Abstract
BACKGROUND:
Although insulin is a well-established therapy that is associated with improved clinical outcomes, adherence and persistence with insulin regimens are poor in patients with type 2 diabetes mellitus (T2DM). Diabetes-related health care costs and the impact of insulin persistence patterns on these health care costs have been previously studied; however, these aspects of insulin therapy have limited data beyond the first year of use and have not been characterized among patients previously naive to basal insulin.
OBJECTIVES:
To (a) describe and compare medical- and pharmacy-related costs, health care resource utilization, and comorbidities and complications during the initial year and second (experienced) year of basal insulin therapy, and (b) describe and compare the impact of continuous versus interrupted basal insulin use during each year.
METHODS:
This was a retrospective observational database analysis using claims from multiple U.S. commercial health plans (Truven Health MarketScan) in previously insulin-naive patients with T2DM who were initiated on basal insulin. Data collected included all-cause and diabetes-related medical and pharmacy costs, health care resource utilization (i.e., number and type of outpatient visits, hospitalization, emergency department [ED] visits), medication use, and preselected comorbidities and complications. This cost analysis described and compared health care costs and resource use between the initial and experienced years and further compared health care costs and resource use between continuers and interrupters within each of those years.
RESULTS:
A total of 23,645 patients were included in the analysis; 12,224 were classified as continuers and 11,421 were classified as interrupters. Among all patients, mean increases from the initial year to the experienced year were observed for all-cause medical costs ($12,690-$13,408; P = 0.048), all-cause pharmacy costs ($6,253-$6,559; P < 0.001), and all-cause health care costs ($18,943-$19,967; P = 0.006), after adjusting for inflation. All-cause pharmacy costs were significantly higher for continuers versus interrupters, but total diabetes-related medical care costs, all-cause ED costs, and all-cause medical costs were significantly lower, resulting in similar all-cause health care costs between continuers and interrupters in both the initial and experienced years. Among all patients, diabetes-related inpatient visits and outpatient primary care physician (PCP) visits, total medical inpatient visits, and total medical outpatient PCP visits were significantly higher in the initial year than in the experienced year; however, there were fewer diabetes-related ED visits in the initial year.
CONCLUSIONS:
Initiation of basal insulin appears to be associated with increased health care costs, and treatment persistence pattern (continuers vs. interrupters) is further correlated with health care expenditures. Although associated with decreased pharmacy costs, interruption of therapy increases medical costs, underscoring the importance of addressing persistence to therapy.
What is already known about this subject
Data regarding the costs associated with the initiation of basal insulin beyond the first year of therapy in the United States are limited.
Data suggest that early discontinuation of insulin and subsequent restarting of insulin are associated with increased acute care costs.
What this study adds
This study provides a direct cost and health care resource utilization analysis associated with basal insulin initiation beyond the first year of therapy using a real-world U.S. commercial database.
The analysis further studies the impact of persistence patterns of basal insulin use on the direct cost of treatment and health care utilization.
Diabetes is associated with a substantial social, clinical, and economic burden. In 2012, the United States had an estimated 29.1 million people diagnosed with diabetes, or 9.3% of the population.1 The associated total cost of diabetes in 2012 was estimated to be $245 billion, including $176 billion in direct medical costs and $69 billion in reduced productivity.2 Thus, appropriate treatment and adherence strategies to mitigate the clinical complications and economic impact of the disease are of critical importance.
Initial treatment for new-onset type 2 diabetes mellitus (T2DM) typically includes the use of metformin along with healthy eating, weight control, increased physical activity, and diabetes education. For those not achieving target glycemic control, other oral antihyperglycemic drugs (OADs), glucagonlike peptide-1 (GLP-1) receptor agonists, or basal insulin are recommended.3,4 The 2015 American Diabetes Association standard of care guidelines state that first-line insulin therapy should be considered (with or without additional agents) in patients with newly diagnosed T2DM and markedly symptomatic and/or elevated blood glucose.5
Despite the well-documented efficacy of insulin, patients are often reluctant to initiate treatment. Barriers include fear of needles, fear of hypoglycemia, and the belief that insulin indicates end-stage disease.6 Because of such concerns, persistence with and adherence to insulin therapy are poor, with high rates of discontinuation and intermittent use.7-11 For instance, in a recent analysis of patients initiating basal insulin by Desai et al. (2015),10 the median time to the first treatment interruption was only 3.3 months.
Furthermore, data regarding the costs associated with insulin initiation are limited.9 Available data suggest that initiation of basal insulin is associated with increased costs12,13 but that early insulin initiation has the potential to produce cost savings over time by improving outcomes.14 Early discontinuation of insulin and subsequent restart is also associated with increased acute care costs.10,15,16 However, a U.S.-based economic analysis of direct costs and health care resource utilization associated with basal insulin initiation in the first and second years of therapy has not been conducted. Additionally, the impact of persistence patterns on the cost of treatment and health care utilization in a real-world commercial database has not been assessed.
The objectives for the current study were therefore to (a) describe and compare medical- and pharmacy-related costs, health care resource utilization, and comorbidities and complications during the initial and second (experienced) year of basal insulin therapy and (b) describe and compare the impact of continuous versus interrupted basal insulin use during each year. Costs were compared during the initial year and second (experienced) year of basal insulin therapy in previously insulin-naive patients to investigate the economic effect of continuous versus interrupted use of basal insulin during each year.
Methods
Study Design and Data Source
This was a retrospective observational database analysis covering the years 2009 through 2013. The study used the Truven Health MarketScan Commercial Claims and Encounters and Medicare Supplemental and Coordination of Benefits databases, which contain employer- and health plan-sourced data, including medical and pharmaceutical claims. These databases represent the health services of approximately 195 million employees, dependents, and retirees in the United States with primary or Medicare supplemental coverage through privately insured fee-for-service, such as preferred and exclusive provider organizations, point-of-service, indemnity plans, health maintenance organizations, consumer-directed health plans, or capitated health plans. The Truven Commercial and Medicare Supplemental databases are generally representative of the treated population in the United States. Medical claims are linked to outpatient prescription drug claims and person-level health plan enrollment information.
Patients
Inclusion Criteria.
The study included all patients diagnosed with T2DM, identified by 2 or more instances of International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) code 250.x0 and 250.x2 or 1 instance of ICD-9-CM code 250.xx plus 1 OAD prescription, with no prescription claims for any insulin in the 12-month pre-index period. The first observed pharmacy claim for basal insulin served as the index date for each patient. Continuous enrollment in the medical and pharmacy benefits for 12 months pre- and 24 months post-index was required. Patients were also required to have basal insulin analog prescription (insulin detemir or insulin glargine) claims for ≥ 2 years (i.e., having at least 1 claim in year 2 in addition to the index claim in year 1).
Exclusion Criteria.
Patients with diagnoses of gestational diabetes; diabetes mellitus complicating pregnancy, childbirth, or the puerperium; nonclinical diabetes; secondary diabetes; pregnancy; or neonatal diabetes mellitus during the pre-period were excluded from the study.
Analyzed Populations
Patients were analyzed for economic outcomes based on treatment duration (initial year vs. experienced year) and persistence patterns (continuers vs. interrupters). The initial year of insulin use was defined as year 1 and the experienced year as year 2. Continuers were defined as patients having no interruption in basal insulin therapy (i.e., no insulin prescription gap ≥ 60 days between the end of 1 and the beginning of another basal insulin prescription) during the post-index period. Interrupters were defined as patients having at least 1 basal insulin claim following the first > 60-day gap during the post-index period. Switching to a different basal insulin (either nonmixed or premixed) was not considered an interruption of basal insulin therapy. Sensitivity analyses were also conducted using insulin prescription gaps of > 30 and > 90 days. The study time period ran from January 1, 2009, to December 31, 2013. The index date identification period was between January 1, 2010, and December 31, 2011, with 24-month follow-up periods between January 1, 2010, and December 31, 2013.
Outcomes and Costs
Data were collected on baseline patient demographics and characteristics, including comorbidities and complications common to patients with T2DM17-21 (preselected based on our understanding of diabetes and diabetes-related claims research and identified using ICD-9-CM codes) and medication use during the 12 months before the index date. Health care resource utilization captured included the number and type of outpatient visits (primary care physician [PCP], specialist), the number and length of inpatient stays, and the number of emergency department (ED) visits. Data on direct costs included all-cause and diabetes-related medical costs that were associated with inpatient, outpatient, and ED visits, and pharmacy-related costs. Both all-cause and diabetes-related health care costs (i.e., health plan and patient out-of-pocket costs) were calculated during the pre-index and post-index periods.
Diabetes-related medical costs were defined as the paid amounts for all medical claims with either a primary or secondary diagnosis of diabetes (ICD-9-CM codes 250.x0 or 250.x2). All-cause medical costs were defined as health plan and patient-paid amounts for all medical services (including inpatient stays, ED visits, outpatient facility visits, physician office visits, and other services) during the relevant period. Diabetes-related pharmacy costs were defined as the sum of paid amounts for all diabetes medications, including insulin, OADs, GLP-1 receptor agonists, and pramlintide. Total diabetes-related health care costs were defined as the sum of diabetes-related pharmacy and medical costs. Total all-cause health care costs were defined as the sum of all-cause pharmacy and medical costs, including laboratory costs. Costs are reported in U.S. dollars (USD) as the actual cost in the year in which they were incurred and inflation-adjusted to 2013 USD.
Prescription fills were used to capture patterns of medication use. Patterns of use included the number of classes used, number of drugs used, and number of prescriptions filled for specific OADs, noninsulin injectable drugs, and insulins.
The primary objectives were to describe and compare the inpatient, outpatient, pharmacy, and total health care costs during the initial year and experienced year and to compare the inpatient, outpatient, pharmacy, and total health care costs between basal insulin continuers and interrupters during the initial year and experienced year. Secondary objectives were to compare medical resource utilization during patients’ initial year and the experienced year and to compare preselected comorbidities and complications for basal insulin continuers versus interrupters during the initial and experienced years.
Statistical Analyses
Patient characteristics and economic and clinical outcomes were described using means and standard deviations. Comparisons of patient demographics and clinical characteristics were conducted using chi-square test for categorical variables and Wilcoxon test for continuous variables. Comparisons of health care costs between the initial year and the experienced year were analyzed using a generalized linear regression model with log-link and gamma distribution of the error term. The dependent variable was cost, and the independent variables were patients’ baseline characteristics and baseline costs.
In the analysis comparing interrupters and continuers (but not in the analysis comparing the initial year with the experienced year), 1:1 greedy propensity score matching was used to adjust for bias between interrupters and continuers. For secondary endpoints, Poisson regression was used to compare the number and type of antihyperglycemic drug classes during patients’ initial year and experienced year. Poisson regression was used to assess the difference in the number and type of antihyperglycemic drug classes. All analyses were carried out using SAS software package 9.2 (SAS Institute, Cary, NC) with statistical significance set to P < 0.05.
Results
Patients
Between January 1, 2010, and December 31, 2011, a total of 393,162 patients were identified as having initiated basal insulin analog therapy. Of these, 26,888 satisfied the inclusion/exclusion criteria. Using an allowable gap of up to 60 days in prescription refills during the index period, 23,645 patients were included in the analysis, with 12,224 patients classified as continuers and 11,421 classified as interrupters (Table 1). The remaining 3,243 patients were considered to have discontinued treatment.
TABLE 1.
Patient Selection for Analyses
| Criteria | N |
|---|---|
| First observed pharmacy claim for basal insulin from January 1, 2010, to December 31, 2011 | 393,162 |
| At least two type 2 diabetes diagnoses from 12 months pre-index to 24 months post-index | 341,656 |
| No diagnoses of gestational, nonclinical, secondary, pregnancy, neonatal diabetes, or diabetes mellitus complicating pregnancy, childbirth, or the puerperium at any time point | 310,188 |
| No prescription claims for any insulins in the 12-month preperiod | 172,126 |
| Continuous enrollment in the medical and pharmacy benefits for 12 months pre- and 24 months post-index | 35,838 |
| Basal long-acting insulin prescription claims for at least 2 years (more than 1 basal insulin prescription claim, including the index prescription, each year) | 26,888 |
| Subgroup: basal insulin continuous user | 12,224 |
| Subgroup: basal insulin intermittent user | 11,421 |
Demographic characteristics of patients included in the analysis are summarized in Table 2. Most of the characteristics were fairly similar between groups, although continuers were slightly older and more likely to be male than were interrupters. There were slight regional differences, with interrupters more likely to be from the South than were continuers.
TABLE 2.
Patient Demographics
| Variable | Continuers (n = 12,224) | Interrupters (n = 11,421) | Total (N = 23,645) | P Valueb |
|---|---|---|---|---|
| Age,a mean (SD) years | 51.8 (9.0) | 50.1 (10.2) | 51.0 (9.7) | < 0.001 |
| Sex, male, n (%, SD) | 7,018 (57.4) | 6,200 (54.3) | 13,218 (55.9) | < 0.001 |
| U.S. region, n (%, SD) | ||||
| North central | 3,480 (28.5) | 2,904 (25.4) | 6,384 (27.0) | < 0.001 |
| Northeast | 1,380 (11.3) | 1,211 (10.6) | 2,591 (11.0) | |
| South | 4,857 (39.7) | 5,220 (45.7) | 10,077 (42.6) | |
| West | 1,829 (15.0) | 1,675 (14.7) | 3,504 (14.8) | |
| Unknown/missing | 678 (5.5) | 411 (3.6) | 1,089 (4.6) | |
| Index basal insulin, n (%, SD) | ||||
| Insulin detemir | 2,946 (24.1) | 2,881 (25.2) | 5,827 (24.6) | 0.045 |
| Insulin glargine | 9,278 (75.9) | 8,540 (74.8) | 17,818 (75.4) | |
| Mode of delivery,a n (%, SD) | ||||
| Pen | 9,045 (74.0) | 8,701 (76.2) | 17,746 (75.1) | 0.001 |
| Vial | 3,165 (25.9) | 2,708 (23.7) | 5,873 (24.8) | |
| Both | 14 (0.1) | 12 (0.1) | 26 (0.1) | |
aOn index date.
bContinuers versus interrupters; chi-square test for categorical variables and Wilcoxon test for continuous variables.
SD = standard deviation.
Economic Outcomes
Costs.
Figure 1 summarizes costs in the initial and experienced years for the total population. In general, there was an increase in costs over the 2 years, with significant differences between the initial and experienced years observed for some of the categories. Among all patients, all-cause medical costs ($12,690-$13,408; P = 0.048), all-cause pharmacy costs ($6,253-$6,559; P < 0.001), and all-cause health care costs ($18,943-$19,967; P = 0.006) significantly increased from the initial year to the experienced year. In contrast, pharmacy costs for OAD use decreased from the initial year to the experienced year ($846-$626; P < 0.001).
FIGURE 1.
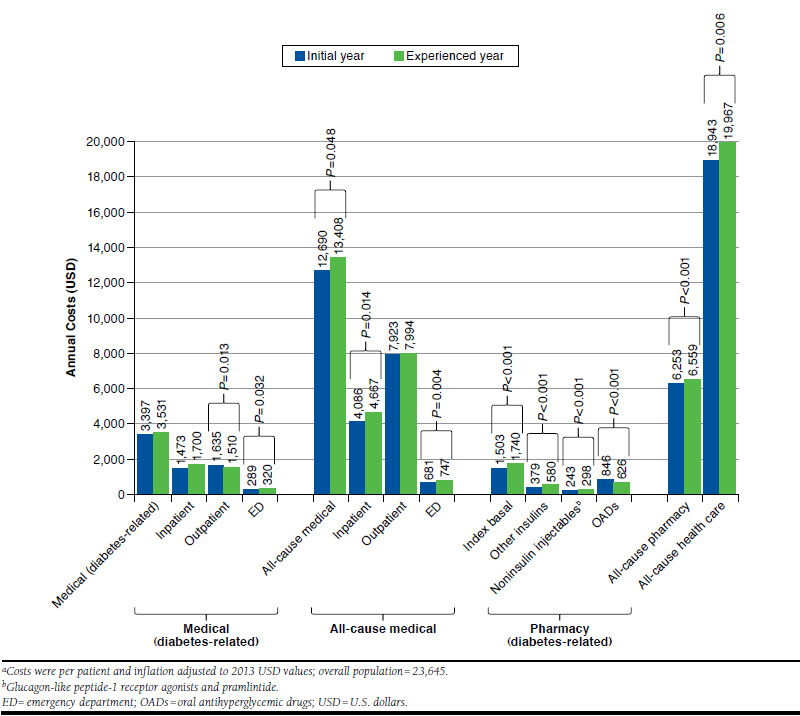
Annual Medical and Drug Costs by Treatment Period for the Overall Populationa
Results showed a similar pattern as above when patients were stratified by insulin persistence patterns (i.e., continuers vs. interrupters), with the experienced year generally associated with higher medical and pharmacy-related costs, except those of OADs, compared with the initial year. Results also showed similar trends when definitions for insulin prescription gaps of > 30 days and > 90 days were used. Among all patients in both the > 30-day and > 90-day analyses, there were nonsignificant increases in diabetes-related medical costs and all-cause medical costs, but there were also statistically significant increases in all-cause pharmacy costs and all-cause total health care costs from the initial year to the experienced year.
A propensity-matched analysis between interrupters and continuers demonstrated that all-cause total health care costs were similar for continuers versus interrupters in both the initial year ($19,333 vs. $18,585; P = 0.132) and the experienced year ($19,797 vs. $19,744; P = 0.917). Costs for continuers versus interrupters after propensity score matching are summarized for the initial year and the experienced year in Figure 2.
FIGURE 2.
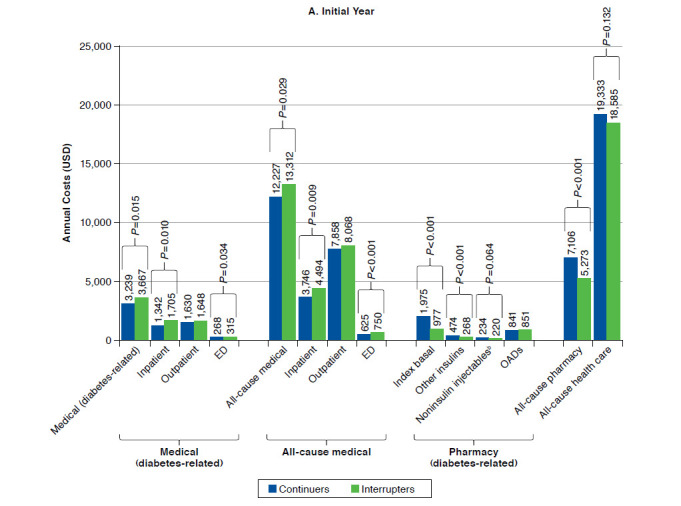
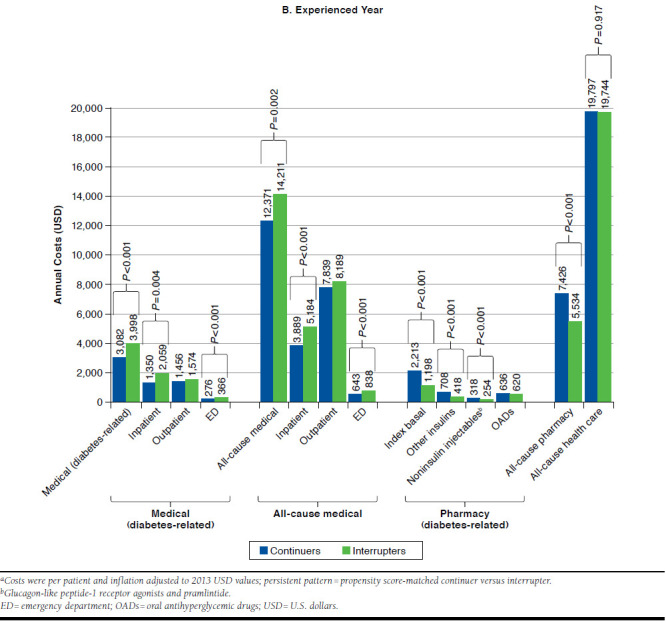
Annual Medical and Drug Costs by Persistence Pattern in the Initial and Experienced Yearsa
A total of 10,285 patients from each cohort were included in the cost analyses. However, significant differences were seen between continuers and interrupters when looking at the individual components of direct costs. All-cause pharmacy costs were significantly higher for continuers than for interrupters in both the initial and the experienced years ($7,106 vs. $5,273; P < 0.001 and $7,426 vs. $5,534; P < 0.001, respectively) as expected. Pharmacy costs were also higher for most antihyperglycemic drug categories among continuers than among interrupters in both periods. These included higher costs for basal insulin ($1,975 vs. $977 in the initial year; P < 0.001, and $2,213 vs. $1,198 in the experienced year; P < 0.001); other insulins ($474 vs. $268 in the initial year; P < 0.001, and $708 vs. $418 in the experienced year; P < 0.001); and noninsulin injectables ($234 vs. $220 in the initial year; P = 0.064, and $318 vs. $254 in the experienced year; P < 0.001).
However, there were significantly lower costs among continuers for total diabetes-related medical care ($3,239 vs. $3,667 in the initial year; P = 0.015, and $3,082 vs. $3,998 in the experienced year; P < 0.001); all-cause ED costs ($625 vs. $750 in the initial year; P < 0.001, and $643 vs. $838 in the experienced year; P < 0.001); and all-cause medical costs ($12,227 vs. $13,312 in the initial year; P = 0.029, and $12,371 vs. $14,211 in the experienced year; P = 0.002). Cost data are also summarized in Appendix A (available in online article).
Resource Utilization.
Medical resource utilization for the overall population is summarized in Table 3. Among all patients who used the relevant medical resource, there were significantly more diabetes-related inpatient visits (0.14 vs. 0.11; P < 0.001); diabetes-related outpatient PCP visits (3.07 vs. 2.53; P < 0.001); all-cause medical inpatient visits (0.35 vs. 0.29; P < 0.001); and all-cause medical outpatient PCP visits (7.29 vs. 6.54; P < 0.001) in the initial year than in the experienced year. However, there were fewer diabetes-related ED visits (0.28 vs. 0.32; P = 0.010) in the initial year than in the experienced year.
TABLE 3.
Medical Resource Utilization (Per Patient Using the Relevant Medical Resource) by Treatment Period for the Total Population and for Each Persistence Pattern Subgroupa
| Category | Total Population N = 23,645 | Continuers n = 12,224 | Interrupters n = 11,421 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Initial Year, Mean (SD) | Experienced Year, Mean (SD) | P Valueb | Initial Year, Mean (SD) | Experienced Year, Mean (SD) | P Valueb | Initial Year, Mean (SD) | Experienced Year, Mean (SD) | P Valueb | |
| Medical (diabetes-related) | |||||||||
| Emergency department, visits | 0.28 (1.019) | 0.32 (1.087) | 0.010 | 0.26 (0.911) | 0.28 (0.974) | 0.490 | 0.32 (1.123) | 0.36 (1.194) | 0.004 |
| Inpatient | |||||||||
| Inpatient visits | 0.14 (0.470) | 0.11 (0.426) | < 0.001 | 0.12 (0.435) | 0.10 (0.387) | < 0.001 | 0.15 (0.506) | 0.13 (0.464) | < 0.001 |
| Inpatient stays, days | 0.47 (2.646) | 0.51 (3.300) | 0.137 | 0.38 (2.296) | 0.42 (2.572) | 0.037 | 0.55 (2.973) | 0.60 (3.931) | 0.934 |
| Outpatient | |||||||||
| Primary care physician | 3.07 (3.107) | 2.53 (2.867) | < 0.001 | 3.24 (3.152) | 2.59 (2.913) | < 0.001 | 2.88 (3.047) | 2.47 (2.814) | < 0.001 |
| Endocrinologist | 0.55 (1.463) | 0.49 (1.285) | 0.526 | 0.57 (1.497) | 0.51 (1.311) | 0.807 | 0.54 (1.426) | 0.48 (1.256) | 0.507 |
| All-cause medical | |||||||||
| Emergency department, visits | 0.83 (2.217) | 0.90 (2.459) | 0.097 | 0.75 (1.930) | 0.78 (2.028) | 0.983 | 0.92 (2.484) | 1.02 (2.845) | 0.021 |
| Inpatient | |||||||||
| Inpatient visits | 0.35 (1.039) | 0.29 (0.964) | < 0.001 | 0.31 (0.957) | 0.26 (0.875) | < 0.001 | 0.39 (1.119) | 0.32 (1.049) | < 0.001 |
| Inpatient stays, days | 0.79 (4.078) | 0.87 (4.882) | 0.924 | 0.65 (3.481) | 0.73 (3.886) | 0.531 | 0.94 (4.627) | 1.02 (5.757) | 0.641 |
| Outpatient | |||||||||
| Primary care physician | 7.29 (7.776) | 6.54 (7.438) | < 0.001 | 7.62 (7.930) | 6.71 (7.741) | < 0.001 | 6.94 (7.593) | 6.35 (7.095) | < 0.001 |
| Endocrinologist | 0.70 (1.779) | 0.63 (1.579) | 0.722 | 0.70 (1.785) | 0.63 (1.579) | 0.922 | 0.70 (1.772) | 0.63 (1.579) | 0.541 |
aAll-cause medical resources, except primary care physician visits (used by 88.9%-92.7% of patients) and emergency department visits (used by 23.0%-31.2% of patients), were used by 11.2%-19.9% of patients across all groups and all time periods.
bComparison between initial year (year 1) and experienced year (year 2) of basal insulin use; Wilcoxon test.
SD = standard deviation.
Results for interrupters and continuers were generally similar to those of the overall population, although continuers also spent more days in inpatient stays in the initial year versus the experienced year and had no difference in diabetes-related ED visits in the 2 time periods. Interrupters had fewer total medical ED visits during the initial year than during the experienced year.
There were differences in medication use between the initial and experienced years among both continuers and interrupters. Overall, mean use of OADs was significantly higher in the initial year than in the experienced year among both continuers (1.3 vs. 1.1; P < 0.001) and interrupters (1.3 vs. 1.1; P < 0.001).
There was also significantly greater use of almost all OAD drug classes in the initial year than in the experienced year among both continuers and interrupters. Among continuers there was higher use of sulfonylureas (34.6% vs. 28.6%; P < 0.001); metformin (51.5% vs. 46.4%; P < 0.001); thiazolidinediones (13.5% vs. 7.0%; P < 0.001); dipeptidyl peptidase-4 (DPP-4) inhibitors (15.7% vs. 13.0%; P < 0.001); fixed-dose OAD combinations (13.8% vs. 11.5%; P < 0.001); and meglitinide analogs (2.4% vs. 1.9%; P = 0.017) in the initial year compared with the experienced year.
Similarly, among interrupters, there was higher use of sulfonylureas (37.0% vs. 31.2%; P < 0.001); metformin (49.1% vs. 43.5%; P < 0.001); thiazolidinediones (15.2% vs. 7.5%; P < 0.001); DPP-4 inhibitors (15.2% vs. 12.8%; P < 0.001); fixed-dose OAD combinations (15.6% vs. 12.7%; P < 0.001); and meglitinide analogs (2.2% vs. 1.9%; P = 0.045) in the initial year compared with the experienced year. In addition, GLP-1 receptor agonists in overall and continuer groups, and insulins in all groups were used by a higher proportion of patients in the experienced year than in the initial year.
Comorbidities and Complications.
Data relating to comorbidities and complications common in patients with T2DM were collected for continuers and interrupters (Appendix B, available in online article). In the baseline period, important comorbidities and complications for the whole patient population included hypertension (56.2%), neuropathy (10.8%), nephropathy (9.5%), obesity (8.8%), hypoglycemia (4.0%), and bariatric surgery (2.2%). Some of these were more common at baseline among the continuers than among interrupters (hypertension: 58.0% vs. 54.3%, P < 0.001; obesity: 9.2% vs. 8.4%, P = 0.018), while others appeared less common among continuers (hypoglycemia: 3.6% vs. 4.3%, P = 0.009; bariatric surgery: 2.0% vs. 2.3%, P = 0.071).
The prevalence of all of these comorbidities and complications increased from the baseline period to the initial year of the study, although the patterns of differences between continuers and interrupters remained. In particular, hypertension (60.6% vs. 56.9%; P < 0.001) and obesity (11.4% vs. 10.1%; P = 0.001) remained higher among continuers than among interrupters during the initial year, while hypoglycemia (5.3% vs. 6.2%; P = 0.003) and bariatric surgery (2.6% vs. 3.2%; P = 0.004) remained lower among continuers.
Discussion
Initiation of basal insulin is associated with overall increased health care costs between the first and second years of therapy. Pharmacy-related costs of most antihyperglycemic agents increased during this time period, with OADs being a notable exception. However, medical resource utilization generally significantly decreased, particularly diabetes-related inpatient and outpatient visits and all-cause medical inpatient and outpatient visits, from the initial year to the experienced year. Conversely, there were fewer diabetes-related ED visits in the initial year than in the experienced year, possibly related to the greater use of insulin (both basal and other insulins) in the experienced year. Longer-term analyses evaluating more than 2 years of treatment with insulin could provide a more indepth understanding of the impact of continued treatment on medical and pharmacy costs.
A further breakdown of the data into persistence patterns also showed a difference in economic implications. As expected, continuous basal insulin therapy was associated with higher all-cause pharmacy-related costs but lower diabetes-related medical costs in both the initial and the experienced years, when compared with interrupted basal insulin therapy. However, the all-cause total health care costs were similar for continuers and interrupters in both periods. This finding suggests that while overall costs are similar despite persistence, the cost categories to which altered (increased or decreased) expenditure is attributed are affected by persistence.
Given that this study was only conducted over a 2-year period after insulin initiation, the authors suspect that a greater impact of persistence on diabetes-related costs might be observed with longer-term data. Owing to the lack of patientor physician-level data to directly confirm persistence in this analysis, future analyses over longer periods of time should be considered to evaluate whether the expected clinical benefits (i.e., better glycemic control, decreased diabetes-related complications) associated with continuous therapy produce economic benefits.
Costs are a reflection of resource utilization, so it is also important to consider the patterns of resource utilization we identified. The change in antihyperglycemic drug use and comorbidities/complications over the course of the study could be explained by clinical practice patterns in diabetes. As observed in this analysis, once basal insulin was initiated, the proportion of patients using most OADs decreased from year 1 to year 2. This was expected, as some oral agents may have been discontinued at initiation of insulin. However, market availability likely affected the usage pattern of the sodiumglucose cotransporter-2 class of medications, which did not follow the pattern of other OADs, reflecting that approval of these agents occurred during that time period.
In contrast to OADs, there was an increase in use of injectable antihyperglycemic agents during the study. Rapid-acting insulin, premixed insulin, and GLP-1 receptor agonist use significantly increased from the initial year to the experienced year. This increase in medication use likely reflects disease progression, which typically requires the use of more injectable agents, and the increased availability of the GLP-1 receptor agonists on the market.
With regard to comorbidities and complications experienced by patients with T2DM, there were some differences between continuers and interrupters during both years of the study. These differences may have at least partially contributed to why patients interrupted or continued treatment. For example, in the pre-period, obesity was significantly higher in continuers than among interrupters, and in the initial year this difference was reversed with a significantly higher rate of bariatric surgery among interrupters than continuers. While hypoglycemia was seen at a higher rate among interrupters in the initial year of insulin use, this group also had higher rates of hypoglycemia present at baseline. The authors hypothesize that this propensity toward hypoglycemia before insulin use may have influenced treatment interruption during the insulin use years.
We found that persistence affected important health care cost categories. In particular, interrupters had significantly higher costs for total diabetes-related medical care and all-cause medical costs. Our study was not designed to identify the reasons for these cost differentials, but we speculate that interrupters may be at increased risk of diabetes-related complications, which could increase these costs. Additional research into clinical outcomes may shed greater insight on this issue.
Nevertheless, it is likely that improved persistence might help improve both patient outcomes and reduce the health care costs identified here. Several publications have described strategies designed to increase persistence and adherence, some of which include improving communication (through motivational interviewing, shared decision making, and patient activation [equipping patients with the skills and confidence to become actively engaged in their health care]); educating patients with respect to the importance of continuing with prescribed treatment and real (vs. perceived) potential for adverse events; simplifying regimens and managing dosing issues; addressing costs; negotiating priorities; and actively monitoring treatment adherence.22-25
Considering the potential negative economic impact of nonadherence or nonpersistence to all stakeholders, the patient, provider, and payer must work in collaboration,24,26 as interventions led by a single practitioner (e.g., nurse or pharmacist) often are associated with limited results.24 Overall, patient-centered, interdisciplinary collaboration is essential to establish, implement, and provide ongoing assessment of a treatment plan that includes strategies for adherence to medication regimens.24
Limitations
Limitations of the study include the nonprospective, noninterventional study design and potential issues of reliability, completeness, and accuracy of claims data that could have been sources of bias. Additionally, unknown and residual confounding could have added bias to the results. Patients or providers were not directly contacted to assess perceived or true treatment persistence; therefore, the conclusions are interpreted from prescription fills within the claims database.
The study was also limited to the use of insulin glargine and insulin detemir (i.e., not neutral protamine Hagedorn insulin), which had the potential to affect persistence pattern behavior given the cost differentiation of human and analog insulins. The study did not compare glycemic control or incidence of adverse events across years or by persistence pattern, which could have further informed treatment patterns and the long-term implications of antihyperglycemic medication use.
Since information about diagnosis or severity of illness was not captured, the relationship between clinical measures such as glycemic control and basal insulin persistence in this study population remains unknown. The effect of differences in comorbidities and complications between groups may also have affected both adherence and the costs we evaluated.
Furthermore, since the study only included patients who were enrolled in commercial plans, the results are applicable only to those patient populations. The impact of plan coverage and socioeconomic factors tied to plan coverage could have been missed due to this inclusion. The use of a claims database also precludes the inclusion of patient-specific factors that could be attributed to interruption of therapy (e.g., switch in insurance plans, changes in plan structure, use of samples, use of insulin beyond coded days supply, or personal preference) or physician-mediated interruption in treatment for clinical efficacy or safety reasons.
In addition, we did not assess the proportions of patients who received insulin from mail order versus retail pharmacies. This could have affected outcomes, since there have been assertions that patients using mail order may have better adherence because their insulin is provided via a schedule of automated shipments.
Conclusions
This study demonstrates increased medical and pharmacy costs associated with the initiation of basal insulin over a 2-year period and higher medical costs associated with interruption of basal insulin therapy. Understanding the reasons behind different patterns of insulin use and associated long-term clinical and economic implications will allow the development and implementation of tools and strategies to improve adherence and persistence with treatment and improve overall treatment outcomes and, at the same time, help reduce medical costs.
Suggestions for future research include a longer in-depth clinical outcome and cost analysis to investigate economic benefits of continuous therapy compared with interrupted use over time. Investigating the patient and provider reasons behind the different persistence patterns would also highlight the potential for an unmet need and uncover further strategies to implement in assisting all stakeholders to improve persistence over time. In practice settings, however, achievement of persistence and clinical outcome goals would also require a multidisciplinary health care team with efforts from payers, providers, and patients alike.
ACKNOWLEDGMENTS
The authors acknowledge Bret Fulton and Caroline Spencer (Rx Communications, Mold, UK) for medical writing assistance funded by Eli Lilly and Company.
APPENDIX A. Annual Medical and Drug Costs by Treatment Period for the Overall Population and by Persistence Pattern During Each Study Perioda
| Category | Overall Population (N = 23,645) | Initial Year | Experienced Year | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Initial Year | Experienced Year | P Valueb | Continuers (n = 10,285) | Interrupters (n = 10,285) | P Value | Continuers (n = 10,285) | Interrupters (n = 10,285) | P Value | |
| Medical (diabetes-related) | 3,397 (12,654) | 3,531 (19,111) | 0.361 | 3,239 (10,955) | 3,667 (14,845) | 0.015 | 3,082 (9,801) | 3,998 (26,505) | < 0.001 |
| Inpatient | 1,473 (9,997) | 1,700 (16,943) | 0.072 | 1,342 (8,429) | 1,705 (12,034) | 0.010 | 1,350 (7,805) | 2,059 (23,879) | 0.004 |
| Outpatient | 1,635 (5,776) | 1,510 (5,329) | 0.013 | 1,630 (5,995) | 1,648 (5,609) | 0.802 | 1,456 (4,666) | 1,574 (6,092) | 0.115 |
| Emergency department | 289 (1,603) | 320 (1,627) | 0.032 | 268 (1,423) | 315 (1,729) | 0.034 | 276 (1,389) | 366 (1,849) | < 0.001 |
| All-cause medical | 12,690 (36,350) | 13,408 (44,583) | 0.048 | 12,227 (34,037) | 13,312 (39,004) | 0.029 | 12,371 (34,208) | 14,211 (51,669) | 0.002 |
| Inpatient | 4,086 (21,210) | 4,667 (29,852) | 0.014 | 3,746 (19,229) | 4,494 (22,688) | 0.009 | 3,889 (19,101) | 5,184 (35,280) | < 0.001 |
| Outpatient | 7,923 (24,684) | 7,994 (26,463) | 0.754 | 7,856 (23,950) | 8,068 (26,193) | 0.550 | 7,839 (23,726) | 8,189 (29,370) | 0.351 |
| Emergency department | 681 (2,512) | 747 (2,628) | 0.004 | 625 (2,181) | 750 (2,837) | < 0.001 | 643 (2,188) | 838 (2,950) | < 0.001 |
| Pharmacy (diabetes-related) | |||||||||
| Index basal insulin | 1,503 (1,059) | 1,740 (1,423) | < 0.001 | 1,975 (1,127) | 977 (617) | < 0.001 | 2,213 (1,546) | 1,198 (987) | < 0.001 |
| Other insulins | 379 (835) | 580 (1,186) | < 0.001 | 474 (977) | 268 (622) | < 0.001 | 708 (1,352) | 418 (886) | < 0.001 |
| Noninsulin injectablesc | 243 (824) | 298 (977) | < 0.001 | 234 (825) | 220 (753) | 0.064 | 318 (1,026) | 254 (868) | < 0.001 |
| OADs | 846 (1,259) | 626 (1,102) | < 0.001 | 841 (1,250) | 851 (1,265) | 0.348 | 636 (1,119) | 620 (1,088) | 0.409 |
| All-cause pharmacyb | 6,253 (6,164) | 6,559 (7,103) | < 0.001 | 7,106 (6,284) | 5,273 (5,889) | < 0.001 | 7,426 (7,245) | 5,534 (6,782) | < 0.001 |
| All-cause total health care | 18,943 (37,661) | 19,967 (45,913) | 0.006 | 19,333 (35,532) | 18,585 (40,231) | 0.132 | 19,797 (36,079) | 19,744 (52,773) | 0.917 |
Note: All data presented as mean (SD).
aCosts were per patient and inflation adjusted to 2013 USD values; persistence pattern = score-matched continuer versus interrupter.
bComparison between initial year (year 1) and experienced year (year 2) of basal insulin use.
cGlucagon-like peptide-1 receptor agonists and pramlintide.
OADs = oral antihyperglycemic drugs; SD = standard deviation; USD = U.S. dollars.
APPENDIX B. Selected Common Comorbidities and Complications for Each Adherence Pattern Subgroup
| Category | Continuers (n = 12,224) % | Interrupters (n = 11,421) % | P Valuea |
|---|---|---|---|
| Prior period | |||
| Cardiovascular disease | 16.9 | 16.1 | 0.106 |
| Cerebrovascular disease | 4.1 | 4.5 | 0.127 |
| Nephropathy/CKD | 9.7 | 9.3 | 0.278 |
| Neuropathy | 10.7 | 10.8 | 0.715 |
| Retinopathy | 5.3 | 5.1 | 0.574 |
| Obesity | 9.2 | 8.4 | 0.018 |
| Bariatric surgery | 2.0 | 2.3 | 0.071 |
| Lower-extremity amputation | 0.2 | 0.3 | 0.249 |
| Depression | 7.0 | 6.8 | 0.416 |
| Hypoglycemia | 3.6 | 4.3 | 0.009 |
| Cognitive disorders/dementia | 3.1 | 3.5 | 0.077 |
| Hypertension | 58.0 | 54.3 | < 0.001 |
| Initial year | |||
| Cardiovascular disease | 17.7 | 17.7 | 0.997 |
| Cerebrovascular disease | 4.6 | 4.7 | 0.632 |
| Nephropathy/CKD | 11.2 | 11.7 | 0.199 |
| Neuropathy | 15.3 | 14.7 | 0.181 |
| Retinopathy | 6.5 | 6.9 | 0.278 |
| Obesity | 11.4 | 10.1 | < 0.001 |
| Bariatric surgery | 2.6 | 3.2 | 0.004 |
| Lower-extremity amputation | 0.4 | 0.6 | 0.018 |
| Depression | 7.5 | 7.4 | 0.759 |
| Hypoglycemia | 5.3 | 6.2 | 0.003 |
| Cognitive disorders/dementia | 3.0 | 3.4 | 0.098 |
| Hypertension | 60.6 | 56.9 | < 0.001 |
| Experienced year | |||
| Cardiovascular disease | 18.1 | 18.1 | 0.995 |
| Cerebrovascular disease | 4.7 | 5.2 | 0.107 |
| Nephropathy/CKD | 11.5 | 12.5 | 0.022 |
| Neuropathy | 16.7 | 15.5 | 0.012 |
| Retinopathy | 7.5 | 8.0 | 0.144 |
| Obesity | 14.0 | 11.3 | < 0.001 |
| Bariatric surgery | 3.4 | 3.6 | 0.336 |
| Lower-extremity amputation | 0.3 | 0.4 | 0.107 |
| Depression | 8.2 | 7.9 | 0.375 |
| Hypoglycemia | 5.5 | 6.1 | 0.068 |
| Cognitive disorders/dementia | 3.3 | 4.1 | 0.001 |
| Hypertension | 63.1 | 60.2 | < 0.001 |
aChi-square test.
CKD = chronic kidney disease.
REFERENCES
- 1.American Diabetes Association.. Statistics about diabetes. 2014. Available at: http://www.diabetes.org/diabetes-basics/statistics/?loc=db-slabnav. Accessed January 30, 2017.
- 2.American Diabetes Association.. Economic costs of diabetes in the U.S. in 2012. Diabetes Care. 2013;36(4):1033-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Inzucchi SE, Bergenstal RM, Buse JB, et al.. Management of hypergly-cemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2015;38(1):140-49. [DOI] [PubMed] [Google Scholar]
- 4.Wright A, Burden AC, Paisey RB, Cull CA, Holman RR;. U.K. Prospective Diabetes Study Group. Sulfonylurea inadequacy: efficacy of addition of insulin over 6 years in patients with type 2 diabetes in the U.K. Prospective Diabetes Study (UKPDS 57). Diabetes Care. 2002;25(2):330-36. [DOI] [PubMed] [Google Scholar]
- 5.American Diabetes Association.. 7. Approaches to glycemic treatment. Diabetes Care. 2015;38(Suppl 1):S41-48. [DOI] [PubMed] [Google Scholar]
- 6.Ng CJ, Lai PS, Lee YK, Azmi SA, Teo CH.. Barriers and facilitators to starting insulin in patients with type 2 diabetes: a systematic review. Int J Clin Pract. 2015;69(10):1050-70. [DOI] [PubMed] [Google Scholar]
- 7.Baser O, Tangirala K, Wei W, Xie L.. Real-world outcomes of initiating insulin glargine-based treatment versus premixed analog insulins among U.S. patients with type 2 diabetes failing oral antidiabetic drugs. Clinicoecon Outcomes Res. 2013;5:497-505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bonafede MM, Kalsekar A, Pawaskar M, et al.. A retrospective database analysis of insulin use patterns in insulin-naïve patients with type 2 diabetes initiating basal insulin or mixtures. Patient Prefer Adherence. 2010;4:147-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ascher-Svanum H, Lage MJ, Perez-Nieves M, et al.. Early discontinuation and restart of insulin in the treatment of type 2 diabetes mellitus. Diabetes Ther. 2014;5(1):225-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Desai U, Kabul S, Ivanova J, et al.. Factors associated with basal insulin persistence after initiation among people with type 2 diabetes mellitus (T2DM). Diabetes. 2015;64(Suppl 1):A223 [Abstract 884-P]. Available at: http://diabetes.diabetesjournals.org/content/64/Supplement_1/A187.full-text.pdf. Accessed February 8, 2017. [Google Scholar]
- 11.Cooke CE, Lee HY, Tong TP, Haines ST.. Persistence with injectable anti-diabetic agents in members with type 2 diabetes in a commercial managed care organization. Curr Med Res Opin. 2010;26(1):231-38. [DOI] [PubMed] [Google Scholar]
- 12.Idris I, Gordon J, Tilling C, Vora J.. A cost comparison of long-acting insulin analogs vs NPH insulin-based treatment in patients with type 2 diabetes using routinely collected primary care data from the UK. J Med Econ. 2015;18(4):273-82. [DOI] [PubMed] [Google Scholar]
- 13.Jones S, Castell C, Goday A, et al.. Increase in direct diabetes-related costs and resource use in the 6 months following initiation of insulin in patients with type 2 diabetes in five European countries: data from the INSTIGATE study. Clinicoecon Outcomes Res. 2012;4:383-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smolen HJ, Murphy DR, Gahn JC, Yu X, Curtis BH.. The evaluation of clinical and cost outcomes associated with earlier initiation of insulin in patients with type 2 diabetes mellitus. J Manag Care Spec Pharm. 2014;20(9):968-84. Available at: http://www.jmcp.org/doi/10.18553/jmcp.2014.20.9.968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anderten H, Dippel FW, Kostev K.. Early discontinuation and related treatment costs after initiation of basal insulin in type 2 diabetes patients: a German primary care database analysis. J Diabetes Sci Technol. 2015;9(3):644-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Perez-Nieves M, Kabul S, Desai U, et al.. Basal insulin persistence, associated factors, and outcomes after treatment initiation among people with type 2 diabetes mellitus in the U.S. Curr Med Res Opin. 2016;32(4):669-80. [DOI] [PubMed] [Google Scholar]
- 17.Pantalone KM, Hobbs TM, Wells BJ, et al.. Clinical characteristics, complications, comorbidities and treatment patterns among patients with type 2 diabetes mellitus in a large integrated health system. BMJ Open Diabetes Res Care. 2015;3(1):e000093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clarke PM, Gray AM, Briggs A, et al.. A model to estimate the lifetime health outcomes of patients with type 2 diabetes: the United Kingdom Prospective Diabetes Study (UKPDS) Outcomes Model (UKPDS no. 68). Diabetologia. 2004;47(10):1747-59. [DOI] [PubMed] [Google Scholar]
- 19.Reijmer YD, Van den Berg E, Ruis C, Kappelle LJ, Biessels GJ.. Cognitive dysfunction in patients with type 2 diabetes. Diabetes Metab Res Rev. 2010;26:507-19. [DOI] [PubMed] [Google Scholar]
- 20.Ali S, Stone MA, Peters JL, Davies MJ, Khunti K.. The prevalence of co-morbid depression in adults with Type 2 diabetes: a systematic review and meta-analysis. Diabet Med. 2006;23(11):1165-73. [DOI] [PubMed] [Google Scholar]
- 21.Dixon JB, Zimmet P, Alberti KG, Rubino F;. International Diabetes Federation Taskforce on Epidemiology and Prevention. Bariatric surgery: an IDF statement for obese Type 2 diabetes. Diabet Med. 2011;28(6):628-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bailey CJ, Kodack M.. Patient adherence to medication requirements for therapy of type 2 diabetes. Int J Clin Pract. 2011;65(3):314-22. [DOI] [PubMed] [Google Scholar]
- 23.Delamater AM. Improving patient adherence. Clin Diabetes. 2006;24(2):71-77. [Google Scholar]
- 24.Vermeire E, Wens J, Van Royen P, Biot Y, Hearnshaw H, Lindenmeyer A.. Interventions for improving adherence to treatment recommendations in people with type 2 diabetes mellitus. Cochrane Database Syst Rev. 2005;(2):CD003638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lerman I. Adherence to treatment: the key for avoiding long-term complications of diabetes. Arch Med Res. 2005;36(3):300-06. [DOI] [PubMed] [Google Scholar]
- 26.Jha AK, Aubert RE, Yao J, Teagarden JR, Epstein RS.. Greater adherence to diabetes drugs is linked to less hospital use and could save nearly $5 billion annually. Health Aff (Millwood). 2012;31(8):1836-46. [DOI] [PubMed] [Google Scholar]


