Abstract
BACKGROUND:
Previous research suggests that weight loss is associated with decreases in health care costs among individuals with type 2 diabetes mellitus (T2DM) and that weight change can affect clinical measures, including hemoglobin A1c (A1c), low-density lipoprotein cholesterol (LDLC), and blood pressure. Previous research has also demonstrated more pronounced impact of weight change among patients with T2DM who are obese and have no evidence of cardiovascular disease (CVD).
OBJECTIVES:
To (a) examine the association between weight change and all-cause and diabetes-related health care costs among patients with T2DM; (b) examine the association between weight change and select clinical measures among patients with T2DM; and (c) analyze a subgroup of obese patients with no previous CVD.
METHODS:
This retrospective, observational cohort study used U.S. insurance claims linked to laboratory and electronic medical records. This study included patients with T2DM aged 18 years or older who added or switched to a nonmetformin antidiabetes medication after metformin monotherapy between January 1, 2007, and June 30, 2012 (date of add/switch was the index date). The primary predictor was percentage weight change (PWC) between a weight measurement at index and a follow-up measurement 6 months later; PWC ranged from negative (weight loss) to positive (weight gain). Outcomes, measured in the 12-month period beginning at the time of follow-up weight measurement, included all-cause and diabetes-related health care costs and achievement of thresholds for A1c, blood pressure, and LDL-C. Multivariable models quantified the association between PWC (linear effect) and study outcomes.
RESULTS:
A total of 1,520 patients (mean age 55 years; 47% female) were included, with 780 patients (mean age 53 years; 51% female) in the subgroup sample. Mean (SD) index weight and PWC were 224.6 (52.8) lbs and +0.2% (4.7%) in the primary analysis, and 241.3 (47.3) lbs and -0.2% (4.6%) in the subgroup sample. In adjusted analyses, decreasing PWC was associated with decreasing diabetes-specific pharmacy costs (P < 0.001) in the primary analysis sample and with decreasing all-cause pharmacy costs (P = 0.018), diabetes-specific total costs (P = 0.039), diabetes-specific medical costs (P = 0.002), and diabetes-specific pharmacy costs (P < 0.001) in the subgroup sample. PWC was not associated with all-cause total health care costs or all-cause medical costs in either sample. In adjusted analyses, decreasing PWC was also associated with increasing odds of attaining the A1c goals of < 6.5% (P < 0.001) and < 7.0% (P < 0.001) in the primary analysis sample and increasing odds of attaining the A1c goals of < 6.5% (P < 0.001), < 7.0% (P < 0.001), and < 8.0% (P = 0.010) in the subgroup sample. PWC was not associated with any of the other clinical measures in either of the study samples.
CONCLUSIONS:
This real-world study suggests that among patients with T2DM, weight loss over a short-term (6-month) period is associated with positive impact on attainment of A1c goals and decreased diabetes-specific pharmacy costs over the subsequent 12 months. In the subset of patients who were obese and had no previus CVD, weight loss over the 6-month period was also associated with decreased all-cause pharmacy costs, diabetes-specific medical costs, and diabetes-specific total health care costs. Future research is warranted to examine whether these associations change over longer-term periods of follow-up.
What is already known about this subject
Weight loss has been shown to improve glycemic control and cardiometabolic risk factors among individuals with type 2 diabetes mellitus (T2DM).
Previous studies suggest that weight loss among individuals with T2DM may reduce health care costs.
Ten-year follow-up of patients in the Look AHEAD (Action For Health in Diabetes) study demonstrated that such associations were most strongly observed among obese patients with T2DM and no cardiovascular disease (CVD).
What this study adds
This study is the first to characterize the economic and clinical impact of weight change among patients with T2DM who specifically added to or switched treatment from metformin, representing a clinical juncture at which a wide variety of potential antidiabetes medications with varying weight change properties are available for second-line treatment.
Subgroup analysis undertaken among obese patients with T2DM and no previous CVD showed a particularly pronounced effect of weight loss on economic outcomes; such patients represent a clinically meaningful subgroup, given that over 50% of patients with T2DM are obese and over 80% of patients with T2DM are free of CVD early (0-10 years) in the course of their disease.
Weight loss increased the likelihood of attainment of common goals for glycemic control among patients with T2DM, underscoring the importance of weight management to the primary goal of glycemic control in the management of diabetes.
Observational studies of patients with type 2 diabetes mellitus (T2DM) have associated obesity and increased weight with a variety of undesirable circumstances, such as reduced glycemic control,1-4 increased risk of cardiovascular disease (CVD),5,6 and increased risk of therapy discontinuation,7 which may consequently add to the clinical and economic burden of T2DM. Indeed, previous studies examining the economic impact of changes in weight among patients with T2DM, or specific subsets thereof, have generally found that weight gain (loss) is associated with increases (decreases) in all-cause and/or diabetes-specific health care utilization and costs.7-10
The American Diabetes Association (ADA) and European Association for the Study of Diabetes (EASD) recommend weight loss for overweight (body mass index [BMI] ≥ 25 kg/m2) and obese (BMI ≥ 30 kg/m2) individuals with diabetes and specifically note that among these patients relatively small changes in body weight that are achieved through intensive lifestyle intervention can improve fitness, glycemic control, and cardiovascular risk factors.11,12 These recommendations are largely based on data collected as part of the Look AHEAD (Action For Health in Diabetes) study, which randomly assigned individuals with T2DM who were at least overweight to an intensive lifestyle intervention (ILI) or a diabetes support and education comparator.13 In that study, weight loss of 5% to < 10% achieved through ILI over a 1-year period was associated with statistically significant improvements in CVD risk factors.14 Although this reduction in risk was not maintained in follow-up of 9.6 years,15 analyses of participants’ 10-year health care cost data demonstrated that ILI reduced health care utilization and costs among patients with no history of CVD.16 The authors concluded that such interventions may be most beneficial to overweight/obese individuals with T2DM before CVD is diagnosed.
The primary objective of this study was to examine the association between weight change and all-cause and diabetes-related health care costs. A secondary objective was to examine the association between weight change and clinical outcomes as defined in the 2014 National Committee for Quality Assurance (NCQA)/Healthcare Effectiveness Data and Information Set (HEDIS) Comprehensive Diabetes Care measures among patients with T2DM. We sought to characterize the economic impact of weight change among patients who added to or switched treatment from metformin, representing a population for which metformin monotherapy may have failed. Furthermore, this point in patient treatment was chosen because, whereas metformin monotherapy is clearly delineated in the ADA and EASD guidelines as first-line therapy for patients with type 2 diabetes, a wide variety of potential anti-diabetes medications with varying weight change properties are available for second-line treatment.12
Subanalyses were also undertaken among obese patients with T2DM and no previous CVD, a clinically meaningful subgroup, given that over 50% of patients with T2DM are obese and over 80% of patients with T2DM are free of CVD early (0-10 years) in the course of their disease.17-20
Methods
Data Source
This study used administrative claims, electronic medical records (EMRs), and laboratory results contained in the Truven Health MarketScan Claims-EMR Linked Database and the MarketScan Lab Database. The MarketScan Claims-EMR Linked Database comprises EMRs from a nationwide network of more than 30,000 health care providers using a common EMR system. These EMRs are linked, at a patient level, to enrollment information; demographic information; and inpatient medical, outpatient medical, and outpatient pharmacy claims data collected from over 300 large, self-insured U.S. employers and over 25 U.S. health plans. The database includes information for individuals who are either under the age of 65 and are the primary insured or a spouse or dependent thereof, or who are Medicare-eligible (primarily representing individuals aged 65 years or older) and have supplemental insurance paid for by their current or former employers. Laboratory results from the MarketScan Lab Database were also linked, at a patient level, to the Claims-EMR Linked Database when possible. All study data in the MarketScan databases are deidentified and fully compliant with the Health Insurance Portability and Accountability Act (HIPAA) of 1996.
All study variables were measured from the databases using International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) diagnosis and procedure codes, Current Procedural Terminology (CPT) codes, Healthcare Common Procedure Coding System (HCPCS) codes, National Drug Code (NDC) numbers, EMR observation key identifiers, and Logical Observation Identifiers Names and Codes (LOINC), as appropriate. Generic names of all study medications and all ICD-9-CM, CPT, HCPCS, EMR identifiers, and LOINC codes used in this study are available in Appendix A (available in online article). Unless otherwise noted, diagnoses and procedures were searched for in all diagnosis/procedure positions on the administrative claims.
Study Sample Selection Criteria and Time Periods
Figure 1 displays a flowchart of the sample selection process. The study sample comprised patients with ≥ 1 outpatient pharmacy claim for metformin that was followed by ≥ 1 outpatient pharmacy claim for a nonmetformin antidiabetes medication between January 1, 2007, and June 30, 2012. The date of the claim for the nonmetformin antidiabetes medication was designated as the index date. Patients were required to be aged 18 years or older on the index date and have continuous enrollment in medical and pharmacy benefits for 6 months before the index date (baseline period) and for ≥ 18 months after the index date (follow-up period). Patients were required to have ≥ 1 outpatient pharmacy claim for metformin, but no outpatient pharmacy claims for any other antidiabetes medication during the baseline period. Patients additionally were required to have ≥ 1 medical claim with a diagnosis of T2DM during the baseline period or on the index date. Patients were excluded if they had ≥ 1 medical claim with a diagnosis or procedure (where applicable) of type 1 diabetes, gestational diabetes, pregnancy, or bariatric surgery during the baseline or follow-up periods.
FIGURE 1.

Sample Selection Process
Patients were required to have ≥ 1 weight measurement within 45 days before to 14 days after the index date (baseline weight), and ≥ 1 weight measurement approximately 6 months later (within 135 to 225 days after the index date; follow-up weight). In addition, ≥ 1 blood pressure and ≥ 1 hemoglobin (A1c) measurement were required in the period spanning 45 days before to 14 days after the index date, as well as at any point on or after the follow-up weight measurement.
For the subgroup of obese patients with no previous CVD, patients were required to have BMI information available in their medical records, be classified as obese using the baseline weight (BMI ≥ 30 kg/m2), 21 and have no medical claims with a diagnosis for CVD—defined as ≥ 1 medical claim with a diagnosis or procedure (where applicable) listed in the “Cardiovascular disease” section of Appendix A—during the baseline period. Within this subgroup, patients were also excluded if they had ≥ 1 medical claim with a diagnosis of cancer (except for nonmelanoma skin cancer) during the baseline or follow-up periods.
Measurement of Primary Independent Variable
The primary independent variable of interest was percentage weight change (PWC) between the baseline weight and the follow-up weight. PWC could range from negative (weight loss) to positive (weight gain). For the purposes of descriptive reporting only, patients were categorized according to whether their PWC indicated (a) weight gain > 3%, or (b) nonweight gain, which included weight gain ≤ 3% and weight loss.22
Measurement of Study Outcomes
Beginning on the date of the follow-up weight measurement, patients’ data for the subsequent 12-month period (outcomes follow-up period) were evaluated to collect information on the study outcomes. Thus, the period during which the PWC was assessed was temporally precedent to the period during which the study outcomes were assessed.
Measurement of NCQA/HEDIS Comprehensive Diabetes Care Outcomes.
The NCQA/HEDIS Comprehensive Diabetes Care outcomes of primary interest were 3 individual measures of A1c control (poor control [> 9.0%], control [< 8.0%], and control [< 7.0%]) and 2 individual measures of blood pressure control (control [< 130/80 mm Hg] and control [< 140/90 mm Hg]). A measure of low-density lipoprotein cholesterol (LDL-C) control (< 100 mg/dL) was examined in the subset of patients for whom LDL-C data were available. Finally, a more stringent measure of A1c control (< 6.5%) was also examined, since it is advocated by the ADA as a reasonable goal for selected patients.23 Consistent with the NCQA/HEDIS specifications, the first available clinical measure that occurred in the outcomes follow-up period was used for analysis.
Measurement of Health Care Cost Outcomes.
All-cause health care costs were measured based on all outpatient pharmacy claims and all inpatient and outpatient medical claims incurred during the outcomes follow-up period. Diabetes-specific costs were measured based on outpatient pharmacy claims for antidiabetes drugs and inpatient and outpatient medical claims with a diagnosis of T2DM (ICD-9-CM 250. x0 and 250.x2) in any diagnosis position incurred during the outcomes follow-up period.24-26
For the all-cause and the diabetes-specific classifications, health care costs were measured for the following mutually exclusive and exhaustive settings of care: inpatient admissions, emergency room (ER) visits, outpatient office visits and services, and pharmacy. The previously mentioned settings of care were distinguished based on place of service, by which the MarketScan databases are organized and classified. Health care costs were expressed in 2013 constant dollars, adjusted using the medical care component of the Consumer Price Index.27
Health care costs included the gross covered payments for all health care services or products, including the patients’ and the payers’ portions of payment.
Measurement of Patient Demographic and Clinical Characteristics
Patient demographics and clinical characteristics measured for this study are listed in Tables 1 and 2. Demographics were measured as of the index date based on insurance enrollment records. Clinical characteristics were measured during the baseline period. Comorbidities were identified based on the presence of ≥ 1 medical claim with an ICD-9-CM diagnosis of interest recorded in any diagnosis position. To reduce the potential for false positives, medical claims associated with services that may be used in the process to rule out conditions (e.g., laboratory claims or radiology claims) were not considered when searching for diagnoses. Medications were identified based on the presence of ≥ 1 outpatient pharmacy claim for the medication of interest. Several indices of health status were measured based on medical claims, including the Deyo adaptation of the Charlson Comorbidity Index, the Adapted Diabetes Complications Severity Index, count of unique 3-digit ICD-9-CM diagnosis codes, count of unique NDC numbers, and total health care expenditures.28-30
TABLE 1.
Patient Index Demographics
| Primary Analysis Sample | Subgroup Sample a | |||||
|---|---|---|---|---|---|---|
| Weight Gain Group n = 325 | Nonweight Gain Groupb n = 1,195 | P Value | Weight Gain Group n = 134 | Nonweight Gain Groupb n = 646 | P Value | |
| Age, mean [SD] | 55.3 [11.2] | 55.1 [10.9] | 0.741 | 53.9 [11.3] | 53.0 [10.0] | 0.368 |
| Age categories, % (n) | 0.086 | 0.061 | ||||
| 18-34 | 4.3 (14) | 2.4 (29) | 6.0 (8) | 3.1 (20) | ||
| 35-44 | 8.9 (29) | 13.8 (165) | 11.2 (15) | 16.4 (106) | ||
| 45-54 | 34.5 (112) | 31.3 (374) | 36.6 (49) | 36.1 (233) | ||
| 55-64 | 34.5 (112) | 35.9 (429) | 28.4 (38) | 33.4 (216) | ||
| 65-74 | 11.7 (38) | 11.6 (139) | 14.2 (19) | 9.4 (61) | ||
| 75+ | 6.2 (20) | 4.9 (59) | 3.7 (5) | 1.5 (10) | ||
| Sex, % (n) | 0.206 | 0.266 | ||||
| Male | 56.0 (182) | 52.1 (622) | 53.7 (72) | 48.5 (313) | ||
| Female | 44.0 (143) | 47.9 (573) | 46.3 (62) | 51.5 (333) | ||
| Geographic region, % (n) | 0.250 | 0.906 | ||||
| Northeast | 21.2 (69) | 23.2 (277) | 23.1 (31) | 22.6 (146) | ||
| North Central | 17.8 (58) | 16.8 (201) | 18.7 (25) | 19.2 (124) | ||
| South | 45.8 (149) | 45.9 (548) | 44.8 (60) | 44.3 (286) | ||
| West | 12.9 (42) | 13.4 (160) | 11.2 (15) | 12.7 (82) | ||
| Unknown | 2.2 (7) | 0.8 (9) | 2.2 (3) | 1.2 (8) | ||
| Insurance plan type, % (n) | 0.091 | 0.954 | ||||
| Basic/major medical | 0.0 (0) | 0.0 (0) | 0.0 (0) | 0.0 (0) | ||
| Comprehensive | 8.6 (28) | 10.6 (127) | 9.0 (12) | 8.7 (56) | ||
| Exclusive provider organization | 0.0 (0) | 0.8 (10) | 0.0 (0) | 0.6 (4) | ||
| Health maintenance organization | 14.2 (46) | 11.8 (141) | 13.4 (18) | 11.3 (73) | ||
| Point of service | 9.2 (30) | 11.5 (137) | 11.2 (15) | 12.4 (80) | ||
| Preferred provider organization | 57.5 (187) | 51.0 (610) | 54.5 (73) | 51.7 (334) | ||
| Point of service with capitation | 0.6 (2) | 2.0 (24) | 0.7 (1) | 2.0 (13) | ||
| Consumer-directed health plan | 4.6 (15) | 5.7 (68) | 5.2 (7) | 5.7 (37) | ||
| High deductible health plan | 2.2 (7) | 1.7 (20) | 2.2 (3) | 1.9 (12) | ||
| Unknown | 3.1 (10) | 4.9 (58) | 3.7 (5) | 5.7 (37) | ||
| Population density, % (n) | 0.402 | 0.972 | ||||
| Urban | 78.8 (256) | 80.2 (958) | 79.1 (106) | 79.4 (513) | ||
| Rural | 19.7 (64) | 19.1 (228) | 19.4 (26) | 19.3 (125) | ||
| Unknown | 1.5 (5) | 0.8 (9) | 1.5 (2) | 1.2 (8) | ||
| Payer, % (n) | 0.452 | 0.023 | ||||
| Commercial | 81.2 (264) | 83.0 (992) | 81.3 (109) | 88.5 (572) | ||
| Medicare | 18.8 (61) | 17.0 (203) | 18.7 (25) | 11.5 (74) | ||
a Sample restricted to patients with BMI ≥ 30 and no evidence of previous cardiovascular disease.
b Weight gain = increase in weight by > 3%; nonweight gain = weight gain ≤ 3% and weight loss.
BMI = body mass index; SD = standard deviation.
TABLE 2.
Patient Baseline Clinical Characteristics
| Primary Analysis Sample | Subgroup Samplea | |||||
|---|---|---|---|---|---|---|
| Weight Gain Group n = 325 | Nonweight Gain Groupb n = 1,195 | P Value | Weight Gain Group n = 134 | Nonweight Gain Groupb n = 646 | P Value | |
| Alc (as %) | ||||||
| Baseline measurement, mean [SD] | 8.8 [2.3] | 8.1 [1.6] | < 0.001 | 8.8 [2.3] | 8.2 [1.6] | < 0.001 |
| Poor control (> 9.0%), % (n) | 36.0 (117) | 21.3 (255) | 0.001 | 36.7 (62) | 22.8 (176) | < 0.001 |
| Control (< 8.0%), % (n) | 47.1 (153) | 56.8 (679) | 0.009 | 45.0 (76) | 55.5 (428) | 0.013 |
| Control (< 7.0%), % (n) | 22.2 (72) | 20.3 (242) | 0.701 | 18.9 (32) | 17.9 (138) | 0.751 |
| Control (< 6.5%), % (n) | 11.4 (37) | 8.8 (105) | 0.373 | 10.7 (18) | 8.0 (62) | 0.271 |
| Blood pressure (mm Hg) | ||||||
| Baseline systolic, mean [SD] | 115.5 [17.1] | 115.5 [17.9] | 0.723 | 115.8 [17.6] | 116.1 [17.8] | 0.855 |
| Baseline diastolic, mean [SD] | 90.8 [16.1] | 91.2 [16.4] | 0.651 | 90.9 [16.3] | 91.9 [16.8] | 0.478 |
| Control (< 130/80 mm Hg), % (n) | 25.8 (84) | 25.5 (305) | 0.693 | 26.6 (45) | 25.6 (197) | 0.772 |
| Control (< 140/90 mm Hg), % (n) | 46.2 (150) | 48.5 (580) | 0.713 | 47.3 (80) | 47.6 (367) | 0.951 |
| LDL-C (mg/dL) c | n = 150 | n = 606 | n = 61 | n = 342 | ||
| Baseline measurement, mean [SD] | 99.6 [33.9] | 96.0 [32.8] | 0.869 | 96.7 [30.3] | 97.4 [32.7] | 0.869 |
| Control (< 100 mg/dL), % (n) | 77.8 (253) | 77.9 (931) | 0.357 | 79.9 (107) | 76.2 (492) | 0.357 |
| Baseline body weight, mean [SD] | ||||||
| Weight (pounds) | 213.2 [48.8] | 227.7 [53.5] | 0.083 | 234.1 [43.3] | 242.7 [48.0] | 0.033 |
| Weight (kilograms) | 96.7 [22.1] | 103.3 [24.3] | 0.083 | 106.2 [19.6] | 110.1 [21.8] | 0.033 |
| Antidiabetes medications at index date, % (n) | ||||||
| Alpha-glucosidase inhibitors | 0.0 (0) | 0.3 (4) | 1.000 | 0.0 (0) | 0.1 (1) | 1.000 |
| Biguanides | 15.7 (51) | 12.2 (146) | 0.003 | 20.1 (34) | 12.3 (95) | 0.008 |
| DPP-4 inhibitors | 11.4 (37) | 17.7 (212) | 0.004 | 8.9 (15) | 17.6 (136) | 0.005 |
| Meglitinides | 0.3 (1) | 1.5 (18) | 0.610 | 0.0 (0) | 0.9 (7) | 0.363 |
| Sulfonylureas | 55.7 (181) | 43.8 (524) | 0.007 | 55.6 (94) | 41.5 (320) | < 0.001 |
| Thiazolidinediones | 12.0 (39) | 9.9 (118) | 0.050 | 13.6 (23) | 10.1 (78) | 0.184 |
| Insulins | 6.2 (20) | 5.4 (65) | 0.774 | 4.7 (8) | 5.3 (41) | 0.757 |
| Glucagon-like peptide-1 receptor agonists | 2.8 (9) | 9.3 (111) | 0.006 | 3.6 (6) | 11.5 (89) | 0.002 |
| Amylin analog | 0.0 (0) | 0.0 (0) | NA | 0.0 (0) | 0.0 (0) | NA |
| SGLT-2 inhibitors | 0.0 (0) | 0.0 (0) | NA | 0.0 (0) | 0.0 (0) | NA |
| Biguanides & DPP-4 inhibitors | 5.5 (18) | 8.9 (106) | 0.239 | 6.5 (11) | 9.2 (71) | 0.260 |
| Biguanides & sulfonylureas | 4.6 (15) | 1.9 (23) | 0.261 | 4.7 (8) | 2.5 (19) | 0.125 |
| Biguanides & meglitinides | 0.0 (0) | 0.0 (0) | NA | 0.0 (0) | 0.0 (0) | NA |
| Biguanides & thiazolidinediones | 3.4 (11) | 1.8 (21) | 0.277 | 4.1 (7) | 1.8 (14) | 0.081 |
| DPP-4 inhibitors & thiazolidinediones | 0.0 (0) | 0.0 (0) | NA | 0.0 (0) | 0.0 (0) | NA |
| Sulfonylureas & thiazolidinediones | 0.3 (1) | 0.2 (2) | 1.000 | 0.0 (0) | 0.3 (2) | 1.000 |
| Microvascular complications, % (n) | ||||||
| Diabetic nephropathy | 1.2 (4) | 1.4 (17) | 0.684 | 1.2 (2) | 1.3 (10) | 1.000 |
| Diabetic retinopathy | 2.8 (9) | 3.4 (41) | 0.602 | 1.8 (3) | 3.9 (30) | 0.176 |
| Diabetic peripheral neuropathy | 4.3 (14) | 3.6 (43) | 0.053 | 5.9 (10) | 3.4 (26) | 0.118 |
| Any microvascular disease (any of above) | 8.0 (26) | 8.1 (97) | 0.399 | 8.3 (14) | 8.2 (63) | 0.961 |
| Other comorbidities, % (n) | ||||||
| Hypertension | 74.2 (241) | 77.2 (922) | 0.879 | 76.3 (129) | 78.1 (602) | 0.620 |
| Dyslipidemia | 67.1 (218) | 64.4 (770) | 0.699 | 64.5 (109) | 62.4 (481) | 0.607 |
| Depression | 4.0 (13) | 5.1 (61) | 0.969 | 4.1 (7) | 5.8 (45) | 0.383 |
| Hypoglycemia | 1.5 (5) | 1.2 (14) | 0.657 | 1.8 (3) | 0.9 (7) | 0.398 |
| DCI, mean [SD] | 1.3 [0.9] | 1.3 [1.0] | 0.790 | 1.2 [0.7] | 1.2 [0.8] | 0.640 |
| ADCSI, mean [SD] | 0.4 [0.9] | 0.4 [0.8] | 0.420 | 0.2 [0.6] | 0.2 [0.5] | 0.259 |
| Unique 3-digit ICD-9-CM codes, mean [SD] | 6.5 [5.2] | 6.4 [4.6] | 0.767 | 6.0 [4.9] | 5.8 [4.0] | 0.491 |
| Unique National Drug Code numbers, mean [SD] | 7.8 [5.2] | 8.1 [5.1] | 0.090 | 8.0 [5.3] | 7.7 [4.8] | 0.451 |
| Total health care expenditures, mean [SD] | $5,255 [$9,549] | $5,083 [$9,882] | 0.174 | $4,326 [$7,510] | $3,730 [$5,786] | 0.251 |
a Sample restricted to patients with BMI ≥ 30 and no evidence of previous cardiovascular disease.
b Weight gain = increase in weight by > 3%; nonweight gain = weight gain ≤ 3% and weight loss.
c Analyses conducted on a subset of patients for whom LDL-C data were available.
Alc = hemoglobin A1c; ADCSI = Adapted Diabetes Complications Severity Index; BMI = body mass index; DCI = Deyo adaptation of Charlson Comorbidity Index;
DPP-4 = dipeptidyl peptidase-4; ICD-9-CM = International Classification of Diseases, Ninth Revision, Clinical Modification; LDL-C = low-density lipoprotein cholesterol;
NA = not applicable; SD = standard deviation; SGLT-2 = sodium/glucose cotransporter 2.
Statistical Analyses
Bivariate analyses were used to descriptively summarize patient demographics, baseline clinical characteristics, and outcomes, stratifying patients by the weight gain and nonweight gain groups. To understand whether patient characteristics differed between the descriptive stratifications, chi-square tests were used to test for statistically significant differences in categorical variables between the cohorts, and t-tests were used to test for statistically significant differences in continuous variables. Hypothesis testing was not completed for the unadjusted analyses of outcomes.
Multivariable generalized linear models were used to examine the adjusted association between a 1 percentage point decrease in PWC (weight loss) and the study outcomes. Models examining the clinical outcomes, all of which were binary, used a logit link and binomial distribution. Models examining health care costs used a log link and gamma distribution. All models adjusted for the following a priori defined specification of covariates: baseline A1c, baseline weight, baseline blood pressure < 140/90 mm Hg, commercial insurance (vs. Medicare supplemental), index year, age category, population density, health plan type, geographic region of residence, sex, baseline or follow-up cancer diagnosis (not included in subanalyses), and baseline clinical characteristics including the Deyo adaptation of the Charlson Comorbidity Index, Adapted Diabetes Complications Severity Index, count of unique 3-digit ICD-9-CM diagnosis codes, count of unique NDC numbers, inpatient stay, ER visit, general practitioner outpatient office visits, cost sharing for outpatient services, cost sharing for outpatient office visits, depression, microvascular disease, CVD (not included in subanalyses), hypertension, dyslipidemia, total health care expenditures, and flags for individual antidiabetes medication classes filled on the index date. The models in which LDL-C control (< 100 mg/dL) was examined also included a covariate for baseline LDL-C value. The absence of multicollinearity among the variables included in the models was verified through the variance inflation factor.31
Absolute adjusted effect estimates, in the form of predicted outcomes at various PWC values while holding other covariates constant, were generated through the recycled prediction method.32 Analyses were conducted using Stata version 12 (StataCorp, College Station, TX). A P value of 0.05 was selected a priori as the maximum P value for which differences were considered statistically significant.
Results
PWC and Patient Characteristics
A total of 1,520 patients were included in the primary analysis sample, and 780 were included in the subgroup sample (Figure 1). Overall, the mean and standard deviation [SD] baseline weight measurement was 224.6 [52.8] lbs for the primary analysis sample and 241.3 [47.3] lbs for the subgroup sample (101.9 [24] kg and 109.5 [21.5] kg, respectively).
The distribution of PWC between baseline and follow-up, ranging from negative (weight loss) to positive (weight gain), is displayed in Figure 2 and Figure 3 for the primary and subgroup samples, respectively. At the follow-up weight measurement taken approximately 6 months after the baseline measurement, the mean [SD] absolute change in weight from baseline was -0.09 [11.1] lbs (-0.04 [5.0] kg), translating to a mean [SD] PWC of 0.2% [4.7%] for the primary analysis sample and -0.7 [11.8] lbs (-0.32 [5.3] kg), translating to a mean [SD] PWC of -0.2% [4.6%] for the subgroup sample.
FIGURE 2.

Distribution of Percentage Weight Change for Primary Sample
FIGURE 3.
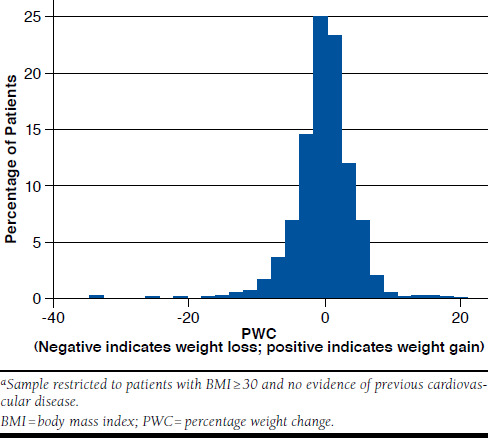
Distribution of Percentage Weight Change for Subgroup Sample
In both samples, demographics were generally similar between the weight gain and nonweight gain groups (Table 1). In both samples, mean baseline A1c was higher among the weight gain versus nonweight gain groups (Table 2). Compared with weight gain groups, a lower proportion of patients in the nonweight gain groups were taking sulfonylureas at index or metformin at index (Table 2); conversely, compared with weight gain groups, a higher proportion of patients in the nonweight gain groups were taking dipeptidyl peptidase-4 inhibitors at index or glucagon-like peptide-1 receptor agonists at index. Other clinical characteristics were generally similar across the groups (Table 2).
NCQA/HEDIS Comprehensive Diabetes Care Outcomes in 12-Month Outcomes Follow-up Period
In adjusted analyses, decreasing PWC (weight loss) was associated with increasing odds of attaining the A1c goals of < 6.5% (P < 0.001) and < 7.0% (P < 0.001) in the primary analysis sample and increasing odds of attaining the A1c goals of < 6.5% (P < 0.001), < 7.0% (P < 0.001), and < 8.0% (P = 0.010) in the subgroup sample (Table 3). PWC was not associated with LDL-C or blood pressure in the primary or subgroup samples.
TABLE 3.
Adjusted NCQA/HEDIS Comprehensive Diabetes Care and Clinical Outcomes During Outcomes Follow-up Period
| Adjusted Effect Estimatea | Adjusted Predicted Probabilities,b % | |||||
|---|---|---|---|---|---|---|
| PWC | ||||||
| -5% | -2.5% | 0% | 2.5% | 5% | ||
| Primary analysis sample | ||||||
| A1c (as %), n = 1,520 | ||||||
| Poor control (> 9.0) | OR = 0.99 (95% CI = 0.95-1.03) P= 0.660 | NS | NS | NS | NS | NS |
| Control (< 8.0) | OR = 1.02 (95% CI = 0.99-1.05) P= 0.240 | NS | NS | NS | NS | NS |
| Control (< 7.0) | OR = 1.05 (95% CI = 1.03-1.09) P < 0.001 | 58.8 | 55.9 | 52.8 | 49.8 | 46.7 |
| Control (< 6.5) | OR = 1.08 (95% CI = 1.05-1.11) P < 0.001 | 43.1 | 39.0 | 35.1 | 31.3 | 27.8 |
| Blood pressure (mm Hg), n = 1,520 | ||||||
| Control (< 130/80 mm Hg) | OR = 1.01 (95% CI = 0.99-1.04) P= 0.380 | NS | NS | NS | NS | NS |
| Control (< 140/90 mm Hg) | OR = 1.00 (95% CI = 0.98-1.03) P= 0.700 | NS | NS | NS | NS | NS |
| LDL-C (mg/dL), n = 756c | ||||||
| Control (< 100 mg/dL) | OR = 1.02 (95% CI = 0.99-1.06) P= 0.210 | NS | NS | NS | NS | NS |
| Subgroup sample d | ||||||
| A1c (as %), n = 780 | ||||||
| Poor control (> 9.0) | OR = 0.96 (95% CI = 0.90-1.03) P= 0.250 | NS | NS | NS | NS | NS |
| Control (< 8.0) | OR = 1.08 (95% CI = 1.02-1.14) P= 0.010 | 86.6 | 84.8 | 82.8 | 80.7 | 78.3 |
| Control (< 7.0) | OR = 1.11 (95% CI = 1.06-1.15) P < 0.001 | 63.2 | 57.9 | 52.5 | 47.1 | 41.7 |
| Control (< 6.5) | OR = 1.09 (95% CI = 1.04-1.12) P < 0.001 | 43.9 | 39.8 | 35.8 | 32.0 | 28.3 |
| Blood pressure (mm Hg), n = 780 | ||||||
| Control (< 130/80 mm Hg) | OR = 0.98 (95% CI = 0.94-1.02) P= 0.410 | NS | NS | NS | NS | NS |
| Control (< 140/90 mm Hg) | OR = 0.97 (95% CI = 0.94-1.01) P= 0.144 | NS | NS | NS | NS | NS |
| LDL-C (mg/dL), n = 403c | ||||||
| Control (< 100 mg/dL) | OR = 1.03 (95% CI = 0.97-1.09) P= 0.287 | NS | NS | NS | NS | NS |
a Association between 1 percentage point decrease in PWC and outcome.
b Adjusted predicted probabilities of attaining A1c goals calculated for outcomes with which the PWC was significantly associated.
c Analyses conducted on a subset of patients for whom LDL-C data were available.
d Sample restricted to patients with BMI ≥ 30 and no evidence of previous cardiovascular disease.
Alc = hemoglobin A1c; BMI = body mass index; CI = confidence interval; LDL-C = low-density lipoprotein cholesterol; NCQA/HEDIS = National Committee for Quality
Assurance/Healthcare Effectiveness Data and Information Set; NS = not significant; OR = odds ratio; PWC = percentage weight change.
The adjusted predicted probabilities of attaining the A1c goals with which the PWC was significantly associated are displayed in Table 3 for varying levels of the PWC in the primary and subgroup samples. For example, in the primary analysis sample, the adjusted predicted probability of attaining the A1c goal of < 6.5% as of the first A1c measurement during the outcomes follow-up period was 39.0% for a PWC of -2.5%, 35.1% for a PWC of 0%, and 31.3% for a PWC of 2.5% (Table 3). Unadjusted results are presented in Appendix B (available in online article).
Health Care Cost Outcomes in 12-Month Outcomes Follow-up Period
In adjusted analyses, decreasing PWC (weight loss) was associated with decreasing diabetes-specific pharmacy costs (P < 0.001) in the primary analysis sample and with decreasing all-cause pharmacy costs (P = 0.018), diabetes-specific total costs (P = 0.039), diabetes-specific medical costs (P = 0.002), and diabetes-specific pharmacy costs (P < 0.001) in the sub-group sample (Table 4). PWC was not associated with all-cause total health care costs or all-cause medical costs in either sample.
TABLE 4.
Adjusted Health Care Cost Outcomes During Outcomes Follow-up Period
| Adjusted Effect Estimatea | Adjusted Predicted Costs, Mean $b | |||||
|---|---|---|---|---|---|---|
| PWC | ||||||
| -5% | -2.5% | 0% | 2.5% | 5% | ||
| Primary analysis sample | ||||||
| All-cause, n = 1,520 | ||||||
| Medical | CR = 1.00 (95% CI = 0.98-1.02) P= 0.920 | NS | NS | NS | NS | NS |
| Pharmacy | CR = 0.99 (95% CI = 0.98-1.01) P= 0.280 | NS | NS | NS | NS | NS |
| Total | CR = 1.00 (95% CI = 0.98-1.02) P= 0.950 | NS | NS | NS | NS | NS |
| Diabetes-specific, n = 1,520 | ||||||
| Medical | CR = 0.99 (95% CI = 0.96-1.03) P=0.760 | NS | NS | NS | NS | NS |
| Pharmacy | CR = 0.97 (95% CI = 0.96-0.99) P < 0.001 | 1,141 | 1,224 | 1,312 | 1,406 | 1,507 |
| Total | CR = 0.99 (95% CI = 0.96-1.01) P= 0.350 | NS | NS | NS | NS | NS |
| Subgroup sample c | ||||||
| All-cause, n = 780 | ||||||
| Medical | CR = 0.99 (95% CI = 0.96-1.02) P= 0.586 | NS | NS | NS | NS | NS |
| Pharmacy | CR = 0.98 (95% CI = 0.96-0.996) P= 0.018 | 4,302 | 4,567 | 4,848 | 5,147 | 5,464 |
| Total | CR = 0.99 (95% CI = 0.96-1.01) P= 0.209 | NS | NS | NS | NS | NS |
| Diabetes-specific, n = 780 | ||||||
| Medical | CR = 0.97 (95% CI = 0.94-0.998) P= 0.030 | 3,096 | 3,340 | 3,603 | 3,887 | 4,193 |
| Pharmacy | CR = 0.97 (95% CI = 0.95-0.99) P= 0.002 | 1,208 | 1,317 | 1,436 | 1,565 | 1,706 |
| Total | CR = 0.96 (95% CI = 0.95-0.98) P < 0.001 | 4,118 | 4,448 | 4,804 | 5,189 | 5,604 |
a Association between 1 percentage point decrease in PWC and outcome.
b Adjusted predicted costs calculated for outcomes with which the PWC was significantly associated.
c Sample restricted to patients with BMI ≥ 30 and no evidence of previous cardiovascular disease.
BMI = body mass index; CI = confidence interval; CR = cost ratio; NS = not significant; PWC = percentage weight change.
The adjusted predicted health care costs with which the PWC was significantly associated are displayed in Table 4 for varying levels of the PWC in the primary and subgroup samples. For example, in the subgroup sample, adjusted mean predicted per-patient diabetes-specific total health care costs during the 12-month outcomes follow-up period were $4,448 for a PWC of -2.5%, $4,804 for a PWC of 0%, and $5,189 for a PWC of 2.5%. Unadjusted results are presented in Appendices C and D (available in online article).
Discussion
This study examined the association between weight change, health care costs, and various clinical outcomes among patients with T2DM, who, as of the index date, had specifically added to or switched treatment from metformin. This study also examined a subgroup of patients who were obese and had no previous CVD. In the primary analysis sample and the subgroup sample, decreasing PWC was found to be significantly associated with increasing odds of attaining several common A1c goals (P < 0.001 for A1c < 7.0 and A1c < 6.5 in the primary analysis sample and subgroup sample; P = 0.01 for A1c < 8.0 in the subgroup sample). Although decreasing PWC was generally associated with decreasing all-cause and diabetes-specific health care costs, this association reached statistical significance in the primary analysis sample for diabetes-specific pharmacy costs (P < 0.001). In the subgroup sample, the association was stronger, reaching statistical significance for all-cause pharmacy costs (P = 0.018) and diabetes-specific medical (P = 0.030), pharmacy (P = 0.002), and total costs (P < 0.001).
To our knowledge, 4 published observational studies have quantified the association between general weight change (i.e., not necessarily resulting from an intervention) and health care utilization and/or costs among patients with T2DM. Yu et al. (2007) analyzed data from 458 U.S. patients with T2DM and reported that 1 percentage point of weight loss over a 6-month period was associated with a 3.6% decrease in total health care costs and a 5.8% decrease in diabetes-specific health care costs in the subsequent year.9 Consistent with the present study’s findings of more pronounced effects of PWC among the subgroup sample of obese patients, Yu et al. found a statistically significant 6.7% (P < 0.01) reduction in diabetes-specific health care costs in obese patients versus a nonstatistically significant 3.5% reduction in nonobese patients.9 Dilla et al. (2012) analyzed self-reported clinical and economic data collected from 738 Spanish patients with T2DM and reported that over a 1-year period, a 1-unit gain in BMI was associated with a 20.0% increase in estimated costs during the same period among BMI gainers, while a 1-unit loss in BMI was associated with an 8.0% decrease in costs among non-BMI gainers.8 Dilla et al. also found more pronounced effects of weight change among patients who were obese, with a 1-unit reduction in BMI associated with a 9.4% decrease in health care costs among obese patients versus 2.7% in nonobese patients.8 Bell et al. (2014) analyzed data from 2,110 patients with T2DM and reported significant associations between weight change over a 6-month period and all-cause total health care costs in the subsequent year but, unlike the present study, found no associations between weight change and all-cause and diabetes-specific pharmacy costs.7 Finally, Davis et al. (2011) analyzed data on 590 Australian patients with T2DM and reported that weight loss ≥ 5% of initial body weight versus weight loss < 5% of initial body weight or weight gain over a mean 4.3-year period was associated with an AUD$100 decrease in diabetes medication costs.10
Thresholds of A1c < 7% (and < 6.5% for select patients) are the primary means to evaluate achievement of glycemic goals among patients with T2DM.23 Observed weight loss was associated with a higher likelihood of achievement of these glycemic goals in the primary population and the subgroup sample (all with P < 0.001 in the primary analysis sample and subgroup sample). These results confirm of the direct impact of moderate weight change on key clinical outcomes for diabetes found in previous research.1-4,33
Associations between PWC and blood pressure or LDL-C were not observed in either of the samples. Such associations might not be expected in the subgroup sample because these individuals did not have evidence of CVD, which is strongly associated with these measures. A lack of observed association overall may also be due to the relatively short window of time over which the weight change and clinical outcomes were measured and may suggest that relatively moderate amounts of weight change do not have the same impact on blood pressure or LDL-C as on A1c.
A multifactorial approach to diabetes care in individuals with CVD includes glucose control, blood pressure management, aspirin therapy, and lipid management with statins, which have been shown to reduce vascular complications and cardiovascular mortality.34 Weight loss, as well as diabetes management, are currently considered after other priorities in the secondary prevention recommendations for individuals with CVD.35-37 Thus, in view of guidelines and the findings of the Look AHEAD study,16 a subpopulation of patients who were obese and had no previous CVD was examined. Given that most of the outcomes observed in this subgroup were favorable, it appears that weight management in a population of patients with T2DM in which primary prevention of CVD is possible may provide more benefit than in a secondary prevention population. Furthermore, the benefits of weight loss in reducing health care costs in patients with T2DM and established CVD may be difficult to detect because such patients may have other comorbidities that are not influenced by weight change and because CVD is a powerful determinant of health care costs.38 In both cohorts, associations between weight loss and decreased costs were observed for health care costs that were diabetes specific. All-cause costs may be driven by multiple factors, including the management of T2DM as well as any variety of comorbidities. It is therefore possible that, in the relatively short term, small amounts of weight change, such as the amount observed in the present study, most strongly impact costs directly related to diabetes care. However, these costs are of great importance, since costs due to diabetes and diabetes-related complications represent a substantial and increasing economic burden to society.39
Taken together with previous research highlighting the favorable association between weight loss, health care costs, and A1c,7-10 the present study adds to a body of knowledge that may have implications for clinical practice. If moderate changes in weight impact health care costs and A1c among patients with T2DM to the extent observed in this study, a focus on weight change early on in the disease course may be warranted. Thus, patients with T2DM may benefit from earlier lines of therapy that are driven not only by glycemic efficacy but also by weight change properties. As outlined in the ADA and EASD guidelines for the management of hyperglycemia in T2DM, weight change properties vary widely across available antidiabetic medication classes.12 For example, among the antidiabetic medications that are most commonly used in the United States, sulfonylureas, thiazolidinediones, and insulins are associated with weight gain; biguanides (metformin) and dipeptidyl peptidase 4 inhibitors are weight neutral; and glucagon-like peptide-1 receptor agonists and sodium-glucose co-transporter 2 inhibitors are associated with weight loss.12
Limitations
The results of this study must be interpreted in light of the study’s limitations. First, the study data do not allow us to understand the source of weight change, which could be related to a broad range of factors, such as patient lifestyle modifications (including diet and exercise), comorbidity, or the weight change properties of the antidiabetes medications that patients were taking. Future research to identify any heterogeneity in the impact of weight loss based on source would be useful in understanding the potential relative values of different weight loss strategies.
Second, administrative claims, EMR, and laboratory records are subject to coding inaccuracies, which can introduce measurement error into the study variables. Despite this, it is unlikely that such coding errors would vary systematically by the PWC. Third, the average duration of diabetes was unknown, so the relative effects of weight change early or late in the disease process could not be evaluated.
Fourth, the PWC was measured over a relatively short period of 6 months after the addition of or switch to a nonmetformin antidiabetes medication. Because this period immediately follows a change to a patient’s antidiabetes medication regimen, it may be relatively unstable with respect to treatment adjustments and the level of interaction that a patient has with health care providers. However, during this 6-month period of time, the interquartile range of the PWC was relatively narrow at -2.2% to 2.2%. Therefore, the results may not be generalizable to longer periods of time or larger gains or losses in weight. Furthermore, the results of this study do not reflect the impact of changes in weight that may or may not be sustained over long periods of time. In a recent study presented by Sabale et al. (2015), initial (1-year) weight gain in Swedish patients who were newly diagnosed with T2DM generally led to longer-term (3-year) increased health care costs even when the initial weight loss was not necessarily sustained.40 Overall, the results of this study suggest that weight changes of only a few percentage points during this short period can be associated with several hundreds of dollars in diabetes-specific health care cost increases or decreases.
Fifth, diabetes-specific cost estimates in this study did not specifically include diabetes supplies, some of which may be paid for out of pocket and which have previously been estimated to represent approximately 1% of diabetes-specific costs in patients with T2DM in 2007.41 Sixth, this study sample was drawn from a broader nonprobability sample and, while larger than those of some similar previous studies, may not be broadly generalizable to the entire U.S. population or individuals in other countries.
Conclusions
This real-world study suggests that among patients with T2DM who had specifically added to or switched treatment from metformin, subsequent weight loss over a short-term (6-month) period was associated with positive impact on attainment of A1c goals and decreased diabetes-specific pharmacy costs over the following 12 months. In the subset of patients who were obese and had no previous CVD, short-term weight loss over the 6-month period was also associated with decreased all-cause pharmacy costs, diabetes-specific medical costs, and diabetes-specific total health care costs. Future research is warranted to examine whether these associations change over longer-term follow-up periods.
APPENDIX A. Study Codes and Medicationsa
| Condition | Type of Code | Codes |
|---|---|---|
| Type 1 diabetes mellitus | ICD-9-CM diagnosis | 250.x1, 250.x3 |
| Gestational diabetes | ICD-9-CM diagnosis | 648.8x |
| Pregnancy | ICD-9-CM diagnosis | 630.xx-648.7x, 648.9x-679.xx, V22.xx-V23.xx, V27.xx, V28.xx, V61.6x-V61.7x, V72.42, V91.xx |
| ICD-9-CM procedure | 72.xx-75.xx | |
| MS-DRG | 765, 766, 767, 768, 770, 774, 775, 777, 778, 779 | |
| CPT | 57022, 58605, 58611, 59000-59899, 76801-76828, 76941, 83661-83664, S0612, S0613, S2400-S2405, S2409, S2411, S8055, 01965, 01966, 0500F, 0501F, 0503F | |
| Bariatric surgery | CPT | 43644, 43645, 43842, 43843, 43845, 43846, 43847, S2083 |
| ICD-9-CM procedure codes ONLY IF also 1 of ICD-9-CM diagnosis codes or MS-DRG codes listed below | 44.95, 44.96, 44.97, 44.98, 44.38, 44.68, 44.31, 44.39, 43.82, 43.89 | |
| ICD-9-CM diagnosis | 278.00, 278.01, V77.8 | |
| MS-DRG | 288 (only prior to October 1, 2007), 619, 620, 621 | |
| Cancer | ICD-9-CM diagnosis | 140.xx-172.xx, 176.xx-184.xx, 188.xx-195.xx, 199.2, 200.xx-209.xx |
| Cardiovascular disease | ||
| Atherosclerosis | ICD-9-CM diagnosis | 440.xx, 437.0x, 414.0x, 414.2x, 414.3x, 414.4x, 433.00, 433.10, 433.20, 433.30, 433.80, 433.90 |
| Stroke | ICD-9-CM diagnosis | 430.xx, 431.xx, 433.x1, 434.xx, 436.xx |
| Myocardial infarction | ICD-9-CM diagnosis | 410.xx |
| Unstable angina pectoris | ICD-9-CM diagnosis | 411.1x |
| Heart failure | ICD-9-CM diagnosis | 428.xx |
| Percutaneous coronary intervention | ICD-9-CM procedure | 00.66, 36.06, 36.07, 17.55 |
| CPT (all years) | 92980, 92981, 92982, 92984, 92995, 92996 | |
| CPT (year 2013 onwards) | 92920, 92921, 92924, 92925, 92928, 92929, 92933, 92934, 92937, 92938, 92941, 92943, 92944 | |
| HCPCS | G0290, G0291 | |
| Coronary artery bypass graft | ICD-9-CM procedure | 36.10, 36.11, 36.12, 36.13, 36.14, 36.15, 36.16, 36.17, 36.19 |
| CPT | 33510, 33511, 33512, 33513, 33514, 33516, 33517, 33518, 33519, 33521, 33522, 33523, 33530, 33533, 33534, 33535, 33536 | |
| HCPCS | S2205, S2206, S2207, S2208, S2209 | |
| Peripheral vascular disease | ICD-9-CM diagnosis | 441.xx, 443.9x, 785.4x, v43.4x |
| ICD-9-CM procedure | 38.48 | |
| Microvascular disease | ||
| Diabetic nephropathy | ICD-9-CM diagnosis | 250.4 |
| Diabetic retinopathy | ICD-9-CM diagnosis | 350.5, 362.0x |
| Diabetic peripheral neuropathy | ICD-9-CM diagnosis | 250.6, 357.2x |
| Hypertension | ICD-9-CM diagnosis | 401.xx, 402.xx, 403.xx, 404.xx, 405.xx |
| Dyslipidemia | ICD-9-CM diagnosis | 272.xx |
| Depression | ICD-9-CM diagnosis | 296.2x, 296.3x, 311.xx |
| Hypoglycemia | ICD-9-CM diagnosis | 251.0x, 251.1x, 251.2x, 250.8x |
| Antidiabetes Medication Classes | Generic Names | |
| Alpha-glucosidase inhibitors | Acarbose, Miglitol | |
| Biguanides | Metformin Hydrochloride | |
| Dipeptidyl peptidase-4 (DPP-4) inhibitors | Alogliptin Benzoate, Linagliptin, Saxagliptin Hydrochloride, Sitagliptin Phosphate | |
| Dopamine receptor agonists | Bromocriptine Mesylate | |
| Meglitinide analogs | Nateglinid, Repaglinide | |
| Sulfonylurea derivatives | Acetohexamide, Chlorpropamide, Glimepiride, Glipizide, Glyburide, Tolazamide, Tolbutamide, Tolbutamide Sodium | |
| Thiazolidinediones | Pioglitazone Hydrochloride, Rosiglitazone Maleate, Troglitazone | |
| Antidiabetes Medication Classes | Generic Names | |
| Insulins | Insulin Aspart, Insulin Aspart Protamine Human/Insulin Aspart, Insulin Glargine Human Recombinant Analog, Insulin Glulisine, Insulin Lispro, Insulin Nph Human Semi-Syn, Insulin Nph Human Semi-Syn/Insulin Reg Human, Semi-Syn, Insulin Reg Human Semi-Syn, Insulin Regular Human, Nph Human Insulin Isophane, Nph Human Insulin Isophane/Insulin Regular Human, Insulin Regular Human/Insulin Release Unit, Insulin Regular Human/Insulin Release Unit/Chamber/Inhaler, Insulin Detemir, Insulin Lispro Protamine & Insulin Lispro, Insulin Human Regular, Insulin Glargine Recombinant, Insulin Aspart Recombinant, Insulin Human Isophane (Nph), Insulin Human Isophane (Nph)/Insulin Human Regular, Insulin Lispro Recombinant, Insulin Lispro/Insulin Lispro Protamine, Insulin Aspart/Insulin Aspart Protamine, Insulin Isophane Nph Bf-Pk, Insulin Isophane Beef, Insulin Isophane Beef Pure, Insulin Isophane Pork Pure, Insulin Protamine Zinc Beef, Insulin Protamine Zn Beef (P), Insulin Protamine Zn Bf-Pk, Insulin Protamine Zn Pork (P), Insulin Reg Hum S-S Buff, Insulin Regular Beef-Pork, Insulin Regular Human Buffered, Insulin Zinc Beef, Insulin Zinc Ext Beef (P), Insulin Zinc Extend Human Rec, Insulin Zinc Extended Beef, Insulin Zinc Extended Bf-Pk, Insulin Zinc Human Rec, Insulin Zinc Human Semi-Syn, Insulin Zinc Prompt Beef, Insulin Zinc Prompt Bf-Pk, Insulin Zinc Prompt Pork Pure, Insulin Zinc Beef Purified, Insulin Zinc Beef Purified/Insulin Zinc Pork Purified, Insulin Zinc Beef-Pork, Insulin Zinc Pork Purified, Insulin Beef, Insulin Pork, Insulin Pork Purified, Insulin Pork Purified/Insulin Isophane Pork Pure, Insulin Pork Reg. Concentrate | |
| Glucagon-like peptide 1 receptor agonists (GLP-1 RA) | Exenatide, Exenatide Microspheres, Liraglutide | |
| Sodium glucose co-transporter 2 (SGLT2) inhibitors | Canagliflozin | |
| Antidiabetes Medication Classes | Generic Names | |
| Metformin/dipeptidyl peptidase-4 | Metformin Hydrochloride/Saxagliptin Hydrochloride, Metformin Hydrochloride/Sitagliptin Phosphate, Linagliptin/Metformin Hydrochloride, Alogliptin Benzoate/Metformin Hydrochloride | |
| Thiazolidinediones /dipeptidyl peptidase-4 | Alogliptin Benzoate/Pioglitazone Hydrochloride | |
| Dipeptidyl peptidase-4 /statins | Simvastatin/Sitagliptin Phosphate | |
| Metformin/Meglitinide | Metformin Hydrochloride/Repaglinide | |
| Metformin/ Sulfonylurea | Glipizide/Metformin Hydrochloride, Glyburide/Metformin Hydrochloride | |
| Thiazolidinediones /sulfonylurea | Glimepiride/Pioglitazone Hydrochloride, Glimepiride/Rosiglitazone Maleate | |
| Metformin/ thiazolidinediones | Metformin Hydrochloride/Pioglitazone Hydrochloride, Metformin Hydrochloride/Rosiglitazone Maleate | |
| Antidiabetes Medication Classes | Generic Names | |
| Renin-angiotensin-aldosterone system (RAAS) blocking agents | Aliskiren Hemifumarate, Azilsartan Medoxomil, Benazepril Hcl, Candesartan Cilexetil, Captopril, Enalapril Maleate, Enalaprilat Dihydrate, Eprosartan Mesylate, Fosinopril Sodium, Irbesartan, Lisinopril, Lisinopril/Dietary Supplement Comb.10, Losartan Potassium, Moexipril Hcl, Olmesartan Medoxomil, Perindopril Erbumine, Quinapril Hcl, Ramipril, Telmisartan, Trandolapril, Valsartan | |
| Beta blockers | Acebutolol Hcl, Atenolol, Betaxolol Hcl, Bisoprolol Fumarate, Carteolol Hcl, Carvedilol, Carvedilol Phosphate, Labetalol Hcl, Metoprolol Succinate, Metoprolol Tartrate, Metoprolol Tartrate/Dietary Supplement Comb.10, Nadolol, Nebivolol Hcl, Penbutolol Sulfate, Pindolol, Propranolol Hcl, Timolol Maleate | |
| Calcium channel blockers | Amlodipine Besylate, Bepridil Hcl, Clevidipine Butyrate, Diltiazem Hcl, Diltiazem Hcl In 0.9 % Sodium Chloride, Diltiazem Hcl/Dextrose 5 % In Water, Diltiazem Malate, Felodipine, Isradipine, Mibefradil Di-Hcl, Nicardipine Hcl, Nicardipine In Dextrose 5 %-Water, Nicardipine In Dextrose Iso-Osmotic, Nicardipine In Sodium Chloride Iso-Osmotic, Nifedipine, Nimodipine, Nisoldipine, Verapamil Hcl | |
| Diruetics | Acetazolamide, Bumetanide, Polythiazide | |
| Vasodilators | Diazoxide, Fenoldopam Mesylate, Hydralazine Hcl, Minoxidil, Nitroprusside Sodium, Tolazoline Hcl | |
| Peripheral vasodilators | Ethaverine Hcl, Isoxsuprine Hcl, Papaverine Hcl | |
| Central alpha-2 receptor agonists | Clonidine, Clonidine Hcl, Guanabenz Acetate, Guanfacine Hcl, Methyldopa, Methyldopate Hcl | |
| Ganglionic blocking, non-depolarizing | Mecamylamine Hcl | |
| Peripheral alpha-1 receptor blockers | Doxazosin Mesylate, Phenoxybenzamine Hcl, Phentolamine Mesylate, Prazosin Hcl, Terazosin Hcl | |
| Postganglionic blockers, antihypertensive | Guanadrel Sulfate, Guanethidine Sulfate | |
| Pulmonary antihypertensive agents | Ambrisentan, Bosentan, Epoprostenol Sodium (Arginine), Epoprostenol Sodium (Glycine), Iloprost, Nitric Oxide, Sildenafil Citrate, Tadalafil, Treprostinil, Treprostinil Sodium, Treprostinil/Nebulizer Accessories, Treprostinil/Nebulizer/Nebulizer Accessories | |
| Reserpine and derivatives | Reserpine | |
| Antidiabetes Medication Classes | Generic Names | |
| Antihypertensive combinations | Aliskiren Hemifumarate/Amlodipine Besylate, Aliskiren Hemifumarate/Amlodipine/Hydrochlorothiazide, Aliskiren Hemifumarate/Hydrochlorothiazide, Aliskiren/Valsartan, Amiloride Hcl/Hydrochlorothiazide, Amlodipine Besylate/Atorvastatin Calcium, Amlodipine Besylate/Benazepril Hcl, Amlodipine Besylate/Olmesartan Medoxomil, Amlodipine Besylate/Valsartan, Amlodipine Besylate/Valsartan/Hydrochlorothiazide, Atenolol/Chlorthalidone, Azilsartan Medoxomil/Chlorthalidone, Benazepril Hcl/Hydrochlorothiazide, Bisoprolol umarate/Hydrochlorothiazide, Candesartan Cilexetil/Hydrochlorothiazide, Captopril/Hydrochlorothiazide, Clonidine Hcl/Chlorthalidone, Deserpidine/Hydrochlorothiazide, Deserpidine/Methyclothiazide, Enalapril Maleate/Diltiazem Malate, Enalapril Maleate/Felodipine, Enalapril Maleate/Hydrochlorothiazide, Eprosartan Mesylate/Hydrochlorothiazide, Fosinopril Sodium/Hydrochlorothiazide, Guanethidine Sulfate/Hydrochlorothiazide, Hydralazine Hcl/Hydrochlorothiazide, Hydralazine Hcl/Reserpine/Hydrochlorothiazide, Irbesartan/Hydrochlorothiazide, Isosorbide Dinitrate/Hydralazine Hcl, Lisinopril/Hydrochlorothiazide, Losartan Potassium/Hydrochlorothiazide, Methyldopa/Chlorothiazide, Methyldopa/Hydrochlorothiazide, Metoprolol Succinate/Hydrochlorothiazide, Metoprolol Tartrate/Hydrochlorothiazide, Moexipril Hcl/Hydrochlorothiazide, Nadolol/Bendroflumethiazide, Olmesartan Medoxomil/Amlodipine Besylate/Hydrochlorothiazide, Olmesartan Medoxomil/Hydrochlorothiazide, Potassium Salicylate/Salicylamide/Caffeine, Prazosin Hcl/Polythiazide, Propranolol Hcl/Hydrochlorothiazide, Quinapril Hcl/Hydrochlorothiazide, Rauwolfia Serpentina/Bendroflumethiazide, Reserpine/Chlorothiazide, Reserpine/Hydrochlorothiazide, Reserpine/Hydroflumethiazide, Reserpine/Methyclothiazide, Reserpine/Polythiazide, Spironolactone/Hydrochlorothiazide, Telmisartan/Amlodipine Besylate, Telmisartan/Hydrochlorothiazide, Timolol Maleate/Hydrochlorothiazide, Trandolapril/Verapamil Hcl, Triamterene/Hydrochlorothiazide, Valsartan/Hydrochlorothiazide | |
| Antidiabetes Medication Classes | Generic Names | |
| Antihyperlipids | Amlodipine Besylate/Atorvastatin Calcium, Aspirin (Calcium Carb & Magnesium Buffers)/Pravastatin, Atorvastatin Calcium, Cerivastatin Sodium, Cholestyramine, Cholestyramine (With Sugar), Cholestyramine/Aspartame, Clofibrate, Colesevelam Hcl, Colesevelam Hydrochloride, Colestipol Hcl, Ezetimibe, Ezetimibe/Simvastatin, Fenofibrate, Fenofibrate Nanocrystallized, Fenofibrate Micronized, Fenofibrate Micronized, Fenofibric Acid, Fenofibric Acid (Choline), Fluvastatin Sodium, Gemfibrozil, Lecithin/Sitosterols/Niacin/Betaine/Chitosan, Lovastatin, Melatonin/Tryptophan/Valerian/Chamomile/Niacin/Inositol/B6, Niacin, Niacin/Lovastatin, Niacin/Simvastatin, Pitavastatin Calcium, Policosanol/Inositol Niacinate/Garlic, Pravastatin Sodium, Rosuvastatin Calcium, Simvastatin, Sitagliptin Phosphate/Simvastatin | |
a Codes measured over time period January 1, 2007-June 30, 2012, unless otherwise noted.
CPT = Current Procedural Terminology; HCPCS = Healthcare Common Procedure Coding System; ICD-9-CM = International Classification of Diseases, Ninth Revision, Clinical Modification; MS-DRG = Medicare Severity Diagnosis-Related Group.
APPENDIX B. Unadjusted NCQA/HEDIS Comprehensive Diabetes Care and Clinical Outcomes During Outcomes Follow-up Period
| Primary Analysis Sample | Subgroup Sample a | |||
|---|---|---|---|---|
| Weight Gain Group n = 325 | Nonweight Gain Groupb n = 1,195 | Weight Gain Group n = 134 | Nonweight Gain Groupb n = 646 | |
| A1c (as %) | ||||
| Follow-up measurement, mean [SD] | 7.4 [1.4] | 7.1 [1.2] | 7.4 [1.3] | 7.1 [1.2] |
| Poor control (> 9.0), % (n) | 10.5 (34) | 6.8 (81) | 11.9 (16) | 6.7 (43) |
| Control (< 8.0), % (n) | 75.4 (245) | 83.0 (992) | 73.9 (99) | 84.4 (545) |
| Control (< 7.0), % (n) | 44.3 (144) | 54.9 (656) | 44.0 (59) | 54.8 (354) |
| Control (< 6.5), % (n) | 24.3 (79) | 32.4 (387) | 24.6 (33) | 31.3 (202) |
| Blood pressure (mm Hg) | ||||
| Follow-up systolic, mean [SD] | 112.3 [16.2] | 112.3 [17.1] | 109.7 [15.5] | 113.4 [17.4] |
| Follow-up diastolic, mean [SD] | 87.7 [15.6] | 88.3 [15.7] | 85.5 [14.9] | 89.6 [16.0] |
| Control (< 130/80 mm Hg), % (n) | 30.8 (100) | 29.8 (356) | 36.6 (49) | 26.9 (174) |
| Control (< 140/90 mm Hg), % (n) | 56.6 (184) | 54.2 (648) | 65.7 (88) | 51.9 (335) |
| LDL-C (mg/dL)c | n = 150 | n = 606 | n = 61 | n = 342 |
| Follow-up measurement, mean [SD] | 92.8 [29.6] | 92.5 [33.0] | 91.5 [27.0] | 92.8 [33.4] |
| Control (< 100 mg/dL), % (n) | 64.7 (97) | 64.2 (389) | 67.2 (41) | 62.0 (212) |
a Sample restricted to patients with BMI ≥ 30 and no evidence of previous cardiovascular disease.
b Weight gain = increase in weight by > 3%; nonweight gain = weight gain ≤ 3% and weight loss.
c Analyses conducted on a subset of patients for whom LDL-C data were available.
Alc = hemoglobin A1c; BMI = body mass index; LDL-C = low-density lipoprotein cholesterol; NCQA/HEDIS = National Committee for Quality Assurance/Healthcare Effectiveness Data and Information Set; SD = standard deviation.
APPENDIX C. Unadjusted All-Cause Health Care Utilization and Costs During Outcomes Follow-up Period
| Primary Analysis Sample | Subgroup Samplea | |||
|---|---|---|---|---|
| Weight Gain Group n = 325 | Nonweight Gain Groupb n = 1,195 | Weight Gain Group n = 134 | Nonweight Gain Groupb n = 646 | |
| Inpatient admissions | ||||
| Patients with an inpatient admission, % (n) | 11.7 (38) | 10.4 (124) | 11.2 (15) | 8.4 (54) |
| Number of inpatient admissions, mean [SD] | 0.2 [0.5] | 0.1 [0.5] | 0.2 [0.6] | 0.1 [0.4] |
| Inpatient expenditures, mean [SD] | $3,694 [$13,938] | $3,149 [$14,371] | $4,674 [$17,585] | $2,131 [$9,791] |
| Emergency room visits | ||||
| Patients with an ER visit, % (n) | 23.7 (77) | 20.8 (249) | 26.1 (35) | 21.4 (138) |
| Number of ER visits, mean [SD] | 0.4 [1.3] | 0.3 [0.8] | 0.5 [1.8] | 0.3 [0.8] |
| ER expenditures, mean [SD] | $312 [$1,195] | $243 [$836] | $395 [$1,621] | $255 [$773] |
| Outpatient office visits and services | ||||
| Patients with outpatient services, % (n) | 100.0 (325) | 100.0 (1,195) | 100.0 (134) | 100.0 (646) |
| Number of outpatient services, mean [SD] | 46.8 [36.1] | 51.9 [43.3] | 46.8 [39.9] | 48.1 [35.7] |
| Outpatient services expenditures, mean [SD] | $5,579 [$7,446] | $6,428 [$13,187] | $4,935 [$5,970] | $5,388 [$11,928] |
| Pharmacy | ||||
| Number of prescription claims, mean [SD] | 42.4 [28.5] | 41.9 [27.2] | 44.0 [28.6] | 41.3 27.9 |
| Prescription drug expenditures, mean [SD] | $3,537 [$3,723] | $4,026 [$4,716] | $4,137 [$4,750] | $3,922 [$4,993] |
| Total cost of health care services | ||||
| Mean [SD] | $13,122 [$18,630] | $13,845 [$24,645] | $14,140 [$21,911] | $11,696 [$18,987] |
| Median | $7,126 | $7,110 | $6,743 | $6,626 |
a Sample restricted to patients with BMI ≥ 30 and no evidence of previous cardiovascular disease.
b Weight gain = increase in weight by > 3%; nonweight gain = weight gain ≤ 3% and weight loss.
BMI = body mass index; ER = emergency room; SD = standard deviation.
APPENDIX D. Unadjusted All-Cause Health Care Utilization and Costs During Outcomes Follow-up Period
| Primary Analysis Sample | Subgroup Samplea | |||
|---|---|---|---|---|
| Weight Gain Group n = 325 | Nonweight Gain Groupb n = 1,195 | Weight Gain Group n = 134 | Nonweight Gain Groupb n = 646 | |
| Inpatient admissions | ||||
| Patients with an inpatient admission, % (n) | 8.3 (27) | 7.4 (89) | 9.0 (12) | 6.0 (39) |
| Number of inpatient admissions, mean [SD] | 0.1 [0.4] | 0.1 [0.4] | 0.1 [0.5] | 0.1 [0.3] |
| Inpatient expenditures, mean [SD] | $2,676 [$12,066] | $2,046 [$10,204] | $3,852 [$16,388] | $1,405 [$7,510] |
| Emergency room visits | ||||
| Patients with an ER visit, % (n) | 8.0 (26) | 7.9 (95) | 6.0 (8) | 8.7 (56) |
| Number of ER visits, mean [SD] | 0.1 [0.5] | 0.1 [0.4] | 0.1 [0.6] | 0.1 [0.4] |
| ER expenditures, mean [SD] | $77 [$439] | $75 [$414] | $47 [$316] | $76 [$402] |
| Outpatient office visits and services | ||||
| Patients with outpatient services, % (n) | 98.8 (321) | 98.6 (1,178) | 98.5 (132) | 98.6 (637) |
| Number of outpatient services, mean [SD] | 16.9 [13.6] | 16.7 [14.0] | 16.6 [15.1] | 16.5 [12.0] |
| Outpatient services expenditures, mean [SD] | $1,406 [$2,643] | $1,597 [$6,934] | $1,176 [$1,855] | $1,585 [$8,754] |
| Pharmacy | ||||
| Number of prescription claims, mean [SD] | 11.0 [7.7] | 10.0 [6.9] | 10.7 [7.2] | 10.1 [6.9] |
| Prescription drug expenditures, mean [SD] | $1,134 [$1,408] | $1,272 [$1,470] | $1,217 [$1,611] | $1,296 [$1,560] |
| Total cost of health care services | ||||
| Mean [SD] | $5,293 [$12,536] | $4,990 [$13,694] | $6,292 [$16,702] | $4,362 [$12,477] |
| Median | $1,949 | $1,953 | $1,897 | $1,885 |
a Sample restricted to patients with BMI ≥ 30 and no evidence of previous cardiovascular disease.
b Weight gain = increase in weight by > 3%; nonweight gain = weight gain ≤ 3% and weight loss.
BMI = body mass index; ER = emergency room; SD = standard deviation.
REFERENCES
- 1.Ross SA, Dzida G, Vora J, Khunti K, Kaiser M, Ligthelm RJ. Impact of weight gain on outcomes in type 2 diabetes. Curr Med Res Opin. 2011;27(7):1431-38. [DOI] [PubMed] [Google Scholar]
- 2.McAdam-Marx C, Bellows BK, Unni S, et al. Determinants of glycaemic control in a practice setting: the role of weight loss and treatment adherence (The DELTA Study). Int J Clin Pract. 2014;68(11):1309-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McAdam-Marx C, Mukherjee J, Bellows BK, et al. Evaluation of the relationship between weight change and glycemic control after initiation of anti-diabetes therapy in patients with type 2 diabetes using electronic medical record data. Diabetes Res Clin Pract. 2014;103(3):402-11. [DOI] [PubMed] [Google Scholar]
- 4.McAdam-Marx C, Bellows BK, Unni S, et al. Impact of adherence and weight loss on glycemic control in patients with type 2 diabetes: cohort analyses of integrated medical record, pharmacy claims, and patient-reported data. J Manag Care Spec Pharm. 2014;20(7):691-700. Available at: http://www.jmcp.org/doi/abs/10.18553/jmcp.2014.20.7.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fox CS, Pencina MJ, Wilson PW, Paynter NP, Vasan RS, D’Agostino RB Sr. Lifetime risk of cardiovascular disease among individuals with and without diabetes stratified by obesity status in the Framingham heart study. Diabetes Care. 2008;31(8):1582-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eeg-Olofsson K, Cederholm J, Nilsson PM, et al. Risk of cardiovascular disease and mortality in overweight and obese patients with type 2 diabetes: an observational study in 13,087 patients. Diabetologia. 2009;52(1):65-73. [DOI] [PubMed] [Google Scholar]
- 7.Bell K, Parasuraman S, Shah M, et al. Economic implications of weight change in patients with type 2 diabetes mellitus. Am J Manag Care. 2014;20(8):e320-29. [PubMed] [Google Scholar]
- 8.Dilla T, Valladares A, Nicolay C, Salvador J, Reviriego J, Costi M. Healthcare costs associated with change in body mass index in patients with type 2 diabetes mellitus in Spain: the ECOBIM study. Appl Health Econ Health Policy. 2012;10(6):417-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yu AP, Wu EQ, Birnbaum HG, et al. Short-term economic impact of body weight change among patients with type 2 diabetes treated with antidiabetes agents: analysis using claims, laboratory, and medical record data. Curr Med Res Opin. 2007;23(9):2157-69. [DOI] [PubMed] [Google Scholar]
- 10.Davis WA, Bruce DG, Davis TM. Economic impact of moderate weight loss in patients with type 2 diabetes: the Fremantle Diabetes Study. Diabet Med. 2011;28(9):1131-35. [DOI] [PubMed] [Google Scholar]
- 11.American Diabetes Association, Bantle JP, Wylie-Rosett J, et al. Nutrition recommendations and interventions for diabetes: a position statement of the American Diabetes Association. Diabetes Care. 2008;31(Suppl 1):S61-78. Available at: http://care.diabetesjournals.org/content/31/Supplement_1/S61.full.pdf+html. Accessed January 27, 2016. [DOI] [PubMed] [Google Scholar]
- 12.Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2012;35(6):1364-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Look AHEAD Research Group, Pi-Sunyer X, Blackburn G, et al. Reduction in weight and cardiovascular risk factors in individuals with type 2 diabetes: one-year results of the Look AHEAD trial. Diabetes Care. 2007;30(6)1374-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wing RR, Lang W, Wadden TA, et al. Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diabetes Care. 2011;34(7):1481-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Look AHEAD Research Group, Wing RR, Bolin P, et al. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med. 2013;369(2):145-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Espeland MA, Glick HA, Bertoni A, et al. Impact of an intensive lifestyle intervention on use and cost of medical services among overweight and obese adults with type 2 diabetes: the action for health in diabetes. Diabetes Care. 2014;37(9):2548-56. Available at: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4140155/. Accessed January 27, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Colosia AD, Palencia R, Khan S. Prevalence of hypertension and obesity in patients with type 2 diabetes mellitus in observational studies: a systematic literature review. Diabetes Metab Syndr Obes. 2013;6:327-38. Available at: www.ncbi.nlm.nih.gov/pmc/articles/PMC3785394/. Accessed February 27, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sluik D, Boeing H, Montonen J, et al. Associations between general and abdominal adiposity and mortality in individuals with diabetes mellitus. Am J Epidemiol. 2011;174(1):22-34. [DOI] [PubMed] [Google Scholar]
- 19.Song SH. Complication characteristics between young-onset type 2 versus type 1 diabetes in a UK population. BMJ Open Diabetes Res Care. 2015;3(1):e000044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zeber J, Parchman ML. Cardiovascular disease in type 2 diabetes: attributable risk due to modifiable risk factors. Can Fam Physician. 2010;56(8):e302-07. [PMC free article] [PubMed] [Google Scholar]
- 21.Kuczmarski RJ, Flegal KM. Criteria for definition of overweight in transition: background and recommendations for the United States. Am J Clin Nutr. 2000;72(5):1074-81. [DOI] [PubMed] [Google Scholar]
- 22.Stevens J, Truesdale KP, McClain JE, Cai J. The definition of weight maintenance. Int J Obes (Lond). 2006;30(3):391-99. [DOI] [PubMed] [Google Scholar]
- 23.American Diabetes Association. Executive summary: standards of medical care in diabetes—2012. Diabetes Care. 2012;35(Suppl 1):S4-S10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang L, Wei W, Miao R, Xie L, Baser O. Real-world outcomes of US employees with T2D mellitus treated with insulin glargine or neutral protamine Hagedorn insulin: a comparative retrospective database study. BMJ Open. 2013;3(4):e002348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wei W, Zhou S, Miao R, et al. Much ado about nothing? A real-world study of patients with type 2 diabetes switching basal insulin analogs. Adv Ther. 2014;31(5):539-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meyers JL, Parasuraman S, Bell KF, Graham JP, Candrilli SD. The high-cost, type 2 diabetes mellitus patient: an analysis of managed care administrative data. Arch Public Health. 2014;72(1):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.U.S. Bureau of Labor Statistics. Consumer Price Index detailed report tables, annual average, 2013. Available from: http://www.bls.gov/cpi/cpid10av.pdf. Accessed January 27, 2016.
- 28.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613-19. [DOI] [PubMed] [Google Scholar]
- 29.Chang HY, Weiner JP, Richards TM, Bleich SN, Segal JB. Validating the adapted Diabetes Complications Severity Index in claims data. Am J Manag Care. 2012;18(11):721-26. [PubMed] [Google Scholar]
- 30.Fowler R, Johnston SS. Comparative performance of risk adjustment measures in a sample of commercially-insured patients under age 65—two simple measures outperform current standards. Value Health. 2010;13(3):A4. [Abstract RM1]. [Google Scholar]
- 31.Greene WH. Econometric Analysis. 4th ed. Upper Saddle River, NJ: Prentice-Hall; 2000. [Google Scholar]
- 32.Kleinman LC, Norton EC. What’s the risk? A simple approach for estimating adjusted risk measures from nonlinear models including logistic regression. Health Serv Res. 2009;44(1):288-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Feldstein AC, Nichols GA, Smith DH, et al. Weight change in diabetes and glycemic and blood pressure control. Diabetes Care. 2008;31(10):1960-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gaede P, Lund-Andersen H, Parving HH, Pedersen O. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med. 2008;358(6):580-91. [DOI] [PubMed] [Google Scholar]
- 35.Smith SC Jr, Benjamin EJ, Bonow RO, et al. AHA/ACCF Secondary Prevention and Risk Reduction Therapy for Patients with Coronary and other Atherosclerotic Vascular Disease: 2011 update: a guideline from the American Heart Association and American College of Cardiology Foundation. Circulation. 2011;124(22):2458-73. [DOI] [PubMed] [Google Scholar]
- 36.University of Michigan Health System. Guidelines for Clinical Care, Ambulatory. Secondary Prevention of Coronary Artery Disease. Updated February 2012. Available at: http://www.med.umich.edu/1info/FHP/practiceguides/cad/cad.pdf. Accessed January 27, 2016.
- 37.American Diabetes Association. (8) Cardiovascular disease and risk management. Diabetes Care. 2015;38(Suppl):S49-57. [DOI] [PubMed] [Google Scholar]
- 38.Nichols GA, Bell TJ, Pedula KL, O’Keeffe-Rosetti M. Medical care costs among patients with established cardiovascular disease. Am J Manag Care. 2010;16(3):e86-e93. [PubMed] [Google Scholar]
- 39.American Diabetes Association. Economic costs of diabetes in the U.S. in 2012. Diabetes Care. 2013;36(4):1033-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sabale U, Bodegard J, Ekman M, et al. Weight change over time and its link to healthcare costs among newly-diagnosed type 2 diabetes patients in Sweden. Abstract presented at: The European Association for the Study of Diabetes 51st Annual Meeting; September 14-18, 2015; Stockholm, Sweden. [Abstract 54]. [Google Scholar]
- 41.Dall TM, Mann SE, Zhang Y, et al. Distinguishing the economic costs associated with type 1 and type 2 diabetes. Popul Health Manag. 2009;12(2):103-10. [DOI] [PubMed] [Google Scholar]


