Abstract
BACKGROUND:
Clinical trials impose exclusion criteria that may limit the generalizability of results.
OBJECTIVES:
To (a) determine the percentage of real-world patients who would qualify for psoriasis randomized controlled trials; (b) ascertain differences between moderate-to-severe psoriasis patients who would be eligible, ineligible, or potentially eligible for clinical trials; and (c) compare their biologic treatment patterns.
METHODS:
Moderate-to-severe psoriasis patients were identified from the U.S. Department of Defense health care database from January 1, 2008, to October 31, 2013. Eligibility classification for psoriasis trials was based on common trial exclusion criteria involving medical conditions and recent treatment history. Patient characteristics and treatment patterns of 4 biologics (adalimumab, etanercept, infliximab, and ustekinumab) were compared between groups. Adherence was measured by medication possession ratio and persistence as continuous time on drug with ≤ 90-day gap between supply times.
RESULTS:
Among 16,284 qualifying psoriasis patients, 4,677 (28.7%) were medically ineligible, and 8,466 (52.0%) had ineligibility-related treatments that could be stopped prior to trial entry; the latter patients were considered potentially eligible for psoriasis trials. Common reasons for medical ineligibility included malignancies and hematologic disorders; treatment ineligibilities included use of topical corticosteroids and phototherapy. Medically ineligible patients were older and had more comorbidities, while potentially eligible patients were younger and healthier than trial-eligible patients. Most treatment patterns were similar across groups, except that, compared with the trial-eligible group, medically ineligible patients had greater adherence to infliximab and potentially trial-eligible patients had greater adherence and persistence to adalimumab.
CONCLUSIONS:
This large real-world study found that patients who may be ineligible for psoriasis trials differ in important respects (e.g., comorbidities, prior treatments) from their trial-eligible counterparts. Regardless of their differences at baseline, adherence, persistence, and switching of biologic medications are largely similar, with few differences noted among groups.
What is already known about this subject
Clinical trials impose many restrictions on eligibility that may result in trial populations not being representative of those receiving the studied drug in the real-world setting.
Real-world treatment adherence and persistence are generally lower than with treatment given under the more tightly controlled conditions of a randomized controlled trial.
What this study adds
Results from this study corroborate what has been observed across other disease domains: that patients who may be ineligible for psoriasis trials differ in important respects (e.g., comorbidities, prior treatments) from their trial-eligible counterparts. Regardless of their differences, adherence, persistence, and switching of biologic medications are largely similar.
In clinical practice, patients with moderate-to-severe psoriasis would be expected to have more comorbidities but similar biologic medication use patterns (e.g., adherence, persistence) to those seen in clinical trial patients.
Psoriasis is a chronic, inflammatory, and proliferative dermatologic disorder that follows a repeated course of remission and relapse. It is characterized by scaly erythematous plaques that are often painful and/or pruritic.1 In addition to skin and joint diseases, psoriasis is also linked to other comorbidities (e.g., autoimmune diseases, cardiovascular disease, metabolic syndrome, skin cancer) and psychological stress (e.g., depression, suicidality) and has a significant impact on patients’ quality of life.2
Several classes of therapy are available to manage psoriasis, including topical agents, phototherapy, nonbiologic oral systemic agents, and biologic agents; clinical guidelines3 and emerging evidence from randomized controlled trials (RCTs) should guide their use. Although RCTs are the “gold standard” for assessing a medicine’s efficacy, they still have limitations.4 The hallmark strength of an RCT is that randomization generally distributes potential confounders similarly across treatment groups; thus, an observed difference between treatment groups is presumed to be causally related to the intervention. A limitation of RCTs is that the very elements that contribute to an unbiased, precise effect estimate (e.g., optimized drug administration and adherence, homogeneous study population) may have a negative effect on the generalizability of results.5 To maximize internal validity and reduce the risk of adverse events, RCTs may include restrictive eligibility criteria,6-10 which may be increasing over time in psoriasis11 and other disease states.12,13 In addition, RCTs have formal study procedures that may not apply to clinical practice; the Psoriasis Area and Severity Index (PASI) is commonly used to assess disease severity in psoriasis clinical trials but has limited use in clinical practice.2 As a result, the real-world applicability of RCT results may be debated.6,8,11,13-20
The objectives of this retrospective cohort study were to (a) determine the percentage of real-world patients who would qualify for psoriasis RCTs; (b) compare differences in baseline characteristics between groups eligible and ineligible for psoriasis RCTs; and (c) assess whether biologic treatment patterns differ based on trial eligibility.
Methods
Data Source
The U.S. Department of Defense (DoD) health care database served as the sole data source for this study and included data from January 1, 2008, to October 31, 2014. The DoD database represents one of the largest health care databases in the United States and includes integrated inpatient, outpatient, and medication data for nearly 10 million active beneficiaries, composed of military personnel, retirees, and their families.21 The database includes administrative claims-level information (e.g., diagnosis and procedure codes, pharmacy drug fills) for care delivered in civilian facilities and claims plus electronic health record-level information (e.g., laboratory results) for care in military facilities. Research data were derived from an approved Naval Medical Center, Portsmouth, Virginia, institutional review board protocol “NMCP.2015.0030.”
Patient Selection
A claims-based psoriasis diagnosis by a dermatologist with evidence of prior receipt of systemic therapy or phototherapy was used as a proxy for moderate-to-severe psoriasis. Qualifying patients met all of the following inclusion criteria: had an outpatient psoriasis diagnosis (International Classification of Diseases, Ninth Revision, Clinical Modification code 696.1) from a dermatologist during the cohort selection period (January 1, 2008, to October 31, 2013), received phototherapy and/or systemic therapy before the index date, had continuous enrollment with medical and pharmacy benefits 12 months pre-index, and were ≥ 18 years as of the index date; the index date was defined as the earliest claim with a psoriasis diagnosis after meeting all inclusion criteria. Exclusion criteria were active-duty military during the study period (restrictions exist for biologic use during active duty) and a psoriasis diagnosis code appearing only on claims with a phototherapy procedure code and not on other dermatologist visit claims (the diagnosis code was frequently observed during chart review to be erroneously entered as justification for treatment; Figure 1).
FIGURE 1.

Patient Attrition
The algorithm for defining psoriasis (claims showing a dermatologist diagnosis plus systemic or phototherapy) was tested via chart review of a random sample of 250 patients treated in military facilities. Results of the chart review indicated that the algorithm had a positive predictive value of 83%.
Patients with moderate-to-severe psoriasis were categorized into a trial-eligible cohort if they had none of a list of RCT exclusion criteria commonly used in trials of medications for moderate-to-severe psoriasis (Table 1); patients with treatment-related exclusion criteria (i.e., received systemic nonbiologic psoriasis therapy within 4 weeks prior to index, biologic agents [etanercept < 28 days; infliximab, adalimumab, or alefacept < 60 days; ustekinumab < 8 months; rituximab or efalizumab < 12 months prior to index], or a live vaccine 12 weeks prior to index) were categorized as potentially trial-eligible, since these patients theoretically could be eligible if the excluded treatment was stopped or if trial enrollment was delayed in the case of vaccine exposure. Patients with the remaining nonmodifiable ineligibility criteria listed in Table 1 were categorized into a medically trial-ineligible cohort.
TABLE 1.
Frequencies of Psoriasis Biologic Trial Ineligibility Criteria Among Moderate-to-Severe Psoriasis Patients
| Baseline Patient Characteristics | Patients with Moderate-to-Severe Psoriasis (n=16,284) | Medically Trial-Ineligible Cohort (n=4,677) | Potentially Trial-Eligible Cohort (n=8,466) |
|---|---|---|---|
| Received systemic nonbiologic psoriasis therapy within 4 weeks prior to index date or topical psoriasis treatment (classes 1-5) within 2 weeks prior to index date, n (%) | |||
| Systemic nonbiologic therapy | |||
| Cyclosporine | 150 (0.9) | 33 (0.7) | 117 (1.4) |
| Oral/IV corticosteroids | 1,204 (7.4) | 362 (7.7) | 842 (9.9) |
| Methotrexate | 1,827 (11.2) | 474 (10.1) | 1,353 (16.0) |
| Oral retinoids (acitretin) | 813 (5.0) | 216 (4.6) | 597 (7.1) |
| Mycophenolate mofetil | 49 (0.3) | 14 (0.3) | 35 (0.4) |
| Thioguanine | 4 (0.02) | 1 (0.02) | 3 (0.04) |
| Hydroxyurea | 25 (0.2) | 21 (0.4) | 4 (0.05) |
| Sirolimus | 4 (0.02) | 3 (0.06) | 1 (0.01) |
| Azathioprine | 66 (0.4) | 32 (0.7) | 34 (0.4) |
| Phototherapy/PUVA, UVB | 2,541 (15.6) | 730 (15.6) | 1,811 (21.4) |
| Topical medications | |||
| Topical corticosteroids (classes 1-5) | 4,233 (26.0) | 1,320 (28.2) | 2,913 (34.4) |
| Anthralin (dithranol) | 1 (0.006) | 1 (0.02) | 0 (0.0) |
| Calcipotriene | 1,424 (8.7) | 380 (8.1) | 1,044 (12.3) |
| Tazarotene | 80 (0.5) | 21 (0.4) | 59 (0.7) |
| Concurrent or recent use of any biologic agent within the specified washout periods, n (%) | |||
| Etanercept < 28 days | 1,504 (9.2) | 333 (7.1) | 1,171 (13.8) |
| Infliximab < 60 days | 313 (1.9) | 95 (2.0) | 218 (2.6) |
| Adalimumab < 60 days | 1,832 (11.3) | 434 (9.3) | 1,398 (16.5) |
| Alefacept < 60 days | 23 (0.1) | 4 (0.09) | 19 (0.2) |
| Ustekinumab <8 months | 82 (0.5) | 20 (0.4) | 62 (0.7) |
| Rituximab < 12 months | 110 (0.7) | 96 (2.1) | 14 (0.2) |
| Efalizumab < 12 months | 283 (1.7) | 73 (1.6) | 210 (2.5) |
| Lymphoproliferative disease or malignant disease < 12 months, n (%) | |||
| Lymphoproliferative disease | 31 (0.2) | 31 (0.7) | 0 (0.0) |
| Malignant disease | 2,248 (13.8) | 2,248 (48.1) | 0 (0.0) |
| Cerebro-cardiovascular, respiratory, hepatic, renal, gastrointestinal, endocrine, hematologic, neurologic, or neuropsychiatric disorders within 12 weeks before index date, n (%) | |||
| Cerebro-cardiovascular | |||
| Myocardial infarction | 39 (0.2) | 39 (0.8) | 0 (0.0) |
| Unstable angina | 47 (0.3) | 47 (1.0) | 0 (0.0) |
| Hypertension diagnosis or at least 1 high BP reading (systolic BP >160 mm Hg or diastolic BP >100 mm Hg) | 316 (1.9) | 316 (6.8) | 0 (0.0) |
| Moderate-to-severe heart failure | 360 (2.2) | 360 (7.7) | 0 (0.0) |
| Cerebrovascular accident | 146 (0.9) | 146 (3.1) | 0 (0.0) |
| Respiratory | 9 (0.06) | 9 (0.2) | 0 (0.0) |
| Hepatic | 311 (1.9) | 311 (6.6) | 0 (0.0) |
| Renal | 519 (3.2) | 519 (11.1) | 0 (0.0) |
| Gastrointestinal disorder | 205 (1.3) | 205 (4.4) | 0 (0.0) |
| Endocrine disorder | 26 (0.2) | 26 (0.6) | 0 (0.0) |
| Hematologic disorder | 998 (6.1) | 998 (21.3) | 0 (0.0) |
| Neurologic or neuropsychiatric disorders | 4 (0.02) | 4 (0.09) | 0 (0.0) |
| Infection either during an inpatient stay or on a claim for IV antibiotics within 12 weeks prior to index, n (%) | |||
| Pneumonia | 45 (0.3) | 45 (1.0) | 0 (0.0) |
| Cellulitis | 62 (0.4) | 62 (1.3) | 0 (0.0) |
| Sepsis | 21 (0.1) | 21 (0.4) | 0 (0.0) |
| Herpes zoster infection or any other clinically apparent varicella-zoster virus infection within 12 weeks prior to index, n (%) | |||
| Herpes zoster | 91 (0.6) | 91 (1.9) | 0 (0.0) |
| Human immunodeficiency virus | 8 (0.05) | 8 (0.2) | 0 (0.0) |
| Herpes zoster infection or any other clinically apparent varicella-zoster virus infection within 12 weeks prior to index, n (%) | |||
| Hepatitis B | 46 (0.3) | 46 (1.0) | 0 (0.0) |
| Abnormal labs within 30 days prior to or on index, n/N (%) | |||
| Neutrophil count < 1,500 cells/µL | 10/647 (1.5) | 10/228 (4.4) | 0/352 (0.0) |
| Lymphocyte count < 800 cells/µL | 14/654 (2.1) | 14/227 (6.2) | 0/357 (0.0) |
| Platelet count < 100,000 cells/µL | 5/811 (0.6) | 5/287 (1.7) | 0/443 (0.0) |
| AST level > 2.5 times the ULN | 11/873 (1.3) | 11/295 (3.7) | 0/492 (0.0) |
| ALT level > 2.5 times the ULN | 20/868 (2.3) | 20/293 (6.8) | 0/490 (0.0) |
| Total WBC count < 3,000 cells/µL | 9/839 (1.1) | 9/295 (3.1) | 0/460 (0.0) |
| Hemoglobin level < 8.5 g/dL for males; <8.0 g/dL for females | 3/837 (0.4) | 3/295 (1.0) | 0/459 (0.0) |
| Serum creatinine level > 2.0 mg/dL | 8/757 (1.1) | 8/291 (2.7) | 0/397 (0.0) |
| Pregnancy | 294 (1.8) | 294 (6.3) | 0 (0.0) |
| Active or latent tuberculosis | 52 (0.3) | 52 (1.1) | 0 (0.0) |
| Hepatitis C | 99 (0.6) | 99 (2.1) | 0 (0.0) |
| Natalizumab or vedolizumab during cohort selection period | 4 (0.02) | 4 (0.09) | 0 (0.0) |
| Live vaccination within 12 weeks prior to index date | 86 (0.5) | 24 (0.5) | 62 (0.7) |
| Inpatient hospitalization within 12 weeks before index date | 502 (3.1) | 502 (10.7) | 0 (0.0) |
| Demyelinating disorder | 51 (0.3) | 51 (1.1) | 0 (0.0) |
| Body temperature ≥ 38°C (100.5°F) at index date | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Presence of at least one of the above (i.e., qualifies as trial-ineligible) | 13,143 (80.7) | 4,677 (100.0) | 8,466 (100.0) |
ALT = alanine aminotransferase; AST = aspartate aminotransferase; BP = blood pressure; IV = intravenous; PUVA = psoralen/ultraviolet A; SD = standard deviation; ULN = upper limit of normal; UVB = ultraviolet B; WBC = white blood cell.
Baseline Variables
Baseline comorbidities were identified using diagnosis and procedure codes from the baseline period, defined as 12 months prior to the index date (i.e., the first claim with an outpatient psoriasis diagnosis by a dermatologist following a claim for systemic therapy or phototherapy) through the index date (inclusive). Baseline variables included demographics, Charlson Comorbidity Index (CCI), psoriasis disease duration (derived from all available claims data before index), other autoimmune conditions, cardiovascular conditions/other comorbidities, and use of medications for psoriasis, including the number of biologics received during the baseline period.
Biologic Usage Measures
Each patient’s index therapy was defined as the first biologic received on or after the index date; eligible biologics included etanercept, adalimumab, infliximab, and ustekinumab. Analyses were conducted for each biologic separately. The following measures were estimated for each biologic initiated from the index date through end of follow-up:
Adherence was estimated using the medication possession ratio (MPR) for up to 12 months following initiation. MPR was calculated by dividing total days’ supply of filled biologic over the number of days following initiation, censoring data for patients who unenrolled from the health plan prior to 12 months after the index date. MPR values were capped at 100% for patients with overlapping prescriptions. MPR ≥ 80% was considered highly adherent.
- Persistence with the index biologic was examined as the continuous time, assessed up to 12 months following initiation, on drug with a ≤ 90-day gap between supply times; each biologic was assigned a supply time of the indicated days between sequential administrations during the maintenance phase of use. The following supply-time calculations pertained only to clinic-administered drugs; for pharmacy-dispensed drugs, days supply data were used.
- The indicated intervals between administrations of etanercept, adalimumab, infliximab, and ustekinumab are 7, 14, 56, and 84 days, respectively, so each administration was assumed to last the corresponding number of days depending on the biologic. A subsequent administration during the indicated period or within the next 90 days continued the treatment episode, counting as continuous use. A gap of 90 or more days after finishing the indicated supply days was considered a discontinuation, as was switching to a different biologic without a continuation of the initial biologic.
Switching was defined as discontinuing the index biologic and starting a different biologic within 90 days. Time to first switch was calculated as the number of days from the index biologic start date to the initiation of the second biologic.
Medication restart was defined as a claim for the same medication following a discontinuation (i.e., a > 90-day gap in treatment), regardless of other biologics that may have been used following the discontinuation. Time to restart was calculated as the number of days from the index biologic stop date to the reinitiation of the same biologic.
Discontinuation of the index biologic prior to end of follow-up was examined for each patient.
Time to start of the first biologic received on or after the index date was also examined.
Statistical Analyses
SAS software version 9.3 (SAS Institute, Cary, NC) was used to conduct the analyses. The number and percentage of patients meeting each trial-ineligibility criteria were tabulated for the full cohort and separately among the 2 subgroups of trial-ineligible patients.
Demographic, clinical, and other baseline variables were summarized for each subgroup and the overall cohort as the counts and percentages for categorical variables and means, medians, standard deviations (SDs), and ranges for continuous variables. Differences between cohorts were assessed using chi-square or Fisher’s exact tests for categorical variables and t-tests or analysis of variance for continuous variables. Nonparametric tests (e.g., Wilcoxon rank-sum test for ordinal variables) were used as appropriate.
Biologic utilization was compared separately for initiators of etanercept, adalimumab, infliximab, and ustekinumab as the index therapy. Adherence, persistence, and switching were examined within each of these 4 groups. Adherence was summarized as continuous values of MPR (mean, SD, median, range) and categorically in intervals of 20%. Adherence was also dichotomized as high (≥ 80%) versus low (< 80%), as is commonly used in medication adherence research.22 The number and percentage of patients who discontinued the index biologic at any time during follow-up, who discontinued and then restarted, and who switched to a different biologic were presented. Chi-square tests were used for the above metrics, with MPR categorized as ≥ 80% versus < 80%. Kaplan-Meier (KM) curves and log-rank tests were used to evaluate time to discontinuation (persistence) of the index biologic, with a separate plot for each drug and separate curves for trial-eligible, medically trial-ineligible, and potentially eligible patients. Median time to each of these events was obtained from the KM plots. For each outcome, pairwise comparisons were made between (1) medically ineligible versus trial-eligible and (2) potentially eligible versus trial-eligible patients.
MPR ≥ 80% was compared between groups using logistic regression modeling, examining all baseline characteristics not used as ineligibility criteria. Univariate models estimated the odds ratio (OR) and 95% confidence intervals (CIs) for each variable. A final, fully adjusted model was constructed using backward selection with P < 0.05 to identify all simultaneously significant predictors of high adherence. Time to treatment discontinuation was similarly modeled, with univariate and multivariable Cox models providing hazard ratios (HRs) and 95% CIs.
Results
Cohort Selection and Trial Eligibility
In total, 147,323 patients in the DoD database had a diagnosis code for psoriasis during the study period (Figure 1). Of these, 77,055 (52.3%) had at least 1 psoriasis diagnosis from a dermatologist, and 18,677 (12.7%) had claims for systemic therapy or phototherapy. Applying additional inclusion and exclusion criteria resulted in a final cohort size of 16,284 patients.
In the claims data, 4,677 (28.7%) patients met 1 or more medical ineligibility criteria for psoriasis biologic trials, while 8,466 (52.0%) had ongoing treatment that made them ineligible for trials as of their index date but who could be potentially eligible if they discontinued that treatment (Table 1). The most common reasons for medical ineligibility were malignant disease during the past year (2,248 patients; 13.8% of all patients and 48.1% of medically ineligible patients) and hematologic disorders during the past year (998 patients; 6.1% of all patients and 21.3% of medically ineligible patients). The most common trial ineligibility criteria in the potentially eligible cohort were topical corticosteroid use within 2 weeks prior to the index date (26.0% [4,233] of all patients; 34.3% [2,913] of potentially eligible patients), phototherapy use (15.6% [2,541] of all patients; 21.4% [1,811] of potentially eligible patients) or methotrexate use (11.2% [1,827] of all patients; 16.0% [1,353] of potentially eligible patients) within the same time frame, and adalimumab use within 60 days prior to the index date (11.3% [1,832] of all patients; 16.5% [1,398] of potentially eligible patients).
Numerous baseline characteristics differed between trial-eligible, medically trial-ineligible, and potentially eligible groups (Table 2). The medically ineligible patients were, on average, 7 years older than the trial-eligible patients (P < 0.0001) but had a similar gender distribution. Compared with the patients who would have been eligible for trials, the medically ineligible group had a higher mean CCI (P < 0.001) and a shorter mean duration of psoriasis (difference of 4.6 months, P < 0.001). All examined comorbidities, except ankylosing spondylitis and psoriatic arthritis, were significantly (P < 0.05) more common among the medically ineligible patients. History of coronary artery bypass graft during the baseline period was not significantly different between groups. The medically ineligible patients were more likely than their trial-eligible counterparts to have received oral/intravenous (IV) or topical corticosteroids (P < 0.001), mycophenolate mofetil (P < 0.001), hydroxyurea (P < 0.001), azathioprine (P = 0.0055), topical corticosteroids (class 1-5; P < 0.001), and phototherapy (P < 0.001) during baseline but less likely to have received oral retinoids (P < 0.001) and methotrexate (P = 0.034). Prior biologic use was more common among the medically ineligible patients, although the specific biologics used showed differential patterns between groups: the medically ineligible patients were more often treated with adalimumab or infliximab than the trial-eligible patients but less likely to be treated with etanercept.
TABLE 2.
Baseline Characteristics of Patients with Moderate-to-Severe Psoriasis Who Would Be Eligible, Potentially Eligible, or Medically Ineligible for Clinical Trials
| Baselinea Patient Characteristics | Trial-Eligible Cohort (n = 3,141) | Medically Trial-Ineligible Cohort (n = 4,677) | P Valueb vs. Trial-Eligible | Potentially Trial-Eligible Cohort (n = 8,466) | P Valueb vs. Trial-Eligible |
|---|---|---|---|---|---|
| Age (years) | |||||
| Mean (SD) | 56.3 (16.1) | 62.9 (15.6) | < 0.0001 | 54.6 (15.9) | < 0.0001 |
| Median (range) | 59 (18-85) | 66 (18-85) | 56 (18-85) | ||
| Age, categorized, n (%) | |||||
| 18 to < 45 | 692 (22.0) | 655 (14.0) | < 0.0001 | 2,054 (24.3) | < 0.0001 |
| 45 to < 55 | 587 (18.7) | 477 (10.2) | 1,736 (20.5) | ||
| 55 to < 65 | 778 (24.8) | 1,025 (21.9) | 2,192 (25.9) | ||
| 65 to < 75 | 725 (23.1) | 1,369 (29.3) | 1,751 (20.7) | ||
| 75+ | 359 (11.4) | 1,151 (24.6) | 733 (8.7) | ||
| Gender, n (%) | |||||
| Male | 1,264 (40.2) | 1,957 (41.8) | 0.16 | 3,386 (40.0) | 0.81 |
| Female | 1,877 (59.8) | 2,720 (58.2) | 5,080 (60.0) | ||
| CCI | |||||
| Mean (SD) | 0.9 (1.4) | 2.6 (2.5) | < 0.0001 | 0.8 (1.3) | 0.0078 |
| Median (range) | 0 (0-10) | 2 (0-17) | 0 (0-11) | ||
| CCI, categorized, n (%) | |||||
| 0-1 | 2,489 (79.2) | 1,811 (38.7) | < 0.0001 | 6,864 (81.1) | 0.013 |
| 2 | 267 (8.5) | 789 (16.9) | 749 (8.8) | ||
| 3 | 190 (6.0) | 672 (14.4) | 421 (5.0) | ||
| 4+ | 195 (6.2) | 1,405 (30.0) | 432 (5.1) | ||
| Disease duration of psoriasis, months | |||||
| Mean (SD) | 33.5 (22.0) | 28.9 (23.3) | < 0.0001 | 28.3 (22.5) | < 0.0001 |
| Median (range) | 39 (0-60) | 30 (0-60) | 27 (0-60) | ||
| Disease duration of psoriasis, categorized, n (%) | |||||
| < 6 months | 621 (19.8) | 1,395 (29.8) | < 0.0001 | 2,334 (27.6) | < 0.0001 |
| 6 to < 12 months | 188 (6.0) | 294 (6.3) | 664 (7.8) | ||
| 12 to < 24 months | 323 (10.3) | 452 (9.7) | 1,017 (12.0) | ||
| 24 to < 36 months | 335 (10.7) | 434 (9.3) | 833 (9.8) | ||
| 36+ months | 1,674 (53.3) | 2,102 (44.9) | 3,618 (42.7) | ||
| Conditions during baseline,a n (%) | |||||
| Depression | 374 (11.9) | 752 (16.1) | < 0.0001 | 1,060 (12.5) | 0.37 |
| Ankylosing spondylitis | 13 (0.4) | 26 (0.6) | 0.38 | 52 (0.6) | 0.2 |
| Rheumatoid arthritis | 274 (8.7) | 620 (13.3) | < 0.0001 | 801 (9.5) | 0.22 |
| Ulcerative colitis | 35 (1.1) | 83 (1.8) | 0.019 | 79 (0.9) | 0.38 |
| Psoriatic arthritis | 502 (16.0) | 817 (17.5) | 0.085 | 1,521 (18.0) | 0.012 |
| Diabetes, uncomplicated | 619 (19.7) | 1,404 (30.0) | < 0.0001 | 1,561 (18.4) | 0.12 |
| Diabetes, complicated | 187 (6.0) | 569 (12.2) | < 0.0001 | 437 (5.2) | 0.093 |
| Dyslipidemia | 1,566 (49.9) | 2,880 (61.6) | < 0.0001 | 4,066 (48.0) | 0.08 |
| Obesity | 277 (8.8) | 623 (13.3) | < 0.0001 | 864 (10.2) | 0.026 |
| Chronic obstructive pulmonary disease | 61 (1.9) | 269 (5.8) | < 0.0001 | 156 (1.8) | 0.73 |
| Cardiac arrhythmias | 218 (6.9) | 859 (18.4) | < 0.0001 | 536 (6.3) | 0.24 |
| Peripheral vascular disorders | 184 (5.9) | 614 (13.1) | < 0.0001 | 371 (4.4) | 0.0009 |
| Percutaneous transluminal coronary angioplasty | 18 (0.6) | 78 (1.7) | < 0.0001 | 36 (0.4) | 0.3 |
| Coronary artery bypass graft | 8 (0.3) | 21 (0.4) | 0.17 | 13 (0.2) | 0.25 |
| Systemic nonbiologic therapy from baselinea up to 4 weeks prior to index date, n (%) | |||||
| Cyclosporine | 48 (1.5) | 58 (1.2) | 0.28 | 115 (1.4) | 0.49 |
| Oral/IV corticosteroids | 779 (24.8) | 1,565 (33.5) | < 0.0001 | 2,313 (27.3) | 0.0064 |
| Methotrexate | 650 (20.7) | 877 (18.8) | 0.034 | 1,481 (17.5) | < 0.0001 |
| Oral retinoids (acitretin) | 357 (11.4) | 308 (6.6) | < 0.0001 | 552 (6.5) | < 0.0001 |
| Mycophenolate mofetil | 12 (0.4) | 55 (1.2) | 0.0002 | 41 (0.5) | 0.47 |
| Thioguanine | 0 (0.0) | 4 (0.09) | 0.15 | 3 (0.04) | 0.57 |
| Hydroxyurea | 2 (0.06) | 52 (1.1) | < 0.0001 | 5 (0.06) | 1 |
| Systemic nonbiologic therapy from baselinea up to 4 weeks prior to index date, n (%) | |||||
| Sirolimus | 1 (0.03) | 6 (0.1) | 0.25 | 1 (0.01) | 0.47 |
| Azathioprine | 27 (0.9) | 74 (1.6) | 0.0055 | 52 (0.6) | 0.15 |
| Phototherapy/PUVA, UVB | 165 (5.3) | 366 (7.8) | < 0.0001 | 671 (7.9) | < 0.0001 |
| Topical medications from baselinea up to 2 weeks prior to index date, n (%) | |||||
| Topical corticosteroids (classes 1-5) | 1,634 (52.0) | 2,918 (62.4) | < 0.0001 | 5,598 (66.1) | < 0.0001 |
| Anthralin (dithranol) | 5 (0.2) | 6 (0.1) | 0.72 | 20 (0.2) | 0.43 |
| Calcipotriene | 725 (23.1) | 1,108 (23.7) | 0.53 | 2,336 (27.6) | < 0.0001 |
| Tazarotene | 48 (1.5) | 90 (1.9) | 0.19 | 193 (2.3) | 0.012 |
| Biologic therapy, n (%) | |||||
| Etanercept from baseline to 28 days before index date | 607 (19.3) | 593 (12.7) | < 0.0001 | 1,422 (16.8) | 0.0014 |
| Infliximab from baseline to 60 days before index date | 33 (1.1) | 135 (2.9) | < 0.0001 | 248 (2.9) | < 0.0001 |
| Adalimumab from baseline to 60 days before index date | 238 (7.6) | 416 (8.9) | 0.039 | 1,058 (12.5) | < 0.0001 |
| Alefacept from baseline to 60 days before index date | 6 (0.2) | 10 (0.2) | 0.83 | 13 (0.2) | 0.66 |
| Ustekinumab from baseline to 8 months before index date | 7 (0.2) | 5 (0.1) | 0.2 | 23 (0.3) | 0.65 |
| Number of previous biologics prescribed during baselinea excluding index date, n (%) | |||||
| 0 | 2,277 (72.5) | 3,208 (68.6) | < 0.0001 | 5,001 (59.1) | < 0.0001 |
| 1 | 837 (26.6) | 1,379 (29.5) | 3,218 (38.0) | ||
| 2 or more | 27 (0.9) | 90 (1.9) | 247 (2.9) | ||
aBaseline period covers 12 months before index date through the index date (inclusive).
bP values were calculated between trial-eligible and trial-ineligible from t-tests for continuous variables and chi-square tests for categorical variables.
CCI = Charlson Comorbidity Index; IV = intravenous; PUVA = psoralen/ultraviolet A; SD = standard deviation; UVB=ultraviolet B.
In contrast to the first comparison, the potentially eligible group was about 2 years younger than the trial-eligible patients (P < 0.001), with a slightly lower mean CCI (P = 0.0078). Different results were also seen for comorbidities: while almost all of the considered comorbidities were more prevalent in the medically ineligible group versus the trial-eligible group, only one comorbidity (obesity) was more common in the potentially eligible group versus trial-eligible patients (P = 0.026); peripheral vascular disease (PVD) was less frequent in the potentially eligible group (P < 0.001). Similar to the medically ineligible group, potentially eligible patients were more likely than their trial-eligible counterparts to have received oral/IV or topical corticosteroids (P = 0.0064) and phototherapy (P < 0.001) during baseline and less likely to have received oral retinoids (P < 0.001) and methotrexate (P < 0.001). The potentially eligible patients were also more likely to have used calcipotriene (P < 0.001) and tazarotene (P = 0.012) than the trial-eligible group. Potentially eligible patients had more baseline treatment with adalimumab or infliximab than the trial-eligible group but were less likely to be treated with etanercept.
Biologic Utilization Patterns
Of the 16,284 patients in the full study cohort, 4,343 (26.7%) initiated a biologic on or after the index date. Adalimumab was most commonly the first biologic started on or after the index date, followed by etanercept, in all 3 cohorts.
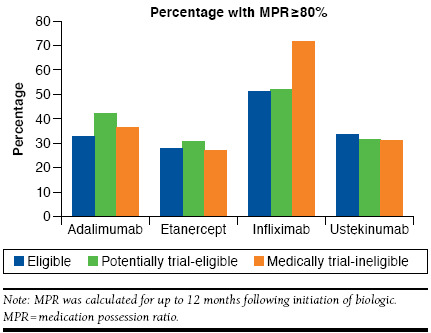
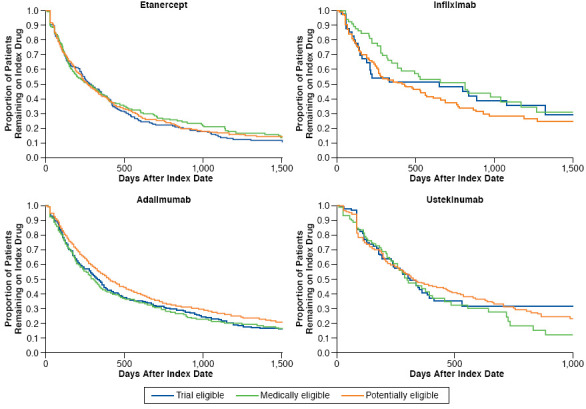
Adherence was similar between the trial-eligible and medically ineligible etanercept initiators and between the trial-eligible and potentially eligible etanercept initiators (Figure 2), with 27.9% of the trial-eligible and 26.9% and 30.5% of medically ineligible and potentially eligible groups, respectively, attaining an MPR ≥ 80%, calculated for up to 12 months following initiation. Frequencies of discontinuing and switching from etanercept were also similar between the trial-eligible (discontinued during the study follow-up period: 80.6%; switched: 16.2%), medically ineligible (discontinued: 75.2%; switched: 16.5%), and potentially eligible (discontinued: 76.2%; switched: 18.3%) groups. Overall, in all eligibility groups, the majority of patients who switched from etanercept moved to adalimumab. Trial-eligible patients had a median treatment duration of 279 days, which was not significantly different from the medically ineligible group (262 days; P = 0.66; Figure 3) or the potentially eligible group (263 days; P = 0.59; Figure 3).
FIGURE 2.
Medication Adherence Among Psoriasis Trial-Eligible and Trial-Ineligible Biologic Treatment Initiators
FIGURE 3.
Kaplan-Meier Plots of Persistence to Biologic Therapy Among Trial-Eligible, Medically Ineligible, and Potentially Eligible Initiators
In the multivariable logistic regression model examining predictors of adherence for etanercept (tabular results not shown), patients 55-64 years old (OR = 1.84; 95% CI = 1.24-2.71; P = 0.0022) and 65-74 years old (OR = 2.29; 95% CI = 1.51-3.48; P < 0.001) had significantly greater odds of high adherence compared with those 18-44 years old; phototherapy within the past year was associated with decreased odds of high adherence (OR = 0.40; 95% CI = 0.19-0.83; P = 0.014). In the Cox model, older age (45-54 years, HR = 0.81, P = 0.041; 55-64 years, HR = 0.82, P = 0.047; 65-74 years, HR = 0.67, P < 0.001; each vs. patients 18-44) was associated with a decreased risk of discontinuation, while male sex (HR = 1.29; P < 0.001) and PVD within the past year (HR = 1.60; P = 0.015) were associated with increased risk of etanercept discontinuation.
Only 234 infliximab initiators were available for analysis, leading to few useful results for this subgroup (Figure 2). The percentage of patients with high adherence (MPR ≥ 80%) was greater for infliximab (57.7% overall vs. 29.0% for etanercept). Medically ineligible patients had greater adherence (71.4%) compared with trial-eligible patients (51.2%; P = 0.032). Median duration of treatment was 651 days for trial-eligible patients, 816 days for medically ineligible patients, and 433 days for potentially eligible patients (P > 0.05; Figure 3). The multivariable model showed that the medically ineligible patients had increased odds of high adherence to infliximab compared with trial-eligible patients (OR = 2.47; 95% CI = 1.09-5.62; P = 0.031). Use of oral/IV corticosteroids within the past year was associated with poorer infliximab adherence (OR = 0.42; 95% CI = 0.24-0.73; P = 0.0019). Only use of oral/IV corticosteroids within the past year was associated with a significant risk of infliximab discontinuation (HR = 1.75; 95% CI = 1.26-2.43; P < 0.001).
Adalimumab initiators comprised the largest treatment cohort (2,443 patients). Trial-eligible and potentially eligible patients had differences in adherence and persistence outcomes (Figure 2). High adherence (MPR ≥ 80%) was seen in 32.7% of trial-eligible patients versus 41.8% of potentially eligible patients (P < 0.001); medically ineligible patients showed no difference (36.3%; P = 0.23) versus the trial-eligible group. Overall discontinuation was more common among the trial-eligible than potentially eligible patients (73.3% vs. 67.7% [P = 0.017], respectively). Switching was not significantly different across groups (trial-eligible: 6.8%; medically ineligible: 9.7%; potentially eligible: 9.4%). Among switchers, the next drug started was most often etanercept or ustekinumab. Trial-eligible patients had a median treatment duration of 331 days; this was not significantly different (P = 0.62) from the medically ineligible patients (307 days) but was significantly shorter than the potentially eligible patients (407 days, P = 0.009; Figure 3). In contrast to the findings for infliximab, the potentially eligible patients had increased odds of high adherence to adalimumab compared with trial-eligible patients (OR = 1.49; 95% CI = 1.21-1.84; P < 0.001). Older age was also associated with greater adherence. Potentially eligible patients also had a significantly decreased risk of adalimumab discontinuation (HR = 0.85; 95% CI = 0.75-0.95; P = 0.006), as did older patients, males, those with a lower CCI, or those with methotrexate use within the past year.
Among the 495 ustekinumab initiators, adherence, discontinuation, and persistence were similar between the trial-eligible and medically ineligible patients. A lower percentage of potentially eligible patients compared with trial-eligible patients switched to a different drug (1.7% and 6.1%, respectively; P = 0.022). Median treatment duration was 309, 298, and 324 days for the trial-eligible, medically ineligible, and trial-ineligible groups, respectively (P > 0.05; Figure 3). In the multivariable logistic regression model for ustekinumab, the only significant predictors of high adherence were age 65-74 compared with age 18-44 (OR = 1.94; P = 0.041), disease duration 18 to < 24 months compared with ≥ 24 months (OR = 0.46; P = 0.044), and tazarotene use within the past year (OR = 16.54; P = 0.01). Significant independent predictors of ustekinumab discontinuation were age 65-74 compared with age 18-44 (OR = 0.61; P = 0.038), psoriatic arthritis in the past year (OR = 1.66; P < 0.001), and uncomplicated diabetes in the past year (OR = 1.46; P = 0.016).
Discussion
This study provides answers to often-posed but seldom-answered questions regarding the differences between RCT and “real-world” psoriasis patients. On the one hand, this study shows that patients who may be ineligible for psoriasis trials differ in important respects (e.g., comorbidities, prior treatments) from their trial-eligible counterparts. On the other hand, regardless of their differences at baseline, adherence, persistence, and switching of biologic medications were largely similar across groups.
The observed higher comorbidity burden, medication burden, and older age in ineligible patients is expected. One surprising finding was the shorter duration of psoriasis among the trial-ineligible patients. Given the slightly older age of trial-ineligible patients, we would expect disease duration to be longer and cannot think of a compelling reason for this difference.
Almost by design, real-world adherence and persistence will be lower than with treatment given under the more tightly controlled conditions of an RCT.23,24 The key question is whether there is something about the patients who do not qualify for RCTs that would make them have higher or lower adherence/persistence to a medicine. To our knowledge, this has not been studied. These results suggest that treatment utilization may vary slightly between psoriasis patients who are/are not eligible for clinical trials of biologics. Infliximab initiators who were trial-ineligible due to medical reasons demonstrated greater adherence to treatment than their trial-eligible counterparts. No such patterns were found for other biologics, however, which suggests that the difference in the relatively small infliximab subgroup may have been simply due to chance. Adalimumab initiators who would have been ineligible due to recent/ongoing treatment but who would be potentially eligible if they stopped treatment had greater adherence and longer time on treatment than trial-eligible patients. Again, this finding was seen only in the adalimumab subgroup and may therefore have been by chance. However, the adalimumab users comprised the largest biologic treatment group, making the findings more robust than for the other biologics. A limitation of medication usage analyses is the small number of patients available for analyses of infliximab and ustekinumab. It is possible that the trial ineligibility criteria involving recent use of psoriasis treatment selected for patients with greater adherence to treatment in the past, who therefore were more likely to continue demonstrating high adherence upon starting a new psoriasis treatment. Older patients also tended to have greater adherence and persistence; this finding is consistent with prior studies of psoriasis patients’ adherence to biologics.25 The degree of biologic initiation among moderate-to-severe psoriasis patients seen in this study is similar to that reported from a medical record review of moderate-to-severe psoriasis patients treated with systemic therapy in which 28.6% were currently receiving or had previously received treatment with a biologic agent at the time of the study.26 A survey of actively practicing U.S. general dermatologists treating 10 or more psoriasis patients per month found that 56%-63% received systemic therapy, including biologics.27 The low proportion of patients considered adherent (i.e., MPR ≥ 80%) in this study was consistent with other studies, which generally show low adherence to psoriasis medications,28,29 although adherence to biologic therapy appears to be greater compared with topical therapies, oral therapies, and phototherapy.28
The algorithm used to identify patients with moderate-to-severe psoriasis demonstrated relatively good performance. A visit to a dermatologist in which a diagnosis of psoriasis was recorded, in combination with use of systemic therapy or phototherapy, identified a cohort in which approximately 83% of patients truly had moderate-to-severe psoriasis. The chart review revealed one important limitation that may be specific to the DoD database: patients undergoing phototherapy for conditions other than psoriasis sometimes had psoriasis entered as the diagnosis. Our study proceeded by excluding patients with no psoriasis diagnosis other than those recorded on phototherapy claims; further validation via additional chart reviews would help to determine the extent to which this should be part of the standard algorithm for identifying moderate-to-severe psoriasis.
Limitations
This study has some limitations that need to be considered. This real-world analysis used surrogates for biologic trial eligibility based on information available in medical claims. Some trial ineligibility criteria may have been erroneously reported in the claims data when the entry of a diagnosis code indicated a condition to be ruled out rather than a definite diagnosis. The prevalence of malignant disease, for example, seems high (13.8% of the full cohort). While some of the trial ineligibility criteria cannot be reliably identified in claims data (e.g., laboratory abnormalities), meaning that some patients may have been misclassified as trial-eligible, it is likely that the present analysis has resulted in a larger portion of patients misclassified as trial-ineligible. The proportion of patients deemed medically ineligible for RCT participation was consistent with a previous study, which found that 29.8% of consecutive systemic- and biologic-treated psoriasis patients from dermatology departments in Spain would be ineligible due to age or existing medical conditions.30
Additional limitations of the study include the imperfect classification of psoriasis status and other diagnoses and the potential that patients may have received pharmacy-dispensed drugs without taking the supply as prescribed. In addition, utilization patterns for patients in the DoD’s health plan may not be representative of all insured patients in the United States. Sample sizes, while large overall, were small for some subgroups, limiting the power for some comparisons.
Conclusions
This study represents a thorough comparison between large populations of patients who may be eligible for biologic trials and those who may be ineligible for the trials but could receive biologics in usual medical care. Many differences existed between these patient groups, most notably a higher prevalence of comorbidities among the trial-ineligible patients, but their treatment utilization patterns were largely similar. Future research should investigate the association between treatment patterns and subsequent health outcomes to determine whether patients ineligible for trials show differences from their trial-eligible counterparts with respect to treatment effectiveness or adverse events.
ACKNOWLEDGMENTS
The authors thank the Navy and Marine Corps Public Health Center for its support during the conduct of this study.
REFERENCES
- 1.Smith C, Chandler D, Hepple P, et al. Psoriasis: assessment and management of psoriasis. Clinical guideline CG153. October 2012. Available at: https://www.nice.org.uk/guidance/cg153. Accessed January 22, 2017.
- 2.Menter A, Gottlieb A, Feldman SR, et al.. Guidelines of care for the management of psoriasis and psoriatic arthritis. Section 1. Overview of psoriasis and guidelines of care for the treatment of psoriasis with biologics. J Am Acad Dermatol. 2008;58(5):826-50. [DOI] [PubMed] [Google Scholar]
- 3.Menter A, Korman NJ, Elmets CA, et al.. Guidelines of care for the management of psoriasis and psoriatic arthritis. Section 3. Guidelines of care for the management and treatment of psoriasis with topical therapies. J Am Acad Dermatol. 2009;60(4):643-59. [DOI] [PubMed] [Google Scholar]
- 4.Manson JE, Shufelt CL, Robins JM. The potential for postrandomization confounding in randomized clinical trials. JAMA. 2016;315(21):2273-74. [DOI] [PubMed] [Google Scholar]
- 5.Booth CM, Tannock IF. Randomised controlled trials and population-based observational research: partners in the evolution of medical evidence. Br J Cancer. 2014;110(3):551-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rothwell PM. External validity of randomised controlled trials: “to whom do the results of this trial apply?” Lancet. 2005;365(9453):82-93. [DOI] [PubMed] [Google Scholar]
- 7.Hampton JR. Size isn’t everything. Stat Med. 2002;21(19):2807-14. [DOI] [PubMed] [Google Scholar]
- 8.Glasziou P, Mant D. Applying results to treatment decisions in primary care. Lancet. 2007:87-88.17223455 [Google Scholar]
- 9.Rothwell PM. Factors that can affect the external validity of randomised controlled trials. PLoS Clin Trials. 2006;1(1):e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van Spall HC, Toren A, Kiss A, Fowler RA. Eligibility criteria of randomized controlled trials published in high-impact general medical journals: a systematic sampling review. JAMA. 2007;297(11):1233-40. [DOI] [PubMed] [Google Scholar]
- 11.Kirsten N, Bulai Livideanu C, Richard MA, et al. Inclusion and exclusion criteria in phase III trials with systemic agents in psoriasis: the external validity of drug development. Br J Dermatol. 2016;175(3):636-38. [DOI] [PubMed] [Google Scholar]
- 12.Kennedy-Martin T, Curtis S, Faries D, Robinson S, Johnston J. A literature review on the representativeness of randomized controlled trial samples and implications for the external validity of trial results. Trials. 2015;16(1):1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zimmerman M, Clark HL, Multach MD, Walsh E, Rosenstein LK, Gazarian D. Have treatment studies of depression become even less generalizable? A review of the inclusion and exclusion criteria used in placebo-controlled antidepressant efficacy trials published during the past 20 years. Mayo Clin Proc. 2015;90(9):1180-86. [DOI] [PubMed] [Google Scholar]
- 14.Battaglia S, Basile M, Spatafora M, Scichilone N. Are asthmatics enrolled in randomized trials representative of real-life outpatients? Respiration. 2015;89(5):383-89. [DOI] [PubMed] [Google Scholar]
- 15.Carter MJ, Fife CE, Walker D, Thomson B. Estimating the applicability of wound care randomized controlled trials to general wound-care populations by estimating the percentage of individuals excluded from a typical wound-care population in such trials. Adv Skin Wound Care. 2009;22(7):316-24. [DOI] [PubMed] [Google Scholar]
- 16.Ha C, Ullman TA, Siegel CA, Kornbluth A. Patients enrolled in randomized controlled trials do not represent the inflammatory bowel disease patient population. Clin Gastroenterol Hepatol. 2012;10(9):1002-07. [DOI] [PubMed] [Google Scholar]
- 17.Hordijk-Trion M, Lenzen M, Wijns W, et al. Patients enrolled in coronary intervention trials are not representative of patients in clinical practice: results from the Euro Heart Survey on Coronary Revascularization. Eur Heart J. 2006;27(6):671-78. [DOI] [PubMed] [Google Scholar]
- 18.Travers J, Marsh S, Williams M, et al. External validity of randomised controlled trials in asthma: to whom do the results of the trials apply? Thorax. 2007;62(3):219-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vashisht P, Sayles H, Cannella AC, Mikuls TR, Michaud K. Generalizability of patients with rheumatoid arthritis in biologic clinical trials. Arthritis Care Res (Hoboken). 2016;68(10):1478-88. [DOI] [PubMed] [Google Scholar]
- 20.Zink A, Strangfeld A, Schneider M, et al. Effectiveness of tumor necrosis factor inhibitors in rheumatoid arthritis in an observational cohort study: comparison of patients according to their eligibility for major randomized clinical trials. Arthritis Rheum. 2006;54(11):3399-407. [DOI] [PubMed] [Google Scholar]
- 21.Dorrance KA, Ramchandani S, Neil N, Fisher H. Leveraging the military health system as a laboratory for health care reform. Mil Med. 2013;178(2):142-45. [DOI] [PubMed] [Google Scholar]
- 22.Peterson AM, Nau DP, Cramer JA, Benner J, Gwadry-Sridhar F, Nichol M. A checklist for medication compliance and persistence studies using retrospective databases. Value Health. 2006;10(1):3-12. [DOI] [PubMed] [Google Scholar]
- 23.Epstein RS. Medication adherence: hope for improvement? Mayo Clin Proc. 2011;86(4):268-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Onzenoort HAW, Menger FE, Neef C, et al. Participation in a clinical trial enhances adherence and persistence to treatment: a retrospective cohort study. Hypertension. 2011;58(4):573-78. [DOI] [PubMed] [Google Scholar]
- 25.Li Y, Zhou H, Cai B, et al. Group-based trajectory modeling to assess adherence to biologics among patients with psoriasis. ClinicoEcon Outcomes Res. 2014;6:197-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Di Lernia V, Ficarelli E. Current therapeutic approaches of psoriasis are affected by age at disease onset. J Dermatolog Treat. 2014;25(1):15-17. [DOI] [PubMed] [Google Scholar]
- 27.Patel V, Horn EJ, Lobosco SJ, Fox KM, Stevens SR, Lebwohl M. Psoriasis treatment patterns: results of a cross-sectional survey of dermatologists. J Am Acad Dermatol. 2008;58(6):964-69. [DOI] [PubMed] [Google Scholar]
- 28.Thorneloe RJ, Bundy C, Griffiths CEM, Ashcroft DM, Cordingley L.. Adherence to medication in patients with psoriasis. A systematic literature review. Br J Dermatol. 2013;168(1):20-31. [DOI] [PubMed] [Google Scholar]
- 29.Doshi JA, Takeshita J, Pinto L, et al. Biologic therapy adherence, discontinuation, switching, and restarting among patients with psoriasis in the U.S. Medicare population. J Am Acad Dermatol. 2016;74(6):1057-65.e1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Garcia-Doval I, Carretero G, Vanaclocha F, et al. Risk of serious adverse events associated with biologic and nonbiologic psoriasis systemic therapy: patients ineligible vs eligible for randomized controlled trials. Arch Dermatol. 2012;148(4):463-70. [DOI] [PubMed] [Google Scholar]


