Abstract
BACKGROUND:
Each year, 6%-20% of U.S. residents are infected by influenza, and more than 200,000 people are hospitalized due to complications related to influenza. In 2003, it was estimated that the direct medical costs for the treatment of influenza were $10.4 billion in the United States.
OBJECTIVES:
To (a) assess the current practice associated with the diagnosis and treatment of influenza-like illnesses (ILIs) in inpatient, ambulatory/outpatient, and emergency room settings and (b) evaluate how the use of rapid influenza diagnostic tests (RIDTs) impacts patient health care utilization and cost in these clinical settings.
METHODS:
For this retrospective cohort study, patients with an influenza-related health care encounter were identified using claims data from a midwestern commercial health insurance plan. In order to select the claims relevant to this study, the corresponding influenza ICD-9-CM codes, GPI codes, and CPT codes for the diagnosis, prescriptions, and procedures were identified and used to detect ILI claims. For the cost analysis of these data, the allowed amount in the billing claims was utilized. Using these data, the median cost, mean cost, minimum cost, and maximum cost were determined for each episode of care. The median costs were compared, and Wilcoxon two-sample tests and Kruskal-Wallis tests with a P value of 0.05 were used as the level of significance.
RESULTS:
Over 32% of the influenza-like illness episodes identified in this study involved empiric antiviral therapy as either treatment (15%) or prophylaxis (17.1%) without an accompanying medical visit. Of patient episodes with a medical visit, patients with an RIDT for influenza received antiviral treatment in 27.5% of the episodes compared with 55% of the episodes for patients with no RIDT. Episodes with a medical visit and an RIDT had statistically significant (P < 0.001) lower median 30-day influenza-related health care costs ($62.46) than episodes with a medical visit but no RIDT ($192.83), as well as with empiric therapy but no accompanying medical visit ($105.64).
CONCLUSIONS:
The results of this analysis for ILI claims over a 2-year period suggest that utilization of RIDTs for influenza may reduce overall influenza-related health care costs and improve proper utilization of anti-influenza medications.
What is already known about this subject
Direct treatment costs for influenza are in the billions of dollars each year.
Current utilization of antiviral therapies is suboptimal.
What this study adds
Over 32% of the influenza-like illness episodes identified in this study involved empiric antiviral therapy as either treatment (15%) or prophylaxis (17.1%) without an accompanying medical visit.
Of patient episodes with a medical visit, patients with a rapid influenza diagnostic test (RIDT) received antiviral treatment in 27.5% of the episodes compared with 55% of the episodes with no RIDT.
Patients with a medical visit and an RIDT had statistically significant (P < 0.001) lower median 30-day influenza-related health care costs ($62.46) than patients who had a medical visit but no RIDT ($192.83), as well as those who received empiric therapy without an accompanying medical visit ($105.64).
Seasonal influenza places a high burden on the U.S. health care system and our society. Each year, 6%-20% of U.S. residents are infected with the influenza virus. Although influenza is typically a self-limiting illness, more than 200,000 people are hospitalized due to complications related to influenza each year.1 In 2003, it was estimated that the direct medical costs for the treatment of influenza were $10.4 billion.2 It was also estimated that 30% of those expenditures originated from outpatient visits.2 One of the main challenges that the U.S. health care system faces with regard to influenza is managing its sporadic and unpredictable activity. The uncertainties of when peak influenza activity will occur, the magnitude of its activity, the efficacy of available vaccines, and the public’s response to influenza in any given year place significant burdens on the health care system, including shortages of oseltamivir (Tamiflu), the preferred pharmacological treatment. These burdens are compounded by potentially unnecessary and inappropriate treatments provided to patients who do not actually have influenza, which could ultimately result in the emergence of resistant strains of the influenza virus; for example, many of the circulating influenza virus strains in the 2008-2009 influenza season were more than 99% resistant to oseltamivir.3
The use of unnecessary and inappropriate antivirals has caused much debate as to how to effectively treat individuals with an influenza-like illnesses (ILIs). According to a recent Cochrane review, antivirals, such as oseltamivir and zanamivir, reduce the time to alleviation of influenza symptoms, but the review explains that the extent of this effect may be small and nonspecific.4 While the benefit of antivirals in patients with influenza may be limited, they remain the best available treatment for influenza to date. The Centers for Disease Control and Prevention (CDC) still currently recommends oseltamivir 75 milligrams (mg) twice a day for 5 days or zanamivir 10 mg twice a day for 5 days for most patients with influenza.5 Yet, a recent study claims that physicians often fail to prescribe antiviral therapy to patients diagnosed with influenza; instead, some of these physicians inappropriately prescribe antibiotics.6 Not only could practices such as these leave patients managing their influenza symptoms for longer periods of time, but it could increase their chances of antibiotic-resistant infections in the future.
One way to decrease the inappropriate use of antivirals in individuals with ILIs who are not infected with the influenza virus and limit the overuse of antibiotics in influenza-positive individuals is to use disease-specific diagnostic tests, such as rapid influenza diagnostic tests (RIDTs), to determine who is most likely to benefit from such agents. RIDTs can be used by practitioners in conjunction with a physical assessment to aid clinical decision making when diagnosing patients presenting with symptoms consistent with ILIs. RIDTs may be utilized by practitioners to improve their abilities to identify patients infected with influenza or rule out influenza as the etiologic agent. Likewise, the results of an RIDT may guide treatment decisions. Although the sensitivity for the detection of the influenza virus with various RIDTs has been documented to range from 50%-70%, the positive predictive value of these tests is increased greatly when tests are used only when influenza activity in the community has been noted.7 In periods of high influenza prevalence, the positive predictive value is high, which means that a positive test result is more likely to identify a patient infected with the influenza virus.7
Community pharmacists may be uniquely positioned to increase access to RIDTs and improve antiviral utilization. Under physician-directed collaborative practice agreements, pharmacists have demonstrated the ability to triage patients with ILIs, referring those at greatest risk for complications to a primary care physician, while conducting physical assessments and RIDTs for patients at low risk for complications.8 These collaborative practice agreements could also allow pharmacists to dispense antivirals per protocol for a positive test result but would prohibit them from dispensing antivirals or other prescription medications, such as antibiotics, without a prescription.
The objectives of this study were to (a) assess the current practice associated with the diagnosis and treatment of ILIs in inpatient, ambulatory/outpatient, and emergency room settings and (b) evaluate how the use of RIDTs impact patient health care utilization (i.e., physician visits, hospitalizations, and prescriptions) and cost in these clinical settings.
Methods
For this retrospective cohort study, patients with an influenza-related health care encounter were identified using claims data from a single midwestern commercial health insurance plan. All individuals aged 19 years or older, with a medical or prescription claim associated with influenza between September 1, 2011, and March 1, 2013, were considered for inclusion in the study. The criterion for inclusion was the presence of 1 or more of the following: a medical claim with an influenza diagnostic code, a medical claim with an influenza test procedure code, or a prescription claim for an anti-influenza medication.
All patients who met the criteria for inclusion were assigned individual study identification numbers by the payer. The patients included individuals from rural, suburban, and urban areas. In order to identify the claims relevant to this study, the corresponding International Classification of Diseases, Ninth Revision, Clinical Modification influenza codes (487.X and 488.X), Generic Product Identifier (GPI) codes (7320001010X, 125046020X, 1250007010X, and 1250408000X), and the Current Procedural Terminology code (87804X) for the diagnosis, prescriptions, and procedures were identified (Table 1) and used to detect ILI claims in the data. All claims were also assigned separate study claim identification numbers. This allowed multiple claims corresponding to a single patient to be properly quantified. To allow for the possibility that patients had multiple distinct episodes of ILIs, an episode was defined as all claims within 30 days of the date of the first claim.
TABLE 1.
Influenza Diagnostic and Procedure Codes
| CPT codes | 87804X | Infectious agent antigen detection by immunoassay with direct optical observation influenza |
| GPI codes | 7320001010X | Amantadine (Symmetrel) |
| 1250406020X | Oseltamivir (Tamiflu) | |
| 1250007010X | Rimantadine (Flumadine) | |
| 1250408000X | Zanamivir (Relenza) | |
| ICD-9-CM codes | 487.X | Influenza |
| 488.X |
CPT = Current ProceduralTerminology; GPI = Generic Product Identifier; ICD-9-CM = International Classification of Diseases, Ninth Revision, Clinical Modification.
Once these codes were extracted, and the claims were assigned study identification numbers, claims with GPI codes were analyzed further for identification as medication prescribed to the patient for treatment or prophylaxis regimens. The differentiating factor between treatment and prophylaxis regimens in this study was the days’ supply. A prescription written for 6 days or fewer was considered a treatment regimen, and a prescription written for 7 or more days was classified as prophylaxis in accordance with CDC recommendations.5 If a prescription claim for anti-influenza medication had a days’ supply greater than 20, the claim was excluded from the study because the claim was not considered an acute influenza episode. Likewise, patients with influenza-related claims during periods of the year not defined as flu season (May through September) were excluded from the study.
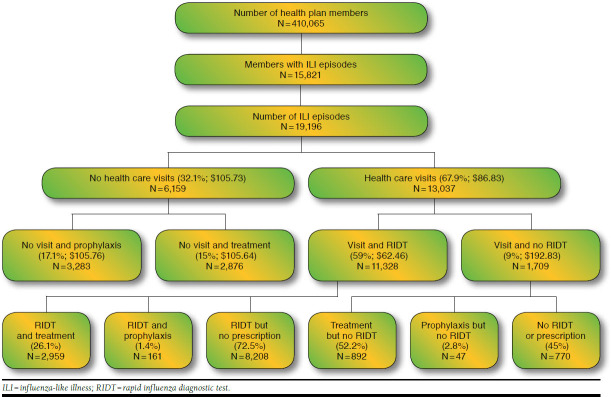
Using the claims data, patient episodes were categorized into the following categories for the purpose of comparison (Figure 1): (a) episodes with a prescription filled for an influenza prophylactic regimen without visiting a health care facility, (b) episodes with a prescription filled for an influenza treatment regimen without visiting a health care facility, and (c) episodes with a health care facility visit (emergency room, inpatient, or ambulatory) for ILI. Patient episodes with no medical claims in the 30 days prior to a prescription fill date were considered to have not visited a health care facility or provider for an ILI.
FIGURE 1.
Claims Data Analysis Flowchart
Patient episodes with a health care facility were further divided into those who received an RIDT during their visit and those who did not receive an RIDT during their visit. Upon identifying whether or not a patient episode included an RIDT during a visit, it was determined if each individual episode was provided care as a treatment regimen, a prophylactic regimen, or no medication for the ILI.
In addition, the clinical setting in which the episode was initially treated, the anti-influenza medication prescribing trends, and the cost associated with each treatment scenario were analyzed. Costs were summed for the 30 days after the initial claim to cover the entire cost of the episode, including potential complications. Cost analysis of these data utilized the allowed amount, which is the sum of patient out-of-pocket costs and the amount paid by the insurer, in the billing claims. Using these data, the median cost, mean cost, minimum cost, and maximum cost were determined for each health care scenario, including all visit, RIDT, and medication costs. The median costs were compared, and Wilcoxon two-sample tests and Kruskal-Wallis tests with a P value of 0.05 were used as the level of significance. Normal distribution of the cost data was rejected using the Kolmogorov Smirnov test, which is why nonparametric tests were used for the cost analysis. All analysis was performed using SAS version 9.2 (SAS Institute, Cary, NC).
Results
In the 2011-2012 and 2012-2013 flu seasons, 19,196 ILI episodes were detected (Figure 1), with over 15,000 episodes occurring during the 2012-13 season. Of those episodes, 3,283 (17.1%) were given prophylaxis without a captured visit to a health care facility or provider, and 2,876 (15%) were given treatment regimens without a captured visit to a health care facility or provider, which means that 32% of the episodes only had a claim for a medication. The remaining 13,037 (67.9%) patient episodes included a visit to a health care facility. While the mean cost for episodes with a health care facility visit was higher than the mean cost for episodes without a health care facility visit due to a few episodes with very high costs (Table 2), the median costs were lower ($87) for episodes with an office visit than for those without ($106). This indicates that episodes with empiric treatment or prophylaxis only were more costly, on average, than those with a provider visit.
TABLE 2.
Cost Analysis of Influenza-like Illness
| Number of Episodes n (%) | Mean Cost $ (SD) | Median Cost (Min Cost, Max Cost, $) | |
|---|---|---|---|
| All influenza-like illnesses | 19,196 (100.0) | 160.54 (666.43) | 105.64 (0, 49,932) |
| Patient episodes with no health care visit | 6,159 (32.1) | 106.15 (37.09) | 105.73a (0, 1,857) |
| But prescribed treatment | 2,876 (15.0) | 102.49 (36.43) | 105.64 (0, 1,857) |
| But prescribed prophylaxis | 3,283 (17.1) | 105.40 (12.76) | 105.76 (0, 316.41) |
| Patient episodes with health care visit | 13,037 (67.9) | 187.23 (807.10) | 86.83a (0, 49,932) |
| And RIDT | 11,328 (59.0) | 153.06 (456.90) | 62.46b (0, 27,111) |
| And no RIDT | 1,709 (9.0) | 413.70 (1,878.35) | 192.83b (3, 49,932) |
a P value < 0.001 using a Kruskal Wallis test to compare median 30-day influenza-related costs between episodes with a health care facility visit and episodes with no visit.
b P value < 0.001 using a Kruskal Wallis test to compare median 30-day influenza-related costs between episodes with a health care facility visit and an RIDT versus those episodes with a visit and no RIDT.
Max = maximum; Min = minimum; RIDT = rapid influenza diagnostic test; SD = standard deviation.
Patient episodes with an initial ILI visit to any health care facility or provider were further divided into 2 subgroups based on whether or not an RIDT was administered. The data revealed that it was more common for episodes with a health care facility visit to include an RIDT than not include an RIDT (Table 2). RIDTs were used in 11,328 (59% of total patient episodes and 87% of episodes with a health care facility visit) of these patient episodes. The remaining 1,709 were patient episodes with a health care facility visit but did not include an RIDT. When comparing the mean and median costs of these patient episodes, the data show a lower cost associated with episodes with an RIDT than those without an RIDT.
Table 3 presents the 30-day influenza-related costs based on place of initial service. Episodes with an initial ILI claim from an inpatient setting had the highest mean and median costs. Episodes initially treated in an emergency room also had higher costs than those occurring in an ambulatory care or clinic setting. Median costs of care were significantly lower for episodes where care originated in an outpatient setting ($63.48), compared with episodes having no influenza-related visits ($105.73).
TABLE 3.
30-Day Cost Analysis Based on Initial Place of Servicea
| Initial Place of Service | Episodes n (%) | Mean Cost $ (SD) | Median Cost (Min Cost, Max Cost, $) |
|---|---|---|---|
| Emergency room | 966 (5.0) | 423.04 (881.38) | 160.97 (0, 10,270) |
| Inpatient | 35 (0.2) | 9,924.07 (8,820.29) | 6,740.36 (2,314, 49,932) |
| Ambulatory/outpatient | 11,918 (62.1) | 139.17 (375.63) | 63.48b (0, 27,111) |
| No visit | 6,159 (32.1) | 106.15 (37.09) | 105.73 (0, 1,857) |
a Place of visit unknown for 118 patient episodes; place of visit for remaining patient episodes indicated in claims.
b P value < 0.001 using a Kruskal Wallis test to compare median 30-day influenza-related costs between episodes with an initial visit in an ambulatory/outpatient setting versus any other setting.
Max = maximum; Min = minimum; SD = standard deviation.
Table 4 shows that 27.5% of the patient episodes presenting to a health care provider as an ILI ultimately received a prescription for either treatment or prophylaxis. On the other hand, episodes without an RIDT were prescribed an anti-influenza medication about 55% of the time—a 27.5 percentage point increase in the number of patient episodes receiving a medication, whether it was for treatment or prophylaxis.
TABLE 4.
Treatment Patterns for Patient Episodes Presenting to a Health Care Provider with an Influenza-like Illness
| n (%) | |
|---|---|
| Patient episodes with RIDT | 11,328 |
| • And treatment regimen | 2,959 (26.1) |
| • And prophylactic regimen | 161 (1.4) |
| • And no medication | 8,208 (72.5) |
| Patient episodes with no RIDT | 1,709 |
| • But with treatment regimen | 892 (52.2) |
| • But with prophylactic regimen | 47 (2.8) |
| • And no medication | 770 (45.0) |
RIDT = rapid influenza diagnostic test.
Discussion
When considering the claims data surrounding health care facility visits and the claims data that are associated with no visits, the results suggest that utilization of an RIDT for influenza may reduce overall influenza-related health care costs. Patient episodes with a visit and an RIDT have statistically significant (P < 0.001) lower median 30-day influenza-related health care costs ($62.46) than patient episodes with a visit but no RIDT ($192.83), as well as those receiving empiric therapy without an accompanying visit ($105.64). The decreased costs for patients who visit a medical facility and receive an RIDT argue that the incorporation of RIDTs in influenza treatment practices may improve the cost-effectiveness of care. It has been proposed that costs associated with management of patients with influenza might be further reduced if RIDTs were used in community pharmacies. As highly accessible health care professionals, community pharmacists could be seen as an alternative from whom patients could receive efficient and quality care for acute illnesses. Since there is no fee charged for a pharmacy consultation, and recent studies have demonstrated that RIDTs can be incorporated into the daily workflow of a community pharmacy, further studies should be conducted to determine the extent of cost reductions that could be realized in this setting.9
In this study, use of RIDTs to direct treatment decisions for influenza is associated with a statistically significant decrease in direct medical costs for patient episodes managed in many settings, except for those who are treated in an inpatient setting or in an emergency room. In these latter settings, patients are likely to be more severely ill or suffering from other illnesses/conditions, which are, in fact, the primary reasons these patients are managed in these higher acuity venues. While these patients are billed for influenza-related claims, it is possible that influenza is not the primary or only reason that the total episode costs are so high.
The results of this study also indicate that a large number of patients receive empiric therapy for influenza. We found that 32% of episodes had a prescription written for either prophylaxis (17.1%) or treatment (15%) with no additional health care encounter within 48 hours of picking up the prescription at the pharmacy. These empiric therapies represent 60.1% of all treatment and prophylaxis prescriptions. This suggests that it is common for health care providers to call in prescriptions for patients without actually seeing them. Additionally, patients receiving care from a clinician who does not use an RIDT to guide treatment decisions are more likely to receive an antiviral subsequent to a visit for an ILI. According to our analysis, anti-influenza medications are prescribed 55% of the time when an RIDT is not performed and only 27.5% of the time when an RIDT is employed to aid in making treatment decisions. When an RIDT is used to aid clinical judgment, treatment decisions are based on evidence rather than “clinical feelings,” a practice that translates into effective antiviral and antimicrobial stewardship. In fact, this study shows that for the patients who visit a health care facility, health care professionals turn to RIDT testing 87% of the time to help determine the proper treatment course for these ILI episodes.
Limitations
As with any retrospective claims analysis, this study is subject to a number of limitations based on the available data. The primary limitation of this study is that it is impossible to determine the true treatment rate for patients without a medical visit (i.e., how many times did a call for ILI result in no further care or treatment). It is also unknown how many patients sought care over the phone and did not receive or fill a prescription. If the treatment rate is comparable to those with office visits, then it is possible that over-the-phone screening is more cost efficient. Another limitation of this study is that it may not account for patients presenting to health care providers with ILI who did not have a diagnosis, a prescription, or test claims. The inclusion of these patients could possibly result in a decreased frequency of patients who did not visit a health care facility but received a treatment regimen.
With the data available for use in this study, it is impossible to determine how many patients actually had influenza, which is why the study refers to patient episodes receiving care for ILIs. It is also not possible to determine the results of RIDTs from the claims data or to know if the decision to treat or not treat was consistently based on RIDT results. Similarly, it is impossible to identify those patients who received positive RIDT results but who were not prescribed a treatment regimen because 48 hours had passed since the influenza symptoms began (no longer making them a candidate for anti-influenza treatment).
Further limitations of this study include many unaccounted-for variables that may have changed the interpretation of the data. Patient comorbidities included in this study were not taken into account and were not available in the data. The presence of certain patient comorbidities could potentially alter the way influenza management would be approached in those patients.
Even with these limitations, this 2-year retrospective study of patients seeking care for ILI demonstrates that the use of diagnostic testing for clinical decision making in the treatment of influenza is relatively common in clinical practice.
Conclusions
This study shows that the use of RIDTs is common in health care practice and proves to be a promising way to improve prescribing patterns of anti-influenza medications and to reduce the costs associated with the medical management of influenza. Payers and policymakers should consider approaches to increase the access and use of rapid diagnostic testing during influenza season as a way to reduce overall economic burden of disease.
Funding Statement
This research is funded in part by a grant from the National Association of Chain Drug Stores Foundation. The authors have no financial interest in any company, product, or service described in this manuscript. Corn and Schmidt contributed to this research study while doctor of pharmacy candidates at the University of Nebraska Medical Center.
REFERENCES
- 1.Thompson WW, Comanor L, Shay DK.. Epidemiology of seasonal influenza: use of surveillance data and statistical models to estimate the burden of disease. J Infect Dis. 2006;194(Suppl 2):S82-91. Available at: http://jid.oxfordjournals.org/content/194/Supplement_2/S82.long. Accessed May 23, 2015. [DOI] [PubMed] [Google Scholar]
- 2.Molinari NA, Ortega-Sanchez IR, Messonnier ML, et al. The annual impact of seasonal influenza in the U.S.: measuring disease burden and costs. Vaccine. 2007;25(27):5086-96. Available at: http://www.sciencedirect.com/science/article/pii/S0264410X07003854. Accessed May 23, 2015. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. FluView: a weekly influenza surveillance report prepared by the Influenza Division. 2008-2009 Influenza Season Summary. January 5, 2008. Available at: http://www.cdc.gov/flu/weekly/weeklyarchives2008-2009/08-09summary.htm. Accessed May 23, 2015.
- 4.Jefferson T, Jones MA, Doshi P.et al. Neuraminidase inhibitors for preventing and treating influenza in healthy adults and children. Cochrane Database Syst Rev. 2014;4:CD008965. Available at: http://onlinelibrary.wiley.com/doi/10.1002/14651858.CD008965.pub4/full. Accessed May 23, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. Influenza antiviral medications: summary for clinicians. March 21, 2014. Updated February 25, 2015. Available at: http://www.cdc.gov/flu/professionals/antivirals/summary-clinicians.htm. Accessed May 23, 2015.
- 6.Havers F, Thaker S, Clippard J.et al. Use of influenza antiviral agents by ambulatory care clinicians during the 2012-2013 influenza season. Clin Infect Dis. 2014;59(6):774-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention. Rapid diagnostic testing for influenza: information for clinical laboratory directors. July 10, 2013. Updated November 5, 2014. Available at: http://www.cdc.gov/flu/professionals/diagnosis/rapidlab.htm. Accessed May 23, 2015.
- 8.Klepser DG, Dering-Anderson A, Morse J, Klepser MK, et al. Innovative rural community pharmacy-based influenza management program. Presented at: 37th Annual National Rural Health Conference; Las Vegas, NV; April 23, 2014. [Google Scholar]
- 9.Klepser DG, Dering-Anderson A, Morse J, et al. Time and motion study of influenza diagnostic testing in a community pharmacy. Innov Pharm. 2014;5(2):Article 159. Available at: http://www.pharmacy.umn.edu/innovations/prod/groups/cop/@pub/@cop/@innov/documents/article/cop_article_478794.pdf. Accessed May 23, 2015. [Google Scholar]