Abstract
BACKGROUND:
Fibromyalgia (FM) affects up to 6% of U.S. adults, resulting in a significant burden on the health care system and poor quality of life for patients. Duloxetine, pregabalin, and milnacipran are approved for management of FM; however, consensus is lacking regarding optimal therapy. Patients with FM taking approved medications often do not experience meaningful symptom relief, and many experience intolerable adverse events.
OBJECTIVE:
To assess treatment patterns associated with available and commonly used medications for the management of FM using U.S. health insurance claims.
METHODS:
This retrospective analysis used the MarketScan claims database to identify adults with a first diagnosis of FM (ICD-9-CM code 729.1) between 2009 and 2011 with continuous health plan enrollment for 12 months pre- and post-index. Medications of interest were pregabalin, gabapentin, duloxetine, milnacipran, cyclobenzaprine, and tramadol. These are 6 of the 8 medications recommended by the American College of Rheumatology (ACR) for treating FM; the other 2 (amitriptyline and venlafaxine) were only included in some initial assessments. The Charlson Comorbidity Index (CCI) was used to assess overall comorbidity burden. Endpoints included proportion of patients treated within 1 year after first diagnosis; initial treatment pattern; adherence over the first-year follow-up period for the medications of interest; and discontinuation, switching, and combination therapy patterns among pain medications of interest at different time points. Proportion of days covered (PDC; defined as number of days in the period when the patient had drug supply divided by the number of days in the period) was used to define adherence, which was categorized as low (PDC < 50%), medium (PDC 50% to < 80%), or high (PDC ≥ 80%). The time to discontinuation (defined as the first drug supply gap ≥ 90 days) was estimated using Kaplan-Meier analysis.
RESULTS:
Overall, 240,144 patients met the inclusion criteria. Patients were predominantly women (68%), had preferred provider organization insurance coverage (68%), and had a CCI score < 1 at baseline (69%). Only 31% (n = 74,738) initiated a treatment with a prescription medication listed in the ACR guidelines, and many patients received less than the recommended dose. Most (n = 70,919) patients initially received monotherapy with one of the 8 prescription medications. Of those who started with ≥ 2 medications (n = 3,819), cyclobenzaprine plus tramadol was the most frequent combination. Adherence was suboptimal for all 6 medications of interest. Duloxetine had the highest mean PDC (59%); for all other agents, mean PDC was < 50%. With the exception of duloxetine, discontinuation rates at 6 months were > 50% for all agents. Alterations in therapy were common. Among patients who discontinued their initial treatment of duloxetine, pregabalin, or milnacipran, approximately one-third had switched treatments within 90 days after their first prescription. For those who maintained their initial treatment agent, approximately 50% of patients added a second pain medication within 1 year of treatment initiation.
CONCLUSIONS:
The evidence suggests that patients with FM often do not receive 1 of the prescription medications recommended by ACR guidelines, and those who do are commonly prescribed lower-than-recommended doses, potentially resulting in poor effectiveness and tolerability. Discontinuation, switching, and addition of new pain medications are common, which may indicate low levels of satisfaction with initial treatment. New therapies with improved effectiveness and better tolerability are urgently needed for patients with FM.
What is already known about this subject
Of those pain therapies approved to manage fibromyalgia (FM), none appear to combine the effectiveness necessary to control symptoms and tolerability needed for long-term use.
Many patients with FM use polypharmacy (including a variety of off-label prescriptions, over-the-counter medications, and nondrug treatments, possibly to address different symptoms.
Treatment discontinuation is prevalent among patients with FM, and common reasons for stopping treatment include the occurrence of adverse events (in up to 40% of patients with FM) and lack of effectiveness (in up to 23% of patients with FM).
What this study adds
We found that more than two thirds of patients diagnosed with FM were not treated with an American College of Rheumatologyrecommended prescription medication for a year after diagnosis.
Of those patients who received treatment, approximately 26% were prescribed FDA-approved therapies, and even fewer received the approved dose. Adherence was suboptimal for all treatments, and discontinuation rates were high.
The results of this analysis show that discontinuation, switching, and addition of new pain medications are common, which may indicate low levels of satisfaction with initial treatment.
Pain is a common symptom reported by patients in primary care and accounts for 40% to 60% of outpatient visits.1 Fibromyalgia (FM), a disorder characterized by widespread chronic musculoskeletal pain, is estimated to affect up to 6% of the adult population in the United States and 3% of adults worldwide.2-5 Women are diagnosed with FM at a rate 3- to 6-fold higher than men6; however, recent research suggests that there are no sex differences in the overall clinical picture of FM (e.g., patient age, time since diagnosis, number of pain sites, and somatic and depressive symptoms).7 The precise cause and pathophysiology of FM is unclear, although it seems to involve disordered afferent processing and might be triggered or exacerbated by biological stressors such as physical trauma, infection, and some forms of psychological stress.8
Establishing an accurate diagnosis of FM is essential to successful disease management. Measures for the classification of FM were first published by the American College of Rheumatology (ACR) in 1990, with an update in 2010, which made the diagnostic criteria easier to use by primary care physicians.5,9,10 A patient is diagnosed with FM if he or she has a widespread pain index (WPI) score ≥ 7 and symptom severity (SS) scale score ≥ 5 or WPI score 3 to 6 and SS scale score ≥ 9, symptoms have been present for at least 3 months, and there is no other disorder present that would explain the pain.9 In addition to pain, patients might also report sleep disturbance and fatigue, as well as mood disturbances, stiffness, and cognitive difficulties.2,3 However, many physicians do not use ACR criteria to diagnose FM.8 The mean time taken to diagnose FM is 5 years, and many people with the condition remain undiagnosed, which might result in suboptimal medical care and potentially incur additional health care costs.2
An estimated 200,000 U.S. hospitalizations annually are associated with FM. Patients commonly have comorbidities (such as cardiac disorders, psychiatric disorders, hypertension, diabetes, and disorders of lipid metabolism) that necessitate additional medical treatment.11 Approximately half of patients diagnosed with FM report some disruption of employment, missing an average of 2 workdays per month because of symptoms.12 Medical costs, drug costs, and indirect costs (e.g., lost productivity at work) associated with FM are estimated to be >$10,000 per patient per year and are nearly twice those of people without FM.13 Patients with FM, compared with other chronic diseases such as rheumatoid arthritis or osteoarthritis, commonly score lower on quality of life measurements and are more likely to have depression and other affective disorders than people without FM.3,14,15
Because the pathogenesis of FM is not fully understood, FM treatments focus on symptom management.16 Of those pain therapies approved to treat FM, none appears to combine the effectiveness necessary to control symptoms with the tolerability needed for long-term use.17,18 Currently, pregabalin (Lyrica, Pfizer, 2007),19 duloxetine (Cymbalta, Eli Lilly, 2008),20 and milnacipran (Savella, Forest Laboratories, 2009)21 have been approved for management of FM.8,22 However, debate continues over the best choice of treatment for FM,23 and there seems to be no consistency in prescribing patterns among physicians.24 Although some patients with FM experience symptom relief with some medications, often the benefits do not outweigh the side effects, and many patients find the adverse events (AEs) intolerable.1,17 Many patients with FM use polypharmacy, possibly to address different symptoms.13,25-27 The most commonly used therapeutic classes are narcotic analgesics, skeletal muscle relaxants, selective serotonin reuptake inhibitors (SSRIs), benzodiazepines, non-benzodiazepine sleep aids, serotonin norepinephrine reuptake inhibitors (SNRIs), and tramadol.13 The use of opioids and nonsteroidal anti-inflammatory drugs (NSAIDs) to manage FM persists, despite the lack of evidence supporting their analgesic effect in FM.8 Treatment discontinuation is prevalent among patients with FM. Clinical trial data from duloxetine FM studies suggest that the 2 most common reasons for stopping treatment were the occurrence of AEs (in up to 40% of patients) and lack of effectiveness (in up to 23% of patients).28
To improve treatment for patients with FM, analyses of real-world evidence are needed to understand current treatment patterns and to examine gaps in achieving treatment goals. We used a health insurance claims database to assess the treatment patterns associated with available and commonly used medications for the management of FM.
Methods
Patients
This retrospective analysis used the MarketScan claims database to identify patients who had a first FM diagnosis code (International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM] code 729.1) in 2009-2011 with a repeat diagnosis (to confirm FM and reduce the possibility of misdiagnosis and exclude rule-out visits) within a year. The 2009-2011 period was selected to provide the most recent data and to include milnacipran, which was approved in January 2009.
Inclusion criteria included the following: patients ≥ 18 years old selected from the MarketScan claims database and patients ≥ 65 years of age identified from the Medicare supplemental database; patients with a first FM diagnosis (ICD-9-CM code 729.1) between April 1, 2009, and December 31, 2011, (primary or secondary diagnosis) with a repeat diagnosis within a year; and continuous enrollment in a health plan during the 12 months preceding the index date (baseline period) and the 12 months after the index date (follow-up period). The first diagnosis date was defined as the index date. Because this analysis focused on patients newly diagnosed with FM, patients with a prior diagnosis of FM were excluded.
Assessments and Endpoints
Eight treatments were assessed, in 4 therapeutic classes, using all National Drug Code numbers associated with the generic names: anticonvulsants (pregabalin and gabapentin [Gralise, Horizant, Neurontin]); antidepressants (duloxetine, milnacipran, amitriptyline [Elavil], and venlafaxine [Effexor]; a muscle relaxant (cyclobenzaprine [Flexeril]); and an opioid analogue (tramadol [Ultram]). ACR treatment recommendations were considered when identifying these prescription medications29; however, only duloxetine, milnacipran, and pregabalin are U.S. Food and Drug Administration (FDA)-approved for the management of FM. These 3 approved medications and 3 other treatments most frequently used (mil-nacipran, cyclobenzaprine, and tramadol) were considered the medications of interest for all evaluations.
Patient baseline characteristics (age, sex, type of claim, comorbidity status) were recorded. To assess patient overall comorbidity burden, the Charlson Comorbidity Index (CCI) was calculated using the algorithm developed by Quan et al. (2011).30 Endpoints included the proportion of patients treated within 1 year after first diagnosis; the initial treatment pattern (including first medication, initial daily dose and frequency, maintenance dose over 1 year, and use of combination therapy); adherence over the first-year follow-up period for the medications of interest; and discontinuation, switching, and combination therapy patterns among pain medications of interest at different time points.
Statistical Analysis
Baseline characteristics were recorded using descriptive statistics. Proportion of days covered (PDC; defined as number of days in the period when the patient had drug supply divided by the number of days in the period) was used to define adherence; this ratio can be multiplied by 100 to yield a percentage.31 Adherence was categorized as low (PDC < 50%), medium (PDC 50% to < 80%), or high (PDC > 80%), as described in previous publications.32-34 The time to discontinuation (defined as the first drug supply gap > 90 days) was estimated using Kaplan-Meier analysis. At any given time, if a patient had discontinued the initial medication and a new medication was filled between the final fill date of the initial medication and 90 days after the discontinuation date, switching of medication was considered to have occurred. Add-on treatment was defined as use of another pain medication in addition to the initial treatment before discontinuation. Only the first switch or first add-on therapy was considered in this analysis.
Results
Patients
A total of 240,144 patients met the inclusion criteria (Figure 1). As expected, the population was composed of more women (68%) than men (32%), and mean (standard deviation [SD]) age was 48 (13.8) years. Overall, 68% of patients had preferred provider organization insurance coverage; 9.6% had a health maintenance organization plan; 6.9% had a point of service (POS) plan; 6.3% had a comprehensive plan; 3.5% had a consumer-driven health plan; and 5.7% had high-deductible health plans, exclusive provider organizations, or POS with capitation.
FIGURE 1.
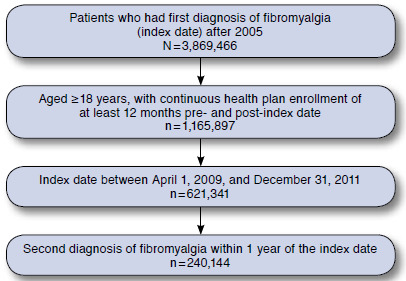
Patient Selection Flowchart
Most patients included in the analysis (69%) had CCI scores < 1; 25% had CCI scores of 1-2; and 6% had CCI scores > 3. The most common comorbidities at baseline were back pain (37%), abdominal pain (17%), chest pain (15%), and headache/migraine (14%). Almost one quarter of patients (23%) were taking prescription NSAIDs at baseline.
Treatment
Only 31% (n = 74,738) of patients initiated a prescription medication of interest, per ACR guidelines (pregabalin, gaba-pentin, duloxetine, milnacipran, cyclobenzaprine, tramadol, amitriptyline, or venlafaxine) within a year after the index date (Figure 2).29 The 3 most frequently dispensed medications were cyclobenzaprine (27%), tramadol (18%), and gabapentin (16%; Table 1). The FDA-approved medications duloxetine, pregabalin, and milnacipran accounted for 14%, 9%, and 4% of treated patients, respectively. Approved medications and/or the treatments most frequently used were considered the medications of interest for the remainder of the evaluation.
FIGURE 2.
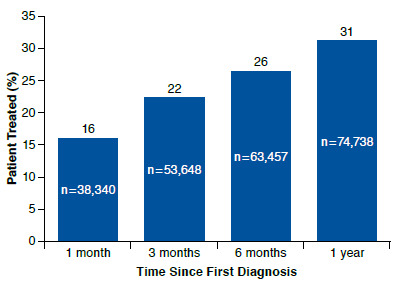
Percentage of Patients Receiving 1 of the 8 Medications of Interest at Each Time Point Since First Diagnosis of Fibromyalgia (N = 240,144)
TABLE 1.
Frequency of Medication Initiation (First Prescription; N = 70,919)
| Medication | Frequency, n (%) |
|---|---|
| Cyclobenzaprine | 19,420 (27) |
| Tramadol | 12,681 (18) |
| Gabapentin | 11,552 (16) |
| Duloxetine | 9,648 (14) |
| Pregabalin | 6,297 (9) |
| Amitriptyline | 4,978 (7) |
| Venlafaxine | 3,677 (5) |
| Milnacipran | 2,666 (4) |
Recommended dosages for approved treatments are 60 mg per day for duloxetine,20 300 mg per day for pregabalin (up to 450 mg per day),19 and 100 mg per day for milnacipran (up to 200 mg per day).21 Although most patients used milnacipran at 100 mg per day, 34% of patients used < 60 mg per day of duloxetine, and 77% used < 300 mg per day of pregabalin. Cyclobenzaprine, tramadol, and gabapentin are not approved for the management of FM, but the usual dosages for other conditions are 15 to 30 mg per day,35 200 to 400 mg per day,36 and 900 to 1,800 mg per day,37 respectively. Most patients in this analysis were receiving lower dosages of these agents; in particular, gabapentin dosing was below 900 mg per day for 53% of patients.
Because the prescribing information recommends that pregabalin dosing begin at 150 mg per day with titration up to 300 mg per day,19 we also evaluated dosing changes for patients starting at 150 mg per day. Among patients with at least 3 prescription fills, when the first dosage was 150 mg per day (n = 1,215), the last dose remained at or below 150 mg per day for 56% (n = 678) of patients. Only 36% (n = 441) of patients followed the recommendation and titrated up to 300 mg per day. The other patients (8%; n = 96) were titrated to > 300 mg per day.
Among 74,738 patients treated with 1 of the 8 prescription medications of interest after diagnosis of FM, 95% (n = 70,919) initially received monotherapy. Of those patients who started with ≥ 2 of such medications (n = 3,819), cyclobenzaprine plus tramadol was the most frequent combination (n = 895; 23%). One quarter of patients (n = 59,133 [25%]) used prescription NSAIDs and 3.0% used celecoxib (n = 7,107) within 1 year after the first diagnosis of FM.
Adherence and Discontinuation
Adherence was suboptimal for all treatments (Table 2). Most patients had low adherence during the first year of treatment for all therapies evaluated. Duloxetine had the highest mean PDC (59%); for all other agents, mean PDC was < 50%. Adherence to duloxetine was generally better than adherence to the other FDA-approved agents and better than adherence to the non-approved agents. Adherence to non-approved medications was extremely low.
TABLE 2.
Adherence to Treatment During First Year of Therapy Based on Proportion of Days Covered
| Treatment | Number | Mean PDCa | High Adherence %b | Medium Adherence %c | Low Adherence %d |
|---|---|---|---|---|---|
| Cyclobenzaprine | 15,388 | 0.20 | 5 | 7 | 88 |
| Gabapentin | 9,605 | 0.44 | 22 | 20 | 58 |
| Tramadol | 9,043 | 0.27 | 9 | 12 | 79 |
| Duloxetine | 8,607 | 0.59 | 38 | 22 | 40 |
| Pregabalin | 5,637 | 0.47 | 24 | 21 | 55 |
| Milnacipran | 2,370 | 0.43 | 20 | 18 | 62 |
aPDC calculated as number of days in the period when the patient had drug supply (i.e., “covered” by medication)/number of days in the period.
bHigh adherence, PDC ≥ 0.8.
cMedium adherence, PDC 0.5 to < 0.8.
dLow adherence, PDC < 0.5.
PDC = proportion of days covered.
Rates of discontinuation were high for most agents (Figure 3A). With the exception of duloxetine, discontinuation rates at 6 months were < 50% for all agents. Because cyclobenzaprine is not recommended for long-term use, it is unsurprising that 63% of patients discontinued treatment (5% switched and 58% discontinued without switching) within 30 days after initiation. Many patients who initiated treatment with tramadol also discontinued within a short period.
FIGURE 3.
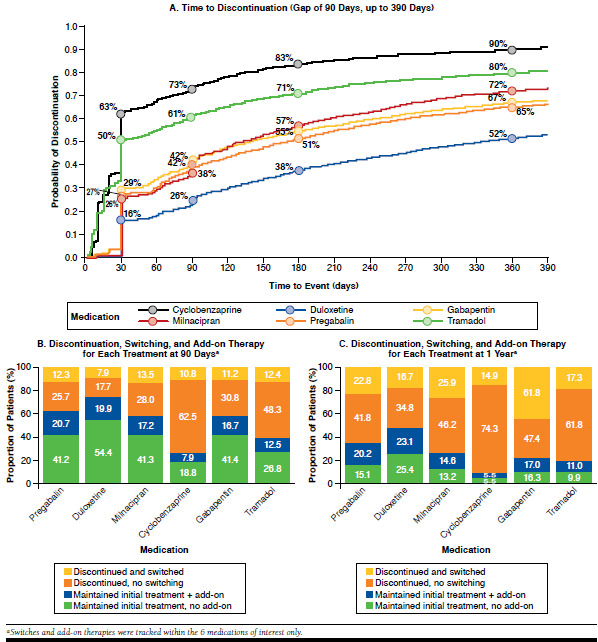
Alterations in Use of the Medications of Interest over the First Year of Therapy
Treatment Alterations
Rates of treatment alterations were high for some agents. Among patients who discontinued initial treatment with duloxetine, pregabalin, or milnacipran, approximately one third had already switched to other ACR-recommended prescription medications of interest within 90 days after their first prescription (Figure 3B). Many patients who discontinued cyclobenzaprine did not switch to another medication. For patients who continued with the initial treatment agent, approximately 50% added a second medication of interest within 1 year of treatment initiation (Figure 3C).
Discussion
Our analysis describes real-world treatment patterns associated with 8 medications of interest for FM using a health insurance claims database. We found that more than two thirds of patients diagnosed with FM were not treated with a prescription medication of interest for a year after diagnosis. Of those who did receive treatment with a medication of interest, 27% were prescribed FDA-approved therapies, and even fewer received the approved dose. Adherence was suboptimal for all treatments of interest, and discontinuation rates were high.
To date, only 3 agents are approved by the FDA for management of FM.8,22 In clinical studies, pregabalin, a nonselective ligand for α2δ subunits of voltage-gated calcium channels, provided significant improvement in FM pain and sleep symptoms compared with placebo.38-40 Typical AEs reported by patients with FM treated with pregabalin include somnolence, dizziness, peripheral edema, and weight gain.41 The SNRI duloxetine has also been shown to improve pain scores, mood, and daily functioning in patients with FM.42-44 The most common AEs with duloxetine were nausea, headache, dry mouth, insomnia, fatigue, constipation, diarrhea, and dizziness.45 The most recently approved treatment for FM, the SNRI milnacipran, has also been shown to be superior to placebo in reducing FM pain and fatigue and in improving mental and physical functioning.46-49 The most frequently reported AEs with milnacipran were nausea, headache, constipation, hot flashes, and dizziness.50 Comparative studies of the 3 treatments indicated that there were no differences in pain relief; however, there were key differences in specific symptom reduction (e.g., sleep disturbances, depressed mood, and fatigue) and drug-specific AEs (e.g., headache, nausea, and diarrhea).18,51
In addition to FDA-approved treatments, various other pain medications are used to treat patients with FM.51-53 In a retrospective analysis of 2,613 patients, the most commonly prescribed medications before and after diagnosis of FM were short-acting opioids (42%-48%) followed by NSAIDs (34%-41%).22 In a survey of > 2,500 people with FM, more than two thirds of respondents indicated that opioids such as hydrocodone and oxycodone were helpful in alleviating symptoms; however, by far most people with FM used over-the-counter (OTC) treatments.54 The exact reasons driving prescription medication use are unknown, and they may include lower costs and ease of obtaining OTC medications, lack of awareness about FM and the available prescription medications, and treatment satisfaction/dissatisfaction.
In this analysis, less than one third of patients (31%) diagnosed with FM were treated with 1 of 8 prescription medications recommended by ACR guidelines within a year of diagnosis. For those who did receive such treatment, the most commonly used agent was cyclobenzaprine. Furthermore, many patients did not receive the treatment dose recommended by the prescribing information. A significant number of patients using pregabalin were using much lower doses than recommended, possibly because of physician concerns about AEs,55 despite evidence that these doses may not be effective in reducing pain severity.43 Although the pregabalin prescribing information recommends initiating treatment at 150 mg per day with uptitration to 300 mg per day,19 our data show that only about 36% of patients received this regimen.
Because FM is a chronic condition, medication should be administered regularly rather than as needed. Adherence and persistence were poor for most of the 6 medications we describe, possibly because of suboptimal dosing patterns. Adherence levels for all medications included in this analysis, with the exception of duloxetine, were < 50%. This might be a result of the medications being prescribed on an as needed basis by physicians; being used as needed by patients, possibly because of affordability issues; or a desire to avoid AEs associated with taking medication. Persistence levels were also low, with patients discontinuing treatment as soon as 4 weeks after initiation. These results are in line with those from previous evaluations of pregabalin and duloxetine, which have reported mean medication possession ratios of 0.7 (duloxetine) and 0.5 (pregabalin).33,56 Similar real-world evaluations of milnacipran are not yet available.
We cannot determine the reason patients discontinued or changed treatments, although poor effectiveness and AEs have been reported previously. Results of long-term safety studies of FDA-approved treatments report discontinuation rates with approved doses of pregabalin of 12% because of AEs and 9% because of other reasons, including lack of effectiveness.57 In placebo-controlled trials of patients with FM, up to one quarter of patients discontinued approved therapies because of AEs (pregabalin, 19%19; duloxetine, 18%-20%20,45; milnacipran, 23%-26%21,58). Similarly, in a real-world, 12-month, observational study of 1,700 patients with FM in the United States and Puerto Rico, 47.7% of pregabalin-treated, 42.4% of duloxetine-treated, and 35.1% of milnacipran-treated patients discontinued their medications at 12 months, with intolerable AEs given as the most common reason in all cohorts (63.4%), followed by lack of effectiveness (30.3%).59 For pregabalin, discontinuation because of an AE is dose-dependent, meaning that patients who require higher doses to control their symptoms might not be able to tolerate the AEs that occur as the dose increases.19 For duloxetine, the discontinuation rates in clinical studies were 8%-17% owing to AEs and 8%-10% owing to lack of effectiveness.42,60 In 1 milnacipran study, discontinuations because of AEs were 20% for 100 mg per day and 27% for 200 mg per day, although discontinuations because of treatment failure remained constant at 11%-12%.47 In our analysis, many patients discontinued treatment early (within 30 days), which might indicate poor tolerability because many AEs occur soon after treatment initiation.
Many patients initiated treatment with cyclobenzaprine, which should not be used for long-term therapy, and subsequently discontinued within a short period without switching to 1 of the 8 medications of interest. Therefore, results from this study suggest that many people with FM are suboptimally treated and likely experience poor pain management. Rates of add-on therapy were also high in the present analysis, suggesting that pain reduction with the initial medication was suboptimal.
Overall, these results indicate that new, more effective, and better-tolerated treatments are necessary for management of FM. A search of the ClinicalTrials.gov database (www.clinicaltrials.gov) shows that multiple drugs are being evaluated for management of FM, including antidepressants, cannabinoids, dopamine agonists, hormones, hypnotics, and neurotropines. It remains to be seen which, if any, of these agents might prove safe and effective.61,62 However, each drug must be evaluated individually because it is clear that even drugs within the same class (pregabalin/gabapentin, duloxetine/milnacipran) can exert differing effects and produce different outcomes.
Limitations
Our analysis has several limitations common to retrospective health insurance claims databases. Only patients with FM with insurance were included in the analysis, and only those with 12-month continuous enrollment before and after the index date were selected. In addition, this analysis focused only on pain medications recommended by the ACR, which are all available by prescription only, and that could be evaluated using a medical claims database. However, it is clear from other studies that many patients with FM use OTC and nonpharmacologic treatments,54 which cannot be evaluated by claims data analysis. Therefore, the effect of adding OTC therapies to prescription medication cannot be assessed. Furthermore, PDC is a proxy measure of adherence to medication regimens, which is based on reimbursed prescriptions, and could be less accurate than measures based on the observed number of pills taken by patients each day. PDC thresholds were based on previously published literature32-34 and equally applied to all medications, regardless of potential instructions to use on an as-needed basis. In the future, a patient and physician surveys might provide insight into use of OTC and nonpharmacologic therapy, the rationale for physician prescribing behavior, and the reasons for patient nonadherence and nonpersistence.
Conclusions
Most patients newly diagnosed with FM are not treated with an FDA-approved medication for a year after diagnosis. Lack of diagnosis and effective pain management might be burdens to the health care system. Patients who are treated with approved therapies might receive a lower-than-recommended dose, potentially resulting in poor effectiveness. Conversely, use of higher doses might be prohibited by poor tolerability. Discontinuation, switching, and use of additional pain medications are common, indicating low levels of satisfaction with initial treatment. The reasons for the high rates of discontinuation and lack of adherence to prescribed medications merit further investigation. Based on this analysis, new therapies with improved effectiveness and better tolerability are urgently needed for FM.
Acknowledgments
The authors acknowledge editorial assistance provided by Sally Mitchell, PhD; Lynn Brown, PhD; and Wm. Lesley R. Castro, PhD, all of ApotheCom, Yardley, Pennsylvania, which was funded by Daiichi Sankyo. The authors also acknowledge Feride Frech-Tamas (Health Economics and Outcomes Research at Daiichi Sankyo, Parsippany, New Jersey) for reviewing the manuscript, and the authors thank Qiaoyi Zhang (Health Economics & Outcomes Research Analytics, Daiichi Sankyo, Parsippany, New Jersey) for help with the study design and manuscript review.
References
- 1. Goldenberg DL, Clauw DJ, Fitzcharles MA. New concepts in pain research and pain management of the rheumatic diseases. Semin Arthritis Rheum. 2011;41(3):319-34. [DOI] [PubMed] [Google Scholar]
- 2. Arnold LM, Clauw DJ, McCarberg BH. Improving the recognition and diagnosis of fibromyalgia. Mayo Clin Proc. 2011;86(5):457-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Centers for Disease Control and Prevention . Fibromyalgia. Available at: http://www.cdc.gov/arthritis/basics/fibromyalgia.htm. Accessed December 13, 2015.
- 4. Lawrence RC, Felson DT, Helmick CG, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58(1):26-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Queiroz LP. Worldwide epidemiology of fibromyalgia. Curr Pain Headache Rep. 2013;17(8):356. [DOI] [PubMed] [Google Scholar]
- 6. Marcus DA. Fibromyalgia: diagnosis and treatment options. Gend Med. 2009;6(Suppl 2):139-51. [DOI] [PubMed] [Google Scholar]
- 7. Hauser W, Kuhn-Becker H, von Wilmoswky H, Settan M, Brahler E, Petzke F. Demographic and clinical features of patients with fibromyalgia syndrome of different settings: a gender comparison. Gend Med. 2011;8(2):116-25. [DOI] [PubMed] [Google Scholar]
- 8. Smith HS, Harris R, Clauw D. Fibromyalgia: an afferent processing disorder leading to a complex pain generalized syndrome. Pain Physician. 2011;14(2):E217-45. [PubMed] [Google Scholar]
- 9. Wolfe F, Clauw DJ, Fitzcharles MA, et al. The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care Res. 2010;62(5):600-10. [DOI] [PubMed] [Google Scholar]
- 10. Wolfe F, Smythe HA, Yunus MB, et al. The American College of Rheumatology 1990 criteria for the classification of fibromyalgia. Report of the Multicenter Criteria Committee. Arthritis Rheum. 1990;33(2):160-72. [DOI] [PubMed] [Google Scholar]
- 11. Haviland MG, Banta JE, Przekop P. Fibromyalgia: prevalence, course, and co-morbidities in hospitalized patients in the United States, 1999-2007. Clin Exp Rheumatol. 2011;29:(6 Suppl 69):S79-87. [PubMed] [Google Scholar]
- 12. Schaefer C, Chandran A, Hufstader M, et al. The comparative burden of mild, moderate and severe fibromyalgia: results from a cross-sectional survey in the United States. Health Qual Life Outcomes. 2011;9:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. White LA, Birnbaum HG, Kaltenboeck A, Tang J, Mallett D, Robinson RL. Employees with fibromyalgia: medical comorbidity, healthcare costs, and work loss. J Occup Environ Med. 2008;50(1):13-24. [DOI] [PubMed] [Google Scholar]
- 14. Arnold LM, Hudson JI, Keck PE, Auchenbach MB, Javaras KN, Hess EV. Comorbidity of fibromyalgia and psychiatric disorders. J Clin Psychiatry. 2006;67(8):1219-25. [DOI] [PubMed] [Google Scholar]
- 15. Picavet HSJ, Hoeymans N. Health-related quality of life in multiple musculoskeletal diseases: SF-36 and EQ-5D in the DMC3 study. Ann Rheum Dis. 2004;63(6):723-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Podolecki T, Podolecki A, Hrycek A. Fibromyalgia: pathogenetic, diagnostic and therapeutic concerns. Pol Arch Med Wewn. 2009;119(3):157-61. [PubMed] [Google Scholar]
- 17. Hauser W, Wolfe F, Tolle T, Uceyler N, Sommer C. The role of antidepressants in the management of fibromyalgia syndrome: a systematic review and meta-analysis. CNS Drugs. 2012;26(4):297-307. [DOI] [PubMed] [Google Scholar]
- 18. Hauser W, Petzke F, Sommer C. Comparative efficacy and harms of duloxetine, milnacipran, and pregabalin in fibromyalgia syndrome. J Pain. 2010;11(6):505-21. [DOI] [PubMed] [Google Scholar]
- 19. LYRICA (pregabalin) capsules CV; oral solution CV. Pfizer Pharmaceuticals. Revised December 2013. Available at: http://labeling.pfizer.com/ShowLabeling.aspx?id=561. Accessed December 14, 2015.
- 20. Cymbalta (duloxetine delayed-release capsules) for oral use. Eli Lilly and Co. Revised June 2015. Available at: http://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=2f7d4d67-10c1-4bf4-a7f2-c185fbad64ba. Accessed December 14, 2015.
- 21. Savella (milnacipran HCl) tablets . Forest Pharmaceuticals. Revised January 2015. Available at: http://pi.actavis.com/data_stream.asp?product_group=1909&p=pi&language=E. Accessed December 14, 2015.
- 22. Sanchez RJ, Uribe C, Li H, et al. Longitudinal evaluation of health care utilization and costs during the first three years after a new diagnosis of fibromyalgia. Curr Med Res Opin. 2011;27(3):663-71. [DOI] [PubMed] [Google Scholar]
- 23. Ablin J, Fitzcharles MA, Buskila D, Shir Y, Sommer C, Hauser W. Treatment of fibromyalgia syndrome: recommendations of recent evidence-based interdisciplinary guidelines with special emphasis on complementary and alternative therapies. Evid Based Complement Alternat Med. 2013;2013:485272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. McNett M, Goldenberg D, Schaefer C, et al. Treatment patterns among physician specialties in the management of fibromyalgia: results of a cross-sectional study in the United States. Curr Med Res Opin. 2011;27(3):673-83. [DOI] [PubMed] [Google Scholar]
- 25. Mease PJ, Dundon K, Sarzi-Puttini P. Pharmacotherapy of fibromyalgia. Best Pract Res Clin Rheumatol. 2011;25(2):285-97. [DOI] [PubMed] [Google Scholar]
- 26. Gilron I, Jensen TS, Dickenson AH. Combination pharmacotherapy for management of chronic pain: from bench to bedside. Lancet Neurol. 2013;12(11):1084-95. [DOI] [PubMed] [Google Scholar]
- 27. Calandre EP, Rico-Villademoros F, Rodriguez-Lopez CM. Monotherapy or combination therapy for fibromyalgia treatment? Curr Rheumatol Rep. 2012;14(6):568-75. [DOI] [PubMed] [Google Scholar]
- 28. Nantz E, Liu-Seifert H, Skljarevski V. Predictors of premature discontinuation of treatment in multiple disease states. Patient Prefer Adherence. 2009;3:31-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. American College of Rheumatology . Fibromylagia. Available at: http://www.rheumatology.org/Practice/Clinical/Patients/Diseases_And_Conditions/Fibromyalgia/. Accessed December 13, 2015.
- 30. Quan H, Li B, Couris CM, et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol. 2011;173(6):676-82. [DOI] [PubMed] [Google Scholar]
- 31. Peterson AM, Nau DP, Cramer JA, Benner J, Gwadry-Sridhar F, Nichol M. A checklist for medication compliance and persistance studies using retrospective databases. Value Health. 2007;10(1):3-12. [DOI] [PubMed] [Google Scholar]
- 32. Yeaw J, Benner JS, Walt JG, Sian S, Smith DB. Comparing adherence and persistence across 6 chronic medication classes. J Manag Care Pharm. 2009;15(9):728-40. Available at: http://www.amcp.org/data/jmcp/728-740.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhao Y, Sun P, Watson P, Mitchell B, Swindle R. Comparison of medication adherence and healthcare costs between duloxetine and pregabalin initiators among patients with fibromyalgia. Pain Pract. 2011;11(3):204-16. [DOI] [PubMed] [Google Scholar]
- 34. Bramley TJ, Gerbino PP, Nightengale BS, Frech-Tamas F. Relationship of blood pressure control to adherence with antihypertensive monotherapy in 13 managed care organizations. J Manag Care Pharm. 2006;12(3):239-45. Available at: http://amcp.org/data/jmcp/Research_239-245.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Flexeril (cyclobenzaprine HCl) tablets . Merck & Co. 2001. Available at: http://www.accessdata.fda.gov/drugsatfda_docs/label/2003/017821s045lbl.pdf. Accessed December 21, 2015.
- 36. Ultram (tramadol hydrochloride) tablets . Ortho Janssen. March 2008. Available at: http://www.accessdata.fda.gov/drugsatfda_docs/label/2009/020281s032s033lbl.pdf. Accessed December 21, 2015.
- 37. Neurontin (gabapentin) capsules, tablets, and oral solution . Parke-Davis, division of Pfizer. July 2012. Available at: http://www.pdr.net/pdrcontent/PDRe_201409110948/mg/pdf/10324.PDF. Accessed December 21, 2015.
- 38. Mease PJ, Russell IJ, Arnold LM, et al. A randomized, double-blind, placebo-controlled, phase III trial of pregabalin in the treatment of patients with fibromyalgia. J Rheumatol. 2008;35(3):502-14. [PubMed] [Google Scholar]
- 39. Pauer L, Winkelmann A, Arsenault P, et al. An international, randomized, double-blind, placebo-controlled, phase III trial of pregabalin monotherapy in treatment of patients with fibromyalgia. J Rheumatol. 2011;38(12):2643-52. [DOI] [PubMed] [Google Scholar]
- 40. Crofford LJ, Rowbotham MC, Mease PJ, et al. Pregabalin for the treatment of fibromyalgia syndrome: results of a randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2005;52(4):1264-73. [DOI] [PubMed] [Google Scholar]
- 41. Smith MT, Moore BJ. Pregabalin for the treatment of fibromyalgia. Exp Opin Pharmacother. 2012;13(10):1527-33. [DOI] [PubMed] [Google Scholar]
- 42. Chappell AS, Littlejohn G, Kajdasz DK, Scheinberg M, D’Souza DN, Moldofsky H. A 1-year safety and efficacy study of duloxetine in patients with fibromyalgia. Clin J Pain. 2009;25(5):365-75. [DOI] [PubMed] [Google Scholar]
- 43. Arnold LM, Zhang S, Pangallo BA. Efficacy and safety of duloxetine 30 mg/d in patients with fibromyalgia: a randomized, double-blind, placebo-controlled study. Clin J Pain. 2012;28(9):775-81. [DOI] [PubMed] [Google Scholar]
- 44. Arnold LM, Clauw D, Wang F, Ahl J, Gaynor PJ, Wohlreich MM. Flexible dosed duloxetine in the treatment of fibromyalgia: a randomized, double-blind, placebo-controlled trial. J Rheumatol. 2010;37(12):2578-86. [DOI] [PubMed] [Google Scholar]
- 45. Choy EH, Mease PJ, Kajdasz DK, et al. Safety and tolerability of duloxetine in the treatment of patients with fibromyalgia: pooled analysis of data from five clinical trials. Clin Rheumatol. 2009;28(9):1035-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Branco JC, Zachrisson O, Perrot S, Mainguy Y. A European multicenter randomized double-blind placebo-controlled monotherapy clinical trial of milnacipran in treatment of fibromyalgia. J Rheumatol. 2010;37(4):851-9. [DOI] [PubMed] [Google Scholar]
- 47. Mease PJ, Clauw DJ, Gendreau RM, et al. The efficacy and safety of milnacipran for treatment of fibromyalgia. a randomized, double-blind, placebo-controlled trial. J Rheumatol. 2009;36(2):398-409. [DOI] [PubMed] [Google Scholar]
- 48. Clauw DJ, Mease P, Palmer RH, Gendreau RM, Wang Y. Milnacipran for the treatment of fibromyalgia in adults: a 15-week, multicenter, randomized, double-blind, placebo-controlled, multiple-dose clinical trial. Clin Ther. 2008;30(11):1988-2004. [DOI] [PubMed] [Google Scholar]
- 49. Geisser ME, Palmer RH, Gendreau RM, Wang Y, Clauw DJ. A pooled analysis of two randomized, double-blind, placebo-controlled trials of milnacipran monotherapy in the treatment of fibromyalgia. Pain Pract. 2011;11(2):120-31. [DOI] [PubMed] [Google Scholar]
- 50. Derry S, Gill D, Phillips T, Moore RA. Milnacipran for neuropathic pain and fibromyalgia in adults. Cochrane Database Syst Rev. 2012;3:CD008244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Choy E, Marshall D, Gabriel ZL, Mitchell SA, Gylee E, Dakin HA. A systematic review and mixed treatment comparison of the efficacy of pharmacological treatments for fibromyalgia. Semin Arthritis Rheum. 2011;41(3):335-45. [DOI] [PubMed] [Google Scholar]
- 52. Smith HS, Bracken D, Smith JM. Pharmacotherapy for fibromyalgia. Front Pharmacol. 2011;2:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Traynor LM, Thiessen CN, Traynor AP. Pharmacotherapy of fibromyalgia. Am J Health Syst Pharm. 2011;68(14):1307-19. [DOI] [PubMed] [Google Scholar]
- 54. Bennett RM, Jones J, Turk DC, Russell IJ, Matallana L. An internet survey of 2,596 people with fibromyalgia. BMC Musculoskelet Disord. 2007;8:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Boomershine CS. Pregabalin for the management of fibromyalgia syndrome. J Pain Res. 2010;3:81-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Sun P, Peng X, Sun S, et al. Direct medical costs and medication compliance among fibromyalgia patients: duloxetine initiators vs. pregabalin initiators. Pain Pract. 2014;14(1):22-31. [DOI] [PubMed] [Google Scholar]
- 57. Arnold LM, Emir B, Murphy TK, et al. Safety profile and tolerability of up to 1 year of pregabalin treatment in 3 open-label extension studies in patients with fibromyalgia. Clin Ther. 2012;34(5):1092-102. [DOI] [PubMed] [Google Scholar]
- 58. Bernstein CD, Albrecht KL, Marcus DA. Milnacipran for fibromyalgia: a useful addition to the treatment armamentarium. Exp Opin Pharmacother. 2013;14(7):905-16. [DOI] [PubMed] [Google Scholar]
- 59. Robinson RL, Kroenke K, Williams DA, et al. Longitudinal observation of treatment patterns and outcomes for patients with fibromyalgia: 12-month findings from the reflections study. Pain Med. 2013;14(9):1400-15. [DOI] [PubMed] [Google Scholar]
- 60. Pangallo BA, Zhang Q, Desaiah D, Perahia DG, Detke MJ, Kennedy SH. Long-term safety of duloxetine during open-label compassionate use treatment of patients who completed previous duloxetine clinical trials. Curr Med Res Opin. 2010;26(11):2643-51. [DOI] [PubMed] [Google Scholar]
- 61. Hauser W, Walitt B, Fitzcharles MA, Sommer C. Review of pharmacological therapies in fibromyalgia syndrome. Arthritis Res Ther. 2014;16(1):201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. xChopra K, Kuhad A, Arora V. Neoteric pharmacotherapeutic targets in fibromyalgia. Expert Opin Ther Targets. 2011;15(11):1267-81. [DOI] [PubMed] [Google Scholar]


