (CY) 2018 for Medicare Advantage (MA) Capitation Rates, Part C and Part D Payment Policies and 2018 Call Letter. February 1, 2017. Available at: https://www.cms.gov/medicare/prescription-drug-coverage/prescriptiondrugcovgenin/performancedata.html. Accessed February 8, 2019.
- 23.Eisenberg W. Medication use performance measures in value-based systems. July 20, 2016. Available at: http://www.achp.org/wp-content/uploads/ACHP-PQA_Webinar_072016.pdf. Acessed February 8, 2019.
- 24.PL Detail-Document . Quality Measures for Pharmacies. Pharmacist’s Letter/Prescriber’s Letter. January 2016. Available at: http://cdn2.hubspot.net/hubfs/229441/Assets/sample_content/PL/2016-07_quality_measures_for_pharmacies.pdf. Accessed February 8, 2019.
- 25.Sherman RE, Anderson SA, Dal Pan GJ, et al. Real-world evidence—what is it and what can it tell us? N Engl J Med. 2016;375(23):2293-97. [DOI] [PubMed] [Google Scholar]


