Abstract
BACKGROUND:
The approval of the first biosimilar in the United States has placed increased pressure on the FDA to provide guidance on the naming convention that will be assigned to current and future biosimilars. The release of the FDA draft guidance on nonproprietary naming of biosimilars in August 2015 established a naming convention for all biologic products, including biosimilars. However, the draft guidance is nonbinding while the FDA continues to receive input from stakeholders, and it does not address the naming convention that will be used for products designated as interchangeable biologics.
OBJECTIVES:
To (a) determine pharmacist perceptions of biosimilar naming conventions and their impact on confidence to dispense biosimilars and (b) measure the burden that is created by laws and regulations requiring pharmacists to complete postdispense notifications.
METHODS:
A cross-sectional survey of 781 members of the Academy of Managed Care Pharmacy and the Hematology/Oncology Pharmacy Association was conducted using an online survey software program.
RESULTS:
Participants reported preferring a biosimilar naming convention that uses a nonproprietary base with a designated suffix (48.1%), compared with the use of a nonproprietary base alone (26.3%), nonproprietary base plus a prefix (14.2%), or a unique brand name (11.4%). However, when participants were asked to report their confidence levels when dispensing a biosimilar in place of the reference biologic, more participants reported high levels of confidence when the products shared the same nonproprietary name (62.9%). A majority of participants (64.9%) reported perceptions of increased burden when required to provide a postdispense notification to prescribers when dispensing biosimilars.
CONCLUSIONS:
According to the survey used in this study, pharmacists prefer the use of a naming convention for biosimilars that includes a nonproprietary proper name with a designated suffix; however, levels of confidence in substituting a biosimilar for the reference biologic are highest when products share the same nonproprietary name. In addition, the results of this study suggest that the naming convention and postdispense notification requirements may affect the willingness of some pharmacists to dispense interchangeable biologics. This effect will be minimized if interchangeable biologics share the same nonproprietary name as the reference biologics.
What is already known about this subject
In March 2015, the first biosimilar in the United States was approved by the FDA.
Despite draft guidance issued by the FDA, specific biosimilar naming conventions remain undefined.
The confidence of pharmacists to dispense an interchangeable biosimilar is higher when the biosimilar and reference biologic share the same nonproprietary name.
What this study adds
Survey results showed that pharmacists preferred a biosimilar naming convention that included the nonproprietary name with a designated suffix.
Pharmacists reported that the type of naming convention affected their confidence in substituting biosimilars for the reference biologic.
Study results showed that reporting requirements affected the willingness of some pharmacists to dispense interchangeable biologics.
The passage of the Drug Price Competition and Patent Term Restoration Act of 1984, also known as the Waxman-Hatch Act, created an abbreviated approval process for small molecule products based predominantly on the ability of generic manufacturers to demonstrate bioequivalence with the reference product.1 The primary purpose of the Waxman-Hatch Act was to expedite the availability of generic drugs so as to increase price competition between branded reference products and less costly generics. This act has been widely successful at creating the intended competition, which has resulted in significant cost savings for small molecule drugs.1 The abbreviated approval process contained in the act, however, cannot be used for biologic agents, since demonstrating bioequivalence was deemed insufficient for such large molecule products.
Before 2009, all prescription drugs produced using biotechnology were required to receive approval from a single U.S. Food and Drug Administration (FDA) application process, known as the Biologic License Application (BLA) pathway, which required manufacturers looking to market follow-on products after patent expiration to complete the same, full-approval process as the reference product manufacturer.2 This requirement left most manufacturers of biologic prescription drugs immune from likely competition from potential follow-on products because of the need for manufacturers to complete the same lengthy and expensive approval process as the reference biologic.
The inclusion of the Biologics Price Competition and Innovation Act of 2009 (BPCIA) into the Affordable Care Act and its passage into law created a new abbreviated approval process for follow-on biologics. Follow-on biologics have since become more commonly known as biosimilars and interchangeable biological products and will be referred to here as “interchangeables.”3 This abbreviated pathway substantially shortened the approval times and reduced the size and length of required clinical trials compared with the standard BLA process and is focused on the ability of manufacturers to show no clinically significant differences in the chemical makeup of the product or safety and efficacy outcomes. The inclusion of a pathway for approving interchangeable products has yet to be defined by the FDA, and guidance for the requirements for approval of these products has not yet been published.
A biosimilar is defined as a biologic agent that is highly similar to the reference product notwithstanding minor difference in clinically inactive components.4 This definition explicitly states that it is not expected that biosimilar manufacturers will be able to create an exact replica but, rather, will produce biologic agents that closely enough resemble the reference product to elicit a response that is clinically the same as the reference product.5 This acceptable variance in the clinically inactive components of these products has been deemed required based on the complexity of the production process of biologics and the complexity of the biologic agents themselves.6
Following the enactment of the BPCIA legislation into law, limited guidance on the approval process and marketing of biosimilars existed.5 In May 2014, the FDA released its initial draft guidance that outlined the approval process and exclusivity standards. In July 2014, the FDA accepted its first biosimilar application, and in March 2015, the first biosimilar was approved by the agency.8 With 1 biosimilar approved, additional applications under review, and an anticipated large number of approvals in the works, several details remained undefined from the original law, including the naming convention that will be used to reference approved biosimilars and interchangeable products.9 Following completion of the data collection phase of this project, the FDA released draft guidance and a proposed rule on naming conventions for biosimilars and biologics in August 2015.10
Historically, small molecule products have relied on a nonproprietary, active ingredient (generic) name assigned by the United States Adopted Names Council to reference the original reference product and subsequent generics.11 This naming structure is intended to establish that products from different manufacturers with the same active ingredient may contain different inactive compounds, but the active component of the product is the same.11
Initially, biologics and the first approved biosimilar worldwide were named using a process much the same as small molecules.12 Biologics were assigned a nonproprietary name based on the active compound, and all future products that shared the same active compound (i.e., protein structure) would share the same nonproprietary name. This approach was altered, however, with the first FDA-approved biosimilar in the United States. The approval of Zarxio, a biosimilar for Neupogen, initially entered the market using a unique trade name with a nonproprietary name that included a base active ingredient name plus a suffix related to the manufacturer’s name, that is, filgrastim-sndz.13
This naming convention was altered with the release of the FDA draft guidance on biosimilar naming in 2015.10 This draft guidance recommends that all previously approved, currently under review, and future biosimilars and current and existing reference biologics be named based on a nonproprietary name with an assigned hyphenated suffix.10 The FDA’s draft guidance reported the intent to designate each approved biologic with a nonproprietary name, including a randomly assigned 4-letter suffix that is devoid of meaning.10 The current draft guidance does not detail the naming requirements for any future biologics that are approved as an interchangeable biologic from the FDA.
The FDA’s draft guidance cited the need for improved pharmacovigilance and to clearly differentiate all products that are not deemed interchangeable as the rationale for assigning unique suffixes to each unique biologic.10 Those supporting the use of a unique biologic qualifier or unique overall name for biosimilars cite the importance of using the unique product names to better track patient- and health care provider-reported adverse events.14 Supporters state that using the same nonproprietary name for biosimilars could result in confusion in the reporting process and make pharmacovigilance studies more difficult and inaccurate. Opponents cite the presence of unique identifiers, such as National Drug Code (NDC) numbers and lot numbers, as reasons why assigning a suffix or other biologic qualifier or unique product name is not necessary. Opponents also cite the potential to create confusion among providers and patients.14 However, data from the FDA Adverse Event Reporting System have suggested an adverse-reporting trend toward continued adverse reports being assigned to the brandname reference product even after a shift in market to primarily using generic products.15
In a previous study that focused on determining the naming preference of biosimilars, pharmacists reported higher levels of confidence when substituting an interchangeable product for the reference product, if each product shared the same nonproprietary (proper) name.16 In addition, pharmacists reported substantially lower levels of confidence if the interchangeable was assigned a unique, proprietary name or a nonproprietary name with either a prefix or suffix.16
The primary objective of this study was to determine pharmacist perceptions regarding biosimilar naming and the impact of the naming convention used for interchangeables on pharmacist willingness to dispense interchangeables. The secondary objective of this study was to measure the anticipated burden that is created by laws and regulations requiring pharmacists to complete postdispense notifications to prescribers after dispensing an interchangeable biologic. The results of this study will help shape the continuing discussion concerning biosimilars and the laws and regulations that govern them.
Methods
This study was conducted using a cross-sectional survey that collected data from May 2015 to June 2015. A web-based, electronic survey was distributed through e-mail and was conducted using the electronic survey engine Qualtrics. This study was reviewed and approved by the Internal Review Board at Chapman University (IRB #1415H152).
Participants were members of the Academy of Managed Care Pharmacy (AMCP) and Hematology/Oncology Pharmacy Association (HOPA) who reported their e-mail addresses to their respective associations. For inclusion in the survey, participants needed to maintain a pharmacist membership with either association. Exclusion criteria were as follows: the removal of student memberships, members without an email address reported, or those holding nonpharmacist membership. Potential participants were e-mailed an invitation to the survey, followed by up to 2 reminder e-mails if they did not complete the survey. A total of 10,673 invitation e-mails were sent, of which 485 e-mails were returned as undeliverable, blocked, or unsubscribed, and an additional 11 respondents requested to be removed from the study. This left 10,177 surveys assumed to have been delivered.
The survey instrument was developed by modifying and adding additional items to an earlier survey that measured pharmacist perceptions of biosimilars.14 Additional items included those focused on determining the participants’ naming preference for biosimilars, level of burden that postdispensing requirements are likely to cause, and whether participant-reported dispensing of biologics occurred at their place of employment.
Measures
Survey items focused on pharmacist perceptions primarily used a 5-point Likert scale. These items varied from asking pharmacists to rank their overall knowledge of biosimilars, with 1 equating to “I know nothing about biosimilars” and 5 equating to “I consider myself an expert on biosimilars.” Questions measuring level of confidence were scored with 1 equating to “Not confident at all” and 5 equating with “Very confident.” Survey items asking participants to rank their confidence based on naming convention included an additional option of “The name of the product would not impact my confidence.” Those choosing this option were removed from the analysis. Participants were also asked to choose from a list of potential naming conventions for hypothetical biosimilars in order to share their most preferred and least preferred naming structure.
Comparison of confidence-level reporting was completed using the Wilcoxon signed-rank test between the naming convention with the highest confidence reported (nonproprietary name only) with each possible other naming convention. In addition, a chi-square test comparing the differences between the nonproprietary name only naming convention with each additional naming convention was conducted using the 5 confidence categories, as well as an analysis based on 2 categories: confident (combination of very confident and moderately confident) and not confident (combination of somewhat confident, minimally confident, and not confident at all). Use of the highest rated confidence naming convention as the reference for statistical analysis allowed for more concise reporting of statistical analysis. Chi-square tests were used to also analyze for potential differences in naming preference between those who were employed at a site that dispensed biologics compared with those who were employed at a site that did not dispense biologics.
The survey also requested information related to the tracking and reporting process. In addition, participants were asked to report how these data were stored and (when applicable) shared with the prescriber. Only participants who reported dispensing biologics at their place of employment were displayed these questions. Finally, demographic questions included employment type, participation in the dispensing process of prescription drugs, likelihood to dispense biosimilars at current practice, years in practice, age, and state of primary practice.
Results
A total of 924 participants (9.1% of assumed delivered invitations) clicked on the link to start the survey. Of those, 851 participants responded to the first question on the survey; however, 70 of those participants completed less than 50% of the survey. Participants completing less than 50% of the survey were eliminated from the analysis, which left 781 participants whose data were included in the survey (response rate = 7.7% and relative completion rate = 84.5%). Not all participants answered all questions, since responding to each question was voluntary. Also, the survey was adaptive, and a number of items were dependent on responses to other questions, resulting in selected items not being displayed to all participants.
The demographic composition of the survey participants is shown in Table 1. The distribution of pharmacists by employment type is similar to that expected based on the e-mail databases that served as the sole means of survey distribution. The majority of participants reported having at least a general knowledge of biosimilars (83.7% rated themselves as generally knowledgeable or higher). Half of the participants (n = 391, 50.2%) reported dispensing biologics at their place of employment, and 389 participants (49.8%) reported that biologics were not dispensed at their place of employment.
TABLE 1.
Key Demographics of Study Participants
| % (n) | |
|---|---|
| Employment type | |
| Managed care organization | 29.8 (233) |
| Hospital | 15 (117) |
| Manufacturer | 10 (78) |
| Large chain pharmacy (>10 pharmacies) | 5.4 (42) |
| Academia | 4.9 (38) |
| Outpatient clinic (dispensing) | 4.7 (37) |
| Specialty pharmacy | 3.3 (26) |
| Outpatient clinic (nondispensing) | 2.4 (19) |
| Independent pharmacy | 1.8 (14) |
| Mail order pharmacy | 1.1 (8) |
| Small chain pharmacy (4-10 pharmacies) | 0.5 (4) |
| Other/not reported | 21.2 (165) |
| Perceived knowledge of biologics | |
| Expert | 4.7 (37) |
| Highly knowledgeable | 29.1 (227) |
| Generally knowledgeable | 49.8 (388) |
| Perceived lack of knowledge | 16.3 (127) |
| Dispenses biologics | |
| Yes | 50.2 (391) |
| No | 49.8 (389) |
| General demographics | |
| Age, in years, mean [SD] | 42.7 [12.7] |
| Years in practice, mean [SD] | 16.7 [12.3] |
SD = standard deviation.
Overall naming preference is reported in Table 2. Participants were asked their preferences for the naming convention to be used for biologic products approved by the FDA as a biosimilar. Results show that participants reported a preference (48.1% overall) for the naming convention that used the nonproprietary (active ingredient) name plus suffix. This preference was also reported by managed care organizations (50.2%) and hospital pharmacists (44.4%). All employment sectors reported a preference for the nonproprietary name plus suffix, except those practicing in specialty pharmacy, who expressed a preference for using strictly the nonproprietary name (38.5% preferred active ingredient only and 34.6% preferred active ingredient plus suffix).
TABLE 2.
Reported Preference of Biosimilar Naming Convention by Employment Type
| Employment Type | Nonproprietary Only % (n) | Nonproprietary Plus Suffix % (n) | Nonproprietary Plus Prefix % (n) | Unique Brand Name % (n) |
|---|---|---|---|---|
| Hospital (n = 116) | 17.9 (21) | 44.4 (52) | 29.1 (34) | 7.7 (9) |
| Outpatient pharmaciesa (n = 124) | 29.8 (37) | 45.2 (56) | 13.7 (17) | 11.3 (14) |
| Specialty pharmacy (n = 26) | 38.5 (10) | 34.6 (9) | 19.2 (5) | 7.7 (2) |
| MCOs (n = 233) | 31.3 (73) | 50.2 (117) | 6.9 (16) | 11.6 (27) |
| Manufacturer (n = 78) | 16.7 (13) | 50.0 (39) | 15.3 (12) | 17.9 (14) |
| Academia (n = 37) | 21.6 (8) | 43.2 (16) | 29.7 (11) | 5.4 (2) |
| Other/unreported (n = 139) | 25.9 (36) | 52.5 (73) | 8.6 (12) | 12.9 (18) |
| Total | 26.3 (198) | 48.1 (362) | 14.2 (107) | 11.4 (86) |
a Includes those reporting independent, small chain, large chain, clinic nondispensing, and clinic dispensing.
MCO = managed care organization.
Those participants reporting preferences for the nonproprietary name plus suffix preferred the use of a suffix tied to the manufacturer name (83.4%), compared with the random assignment of a 4-letter suffix (16.6%). Nonproprietary (active ingredient) name only was the second most preferred naming convention, with 26.3% of participants selecting it. Overall, the least preferred naming conventions included the use of unique brand names and the use of a nonproprietary names plus a prefix (Table 3). Hospital pharmacists and pharmacists representing academia preferred the use of a prefix plus nonproprietary name (29.1% and 29.7%, respectively) over the nonproprietary name only convention (17.9% and 21.6%, respectively; Figure 2).
TABLE 3.
Reported Least Preferred Biosimilar Naming Convention by Employment Type
| Employment Type | Nonproprietary Only % (n) | Nonproprietary Plus Suffix % (n) | Nonproprietary Plus Prefix % (n) | Unique Brand Name % (n) |
|---|---|---|---|---|
| Hospital (n = 116) | 37.6 (44) | 4.3 (5) | 20.5 (24) | 36.8 (43) |
| Outpatient pharmaciesa (n = 122) | 20.3 (25) | 15.5 (19) | 26.0 (32) | 37.4 (46) |
| Specialty pharmacy (n = 26) | 26.9 (7) | 19.2 (5) | 26.9 (7) | 26.9 (7) |
| MCOs (n = 233) | 18.0 (42) | 4.3 (10) | 30.5 (71) | 46.8 (109) |
| Manufacturer (n = 78) | 39.7 (31) | 7.7 (6) | 25.6 (20) | 25.6 (20) |
| Academia (n = 37) | 23.7 (9) | 10.5 (4) | 5.2 (2) | 60.5 (23) |
| Other/unreported (n = 139) | 19.5 (32) | 5.5 (9) | 23.3 (38) | 36.0 (59) |
| Total | 25.4 (190) | 7.7 (58) | 25.9 (194) | 40.9 (307) |
a Includes those reporting independent, small chain, large chain, clinic nondispensing, and clinic dispensing.
MCO = managed care organization.
FIGURE 2.
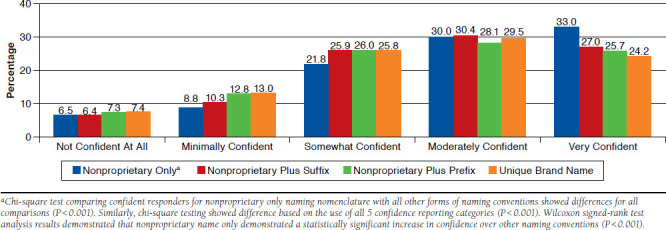
Confidence of Pharmacists in Dispensing an Interchangeable Biologic Based on Naming Convention Use (N = 619)
A total of 383 participants who responded to these survey items indicated that they did not dispense biologics at their place of employment, and 369 participants reported dispensing biologics. Figure 1 shows naming preferences of those participants who worked at a pharmacy that dispensed biologic agents, compared with those participants who did not work at a pharmacy that dispensed biologics. Naming convention preference here remained with the active ingredient plus a suffix (45.3% and 50.9%, respectively), and based on a chi-square analysis (P = 0.082), no distinguishable difference existed between those dispensing biologics, suggesting that the involvement in the dispensing of these products did not affect the overall naming preferences.
FIGURE 1.
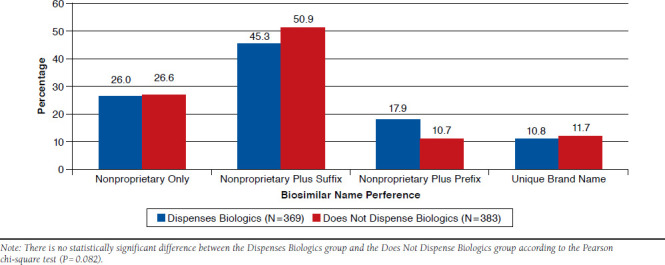
Naming Preference Dependent on Reports of Dispensing Biologics (N = 752)
Although pharmacists reported a preference for the use of a naming convention that included the nonproprietary name plus a suffix for biosimilars overall, when asked to rank their confidence in dispensing an interchangeable biosimilar based on naming conventions, the use of a nonproprietary name only was related to highest levels of confidence, with 62.9% of participants reporting “very confident” or “moderately confident” when substituting an interchangeable (chi-square analysis between confident and not confident responders based on naming convention, P < 0.001; Figure 2). When asked to rank confidence levels when using a nonproprietary name plus a suffix, 57.4% of participants reported being confident. Use of a nonproprietary name plus a prefix was associated with 53.8% of participants being confident, and the use of a unique brand name was associated with 53.7% of participants being confident. In addition, when comparing confidence based on the assigned 5 categories of confidence, the nonproprietary-only naming convention remained associated with more participants reporting higher levels of confidence (P < 0.001). A Wilcoxon signed-rank test further demonstrated that the use of a nonproprietary-only name was related to higher levels of confidence when compared with all other naming conventions (P < 0.001 across all comparisons).
Participants who reported that biologics were dispensed at their place of employment were asked to share what product-related data were collected and stored when dispensing biologics. Based on participants who reported this information (n = 357), 77.9% (n = 278) reported recording NDC numbers related to the product dispensed. Of those who reported that NDC numbers were not recorded (n = 79), 32.9% (n = 26) reported that they recorded a combination of J-code or Healthcare Common Procedure Coding System codes or the nonproprietary name and the manufacturer. Of those participants who reported that NDC numbers were not reported (n = 79), 18.1% (n = 14) reported that they were unaware of what data were recorded. This left 10.9% (n = 39) of participants who reported potentially insufficient data to determine which product was dispensed.
Participants were also asked how they recorded these data, and responses primarily included barcode scanning (33.5%), typing product name into an electronic prescription record (24.8%), and selecting the product in a drop-down window (21.7%). A total of 46.5% of participants reported that some or all of this recorded information was shared with the prescribers. The remaining participants were split between reporting that no data were shared (24.2%), unsure if any data were shared (8.7%), and nonresponders (20.6%).
Finally, participants were asked to report the level of burden they perceived if they were required to provide a postdispensing notification to prescribers whenever a biosimilar was dispensed (Figure 3). A majority of participants reported some to substantial burden (64.8%) resulting from such a requirement. When asked if a postdispensing reporting requirement would affect their likelihood of dispensing a biosimilar, many reported that it would not have an effect (44.4%). In addition, 27.7% participants stated that it would make them less likely to dispense a biosimilar or were unsure of its effect currently (24.3%; Figure 4).
FIGURE 3.
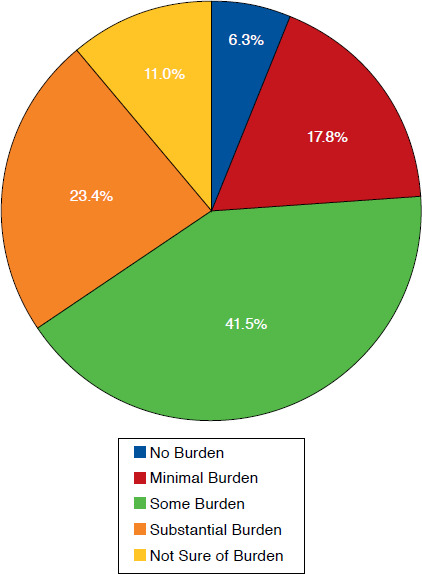
Pharmacist-Reported Level of Burden Associated with Postdispensing Notification Requirements When Dispensing a Biosimilar (N = 381)
FIGURE 4.
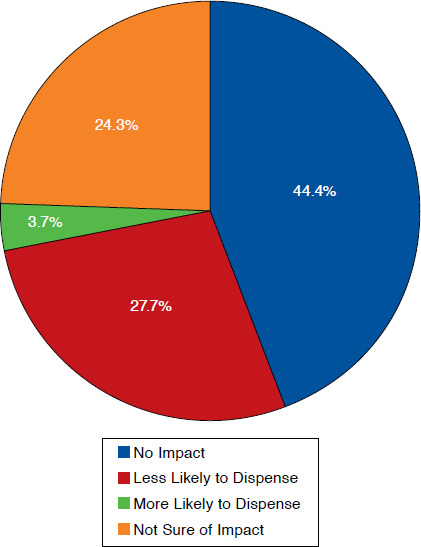
Effect of Likelihood to Dispense Biosimilar If Postdispensing Notification to Prescriber Is Required (N = 383)
Discussion
The nonproprietary name plus a suffix was the biosimilar naming convention most preferred by pharmacists completing the survey used in this study. Although pharmacists did report a preference for using a nonproprietary name plus a suffix for biosimilars, they did not demonstrate increased confidence when dispensing an interchangeable biologic using this naming convention. Higher levels of confidence were reported when the interchangeable product shared the same nonproprietary name with the reference biologic.
Because pharmacists reported that they are more confident substituting interchangeables when products share the same nonproprietary name, the use of a unique name for each interchangeable may reduce pharmacist confidence in dispensing products using other naming conventions. It is, however, unclear from the information gathered in this study what effect lowered pharmacist confidence would have on the dispensing and use of interchangeables, but the results of this study suggest that using the nonproprietary name only as the naming convention for interchangeables could have a positive effect on pharmacist attitudes.
Responses to questions regarding the recording of data suggest that NDC numbers are the most reported product specific information recorded at the time of dispensing. NDC numbers are product specific and allow for pharmacovigilance tracking similar to that of assigning a unique suffix to each product. To further demonstrate the frequent use of NDC numbers, participants reported recording nonproprietary names less frequently than NDC numbers (70.5% vs. 77.9%). These responses suggest that the inclusion of a suffix may not achieve the goals outlined by the designation of a suffix.
As states consider enacting laws requiring pharmacists to complete either predispense or postdispense reporting to prescribers when dispensing a biosimilar, legislators and regulators should consider potential increased burden on pharmacists and what value this will bring to the patient. The results of this study suggest that many pharmacists believe such requirements are adding burden, and many may be less likely to substitute approved interchangeable biosimilars, if required to complete postdispense reporting.
Unfortunately, no research has been conducted as to how prescribers will use this information or how such data will be incorporated into patient medical records. The current proposed bills and already enacted state laws requiring pre- or postdispensing reporting do not require prescribers to share any clinical information with pharmacists. In addition, the laws generally do not require prescribers to maintain the information that is provided. These one-sided communication requirements do not promote team-based care and lack reporting requirements that will help inform pharmacists as to whether patients may benefit from the dispensing of a specific product.
Limitations
There are limitations to this study that need to be considered. First, the methods used to initiate this survey limited responses to pharmacists who were members of HOPA or AMCP at the time of the survey launch, which limits the generalizability of the study to pharmacists as a whole. Second, the survey was conducted as a single cross-sectional study, which does not allow for longitudinal analysis of pharmacist perceptions related to biosimilars. Third, the methods used to deliver the e-mail invitation to the survey did not allow for survey receipt tracking. Response rates were based on the assumption that all e-mails not rejected or returned were delivered to the intended recipient. The use of e-mail tracking and receipt confirmation would have helped to define which invitees actually received the survey. Finally, the use of a close-ended online survey limited the ability of participants to provide researchers with additional information related to the rationale for the responses provided in the survey.
Conclusions
The results of this study update the preferences and perceptions of pharmacists regarding biosimilars from previous studies. The findings are intended to show regulators and legislators the importance of establishing a naming convention that encourages acceptance of biosimilars in the United States, as well as offers the necessary safety and pharmacovigilance tracking to ensure proper use. Regarding biosimilar naming conventions, participants in this study reported a preference for using the nonproprietary name plus a suffix; however, pharmacist confidence levels in substituting an interchangeable product with the reference biologic were highest when both products shared the same nonproprietary name. This finding suggests that, as biosimilars are considered by the FDA for approval with the designation as interchangeables, it may be beneficial for these products to share the same nonproprietary name. If such a naming convention is not adopted, additional efforts may be required to ensure that the dispensing pharmacists are conveying appropriate information to patients who will be using these products.
The potential requirement for pharmacists to provide postdispensing notification to prescribers after dispensing a biosimilar or interchangeable product was reported by survey participants to place increased burden on dispensing pharmacists. Such requirements need to be evaluated to determine their usefulness, and states that do implement these requirements need to evaluate their effects during implementation. This evaluation should include the potential effects on the willingness of pharmacists to dispense interchangeable products, prescriber use of the reported information, and the increased workload that these requirements create.
This project suggests the need for continued provision of educational support for pharmacists who are involved with biologics, biosimilars, and interchangeable biologics. Although pharmacists report a general knowledge of these products, there remain varying opinions and knowledge levels across the profession. It is vital that pharmacists have the necessary knowledge and expertise in this area so that they can take their place as medication experts within the health care team.
References
- 1.U.S. Food and Drug Administration. Abbreviated new drug application (ANDA): generics. July 14, 2015. Available at: http://www.fda.gov/Drugs/Development%20ApprovalProcess/HowDrugsareDevelopedandApproved/ApprovalApplications/AbbreviatedNewDrugApplicationANDAGenerics. Accessed June 17, 2016.
- 2.Patient Protection and Affordable Care Act. 42 U.S.C. § 18001 (2010).
- 3.U.S. Food and Drug Administration. Information on biosimilars. August 27, 2015. Available at: http://www.fda.gov/Drugs/DevelopmentApprovalProcess/HowDrugsareDevelopedandApproved/ApprovalApplications/TherapeuticBiologicApplications/Biosimilars/. Accessed June 17, 2016.
- 4.U.S. Food and Drug Administration. Implementation of Biologics Price Competition and Innovation Act of 2009. March 10, 2011. Available at: http://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/ucm215089.htm. Accessed June 17, 2016.
- 5.U.S. Food and Drug Administration. Scientific considerations in demonstrating biosimilarity to a reference product. Guidance for industry. April 2015. Available at: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM291128.pdf. Accessed June 17, 2016.
- 6.U.S. Food and Drug Administration. Quality considerations in demonstrating biosimilarity of a therapeutic protein product to a reference product. Guidance for industry. April 2015. Available at: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM291134.pdf. Accessed June 17, 2016.
- 7.Wolgemuth L, Jeffries JA.. Alexander, Hatch call on Burwell to immediately release guidance on biosimilar drugs. U.S. Senate Committee on Health, Education, Labor and Pensions. August 1, 2014. Available at: http://www. help.senate.gov/chair/newsroom/press/alexander-hatch-call-on-burwell-to-immediately-release-guidance-on-biosimilar-drugs. Accessed June 17, 2016.
- 8.Chustecka Z. First biosimilar approved in US: filgrastim-sndz (Zarxio). Medscape. March 6, 2014. Available at: http://www.medscape.com/view-article/841021. Accessed June 17, 2016.
- 9.U.S. Food and Drug Administration. FDA approves first biosimilar product Zarxio. March 6, 2015. Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm436648.htm. Accessed Juned 17, 2016.
- 10.U.S. Food and Drug Administration. Nonproprietary naming of biological products. Guidance for industry (Draft guidance). August 2015. Available at: http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryin-formation/guidances/ucm459987.pdf. Accessed June 17, 2016.
- 11.U.S. Food and Drug Administration. How FDA reviews proposed drug names. Available at: http://www.fda.gov/downloads/drugs/drugsafety/medication errors/ucm080867.pdf. Accessed June 17, 2016.
- 12.American Medical Association. Naming biologics. 2015. Available at: http://www.ama-assn.org/ama/pub/physician-resources/medical-science/united-states-adopted-names-council/naming-guidelines/naming-biologics.page?. Accessed June 17, 2016.
- 13.Zarxio (filagrastim-sndz) injection, for subcutaneous or intravenous use. Sandoz. March 2015. Available at: http://www.accessdata.fda.gov/drugsatfda_docs/label/2015/125553lbl.pdf. Accessed June 17, 2016.
- 14.Paradise J. The legal and regulatory status of biosimilars: how product naming and state substitution laws may impact the United States healthcare system. Am J Law Med. 2015;41(1):49-84. [DOI] [PubMed] [Google Scholar]
- 15.Grampp G, Felix T.. Pharmacovigilance considerations for biosimilars in the USA. BioDrugs. 2015;29(5):309-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fernandez-Lopez S, Kazzaz D, Bashir M, McLaughlin T.. Assessment of pharmacists’ views on biosimilar naming conventions. J Manag Care Spec Pharm. 2015;21(3):188-95. Available at: http://www.jmcp.org/doi/10.18553/jmcp.2015.21.3.188. [DOI] [PMC free article] [PubMed] [Google Scholar]


