Abstract
BACKGROUND:
Previous studies have shown an increased risk of pneumonia with benzodiazepines (BZD) and an increased risk of any infection with non-BZD hypnotics, but no analysis has specifically investigated the risk of pneumonia with non-BZD hypnotic use.
OBJECTIVE:
To evaluate the risk of pneumonia associated with non-BZD hypnotic use in the elderly.
METHODS:
This was a retrospective case-control study of members aged 65 years and older enrolled in an integrated health care system. Cases were identified as patients aged 65 years and older with a diagnosis of pneumonia from January 2011 to December 2012. Controls were matched in a 4:1 ratio to cases based on age, gender, and active enrollment. Non-BZD hypnotic exposure was evaluated for all cases and controls 1 year before the index date. Proximity of exposure to index date and duration of use were analyzed. Conditional logistic regression adjusted for covariates was performed.
RESULTS:
We identified 51,029 cases with pneumonia and matched 188,391 controls without pneumonia. Of the cases with pneumonia, 5.5% (2,790) of cases had exposure to a non-BZD hypnotic, compared with 3.4% (6,345) of controls. Non-BZD hypnotic exposure was associated with an increased risk of pneumonia (OR = 1.14; 95% CI = 1.08-1.20). When exposure was stratified by proximity to index date, only current exposure was associated with an increased risk of pneumonia (OR = 1.27; 95% CI = 1.18-1.36). Short-term exposure was associated with a relatively higher risk of pneumonia (OR = 1.57; 95% CI = 1.39-1.77) compared with long-term use (OR = 1.16; 95% CI = 1.06-1.25).
CONCLUSIONS:
Current use of non-BZD hypnotics in older adults is associated with an increased risk of pneumonia. The findings of this study provide additional support for reducing the use of non-BZD hypnotics in older adults and for pursuing safer alternatives for treating insomnia.
What is already known about this subject
The risk of adverse events associated with nonbenzodiazepine (non-BZD) hypnotics is well documented.
An increased risk of infections with the use of BZDs and selected non-BZD hypnotics has been shown in previous studies.
Despite the designation of non-BZD hypnotics as a high-risk medication, use of these agents remains persistently high among older adults, and their associated complications account for a significant proportion of emergency department visits.
What this study adds
This study evaluates the risk of pneumonia in a large population of older adults in an integrated health care system.
Pneumonia is an adverse event of significant clinical concern in older adults but has not been specifically evaluated for non-BZD hypnotics.
Study findings provide additional rationale for reducing the prescribing of high-risk medications for older adults.
Nonbenzodiazepine (non-BZD) hypnotics are indicated for the treatment of insomnia and include the agents zolpidem, eszopiclone, and zaleplon. Compared with BZDs, non-BZD hypnotics exhibit greater specificity for omega1 γ-aminobutyric acid (GABA) receptors, which are responsible for sedation.1 Traditionally, BZDs were the preferred medical treatment for insomnia but have fallen out of favor in recent years because of concerns regarding abuse and addiction potential.2-8 Combined with a rising trend in the diagnosis and treatment of insomnia, non-BZD hypnotics have filled this prescribing void and are now among the most widely prescribed medications in adults. One study using data from the National Ambulatory Medical Care Survey reported that non-BZD hypnotic prescribing increased 350% from 1999 to 2010—the same time period that BZD use plateaued.9,10
Although historically considered safer than BZDs, non-BZD hypnotics are high-risk medications nonetheless, and their use by older adults should be avoided.11,12 Adverse events associated with non-BZD hypnotics are well documented and include dizziness, drowsiness, falls, fracture, cognitive impairment, delirium, parasomnias, motor vehicle accidents, and mortality.13-17 Notably, in a study examining emergency department visits involving adverse events related to psychiatric medications, zolpidem was implicated in 11.5% of all adult psychiatric medication adverse event emergency department visits and in 21.0% of visits involving older adults. In both instances, zolpidem accounted for significantly more emergency department visits than any other psychotropic medication.18
Citing little benefit for improved sleep latency and duration, the American Geriatrics Society Beers Criteria advises to avoid the use of non-BZD hypnotics, with their most recent update removing the caveat for chronic 90-day use and discouraging use of any duration.19 The Centers for Medicare & Medicaid (CMS) and the National Committee for Quality Assurance urge similar caution regarding the inappropriate and indiscriminate use of these agents.20 Unfortunately, not only are older adults more likely to experience poor sleep quality, they are also mores susceptible to medication-related adverse events because of age-related pharmacokinetic and pharmacodynamic changes.
Previous studies investigating the risk for pneumonia with the use of agents with GABA-agonist properties have found conflicting results.21-25 These studies focused primarily on the use of BZDs and showed an increased risk of pneumonia, although this association was not consistently confirmed in subsequent studies in other populations.22-24 While these studies were not designed to determine the physiologic mechanism underlying this association, the study authors hypothesized that this increased risk could be attributed to the immunomodulatory effects of GABA receptor agonism or an increased risk of aspiration with gastroesophageal relaxation or respiratory depression.21,23,26
No published studies have evaluated the risk of pneumonia associated specifically with the use of non-BZD hypnotics in older adults. The purpose of this study was to investigate this question through a retrospective case-control study using data from a large integrated health care system.
Methods
Study Design and Population
This was a retrospective case-control study that analyzed data from Kaiser Permanente Southern California (KPSC) and Northern California (KPNC) regions. The base population was defined as KPSC and KPNC members aged 65 years and older during the study period January 2011-December 2012. The study used data from the electronic medical record, including patient enrollment, diagnoses, outpatient visits, hospital admissions, emergency department visits, and outpatient drug prescriptions. This study was approved by KPSC and KPNC institutional review boards.
Cases and controls were identified from within the base population (Figure 1). Cases were defined as patients with a documented diagnosis of pneumonia during the study period. Pneumonia was defined based on the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes, which included pneumonias of bacterial and viral etiologies (ICD-9-CM codes 480.x-486.x and 487.0). The date on which pneumonia was first diagnosed was defined as the index date.
FIGURE 1.
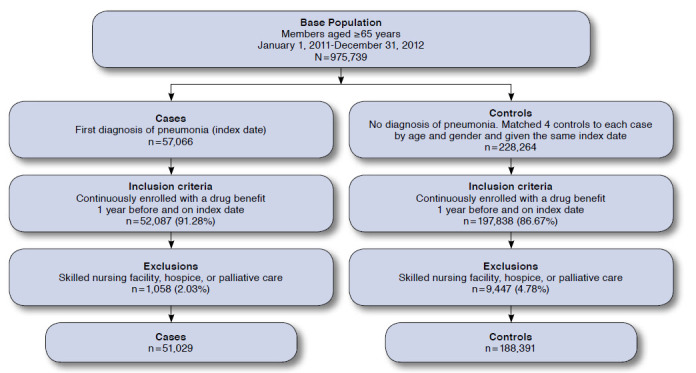
Cohort Selection Flowchart
Controls were randomly selected from the base population that did not have pneumonia and matched in a 4:1 ratio for each case, based on age, gender, and active enrollment. Matching was performed separately for each health plan region (north and south). Controls were assigned the same index date as their matching cases. Any member selected as a control for a case on one date could become a control for another case occurring on a later index date, as long as the member remained in the base population and was also at risk of becoming a case.27 Cases and controls were identified without previous knowledge of exposure to any non-BZD hypnotics.
For study inclusion, all patients had to have continuous enrollment and a drug benefit for 1 year before the index date and be alive and enrolled on the index date. Patients were excluded if they were in a long-term care facility, nursing home, or receiving palliative and hospice care within 1 year of the index date, which resulted in some cases no longer having a 4:1 match.
The following agents were identified and included as non-BZD hypnotics: zolpidem, zaleplon, and eszopiclone. Although non-BZD hypnotics were the focus of this study, we also in explored the use of BZDs and the risk of pneumonia. BZD exposure was included to compare risk with previous studies. Based on prescription volume at our institution, we selected the most commonly prescribed BZDs for analysis: alprazolam, clonazepam, lorazepam, and temazepam, which represent 89% of all benzodiazepine use at our institution. Furthermore, we performed subanalyses on each of these BZDs to explore variations in risk by individual agent.
Drug exposure was stratified by proximity of sold date to index date, with exposure classified as “current” if the most recent prescription was sold within 30 days of, and overlapped, the index date. “Recent” exposure included prescriptions within 31-90 days of the index date, and prescriptions beyond 90 days were considered “remote.” Consistent with the British National Formulary prescription guidelines, these definitions of drug exposure were used to compare our analysis with that of the case-control study of BZDs.23 Duration of use, measured by cumulative drug days supply, was analyzed for short-term (≤ 90 days) and long-term use (> 90 days) among current users.
Covariates
We collected and evaluated information on concomitant medications and comorbid conditions for cases and controls in order to control for potential differences between the groups. We included covariates known to affect the risk of pneumonia, whether positively and negatively, including previous episode of pneumonia, acute myocardial infarction, asthma, chronic obstructive pulmonary disease (COPD), upper respiratory tract infection, anticonvulsants, antidepressants, antipsychotics, BZDs, mood stabilizers, opioids, and acid suppressive therapy. Covariates were identified during the year before the index date and were measured using ICD-9-CM codes and prescription records (see Appendix, available in online article). The Diagnostic Cost Group (DxCG) score was also used as a covariate. DxCG measures comorbidity and includes patient encounter-based indicators for a variety of chronic conditions, including coronary heart disease, heart failure, diabetes, hypertension, cancer, and human immunodeficiency virus. The DxCG score is similar to the risk-adjustment method used by the CMS.28
Statistical Analysis
A descriptive analysis was performed to compare baseline characteristics for cases and controls. Pearson χ2 tests were used to compare unadjusted rates of pneumonia, and t-tests were used to detect differences in means for normally distributed variables, while the Wilcoxon rank-sum test was applied to variables that did not meet the normality assumption. Conditional logistic regression was performed to estimate the odds ratio (ORs) for pneumonia with 95% confidence intervals (CI) for patients who used a non-BZD hypnotic, compared with those without non-BZD hypnotic use. The same regression model was run to calculate OR estimates with 95% CIs for proximity of exposure to index date and for duration of exposure compared with no use. Pre-specified covariates used in the final models included DxCG scores, region (north or south), previous episode of pneumonia, acute myocardial infarction, asthma, COPD, upper respiratory tract infection, alcoholism, smoking, neurologic disorders, dementia, swallowing disorder, nausea and vomiting, anticonvulsants, antidepressants, antipsychotics, mood stabilizers, opioids, and acid suppressive therapy. In the non-BZD models, previous BZD use was included as a covariate. In the BZC models, non-BZDs were used as a covariate. Similar regression models were run separately for the 4 BZDs, adjusting for the same covariates and previous non-BZD hypnotic use. Unadjusted and adjusted ORs are presented. A P value of 0.05 or less was considered to be statistically significant. Statistical Analysis System (SAS), version 9.2, was used to conduct the statistical analysis (SAS Institute, Carey, NC).
Results
From a base population of 975,739 older adults, we identified 51,029 cases of pneumonia and 188,391 matching controls (Figure 1). The mean age for both groups was 77 years, and 52% of patients were female. As expected, cases with pneumonia had significantly higher rates of medication usage and concomitant conditions than controls (P < 0.001 for all covariates; Table 1).
TABLE 1.
Characteristics of Cases and Matched Controls
| Covariates | Cases n = 51,029 | Controls n = 188,391 | |
|---|---|---|---|
| DxCG risk score, median; mean [± SD] | 1.3; 1.6 [± 1.8] | 0.8; 0.9 [± 1.2] | |
| Concomitant medical conditions, n (%) | |||
| Previous pneumonia | 3,132 (6.1) | 2,148 (1.1) | |
| Asthma | 10,807 (21.2) | 18,261 (9.7) | |
| Chronic obstructive pulmonary disease | 19,810 (38.8) | 28,626 (15.2) | |
| Upper respiratory tract infection | 10,210 (20.0) | 27,444 (14.6) | |
| Smoking | 12,143 (23.8) | 30,571 (16.2) | |
| Alcohol | 881 (1.7) | 2,053 (1.1) | |
| Acute myocardial infarction | 2,860 (5.6) | 3,658 (1.9) | |
| Dementia | 3,150 (6.2) | 7,413 (3.9) | |
| Swallowing disorders | 3,316 (6.5) | 6,300 (3.3) | |
| Nausea | 5,837 (11.4) | 11,111 (5.9) | |
| Parkinson’s disease | 1,117 (2.2) | 2,795 (1.5) | |
| Concomitant medications, n (%) | |||
| Anticonvulsants | 4,433 (8.7) | 9,030 (4.8) | |
| Antidepressants | 15,238 (29.9) | 35,710 (19.0) | |
| Antipsychotics | 2,955 (5.8) | 4,892 (2.6) | |
| Benzodiazepines | 10,200 (20.0) | 23,778 (12.6) | |
| Mood stabilizers | 450 (0.9) | 861 (0.5) | |
| Opioids | 21,857 (42.8) | 53,336 (28.3) | |
| Proton pump inhibitors | 13,751 (27.0) | 32,412 (17.2) | |
| H2 receptor antagonists | 7887 (15.5) | 21,411 (11.4) | |
Note: Differences between cases and controls for covariates above had P values < 0.05.
DxCG = Diagnostic Cost Groups comorbidity score; SD = standard deviation.
A total of 2,790 (5.5%) cases and 6,345 (3.4%) controls had non-BZD hypnotic exposure (Table 2). Zolpidem was the most commonly used agent (98.5%), whereas zaleplon (0.3%) and eszopiclone (1.2%) had low rates of use; this was consistent with the KPSC and KPNC formulary.
TABLE 2.
Risk of Pneumonia with Use of Non-BZDs and BZDs Compared with No Use
| Cases n (%) | Controls n (%) | Unadjusted Odds Ratio (95% CI) | Adjusteda Odds Ratio (95% CI) | P Value | |
|---|---|---|---|---|---|
| Non-BZDs | 2,790 (5.5) | 6,345 (3.4) | 1.68 (1.60-1.75) | 1.14 (1.08-1.20) | < 0.001 |
| BZDs | 10,258 (20.1) | 23,938 (12.7) | 1.75 (1.70-1.79) | 1.16 (1.13-1.20) | < 0.001 |
| Alprazolam | 1,625 (3.2) | 3,812 (2.0) | 1.61 (1.52-1.71) | 1.12 (1.05-1.20) | < 0.001 |
| Clonazepam | 922 (1.8) | 2,276 (1.2) | 1.52 (1.40-1.64) | 0.99 (0.91-1.08) | 0.863 |
| Lorazepam | 2,201 (4.3) | 5,194 (2.8) | 1.59 (1.51-1.67) | 1.06 (1.00-1.12) | 0.048 |
| Temazepam | 2,126 (4.2) | 5,034 (2.7) | 1.59 (1.51-1.67) | 1.15 (1.09-1.22) | < 0.001 |
a Adjusted for covariates listed in the Appendix (available in online article).
BZD = benzodiazepine; CI = confidence interval.
Unadjusted and adjusted ORs comparing non-BZD hypnotic use and no use are shown in Table 2. When adjusted for covariates, non-BZD hypnotic use was associated with a 14% increased risk of pneumonia compared with no use (OR = 1.14; 95% CI = 1.08-1.20; P < 0.001). Because zolpidem accounted for most of the non-BZD hypnotic use, we conducted a sensitivity analysis that excluded nonzolpidem users and found similar results (OR = 1.14; 95% CI = 1.08-1.19; P < 0.001). When examining relative risk of exposure by proximity to index date, current exposure had a significantly higher risk of pneumonia (OR = 1.27; 95% CI = 1.18-1.36; P < 0.001; Table 3). Short-term use was associated with a higher relative risk for pneumonia (OR = 1.57; 95% CI = 1.39-1.77; P < 0.001) than long-term use (OR = 1.16; 95% CI = 1.06-1.25; P < 0.001).
TABLE 3.
Risk of Pneumonia with Use of Non-BZDs and BZDs by Proximity to Index Date
| Cases n (%) | Controls n (%) | Unadjusted Odds Ratio (95% CI) | Adjusteda Odds Ratio (95% CI) | P Value | |
|---|---|---|---|---|---|
| Current use | |||||
| Non-BZDs | 1,535 (3.0) | 3,061 (1.6) | 1.92 (1.80-2.04) | 1.27 (1.18-1.36) | < 0.001 |
| BZDs | 5,956 (11.7) | 12,225 (6.5) | 1.98 (1.92-2.05) | 1.28 (1.23-1.33) | < 0.001 |
| Alprazolam | 945 (1.9) | 1,965 (1.0) | 1.82 (1.68-1.97) | 1.22 (1.21-1.33) | < 0.001 |
| Clonazepam | 627 (1.3) | 1,521 (0.8) | 1.65 (1.51-1.81) | 1.06 (0.95-1.17) | 0.301 |
| Lorazepam | 1,186 (2.3) | 2,464 (1.3) | 1.80 (1.68-1.93) | 1.15 (1.06-1.24) | < 0.001 |
| Temazepam | 1,398 (2.7) | 2,912 (1.6) | 1.80 (1.69-1.92) | 1.28 (1.19-1.37) | < 0.001 |
| Recent use | |||||
| Non-BZDs | 396 (0.8) | 982 (0.5) | 1.53 (1.36-1.72) | 1.13 (1.00-1.29) | 0.061 |
| BZDs | 1,508 (3.0) | 3,699 (2.0) | 1.66 (1.57-1.77) | 1.15 (1.07-1.22) | < 0.001 |
| Alprazolam | 255 (0.5) | 607 (0.3) | 1.59 (1.37-1.84) | 1.09 (0.92-1.28) | 0.316 |
| Clonazepam | 87 (0.2) | 303 (0.2) | 1.06 (0.84-1.35) | 0.71 (0.55-0.93) | 0.011 |
| Lorazepam | 366 (0.7) | 856 (0.5) | 1.60 (1.41-1.81) | 1.14 (0.99-1.30) | 0.066 |
| Temazepam | 255 (0.5) | 655 (0.4) | 1.47 (1.27-1.70) | 1.11 (0.95-1.30) | 0.199 |
| Remote use | |||||
| Non-BZDs | 859 (1.7) | 2,302 (1.2) | 1.42 (1.31-1.53) | 0.96 (0.88-1.05) | 0.367 |
| BZDs | 2,794 (5.5) | 8,014 (4.3) | 1.42 (1.36-1.49) | 0.99 (0.94-1.04) | 0.726 |
| Alprazolam | 425 (0.8) | 1,240 (0.7) | 1.29 (1.16-1.44) | 0.96 (0.85-1.09) | 0.539 |
| Clonazepam | 163 (0.3) | 452 (0.2) | 1.36 (1.14-1.63) | 0.96 (0.79-1.17) | 0.702 |
| Lorazepam | 649 (1.3) | 1,874 (1.0) | 1.30 (1.19-1.42) | 0.91 (0.82-1.00) | 0.054 |
| Temazepam | 473 (0.9) | 1.467 (0.8) | 1.21 (1.09-1.34) | 0.92 (0.82-1.03) | 0.141 |
a Adjusted for covariates listed in the Appendix (available in online article).
BZD =benzodiazepine; CI = confidence interval.
Similar to non-BZD hypnotics, BZD use was also more frequently observed in cases (20.1%) compared with controls (12.7%). All BZD use was associated with a higher risk of similar magnitude (OR = 1.16; 95% CI = 1.13-1.20; P < 0.001), which was driven by current users (OR = 1.28; 95% CI = 1.23-1.33; P < 0.001; Table 3). Likewise, short-term use of BZDs was associated with a higher relative risk (OR = 1.69; 95% CI = 1.58-1.80; P < 0.001) compared with long-term use (OR = 1.14; 95% CI = 1.09-1.19; P < 0.001; Table 4).
TABLE 4.
Risk of Pneumonia with Use of Non-BZDs and BZDs by Duration of Use
| Cases n (%) | Controls n (%) | Unadjusted Odds Ratio (95% CI) | Adjusteda Odds Ratio (95% CI) | P Value | |
|---|---|---|---|---|---|
| Short-term use | |||||
| Non-BZDs | 502 (1.0) | 874 (0.5) | 2.18 (1.95-2.43) | 1.57 (1.39-1.77) | < 0.001 |
| BZDs | 1,758 (3.5) | 3,006 (1.6) | 2.31 (2.18-2.45) | 1.69 (1.58-1.80) | < 0.001 |
| Alprazolam | 323 (0.6) | 552 (0.3) | 2.22 (1.94-2.55) | 1.61 (1.38-1.87) | < 0.001 |
| Clonazepam | 110 (0.2) | 183 (0.1) | 2.29 (1.80-2.90) | 1.60 (1.23-2.08) | < 0.001 |
| Lorazepam | 508 (1.0) | 869 (0.5) | 2.18 (1.96-2.44) | 1.52 (1.34-1.72) | < 0.001 |
| Temazepam | 361 (0.7) | 580 (0.3) | 2.33 (2.04-2.66) | 1.80 (1.56-2.09) | < 0.001 |
| Long-term use | |||||
| Non-BZDs | 1,033 (2.0) | 2,187 (1.2) | 1.79 (1.66-1.93) | 1.16 (1.06-1.25) | < 0.001 |
| BZDs | 4,198 (8.2) | 9,219 (4.9) | 1.79 (1.72-1.86) | 1.14 (1.09-1.19) | < 0.001 |
| Alprazolam | 622 (1.2) | 1,413 (0.8) | 1.66 (1.51-1.82) | 1.08 (0.98-1.20) | 0.132 |
| Clonazepam | 562 (1.1) | 1,338 (0.7) | 1.56 (1.42-1.73) | 0.99 (0.88-1.10) | 0.811 |
| Lorazepam | 678 (1.3) | 1,595 (0.9) | 1.58 (1.44-1.73) | 0.96 (0.87-1.06) | 0.438 |
| Temazepam | 1,037 (2.0) | 2,332 (1.2) | 1.66 (1.54-1.79) | 1.16 (1.06-1.25) | < 0.001 |
a Adjusted for covariates listed in the Appendix (available in online article).
BZD = benzodiazepine; CI = confidence interval.
When looking at specific BZDs, there was a small but significant increased risk of pneumonia with use of alprazolam (OR = 1.12; 95% CI = 1.05-1.20; P < 0.001); lorazepam (OR = 1.06; 95% CI = 1.00-1.12; P = 0.048); and temazepam (OR = 1.15; 95% CI = 1.09-1.22; P < 0.001), but not with clonazepam (OR = 0.99; 95% CI = 0.91-1.08; P = 0.863; Table 2). Patterns of increased risk of pneumonia were generally consistent with non-BZD hypnotics and for specific BZDs (e.g., alprazolam and lorazepam).
Discussion
While an increased risk of pneumonia with the use of BZDs has been generally demonstrated in previous studies, this population-based study has demonstrated a similarly increased risk of pneumonia with non-BZD hypnotic use among older adults. Current users of non-BZD hypnotics were at the highest risk. Older adults with short-term prescriptions were at higher risk than those with long-term prescriptions, who may have developed physiologic tolerance with prolonged use.
BZD use was also associated with pneumonia risk in a manner consistent not only with non-BZD hypnotic use in this study but also with BZD use in other studies. For instance, a meta-analysis drawing from several sources of data, including U.S. Food and Drug Administration New Drug Application documents, demonstrated an increased risk of any type of infection with the use of non-BZD hypnotics (relative risk = 1.44; 95% CI = 1.25-1.64; P < 0.001), driven primarily by eszopiclone and zolpidem.21 However, in a population-based case-control study in older adults in an integrated health system, BZD use was not associated with pneumonia risk (OR = 1.08; 95% CI = 0.80-1.47).22 A nested case-control study using data from a primary care patient database in the United Kingdom showed that exposure to BZDs was associated with an increased risk of pneumonia (OR = 1.54; 95% CI = 1.42-1.67), as well as an increased risk of 30-day (hazard ratio [HR] = 1.22; 95% CI = 1.06-1.39) and long-term mortality (HR = 1.32; 95% CI = 1.19-1.47). In addition, the United Kingdom analysis for zopiclone found similar results for pneumonia (HR = 1.98; 95% CI = 1.49-2.64) and long-term mortality (HR = 1.97; 95% CI = 1.18-3.28), although not for 30-day mortality (HR = 1.17; 95% CI = 0.53-2.62).23 When a similar but separate analysis of BZD use was conducted in a Taiwanese population, this association was not confirmed.24
Interestingly, clonazepam did not have an elevated risk in contrast to alprazolam, lorazepam, and temazepam. Whereas BZDs such as alprazolam and temazepam are typically prescribed for anxiolytic and hypnotic purposes, respectively, clonazepam also has a significant role in managing epilepsy. Whether the risk of specific BZD agents varies by prescribed indication could present an interesting hypothesis for future investigation.
Limitations
This study has several limitations. First, residual confounding is always a possibility in observational studies. We sought to account for this by adjusting for covariates that could increase risk for pneumonia, including chronic lung diseases, acid-suppressive therapies, and conditions that may predispose one to aspirate. Because of inconsistencies in data reporting and collection, we were unable to incorporate additional factors that could increase the risk of pneumonia into this model. Conditions (e.g., asplenia and organ transplantation); medical procedures (e.g., ventilators and endotracheal intubation); and drug therapies (e.g., glucocorticoids, cytotoxic chemotherapies, and biologics targeting inflammatory cytokines) with immunosuppressive effects constitute several examples, and their omission could result in biased exposure effect estimates.
Second, because this analysis relied on data from clinical and administrative databases, some information could not be readily obtained. Exposure was based on pharmacy dispense records, which may not consistently reflect actual patient usage, particularly for a medication used on an as-needed dosing schedule. Although we tried to avoid the potential for exposure misclassification through the analysis by proximity to the index date as well as duration, misclassification cannot be ruled out and may have accounted for the associations we observed. Because zolpidem accounted for the vast majority of non-BZD drug usage at our institution, it may be difficult to accurately evaluate the risk of pneumonia with other non-BZD hypnotics. There may also have been other variables that were possibly not considered or unable to be captured (e.g., adherence to sleep hygiene measures and taking over-the-counter supplements such as melatonin). Finally, generalizability could be limited, since only individuals within a single health care system in California were studied. Since this health care system is composed of several million patients from diverse geographical areas within the state and from varied socioeconomic backgrounds, this limitation may not be profound.
Although the potential harms are well known, non-BZD hypnotic use remains persistently high in older adults. While short-term users were at more of an increased risk for pneumonia than long-term users, long-term users were still at a slightly higher risk, and residual risk can persist even with the development of tolerance. In a meta-analysis that evaluates various hypnotics for chronic insomnia, even though BZDs and non-BZD hypnotics were effective for chronic insomnia in terms of sleep onset latency, patients were also at a higher risk of harm compared with placebo, whether using BZDs (risk difference [RD] = 0.15), non-BZD hypnotics (RD = 0.07), or antidepressants (RD = 0.09).11 Considering the potential adverse effects of any long-term pharmacologic treatment in older adults, more research is needed to identify and promote safer alternatives for insomnia.
Conclusions
This study’s findings provide additional rationale for reducing the use of high-risk medications, such as non-BZD hypnotics, in older adults. Further studies should be performed to understand the underlying physiologic and pharmacologic mechanisms and validate a possible causal relationship between non-BZD hypnotics and pneumonia. In addition to supporting national quality and safety initiatives with the aim of promoting appropriate medication use in older adults, we hope that this study will facilitate fruitful discussions among prescribers and patients as providers inform their patients of the risks of non-BZD hypnotic use and that both providers and patients will carefully weigh the risks and benefits before initiating or continuing treatment with these medications.
Acknowledgments
The authors thank the following people for their support of this research project: Mirta Millares, PharmD; Jane Takagi, PharmD; Joanne Schottinger, MD; Steve Steinberg, MD; Denis Matsuoka, PharmD; Gene Nakagawa, PharmD; Howard Fullman, MD; Michael Kanter, MD; and Jeffrey Brettler, MD.
APPENDIX. Definition of Study Covariates
| Medical Conditions | ICD-9-CM Codes |
| Previous pneumonia | 480.xx-486.xx, 487.0 |
| Asthma | 493.xx |
| Chronic obstructive pulmonary disease | 490.xx-492.xx, 494.xx-496.xx |
| Upper respiratory tract infection | 473.xx, 477.xx |
| Smoking | 305.1, V15.82 |
| Alcoholism | 303.xx |
| Acute myocardial infarction | 410.xx |
| Dementia | 290.xx, 294.1, 331.0 |
| Swallowing disorders | 438.82, 787.2, V41.6 |
| Nausea | 564.3, 787.0 |
| Parkinson’s disease | 332.xx |
| Medication Covariates | Generic Drug Names |
| Anticonvulsants | Carbamazepine, ethotoin, ezogabine, felbamate, fosphenytoin, gabapentin, lacosamide, lamotrigine, levetiracetam, mephenytoin, oxcarbazepine, paramethadione, phenacemide, phenytoin, pregabalin, primidone, rufinabide, tiagabine, topiramate, trimethadione, vigabatrin, zonisamide |
| Antidepressants | Amitriptyline, amoxapine, bupropion, citalopram, clomipramine, desipramine, desvenlafaxine, doxepin, duloxetine, escitalopram, fluoxetine, fluvoxamine, imipramine, mirtazapine, nefazodone, nortriptyline, paroxetine, phenelzine, protriptyline, sertraline, tranylcypromine, trazodone, trimipramine, venlafaxine, vilazodone |
| Antipsychotics | Aripiprazole, chlorpromazine, chlorprothixene, clozapine, fluphenazine, haloperidol, iloperidone, loxapine, lurasidone, olanzapine, paliperidone, perphenazine, pimozide, prochlorperazine, promazine, quetiapine, risperidone, thioridazine, thiothixene, trifluoperazine, ziprasidone |
| Benzodiazepines (used as a covariate in the non benzodiazepine regressions) | Alprazolam, clonazepam, diazepam, flurazepam, lorazepam, oxazepam, temazepam, triazolam |
| Mood stabilizers | Lithium, divalproex sodium, valproate sodium, valproic acid |
| Nonbenzodiazepine hypnotics (used as a covariate in the benzodiazepine regressions) | Eszopiclone, zaleplon, zolpidem |
| Narcotics | Acetaminophen/codeine, aspirin/codeine, alfentanil, buprenorphine, codeine, fentanyl, hydrocodone/acetaminophen, hydromorphone, levorphanol, meperidine, methadone, morphine, oxycodone, oxycodone/acetaminophen, oxymorphone, propoxyphene, remifentanil, tramadol |
| Proton pump inhibitors | Dexlansoprazole, esomeprazole, lansoprazole, omeprazole, pantoprazole, rabeprazole |
| H2 receptor antagonists | Cimetidine, famotidine, nizatidine, ranitidine |
| Diagnostic Cost Group score | Range 0-30 |
ICD-9-CM = International Classification of Diseases, Ninth Revision, Clinical Modification.
References
- 1.Terzano MG, Rossi M, Palomba V, Smerieri A, Parrino L.. New drugs for insomnia: comparative tolerability of zopiclone, zolpidem, and zaleplon. Drug Saf. 2003;26(4):261-82. [DOI] [PubMed] [Google Scholar]
- 2.Lader M. Zopiclone: is there any dependence and abuse potential? J. Neurol. 1997;244(4 Suppl 1):S18-22. [DOI] [PubMed] [Google Scholar]
- 3.Rush CR. Behavior pharmacology of zolpidem relative to benzodiazepines: a review. Pharmacol Biochem Behav. 1998;61(3):253-69. [DOI] [PubMed] [Google Scholar]
- 4.Darcourt G, Pringuey D, Salliere D, Lavoisy J.. The safety and tolerability of zolpidem—an update. J Psychopharmacol. 1999;13(1):81-93. [DOI] [PubMed] [Google Scholar]
- 5.Hajak G. A comparative assessment of the risks and benefits of zopiclone: a review of 15 years’ clinical experience. Drug Saf. 1999;21(6):457-69. [DOI] [PubMed] [Google Scholar]
- 6.Sokya M, Bottlender R, Moller HJ.. Epidemiological evidence for a low abuse potential of zolpidem. Pharmacopsychiatry. 2000;33(4):138-41. [DOI] [PubMed] [Google Scholar]
- 7.Neubauer DN. Pharmacologic approaches for the treatment of chronic insomnia. Clin Cornerstone. 2003;5(3):16-27. [DOI] [PubMed] [Google Scholar]
- 8.Rasu RS, Shenolikar RA, Nahata MC, Balkrishnan R.. Physician and patient factors associated with the prescribing of medications for sleep difficulties that are associated with high abuse potential or are expensive: an analysis of data from the National Ambulatory Medical Care Survey for 1996-2001. Clin Ther. 2005(12);27:1970-79. [DOI] [PubMed] [Google Scholar]
- 9.Moloney ME, Konrad TR, Zimmer CR.. The medicalization of sleeplessness: a public health concern. Am J Public Health. 2011;101(8):1429-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ford ES, Wheaton AG, Cunningham TJ, Giles WH1, Chapman DP, Croft JB.. Trends in outpatient visits for insomnia, sleep apnea, and prescriptions for sleep medications among U.S. adults: findings from the National Ambulatory Medical Care Survey 1999-2010. Sleep. 2014;37(8):1283-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buscemi N, Vandermeer B, Friesen C, et al. The efficacy and safety of drug treatments for chronic insomnia in adults: a meta-analysis of RCTs. J Gen Intern Med. 2007;22(9):1335-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holbrook AM, Crowther R, Lotter A, Cheng C, King D.. Meta-analysis of benzodiazepine use in the treatment of insomnia. CMAJ. 2000;162(2):225-33. [PMC free article] [PubMed] [Google Scholar]
- 13.Glass J, Lanctot KL, Herrmann N, Sproule BA, Busto UE.. Sedative hypnotics in older people with insomnia: meta-analysis of risks and benefits. BMJ. 2005;331(7526):1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Southworth MR, Kortepeter C, Hughes A.. Nonbenzodiazepine hypnotic use and cases of “sleep driving.” Ann Intern Med. 2008;148(6):486-87. [DOI] [PubMed] [Google Scholar]
- 15.Berry SD, Lee Y, Cai S, Dore DD.. Nonbenzodiazepine sleep medication use and hip fractures in nursing home residents. JAMA Intern Med. 2013;173(9):754-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Diem SJ, Ewing SK, Stone KL, et al. Use of non-benzodiazepine sedative hypnotics and risk of falls in older men. J Gerontol Geriatr Res. 2014;3(3):158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weich S, Pearce HL, Croft P, et al. Effect of anxiolytic and hypnotic drug prescriptions on mortality hazards: retrospective cohort study. BMJ. 2014;348:1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hampton LM, Daubresse M, Chang HY, Alexander GC, Budnitz DS.. Emergency department visits by adults for psychiatric medication adverse events. JAMA Psychiatry. 2014;71(9):1006-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2015;63(11):2227-46. [DOI] [PubMed] [Google Scholar]
- 20.National Committee for Quality Assurance. HEDIS 2015. Volume 1: narrative. Available at: http://www.ncqa.org/hedis-quality-measurement/hedis-measures/hedis-2015. Accessed June 27, 2016.
- 21.Joya FL, Kripke DF, Loving RT, Dawson A, Kline LE.. Meta-analyses of hypnotics and infections: eszopiclone, ramelteon, zaleplon, and zolpidem. J Clin Sleep Med. 2009;5(4):377-83. [PMC free article] [PubMed] [Google Scholar]
- 22.Dublin S, Walker RL, Jackson ML, et al. Use of opioids or benzodiazepines and risk of pneumonia in older adults: a population-based case-control study. J Am Geriatr Soc. 2011;59(10):1899-907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Obiora E, Hubbard R, Sanders RD, Myles PR.. The impact of benzodiazepines on occurrence of pneumonia and mortality from pneumonia: a nested case-control and survival analysis in a population-based cohort. Thorax. 2013;68(2):163-70. [DOI] [PubMed] [Google Scholar]
- 24.Iqbal U, Syed-Abdul S, Nguyen PA, Jian WS, Li YC.. The impact of benzodiazepines on occurrence of pneumonia and mortality from pneumonia: a nested case-control and survival analysis in a population-based cohort. Thorax. 2013;68(6):591-92. [DOI] [PubMed] [Google Scholar]
- 25.Sanders RD, Godlee A, Fujimori T, et al. Benzodiazepine augmented γ-amino-butyric acid signaling increases mortality from pneumonia in mice. Crit Care Med. 2013;41(7):1627-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Campbell-Taylor I. Benzodiazepines and pneumonia or aspiration pneumonitis. Thorax. 2013;68(6):591. [DOI] [PubMed] [Google Scholar]
- 27.Rothman KJ, Greenland S.. Case-control studies. In: Rothman KJ, Greenland S, eds.. Modern Epidemiology. 2nd ed. Philadelphia, PA: Lippincott-Raven Publishers; 1998:93-114. [Google Scholar]
- 28.Ash AS, Ellis RP, Pope GC, et al. Using diagnoses to describe populations and predict costs. Health Care Financ Rev. 2000;21(3):7-28. Available at: http://www.cms.gov/HealthCareFinancingReview/Downloads/00springpg7.pdf. Accessed June 27, 2016. [PMC free article] [PubMed] [Google Scholar]


