Abstract
BACKGROUND:
Health plans use formulary restrictions (e.g., prior authorization, step therapy, tier change, nonformulary status) in an effort to control cost and promote quality, safety, and appropriate prescription utilization. Some Medicare payers perceive that the inclusion of certain agents, such as branded oxycodone HCl extended-release tablets (OERs), on their formularies is associated with attracting high-cost members to the plan.
OBJECTIVE:
To evaluate disenrollment rates, patient migration, and subsequent health care costs among OER users who disenrolled from a national Medicare Advantage Prescription Drug plan (study-MAPD) in the plan year following OER nonformulary restriction.
METHODS:
A retrospective, longitudinal cohort study using IMS pharmacy and medical claims data between July 1, 2011, and December 31, 2014, was conducted. In the study-MAPD, adults aged ≥ 18 years who were chronic OER users with ≥ 2 OER claims 6 months before the nonformulary restriction date on January 1, 2013 (index date) and with continuous activity in pharmacy and medical claims for 6 months pre- and post-index were included in the study. Comparison years of 2012 and 2014 prerestriction/postrestriction were selected. All groups were followed for 6 months postindex. Year-to-year disenrollment rates of OER patients and the overall plan, as well as patient characteristics and costs of those who disenrolled from and those who remained with the plan, were measured. Costs were compared using a difference-in-differences approach.
RESULTS:
This study identified 2,935 eligible OER users from the study-MAPD population after imposing nonformulary restrictions on OERs on January 1, 2013. Mean age was 62.1 years, and 59.8% were female. The mean Charlson Comorbidity Index score was 1.83 for those 1,001 patients with medical claims data. For comparison years 2012 (prerestriction) and 2014 (postrestriction), 2,248 and 2,222 OER patients were identified, respectively. Patient characteristics were similar across patient cohorts in all 3 study years. Disenrollment rates for OER users (12.9%, 5.5%, and 14.3% for years 2012, 2013, and 2014, respectively) were lower or similar to those of the overall plan (18.3%, 7.6%, and 14.1%, respectively, for the same 3 years). Approximately 40% of OER users who disenrolled from the study-MAPD migrated to plans also imposing a nonformulary restriction on OERs, while about 25% moved to plans with less restrictive OER coverage. The majority (59.9%) of patients continued OER use irrespective of their disenrollment from the study-MAPD in 2013. Although a nonsignificant decrease ($117; P = 0.340) in per patient per month (PPPM) cost was observed among OER patients postrestriction (from 2012 to 2013), the difference-in-difference analysis indicated a net postrestriction increase of $124 (P = 0.461) in PPPM for OER patients.
CONCLUSIONS:
This study found little evidence to support any consistent directional effect on patient enrollment behavior as a result of an OER non-formulary restriction. Implementation of an OER nonformulary restriction did not lead to higher OER patient disenrollment or lower patient costs in the study-MAPD.
What is already known about this subject
Access restrictions such as prior authorization, step therapy, tier change, and not covered/nonformulary edits are used to ensure appropriate utilization and control costs.
Opioid pain medications are subject to access restrictions for clinical and economic reasons.
Access restrictions on oxycodone HCl extended-release tablets (OERs) have not consistently led to net cost savings.
What this study adds
Removing OERs from the formulary was not found to increase chronic OER patient disenrollment from plans imposing such restrictions.
OER patients who disenrolled from plans imposing OER restrictions did not consistently seek out less restrictive plans following imposition of the nonformulary restriction.
There was no consistent or substantial effect on health care costs among OER patients following the OER restriction.
Managed care organizations implement formulary access restrictions (e.g., prior authorization [PA], step therapy, tier change, nonformulary status) and other interventions (e.g., drug utilization review [DUR]) in an effort to influence use and improve safety and clinical and/or economic outcomes among members.1 When designed and applied effectively, research has demonstrated that clinical utilization management can improve outcomes while ensuring appropriate utilization of drug products. For example, a recent study showed that opioid analgesics cause some of the most common adverse drug events that lead to emergency department visits in the United States,2 and DUR can potentially reduce unsafe opioid use.3 Likewise, certain types of utilization management have proven effective in lowering costs.4-6 Although many formulary restrictions are instituted for both clinical and economic reasons, when a class has numerous agents with similar indications and safety precautions, a payer may choose to impose a formulary restriction on certain agents in order to contain costs. However, other policies designed to control expenditures, such as the use of highly restrictive formularies, may disproportionately affect severely ill patients, decrease access to essential medications, and increase the use of expensive medical care.7,8
Whatever the basis for the restriction, however, payers must consider the economic effect beyond the cost of drug products, such as administrative costs, medical costs, and potential effects on member enrollment. Past studies have shown that placing pain medications such as oxycodone HCl extended-release tablets (OERs) on a higher tier or requiring a PA did not translate into net cost savings,9 as it increased costs related to medical office visits without an offsetting savings in pharmacy costs. A comparison between plans imposing and those not imposing a PA on OERs has demonstrated that modest market share changes resulting from the policy were offset by rebate losses and administrative costs leading to cost neutrality.10
In addition, Medicare Part D payers must also consider yearly member migration in designing a clinically sound and cost-effective formulary. Unlike traditional group plans that often enroll large populations for multiple years, most Medicare Part D plans enroll individuals. Using the Medicare Planfinder, beneficiaries can compare formularies, premiums, and out-of-pocket prices before signing up for a plan. This individual selection requires that Medicare plans also consider the cost of members who may migrate to a plan and the potential of attracting more costly members based on inclusion of specific drug products. This concept, known as adverse selection,11,12 has been raised as a potential concern since the inception of Medicare Part D.13 However, research evaluating the effect of formulary changes on member enrollment patterns is quite limited.
Since adverse selection is based on perceived member behavior, no direct metric for measurement exists. Undoubtedly, numerous factors contribute to individual enrollment decisions, making it nearly impossible to pinpoint a single formulary feature that influences most members’ choices. Still, some interpretations of adverse selection would attribute enrollment decisions to placement of a single agent on formulary.
The purpose of this study was to examine retrospective claims data for evidence of trends that would that indicate that enrollment decisions are being made as a result of a formulary exclusion of a single drug product. OER is a commonly prescribed opioid for the management of severe pain and is subject to various access restrictions.14 Using a national Medicare Advantage Prescription Drug (MAPD) plan that removed OERs from its formulary at the beginning of 2013, this study evaluated enrollment patterns and health care costs from a payer perspective associated with OER users before and after formulary exclusion.
Methods
Data Source
This analysis used anonymized IMS patient-centric pharmacy and medical open-source claims databases. The pharmacy data capture information on adjudicated dispensed prescriptions sourced from retail, mail, long-term care, and specialty pharmacies, with 86% coverage of the retail channel, 55% of standard mail-service, and 40%-70% of specialty pharmacy volume.
The medical data are from medical claims that provide patient-level diagnoses and procedures for visits to U.S. office-based physicians, ambulatory care sites, and general health care sites and unadjudicated charge data. The medical database receives approximately 1 billion claims per year submitted by over 860,000 practitioners in the United States.
Patient Selection
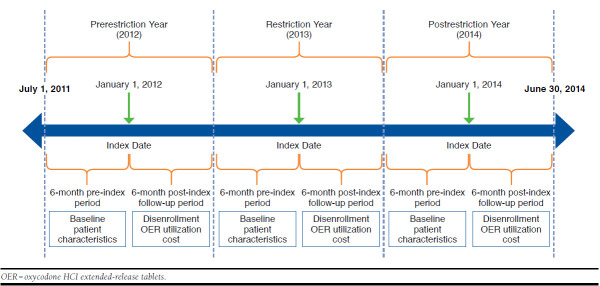
Adult patients aged ≥ 18 years with continuous prescription activity in the pharmacy database from a large national MAPD plan (study-MAPD) during the 6 months pre-index and observed in the database during the 6 months post-index period were identified. For this study, 3 relevant index dates were identified: January 1, 2012; January 1, 2013; and January 1, 2014. Since the study-MAPD removed OER from its formulary (nonformulary status) on January 1, 2013, this was the index date for the restriction year cohort. Two comparator cohorts were identified, with January 1, 2012 (prerestriction year) and January 1, 2014 (post-restriction year, where OER nonformulary status was still in place), as their index dates.
Qualifying OER users were patients who had at least 2 claims for an OER in the 6-month pre-index period and were required to be observed in pharmacy claims for the 6-month post-index period. Appendix A (available in online article) represents a schematic description of the study design.
Patient Characteristics
Patient characteristics (demographic and clinical) were measured at baseline (index date and 6 months pre-index). Clinical characteristics are reported for a subset of patients with both pharmacy and medical claims data. Comorbid conditions were identified using International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes. Charlson Comorbidity Index (CCI) scores were calculated using the Dartmouth-Manitoba adaptation.15,16 Clinical characteristics such as most prevalent comorbidities were compared between those who disenrolled and those who remained in the study-MAPD after the change to nonformulary status.
Effect of OER Restriction on Patient Enrollment Status
Year-to-year disenrollment rates for OER patients and overall plan level were calculated as the proportions of patients with no pharmacy or medical claims from the study-MAPD during the post-index period. Patients disenrolling from the study-MAPD were followed to evaluate enrollment in other plans and the OER formulary status in those plans. A subset of patients with observed OER use in the post-index period was analyzed to evaluate if plan migration was sensitive to OER restriction.
OER Utilization Status After Restriction Period
To evaluate the effect of OER nonformulary status, pain medication claims after the restriction period were tracked and grouped into continued OER use, switched to other long-acting opioids (LAOs), or discontinued all LAOs.
Health Care Costs
Health care costs as billed charges were reported as per patient per month (PPPM) total costs, costs of medical claims, and costs of pharmacy claims during the 6-month pre- and post-index periods for the subset of OER users with both pharmacy and medical claims data. Pharmacy costs were further categorized into LAO costs, short-acting opioid (SAO) costs, and nonopioid costs. Costs were reported for each of the 3 index years. Six-month post-index costs were compared for patients remaining with the study-MAPD to those disenrolling. The difference in mean cost between pre- and post-index costs was assessed. All costs were adjusted to 2014 U.S. dollars using the medical care component of the Consumer Price Index for All Urban Consumers.
Statistical Analysis
Analyses and comparisons on disenrollment rates, patient characteristics, and costs between restriction and non-restriction cohort years, as well as between disenrolled and remaining OER patients, were conducted. A difference-in-difference analysis was conducted to evaluate the net year-over-year cost differences. First, PPPM (pre- and post-index and remained vs. disenrolled patients) was calculated for the restriction year (2013) and the 2 comparator years (2012 and 2014). Differences in PPPM between the restriction and comparator years were used to evaluate the net impact of the OER nonformulary restriction. Chi-square test for categorical variables and Wilcoxon signed-rank test for continuous variables were used to test if differences were statistically significant. Level of significance was tested at α = 0.05. All analyses were conducted using SAS version 9.2 (SAS Institute, Cary, NC).
Results
Demographic and Clinical Characteristics
This study identified 2,935 eligible OER users (≥ 2 OER claims in 6-month pre-index period) from the study-MAPD for restriction year 2013, 2,248 users for year 2012, and 2,222 users for year 2014. Table 1 describes baseline patient characteristics. Mean age was 63.2, 62.1, and 60.9 years for cohorts indexed to 2012, 2013, and 2014, respectively. There were more female than male patients (approximately 60% vs. 40%) in all 3 index years. A majority of the study patients (55.9%, 57.2%) resided in the West region of the United States among the cohorts indexed to 2012 and 2013, while for 2014, 42.9% resided in the South, and 42.8% resided in the West.
TABLE 1.
Patient Characteristics (Restriction Year 2013: Disenrolled vs. Remained with the Study-MAPD)
| All Patients in 2013 | Disenrolled During Postrestriction Period | Remained with Plan During Postrestriction Period | P Value (Remained vs. Disenrolled) | |
|---|---|---|---|---|
| Sample: Patients with Pharmacy Claims | ||||
| Sample size (n) | 2,935 | 162 | 2,773 | |
| Baseline demographic characteristics | ||||
| Age at index | ||||
| Mean | 62.1 | 61.4 | 62.2 | 0.457 |
| Median | 62.0 | 61.0 | 62.0 | 0.599 |
| SD | 12.8 | 13.5 | 12.8 | |
| Gender, n (%) | ||||
| Female | 1,754 (59.8) | 97 (59.9) | 1,657 (59.8) | |
| Male | 1,166 (39.7) | 64 (39.5) | 1,102 (39.7) | |
| Unknown | 15 (0.5) | 1 (0.6) | 14 (0.5) | |
| Region, n (%) | ||||
| Midwest | 137 (4.7) | 8 (4.9) | 129 (4.7) | 0.284 |
| Northeast | 113 (3.9) | 10 (6.2) | 103 (3.7) | |
| South | 883 (30.1) | 42 (25.9) | 841 (30.3) | |
| West | 1,679 (57.2) | 92 (56.8) | 1,587 (57.2) | |
| Unknown | 123 (4.2) | 10 (6.2) | 113 (4.1) | |
| Baseline clinical characteristics | ||||
| Sample size, n-linked subset with diagnosis data, n (%) | 1,001 (34.1) | 60 (37.0) | 941 (33.9) | |
| CCI scores | ||||
| Mean | 1.83 | 2.08 | 1.81 | 0.333 |
| Median | 1.00 | 1.50 | 1.00 | |
| SD | 2.11 | 2.32 | 2.10 | |
| Top comorbidities, n (%) | ||||
| Osteoarthritis | 664 (66.3) | 36 (60.0) | 628 (66.7) | 0.284 |
| Hypertension | 487 (48.7) | 34 (56.7) | 453 (48.1) | 0.200 |
| Chronic pain/fibromyalgia | 341 (34.1) | 15 (25.0) | 326 (34.6) | 0.126 |
| Diabetes | 285 (28.5) | 10 (16.7) | 275 (29.2) | 0.037 |
| Dyslipidemia | 269 (26.9) | 23 (38.3) | 246 (26.1) | 0.039 |
| Sleep disorders | 164 (16.4) | 9 (15.0) | 155 (16.5) | 0.765 |
| Myocardial infarction/coronary artery disease | 149 (14.9) | 8 (13.3) | 141 (15.0) | 0.728 |
| Depression | 142 (14.2) | 8 (13.3) | 134 (14.2) | 0.845 |
| Alcohol/drug abuse | 125 (12.5) | 6 (10.0) | 119 (12.6) | 0.548 |
| Cardiac arrhythmia | 114 (11.4) | 5 (8.3) | 109 (11.6) | 0.442 |
| Rheumatologic disease (SLE, RA, AS, PSA) | 114 (11.4) | 4 (6.7) | 110 (11.7) | 0.235 |
AS = ankylosing spondylitis; CCI = Charlson Comorbidity Index; MAPD = Medicare Advantage Prescription Drug plan; PSA = psoriatic arthritis; RA = rheumatoid arthritis; SD = standard deviation; SLE = systemic lupus erythematosus.
Only a subset of pharmacy patients was linkable to medical claims where diagnosis and clinical data were available. The subsets consisted of 762 patients in 2012, 1,001 in 2013, and 832 in 2014. For the 1,001 patients with diagnosis data in the restriction year 2013, the mean CCI score was 1.83 (comparable with the general population at similar age [mean = 62.1] with 1 to 2 nonsevere chronic conditions such as diabetics and chronic obstructive pulmonary disease), with hypertension (48.7%), chronic pain/fibromyalgia (34.1%), diabetes (28.5%), dyslipidemia (26.9%), and sleep disorders (16.4%) being the top 5 comorbidities. A non-negligible proportion of patients was observed to have depression and/or alcohol/drug abuse (14.2% and 12.5%, respectively). Similar demographic and clinical characteristics were observed in the 2012 and 2014 comparison cohorts.
In the restriction year 2013, the overall comorbidity burden was found to be not significantly higher among the disenrolled OER patients compared with those remaining with the plan postrestriction (CCI: 2.08 vs. 1.81; P = 0.333). When segmented by individual comorbidities, the disenrolled group had a significantly lower rate of diabetes (16.7% vs. 29.2%; P = 0.037) but a significantly higher rate of dyslipidemia (38.3% vs. 26.1%; P = 0.039). However, this should be interpreted in the context of the low counts of patients with diabetes (n = 10) and dyslipidemia (n = 23) in the disenrolled group. No significant differences in common pain-related comorbidities were observed between patients remaining with the study-MAPD and those who disenrolled (osteoarthritis, 66.7% vs. 60.0%, P = 0.284; chronic pain/fibromyalgia 34.6% vs. 25.0%, P = 0.126; and rheumatologic disease, 11.7% vs. 6.7%, P = 0.235).
Effect of OER Restriction on Patient Disenrollment
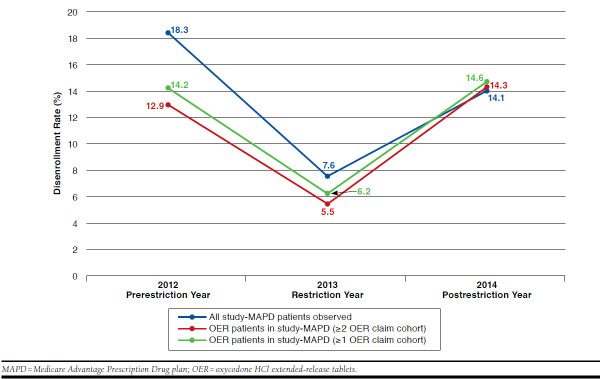
As indicated in Figure 1, the disenrollment rate among OER patients (≥ 2 OER claims in the pre-index period) at the time of removal of OER from the study-MAPD formulary (January 1, 2013) was 5.5% (n = 60) compared with 12.9% (n = 99) in 2012 when OER formulary status was unrestricted and 14.3% (n = 106) in 2014, 1 year after OERs were removed from the formulary. Similar disenrollment rates were also observed in a broader OER patient cohort with only ≥ 1 OER claim in the pre-index 6-month period. As shown in Figure 1, increases and decreases in year-to-year disenrollment rate estimates between the chronic OER user and overall plan level paralleled each other very closely (18.3% in 2012, 7.6% in 2013, and 14.1% in 2014 overall disenrollment rates compared to 12.9% in 2012, 5.5% in 2013, and 14.3% in 2014 among chronic OER users). In a similar trend, disenrollment rates among OER users with ≥ 1 OER claim post-index were 14.2%, 6.2%, and 14.6% for years 2012, 2013, and 2014, respectively.
FIGURE 1.
Study-MAPD Disenrollment Rates (2012-2014)
Patient Migration Following Study-MAPD Disenrollment
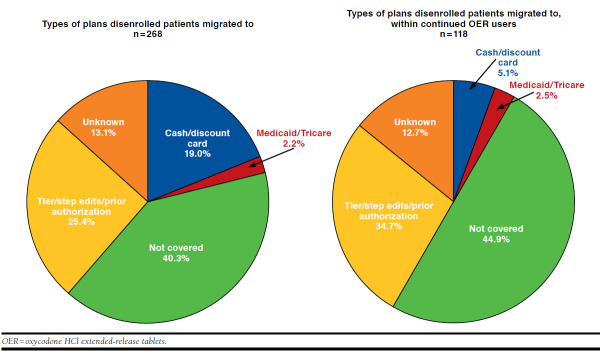
Approximately 25.0% of patients who disenrolled from the study-MAPD following the nonformulary edit switched to a plan without a nonformulary restriction, such as a PA (2.6%) or step edits (1.1%), while about 1 in 5 (21.6%) of those who disenrolled switched to a plan with a tiered formulary with no evidence of a formulary restriction (Figure 2). However, a large proportion (40.3%) migrated to a plan where OERs were also nonformulary. Examining the subset of patients who had OER claims following disenrollment from the study-MAPD, it was found that 32.2% migrated to health plans with a tiered formulary (no apparent restriction) while 44.9% moved to plans where OER was nonformulary.
FIGURE 2.
Migration Pattern of Disenrolled Patients
OER Use After Restriction Period
A majority of patients had at least 1 OER claim after January 1, 2013 (restriction year), irrespective of whether they disenrolled from the study-MAPD that removed OER from the formulary, with 59.8% of those remaining and 59.9% of those disenrolling filling at least 1 OER after the nonformulary restriction. The switch rate to an alternate LAO was 23.2% in the group remaining in the study-MAPD and 11.1% in the group that disenrolled. The rate of complete discontinuation from all LAOs/OERs was 17.0% among those who remained with the study-MAPD and 29.0% among those disenrolled in the restriction year. Notably, in the 6-month post-index period following the restriction, 49.4% of those who disenrolled and 45.1% of those who remained had at least 2 OER claims, which indicates at least 1 additional OER prescription beyond the mandated 1 transition refill in Medicare plans (see Appendix B, available in online article).
Cost Analyses by Plan Enrollment Status
In restriction year 2013, OER patients remaining in the study-MAPD (N = 941) had a higher unadjusted mean 6-month total cost ($17,285) than those disenrolling (n = 60, $13,338). A nonsignificant post-nonformulary PPPM decrease of $117 (P = 0.340) from 6 months pre- to 6 months post-index was observed during this period for all study-MAPD patients remaining in the plan. There was a nonsignificant postrestriction decrease ($10; P = 0.252) in LAO cost. However, increases in PPPM nonopioid ($59; P = 0.011) and SAO costs ($9; P = 0.001) resulted in a significant increase of $58 (P = 0.042) in overall PPPM pharmacy cost (from $1,201 in prerestriction to $1,259 in postrestriction periods; mean 6-month total pharmacy costs were $7,207 and $7,556 in prerestriction and postrestriction periods, respectively; Table 2).
TABLE 2.
Effect of OER Access Restriction on Costs: Restriction Year 2013
| Health Care Charges, $ | Mean | SD | PPPM | Mean | SD | PPPM | Mean | SD | PPPM | Post-index Minus Pre-index Patients Remained | Remained Minus Disenrolled | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre-Index 6-Month Period (All Patients) N = 1,001 | Postrestriction 6-Month Period (Remained with Plan During Postrestriction Period) n = 941 | Postrestriction 6-Month Period (Disenrolled During Postrestriction Period) n = 60 | Change in PPPM | P Value | Change in PPPM | P Value | |||||||
| Total | 17,985 | 21,757 | 2,997 | 17,285 | 22,475 | 2,881 | 13,338 | 15,242 | 2,223 | -117 | 0.340 | $658 | 0.180 |
| Office visits | 10,778 | 20,465 | 1,796 | 9,728 | 20,969 | 1,621 | 7,375 | 13,690 | 1,229 | -175 | 0.149 | $392 | 0.391 |
| All prescriptions | 7,207 | 7,450 | 1,201 | 7,556 | 7,639 | 1,259 | 5,963 | 6,584 | 994 | 58 | 0.042 | $265 | 0.114 |
| LAO prescriptions | 2,451 | 2,726 | 409 | 2,392 | 3,330 | 399 | 1,530 | 2,402 | 255 | -10 | 0.252 | $144 | 0.048 |
| Nonopioid prescriptions | 4,321 | 5,810 | 720 | 4,675 | 5,510 | 779 | 4,026 | 5,239 | 671 | 59 | 0.011 | $108 | 0.375 |
| SAO prescriptions | 435 | 2,373 | 73 | 489 | 2,291 | 82 | 407 | 563 | 68 | 9 | 0.001 | $14 | 0.782 |
Note: All charges were adjusted to 2014 U.S. dollars using the Consumer Price Index.
LAO = long-acting opioid; OER = oxycodone HCl extended-release tablets; PPPM = per patient per month; SAO = short-acting opioid; SD = standard deviation.
A difference-in-differences approach indicated a nonsignificant net postrestriction (2012 to 2013) PPPM total cost increase of $124 (P = 0.461) among OER patients (Table 3). This was accompanied by a significant net decrease in pharmacy cost (-$70; P = 0.048) accompanied by a significant net decrease in LAO cost (-$48; P = 0.001) following the nonformulary status in 2013. The net change in PPPM total cost in 2014 following the OER nonformulary restriction, compared with the restriction year (2013), was a nonsignificant increase of $196 (P = 0.303).
TABLE 3.
Effect of OER Access Restriction on Costs: Difference-in-Difference Analysis
| Comparison: Pre- vs. Post-Index Periods | |||||||
|---|---|---|---|---|---|---|---|
| Health Care Charges | Change in PPPM (Patients Remained in Plan in Postindex Period vs. All Patients in the Pre-index Period) | Difference in PPPM Change | |||||
| Control Year 2012a | Restriction Year 2013 | Control Year 2014a | 2013-2012 | 2014-2013 | P Value 2013-2012 | P Value 2014-2013 | |
| n | 762 | 1,001 | 832 | ||||
| Total, $ | -241 | -117 | 79 | 124 | 196 | 0.461 | 0.303 |
| Office visits, $ | -370 | -175 | -40 | 195 | 135 | 0.221 | 0.495 |
| All prescriptions, $ | 129 | 58 | 119 | -70 | 61 | 0.048 | 0.072 |
| LAO prescriptions | 39 | -10 | -14 | -48 | -4 | 0.001 | 0.833 |
| Nonopioid prescriptions | 87 | 59 | 100 | -28 | 41 | 0.299 | 0.187 |
| SAO prescriptions | 3 | 9 | 33 | 6 | 24 | 0.210 | < 0.0001 |
| Comparison: Remained vs. Disenrolled Patients | |||||||
| Health Care Charges | Change in PPPM Postrestriction Period (Remained, Disenrolled) | Change in PPPM (Difference in Difference) | |||||
| Control Year 2012a | Restriction Year 2013 | Control Year 2014a | 2013-2012 | 2014-2013 | P Value 2013-2012 | P Value 2014-2013 | |
| n (remained/disenrolled) | 663/99 | 941/60 | 726/106 | ||||
| Total, $ | 11 | 658 | 616 | 646 | -42 | 0.249 | 0.944 |
| Office visits, $ | 26 | 392 | 448 | 366 | 56 | 0.471 | 0.916 |
| All prescriptions, $ | -14 | 265 | 168 | 280 | -98 | 0.210 | 0.709 |
| LAO prescriptions | 56 | 144 | 14 | 87 | -130 | 0.361 | 0.163 |
| Nonopioid prescriptions | -83 | 108 | 121 | 191 | 13 | 0.263 | 0.953 |
| SAO prescriptions | 12 | 14 | 33 | 2 | 20 | 0.976 | 0.738 |
Note: All charges were adjusted to 2014 U.S. dollars using the Consumer Price Index.
aTwo comparator cohorts were identified with January 1, 2012, and January 1, 2014, as their index dates.
LAO = long-acting opioid; OER = oxycodone HCl extended-release tablets; PPPM = per patient per month; SAO = short-acting opioid.
There were no significant differences in overall cost between those who remained with the study-MAPD and those who disenrolled in the restriction year (2013) or the prerestriction (2012) and postrestriction (2014) years. The PPPM total cost for those who remained in the study-MAPD was $2,881 in 2013, while the same for those who disenrolled was $2,223. OER patients who remained in the study-MAPD had a nonsignificantly higher PPPM total cost ($658, P = 0.180) compared with the disenrolled OER patients (Table 2). Difference-in-difference analysis also indicated a net nonsignificant difference in PPPM total cost between OER patients remaining vs. disenrolled from the study-MAPD for the 2 postrestriction years (2013 and 2014; Table 3).
Discussion
Chronic pain patients are clinically complex and often present challenges in formulary management. Medications that treat chronic pain such as OERs are managed in various ways by payers, yet decisions may rely on anecdotal or nongeneralizable data. If an OER access restriction encouraged migration away from a health plan among the sicker patient population, we would expect healthier patients to remain in the plan following a restriction. Based on the observations in the current study accounting for CCI and comorbidities of the patients disenrolling versus remaining in the study-MAPD after the restriction, there was little evidence that the OER nonformulary restriction was associated with patients with higher burden of disease migrating out of the study-MAPD. Both groups were similar in their CCI measure (CCI: 2.08 vs. 1.81; P = 0.333 for disenrolled and remaining OER patients, respectively). It is interesting to note that, although not statistically significant, some of the common comorbidities, including pain-related comorbidities (osteoarthritis, chronic pain/fibromyalgia, rheumatologic diseases, sleep disorder, myocardial infarction/coronary artery disease, depression, and alcohol/drug abuse), were slightly higher in OER patients remaining in the study-MAPD. However, this should be interpreted in the context that patient counts for these comorbidities were low in the disenrolled group. Further research is needed to understand the level of illness and comorbidities among enrolled and disenrolled patients for the overall plan.
Lower disenrollment rates for OER users in 2012 (prerestriction year) and 2013 (restriction year) compared to the overall plan-level disenrollment rates indicate that the OER restriction did not lead a disproportionately higher portion of OER users to disenroll from the study-MAPD at the time of the formulary restriction. It is also worth noting that disenrollment rates at both the plan and OER user level in 2013 were much lower than those in 2012 and 2014. This might indicate a market or study-MAPD specific event, but the exact reason was unknown and beyond the scope of this study. In addition, the OER nonformulary restriction did not seem to have major impact on the use of OERs, which suggests that patients and prescribers were not proactively deterred from the medication by a nonformulary restriction. This study found that a majority of patients continued to fill at least 1 OER prescription following the nonformulary restriction, irrespective of whether they disenrolled from the study-MAPD. To account for potential transition OER fills, this study also conducted sensitivity analyses by requiring 2 OER claims in the postrestriction period for patients to be considered continued OER users. Taking into account any transition fill, it was observed that about half of the patients who disenrolled in 2013 had ≥ 2 OER claims compared to 45.1% of those who remained with the study-MAPD, indicating that many patients and prescribers were willing to take necessary steps to obtain a formulary exception for this treatment. Further analysis is needed to understand whether patients with a medical need for chronic pain control are less likely to change health plans, even in the face of an OER nonformulary restriction. It is interesting to note that the switch rate to an alternate LAO (as opposed to SAO switch or complete discontinuation of opioids) was higher in the group remaining in the study-MAPD (23.2%) compared with the disenrolled group (11.1%). This might suggest that many chronic OER users remaining in the plan switched to an alternate LAO due to a medical need for long-term pain relief. We also observed that the rate of complete discontinuation from all LAOs (including OERs) was lower in the group remaining (17.0%) versus those disenrolled (29.0%).
Migration patterns to alternate health plans following the nonformulary restriction were informative. While examining patients with OER claims after disenrollment from the study-MAPD, it was found that 32.2% had migrated to health plans with a less restrictively tiered formulary; however, 44.9% migrated to plans that also had a nonformulary restriction on OERs. The reason why these patients moved from one plan imposing a formulary restriction on OERs to another plan where OERs were not covered is intriguing. Further research is needed to evaluate if the OER restriction led some chronic OER users to seek plans with greater OER access, as this study was not designed to examine detailed reasons for patients switching health plans.
Similar to findings from earlier studies to the current study found that an OER nonformulary restriction did not appear to achieve any net cost savings.9,10 The perception that imposing formulary restrictions will drive high-cost patients away was not supported by the current study findings. During restriction year 2013, OER patients who remained with the study-MAPD actually had a higher unadjusted cost (mean 6-month total cost of $17,285 vs. $13,338) than the patients who disenrolled from the plan, though the difference ($658) in PPPM cost was not statistically significant (P = 0.180). This should also be interpreted in the context that only 60 disenrolled patients were available for this cost comparison, as the majority (94%) reenrolled in the study-MA-DP plan even after the restriction was imposed. After taking into account the cost changes between the remaining versus disenrolled OER patients in the prerestriction year (2012), patients who remained with the plan were observed to have $3,876 higher mean 6-month cost compared to those who left the study-MAPD using the difference-in-differences approach. Although this difference should be validated with a larger sample, this preliminary analysis indicates that an OER restriction would not lead to migration of patients with a higher disease burden away from a health plan imposing OER formulary restrictions.
Limitations
The results of this study should be interpreted in light of its limitations. Since not all of the study-MAPD OER patients were included in this analysis because of inclusion/exclusion criteria, results may only be interpreted in the context of the study-MAPD OER patients in this study sample and may not be generalized to all the study-MAPD patients or other health plans with similar OER restrictions.
This study analyzed the migration patterns, OER utilization, and cost of the disenrolled patients, and the results should be interpreted in the context of overall low disenrollment rates, typical of some Medicare plans. Additionally, this study used a difference-in-differences approach (by comparing the restriction year to nonrestriction years) in an attempt to estimate the net effect of the OER restriction on disenrollment rate and OER patient risk pool. However, study findings could still be confounded or biased by other unadjusted and/or unobservable factors such as the formulary status of other LAOs, plan design changes, and other market events that might affect the use of LAOs within and outside the study-MAPD.
Since rebates are not captured in claims databases, the net cost to the plan may not be reflected completely in the current analysis. Therefore, these analyses could not account for any rebate lost as a result of increased restriction. Information on hospital encounters was not available through the claims databases used and was not included in the current study, so actual costs might have been underestimated. Missing data are possible due to out-of-network enrollees, unrecorded observations, and similar artifacts.
Like any retrospective analysis, this study demonstrates associations but cannot infer causality. Charges for office visits were available in the medical claims database, which are not adjudicated costs, so might not provide accurate cost estimates, although these were applied uniformly across all groups. Finally, the 6-month follow-up period may not reflect the long-term impact of access restrictions on LAOs.
Conclusions
This analysis indicated that, compared with the overall study-MAPD, disproportionate migration with other plans of chronic OER users was not observed after an OER nonformulary restriction was implemented, and no evidence supported the assumption that imposing an OER nonformulary restriction would lead to more severe and/or more costly patients leaving the plan. The cost analysis demonstrated that an OER restriction did significantly reduce pharmacy/LAO prescriptions costs but not overall health care costs. This study also found that a majority of patients continued to use OERs after the nonformulary restriction, regardless of whether they remained in or disenrolled from the study-MAPD. Further analysis is warranted to evaluate the increases in pharmacy and medical costs and utilization before and after the OER restriction in nonopioid users to determine if market factors could have influenced results.
ACKNOWLEDGMENTS
The authors acknowledge Kainan Sun, who provided programming and advanced analytical assistance throughout the course of this study, and Kunal Saxena and Katharine Coyle, who provided literature review support on behalf of QuintilesIMS.
APPENDIX A. Study Schema
APPENDIX B. OER Utilization in the Post-index Period by Enrollment Status
| Prerestriction Year 2012 | Restriction Year 2013 | Postrestriction Year 2014 | |
|---|---|---|---|
| n (%) | n (%) | n (%) | |
| OER patients in study-MAPD, n | 2,248 | 2,935 | 2,222 |
| ≥ 1 claim (OER or LAO) in postperiod | |||
| Disenrolled | 289 | 162 | 318 |
| ≥ 1 OER claim postrestriction | 209 (72.3) | 97 (59.9) | 222 (69.8) |
| Switched to other LAO | 16 (5.5) | 18 (11.1) | 29 (9.1) |
| Discontinued all LAO | 64 (22.1) | 47 (29.0) | 67 (21.1) |
| Remained | 1,959 | 2,773 | 1,904 |
| ≥ 1 OER claim postrestriction | 1,576 (80.4) | 1,659 (59.8) | 1,473 (77.4) |
| Switched to other LAO | 86 (4.4) | 642 (23.2) | 183 (9.6) |
| Discontinued all LAO | 297 (15.2) | 472 (17.0) | 248 (13.0) |
| ≥ 2 claims (OER or LAO) in postperiod | |||
| Disenrolled | 289 | 162 | 318 |
| Continued with ≥ 2 OER claims | 182 (63.0) | 80 (49.4) | 182 (57.2) |
| Switched to other LAO (≥ 2 other LAO claims) | 11 (3.8) | 17 (10.5) | 23 (7.2) |
| Discontinued all LAOs | 64 (22.1) | 47 (29.0) | 67 (21.1) |
| Other (< 2 OER or LAO claims) | 32 (11.1) | 18 (11.1) | 46 (14.5) |
| Remained | 1,959 | 2,773 | 1,904 |
| ≥ 2 OER claims postrestriction | 1,455 (74.3) | 1,252 (45.1) | 1,264 (66.4) |
| Switched to other LAO | 69 (3.5) | 561 (20.2) | 156 (8.2) |
| Discontinued all LAO | 297 (15.2) | 472 (17.0) | 248 (13.0) |
| Other (< 2 OER or LAO claims) | 138 (7.0) | 488 (17.6) | 236 (12.4) |
LAO = long-acting opioid; MAPD = Medicare Advantage prescription drug plan; OER = oxycodone HCl extended-release.
REFERENCES
- 1.Academy of Managed Care Pharmacy. Common practice in formulary management systems. 2000. Available at: http://www.amcp.org/WorkArea/DownloadAsset.aspx?id=9274. Accessed May 15, 2017.
- 2.Shehab N, Lovegrove MC, Geller AI, et al. . U.S. emergency department visits for outpatient adverse drug events, 2013-2014. JAMA. 2016;316(20):2115-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Qureshi N, Wesolowicz LA, Liu CM, Lin AT. Effectiveness of a retrospective drug utilization review on potentially unsafe opioid and central nervous system combination therapy. J Manag Care Spec Pharm. 2015;21(10):938-44. Available at: http://www.jmcp.org/doi/10.18553/jmcp.2015.21.10.938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huskamp HA, Epstein AM, Blumenthal D. The impact of a national prescription drug formulary on prices, market share, and spending: lessons for Medicare? Health Aff (Millwood). 2003;22(3):149-58. [DOI] [PubMed] [Google Scholar]
- 5.Horn SD, Sharkey PD, Phillips-Harris C.. Formulary limitations and the elderly: results from the Managed Care Outcomes Project. Am J Manag Care. 1998;4:1105-13. [PubMed] [Google Scholar]
- 6.Huskamp HA, Deverka PA, Epstein AM, Epstein RS, Mcguigan KA, Frank RG.. The effect of incentive-based formularies on prescription-drug utilization and spending. N Engl J Med. 2003;349(23):2224-32. [DOI] [PubMed] [Google Scholar]
- 7.Soumerai SB, McLaughlin TJ, Ross-Degnan D, Casteris CS, Bollini P.. Effects of limiting Medicaid drug-reimbursement benefits on the use of psychotropic agents and acute mental health services by patients with schizophrenia. N Engl J Med. 1994;331(10):650-55. [DOI] [PubMed] [Google Scholar]
- 8.Soumerai SB. Benefits and risks of increasing restrictions on access to costly drugs in Medicaid. Health Aff (Millwood). 2004;23(1):135-46. [DOI] [PubMed] [Google Scholar]
- 9.Ben-Joseph R, Chen CC, De AP, Wade RL, Shah D.. Consequences of patient access restrictions to branded oxycodone hydrochloride extended-release tablets on healthcare utilization and costs in U.S. health plans. J Med Econ. 2014;17(10):708-18. [DOI] [PubMed] [Google Scholar]
- 10.Howard JC, Chen CC, De AP, Wehler E, Wade RL.. Impact of a prior authorization program on an extended release opioid market share and pharmacy costs: a comparison among two national commercial payers. J Manag Care Spec Pharm. 2016;22(4-a Suppl):S79 [Abstract G50]. Available at: http://www.jmcp.org/doi/pdf/10.18553/jmcp.2016.22.4.S1. [Google Scholar]
- 11.Cutler DM, Reber S.. Paying for health insurance: the tradeoff between competition and adverse selection. National Bureau of Economic Research. Working Paper No. 5796. October 1996. Available at: http://www.nber.org/papers/w5796.pdf. Accessed May 15, 2017.
- 12.Cutler D, Lincoln B, Zeckhauser R.. Selection stories: understanding movement across health plans. J Health Econ. 2010;29(6):821-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Riley G, Levy J, Montgomery M.. Adverse selection in the Medicare Prescription Drug Program. Health Aff (Millwood). 2009; 28(6)1826-37. [DOI] [PubMed] [Google Scholar]
- 14.Oxycontin (oxycodone hydrochloride) extended-release tablets, for oral use, CII. Purdue Pharma. Revised August 2015. Available at: http://www.accessdata.fda.gov/drugsatfda_docs/label/2015/022272s027lbl.pdf. Accessed May 15, 2017.
- 15.Ghali WA, Hall RE, Rosen AK, Ash AS, Moskowitz MA.. Searching for an improved clinical comorbidity index for use with ICD-9-CM administrative data. J Clin Epidemiol. 1996;49(3):273-78. [DOI] [PubMed] [Google Scholar]
- 16.Charlson ME, Pompei P, Ales KL, MacKenzie CR.. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-83. [DOI] [PubMed] [Google Scholar]