Abstract
BACKGROUND:
U.S. specialty drug spend is expected to reach $400 billion by 2020, with significant growth in oncology. New oral oncology approvals have allowed for more convenient outpatient administration compared with physician-administered chemotherapies; however, patients may encounter challenges with adherence when taking medications at home. Emerging medication adherence technology (MAT) attempts to provide at-home adherence support, and while one such technology, smart pill bottles (SPB), claims to improve medication adherence, few studies have formally assessed their effects.
OBJECTIVES:
To assess the effect of an SPB with pharmacist intervention on medication adherence in adult patients with multiple myeloma (MM) new to lenalidomide therapy (≤ 5 cycle dispenses). Secondary objectives were to evaluate treatment cycles completed, evaluate the significance of real-time pharmacist engagement (intervention group only), determine the incremental cost-effectiveness ratio (ICER), and evaluate patient satisfaction and likelihood to use an SPB.
METHODS:
This prospective, random assignment, single-site, and single-blinded study recruited 40 adult patients diagnosed with MM new to lenalidomide at a specialty pharmacy. Recruitment was completed January-February 2016, and the length of study was 6 months. Participants were randomized 1:1 between the intervention and control groups. The intervention group received lenalidomide in activated SPBs with light, chimes, text message reminders, and pharmacist follow-up if weekly SPB adherence rates dropped below 80%. The control group received lenalidomide in identical SPBs with all alerts deactivated. SBPs contained cellular capabilities, enabling around-the-clock data transmission and captured data upon bottle-uncapping events. Patient adherence was calculated by dividing the number of bottle-uncapping events by the total number of doses supplied for each dosing cycle. Lenalidomide cycles completed and pharmacist outreach to the same patient were counted to determine pharmacist intervention. The ICER was calculated to determine SPB cost-effectiveness, and a Likert scale survey was given to the intervention group to evaluate patient satisfaction with the full-service SPB.
RESULTS:
Sixteen participants in each arm completed the study; 4 patients in each arm were lost to follow-up. Median adherence was improved for the intervention group compared with the control group (median = 100% vs. 87.4%; P = 0.001). The ICER per patient percentage adherence increase was found to be $96.03. Sixty percent of patients in the intervention group who responded to the post-satisfaction survey rated the full SPB service very positively.
CONCLUSIONS:
In this study, SPB interventions were associated with increased medication adherence and patient satisfaction. This pilot also provides empirical data on the cost-effectiveness of adherence technology used in a specialty pharmacy oncology setting.
What is already known about this subject
Oral oncology medications provide ease of administration, but many are still associated with a wide range of adherence, from 46%-100%.
Recent literature has shown conflicting results regarding whether text message programs and other technologies increase medication adherence in chronic disease states.
What this study adds
The smart pill bottle (SPB) pilot program effectively increased adherence in multiple myeloma patients new to oral lenalidomide treatment in a real-world specialty pharmacy setting.
At an estimated cost of $1,210 per patient, this program yielded an ICER of $96.03 per percentage adherence gained.
Sixty percent of intervention program participants who were surveyed rated the full SPB program very positively.
Multiple myeloma (MM) is a hematologic malignancy that originates in bone marrow and affects immunoglobulin-producing B lymphocytes.1 According to the National Cancer Institute’s Surveillance, Epidemiology, and End Results Program, MM is a relatively uncommon cancer, with 30,770 new cases and 12,770 MM-related deaths estimated for 2018.2
Treatment of MM may include surgery, radiation therapy, stem cell transplant, chemotherapy, and targeted therapy.3 Drug choices, including bortezomib, carfilzomib, lenalidomide, and others, often depend on a patient’s MM staging, candidacy for a stem cell transplant, and treatment history.3 Lenalidomide is an oral antineoplastic agent that has U.S. Food and Drug Administration (FDA) approval for several cancers, including MM in combination with dexamethasone and MM maintenance therapy after stem cell transplant.4 The goal of therapy in these indications is for patients to take lenalidomide until disease progression or unacceptable toxicity occurs; landmark trials have shown average treatment durations of 38-50 months.5,6 An FDA-mandated Risk Evaluation and Mitigation Strategy (REMS) program enrolls patients, prescribers, and dispensing pharmacies to communicate required lenalidomide drug information to patients.7 Due to this, lenalidomide is only available in a select network of pharmacies.
High adherence has been shown to be an independent predictor of achieving complete tumor response.8,9 However, a systematic review found that oral oncology medication adherence rates vary widely in the literature, with rates reported from 46%-100% depending on study patient sample, medication type, time frame, assessment measure, and how adherence was calculated.10 Gupta et al. (2018) used a validated adherence survey, the Morisky Medication Adherence Scale, to describe the effects of adherence in patients with MM. Results found a significant improvement in multiple functional areas in patients with higher adherence. Patients with higher adherence rates were also found to have higher functional and MM well-being scores and lower out-of-pocket costs.11
Adherence technologies such as text messaging and medication adherence technology (MAT) have been studied specifically for oral anticancer medications. A 12-week observational study used a MAT pill bottle cap to measure adherence for 90 patients with various cancers. While the average adherence rate was 89.3%, the lowest quartile had an average adherence rate of only 67.8%.12 Another study measuring the effect of text messaging on weekly adherence rates for patients taking oral anticancer medication found no statistical difference between the text message intervention and control groups. As the text message patient group did report reading and being satisfied with the intervention, the authors concluded that proof of concept was achieved with further research needed.13
In another study, a low-cost medication reminder device failed to improve patient adherence in a population taking up to 3 medications for common chronic diseases. The authors noted that MAT devices might have been more effective if pharmacists had intervened to target low-adherence populations.14 To date, no published studies have assessed the role of MATs in oral anticancer therapy with pharmacist interventions in a specialty pharmacy setting.
Pharmacist engagement in medication therapy has demonstrated improvement in health care outcomes, including mitigation of drug costs and improvement in adherence, when actively involved with patient care.15 Pharmacist interventions have been shown to improve medication management and adherence in heart failure, hypertension and glycemic control, dyslipidemia, and health literacy.16,17 While less often studied in oncology, pharmacist interventions were linked with improvement in patient-reported quality of life scores in patients with breast cancer.18
The objective of this study was to quantify the effect of a smart pill bottle (SPB) and targeted pharmacist interventions when notified of missed uncapping events on medication adherence in MM patients taking oral lenalidomide. Lenalidomide was chosen because it is an oral oncology medication patients take from home, with a complicated time-on time-off cycle dosing regimen, and it has a high-touch REMS program, providing multiple points of contact between clinicians, pharmacists, and patients. An SPB that combined multiple MAT strategies, including real-time light alerts, dose sound notifications, and text message dose reminders, and that has built-in electronic medication monitoring sensors designed to yield an accurate representation of adherence rates was chosen for evaluation.19
Methods
Study Design and Eligibility
This prospective, single-site, randomized, single-blind, real-world study monitored medication adherence using an SPB in patients with MM receiving lenalidomide. The study included men and women aged over 18 years and diagnosed with MM who were new to lenalidomide therapy, defined as 5 or fewer cycles of lenalidomide, at Avella Specialty Pharmacy. This number was chosen to increase enrollment in the study time frame but still capture patients naive to therapy to receive adherence support from the beginning of care. Study participants were included if they had access to a cellular phone, were able to unscrew a child safety cap, and were willing to transfer medications between pill bottles. The SPBs were provided at no cost to participants. Informed consent, confirmed MM diagnosis, and inclusion in the lenalidomide REMS program were required. The study protocol was approved by the New England Independent Review Board.
Study Procedures
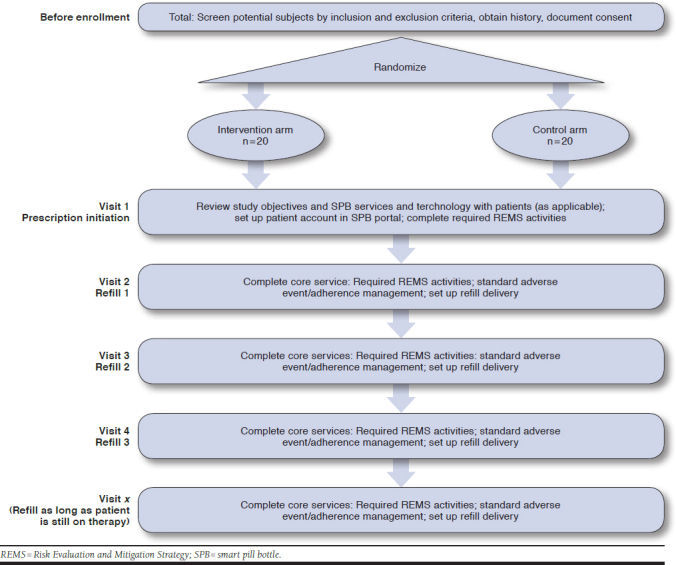
All lenalidomide prescriptions were processed by pharmacy technicians following Avella’s standard operating procedures. After delivery was scheduled, the technician transferred the patient to a pharmacist to complete the REMS counseling. Following the counseling, the pharmacist asked the patient a series of inclusion criteria questions to assess the patient’s willingness and ability to join the study. After recruitment of the patient, randomization occurred 1:1 between intervention and control groups. The counseling pharmacist filled out an SPB enrollment form and explained how the SPB worked, including activating the device by pulling the tab to turn the battery on. Patients were blinded to their intervention or control arm statuses. A completed SPB enrollment form included deidentified patient identification, date of birth, phone number, usual time of day for medication administration, and expected start date. The enrollment form followed the prescription as it moved through workflows and to the fulfillment area of the pharmacy.
The fulfillment pharmacist was responsible for placing dispensed medication into SPBs and documenting SPB serial numbers for each prescription. Each device had a unique serial number for tracking purposes, which allowed the pharmacy to verify each serial number and ensure successful delivery and device activation. SPB prescriptions were collected at the end of each business day and individual patient profiles were created on the SPB online portal using the script information (patient identification, serial number, phone number, medication dose time, and start date). The SPB was packaged and shipped directly to the patient’s home. After enrollment, core patient services of completing required REMS activities and setting up refill delivery were provided to both arms throughout the course of the study (Appendix A, available in online article).
Patients enrolled in the intervention arm received their bottles with all alerts activated. These alerts included a glowing light-emitting diode (LED) strip on the bottle that started 1 hour before dose time and continued throughout each 2-hour dosing window, a single chime when the medication dose was scheduled and every 15 minutes throughout the dosing window, and a text message or phone call 2 hours after any missed doses. The control arm received identical SPBs with all alerts deactivated. SPBs in both arms were turned on to measure adherence by capturing time stamps of each bottle uncapping; the bottles then transmitted data via cellular network, allowing the pharmacy to view real-time dosing data.
Lenalidomide for MM is typically dosed in a 28 day-cycle, with 1 dose daily for 21 days and no doses for 7 days. All SPBs were programmed to account for the 7-day off period as rest days in the cycle and were not marked against adherence; similarly, intervention group SPBs were programmed to not alert for medication reminders during these times.
Data collected from each SPB included the SPB unique identification, date enrolled, start date of medication, dose reminder time, dose taken time, dose medication outcome, date discontinued from medication, and total days active in the period. Dose medication outcomes included whether the dose was on time (dose taken within 1 hour before or after dose taken time that was set in the portal), taken early (> 1 hour before dosing time within the same day), taken late (> 1 hour after dosing time but within the same day), missed for that day, or therapy was paused (no alerts generated, such as when a patient was in the hospital or doses held). A weekly report showing the last 7 days of SPB/medication use was available on the portal.
A pharmacist follow-up call was planned if intervention arm patients missed 2 or more doses within a 7-day period in order to keep the pharmacy updated with potential treatment status and changes. Follow-up calls were considered effective if the patient did not require a second follow-up due to 2 missed doses over 7 days during the remainder of the current cycle and the next future cycle.
Study Outcomes and Calculations
The primary outcome of this study was to detect a significant difference in adherence rates between the intervention arm and the control arm after 6 months of combined SPB use with pharmacist intervention. Each bottle-uncapping event was captured and registered in the SPB online portal in real time. The primary outcome was calculated as an adherence percentage20,21:
Adherence (%) = (Total number of daily bottle-uncapping events from enrollment to end of study period)/(Total days active from enrollment to end of study period)
Secondary outcomes of this study included calculation of the incremental cost-effectiveness ratio (ICER) to determine SPB cost-effectiveness, number of lenalidomide interim cycles completed after SBP enrollment, effectiveness of intervention arm pharmacist follow-up, and patient satisfaction levels. An interim dosing window analysis was performed at the 3-month mark using uncapping data for 2 participants in each arm with high adherence (> 95%).
The ICER was calculated to determine SPB cost-effectiveness and the cost of the program per patient for additional adherence percentage increase. The ICER was calculated by the difference in cost over study time period between intervention and control arms divided by the difference in their adherence effects. The cost of the intervention arm was calculated directly from the pharmacy system by adding the cost of the SPB + monthly service charges + potential charge of the pharmacist’s time who was conducting the intervention. Typical dispensing and shipping costs were equal in both arms. The ICER calculation is as follows:
ICER = (Cost in intervention arm – cost in control arm)/(Effect in intervention arm – effect in control arm)
A nonvalidated post-study patient satisfaction survey asked intervention arm participants over the phone at the end of their 6-month study period to rate and provide feedback for their experiences using SPBs. Four questions used a Likert scale from 1 to 5, and 3 questions were open-ended. Patients in the control arm were not interviewed for satisfaction as their SPB had no additional function or services than a normal prescription vial to evaluate.
Statistical Analysis
Twenty patients in each study arm provided the 80% power to detect a difference in median adherence of 10%. Median adherence levels for both arms were reported and analyzed via Mann-Whitney U tests. Median analysis was used to account for potential outlier data skewing results with a small patient population. Mean adherence for both arms were also reported and analyzed via t-test. Other descriptive statistics were used to review secondary outcomes. All tests were 2-sided with a 5% level of significance. An ad hoc analysis of dosing times was conducted during the 3-month preliminary data review. All analyses were conducted using Minitab Statistical Software, version 18 (Minitab, State College, PA).
Results
Patient Characteristics
From January-February 2016, 40 patients with MM new to lenalidomide (≤ 5 cycles) were recruited for the study and randomly allocated to the intervention arm or control arm. Sixteen patients from each arm completed at least 1 full cycle and were included in the per-protocol 6-month analyses. Baseline characteristics were generally balanced between the intervention and control groups (Table 1). The average age in the intervention arm was 70 years compared with the control arm, which was 73 (P = 0.24). The intervention group consisted of 12 males (60%), and the control group consisted of 10 (50%) males (P = 0.525). Finally, insurance payer mix for the intervention group was 12 Medicare patients, 5 commercial patients, and 3 Tricare patients. The payer mix for the control group was 11 Medicare patients, 7 commercial patients, 1 Tricare patient, and 1 Medicaid patient (P = 0.73).
TABLE 1.
Baseline Patient Characteristics
| Intervention Arm (n = 20) | Control Arm (n = 20) | |
|---|---|---|
| Mean age,a years ± SD | 69 ± 10.6 | 73 ± 11 |
| Male,b n (%) | 12 (60) | 10 (50) |
| Insurance type,c n (%) | ||
| Government (Medicare, Medicaid, Tricare) | 15 (75) | 13 (65) |
| Commercial | 5 (25) | 7 (35) |
aT-test; P = 0.24.
bChi square test; P = 0.525.
cFisher’s exact test; P = 0.73.
SD = standard deviation.
The flow of patients through the study is shown in Figure 1. According to the study’s per-protocol analysis, patients who completed at least 1 full cycle were included in the analysis.
FIGURE 1.
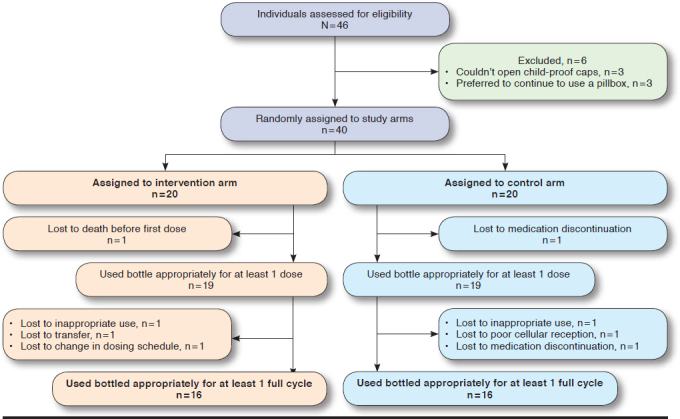
Patient Enrollment
Adherence Rates
After 6 months of data collection, the intervention arm demonstrated a significantly higher median adherence of 100% compared with the control arm median adherence of 87.4% (P = 0.001). The lowest reported intervention arm adherence rate was 91.8%, which was higher than the median control group adherence rate of 87.4% (Table 2).
TABLE 2.
Patient Adherence Rates
| Patient | Adherence (%) | Cycles | Patient | Adherence (%) | Cycles |
|---|---|---|---|---|---|
| Intervention 1 | 98.9 | 6 | Control 1 | 89.1 | 7 |
| Intervention 2 | 100.0 | 4 | Control 3 | 77.3 | 1 |
| Intervention 3 | 100.0 | 2 | Control 4 | 100.0 | 3 |
| Intervention 4 | 95.0 | 1 | Control 5 | 78.6 | 3 |
| Intervention 6 | 100.0 | 1 | Control 6 | 76.2 | 1 |
| Intervention 8 | 95.2 | 1 | Control 8 | 85.7 | 1 |
| Intervention 9 | 100.0 | 1 | Control 9 | 77.1 | 3 |
| Intervention 10 | 100.0 | 5 | Control 10 | 100.0 | 5 |
| Intervention 11 | 98.8 | 4 | Control 11 | 100.0 | 3 |
| Intervention 12 | 91.8 | 4 | Control 12 | 98.1 | 4 |
| Intervention 13 | 100.0 | 3 | Control 13 | 98.0 | 3 |
| Intervention 14 | 100.0 | 6 | Control 15 | 76.9 | 3 |
| Intervention 16 | 100.0 | 3 | Control 16 | 50.0 | 1 |
| Intervention 17 | 100.0 | 5 | Control 18 | 100.0 | 1 |
| Intervention 18 | 98.9 | 4 | Control 19 | 69.8 | 3 |
| Intervention 20 | 99.1 | 4 | Control 20 | 91.0 | 4 |
| Median | 100a | 4b | Median | 87.4a | 3b |
| Mean | 98.6 ± 2.4c | 3.4 ± 1.8d | Mean | 85.5 ± 14.5c | 2.9 ± 1.7d |
| Intervention arm > 95% adherence, n (%)e | 14 (87.5) | Control arm > 95% adherence, n (%)b | 2 (12.5) | ||
aMann-Whitney U test; P = 0.001.
bMann-Whitney U test; P = 0.30.
cT-test; P = 0.002.
dT-test; P = 0.41.
eChi square test; P = 0.003.
Table 2 also shows the number of patients with high adherence rates (defined as > 95%) for each arm. Fourteen of 16 intervention arm patients had adherence rates > 95%, and 6 of 16 control patients had adherence rates > 95% (P = 0.003).
Treatment Cycles Completed
The intervention arm completed a greater number of cycles using the SPB within the study time frame than the control arm (median = 4 vs. 3, P = 0.30; mean = 3.4 ± 1.8 vs. 2.9 ± 1.7, P = 0.41; Table 2). Each cycle consisted of 28 total days, with 21 days on and 7 days off.
Pharmacist Follow-Up
One patient in the intervention arm missed 2 doses within a 7-day period, requiring a pharmacist follow-up call per study protocol. In this follow-up call, the pharmacist assessed the reasons for missed doses and encouraged improved adherence; for the remainder of the study, this patient did not require further pharmacist follow-up calls. The total intervention time took 15 minutes. Although not eligible for pharmacist follow-up calls, 7 control arm patients met the same follow-up criteria.
Cost-Effectiveness
An annual cost of $1,210 per patient, with 1 pharmacist intervention and median adherence improvement of 12.6%, was found in the intervention arm compared with the control arm. Therefore, the ICER per percentage adherence increase was found to be $96.03. This ICER assumed all non-SPB therapy costs to be equivalent in SPB adopters and nonadopters because both arms received the same core services and refill delivery process.
Patient Satisfaction
Patient satisfaction was measured using a nonvalidated survey and conducted over the phone to intervention arm participants at the end of their 6-month study period.
Two questions received a rating of “5 – strongly agree” from all 9 responses: “(1) the full smart pill bottle service was easy to set up” and “(2) the full smart pill bottle service was easy to use.” Question 3 assessed patients’ willingness to participate in a free SPB service again and had 9 responses averaging 4.67; question 4 asked patients for an overall program rating and had 10 responses averaging 4.5. Questions 5-7 were free form and asked patients for additional feedback regarding SPB storage, the most positive program features, and areas for improvement (Appendix B, available in online article).
Interim Dose Timing Analysis
After noting the significant differences in adherence rates after a 3-month interim analysis, a dosage window analysis was initiated. Dosage times for 2 highly adherent patients (> 95%) from the intervention and control arms each were graphed in a scatterplot. The bars represent the dosing window that the patient stated they would take lenalidomide each day and when the SPB light/chime reminders would occur. The dot is the actual time of day that the SPB was opened, designating when the dose was taken. While all 4 patients had > 95% adherence, showing that they did not miss the daily dose, patients in the intervention arm had more regulated behavior and took a greater proportion of doses during the optimal 2-hour dosing window (83% vs. 22%; P < 0.001; Figure 2).
FIGURE 2.
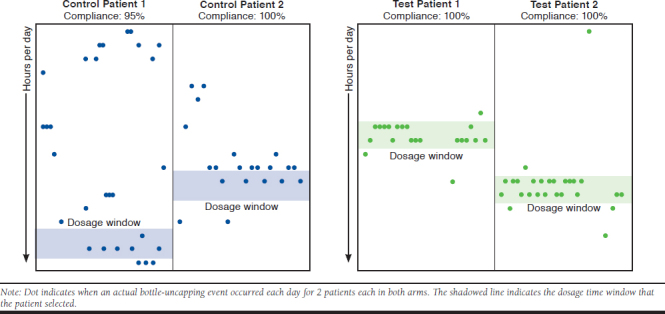
Daily Dosage Timing for Patients with High Adherence (> 95%)
Discussion
Although ideal adherence goals may be disease-specific, they are most often benchmarked at 80%, reflecting a general consensus surrounding clinical response rates.22 Our control group had an unexpectedly high median adherence rate of 87.4%, with 7 of 16 patients under the 80% benchmark compared with zero patients under 80% for the intervention group. We speculate that this is the result of a specialty pharmacy/REMS program model with greater patient-pharmacist contact and tighter therapy control; similar improved adherence has been noted when offering specialty pharmacy services to Medicare Part D beneficiaries, retail patients, and health-system patients.23-25
While little published data exist concerning adherence rates in MM patients taking lenalidomide, a 2017 poster presentation analyzing 64 Medicare patients found a median medication possession ratio (MPR) of 92%, with 14.5% of patients under 80% adherence.26 The MPR uses refill claim records to calculate adherence, but authors note that MPR may overestimate adherence. Our study had 19 (38%) Medicare patients. These adherence results are higher than our control group but lower than our intervention arm, possibly showing that providing MAT can help improve patient adherence behavior in a Medicare population. However, we have to also consider that differences may be attributed to both studies having a small sample size and using uncapping electronic data compared with claims records for adherence metrics.
Our study demonstrates a possible link between increased adherence due to SPB programs and tighter dosing windows. Patients in the intervention arm had consistent dosing behavior, while control patients had more variable dosing, which could lead to lower ongoing compliance, decreased efficacy, and/or increased risk of side effects.27 Although there is a paucity of literature on outcomes in oncology associated with dose timing, several studies have examined dosing windows in patients taking oral antiretroviral therapy for human immunodeficiency virus. MAT programs have been associated with tighter dosing windows, and tighter dosing windows have been associated with improved adherence and improved outcomes as measured by viral suppression.28-30
In addition to improved adherence, the intervention arm completed more cycles using the SPB when measured by the median (4 vs. 3) and mean (3.38 vs. 2.88). Due to the short time frame of this study compared with the typical length of lenalidomide therapy for an MM patient, we did not expect significant results, but this directionally shows improvements in duration of therapy and engagement in using an SPB when fully active in the intervention arm.
If these results are used as estimates in future SPB programs, enrolled patients may be expected to use the SPB and remain on therapy for roughly 17% longer than nonenrolled patients. When allocating pharmacy resources, future SPB programs may consider pharmacist follow-up calls in roughly 6% of enrolled patients and improved outcomes based on increased adherence and tighter dosing windows. The complexity and toxicity of the drug, as well as the disease state and patient status for allocation of pharmacy resources, will also need to be factored into future SPB programs.
Patients reported high satisfaction levels for this SPB program. Of note are the 9 unanimous “5 – strongly agree” ratings for ease of SPB set-up and ease of use. Patients’ open-ended responses included “never forgetting,” “cool technology with lights and sounds,” and “remember a little bit better” when asked what was the most useful, helpful, or enjoyable part of the SPB program. When asked what could be improved or changed with the program, 3 patients answered that no changes were needed; however, 1 patient did request that the SPB chime be louder for those hard of hearing, and 1 patient stated that he would only use it again if it were free. Overall, this feedback shows that the SPB was a well-received adherence program for MM patients, and few changes are needed for program improvement. Future studies evaluating patient satisfaction are warranted to see if this finding translates to patients taking SPBs with other oral treatment regimens. Additionally, payment for the SPB program needs to be taken into consideration.
Adherence-based cost measures are documented in bisphosphonates for osteoporosis and in cardiovascular medications but have not been documented in oncology.31,32 In treatment of breast cancer, improved adherence (> 80% vs. ≤ 80%) to tamoxifen has been linked to improved value per quality-adjusted life-year (QALY).33 Lenalidomide costs roughly $15,000 per month. Keeping an MM patient adherent for 1 full month was found to lower out-of-pocket costs.11 Expanding this to chronic disease states could potentially decrease wasted medication and health care expenditures by billions of dollars.34
Of the 12 new medications approved to treat cancers in 2017, 8 were available in oral formulations, with monthly costs of oral oncology medications ranging as high as $10,000-$15,000.35,36 Poor adherence in oral oncology medications has been linked to increased nonpharmacy health care utilization and decreased treatment effectiveness.10,37 As health care spend in the United States eclipses $3.4 trillion and is projected to increase to nearly 20% of the gross domestic product by 2026, MATs that allow for targeted pharmacist clinical intervention to help improve medication adherence may play a growing role in health care cost savings as one pharmacy management strategy.38,39 Future research with a longer data collection period and a larger patient cohort could further validate our study results.
Insurance plans that identify patients at risk of low adherence could optimize cost-effectiveness of SPB programs—over the course of 15 completed cycles, 7 control group patients combined for 54.1 adherence percentage points under the 80% adherence goal. At the reported annual SPB of $96.03 per percentage adherence gained, an incremental cost of $5,195.22 would move these 7 patients to the 80% adherence goal threshold. As the pricing was based on current SPB contracting, the volume of SPB ordered could reduce pricing. Future SPB programs could target inclusion toward patients with increased risk of low adherence to allow for targeted intervention from SPBs and pharmacist support as a value-based benefit design model.40
Limitations
This prospective study has some limitations to consider. This was a single-site study at a specialty pharmacy, so results may not be generalizable to patients taking other oral oncolytic therapies at other pharmacy sites. Additionally, adherence was measured by tracking the number of bottle-uncapping occurrences within the study time frame, but that does not necessarily mean that the patient ingested the lenalidomide dose, since direct observation or therapeutic blood monitoring was not a part of the study protocol.
Although our study originally recruited 20 participants in each arm to meet 80% power, 4 participants from each arm did not complete a full cycle and were thus excluded from our analysis (Figure 1). Additionally, we discussed other adherence cost-effective measures reported in studies compared with ICER findings in our study. Since most oncology studies report ICER per QALY, we are unable to directly benchmark our reported ICER against the literature.
Follow-up calls were only planned for the intervention arm. Although we anticipated additional follow-up calls would increase control arm adherence rates, our study was designed to mimic a real-world specialty pharmacy setting with no means to assess missed daily doses for unenrolled patients. To improve on this study, a crossover design could have been used to see if improvement in adherence was found in the control arm when SPB alerts were fully activated.
Full patient demographics, potential concomitant disease states, social differences such as language and ethnicity, and duration of MM diagnosis were not reported due to incomplete patient chart notes. Additionally, level and quality of provider involvement were not accounted for in this study. Baseline patient disease, socioeconomic factors, and/or provider office therapy protocol differences between arms could affect overall patient outcome result findings.
Conclusions
Considering the improved adherence and number of cycles using the SPB, targeted pharmacist follow-ups, and cost-effectiveness, specialty pharmacy-based SPBs with pharmacist-targeted interventions may have a role in managing oral oncology medication adherence. Based on ICER findings, there is an opportunity for a value-based contracting model with an SPB program to lower health care costs. While the study population was small, we postulate that other disease states with oral medication therapy requiring tight dosing windows (e.g., transplant immunosuppressants, opioids, or novel oral anticoagulants), complicated dosing regimens, or those with low adherence rates may benefit from SPB and pharmacist-targeted intervention programs.
ACKNOWLEDGMENTS
Writing support was provided by Logan Cast, PharmD (Avella Specialty Pharmacy).
APPENDIX A. Study Timeline Schematic
APPENDIX B. Patient Satisfaction Likert Survey Question Results
| Questions | Responses | Strongly Agree % | Agree % | Neutral % | Disagree % | Strongly Disagree % | ||
|---|---|---|---|---|---|---|---|---|
| 1. The full SPB service was easy to set up | n = 9 | 100 | 0 | 0 | 0 | 0 | ||
| 2. The full SPB service was easy to use | n = 9 | 100 | 0 | 0 | 0 | 0 | ||
| Very Likely % | Likely % | Neutral % | Unlikely % | Very Unlikely % | ||||
| 3. If you were given the option to use the free SPB service again, how likely would you be to enroll in the program? | n = 9 | 90 | 10 | 0 | 0 | 0 | ||
| Very Positive % | Positive % | Neutral % | Slightly Negative % | Negative % | ||||
| 4. How would you rate the full SPB service (including the automated reminders, the live support calls from care team) | n = 10 | 60 | 10 | 30 | 0 | 0 | ||
| Open-ended question replies | ||||||||
| 5. Where do you normally store and use the SPB (e.g., medicine cabinet, kitchen sink area)? | n = 7 | Room (n = 1) | Kitchen (n = 3) | Medicine cabinet (n = 1) | Bedroom (n = 1) | Living room (n = 1) | ||
| 6. In your opionion, what was the most useful, helpful, or enjoyable part of the SPB program? | n = 7 | Remember a little bit better (n = 1) | “Cute” idea (n = 1) | Cool technology, lights/sounds/text effects (n = 1) | Never forget (n = 1) | Everything (n = 1) | Kept patient on track (n = 1) | Alarm every morning as reminder (n = 1) |
| 7. If you could improve or change one aspect of the SPB program, what would it be? | n = 5 | No changes needed (n = 3) | Wasn’t used to bottle/had own routine (n = 1) | Ring chime a little louder/different decibel for older patients (n = 1) | ||||
Note: Patients 4 and 7 were deceased at follow-up contact. Patients 2, 3, 8, 9, 11, 15, 16, and 19 were unable to be reached by telephonic outreach. SPB = smart pill bottle.
REFERENCES
- 1.Mahindra A, Hideshima T, Anderson KC. Multiple myeloma: biology of the disease. Blood Rev. 2010;S5-11. [DOI] [PubMed] [Google Scholar]
- 2.National Cancer Institute . Cancer stat facts: myeloma. Available at: https://seer.cancer.gov/statfacts/html/mulmy.html. Accessed September 25, 2019.
- 3.Kumar SK, Callander NS, Alsina M, et al. NCCN Guidelines Insights. Multiple myeloma, version 3.2018: featured updates to the NCCN Guidelines. J Natl Compr Canc Netw. 2018:16(1):11-20. [DOI] [PubMed] [Google Scholar]
- 4.Revlimid (lenalidomide) capsules, for oral use . Celgene. Revised May 2019. Available at: https://media.celgene.com/content/uploads/revlimid-pi.pdf. Accessed October 7, 2019.
- 5.Dimopoulos MA, Chen C, Spencer A, et al. Long-term follow-up on overall survival from the MM-009 and MM-010 phase III trials of lenalidomide plus dexamethasone in patients with relapsed or refractory multiple myeloma. Leukemia. 2009;23(11):2147-52. [DOI] [PubMed] [Google Scholar]
- 6.Jagannath S, Abonour R, Durie BGM, et al. Impact of post-ASCT maintenance therapy on outcomes in patients with newly diagnosed multiple myeloma in Connect MM. Blood Adv. 2018;2(13):1608-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bandenburg NA, Bwire R, Freeman J, et al. Effectiveness of Risk Evaluation and Mitigation Strategies (REMS) for lenalidomide and thalidomide: patient comprehension and knowledge retention. Drug Saf. 2017;40(4):333-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marin D, Bazeos A, Mahon FX, et al. Adherence is the critical factor for achieving molecular responses in patients with chronic myeloid leukemia who achieve complete cytogenetic responses on imatinib. J Clin Oncol. 2010;28(14):2381-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ruddy K, Mayer E, Partridge A. Patient adherence and persistence with oral anticancer treatment. CA Cancer J Clin. 2009;59(1):56-66. [DOI] [PubMed] [Google Scholar]
- 10.Greer JA, Amoyal N, Nisotel L, et al. A systematic review of adherence to oral antineoplastic therapies. Oncologist. 2016;21(3):354-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gupta S, Abouzaid S, Libert R, et al. Assessing the effect of adherence on patient-reported outcomes and out of pocket costs among patients with multiple myeloma. Clin Lymphoma Myeloma Leuk. 2018;18(3):210-18. [DOI] [PubMed] [Google Scholar]
- 12.Jacobs JM, Pensak NA, Sporn NJ, et al. Treatment satisfaction and adherence to oral chemotherapy in patients with cancer. Am Soc Clin Oncology. 2017;13(5):e474-85. [DOI] [PubMed] [Google Scholar]
- 13.Spoelstra SL, Given GW, Sikorskii A, et al. Proof of concept of a mobile health short message service text message intervention that promotes adherence to oral anticancer agent medications: a randomized controlled trial. Telemed J E Health. 2016;22(6):497-506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Choudhry NK, Krumme AA, Ercole PM, et al. Effect of reminder devices on medication adherence: the REMIND randomized clinical trial. JAMA Intern Med. 2017;177(5):624-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dalton K, Byrne S. Role of the pharmacist in reducing healthcare costs: current insights. Integr Pharm Res Pract. 2017;6:37-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parajuli DR, Franzon J, McKinnon RA, et al. Role of the pharmacist for improving self-care and outcomes in heart failure. Curr Heart Fail Rep. 2017;14(2):78-86. [DOI] [PubMed] [Google Scholar]
- 17.Omboni S, Caserini M. Effectiveness of pharmacist’s intervention in the management of cardiovascular diseases. Open Heart. 2018;5(1):e000687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tanaka K, Hori A, Tachi T, et al. Impact of pharmacist counseling on reducing instances of adverse events that can affect the quality of life of chemotherapy outpatients with breast cancer. J Pharm Health Care Sci. 2018;4(9):1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aardex Group . Medication adherence monitoring and management. Available at: https://www.aardexgroup.com. Accessed September 25, 2019.
- 20.Lizheng S, Liu J, Fonseca V, et al. Correlation between adherence rates measured by MEMS and self-reported questionnaires: a meta-analysis. Health Qual Life Outcomes. 2010;8:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van den Boogaard J, Lymio RA, Boeree MJ, et al. Electronic monitoring of treatment adherence and validation of alternative adherence measures in tuberculosis patients: a pilot study. Bull World Health Organ. 2011;89(9):632-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peterson AM, Nau DP, Cramer JA, et al. A checklist for medication compliance and persistence studies using retrospective databases. Value Health. 2007;10(1):3-12. [DOI] [PubMed] [Google Scholar]
- 23.Kale HP, Patel AM, Carroll NV. A comparison of pharmacy dispensing channel use and adherence to specialty drugs among Medicare Part D beneficiaries. J Manag Care Spec Pharm. 2018;24(4):317-26. Available at: https://www.jmcp.org/doi/10.18553/jmcp.2018.24.4.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moore JM, Matlin OS, Lotvin AM, et al. The adherence impact of a program offering specialty pharmacy services to patients using retail pharmacies. J Am Pharm Assoc. 2016;56(1):47-53. [DOI] [PubMed] [Google Scholar]
- 25.Bagwell A, Kelley T, Carver A, et al. Advancing patient care through specialty pharmacy services in an academic health system. J Manag Care Spec Pharm. 2017;23(8):815-20. Available at: https://www.jmcp.org/doi/full/10.18553/jmcp.2017.23.8.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wildes T, Fiala MA, Vij R. Adherence to lenalidomide in older patients with multiple myeloma (MM): a SEER-Medicare analysis. Poster presented at: American Society of Hematology 59th Annual Meeting and Exposition; December 9-12, 2017; Atlanta, GA. [Google Scholar]
- 27.Andrews LM, Li Y, De Winter BCM, et al. Pharmacokinetic considerations related to therapeutic drug monitoring of tacrolimus in kidney transplant patients. Expert Opin Drug Metab Toxicol. 2017;13(12):1225-36. [DOI] [PubMed] [Google Scholar]
- 28.Moore DJ, Poquette A, Casaletto KB, et al. Individualized texting for adherence building (iTAB): improving antiretroviral dose timing among HIV-infected persons with co-occurring bipolar disorder. AIDS Behav. 2015;9(3):459-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gill CJ, DeSliva MB, Hamer DH, et al. Novel approaches for visualizing and analyzing dose-timing data from electronic drug monitors, or “how the ‘broken window’ theory pertains to ART adherence.” AIDS Behav. 2015;19(11):2057-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gill CJ, Sabin LL, Hamer DH, et al. Importance of dose timing to achieving undetectable viral loads. AIDS Behav. 2010;14(4):785-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hiligsmann M, McGowan B, Bennett K, et al. The clinical and economic burden of poor adherence and persistence with osteoporosis medications in Ireland. Value Health. 2012;15(5):604-12. [DOI] [PubMed] [Google Scholar]
- 32.Smith DH, O’Keeffe-Rosetti M, Owen-Smith AA, et al. Improving adherence to cardiovascular therapies: an economic evaluation of a randomized pragmatic trial. Value Health. 2016;19(2):176-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McCowan C, Wang S, Thompson AM, et al. The value of high adherence to tamoxifen in women with breast cancer: a community-based cohort study. Br J Cancer. 2013;109:1172-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Iuga AO, McGuire MJ. Adherence and health care costs. Risk Manag Healthc Policy. 2014;4:35-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.U.S. Food and Drug Administration. Center for Drug Evaluation and Research . Novel drug approvals for 2017. February 2, 2018. Available at: https://www.fda.gov/drugs/developmentapprovalprocess/druginnovation/ucm537040.htm. Accessed September 25, 2019.
- 36.Express Scripts . 2018 drug trend report. February 2019. Available at: https://my.express-scripts.com/rs/809-VGG-836/images/Express%20Scripts%202018%20Drug%20Trend%20Report.pdf. Accessed September 25, 2019.
- 37.Cutler RL, Fernandez-Llimos F, Frommer M, et al. Economic impact of medication non-adherence by disease groups: a systematic review. BMJ Open. 2018;8(1):e016982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Centers for Medicare & Medicaid Services . National health expenditure projections 2018-2027. Available at: https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/NationalHealthExpendData/Downloads/ForecastSummary.pdf. Accessed September 25, 2019.
- 39.Fleming WK. Pharmacy management strategies for improving drug adherence. J Manag Care Pharm. 2008;14(6 Supp B):16-20. Available at: https://www.jmcp.org/doi/abs/10.18553/jmcp.2008.14.S6-B.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mahoney JJ. Value-based benefit design: using a predictive modeling approach to improve compliance. J Manag Care Pharm. 2008;14(6 Supp B):3-8. Available at: https://www.jmcp.org/doi/abs/10.18553/jmcp.2008.14.S6-B.3. [DOI] [PMC free article] [PubMed] [Google Scholar]


