Abstract
Most studies of human immunodeficiency virus type 1 (HIV-1)-specific cytotoxic T lymphocytes (CTL) have been confined to the evaluation of these effector cells in the peripheral blood. What has not been clear is the extent to which CTL activity in the blood actually reflects this effector cell function in the lymph nodes, the major sites of HIV-1 replication. To determine the concordance between CTL activity in lymph nodes and peripheral blood lymphocytes (PBL), CTL specific for simian immunodeficiency virus of macaques (SIVmac) have been characterized in lymph nodes of infected, genetically selected rhesus monkeys by using both Gag peptide-specific functional CTL assays and tetrameric peptide-major histocompatibility complex (MHC) class I molecule complex staining techniques. In studies of six chronically SIVmac-infected rhesus monkeys, Gag epitope-specific functional lytic activity and specific tetrameric peptide-MHC class I staining were readily demonstrated in lymph node T lymphocytes. Although the numbers of tetramer-binding cells in some animals differed from those documented in their PBL, the numbers of tetramer-binding cells from these two different compartments were not statistically different. Phenotypic characterization of the tetramer-binding CD8+ lymph node T lymphocytes of the infected monkeys demonstrated a high level of expression of the activation-associated adhesion molecules CD11a and CD49d, the Fas molecule CD95, and MHC class II-DR. These studies documented a low expression of the naive T-cell marker CD45RA and the adhesion molecule CD62L. This phenotypic profile of the tetramer-binding lymph node CD8+ T cells was similar to that of tetramer-binding CD8+ T cells from PBL. These observations suggest that characterization of AIDS virus-specific CTL activity by sampling of cells in the peripheral blood should provide a reasonable estimation of CTL in an individual’s secondary lymphoid tissue.
CD8+ cytotoxic T lymphocytes (CTL) are important in containing the spread of human immunodeficiency virus type 1 (HIV-1) in infected individuals. Studies have shown that virus-specific CD8+ CTL can inhibit AIDS virus replication in autologous CD4+ T lymphocytes in vitro, probably by release of chemokines and cytokines, as well as by lysis of infected cells (35, 36). In vivo the containment of HIV-1 replication that occurs during the period of primary infection coincides temporally with the generation of virus-specific CTL (8, 17, 29). Finally, a potent CTL response is correlated with low virus load and a stable clinical status in individuals chronically infected with HIV-1 (25, 27).
HIV-1 replication occurs predominantly in the lymph nodes of the infected individual (30). However, most studies of HIV-1-specific CTL have been confined to the evaluation of these effector cells in the peripheral blood. It is not clear to what extent CTL activity in the blood actually reflects this effector cell function at the major sites of HIV-1 replication. An extensive evaluation of CTL in lymph nodes of HIV-1-infected humans has not been undertaken, at least in part because of the numerous surgical procedures that would be required for such a study. The use of such procedures in clinically stable individuals might be difficult to rationalize.
The simian immunodeficiency virus (SIV)-infected macaque provides an ideal animal model in which to examine AIDS virus-specific CTL in lymph nodes. SIVmac-infected rhesus monkeys develop a disease with remarkable similarities to HIV-1-induced disease in humans (19, 20). SIVmac-specific CTL are readily detected in infected monkeys by functional killing assays (21, 38). We have made use of a dominant CTL response to the SIVmac Gag epitope p11C, C-M in rhesus monkeys expressing the major histocompatibility complex (MHC) class I molecule Mamu-A*01 to explore the role of CTL in the immunopathogenesis of AIDS (1, 22). In the present study, CTL specific for SIVmac have been characterized in lymph nodes of infected, Mamu-A*01+ rhesus monkeys using both Gag peptide-specific functional CTL assays and tetrameric peptide-MHC class I molecule complex staining techniques (2, 6, 12, 18, 24, 27).
MATERIALS AND METHODS
Animals and viruses.
EDTA-anticoagulated blood samples and lymph node biopsies were obtained from rhesus monkeys (Macaca mulatta) experimentally infected with uncloned SIVmac strain 251. These animals were maintained in accordance with the guidelines of the Committee on Animals for the Harvard Medical School and the Guide for the Care and Use of Laboratory Animals (25a).
Selection of Mamu-A*01+ rhesus monkeys.
Rhesus monkeys were screened for the presence of the Mamu-A*01 allele by a PCR-based technique (16). EDTA-preserved whole blood from macaques was subject to Ficoll diatrizoate density gradient centrifugation to isolate leukocytes, and the washed cell pellets were resuspended in 200 μl of phosphate-buffered saline. DNA extraction was then carried out with a QIAmp blood kit (QIAgen, Inc., Chatsworth, Calif.). PCR was performed with 200 to 500 μg of extracted DNA by using allele-specific primers in a 50-μl reaction mixture consisting of 60 mM Tris, 2 mM MgCl2, 15 mM ammonium sulfate, 2 mM deoxynucleoside triphosphates (0.5 mM each), and 5 U of Taq polymerase (pH 8.5). Primers A*01/F (5′-GAC AGC GAC GCC GCG AGC CAA-3′) and A*01/R (5′-CGCT GCA GCG TCT CCT TCC CC-3′) were used at a final concentration of 800 nM each. Two additional primers specific for a conserved MHC class II sequence (based on the macaque homolog of HLA DRB3) were included in the reaction as internal positive controls. Primers 5′MDRB (5′-GCC TCG AGT GTC CCC CCA GCA CGT TTC-3′) and 3′MDRB (5′-GCA AGC TTT CAC CTC GCC GCT G-3′) were used at a final concentration of 680 nM each. PCR was carried out with a Perkin-Elmer GeneAmp system 9600 thermocycler (Perkin-Elmer, Inc., Norwalk, Conn.). Samples were denatured at 96°C for 2 min followed by 5 cycles of 25 s at 96°C and 60 s at 72°C; 21 cycles of 25 s at 96°C, 50 s at 67°C, and 45 s at 72°C; and 4 cycles of 25 s at 96°C, 60 s at 55°C, and 80 s at 72°C. PCR products were analyzed by electrophoresis in 2% agarose gels. Ten microliters of each reaction mixture was loaded per lane.
Potential Mamu-A*01-positive animals were identified by the presence of two bands: 685-bp amplified product and a 260-bp band. DNA sequence analysis was then performed with all potential positive samples to confirm nucleotide sequence identity with the published Mamu-A*01 prototype sequence (22). Prior to sequencing, amplified DNA was treated with 1 U of shrimp alkaline phosphatase and 10 U of exonuclease I per reaction for 15 min at 37°C followed by 15 min at 80°C. The sequencing templates were then purified with a QIAquick PCR purification kit (QIAgen, Inc.). For each template, 70 ng of DNA was used for PCR sequencing together with 5 pmol of primer. Four PCR primers were used: A*01/F and A*01/R, whose sequences are given above, and A*01-Int2/F (5′-TTC ATT TTC AGT TGA GG-3′) and A*01-Int2/R (5′-GGA GGG GTC GTG ACC TGC-3′). Sequencing was carried out at a central sequencing facility on an ABI-373 stretch DNA sequencing machine, using ABI AmpiTaq FS dye terminator chemistry (Perkin-Elmer, Inc.). The six animals used in this study, which were genotypically Mamu-A*01 positive, based on the screening described above, were also positive by functional CTL assay as described previously (18).
Staining and phenotypic analysis of p11C, C-M-specific CD8+ T lymphocytes.
Soluble tetrameric Mamu-A*01/p11C, C-M complex was made as described by Kuroda et al. (18). Phycoerythrin (PE)-labeled ExtrAvidin (Sigma Chemical Co., St. Louis, Mo.) or Alexa 488-labeled NeutrAvidin (Molecular Probe, Eugene, Oreg.) was mixed with biotinylated Mamu-A*01/p11C, C-M complex at a 1:4 molar ratio to produce the tetramers. The monoclonal antibodies (MAbs) used for this study were directly coupled to fluorescein isothiocyanate (FITC), PE-Texas red (ECD), or allophycocyanin (APC). The following MAbs were used: anti-CD8α(Leu2a)-FITC and anti-CD62L(Leu8)-PE (Becton Dickinson, San Jose, Calif.), anti-CD8αβ(2ST8-5H7)-ECD, anti-CD11a(25.3.1)-PE, anti-CD28(4B10)-PE, anti-CD45RA(2H4)-PE, anti-CD49d(HP2/1)-PE and anti-HLA-DR(I3)-PE (Beckman Coulter, Inc., Miami, Fla.), anti-CD95(DX2)-PE (Caltag, Burlingame, Calif.). The MAb FN18, which recognizes rhesus monkey CD3, a gift from D. M. Neville, Jr., National Institutes of Health, Bethesda, Md., was directly coupled to APC. The three reagents Alexa 488-coupled tetrameric Mamu-A*01/p11C, C-M complex, anti-CD8αβ-ECD, and anti-rhesus monkey CD3-APC were used with either anti-CD11a-PE, anti-CD28-PE, anti-CD45RA-PE, anti-CD49d-PE, anti-CD62L-PE, anti-CD95-PE, or anti-HLA-DR-PE to perform four-color flow cytometric analyses. The PE-coupled tetrameric Mamu-A*01/p11C, C-M complex was used with anti-CD8α-FITC in conjunction with anti-CD8αβ-ECD and anti-rhesus monkey CD3-APC. Alexa 488- or PE-coupled tetrameric Mamu-A*01/p11C, C-M complex (0.5 μg) was used in conjunction with the directly labeled MAbs to stain either 100 μl of fresh whole blood, 5 × 105 single cells from lymph nodes, or 5 × 105 lymphocytes isolated by density gradient centrifugation over Ficoll-Hypaque following in vitro culture. Samples were analyzed on a Coulter EPICS® Elite ESP as described previously (18). Statistical evaluation of the results of the phenotypic analyses was performed with the Wilcoxon matched-pairs test (37), and the results were analyzed with Microsoft Excel software version 5.0 (Microsoft Corp., Redmond, Wash.) and PRISM software version 2.01 (GraphPad Software, San Diego, Calif.). Data presentation was performed with WinMDI software version 2.7 (Joseph Trotter, La Jolla, Calif.) and Microsoft PowerPoint software version 4.0c (Microsoft Corp.).
Cytotoxicity assay.
Autologous B-lymphoblastoid cell lines (B-LCL) were used as target cells in functional CTL assays. B-LCL were incubated with 5 μg of p11C, C-M (CTPYDINQM) or the negative control peptide p11B (ALSEGCTPYDIN) per ml for 90 min during 51Cr labeling. For effector cells, peripheral blood mononuclear cells (PBMC) or single cells isolated from lymph nodes of monkeys chronically infected with SIVmac were cultured for 3 days at 2 × 106 cells/ml with concanavalin A (ConA [5 μg/ml]) (Sigma Chemical Co.), washed, and then maintained for another 7 to 11 days in medium supplemented with recombinant human interleukin 2 (IL-2 [20 U/ml]) (provided by Hoffman-La Roche, Nutley, N.J.). Alternatively, PBMC or single cells isolated from lymph nodes were cultured for 3 days at a density of 3 × 106 cells/ml in the presence of 1 μg of the peptide p11C, C-M per ml. Cells were then maintained for another 7 to 11 days in medium supplemented with recombinant human IL-2 (20 U/ml) as described above. PBMC or lymph node cells cultured according to one of these two protocols were then centrifuged over Ficoll-Hypaque (Ficopaque; Pharmacia Chemical Co., Piscataway, N.J.) and assessed as effector cells in a standard 51Cr release assay with U-bottom microtiter plates containing 104 target cells with effector cells at different effector/target cell (E/T) ratios. All wells were established and assayed in duplicate. Plates were incubated in a humidified incubator at 37°C for 4 h. Specific release was calculated as [(experimental release − spontaneous release)/(maximum release − spontaneous release)] × 100. Spontaneous release was less than 20% of maximal release with detergent (1% Triton X-100; Sigma Chemical Co.) in all assays.
RESULTS
Lymph node T cells of SIVmac-infected rhesus monkeys contain virus-specific CTL.
Lymph nodes of six chronically SIVmac-infected rhesus monkeys were evaluated for virus-specific CTL by using both functional and tetramer binding assays. Since the monkeys selected for these studies all shared the MHC class I allele Mamu-A*01, assays were done to measure T-lymphocyte recognition of the Mamu-A*01-restricted, dominant Gag CTL epitope p11C, C-M. Functional assays to measure lysis of autologous target cells expressing p11C, C-M were done by using effector cells expanded by in vitro cultivation with either ConA or p11C, C-M (Table 1). p11C, C-M-specific lytic activity was detected in both the lectin- and antigen-stimulated lymph node cells. The relative magnitude of this lytic activity in lymph nodes compared to that in simultaneously sampled PBL differed in each animal studied (Table 1). However, the functional CTL activity in the lymph nodes was not consistently greater, nor was it less than that seen in PBL.
TABLE 1.
Lymph node lymphocytes of SIVmac-infected rhesus monkeys demonstrate Gag epitope-specific CTL function
| Monkeya | % p11C, C-Mb-specific lysisc
|
|||
|---|---|---|---|---|
| PBL
|
Lymph node
|
|||
| ConA–IL-2 expanded (50/25/13)d | p11C, C-M expanded (5/3/1) | ConA–IL-2 expanded (50/25/13) | p11C, C-M expanded (5/3/1) | |
| GL9 | 13/07/04 | 40/35/30 | 57/52/39 | 27/21/15 |
| 3K1 | 25/16/08 | 45/34/28 | 07/04/02 | 12/07/05 |
| 403 | 43/40/32 | 60/60/56 | 41/34/29 | NTe |
| 138 | 18/13/06 | 68/66/58 | 50/46/40 | 38/34/26 |
| 575 | 11/07/05 | 14/09/05 | 08/04/01 | 16/10/06 |
| 154 | 39/27/14 | 60/58/48 | 15/08/04 | NT |
Chronically SIVmac-infected, Mamu-A*01+ rhesus monkeys.
p11C, C-M represents the optimal 9-amino-acid fragment of the SIV Gag 12-amino-acid peptide p11C.
Percent p11C, C-M-specific lysis was calculated as percent specific release by p11C, C-M-pulsed MHC class I-matched target cells minus the average of the percent release by control peptide p11B-pulsed target cells. The background level of p11B peptide-specific lysis was always less than 5% of the spontaneous release.
E/T ratio.
NT, not tested.
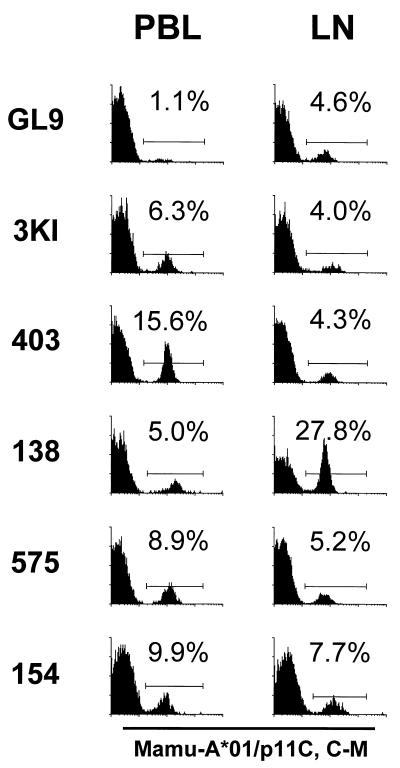
Binding of tetrameric Mamu-A*01/p11C, C-M complex to lymph node CD8αβ+ T cells was also assessed (Fig. 1 and Table 2). Flow cytometric analysis of freshly isolated lymph node cells demonstrated tetramer binding for all six animals that were evaluated, with 4.0 to 27.8% of lymph node CD8αβ+ T cells staining positively. Simultaneously sampled CD8αβ+ peripheral blood T lymphocytes also bound the tetramer in each monkey. However, as was seen in the functional studies, the percentage of CD8αβ+ T cells that bound the tetramer frequently differed in these two anatomic compartments in each monkey. Application of the Wilcoxon matched-pairs test to the lymph node and PBL tetramer binding data did not indicate a statistically significant difference in the number of SIVmac Gag-specific CTL present in these anatomic compartments.
FIG. 1.
Tetrameric Mamu-A*01/p11C, C-M complex binds to CD8αβ+ T cells from the peripheral blood and lymph nodes of SIVmac-infected, Mamu-A*01+ rhesus monkeys. PBL and lymph node lymphocytes (LN) from six Mamu-A*01+ SIVmac-infected monkeys (GL9, 3KI, 403, 138, 575, and 154) were assessed. Flow cytometric analysis was performed with gated CD8αβ+ CD3+ T cells stained with PE-coupled tetrameric Mamu-A*01/p11C, C-M complex.
TABLE 2.
Binding of the tetrameric Mamu-A*01/p11C, C-M complex to lymph node CD8+ T cells of SIVmac-infected, Mamu-A*01+ rhesus monkeys
| Monkeya | % CD8αβ+ T cells stained with Mamu-A*01/p11C, C-Mb
|
|||||
|---|---|---|---|---|---|---|
| PBL
|
Lymph node
|
|||||
| Fresh | ConA–IL-2 expanded | p11C, C-Mc expanded | Fresh | ConA–IL-2 expanded | p11C, C-M expanded | |
| GL9 | 1.1 | 1.5 | 62.0 | 4.6 | 9.5 | 35.5 |
| 3KI | 6.3 | 4.3 | 45.0 | 4.0 | 1.0 | 18.5 |
| 403 | 15.6 | 16.3 | 74.9 | 4.3 | 11.9 | NTd |
| 138 | 5.0 | 3.5 | 77.2 | 27.8 | 20.0 | 61.8 |
| 575 | 8.9 | 4.0 | 18.0 | 5.2 | 1.0 | 17.0 |
| 154 | 9.9 | 3.6 | 65.0 | 7.7 | 1.4 | NT |
Chronically SIVmac-infected, Mamu-A*01+ rhesus monkeys.
Staining with PE-coupled tetrameric Mamu-A*01/p11C, C-M complex on gated CD8αβ T cells.
p11C, C-M represents the optimal 9-amino-acid fragment of the SIV Gag 12-amino-acid peptide p11C.
NT, not tested.
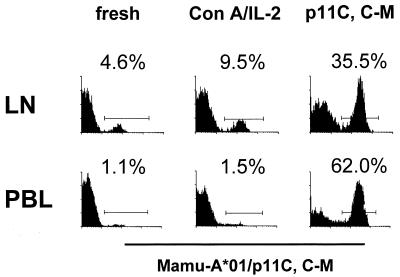
In vitro stimulation with peptide antigen clearly expanded the representation of functional CTL and tetramer-binding cells in both the lymph node and PBL populations (Fig. 2 and Table 2). Interestingly, the antigen-stimulated in vitro expansion of these CTL was in general less marked in the lymph node cells than in the PBL.
FIG. 2.
Tetrameric Mamu-A*01/p11C, C-M complex binds to in vitro expanded T cells from a lymph node of a SIVmac-infected, Mamu-A*01+ rhesus monkey. A whole-blood specimen (PBL) and a single-cell suspension of lymph node lymphocytes (LN) from a Mamu-A*01+ SIVmac-infected rhesus monkey (GL9) were stained with PE-coupled tetrameric Mamu-A*01/p11C, C-M complex and analyzed by flow cytometry with gating on CD8αβ+ CD3+ T cells. Cells were also expanded for 12 days in IL-2-containing medium, after stimulation either with ConA or with p11C, C-M peptide. The cells were again similarly stained and analyzed by flow cytometry.
Phenotypic characterization of tetrameric Mamu-A*01/p11C, C-M binding CD8+ T cells in lymph nodes and peripheral blood.
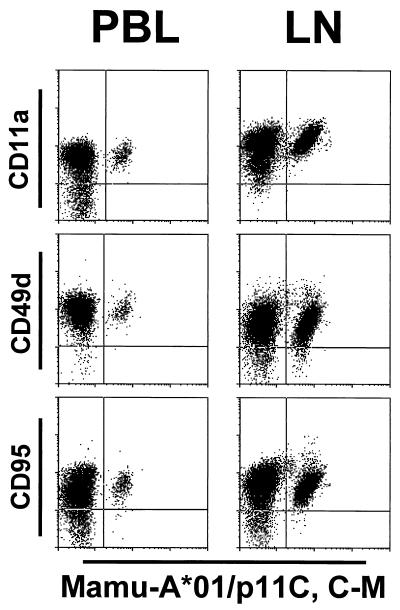
The phenotypes of the CD8αβ+ tetramer-binding T cells in lymph nodes and PBL of SIVmac-infected Mamu-A*01+ rhesus monkeys were analyzed by four-color flow cytometry. The expression of CD11a, CD28, CD45RA, CD49d, CD62L, CD95, and MHC class II-DR was investigated on tetramer-binding and nonbinding CD8αβ+ T cells from PBL and lymph nodes (Fig. 3 and 4 [and see Tables 4 and 5]). To establish reference values, the expression of these molecules was also analyzed on CD8αβ+ T cells from PBL and lymph nodes of four uninfected rhesus monkeys (Table 3).
FIG. 3.
Phenotypic characterization of tetrameric Mamu-A*01/p11C, C-M complex-binding CD8αβ+ T cells. A whole-blood specimen (PBL) and a single-cell suspension of lymph node lymphocytes (LN) from the Mamu-A*01+, SIVmac-infected rhesus monkey 138 were stained with Alexa 488-coupled tetrameric Mamu-A*01/p11C, C-M complex and four different PE-coupled MAbs (anti-CD28, anti-CD45RA, anti-CD62L, and anti-HLA-DR). Percentages of cells in the different quadrants are indicated.
FIG. 4.
Phenotypic characterization of tetrameric Mamu-A*01/p11C, C-M complex binding CD8αβ+ T cells. A whole-blood specimen (PBL) and a single-cell suspension of lymph node lymphocytes (LN) from the Mamu-A*01+, SIVmac-infected rhesus monkey 138 were stained with Alexa 488-coupled tetrameric Mamu-A*01/p11C, C-M complex and three different PE-coupled MAbs (anti-CD11a, anti-CD49d, and anti-CD95).
TABLE 4.
Phenotypic characterization of CD8αβ+ T cells of SIVmac-infected rhesus monkey
| Cell subset | % Cells staining in SIV-infected, Mamu-A*01+ monkeya:
|
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GL9
|
3KI
|
403
|
138
|
575
|
154
|
Median
|
||||||||
| PBL | LN | PBL | LN | PBL | LN | PBL | LN | PBL | LN | PBL | LN | PBL | LN | |
| CD11a+ | 98.6 | 76.4 | 98.8 | 82.5 | 71.4 | 67.1 | 79.8 | 95.0 | 73.6 | 73.8 | 79.3 | 56.3 | 74.6 | 75.1 |
| CD28+ | 16.3 | 90.9 | 36.5 | 69.6 | 46.9 | 76.5 | 27.6 | 74.0 | 22.2 | 37.4 | 35.9 | 78.1 | 30.8 | 75.3 |
| CD45RA+ | 43.8 | 22.4 | 31.6 | 35.6 | 34.8 | 32.0 | 82.4 | 35.1 | 53.7 | 32.4 | 21.3 | 47.3 | 39.3 | 33.8 |
| CD49d+ | 99.3 | 89.9 | 93.1 | 66.7 | 82.5 | 82.6 | 98.3 | 91.1 | 89.4 | 69.2 | 95.8 | 95.6 | 94.5 | 86.3 |
| CD62L+ | 2.7 | 37.7 | 14.6 | 34.3 | 28.6 | 43.8 | 20.6 | 14.7 | 16.9 | 25.2 | 25.2 | 41.0 | 18.8 | 36.0 |
| CD95+ | 97.5 | 79.9 | 98.7 | 92.5 | 84.0 | 64.6 | 89.5 | 75.9 | 91.3 | 83.9 | 96.2 | 61.4 | 93.8 | 77.9 |
| MHC class II DR+ | 57.1 | 34.8 | 75.1 | 79.5 | 11.2 | 34.4 | 19.7 | 64.3 | 45.3 | 62.6 | 63.1 | 64.6 | 51.2 | 63.5 |
Values represent the percentages of PBL and lymph node (LN) cells staining for expression of CD11a, CD28, CD45RA, CD49d, CD62L, CD95, and MHC class II-DR gated on CD8αβ+ T cells.
TABLE 5.
Phenotypic characterization of tetrameric Mamu-A*01/p11C, C-M-binding CD8αβ+ T cells of SIVmac-infected, Mamu-A*01+ rhesus monkeys
| Cell class subset | % Cells staining in SIV-infected, Mamu-A*01+ monkeya:
|
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GL9
|
3KI
|
403
|
138
|
575
|
154
|
Median
|
||||||||
| PBL | LN | PBL | LN | PBL | LN | PBL | LN | PBL | LN | PBL | LN | PBL | LN | |
| CD11a+ | 100 | 97.8 | 100 | 100 | 98.7 | 97.7 | 100 | 100 | 98.9 | 98.1 | 96.0 | 97.4 | 99.5 | 98.0 |
| CD28+ | 54.5 | 89.1 | 4.8 | 60.0 | 24.4 | 93.0 | 52.0 | 86.3 | 1.1 | 17.3 | 5.1 | 63.6 | 14.8 | 75.0 |
| CD45RA+ | 9.1 | 17.4 | 3.2 | 20.0 | 12.8 | 23.3 | 12.0 | 3.2 | 39.3 | 13.5 | 5.1 | 27.3 | 10.6 | 18.7 |
| CD49d+ | 100 | 97.8 | 98.4 | 97.5 | 99.4 | 97.7 | 100 | 97.1 | 98.9 | 94.2 | 98.0 | 100 | 99.2 | 97.6 |
| CD62L+ | 0 | 23.9 | 0 | 20.0 | 3.8 | 30.2 | 12.0 | 2.9 | 1.1 | 11.5 | 2.0 | 10.4 | 1.6 | 15.8 |
| CD95+ | 100 | 93.5 | 98.4 | 100 | 97.4 | 97.7 | 98.0 | 97.8 | 91.0 | 98.1 | 98.0 | 97.4 | 98.0 | 97.8 |
| MHC class II-DR+ | 63.6 | 28.3 | 90.5 | 97.5 | 26.9 | 60.5 | 34.0 | 86.7 | 36.0 | 92.3 | 83.8 | 96.1 | 49.8 | 89.5 |
Values represent the percentages of the Mamu-A*01/p11C, C-M complex population staining for expression of CD11a, CD28, CD45RA, CD49d, CD62L, CD95, and MHC class II-DR in PBL and in lymph node (LN) cells gated on CD8αβ+ T cells.
TABLE 3.
Phenotypic characterization of CD8αβ+ T cells in uninfected rhesus monkeys
| Cell subset | % Cells staining in SIV-negative monkeya:
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 50
|
162
|
207
|
270
|
Median
|
||||||
| PBL | LN | PBL | LN | PBL | LN | PBL | LN | PBL | LN | |
| CD11a+ | 34.0 | 19.3 | 43.6 | 22.5 | 63.2 | 24.8 | 56.7 | 32.8 | 50.2 | 23.7 |
| CD28+ | 79.4 | 97.1 | 68.2 | 96.1 | 48.0 | 80.5 | 60.6 | 87.1 | 64.4 | 91.6 |
| CD45RA+ | 65.2 | 79.2 | 72.1 | 75.9 | 48.1 | 70.7 | 68.2 | 69.0 | 66.7 | 73.3 |
| CD49d+ | 18.4 | 12.4 | 29.6 | 11.2 | 32.1 | 8.6 | 17.4 | 13.0 | 24.0 | 11.8 |
| CD62L+ | 67.9 | 66.7 | 47.9 | 65.6 | 34.9 | 57.4 | 62.2 | 61.3 | 55.1 | 63.5 |
| CD95+ | 34.3 | 12.3 | 44.1 | 16.2 | 62.7 | 16.8 | 49.1 | 24.8 | 46.6 | 16.5 |
| MHC class II-DR+ | 24.6 | 35.1 | 37.4 | 34.0 | 35.7 | 41.0 | 37.2 | 33.3 | 36.5 | 34.6 |
Values represent the percentages of PBL and lymph node (LN) cells staining for expression of CD11a, CD28, CD45RA, CD49d, CD62L, CD95, and MHC class II-DR gated on CD8αβ+ T cells.
CD28 expression by CD8αβ+ T cells from PBL of infected monkeys was lower than that of uninfected monkeys (medians of 30.8 and 64.4%, respectively); CD28 expression was high on CD8αβ+ T cells from lymph nodes of both of these groups of animals (medians of 91.6% in uninfected animals and 75.3% in infected animals) (Tables 3 and 4). As described previously, CD28 expression was heterogeneous on tetramer-binding peripheral blood T cells (18). The level of CD28 expression on lymph node CD8αβ+ tetramer binding as well as unselected CD8αβ+ T cells was high, with median positivities of 75 and 75.3%, respectively. Naive T cells have previously been shown to have a high mean fluorescence in expression of CD45RA and coexpress the CD62L molecule (31, 32). The tetramer-binding CD8αβ+ T cells were predominantly intermediate positive or negative for CD45RA expression in lymph nodes and PBL. (No anti-CD45RO MAb is available for staining of rhesus monkey lymphocytes.) Only a minor subset of tetramer-binding cells from both the lymph node and PBL compartments (less than 30.2 and 12%, respectively) demonstrated staining for CD62L. The patterns of MHC class II-DR expression on CD8αβ+ lymph node lymphocytes and PBL were similar. The level of MHC class II-DR expression by tetramer-binding cells was higher in lymph nodes than in PBL, but this difference was not statistically significant (P = 0.219). The CD8αβ+ T cells of the four uninfected monkeys and the tetramer-binding cells of the six infected monkeys were also assessed for CD11a, CD49d, and CD95 expression. Lymphocyte expression of these molecules was higher in infected than in uninfected animals (Tables 3 and 4). The difference in expression of these molecules by CD8αβ+ T cells of infected and uninfected animals was greater in lymph node than in PBL populations (Tables 3 and 4). Data from cells of a representative infected animal stained with the three MAbs are shown in Fig. 4. A remarkably large percentage of tetramer-binding CD8αβ+ T lymphocytes from all of the infected animals showed a high level of expression of CD11a, CD49d, and CD95 in both anatomic compartments (Fig. 4 and Table 5).
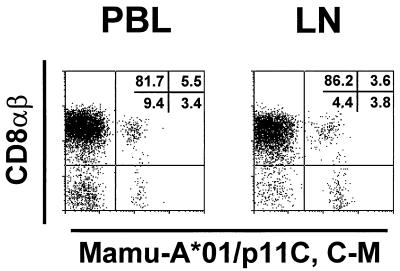
As we previously demonstrated (18), tetramer-binding T cells predominantly expressed the CD8αβ heterodimer. In five of the six infected animals evaluated in this study, tetramer-binding cells were greater than 95% CD8αβ positive. However, we found that CD8αβ− CD8αα+ lymphocytes of one animal bound the p11C, C-M tetramer (Fig. 5). This was seen not only in PBL but also in lymph node cells. The percentages of the tetramer-binding CD8αα+ T cells in PBL and lymph node were 38 and 51%, respectively. No phenotypic difference was seen in CD8αα+ tetramer-binding T cells compared with the CD8αβ+ tetramer-binding T cells of this animal.
FIG. 5.
Tetrameric Mamu-A*01/p11C, C-M complex binds to CD8αβ+ and CD8αα T cells from a whole-blood specimen (PBL) and a single-cell suspension of lymph node lymphocytes (LN) of a SIVmac-infected, Mamu-A*01+ rhesus monkey. Cells from monkey 3KI were gated on CD8α+ and CD3+ T cells and were analyzed for binding of anti-CD8αβ+ and tetrameric Mamu-A*01/p11C, C-M complex.
DISCUSSION
These studies demonstrate that virus-specific CTL are present in lymph nodes of SIVmac-infected rhesus monkeys. This is shown by the results of both SIVmac Gag epitope-specific functional CTL assays and MHC class I-Gag peptide tetramer staining. Differences in the levels of Gag-specific CTL in lymph nodes and PBL of individual monkeys were observed. However, there was no consistent pattern in these differences. Occasional animals such as no. 138 did evidence a striking difference in the amount of CTL in the lymph node and PBL compartments. Such animals, however, appear to be exceptional among the monkeys studied to date. In fact, including the data from all monkeys that we have analyzed, no statistically significant differences were noted in the number of tetramer-positive CD8+ T cells detected in these two anatomic compartments. This suggests that the determination of AIDS virus-specific CTL activity by sampling of cells in the peripheral blood should provide a reasonable estimation of CTL in an individual’s secondary lymphoid tissue.
In vitro peptide stimulation was consistently less efficient in expanding tetramer-binding lymphocyte populations from lymph nodes than those from peripheral blood. A number of properties of these cell populations might account for that difference. Lymph node and PBMC populations may differ in the accessory cells they contain. Those accessory cells or the lymphocytes themselves may differ in their expression of costimulatory molecules. Antigen stimulation may also drive the secretion of different cytokines from those distinct cell populations. Finally, while no significant phenotypic differences were seen in tetramer-binding peripheral blood and lymph node lymphocytes, the functional states of these cells might be different.
The phenotypic profile of CD8+ peripheral blood T cells of SIVmac-infected rhesus monkeys is remarkably similar to that described for CD8+ PBL of HIV-1-infected humans (13, 14, 15, 28, 33). In comparing CD8+ peripheral blood T cells of infected monkeys to those of uninfected monkeys, an increase is seen in the expression of the adhesion molecules CD11a and CD49d, the Fas molecule CD95, and MHC class II-DR, all molecules whose expression are associated with cellular activation. A decrease in the expression of the naive T-cell marker CD45RA, the costimulatory molecule CD28, and the adhesion molecule CD62L (7, 9, 31, 32) is also seen in CD8+ peripheral blood T cells of these infected monkeys. The similarity in the phenotypic changes of CD8+ T cells from SIVmac-infected rhesus monkeys and HIV-1-infected humans suggests that the phenotypic profile of lymph node CD8+ T cells in the infected monkeys should be predictive of the changes that occur in lymph nodes of infected humans. Thus, as seen in the infected monkeys, we would expect the coexpression of these molecules by human CD8+ lymph node T cells to mirror that seen in PBL.
The phenotypic appearance of the tetramer-binding CD8+ T lymphocytes in both the lymph nodes and peripheral blood was similar to that of other CD8+ T cells. Thus, an increase in expression of CD11a, CD49d, CD95, and MHC class II-DR and a decrease in expression of CD62L and CD45RA were seen. Furthermore, of all of the cell surface molecules that were studied, only CD28 expression was greater on the tetramer-binding CD8+ T cells in lymph nodes than in PBL. The ramifications of the difference between CD28 expression by tetramer-binding CD8+ T lymphocytes in these anatomic compartments are unclear. Although it has been reported that a low level of expression of CD28 by T lymphocytes is associated with a decrease in the functional activity of these cells (3, 5), AIDS virus-specific CTL activity has been demonstrated in CD8+ CD28− T cells (10, 11). These observations notwithstanding, the tetramer-binding CD8+ T lymphocytes in lymph nodes are likely to employ the CD28 molecule when functionally active.
The CD8 molecule is composed of two distinct polypeptide chains that pair on the cell surface either as a CD8αα homodimer or as a CD8αβ heterodimer (4, 23, 26, 34). These forms of the CD8 molecule are differentially expressed on functionally distinct CD8+ lymphocyte subsets. As expected, the tetramer-binding T cells were, in most instances, found in the CD8αβ+ CD3+ T-cell subpopulation. The finding of a significant number of tetramer-binding T lymphocytes in the CD8αα+ CD8αβ− cell population of an infected monkey may indicate that two distinct populations of CTL with distinct T-cell receptor repertoires and functional capabilities exist in this animal.
Finally, the percentage of tetramer-binding CD8αβ+ T cells in both lymph node and peripheral blood cell populations was consistently in accord with the functional CTL activity of these cells. This observation underscores the utility of the tetramer technology for studying AIDS pathogenesis in the SIVmac-infected rhesus monkey.
ACKNOWLEDGMENTS
We thank Evelyn Gould for assistance with preparation of the manuscript.
This work was supported by NIH grants AI 85343 and AI 20729.
REFERENCES
- 1.Allen T M, Sidney J, del Guercio M F, Glickman R L, Lensmeyer G L, Wiebe D A, DeMars R, Pauza C D, Johnson R P, Sette A, Watkins D I. Characterization of the peptide binding motif of a rhesus MHC class I molecule (Mamu-A*01) that binds an immunodominant CTL epitope from simian immunodeficiency virus. J Immunol. 1998;160:6062–6071. [PubMed] [Google Scholar]
- 2.Altman J D, Moss P A H, Goulder P J R, Barouch D H, McHeyzer-Williams M G, Bell J I, McMichael A J, Davis M M. Phenotypic analysis of antigen-specific T lymphocytes. Science. 1996;274:94–96. [PubMed] [Google Scholar]
- 3.Azuma M, Phillips J H, Lanier L L. CD28− T lymphocytes. Antigenic and functional properties. J Immunol. 1993;150:1147–1159. [PubMed] [Google Scholar]
- 4.Baume D M, Caligiuri M A, Manley T J, Daley J F, Ritz J. Differential expression of CD8 alpha and CD8 beta associated with MHC-restricted and non-MHC-restricted cytolytic effector cells. Cell Immunol. 1990;131:352–365. doi: 10.1016/0008-8749(90)90260-x. [DOI] [PubMed] [Google Scholar]
- 5.Borthwick N J, Bofill M, Gombert W M, Akbar A N, Medina E, Sagawa K, Lipman M C, Johnson M A, Janossy G. Lymphocyte activation in HIV-1 infection. II. Functional defects of CD28− T cells. J Acquired Immune Defic Syndr. 1994;8:431–441. doi: 10.1097/00002030-199404000-00004. [DOI] [PubMed] [Google Scholar]
- 6.Callan M F, Tan L, Annels N, Ogg G S, Wilson J D, O’Callaghan C A, Steven N, McMichael A J, Rickinson A B. Direct visualization of antigen-specific CD8+ T cells during the primary immune response to Epstein-Barr virus in vivo. J Exp Med. 1998;187:1395–1402. doi: 10.1084/jem.187.9.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Caruso A, Licenziati S, Canaris A D, Cantalamessa A, Fiorentini S, Ausenda S, Ricotta D, Dima F, Malacarne F, Balsari A, Turano A. Contribution of CD4+, CD8+CD28+, and CD8+CD28− T cells to CD3+ lymphocyte homeostasis during the natural course of HIV-1 infection. J Clin Investig. 1998;101:137–144. doi: 10.1172/JCI195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen Z W, Kou Z C, Lekutis C, Shen L, Zhou D, Halloran M, Li J, Sodroski J, Lee-Parritz D, Letvin N L. T cell receptor Vβ repertoire in an acute infection of rhesus monkeys with simian immunodeficiency viruses and a chimeric simian-human immunodeficiency virus. J Exp Med. 1995;182:21–31. doi: 10.1084/jem.182.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choremi-Papadopoulou H, Viglis V, Gargalianos P, Kordossis T, Iniotaki-Theodoraki A, Kosmidis J. Downregulation of CD28 surface antigen on CD4+ and CD8+ T lymphocytes during HIV-1 infection. J Acquired Immune Defic Syndr. 1994;7:245–253. [PubMed] [Google Scholar]
- 10.Couëdel-Courteille A, Le Grand R, Tulliez M, Guillet J-G, Venet A. Direct ex vivo simian immunodeficiency virus (SIV)-specific cytotoxic activity detected from small intestine intraepithelial lymphocytes of SIV-infected macaques at an advanced stage of infection. J Virol. 1997;71:1052–1057. doi: 10.1128/jvi.71.2.1052-1057.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fiorentino S, Dalod M, Olive D, Guillet J-G, Gomard E. Predominant involvement of CD8+CD28− lymphocytes in human immunodeficiency virus-specific cytotoxic activity. J Virol. 1996;70:2022–2026. doi: 10.1128/jvi.70.3.2022-2026.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gallimore A, Glithero A, Godkin A, Tissot A C, Pluckthun A, Elliott T, Hengartner H, Zinkernagel R. Induction and exhaustion of lymphocytic choriomeningitis virus-specific cytotoxic T lymphocytes visualized using soluble tetrameric major histocompatibility complex class I-peptide complexes. J Exp Med. 1998;187:1383–1393. doi: 10.1084/jem.187.9.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Giorgi J V. Phenotype and function of T cells in HIV disease. In: Gupta S, editor. Immunology of HIV infection. New York, N.Y: Plenum Press; 1996. p. 181. [Google Scholar]
- 14.Giorgi J V, Boumsell L, Autran B. Reactivity of workshop T-cell section mAb with circulating CD4+ and CD8+ cells in HIV disease and following in vitro activation. In: Schlossman S F, Boumsell L, Gilks W, Harlan J M, Kishimoto T, Morimoto C, Ritz J, Shaw S, Silverstein R, Springer T, Tedder T F, Todd R F, editors. Leucocyte typing V, white cell differentiation antigens. Vol. 1. Oxford, United Kingdom: Oxford University Press; 1995. p. 446. [Google Scholar]
- 15.Ho H N, Hultin L E, Mitsuyasu R T, Matud J L, Hausner M A, Bockstoce D, Chou C C, O’Rourke S, Taylor J M, Giorgi J V. Circulating HIV-specific CD8+ cytotoxic T cells express CD38 and HLA-DR antigens. J Immunol. 1993;150:3070–3079. [PubMed] [Google Scholar]
- 16.Knapp L A, Lehmann E, Piekarczyk M S, Urvater J A, Watkins D I. A high frequency of Mamu-A*01 in the rhesus macaque detected by polymerase chain reaction with sequence-specific primers and direct sequencing. Tissue Antigens. 1997;50:657–661. doi: 10.1111/j.1399-0039.1997.tb02927.x. [DOI] [PubMed] [Google Scholar]
- 17.Koup R A, Safrit J T, Cao Y, Andrews C A, McLeod G, Borkowsky W, Farthing C, Ho D D. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J Virol. 1994;68:4650–4655. doi: 10.1128/jvi.68.7.4650-4655.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuroda M J, Schmitz J E, Barouch D H, Craiu A, Allen T M, Sette A, Watkins D I, Forman M A, Letvin N L. Analysis of Gag-specific cytotoxic T lymphocytes in simian immunodeficiency virus-infected rhesus monkeys by cell staining with a tetrameric major histocompatibility complex class I/peptide complex. J Exp Med. 1998;187:1373–1381. doi: 10.1084/jem.187.9.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Letvin N L, Daniel M D, Sehgal P K, Desrosiers R C, Hunt R D, Waldron L M, MacKey J J, Schmidt D K, Chalifoux L V, King N W. Induction of AIDS-like disease in macaque monkeys with T-cell tropic retrovirus STLV-III. Science. 1985;230:71–73. doi: 10.1126/science.2412295. [DOI] [PubMed] [Google Scholar]
- 20.Letvin N L, King N W. Immunologic and pathologic manifestations of the infection of rhesus monkeys with simian immunodeficiency virus of macaques. J Acquired Immune Defic Syndr. 1990;3:1023–1040. [PubMed] [Google Scholar]
- 21.Miller M D, Lord C I, Stallard V, Mazzara G P, Letvin N L. The gag-specific cytotoxic T lymphocytes in rhesus monkeys infected with the simian immunodeficiency virus of macaques. J Immunol. 1990;144:122–128. [PubMed] [Google Scholar]
- 22.Miller M D, Yamamoto H, Hughes A L, Watkins D I, Letvin N L. Definition of an epitope and MHC class I molecule recognized by gag-specific cytotoxic T lymphocytes in SIVmac-infected rhesus monkeys. J Immunol. 1991;147:320–329. [PubMed] [Google Scholar]
- 23.Moebius U, Kober G, Griscelli A L, Hercend T, Meuer S C. Expression of different CD8 isoforms on distinct human lymphocyte subpopulations. Eur J Immunol. 1991;21:1793–1800. doi: 10.1002/eji.1830210803. [DOI] [PubMed] [Google Scholar]
- 24.Murali-Krishna K, Altman J D, Suresh M, Sourdive D J, Zajac A J, Miller J D, Slansky J, Ahmed R. Counting antigen-specific CD8 T cells: a reevaluation of bystander activation during viral infection. Immunity. 1998;8:177–187. doi: 10.1016/s1074-7613(00)80470-7. [DOI] [PubMed] [Google Scholar]
- 25.Musey L, Hughes J, Schacker T, Shea T, Corey L, McElrath M J. Cytotoxic-T-cell responses, viral load, and disease progression in early human immunodeficiency virus type 1 infection. N Engl J Med. 1997;337:1267–1274. doi: 10.1056/NEJM199710303371803. [DOI] [PubMed] [Google Scholar]
- 25a.National Institutes of Health. Guide for the care and use of laboratory animals. U.S. Department of Health and Human Services publication no. 82-23. Bethesda, Md: National Institutes of Health; 1985. [Google Scholar]
- 26.Norment A M, Littman D R. A second subunit of CD8 is expressed in human T cells. EMBO J. 1988;7:3433–3439. doi: 10.1002/j.1460-2075.1988.tb03217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ogg G S, Jin X, Bonhoeffer S, Dunbar P R, Nowak M A, Monard S, Segal J P, Cao Y, Rowland-Jones S L, Cerundolo V, Hurley A, Markowitz M, Ho D D, Nixon D F, McMichael A J. Quantitation of HIV-1-specific cytotoxic T lymphocytes and plasma load of viral RNA. Science. 1998;279:2103–2106. doi: 10.1126/science.279.5359.2103. [DOI] [PubMed] [Google Scholar]
- 28.Palmer S, Hamblin A S. Increased CD11/CD18 expression on the peripheral blood leucocytes of patients with HIV disease: relationship to disease severity. Clin Exp Immunol. 1993;93:344–349. doi: 10.1111/j.1365-2249.1993.tb08183.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pantaleo G, Demarest J F, Soudeyns H, Graziosi C, Denis F, Adelsberger J W, Borrow P, Saag M S, Shaw G M, Sekaly R P, et al. Major expansion of CD8+ T cells with a predominant V beta usage during the primary immune response to HIV. Nature. 1994;370:463–467. doi: 10.1038/370463a0. [DOI] [PubMed] [Google Scholar]
- 30.Pantaleo G, Graziosi C, Demarest J F, Butini L, Montroni M, Fox C H, Orenstein J M, Kotler D P, Fauci A S. HIV infection is active and progressive in lymphoid tissue during the clinically latent stage of disease. Nature. 1993;362:355–358. doi: 10.1038/362355a0. [DOI] [PubMed] [Google Scholar]
- 31.Rabin R L, Roederer M, Maldonado Y, Petru A, Herzenberg L A. Altered representation of naive and memory CD8 T cell subsets in HIV-infected children. J Clin Investig. 1995;95:2054–2060. doi: 10.1172/JCI117891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roederer M, Dubs J G, Anderson M T, Raju P A, Herzenberg L A. CD8 naive T cell counts decrease progressively in HIV-infected adults. J Clin Investig. 1995;95:2061–2066. doi: 10.1172/JCI117892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schmitz J E, Forman M A, Lifton M A, Concepcion O, Reimann K A, Crumpacker C S, Daley J F, Gelman R S, Letvin N L. Expression of the CD8αβ-heterodimer on CD8+ T lymphocytes in PBL of HIV− and HIV+ individuals. Blood. 1998;92:198–206. [PubMed] [Google Scholar]
- 34.Terry L A, DiSanto J P, Small T N, Flomenberg N. Differential expression and regulation of the human CD8 alpha and CD8 beta chains. Tissue Antigens. 1990;35:82–91. doi: 10.1111/j.1399-0039.1990.tb01761.x. [DOI] [PubMed] [Google Scholar]
- 35.Tsubota H, Lord C I, Watkins D I, Morimoto C, Letvin N L. A cytotoxic T lymphocyte inhibits acquired immunodeficiency syndrome virus replication in peripheral blood lymphocytes. J Exp Med. 1989;169:1421–1434. doi: 10.1084/jem.169.4.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Walker C M, Moody D J, Stites D P, Levy J A. CD8+ lymphocytes can control HIV infection in vitro by suppressing virus replication. Science. 1986;234:1563–1566. doi: 10.1126/science.2431484. [DOI] [PubMed] [Google Scholar]
- 37.Wilcoxon F, Wilcox R A. Some rapid approximate statistical procedures. Pearl River, N.Y: Lederle Laboratories; 1964. p. 59. [Google Scholar]
- 38.Yamamoto H, Ringler D J, Miller M D, Yasutomi Y, Hasunuma T, Letvin N L. Simian immunodeficiency virus-specific cytotoxic T lymphocytes are present in the AIDS-associated skin rash in rhesus monkeys. J Immunol. 1992;149:728–734. [PubMed] [Google Scholar]