Abstract
BACKGROUND:
Treatment for overactive bladder (OAB) remains suboptimal, in part because of patient nonadherence to medications. Primary nonadherence is when patients fail to pick up their initial prescriptions.
OBJECTIVE:
To measure primary nonadherence to OAB medications within 30 days of a first OAB prescription order using electronic medical records from a U.S. managed care health care system
METHODS:
A retrospective cohort study was conducted using electronic medical records from the Kaiser Permanente Southern California (KPSC) database to identify patients with new OAB prescriptions between January 1, 2007, and December 31, 2013. The index date was defined as the first order of an OAB prescription. Patients had to be aged ≥ 18 years on the index date and were required to have 12 months of continuous membership with drug benefit eligibility before, during, and after the index date. Patients were defined as primary nonadherent if they did not pick up their new OAB prescriptions within 30 days of the order date. Descriptive statistics and a multivariable logistic regression analysis with backward selection were conducted to identify factors associated with patients who were primary nonadherent versus adherent.
RESULTS:
There were 9,050 patients with a new OAB prescription order; 1,662 (18%) of these were primary nonadherent. Patients with primary nonadherence were younger in age (56.9 [SD ± 16.0] years vs. 63.9 [SD ± 14.8] years; P < 0.001) and more likely to have commercial insurance (65.9% vs. 46.2%; P < 0.001). They also had lower mean Charlson Comorbidity Index (CCI) scores (1.99 vs. 2.70; P < 0.001), fewer OAB-related comorbidities, fewer concomitant medications (P < 0.005), and fewer overall prescriptions dispensed in the previous 12 months (P < 0.001) compared with adherent patients. Significant factors such as commercial insurance (P = 0.013), race other than white (P = 0.020), CCI = 0 versus CCI ≥ 2 (P = 0.001), urinary tract infections (P < 0.001), and falls (P = 0.047) were associated with a higher likelihood of primary nonadherence versus adherence.
CONCLUSIONS:
Nearly 1 in 5 patients did not pick up their new OAB medications within 30 days of the order date. Knowledge of factors associated with primary nonadherence may inform strategies for improving management of OAB.
What is already known about this subject
Population-based studies indicate that the prevalence of overactive bladder (OAB) in the United States is 27.2% in men and 43.1% in women.
Effective medications for OAB are available in the United States, but treatment remains suboptimal due in part to patient nonadherence to medications.
The majority of research on medication adherence in the OAB population has focused on secondary nonadherence.
What this study adds
Individuals who were primary nonadherent were compared with those who were primary adherent, and factors associated with primary nonadherence to OAB medications were identified.
Patients who were primary nonadherent were female, younger, had fewer comorbid conditions, were healthier, and had less concomitant medications and prescriptions dispensed in the past year compared with adherent patients.
Factors such as female gender, commercial insurance, race other than white, Charlson Comorbidity Index scores, depression, urinary tract infection, skin conditions, falls, vulvovaginitis, and substance abuse were associated with higher likelihood of primary nonadherence versus primary adherence.
Overactive bladder (OAB) is a symptom-driven condition characterized by urinary urgency, usually with frequency and nocturia, with or without urgency incontinence, in the absence of urinary tract infection (UTI) or other obvious pathology.1 OAB represents a substantial burden of illness, since it effects physical, psychological, occupational, and social aspects of daily living.2 Individuals with OAB are at greater risk for falls with or without bone fracture, UTIs, and nursing home admissions.3,4 OAB has a clinically significant negative effect on an individual’s health-related quality of life, quality of sleep, productivity, and mental health.2,5
Population-based studies indicate that the prevalence of OAB in the United States is 27.2% in men and 43.1% in women.6 The incidence of OAB is highest in the elderly.6 In the coming decades, the economic and humanistic burden of OAB is expected to increase in the United States, since the proportion of individuals aged > 65 years is predicted to rise from 13% in 2010 to > 20% in 2050.7 In 2007, total costs of OAB (direct and nonmedical) in the United States were estimated at $65.9 billion.8 These costs are projected to reach $82.6 billion in 2020. As the population ages, the increasing costs of OAB are likely to be driven by direct medical costs.8 In the United States, this may create a substantial financial challenge to the Medicare program, which is responsible for the health care of most Americans aged ≥ 65 years.8
Treatment of OAB is aimed at managing symptoms and OAB-related comorbidities. Effective medications for OAB are available in the United States, but treatment remains suboptimal, in part because of patient nonadherence to medications.9 The majority of research on medication adherence has focused on secondary nonadherence, which occurs when a patient discontinues a medication after filling the initial prescription. Primary nonadherence occurs when a prescription for a new medication is written for a patient, but the prescription is never filled or dispensed. The patient may not take the prescription to the pharmacy, or the patient may take the prescription to the pharmacy but not pick up the medication.10 The advent of electronic medical records (EMRs) has improved tracking of medication records, which facilitates quantification of primary nonadherence.10,11
Recent reports suggest that primary nonadherence to medication for chronic diseases is prevalent,12-16 ranging from 15.4% of patients who do not pick up their statin prescriptions within 90 days of the order date to 29.5% of women aged ≥ 55 years who do not pick up their bisphosphonate prescriptions within 60 days of the order date.12,13 Failure to initiate a medication for a chronic condition may lead to poor patient outcomes and substantial long-term health care costs.17 Previous studies on nonadherence in OAB have investigated secondary nonadherence and other treatment patterns, such as switching, discontinuation, and persistence.9 Identifying and understanding the factors associated with primary nonadherence to OAB medication will inform the development of targeted interventions to improve the use of pharmacotherapy and provide an important opportunity for clinicians and health plans to improve quality of care.
The objective of this study was to measure primary nonadherence to OAB medications within 30 days of a first OAB prescription order using EMRs from a U.S. managed care health care system. Individuals who were primary nonadherent were compared with those who were adherent, and factors associated with primary nonadherence to OAB medications were identified.
Methods
Study Setting and Data
This was a retrospective cohort study of patients with new OAB prescriptions in the Kaiser Permanente Southern California (KPSC) database. KPSC is a nonprofit, integrated health services delivery system with approximately 4 million health plan members; it is the largest of 8 regions in Kaiser Permanente. In 2015, an estimated 1.9% of patients in the KPSC database had an OAB diagnosis. The population served by KPSC is socioeconomically diverse and broadly representative of the racial/ethnic groups living in Southern California.18,19 The KPSC database is drawn from the KPSC system of approximately 14 hospitals and 197 outpatient clinics, which are facilities that provide integrated, comprehensive medical services. Each KPSC member has a unique medical record number that can be used to link various clinical and administrative databases, including membership, drug benefits, medical and hospital encounters, laboratory test results, and pharmacy-dispensing records. All aspects of patient care and interactions with the health care delivery system are captured in a continuously updated, comprehensive EMR system, and care delivered in non-KPSC settings is also captured by the claims system. This information is available for research purposes. All data used in the current study were de-identified. The study was approved by the institutional review board for KPSC.
Design and Study Population
Patients with new OAB prescription orders between January 1, 2007, and December 31, 2013, were identified. The index date was defined as the first order of an OAB prescription within that time period. Inclusion criteria were age ≥ 18 years on the index date and continuous membership and drug benefit eligibility for 12 months before and after the index date. Exclusion criteria were history of a dispensed or ordered OAB prescription within the 12 months before the index date or 2 or more outpatient or 1 or more inpatient International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) diagnosis codes of cancer, chemotherapy use, hospice, radiation, dialysis, chronic kidney disease stage 5 or glomerular filtration rate of < 15 mL/min, or any organ transplant surgery within the 12 months before the index date (Figure 1). These exclusion criteria ensured the identification of new OAB prescription orders and the preclusion of patients with severe comorbidities, which may effect initial adherence. Only electronic orders intended to be dispensed at a KPSC pharmacy were included, since we were not able to determine whether paper prescriptions were picked up at a non-KPSC pharmacy.13
FIGURE 1.
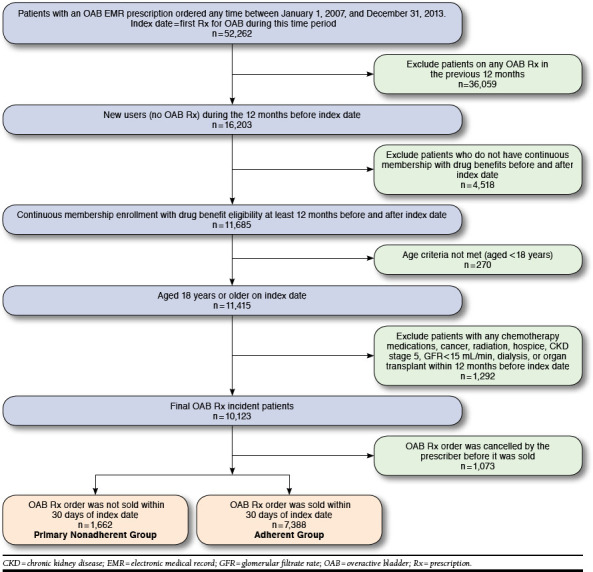
OAB Study Population Attrition Diagram
Medication Orders
Prescription orders are placed and routed electronically to the KPSC Pharmacy Information Management System. Prescriptions sold through KPSC pharmacies are linked with EMR orders through an established electronic interface, allowing for the identification of those patients who had a prescription ordered but who never picked it up. The system also captures cancelled orders, which were identified and excluded.
Primary and Secondary Outcomes of Interest
The primary outcome was to assess the percentage of patients who were primary nonadherent to their new OAB prescription orders. Primary nonadherence was defined as the failure to pick up a new prescription within 30 days of the index date, and adherence was defined as picking up a new prescription within 30 days of the index date. In the absence of a standardized definition of primary nonadherence, these definitions of primary nonadherence and adherence were considered appropriate for this study, since they were based on clinical input from pharmacists and urologists. The definition was conservative, considering that evidence shows that most patients pick up their medications within 2 weeks of a prescription order.20,21
The secondary outcome was to identify patient factors, including gender, age, race, Charlson Comorbidity Index (CCI) score, OAB-related comorbidities, risk factors for OAB, concomitant medications, body mass index (BMI), and average electronic number of any previous prescriptions filled from the KPSC pharmacy database, associated with primary nonadherence versus adherence. Risk factors for OAB were identified through a literature search and were confirmed by KPSC physicians.4,5,22-26
Statistical Analyses
A patient-level analysis was conducted, where the primary outcome of interest was primary nonadherence. Primary nonadherent patients were compared with adherent patients. Descriptive statistics were used to assess baseline differences between the primary nonadherent and adherent patient groups using a two-sided t-test for continuous variables, and tests of proportions were used to compare categorical variables. If the frequency of the variable was < 5, then Fisher’s exact chi-square test was used. Age was calculated using date of birth closest to the index date. Baseline factors such as comorbidities for CCI, OAB-related comorbidities, and risk factors were identified using ICD-9-CM codes during the 12 months before the index date where the discharge diagnosis code was in the primary or secondary position. BMI was identified from medical claims where it is input electronically, and the closest BMI before the index date was used. Concomitant therapies were identified 12 months before the index date as dispensed and sold prescriptions to the patient using the pharmacy claims database (Table 1). A stepwise logistic regression model using backward selection was used to identify factors associated with primary nonadherent versus adherent patients. We used a stepwise method (Pentry ≤ 0.30, Pstay ≤ 0.35) to select variables.
TABLE 1.
Baseline Characteristics of Adherent and Primary Nonadherent Patients with OAB
| Baseline Patient and Clinical Characteristics | All Patients | Adherent Patients | Primary Nonadherent Patients | P Value |
|---|---|---|---|---|
| Number | N = 9,050 | n = 7,388 | n = 1,662 | |
| Female, n (%) | 6,599 (72.9) | 5,321 (72.0) | 1,278 (76.9) | < 0.0001 |
| Age (years) mean (SD) | 62.6 (15.3) | 63.9 (14.8) | 56.9 (16.0) | < 0.0001 |
| Age categories, years, n (%) | < 0.0001 | |||
| 18 to < 40 | 706 (7.8) | 482 (6.5) | 224 (13.5) | |
| 40 to < 65 | 3,845 (42.5) | 2,968 (40.2) | 877 (52.8) | |
| 65 to < 75 | 2,308 (25.5) | 1,992 (27.0) | 316 (19.0) | |
| ≥ 75 | 2,191 (24.2) | 1,946 (26.3) | 245 (14.7) | |
| Insurance type, n (%) | < 0.0001 | |||
| Commercial | 4,511 (49.8) | 3,416 (46.2) | 1,095 (65.9) | |
| Dual eligibility | 3 (0.0) | 3 (0.0) | 0 (0.0) | |
| Medicaid | 118 (1.3) | 99 (1.3) | 19 (1.1) | |
| Medicare | 4,418 (48.8) | 3,870 (52.4) | 548 (33.0) | |
| Race, n (%) | < 0.0001 | |||
| Asian/Pacific Islander | 647 (7.2) | 503 (6.8) | 144 (8.7) | |
| Black | 977 (10.8) | 762 (10.3) | 215 (12.9) | |
| Hispanic | 2,474 (27.3) | 1,913 (25.9) | 561 (33.8) | |
| White | 4,420 (48.8) | 3,823 (51.7) | 597 (35.9) | |
| Othera | 532 (5.9) | 387 (5.2) | 145 (8.7) | |
| Comorbidities (CCI) | < 0.0001 | |||
| CCI mean (SD) | 2.57 (2.4) | 2.70 (2.5) | 1.99 (2.2) | < 0.0001 |
| Score = 0, n (%) | 1,375 (15.2) | 1,008 (13.6) | 367 (22.1) | < 0.0001 |
| Score = 1, n (%) | 2,540 (28.1) | 1,996 (27.0) | 544 (32.7) | |
| Score ≥ 2, n (%) | 5,135 (56.7) | 4,384 (59.3) | 751 (45.2) | |
| Median | 2 | 2 | 1 | |
| OAB-related comorbidities,b n (%) | ||||
| Benign prostatic hyperplasia | 1,206 (13.3) | 1,054 (14.3) | 152 (9.1) | < 0.0001 |
| Depression | 2,210 (24.4) | 1,892 (25.6) | 318 (19.1) | < 0.0001 |
| Urinary tract infection | 1,890 (20.9) | 1,567 (21.2) | 323 (19.4) | 0.1076 |
| Skin infections | 461 (5.1) | 397 (5.4) | 64 (3.8) | 0.0107 |
| Falls, fractures, or related injuries | 2,318 (25.6) | 1,906 (25.8) | 412 (24.8) | 0.3944 |
| Vulvovaginitis | 486 (5.4) | 371 (5.0) | 115 (6.9) | 0.0019 |
| Risk factors of OAB,b n (%) | ||||
| Alzheimer’s disease/dementia | 318 (3.5) | 276 (3.7) | 42 (2.5) | 0.0156 |
| Congestive heart failure | 296 (3.3) | 272 (3.7) | 24 (1.4) | < 0.0001 |
| Diabetes mellitus | 1,747 (19.3) | 1,517 (20.5) | 230 (13.8) | < 0.0001 |
| Diuretic use | 2,051 (22.7) | 1,806 (24.4) | 245 (14.7) | < 0.0001 |
| Epilepsy | 80 (0.9) | 70 (0.9) | 10 (0.6) | 0.1736 |
| Hypertension | 4,742 (52.4) | 4,117 (55.7) | 625 (37.6) | < 0.0001 |
| Ischemic heart disease | 808 (8.9) | 708 (9.6) | 100 (6.0) | < 0.0001 |
| Multiple sclerosis | 183 (2.0) | 163 (2.2) | 20 (1.2) | 0.0087 |
| Obesity | 3,708 (41.0) | 3,026 (41.0) | 682 (41.0) | 0.9543 |
| Parkinson’s disease | 139 (1.5) | 128 (1.7) | 11 (0.7) | 0.0013 |
| Spinal cord injury | 11 (0.1) | 9 (0.1) | 2 (0.1) | 0.9871 |
| Substance abuse | 892 (9.7) | 724 (9.8) | 168 (10.1) | 0.7029 |
| Stroke | 327 (3.6) | 287 (3.9) | 40 (2.4) | 0.0035 |
| Urethral stricture or urinary obstruction | 128 (1.4) | 112 (1.5) | 16 (1.0) | 0.0844 |
| BMI information | ||||
| BMI kg/m2, mean (SD) | 29.3 (6.53) | 29.4 (6.57) | 29.1 (6.35) | 0.0853 |
| Underweight < 18.5 kg/m2, n (%) | 119 (1.3) | 94 (1.3) | 25 (1.5) | < 0.0001 |
| Normal BMI 18.5 to < 25 kg/m2, n (%) | 2,176 (24.0) | 1,749 (23.7) | 427 (25.7) | |
| Overweight BMI ≥ 25 to < 30 kg/m2, n (%) | 2,961 (32.7) | 2,404 (32.5) | 557 (33.5) | |
| Obese BMI ≥ 30 kg/m2, n (%) | 3,414 (37.7) | 2,775 (37.6) | 639 (38.4) | |
| Unknown, n (%) | 380 (4.2) | 366 (4.9) | 14 (0.8) | |
| Previous other OAB therapies, n (%) | ||||
| Botox injection | 5 (0.1) | 3 (0.0) | 2 (0.1) | 0.2296 |
| Surgical therapy | 1,552 (17.1) | 1,241 (16.8) | 311 (18.7) | 0.0613 |
| Concomitant medications, n (%) | ||||
| Anticholinesterase/anticholinergic medications | 203 (2.2) | 177 (2.4) | 26 (1.6) | 0.0386 |
| PDE5 inhibitors | 417 (4.6) | 373 (5.1) | 44 (2.7) | < 0.0001 |
| 5-alpha reductase inhibitors | 380 (4.2) | 342 (4.6) | 38 (2.3) | < 0.0001 |
| Antihypertensives | 3,813 (42.1) | 3,320 (44.9) | 493 (29.7) | < 0.0001 |
| Antidiabetics | 1,509 (16.7) | 1,313 (17.8) | 196 (11.8) | < 0.0001 |
| Antihyperlipidemics | 4,037 (44.6) | 3,501 (47.4) | 536 (32.3) | < 0.0001 |
| Previous number of unique medications | ||||
| Average number of Rxs per patient, mean (SD) | 10.6 (6.43) | 11.2 (6.48) | 7.6 (5.22) | < 0.001 |
| Number of medications (median) | 10 | 10 | 6 | < 0.0001 |
| 0, n (%) | 99 (1.1) | 0 (0.0) | 99 (5.9) | < 0.0001 |
| 1-4, n (%) | 1,415 (15.6) | 902 (12.2) | 513 (30.9) | < 0.0001 |
| 5-9, n (%) | 3,032 (33.5) | 2,441 (33.0) | 591 (35.6) | 0.0493 |
| 10-15, n (%) | 2,771 (30.6) | 2,443 (33.1) | 328 (19.7) | < 0.0001 |
| 16+, n (%) | 1,733 (19.1) | 1,602 (21.7) | 131 (7.9) | < 0.0001 |
aOther is multi, other, unknown.
bRisk factors and comorbidities were identified based on ICD-9-CM codes.
BMI = body mass index; CCI = Charlson Comorbidity Index; ICD-9-CM = International Classification of Diseases, Ninth Revision, Clinical Modification; OAB = overactive bladder; PDE5 = phosphodiesterase type 5; Rx = prescription; SD = standard deviation.
All data were analyzed using SAS version 9.3 (SAS Institute, Cary, NC). P values < 0.05 were considered to be statistically significant.
Results
During the study period, 52,262 patients received an OAB prescription order. Of these, 9,050 patients met the inclusion criteria. Most of the exclusions resulted from patients who had an OAB prescription within the 12 months before the index date (Figure 1). Of the 9,050 included patients, 1,662 (18%) were identified as primary nonadherent.
The unadjusted baseline characteristics of the study population, stratified by adherence status, are shown in Table 1. Univariate analysis revealed statistically significant differences between primary nonadherent patients and adherent patients. Primary nonadherent patients were younger (mean age 56.9 [standard deviation ± 16.0] years vs. 63.9 [SD ± 14.8] years, P < 0.0001); less likely to be white (35.9% vs. 51.7%, P < 0.001); and more likely to have commercial insurance (65.9% vs. 46.2%, P < 0.001). Primary nonadherent patients had fewer comorbid conditions overall; were healthier (mean CCI score 1.99 vs. 2.70, P < 0.001); were less likely to have OAB-related comorbidities, including benign prostatic hyperplasia (9.1% vs. 14.3%, P < 0.001), depression (19.1% vs. 25.6%, P < 0.001), and skin infections (3.8% vs. 5.4%, P = 0.010); and had fewer risk factors associated with OAB, including Alzheimer’s disease/dementia (2.5% vs. 3.7%, P = 0.015), congestive heart failure (1.4% vs. 3.7%, P < 0.001), diabetes mellitus (13.8% vs. 20.5%, P < 0.001), diuretic use (14.7% vs. 24.4%, P < 0.001), hypertension (37.6% vs. 55.7%, P < 0.001), ischemic heart disease (6.0% vs. 9.58%, P < 0.001), multiple sclerosis (1.2% vs. 2.2%, P = 0.008), Parkinson’s disease (0.7% vs. 1.7%, P = 0.001), and stroke (2.4% vs. 3.9%, P = 0.003). Primary nonadherent patients also had fewer concomitant medications and prescriptions dispensed within the 12 months before the index date (P < 0.005).
Index OAB therapies prescribed to patients are shown in Table 2. Oxybutynin, in various forms, accounted for 74.8% of all patients’ prescriptions, while trospium, in various forms and the second most frequently prescribed medication, accounted for 19% of all prescriptions. Significantly less oxybutynin (capsule, tablet, and syrup) and significantly more trospium was prescribed to primary nonadherent patients, compared with adherent patients (oxybutynin, 38.2% vs. 43.6%, P < 0.001; trospium, 21.1% vs. 16.2%, P < 0.001). In terms of prescriber specialties, primary care, obstetrics/gynecology (OB/GYN), and urology accounted for 93.2% of the prescriptions (Table 2). Of these specialties, only OB/GYN had a significantly higher proportion of primary nonadherent patients than adherent patients (proportion of primary nonadherent patients 25.6% vs. proportion of adherent patients 17.1%, P < 0.001).
TABLE 2.
Index OAB Therapies and Prescriber Specialty for Adherent and Primary Nonadherent Patients
| Index OAB Therapies | All Patients | Adherent Patients | Primary Nonadherent Patients | P Value |
|---|---|---|---|---|
| Index therapy, n (%) | N = 9,050 | n = 7,388 | n = 1,662 | |
| Darifenacin | 60 (0.7) | 50 (0.7) | 10 (0.6) | 0.7332 |
| Fesoterodine | 3 (0.0) | 2 (0.0) | 1 (0.1) | 0.4560 |
| Mirabegron | 8 (0.1) | 5 (0.1) | 3 (0.2) | 0.1682 |
| Oxybutynin (capsule/tablet/syrup) | 3,853 (42.6) | 3,218 (43.6) | 635 (38.2) | < 0.0001 |
| Oxybutynina | 2,230 (24.6) | 1,793 (24.3) | 437 (26.3) | 0.0835 |
| Oxybutynin patch (Oxytrol) | 678 (7.5) | 547 (7.4) | 131 (7.8) | 0.5035 |
| Oxybutynin gel (Gelnique) | 5 (0.1) | 5 (0.1) | 0 (0.0) | 0.5921 |
| Solifenacin | 105 (1.2) | 86 (1.2) | 19 (1.1) | 0.9428 |
| Tolterodine | 105 (1.2) | 96 (1.3) | 9 (0.5) | 0.0091 |
| Tolterodinea | 283 (3.1) | 249 (3.4) | 34 (2.1) | 0.0051 |
| Trospium | 1,550 (17.1) | 1,200 (16.2) | 350 (21.1) | < 0.0001 |
| Trospiuma | 170 (1.9) | 137 (1.9) | 33 (1.9) | 0.7219 |
| Prescriber specialty, n (%) | N = 9,050 | n = 7,388 | n = 1,662 | |
| Primary careb | 3,804 (42.0) | 3,136 (42.4) | 668 (40.2) | 0.0925 |
| OB/GYN | 1,693 (18.7) | 1,267 (17.1) | 426 (25.6) | < 0.0001 |
| Urology | 2,943 (32.5) | 2,428 (32.9) | 515 (31.0) | 0.1399 |
| Emergency medicine | 81 (0.9) | 71 (1.0) | 10 (0.6) | 0.1599 |
| Gerontology | 9 (0.1) | 7 (0.1) | 2 (0.1) | 0.7641 |
| Neurology | 142 (1.6) | 124 (1.7) | 18 (1.1) | 0.0776 |
| Surgery | 13 (0.1) | 8 (0.1) | 5 (0.8) | < 0.0001 |
| Other | 365 (4.0) | 347 (4.7) | 18 (1.1) | < 0.0001 |
aExtended release.
bPrimary care (internal and family medicine).
OAB = overactive bladder; OB/GYN = obstetrics and gynecology.
In the multivariate logistic regression model (Table 3), a lower CCI (CCI = 0 vs. CCI ≥ 2) was associated with 70.6% higher odds of primary nonadherence. Commercial insurance (24.2%); any other race than white (34.8%-61.4%); OAB-related comorbidities; UTIs (34.3%); and falls, fractures, or related injuries (28.9%) were associated with higher odds of primary nonadherence. OAB risk factors of age, hypertension, and multiple sclerosis were associated with lower odds of primary nonadherence. The final model had a c-statistic of 0.736.
TABLE 3.
Multivariate Logistic Regression of Factors Associated with Primary Nonadherence
| Odds Ratio Estimates | Point Estimate | 95% Wald | |
|---|---|---|---|
| Effect | Confidence Limits | ||
| Female vs. male | 1.085 | 0.882 | 1.334 |
| Age | 0.981 | 0.974 | 0.988 |
| BMI normal | 0.873 | 0.735 | 1.044 |
| Prior prescription number | 0.865 | 0.849 | 0.881 |
| Commercial insurance vs. others | 1.242 | 1.104 | 1.535 |
| White (reference) | 1.000 | ||
| Asian | 1.614 | 1.243 | 2.096 |
| Black | 1.556 | 1.235 | 1.962 |
| Hispanic | 1.348 | 1.137 | 1.598 |
| Other | 1.499 | 1.139 | 1.974 |
| CCI = 0 vs. CCI ≥ 2 | 1.706 | 1.566 | 1.879 |
| CCI = 1 vs. CCI ≥ 2 | 0.861 | 0.722 | 1.027 |
| Benign prostatic hyperplasia | 0.963 | 0.726 | 1.275 |
| Depression | 1.072 | 0.898 | 1.281 |
| Urinary tract infections | 1.343 | 1.122 | 1.609 |
| Skin infections | 1.297 | 0.923 | 1.824 |
| Falls, fractures, or related injuries | 1.289 | 1.094 | 1.518 |
| Vulvovaginitis | 1.044 | 0.773 | 1.409 |
| Epilepsy | 0.549 | 0.212 | 1.439 |
| Hypertension | 0.418 | 0.229 | 0.764 |
| Ischemic heart disease | 0.321 | 0.182 | 1.081 |
| Multiple sclerosis | 0.472 | 0.254 | 0.879 |
| Parkinson’s disease | 0.844 | 0.396 | 1.781 |
| Spinal cord injury | 0.722 | 0.138 | 3.781 |
| Substance abuse | 1.102 | 0.868 | 1.394 |
| Stroke | 0.784 | 0.468 | 1.312 |
| Urethral stricture or urinary obstruction | 0.974 | 0.532 | 1.789 |
BMI = body mass index; CCI = Charlson Comorbidity Index.
Discussion
To the best of our knowledge, this is the first study to evaluate primary nonadherence in patients with an OAB prescription order in an integrated health care delivery system. The distribution of index OAB therapies in this study was driven by the formulary design at KPSC. The percentage of primary nonadherence to OAB medications was 18% within 30 days of the prescription order date. Primary nonadherent patients were mostly female, younger, had fewer comorbid conditions, were healthier, and had fewer concomitant medications and prescriptions dispensed in the past year compared with adherent patients. Factors such as commercial insurance, race other than white, CCI = 0 vs. CCI ≥ 2, UTIs, and falls were more likely to be associated with primary nonadherence versus adherence and were found to be statistically significant.
Primary nonadherence has been investigated in a variety of chronic conditions, providing a range of results. In patients with cardiovascular disease or osteoporosis, primary nonadherence rates were 15.4% for statins within a 90-day study window and 29.5% for bisphosphonates within 60 days of the prescription order date, respectively.12,13 Across 10 therapeutic drug groups, primary nonadherence rates were 9.8% within 14 days of the prescription order date, reaching 22.4% for osteoporosis medications and 22.3% for antihyperlipidemics.16 In patients prescribed an antihypertensive, an antidiabetic, or an antihyperlipidemic, primary nonadherence rates were 7%, 11%, and 13%, respectively, within an 18-month period.27 In the clinical setting, 16.6% of patients with type 2 diabetes were initially nonadherent to metformin.15 These results are comparable to this analysis of OAB patients.
Notably, all these studies were conducted at Kaiser Permanente. The closed network health care delivery systems used in the analyses capture patient health care utilization data in EMRs, including the ordering and dispensing of medications; therefore, they are ideally suited for measuring primary nonadherence rates. Increasing use of electronic prescribing and EMRs will facilitate the identification of primary nonadherence rates for more chronic conditions in a variety of health care settings.12 Differences in findings between the current and previous studies are likely because of dissimilar patient populations and definitions of primary nonadherence.12,13,15,16,27
A standardized definition of primary nonadherence may aid in future research. Evidence shows that most patients pick up their medications within the first 2 weeks of the index date20,21; therefore, a study period of 14-21 days may be most appropriate. The use of 30 days in this study was a conservative approach and was based on clinical input from pharmacists and urologists. A standardized definition of primary nonadherence will require appropriate validation across different patient populations and prescribed drugs before it can be applied in clinical research.
Patient-reported reasons for OAB medication discontinuation include side effects, low efficacy, insurance limits, and lifestyle incompatibility/inconvenience but do not include cost, suggesting that patients may need more education and support from physicians to encourage persistence.28 The most common reasons reported by cardiovascular patients for primary nonadherence were general concerns about taking the medication, a decision to try lifestyle modifications, and fear of side effects, and some patients reported inadequate health literacy.29 In adults with chronic disease, reasons for not filling a new prescription and nonpersistence include financial hardship, fear of side effects, general concerns about medications, lack of perceived need, change in health benefits, and the belief that the condition is not life threatening.30 Predictors of primary nonadherence identified in previous research include nonformulary status of medications, residence in a low-income neighborhood, method of prescription transmission to the pharmacy, medication class, and cost; similar factors should be investigated in future studies of primary nonadherence in OAB.14,31,32
Characteristics of primary nonadherent patients vary across studies. Patients with primary nonadherence to statins were younger and healthier, with fewer comorbid conditions, concurrent prescriptions, hospitalizations, and clinic and emergency department visits than patients who were adherent.12 In osteoporosis patients, older age and emergency department visits were associated with increased odds of primary nonadherence, while prescription medication use and hospitalization were associated with lower odds.13
This study suggests that primary nonadherent OAB patients are usually young, otherwise healthy, females. This demographic may perceive little benefit from taking OAB medication and assume lifestyle changes are adequate to manage the condition.33 Older OAB patients with more comorbid conditions may have greater interactions with their health care providers, which could effectively improve medication adherence.
These observations indicate that interventions to improve primary nonadherence to OAB medication should focus on younger patients, even though OAB is considered more prevalent in the older population. Emerging evidence suggests that strategies to support medication adherence should be personalized. In patients with multiple chronic diseases, adherence may be improved by financial assistance, such as reducing drug cost sharing.34 Copayment should be investigated in future studies, since it may be a strong predictor of primary nonadherence. Younger patients and other patients interested in digital solutions may be engaged with text alerts and reminders.35 KPSC currently uses digital solutions for primary nonadherence in other disease areas.
Early detection of primary nonadherence and appropriate interventions have the potential to alleviate some of the clinical, economic, and humanistic burden of chronic diseases. Interventions that have successfully reduced primary nonadherence include telephone calls from pharmacists encouraging patients to pick up their prescriptions and educating them on the importance of their treatment and an automated reminder in the form of a telephone call followed by a letter reinforcing the reasons to use medications.36,37
The findings of this study suggest that efforts to improve follow-up care and increase interactions between physicians, pharmacists, and patients could reduce primary nonadherence rates. Within an integrated system, such an interaction may be as simple as creating an automated call to patients who are not picking up their prescriptions. The factors associated with primary nonadherence identified in this analysis may act as a guide to health care providers when targeting such calls; however, effective interventions should be patient specific.
Further studies are needed to better understand barriers to initial prescription pick up and to inform the development of effective interventions. Knowledge of primary nonadherence rates is valuable for health care systems, health care providers, and researchers. It provides the first step towards identifying patient-, physician-, and health system-level factors associated with primary nonadherence, all of which have important implications when developing interventions. Health care systems, pharmacies, primary care providers, obstetricians, gynecologists, and urologists should be aware that 18% of OAB patients are not picking up their initial prescriptions and should be provided with opportunities to propose and implement interventions and contribute to future research on primary nonadherence.
Limitations
This study has a number of limitations. First, the limitations associated with retrospective analyses exist when conducting observational studies of medication adherence. In this study, patients were not interviewed to determine reasons for nonadherent behavior. Second, several factors noted as predictors of primary nonadherence in studies of other prescribed drugs were not assessed in this study, which may have affected the logistic regression model. Third, the analysis was restricted to patients with a prescription drug benefit; therefore, the results may not be applicable to individuals without a drug benefit. Fourth, prescriptions filled at a non-KPSC pharmacy were not captured. In a previous study conducted at KPSC, 20% of primary nonadherent patients reported filling their statin prescription at a non-KPSC pharmacy.29 Fifth, claims data do not capture nonpharmacologic interventions that primary nonadherent OAB patients may have used instead of pharmacotherapy. Sixth, although KPSC membership is socioeconomically diverse, this study only sampled patients from 1 integrated health plan and in 1 geographic region, so the results may differ from those in other regions and from other types of managed care organizations. Finally, these results may have limited generalizability to patient populations in nonintegrated health care systems.
This is the first study reporting on primary nonadherence to OAB medications, and it has a number of strengths. The study was able to identify a relatively large and racially diverse population of patients with new OAB prescription orders; KPSC members generally reside within 7 of the most populous counties in Southern California.12 In addition, the study captured a comprehensive set of clinical and demographic characteristics for patients and information on prescriber specialties, allowing a number of factors potentially associated with primary nonadherence to OAB medications to be identified. This study was able to capture all prescription transactions between the EMR and the dispensing pharmacy and to report on all sold prescriptions, which minimized the risk of incorrectly identifying patients who filled their prescriptions at a non-KPSC pharmacy as primary nonadherent.
Conclusions
In this study, we found that nearly 1 in 5 patients did not pick up their new order of OAB medication within 30 days of the order date. Primary nonadherence has been recognized as a substantial health care burden, and it is important to understand barriers that result in patients not picking up their new prescription orders. Future research is needed to determine patient-specific reasons for primary nonadherence. This study found that younger patients without significant risk factors were highly associated with primary nonadherence to OAB medications. Novel interventions are needed to improve primary nonadherence in the OAB population.
References
- 1.Haylen BT, de Ridder D, Freeman RM, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Neurourol Urodyn. 2010;29(1):4-20. [DOI] [PubMed] [Google Scholar]
- 2.Tubaro A. Defining overactive bladder: epidemiology and burden of disease. Urology. 2004;64(6 Suppl 1):2-6. [DOI] [PubMed] [Google Scholar]
- 3.Qin L, Luo X, Zou KH, Snedecor SJ. Economic impact of using fesoterodine for the treatment of overactive bladder with urge urinary incontinence in vulnerable elderly population in the United States. J Med Econ. 2016;19(3):229-35. [DOI] [PubMed] [Google Scholar]
- 4.Wagner TH, Hu TW, Bentkover J, et al. Health-related consequences of overactive bladder. Am J Manag Care. 2002;8(19 Suppl):S598-607. [PubMed] [Google Scholar]
- 5.Stewart WF, Van Rooyen JB, Cundiff GW, et al. Prevalence and burden of overactive bladder in the United States. World J Urol. 2003;20(6):327-36. [DOI] [PubMed] [Google Scholar]
- 6.Coyne KS, Sexton CC, Vats V, Thompson C, Kopp ZS, Milsom I. National community prevalence of overactive bladder in the United States stratified by sex and age. Urology. 2011;77(5):1081-87. [DOI] [PubMed] [Google Scholar]
- 7.Ortman JM, Velkoff VA. An aging nation: the older population in the United States. Population estimates and projections. U.S. Census Bureau. May 2014. Available at: https://www.census.gov/prod/2014pubs/p25-1140.pdf. Accessed March 6, 2017.
- 8.Ganz ML, Smalarz AM, Krupski TL, et al. Economic costs of overactive bladder in the United States. Urology. 2010;75(3):526-32.e18. [DOI] [PubMed] [Google Scholar]
- 9.Sexton CC, Notte SM, Maroulis C, Dmochowski RR, Subramanian D, Coyne KS. Persistence and adherence in the treatment of overactive bladder syndrome with anticholinergic therapy: a systematic review of the literature. Int J Clin Pract. 2011;65(5):567-85. [DOI] [PubMed] [Google Scholar]
- 10.Joyce GF. Understanding primary nonadherence. Am J Pharm Benefits. 2010;2(2):109-10. [PMC free article] [PubMed] [Google Scholar]
- 11.Solomon MD, Majumdar SR. Primary non-adherence of medications: lifting the veil on prescription-filling behaviors. J Gen Intern Med. 2010;25(4):280-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheetham TC, Niu F, Green K, et al. Primary nonadherence to statin medications in a managed care organization. J Manag Care Pharm. 2013;19(5):367-73. Available at: http://www.jmcp.org/doi/10.18553/jmcp.2013.19.5.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reynolds K, Muntner P, Cheetham TC, et al. Primary non-adherence to bisphosphonates in an integrated healthcare setting. Osteoporos Int. 2013;24(9):2509-17. [DOI] [PubMed] [Google Scholar]
- 14.Tamblyn R, Eguale T, Huang A, Winslade N, Doran P. The incidence and determinants of primary nonadherence with prescribed medication in primary care: a cohort study. Ann Intern Med. 2014;160(7):441-50. [DOI] [PubMed] [Google Scholar]
- 15.Nichols GA, Conner C, Brown JB. Initial nonadherence, primary failure and therapeutic success of metformin monotherapy in clinical practice. Curr Med Res Opin. 2010;26(9):2127-35. [DOI] [PubMed] [Google Scholar]
- 16.Shin J, McCombs JS, Sanchez RJ, Udall M, Deminski MC, Cheetham TC. Primary nonadherence to medications in an integrated healthcare setting. Am J Manag Care. 2012;18(8):426-34. [PubMed] [Google Scholar]
- 17.Sokol MC, McGuigan KA, Verbrugge RR, Epstein RS. Impact of medication adherence on hospitalization risk and healthcare cost. Med Care. 2005;43(6):521-30. [DOI] [PubMed] [Google Scholar]
- 18.Koebnick C, Langer-Gould AM, Gould MK, et al. Sociodemographic characteristics of members of a large, integrated health care system: comparison with US Census Bureau data. Perm J. 2012;16(3):37-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Derose SF, Contreras R, Coleman KJ, Koebnick C, Jacobsen SJ. Race and ethnicity data quality and imputation using U.S. Census data in an integrated health system: the Kaiser Permanente Southern California experience. Med Care Res Rev. 2013;70(3):330-45. [DOI] [PubMed] [Google Scholar]
- 20.Karter AJ, Parker MM, Moffet HH, et al. New prescription medication gaps: a comprehensive measure of adherence to new prescriptions. Health Serv Res. 2009;44(5 Pt 1):1640-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liberman JN, Hutchins DS, Popiel RG, et al. Determinants of primary nonadherence in asthma-controller and dyslipidemia pharmacotherapy. Am J Pharm Benefits. 2010;2(2):111-18.24466385 [Google Scholar]
- 22.Darkow T, Fontes CL, Williamson TE. Costs associated with the management of overactive bladder and related comorbidities. Pharmacotherapy. 2005;25(4):511-19. [DOI] [PubMed] [Google Scholar]
- 23.Zorn BH, Montgomery H, Pieper K, et al. Urinary incontinence and depression. J Urol. 1999;162(1):82-84. [DOI] [PubMed] [Google Scholar]
- 24.Stach-Lempinen B, Hakala A, Laippala P, et al. Severe depression determines quality of life in urinary incontinent women. Neurourol Urodyn. 2003;22(6):563-68. [DOI] [PubMed] [Google Scholar]
- 25.Brown JS, McGhan WF, Chokroverty S. Comorbidities associated with overactive bladder. Am J Manag Care. 2000;6(Suppl 11):S574-79. [PubMed] [Google Scholar]
- 26.Brown JS, Vittinghoff E, Wyman JF, et al. Urinary incontinence: does it increase risk for falls and fractures? Study of Osteoporotic Fractures Research Group. J Am Geriatr Soc. 2000;48(7):721-25. [DOI] [PubMed] [Google Scholar]
- 27.Raebel MA, Ellis JL, Carroll NM, et al. Characteristics of patients with primary non-adherence to medications for hypertension, diabetes, and lipid disorders. J Gen Intern Med. 2012;27(1):57-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schabert VF, Bavendam T, Goldberg EL, Trocio JN, Brubaker L. Challenges for managing overactive bladder and guidance for patient support. Am J Manag Care. 2009;15(4 Suppl):S118-22. [PubMed] [Google Scholar]
- 29.Harrison TN, Derose SF, Cheetham TC, et al. Primary nonadherence to statin therapy: patients’ perceptions. Am J Manag Care. 2013;19(4):e133-39. [PubMed] [Google Scholar]
- 30.McHorney CA, Spain CV. Frequency of and reasons for medication non-fulfillment and non-persistence among American adults with chronic disease in 2008. Health Expect. 2011;14(3):307-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fischer MA, Stedman MR, Lii J, et al. Primary medication nonadherence: analysis of 195,930 electronic prescriptions. J Gen Intern Med. 2010;25(4):284-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fischer MA, Choudhry NK, Brill G, et al. Trouble getting started: predictors of primary medication nonadherence. Am J Med. 2011;124(11):1081. e9-22. [DOI] [PubMed] [Google Scholar]
- 33.Gadkari AS, McHorney CA. Medication nonfulfillment rates and reasons: narrative systematic review. Cur Med Res Opin. 2010;26(3):683-705. [DOI] [PubMed] [Google Scholar]
- 34.Choudhry NK, Avorn J, Glynn RJ, et al. Full coverage for preventive medications after myocardial infarction. N Engl J Med. 2011;365(22):2088-97. [DOI] [PubMed] [Google Scholar]
- 35.Wald DS, Bestwick JP, Raiman L, Brendell R, Wald NJ, Walker N. Randomised trial of text messaging on adherence to cardiovascular preventive treatment (INTERACT trial). PLoS ONE. 2014;9(12):e114268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fischer MA, Choudhry NK, Bykov K, et al. Pharmacy-based interventions to reduce primary medication nonadherence to cardiovascular medications. Med Care. 2014;52(12):1050-54. [DOI] [PubMed] [Google Scholar]
- 37.Derose SF, Green K, Marrett E, et al. Automated outreach to increase primary adherence to cholesterol-lowering medications. JAMA Intern Med. 2013;173(1):38-43. [DOI] [PubMed] [Google Scholar]


