Abstract
BACKGROUND:
The United States is currently experiencing an opioid abuse epidemic. Many policies and programs have been implemented at local, state, and national levels in an attempt to decrease prescription opioid addiction and overdose. On August 1, 2014, Colorado Medicaid implemented a policy change that limited the quantity of short-acting opioids (SAOs) that could be filled through the Medicaid benefit to no more than 4 tablets per day, or 120 tablets in 30 days.
OBJECTIVE:
To compare mean total daily dose (TDD) of opioids purchased by Kaiser Permanente Colorado (KPCO) Medicaid patients before and after implementation of the Medicaid SAO quantity limit.
METHODS:
This investigation used a pre/post study design to compare opioid use in Medicaid-eligible patients during the 90 days before implementation of the Medicaid quantity limit on August 1, 2014, and 90 days after full implementation of the change on December 31, 2014. The study was conducted at KPCO, an integrated care delivery system providing medical care for approximately 615,000 patients, of which over 54,000 are Medicaid members. Electronic medical and pharmacy records were used to identify patients and assess medication use.
RESULTS:
There was a small difference in opioid use in the population of Medicaid opioid users as evidenced by the median TDD of oral morphine equivalents (OME) purchased decreasing from 6.8 mg (IQR = 2.2-25.8) in the pre-implementation period to 6.6 mg (IQR = 1.7-24.0) in the postimplementation period (P = 0.027). The proportion of patients purchasing more than 120 mg OME per day and the proportion of patients purchasing long-acting opioids (LAOs) did not change significantly from the pre- to postimplementation period (OME > 120 of 4.2% vs. 3.6%, respectively, P = 0.290; LAO use of 12.9% vs. 13.6%, respectively, P = 0.465).
CONCLUSIONS:
This study found a statistically significant 3% decrease of 0.2 mg OME per day in the primary study population. A 24% reduction of 10 mg OME per day before and after implementation of the Medicaid SAO quantity limit was found in those patients identified as exceeding the Medicaid SAO quantity limit at baseline. These patients tended to be purchasing low to moderate total daily doses of opioids at baseline.
What is already known about this subject
In 2012, health care providers ordered 259 million opioid pain prescriptions; in 2014, the Centers for Disease Control and Prevention reported that more than 14,000 people died that year from prescription opioid overdose.
Many factors, such as prescriptions for high total daily doses of opioid medications, prescriptions from multiple providers, use of multiple pharmacies, and concurrent use of benzodiazepines, contribute to an increased risk of prescription drug overdose.
What this study adds
A statistically significant 24% decrease in mean total daily dose (TDD) of opioids before and after implementation of the Medicaid short-acting opioid quantity limit was seen in patients who exceeded the quantity limit at baseline.
Patients exceeding the Medicaid short-acting opioid quantity limit at baseline do not necessarily also exceed the high-risk threshold of 100 mg-120 mg OME per day.
A policy focused on reducing TDD of OME in those patients known to be at highest risk due to TDD of OME > 100 mg per day rather than quantity limits of short-acting opioids may be more beneficial in reducing risk of opioid-related overdose.
In 2012, health care providers prescribed 259 million opioid pain medications.1 In 2014, the Centers for Disease Control and Prevention reported that more than 14,000 people died that year from prescription opioid overdose.2 In the state of Colorado, 35% of all deaths from drug poisoning in 2013 involved prescription painkillers.3 These prescription drugs are not necessarily obtained illegally but are prescribed for an individual patient as part of routine medical care. A North Carolina study noted that about half of the patients who died of a drug overdose had filled a prescription for at least 1 drug that contributed to their deaths within the previous 60 days. Another study based on an insurance claims database found that over two thirds of nonfatal opioid overdoses involved patients with a valid opioid prescription.4
Many patient-specific factors are associated with an increased risk of prescription drug overdose, including male gender, age between 35 and 45 years, white and American Indian/Alaska Native ethnicity, low socioeconomic status, presence of mental health conditions, prescriptions for high total daily doses of opioid medications, prescriptions from multiple providers, use of multiple pharmacies, and concurrent use of benzodiazepines.4 Several of these factors can be addressed at a state or systems level, such as total daily medication dose prescribed, use of multiple providers and/or pharmacies, and the combination of opioids and benzodiazepines.
On August 1, 2014, Colorado Medicaid implemented a pharmacy benefit change that limited the quantity of short-acting opioids (SAOs) that could be reimbursed to no more than 4 tablets per day, or 120 tablets in 30 days. Four types of prior authorizations (PAs) to allow tablet quantities above this limit were available as part of policy implementation. All PA requests required either prescriber or pharmacy initiation. Lifetime PAs were permitted for patients with sickle cell disease or terminal illness (e.g., receiving hospice or palliative care services). Temporary PAs for up to 6 months were available for patients exceeding the Medicaid quantity limit at baseline to allow health care providers time to individually address each patient’s pain medication regimen. Longer-term PAs could be requested if the prescriber felt the pain diagnosis required doses exceeding the new limit and could not be addressed using long-acting opioids (LAOs) or would require a taper exceeding 6 months. Finally, PAs were available for acute pain situations, as defined by the prescriber. The acute pain PA allowed use of more than 4 tablets per day; however, the limit of 120 tablets in 30 days restriction remained in these situations.5 Between August 1, 2014, and December 31, 2014, Kaiser Permanente Colorado (KPCO) providers, clinical pharmacy specialists, and clinic staff worked with individual patients to evaluate and adjust pain management regimens that did not comply with the Colorado Medicaid quantity limit.
The purpose of this study was to compare mean total daily dose (TDD) of opioids purchased by KPCO Medicaid patients before and after implementation of the Medicaid SAO quantity limit.
Methods
Study Design and Data Collection
This investigation used a pre/post study design to compare opioid use in Medicaid-eligible patients during the 90 days before enactment of the Medicaid quantity limit on August 1, 2014, and 90 days after full implementation of the change on December 31, 2014. The study was conducted at KPCO, an integrated care delivery system providing medical care for approximately 615,000 patients, of which over 54,000 are Medicaid members. Electronic medical and pharmacy records were used to identify patients and assess medication use. Pharmacy records were queried using Generic Product Identifier (GPI) codes. Colorado Medicaid did not provide a list of opioids that would be affected by the SAO quantity limit; therefore, all oral SAO formulations in KPCO pharmacy records were queried using GPI codes. All oral and transdermal LAO were also queried using this method, which resulted in 703 unique SAO and LAO products. Tramadol and buprenorphine were excluded from analysis to align with the Colorado Medicaid policy. Because of KPCO’s formulary management system, of the 703 products queried, only 48 unique products were sold at KPCO to patients who met study inclusion criteria (Appendix A, available in online article). The KPCO institutional review board approved all study activities.
Study Participants
For the primary analysis, patients were eligible for inclusion if they were (a) aged 18 years and older; (b) purchased at least 1 SAO prescription at a KP pharmacy using the Medicaid benefit during either the pre-implementation (May 3, 2014-July 31, 2014) or postimplementation period (January 1, 2015-March 31, 2015); and (c) were continuously enrolled in a KPCO insurance plan between May 3, 2014, and March 31, 2015, the end of the postimplementation period. These patients were termed the “primary study population.”
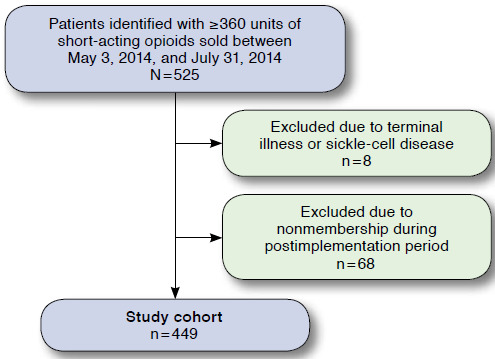
A secondary a priori analysis of Medicaid patients who exceeded the SAO limit at baseline was undertaken to assess the effect of the quantity limit on mean TDD of opioids purchased within the affected population. Patients were eligible for inclusion if they were (a) aged 18 years and older; (b) purchased at least 2 prescriptions through a Medicaid benefit for an SAO during the pre-implementation period May 3, 2014-July 31, 2014); (c) had a total days supply sold during the period of at least 360 units of SAOs; and (d) were continuously enrolled in a KPCO insurance plan between May 3, 2014, and March 31, 2015, the end of the postimplementation period. Patients were excluded from the Medicaid quantity limit and the study if they had sickle cell disease or a terminal illness, which was identified using International Classification of Diseases, Ninth Revision, Clinical Modification codes and verified by manual chart review. Patients were also excluded if they received a one-time PA to exceed the opioid quantity limit for acute pain, such as for a surgical procedure or motor vehicle accident, as determined by the prescriber. The remaining patients were termed the “secondary study population” (Figure 1).
FIGURE 1.
Patients Included in Secondary Analysis
Study Outcomes
The primary study outcome was the mean TDD of all oral and transdermal opioids purchased during the pre-implementation period versus the postimplementation period, expressed as oral morphine equivalents (OME; Appendix B, available in online article).
Opioid doses were converted to OMEs using a standardized conversion strategy (Appendix B).6 Mean TDD was calculated by dividing the total dose (as OME) sold by the days in the study period. If no opioids were purchased, the TDD was zero. Total dose on hand was calculated for each study period by summing prescription purchases during the study period plus fractions of purchases made previously whose days supply extended into the study period, and truncating purchases made near the end of the period whose days supply exceeded the length of the period. For example, for a patient purchasing 4 prescriptions for hydrocodone/acetaminophen that completely or partially covered the study period, the total number of tablets counted towards the mean daily OME would be calculated as shown in Table 1.
TABLE 1.
Sample Calculation for Daily OME
| Prescription Purchase Dates for Hydrocodone/Acetaminophen, #60 Tablets | Days Supply (per Prescription Instructions) | Number of Days Prescription Overlapped with Study Period (May 1, 2014-July 31, 2014) | Number of Tablets Counted Towards Daily OME |
|---|---|---|---|
| April 20, 2014 | 30 | May 1-19 = 19 | 19/30 × 60 = 38 |
| May 30, 2014 | 30 | all | 60 |
| June 21, 2014 | 30 | all | 60 |
| July 17, 2014 | 30 | July 17-31 = 14 | 14/30 × 60 = 28 |
| Total number of dosage units used within the study period: 186 | |||
OME = oral morphine equivalents.
Secondary outcomes included the proportion of patients whose mean TDD of opioids in OME was > 120 mg per day during the study period and the percentage of patients purchasing any LAO. The proportion of patients purchasing 1 or more adjuvant nonopioid pain medications was also assessed in the secondary study population.
Statistical Analysis
For the primary analysis of all Medicaid SAO users, continuous outcomes were compared using Wilcoxon’s two-sample test, and categorical outcomes were compared using chi square tests. For the primary analysis of opioid use in the secondary study population, continuous data were expressed as a median with interquartile range (IQR) and were compared using Wilcoxon’s signed-rank test. Categorical data were expressed as percentages and compared using McNemar’s test. The α level was set at 0.05. Data analyses were performed using SAS, version 9.2 (SAS Institute, Carey, NC).
Results
A total of 2,471 patients in the pre-implementation period and 2,721 patients in the postimplementation period with KPCO Medicaid insurance coverage purchased at least 1 opioid prescription during the study period and were included in the primary analysis of all Medicaid SAO users.
A total of 525 patients were identified who were (a) aged 18 years and older; (b) purchased at least 1 SAO prescription at a KP pharmacy using a Medicaid benefit during either the pre-implementation period (May 3, 2014-July 31, 2014) or postimplementation period (January 1, 2015-March 31, 2015); and (c) were continuously enrolled in a KPCO insurance plan between May 3, 2014, and March 31, 2015, the end of the postimplementation period. Of these, 8 patients were excluded because of sickle cell disease or terminal illness, and 68 patients were excluded due to lack of continuous membership, leaving 449 patients available for the secondary analysis.
In the primary study population, the mean age of patients in the pre-implementation period was 42.3 years and 43.5 years in the postimplementation period (P = 0.541). A statistically, although not clinically significant, difference was seen in the percentage of the population that was male, with 32.4% in the pre-implementation period and 32.9% in the postimplementation period (P < 0.001). In the secondary study population, the mean age was 45.2 years, and 34.3% of the population was male. The most common problem designations associated with opioid prescriptions were chronic pain and low back pain (Table 2).
TABLE 2.
Selected Baseline Demographics for Study Population
| Primary study population patients | |||
|---|---|---|---|
| Characteristics | Preperiod N = 2,472 | Postperiod N = 2,721 | P Value |
| Age, years (mean) | 42.3 | 43.5 | 0.541 |
| Male, n (%) | 801 (32.4) | 895 (32.9) | < 0.001 |
| Secondary study population patients (N = 499) | |||
| Age, years (mean) | 45.2 | ||
| Male, n (%) | 154 (34.3) | ||
| Secondary study population patients: most common problem list designations throughout study duration (patients may have more than 1 designation), % | |||
| Chronic pain | 24.1 | ||
| Low back pain | 11.3 | ||
| Spine/lumbar/cervical pain | 9.3 | ||
| Joint pain | 6.7 | ||
| Arthritis | 5.4 | ||
| Cancer | 2.3 | ||
| Nerve pain | 2.3 | ||
| Abdominal/pelvic pain | 2.2 | ||
| Infection | 2.1 | ||
In the primary study population, the median TDD of OME purchased decreased from 6.8 mg (IQR = 2.2-25.8) in the pre-implementation period to 6.6 mg (IQR = 1.7-24.0) in the postimplementation period (P = 0.027; Table 3). The proportion of patients purchasing more than 120 mg OME per day and the proportion of patients purchasing LAOs did not change significantly from the pre- to postimplementation period (OME > 120 of 4.2% vs. 3.6%, respectively, P = 0.290; LAO use of 12.9% vs. 13.6%, respectively, P = 0.465; Table 3).
TABLE 3.
Outcomes by Study Group
| Primary Study Population | Secondary Study Population | |||||
|---|---|---|---|---|---|---|
| Preperiod n = 2,471 | Postperiod n = 2,721 | P Value | Preperiod n = 449 | Postperiod n = 449 | P Value | |
| Median TDD opioids in OME, median (IQR) | 6.8 (2.2-25.8) | 6.6 (1.7-24.0) | 0.027 | 42.2 (27.5-76.5) | 32.2 (21.0-62.0) | < 0.001 |
| Median TDD LAO in OME, median (IQR) | 0.0 (0.0-0.0) | 0.0 (0.0-0.0) | 0.429 | 0.0 (0-4.7) | 0.0 (0-25.0) | 0.059 |
| Patients purchasing any LAO, n (%) | 319 (12.9) | 370 (13.6) | 0.465 | 118 (26.3) | 134 (29.8) | 0.060 |
| Patients purchasing > 120 mg OME per day, n (%) | 104 (4.2) | 99 (3.6) | 0.290 | 61 (13.6) | 53 (11.8) | 0.201 |
IQR = interquartile range; LAO = long-acting opioid; OME = oral morphine equivalents; TDD = total daily dose.
In the secondary study population, the TDD of OME decreased from 42.2 mg (IQR = 27.5-76.5) in the pre-implementation period to 32.2 mg (IQR = 21.0-62.0) in the postimplementation period (P < 0.001; Table 3). The proportion of patients purchasing at least 1 adjuvant nonopioid pain medication decreased from 65.9% in the pre-implementation period to 55.0% in the post-implementation period (P < 0.001, Tables 3 and 4). The proportion of patients purchasing cumulative opioid doses exceeding 120 mg of OME per day or utilizing a LAO between the pre- and post-implementation periods was not significantly different (OME > 120 of 13.6% vs. 11.8%, respectively, P = 0.201; LAO use of 26.3% vs. 29.8%, respectively, P = 0.060; Table 3).
TABLE 4.
Medication Use for Secondary Study Population
| Preperiod n = 449 | Postperiod n = 449 | P Value | |
|---|---|---|---|
| Short-acting opioids, n (%) | |||
| Oxycodonea | 348 (77.5) | 312 (69.5) | |
| Hydrocodonea | 121 (26.9) | 95 (21.2) | |
| Hydromorphonea | 34 (7.6) | 27 (6.0) | |
| Morphinea | 7 (1.6) | 7 (1.6) | |
| Codeinea | 3 (0.7) | 4 (0.9) | |
| Long-acting opioids, n (%) | |||
| Any long-acting opioid | 118 (26.3) | 134 (29.8) | 0.060 |
| Morphine SRa | 77 (17.1) | 87 (19.4) | |
| Methadonea | 24 (5.3) | 22 (4.9) | |
| Fentanyla | 17 (3.8) | 20 (4.5) | |
| Oxycodone SRa | 9 (2.0) | 11 (2.4) | |
| Oxymorphonea | 1 (0.2) | 0 (0.0) | |
| Adjuvant nonopioid,a n (%) | |||
| Any adjuvant nonopioid | 296 (65.9) | 247 (55.0) | < 0.001 |
| Cyclobenzaprine | 121 (26.9) | 104 (23.2) | |
| SSRI | 81 (18.0) | 66 (14.7) | |
| TCA | 75 (16.7) | 64 (14.3) | |
| NSAID | 75 (16.7) | 67 (14.9) | |
| Gabapentin | 65 (14.5) | 55 (12.2) | |
| SNRI | 59 (13.1) | 37 (8.2) | |
| Lidocaine | 36 (8.0) | 25 (5.6) | |
| Pregabalin | 10 (2.2) | 12 (2.7) | |
| Carbamazepine | 6 (1.3) | 2 (0.4) | |
aAdds up to > 100%; patients could receive more than 1 agent.
NSAID = nonsteroidal anti-inflammatory drug; SNRI = serotonin/norepinephrine reuptake inhibitor; SR = sustained-release; SSRI = selective serotonin reuptake inhibitor; TCA = tricyclic antidepressant.
Discussion
This study found a statistically significant 3% decrease of 0.2 mg OME per day in the primary study population. Within the secondary study population, a 24% decrease of 10 mg OME per day was seen in those patients who exceeded the Medicaid SAO quantity limit as baseline. At baseline, the secondary study population was purchasing low to moderate total daily doses of opioids (median OME 42.2 mg/day, IQR = 27.5-76.5 mg/day) despite exceeding the Medicaid SAO quantity limit. Recent studies have demonstrated a 7- to 9-fold increased risk of overdose when taking 100 mg of OME per day or more, suggesting that identification of patients taking opioids over a given threshold may be a better indicator of overdose risk than tablet quantity.7,8 Several organizations recommend identifying patients at highest risk for opioid overdose based on an OME per day threshold, but there is variability in the threshold recommended. The Centers for Medicare & Medicaid Services and the Colorado Department of Regulatory Agencies’ Quad-Regulator Boards recommend a threshold of 120 mg OME per day.9,10 Recently, the Centers for Disease Control and Prevention recommended a threshold of 90 OME per day, where opioids should not be further titrated without careful justification based on diagnosis, benefits, and risks.1
In this study, we analyzed the proportion of patients purchasing any combination of opioids at a threshold of 120 mg OME per day to align with the recommendations from the Colorado Department of Regulatory Agencies’ Quad-Regulator Boards and found a nonsignificant 0.6% decrease in the primary study population and a nonsignificant 1.8% decrease in the secondary study population after implementing the Medicaid quantity limit. Patients exceeding the SAO quantity limit imposed by Medicaid did not necessarily also exceed the high-risk threshold of 100 mg to 120 mg OME per day. Given this, use of tablet quantity to identify patients who require review and possible modification of an opioid regimen is likely to include some low- to moderate-risk patients. To our knowledge, this is the first study to assess the effect on TDD of OME by a state- or systems-level policy designed exclusively to target opioid quantity limits.
A policy focused on reducing TDD of OME in those patients known to be at highest risk because of TDD of OME > 100 mg per day rather than quantity limits of SAO may be more beneficial in reducing risk of opioid-related overdose. Implementation of an opioid dosing guideline in 2007 in Washington State recommended that providers seek consultation with a pain medicine expert for patients with noncancer pain on opioid doses of 120 mg or more TDD of OME who are not experiencing significant pain relief. A study assessing the Washington State workers’ compensation system following implementation of this guideline demonstrated a 35% decrease in the proportion of patients on 120 mg or more TDD of OME.11
This study also sought to examine if implementation of the Medicaid SAO quantity limit affected use of adjuvant nonopioid pain medications. Surprisingly, the results showed a statistically significant decrease of 10.9% in purchases of nonopioid pain medications in the post-implementation period, compared with the pre-implementation period within the secondary study population. We believe this occurred because, at KPCO, implementation of the Medicaid quantity limit encouraged collaborative reassessment of a patient’s pain management regimen between patient and provider. Many nonopioid pain medications can be difficult to tolerate and costly and often require trial and error to identify an effective agent. Revisiting the patient’s pain medication regimen could have identified some of these issues and resulted in discontinuation of ineffective or poorly tolerated adjuvant agents.
Limitations
Study limitations include use of a pre/post study design and lack of a control group, which increases the likelihood that the change seen is caused by a confounding factor. We believe that the significant resources devoted to coming into compliance with the law are the most likely causes of the short-term reduction in opioid use observed, but whether this effect will persist without continuation of such efforts is unknown. Additional limitations include exclusion of tramadol from TDD of OME to align with the Medicaid SAO quantity limit and an inability to capture data for prescription purchases made outside of the KPCO pharmacy system. Previous research has shown that around 95% of prescriptions at KPCO are filled within the KPCO pharmacy system.12 There were no external factors identified that varied between the study periods that would make external prescription filing more or less attractive between the pre- and postimplementation period. We were also unable to assess health outcomes such as nonfatal or fatal overdose and health care utilization, given the short duration of the pre- and postimplementation periods.
Conclusions
This study found a statistically significant 3% decrease of 0.2 mg OME per day in the primary study population. A 24% reduction of 10 mg OME per day was found in the secondary study population before and after implementation of the Medicaid SAO quantity limit; however, these patients tended to be purchasing low to moderate total daily doses of opioids at baseline. For the primary and secondary study populations, no change was seen in the percentage of patients purchasing greater than 120 mg per day of OME, a threshold commonly used to identify patients at highest risk of opioid overdose. If the goal is to decrease risk of opioid overdose, future insurer and pharmacy benefit manager strategies should focus on reducing opioid doses below a threshold of 100 mg to 120 mg of OME per day rather than SAO quantity limits.
APPENDIX A. GPI Codes for Opioids Sold to Study Participants and for Pain Medication
| SA and LA Opioid Oral and Transdermal GPI Codes Sold to Study Participants | |
|---|---|
| Generic Product Name | GPI Code |
| Codeine 30 mg | 65100020200310 |
| Fentanyl 12 mcg | 65100025008610 |
| Fentanyl 25 mcg | 20 |
| Fentanyl 50 mcg | 30 |
| Fentanyl 75 mcg | 40 |
| Fentanyl 100 mcg | 50 |
| Hydromorphone 2 mg | 65100035100310 |
| Hydromorphone 4 mg | 320 |
| Hydromorphone 1 mg/mL | 920 |
| Methadone 5 mg | 65100050100305 |
| Methadone 10 mg | 10 |
| Methadone 10 mg/mL | 1310 |
| Methadone 5 mg/mL | 2010 |
| Morphine 15 mg | 65100055100310 |
| Morphine 30 mg | 315 |
| Morphine ER 15 mg | 415 |
| Morphine ER 30 mg | 432 |
| Morphine ER 60 mg | 445 |
| Morphine ER 100 mg | 460 |
| Morphine 20 mg/mL | 2090 |
| Oxycodone 5 mg | 65100075100310 |
| Oxycodone 10 mg | 320 |
| Oxycodone 15 mg | 325 |
| Oxycodone 20 mg | 330 |
| Oxycodone 30 mg | 340 |
| Oxycodone 20 mg/mL | 1320 |
| Oxycodone 5 mg/5mL | 2005 |
| Oxycodone CR 10 mg | A710 |
| Oxycodone CR 15 mg | A715 |
| Oxycodone CR 20 mg | A720 |
| Oxycodone CR 40 mg | A740 |
| Oxycodone CR 60 mg | A760 |
| Oxycodone CR 80 mg | A780 |
| Oxymorphone 5 mg | 65100080107405 |
| Oxymorphone ER 5 mg | A705 |
| Oxymorphone ER 10 mg | A710 |
| Tylenol w/codeine 300/30 mg | 65991002050315 |
| Tylenol w/codeine 300/60 mg | 320 |
| Tylenol w/codeine 120/12 mg/5mL | 2020 |
| Oxycodone w/acetaminophen 5 mg | 65990002200310 |
| Oxycodone w/acetaminophen 7.5 mg | 27 |
| Oxycodone w/acetaminophen 10 mg | 35 |
| Hydrocodone-acetaminophen 10 mg | 65991702100305 |
| Hydrocodone-acetaminophen 5 mg | 356 |
| Hydrocodone-acetaminophen 7.5 mg | 358 |
| Hydrocodone-acetaminophen 7.5 mg | 2015 |
| Hydrocodone-ibuprofen 7.5 mg | 500320 |
| Tapentadol 100 mg | 65100091100340 |
| Adjuvant Pain Medication GPI Codes | |
| Name of Drug | GPI Code |
| Tricyclic antidepressants (e.g., nortriptyline, amitriptyline) | 582000* |
| Pregabalin | 7260005700* |
| Gabapentin | 72600030000* |
| Nonsteroidal anti-inflammatory drugs (e.g., ibuprofen, meloxicam) | 66100* |
| Acetaminophen | 64200010000 |
| Lidocaine | 90850060* |
| SSRI | 581600* |
| SNRI | 581800* |
| Cyclobenzaprine | 751000* |
| Carbamazepine | 7260002000* |
| Capsaicin | 90850025* |
GPI = Generic Product Identifier; LA = long acting; SA = short acting; SNRI = serotonin/norepinephrine reuptake inhibitor; SSRI = selective serotonin reuptake inhibitor.
APPENDIX B. Oral/Transdermal Morphine Equianalgesic Conversion Table
| Product | Ratio of Product: Oral Morphine |
|---|---|
| Codeine | 20:3 |
| Fentanyl | 1:2 |
| Hydromorphone | 1:4 |
| Methadone | 1:3 |
| Morphine | 1:1 |
| Oxycodone | 2:3 |
| Oxymorphone | 1:3 |
| Hydrocodone | 1:1 |
| Tapentadol | 1:2 |
Adapted from: McPherson ML. Demystifying Opioid Conversion Calculations: A Guide for Effective Dosing.6
REFERENCES
- 1.Dowell D, Haegerich TM, Chou R.. CDC guideline for prescribing opioids for chronic pain–United States, 2016. MMWR Recomm Rep. 2016;65(1):1-49. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention.. Prescription opioid overdose data. Updated December 16, 2016. Available at: http://www.cdc.gov/drugoverdose/data/overdose.html. Accessed January 30, 2017.
- 3.Colorado Consortium for Prescription Drug Abuse Prevention.. The problem: Colorado statistics. TakeMedsSeriously.org. Available at: http://takemedsseriously.org/the-problem/colorado-statistics/. Accessed January 30, 2017.
- 4.Haegerich TM, Paulozzi LJ, Manns BJ, et al.. What we know, and don’t know, about the impact of state policy and systems-level interventions on prescription drug overdose. Drug Alcohol Depend. 2014;145:34-47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Colorado Department of Health Care Policy & Financing.. Communication regarding clarification of new short-acting oral opioid policy. August 25, 2014. Available at: https://www.colorado.gov/pacific/sites/default/files/Stakeholder%20communication%20regarding%20new%20short-acting%20opioid%20policy.pdf. Accessed January 30, 2017.
- 6.McPherson ML. Demystifying Opioid Conversion Calculations: A Guide for Effective Dosing. Bethesda, MD: American Society of Health-System Pharmacists; 2010. [Google Scholar]
- 7.Dunn KM. Opioid prescriptions for chronic pain and overdose: a cohort study. Ann Intern Med. 2010;152(2):85-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bohnert ASB, Valenstein M, Bair MJ, et al.. Association between opioid prescribing patterns and opioid overdose-related deaths. JAMA. 2011;305(13):1315-21. [DOI] [PubMed] [Google Scholar]
- 9.Centers for Medicare & Medicaid Services.. Supplemental guidance related to improving drug utilization review controls in Part D [Memorandum]. September 6, 2012. Available at: https://www.cms.gov/Medicare/Prescription-Drug-Coverage/PrescriptionDrugCovContra/Downloads/HPMSSupplementalGuidanceRelated-toImprovingDURcontrols.pdf. Accessed January 30, 2017.
- 10.Colorado Department of Regulatory Agencies, Division of Professions and Occupations.. Open letter to the general public on the Quad-Regulator joint policy for prescribing and dispensing opioids. October 15, 2014. Available at: https://drive.google.com/file/d/0B-K5DhxXxJZbd01vVXdTTklZLVU/view?pref=2&pli=1. Accessed January 30, 2017.
- 11.Franklin GM, Mai J, Turner J, Sullivan M, Wickizer T, Fulton-Kehoe D.. Bending the prescription opioid dosing and mortality curves: impact of the Washington State opioid dosing guideline. Am J Ind Med. 2012;55(4):325-31. [DOI] [PubMed] [Google Scholar]
- 12.Delate T, Albrecht G, Olson, KL.. Out-of plan pharmacy use by members of a managed care organization. Perm J. 2012;16(2):14-19. [DOI] [PMC free article] [PubMed] [Google Scholar]


