Abstract
BACKGROUND:
Pazopanib was noninferior to sunitinib in progression-free survival in a phase III, open-label, randomized clinical trial comparing the efficacy and safety of the 2 drugs for treatment of patients with advanced renal cell carcinoma (RCC). A secondary analysis of this trial conducted on patient-reported health care resource utilization (HCRU) endpoints revealed significantly fewer monthly telephone consultations and emergency department visits among patients treated with pazopanib over the first 6 months of treatment.
OBJECTIVES:
To (a) compare total costs of HCRU and adverse events (AEs) in patients with advanced RCC receiving first-line pazopanib or sunitinib from the phase III clinical trial and (b) perform a post hoc economic analysis that applied direct medical care and pharmacy unit costs, obtained from the Truven Health MarketScan Databases, to HCRU and AE rates.
METHODS:
Total HCRU costs included components for provider contacts, diagnostics, hospitalizations, procedures, and study/nonstudy drugs. Patients were stratified by the presence or absence of an AE in order to estimate costs attributable to AEs. Costs were adjusted to 2013 U.S. dollars. The highest 1% of cost outliers were equally excluded from each group. Univariate (t-test and Kaplan-Meier sample average [KMSA]) and multivariate (using treatment group and region as covariates) analyses were performed.
RESULTS:
A total of 906 patients (pazopanib, n = 454; sunitinib, n = 452) reported HCRU; higher rates were observed for sunitinib. In unadjusted cost analyses, the mean total costs for pazopanib-treated patients were 8.0% lower than those treated with sunitinib ($80,464 vs. $86,886; P = 0.20). The difference in KMSA-estimated costs was significantly higher for sunitinib versus pazopanib ($156,128 vs. $143,585; P = 0.003). Adjusted cost differences between arms consistently suggested higher costs for sunitinib. Among patients who experienced ≥ 1 AE, costs were $8,118 higher for pazopanib-treated patients and $14,343 for sunitinib-treated patients.
CONCLUSIONS:
The findings suggest that health care costs were lower among patients with advanced RCC treated first-line with pazopanib versus sunitinib.
What is already known about this subject
Few cost analyses of oncolytic treatments have been performed from a U.S. health care perspective, although costs are an important part of treatment decisions for patients, payers, and economic evaluations.
Total costs of treatment using targeted agents for advanced renal cell carcinoma (RCC) are known to differ partly because of differences in the incidence of associated adverse events (AEs) and their impact on health care resources used to treat them.
What this study adds
A phase III clinical trial, COMPARZ (Comparing the Efficacy, Safety and Tolerability of Pazopanib versus Sunitinib), provided an opportunity to assess, quantify, and evaluate health care resource utilization and AEs alongside costs in patients with advanced RCC receiving first-line treatment with pazopanib versus sunitinib.
This study links health care cost estimates to utilization in order to help payers make more informed decisions for managing advanced RCC in the United States.
Kidney cancer accounts for approximately 4% of all cancers in the United States, with an estimated 63,920 new cases and 13,680 deaths in 2014.1 Approximately 92% of kidney cancer cases are renal cell carcinoma (RCC).2 Advances in understanding of the biology and genetics of RCC have led to novel targeted approaches for treatment of the disease. The tyrosine kinase inhibitors sunitinib (Sutent, Pfizer Inc.) and pazopanib (Votrient, GlaxoSmithKline) are approved in the United States for the treatment of advanced RCC.3,4 Sunitinib is administered orally as 50 milligrams (mg) once daily in a 4-week-on/2-week-off schedule,3 while pazopanib has a continuous daily oral dosing regimen of 800 mg.4 Both are considered first-line treatment options for RCC.5 Sunitinib and pazopanib have similar mechanisms of action and showed efficacy in separate phase III trials with differences in the adverse event (AE) profiles.6-9
In a multicountry, phase III, open-label, randomized trial—COMPARZ (Comparing the Efficacy, Safety and Tolerability of Pazopanib versus Sunitinib; ClinicalTrials.gov identifier: NCT00720941)—that compared the efficacy and safety of pazopanib with sunitinib for treatment of patients with advanced RCC, results showed that pazopanib was noninferior to sunitinib in progression-free survival.10 However, analysis of the secondary endpoint of health care resource utilization (HCRU) revealed fewer monthly telephone consultations (P = 0.04) and emergency department visits (P = 0.003) among patients treated with pazopanib over the first 6 months of treatment.10
Since costs are an increasingly important component of treatment decisions for patients and payers, we conducted a post hoc evaluation of costs in patients with advanced RCC receiving first-line treatment with pazopanib and sunitinib in the COMPARZ trial. Specifically, we evaluated the costs associated with HCRU and AEs from a U.S. health care system perspective.
Methods
Objectives
The primary objective was to compare total costs of patient-reported HCRU in patients with advanced RCC receiving first-line treatment with pazopanib or sunitinib in the COMPARZ trial. A secondary objective was to compare total costs of AEs in the same patient population.
Study Design
Clinical trial data from patients assigned to each treatment arm as they were randomized (the intent-to-treat population) were used for a post hoc analysis of direct medical care costs applied to the HCRU rates. Patient-level assessment of unit cost estimates based on the diagnostic, laboratory, prescription medications, and procedural codes for each HCRU were assigned price weights based on estimates from the Truven Health MarketScan Commercial and Medicare Supplemental Databases for the period of 2008 to 2011.11 Total costs were then determined as the sum of costs for individual health care resources used during the observation period in the clinical trial.
We also stratified patients from the clinical trial on the presence or absence of grade 3 or grade 4 AEs (not including laboratory abnormalities) with ≥ 2% incidence. These reported AEs were similarly assigned price weights based on the MarketScan databases. A separate cost analysis was undertaken to estimate the incremental impact of AEs on total costs.
The clinical trial population consisted of 1,110 patients enrolled from 14 countries in Europe, Asia, Australia, and North America. Inclusion and exclusion criteria are detailed elsewhere.10 Briefly, the study included adults aged ≥ 18 years with a diagnosis of advanced/metastatic RCC with no prior systemic treatment and measurable disease per Response Evaluation Criteria in Solid Tumors, version 1.0.10 Patients also had Karnofsky performance status ≥ 70; adequate hematologic, renal, and hepatic function; and provided written, informed consent.
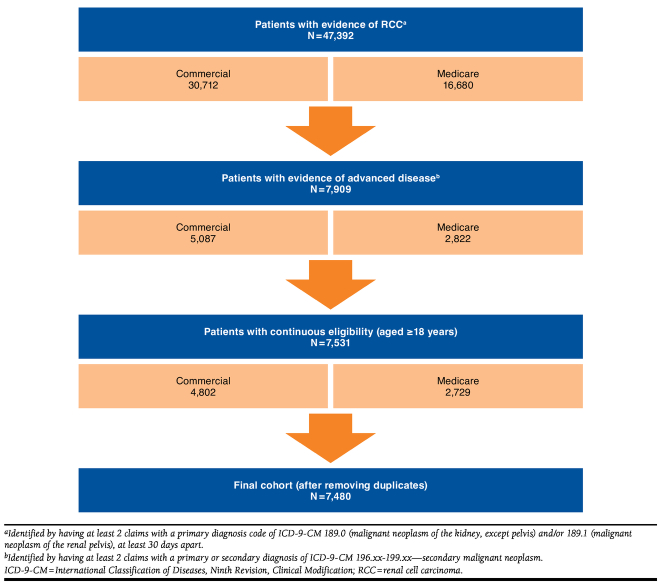
The MarketScan database population consisted of 7,480 adult patients (aged ≥ 18 years) with evidence of advanced RCC (≥ 2 claims with a primary diagnosis code of International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM] 189.0 or 189.1 and ≥ 2 claims with a primary or secondary diagnosis of ICD-9-CM 196.xx-199.xx) who were continuously enrolled in their health plans for at least 1 month following diagnosis.
No institutional review board approval was performed, since this study was a post hoc costing analysis of a previously approved clinical trial using de-identified records.
Rates of HCRU and AEs
HCRU was collected prospectively in the clinical trial via patient self-report at day 28 (± 3 days) of every 6-week cycle through cycle 9. Beginning with cycle 10, HCRU was collected at day 42 (± 3 days) of every cycle until treatment discontinuation. The clinical trial also reported grades 3 and 4 AEs with ≥ 2% incidence in either treatment arm. The HCRU components and AEs examined in this study are provided in Table 1 and Appendices A and B (available in online article), respectively.
TABLE 1.
Components of Total Health Care Resource Use and Costs Used in Economic Analysis
Medical and Ambulatory Services
|
Pharmacy Services
|
Total Health Care Resource Use Costs
|
Costs
Mean unit costs (noncapitated claims > $1) at the service level were estimated across all individuals with RCC in the MarketScan databases after excluding observations more than 2 standard deviations (SD) outside the mean. Categories of HCRU with less than 30 claims were expanded to the entire adult MarketScan database population. Total costs for the parameters listed in Table 1 were estimated by multiplying reported HCRU by standardized price weights from the MarketScan database and were tracked from treatment initiation to study discontinuation or death. Medical and outpatient prescription costs were assigned U.S. national average unit costs based on adjudicated claims. Cost of sunitinib and pazopanib were based on wholesale acquisition cost reference prices as of July 2013 for sunitinib and January 2013 for pazopanib. All unit cost estimates were adjusted to 2013 U.S. dollars using the medical care component of the Consumer Price Index (Appendices A and B, available in online article).
Statistical Analyses
HCRU Analyses.
Mean HCRU per month in each arm was calculated by summing patients’ HCRU and dividing by the mean follow-up period for the entire intent-to-treat population. Means of HCRU categories were estimated for each treatment, and the difference in means was tested using the Student’s t-test.
Unadjusted Costs.
Neither the clinical trial nor this economic analysis were powered to test individual differences between HCRU components and corresponding costs. Initial analyses on total HCRU costs revealed extreme cost values in both treatment groups influencing estimation of the mean and variance. Upon further examination of the data, the specific HCRU observed for these cost outliers were found to be unrelated to the disease or treatment. Therefore, the most extreme cost estimates (1% of patients) were excluded from both groups for interpretation of the cost analysis. Mean (raw/untransformed) total costs for each study arm were initially compared using the parametric Student’s t-test. Total costs for each study arm were also estimated using the Kaplan-Meier sample average (KMSA) estimation technique,12,13 which addresses rightcensoring (i.e., patients with truncated follow-up periods) and nonstandard distributions (i.e., small numbers of individuals with very high costs). HCRU in the clinical trial was only measured over the treatment period; therefore, survival weights were calculated based on treatment discontinuation with a follow-up time of 40 months. Confidence intervals (CIs) for the KMSA were generated by bootstrapping 2,000 independent samples. The 2.5 and 97.5 percentiles for each treatment, as well as the difference between treatments, were calculated as the 95% CI. The P value for the difference was calculated as the proportion of bootstrapped samples that had a difference more extreme than the difference in the complete sample.
Adjusted Costs.
The randomization scheme for the clinical trial accounted for age, sex, disease-risk classification, and stage at diagnosis. To account for factors that could influence the cost analysis, a prespecified adjusted evaluation using a multivariate generalized linear model (GLM) approach was employed to account for clustering within different geographic regions. In this model, treatment group (sunitinib as reference) and region (North America as reference, Europe, Asia, Australia) were used as covariates, with total costs as the dependent variable.14 Because the distribution of costs was skewed even after trimming the extreme cost values, 2 additional methods were used to test for differences in costs between groups.15 First, the costs were transformed using the natural log value of each of the total cost measures. For values where the cost was zero, $10 was added to the value before taking the natural log. Ordinary least-squares (log OLS) regression was then used to regress the treatment group and region variables on the natural log of total costs.16 As a second analytic approach, a GLM with a gamma distribution and log-link function was used.16 Finally, the population was stratified by the presence of at least one grade 3 or grade 4 AE, and total costs were estimated for those with and without AEs using a GLM including treatment group (sunitinib as reference) and region (North America as reference, Europe, Asia, Australia) as covariates.
Grades 3 and 4 AEs considered most likely to influence resource use and cost and that could be identified by ICD-9-CM diagnosis codes from the MarketScan databases were considered (Appendix B, available in online article). No missing data were noted in the analyses; therefore, no imputations were undertaken. All analyses were performed in SAS 9.3 (SAS Institute Inc, Cary, NC).
Results
Demographics
The 1,110 enrolled patients in the clinical trial were similar between treatment arms, predominantly males (73.2%), which is consistent with the RCC incidence in the population at large,17 with a mean age of 61 years. Nearly all patients (97.8%) had advanced/metastatic or stage IV disease at screening, and most were in the intermediate-risk category (58.6% per Memorial Sloan Kettering Cancer Risk Category; 54.7% per Heng Risk Category), with similar numbers in the favorable-, intermediate-, and poor-risk categories between treatment arms. Additionally, most patients had good performance status (76% with score of 90 or 100). The mean follow-up duration for the clinical trial period was 19.2 months.10 A total of 906 patients (pazopanib, n = 454; sunitinib, n = 452) reported HCRU and were included in the final costing analyses. Overall monthly HCRU was assessed for the entire clinical trial period for an overall mean (± SD) of 323.7 days (± 285.6 days; approximately 10.6 months); 318.1 days (± 282.9 days; 10.4 months) for pazopanib; and 329.2 days (± 288.4 days; 10.8 months) for sunitinib. Of patients in the MarketScan databases cohort used for the unit cost estimations (n = 7,480), the mean age (± SD) of all pazopanib users with RCC was 62.8 (± 11.4) years, and 67.6% were males (Figure 1).
FIGURE 1.
Selection of Patients from the MarketScan Commercial and Medicare Supplemental Databases for Unit Cost Estimation
Health Care Resource Use
Significantly higher monthly HCRU was observed for patients receiving sunitinib compared with those receiving pazopanib (Appendix C, available in online article) for the number of radiological visits (sunitinib: 0.072 ± 0.19; pazopanib: 0.050 ± 0.13; P = 0.045) and emergency department visits (sunitinib: 0.035 ± 0.07; pazopanib: 0.022 ± 0.05; P = 0.003). Although no other parameters showed significant differences between cohorts, there was a trend toward higher rates of HCRU for sunitinib compared with pazopanib across the other categories of use.
Unadjusted Total HCRU Costs
A small number of patients (4 patients in each group) constituted extreme cost outliers in both arms of the study. In the pazopanib arm, costs for the top 1% of patients averaged $320,871 versus a median cost of $4,639. In the sunitinib arm, costs for the top 1% of patients averaged $203,014 versus a median cost of $7,281. After accounting for the outliers, the mean total unadjusted costs (± standard error) for patients using pazopanib (n = 450) were $80,464 (± $75,432) compared with $86,886 (± $75,291) in patients using sunitinib (n = 448)—a difference of $6,422 (P = 0.20) or 8.0% (Table 2).
TABLE 2.
Average Total Health Care Resource Use, Unadjusted Costs
| Pazopanib (n = 450, $) | Sunitinib (n = 448, $) | Difference ($) | P Value (t-test) | |
|---|---|---|---|---|
| Mean | 80,464 | 86,886 | -6,422 | 0.20 |
| SD | 75,432 | 75,291 | 16,293 | |
| Minimum | 480 | 899 | NA | |
| Maximum | 402,881 | 351,342 | NA |
NA = not applicable; SD = standard deviation.
A series of 6 t-tests were performed on the cost components of the total HCRU unadjusted costs to aid in discerning the origin of the total cost difference observed, which are presented in Figure 2. Differences in the costs of hospitalizations ($772 higher for sunitinib; P = 0.068; Figure 2A) and study drug ($5,016 higher for sunitinib; P = 0.257; Figure 2B) accounted for a majority of total HCRU costs between the 2 treatment groups. With the exception of nonstudy drug costs (Figure 2B), costs related to treatment with pazopanib were less expensive than those related to treatment with sunitinib.
FIGURE 2.
Average Total Health Care Resource Use, Unadjusted Costs by Component
The overall KMSA-estimated difference was $12,543 lower costs for pazopanib ($143,585; 95% CI = $97,353-$148,005) versus sunitinib ($156,128; 95% CI = $105,128-$157,231), which was statistically significant (P = 0.003).
Adjusted Total HCRU Costs
Results of each of the multivariate models (i.e., OLS with logged outcome, GLM with gamma distribution and log-link) demonstrated consistent higher costs for sunitinib when compared with pazopanib, although only the OLS with logged outcome was statistically significant. Across all analyses, region-specific differences were not significant in the multivariate models (Appendices D-F, available in online article).
AEs and Associated Costs
In patients who experienced at least one grade 3 or grade 4 AE reported in Appendix B (available in online article), stratified GLM regression estimated costs were $8,118 higher for pazopanib-treated patients and $14,343 higher for sunitinib-treated patients (Table 3).
TABLE 3.
Total Cost Regression (GLM) Stratified by Adverse Events
| Parameter | Estimate ($) | 95% CI ($) | P Value | |
|---|---|---|---|---|
| Average total costs without AEs (n = 536) | ||||
| Intercept | 71,235 | 59,325 | 83,145 | < 0.001 |
| Pazopanib | -1,916 | -14,259 | 10,428 | 0.761 |
| Sunitinib | Reference | NA | NA | NA |
| Europe | 14,748 | 455 | 29,040 | 0.044 |
| Asia | 13,413 | -3,390 | 30,216 | 0.118 |
| Australia | 6,690 | -21,878 | 35,258 | 0.646 |
| North America | Reference | NA | NA | NA |
| Average total costs with AEs (n = 362) | ||||
| Intercept | 85,578 | 72,152 | 99,004 | < 0.001 |
| Pazopanib | -8,141 | -23,407 | 7,124 | 0.297 |
| Sunitinib | Reference | NA | NA | NA |
| Europe | 11,449 | -6,346 | 29,244 | 0.208 |
| Asia | 11,803 | -8,631 | 32,237 | 0.258 |
| Australia | 6,625 | -25,423 | 38,674 | 0.686 |
| North America | Reference | NA | NA | NA |
AEs = adverse events; CI = confidence interval; GLM = generalized linear model; NA = not applicable.
Discussion
We conducted a post hoc analysis of the COMPARZ clinical trial data evaluating costs associated with HCRU and AEs in patients with advanced RCC receiving first-line treatment with pazopanib versus sunitinib from a U.S. health care system perspective. Radiology and emergency department visits were significantly greater for patients in the sunitinib arm compared with pazopanib-treated patients. The mean total unadjusted HCRU costs were 8.0% higher for sunitinib than pazopanib (P = 0.20) by t-test, and the difference in KMSA-estimated costs was significantly (P = 0.003) higher for sunitinib versus pazopanib ($12,543). Additionally, multivariate analyses (adjusting for treatment and region) suggested total HCRU costs for pazopanib-treated patients were less than for sunitinib-treated patients.
Because the clinical trial was not designed to test differences in total costs, some method of adjustment to account for extreme cost outliers is necessary for interpretation of the cost analysis.18 In this study, the cost differences between outliers and the rest of the sample were extreme, with 69-fold and 27-fold differences between the top 1% of patients (n = 4 in each group) and the remainder of the sample, respectively. If treatment-related effects were underlying factors for these high-cost patients, it would be inappropriate to remove them from the analysis, and one would have to conclude that the study was underpowered to detect a cost difference between groups. If the high costs were due to events unrelated to treatment, then removing these individuals from the cost analysis is appropriate.
Multivariate analyses were also undertaken to address factors not accounted for in randomization that could influence costs and the remaining skewing of the data. First, a GLM regression was employed to adjust for region. The dataset did not show significant region-specific effects or lower HCRU costs in the pazopanib arm. A GLM using the gamma distribution and a log link with the same covariates to better adjust for remaining skewing also did not show region-specific effects or lower costs for the pazopanib arm. When we log-transformed the dependent variable to improve its distribution and performed an OLS regression, we found no significant differences by region and significantly lower costs for the pazopanib arm. Multivariate regressions do not account for patient censoring over the follow-up period. To determine if accounting for censoring influences the results, we conducted a KMSA estimator analysis, which showed that total HCRU cost for patients in the sunitinib arm was $12,543 more than patients in the pazopanib arm.
Consistent with previous studies,19,20 this study also indicates that there were additional costs between $8,118 and $14,343 for patients who experienced at least 1 AE during treatment.
Costs are an important part of treatment decisions for patients, payers, and economic evaluations; however, few cost analyses with oncolytic treatments have been performed from a U.S. health care perspective.21 Total costs of treatments using targeted agents for advanced RCC are known to differ, in part, because of differences in the incidence of associated AEs and their impact on health care resources used to treat them.22,23 Detailed information including the cost of drug treatments, side effects, and outcomes such as HCRU would help payers to make more informed decisions for managing advanced RCC in the United States. The COMPARZ clinical trial provided an opportunity to assess, quantify, and evaluate HCRU and AEs alongside costs in patients with advanced RCC receiving first-line treatment with pazopanib versus sunitinib.
Limitations
The results of this study should be evaluated with the acknowledgment of limitations. First, the COMPARZ trial was designed to test clinical and not economic endpoints. Second, this was a global study, and medical and pharmacy resource utilization are highly localized, raising concerns about external validity (i.e., representativeness) when pooling data across regions for cost analysis. Region-specific analyses did not suggest differences, but this could be due to the relatively low number of participants in some regions included in the study. Finally, actual resource utilization, treatment, or AE costs were not collected during the clinical trial; thus, the estimated unit costs may not accurately represent the actual costs incurred to treat those patients in the trial. Furthermore, because these cost estimates are influenced by the design of the clinical trial, they may not represent what may be experienced in a “real-world” setting.
Implications for Future Research
Findings in this study suggest HCRU and AE costs were significantly lower among patients with advanced RCC treated with pazopanib compared with sunitinib. To establish whether this translates into lower costs for patients treated with pazopanib in practice, future studies should directly collect and evaluate costs for patients treated with these agents outside of a clinical trial setting.
Conclusions
Based on a costing analysis of data on HCRU and grades 3 and 4 AEs from a clinical trial, our results showed consistently lower health care costs for patients with advanced RCC treated with first-line pazopanib compared with sunitinib.
ACKNOWLEDGMENTS
The authors wish to acknowledge individuals who contributed and provided critical review during the development of this manuscript. Nate Connors, PhD, and Nancy Price, PhD, of AOI Communications, L.P., provided medical writing and editorial assistance, which was funded by GSK.
APPENDIX A. Health Care Resource Utilization Components for Medical/Ambulatory Services and Unit Costs Estimated from Truven MarketScan Databases
| Medical and Ambulatory Service | Number of Observations | Mean Unit Costs ($) a | Standard Deviation ($) | Minimum ($) | Maximum ($) |
|---|---|---|---|---|---|
| Providers | |||||
| Primary care physician visit | 92,590 | 104.20 | 50.85 | 1.21 | 392.24 |
| Nurse practitioner/physician’s assistant/nurse visit | 687 | 90.56 | 42.74 | 12.11 | 209.91 |
| Telephone consultation | 9,644 | 54.60 | 47.57 | 1.05 | 412.31 |
| Medical/surgical specialist visit | |||||
| Allergy and immunology | 385 | 78.76 | 71.79 | 1.18 | 301.93 |
| Cardiovascular disease | 1,026 | 758.18 | 797.38 | 5.82 | 4,771.57 |
| Dermatology | 3,778 | 99.49 | 79.43 | 1.45 | 502.97 |
| Endocrinology and metabolism | 2,264 | 73.75 | 61.01 | 1.15 | 277.85 |
| Gastroenterology | 4,025 | 164.26 | 130.21 | 1.05 | 654.42 |
| Gynecology | 1,429 | 95.89 | 108.28 | 1.03 | 861.87 |
| Hematology | 68,649 | 147.70 | 292.35 | 1.00 | 1,876.90 |
| Infectious diseases | 4,272 | 98.74 | 89.10 | 1.22 | 1,096.72 |
| Internal medicine | 71,264 | 102.67 | 111.48 | 1.00 | 980.46 |
| Nephrology | 8,646 | 119.51 | 91.72 | 1.13 | 534.91 |
| Neurological surgery | 3,639 | 679.62 | 941.74 | 1.46 | 5,436.82 |
| Neurology | 3,665 | 169.39 | 195.78 | 1.06 | 1,690.75 |
| Obstetrics and gynecology | 1,429 | 95.89 | 108.28 | 1.03 | 861.87 |
| Oncology, medical | 141,680 | 152.66 | 277.96 | 1.00 | 1,623.26 |
| Ophthalmology | 4,200 | 101.67 | 80.12 | 1.18 | 624.32 |
| Orthopedic surgery | 8,700 | 183.18 | 303.59 | 1.02 | 1,850.92 |
| Pathology | 66,756 | 37.18 | 61.19 | 1.01 | 374.75 |
| Physical medicine and rehabilitation | 3,358 | 103.17 | 77.25 | 1.06 | 609.08 |
| Plastic surgery | 546 | 420.18 | 610.12 | 3.43 | 2,914.14 |
| Podiatric medicine (podiatry) | 2,961 | 73.40 | 54.39 | 1.12 | 374.62 |
| Preventative medicine | 87 | 148.05 | 219.08 | 5.13 | 1,048.69 |
| Psychiatry | 1,283 | 118.14 | 74.81 | 4.20 | 766.08 |
| Pulmonary disease | 8,865 | 124.46 | 96.62 | 1.40 | 524.61 |
| Rheumatology | 758 | 68.77 | 64.02 | 1.57 | 280.08 |
| Home health care visit | 19,632 | 769.54 | 1,806.12 | 1.39 | 17,813.70 |
| Diagnostics | |||||
| Laboratory visit | 367,437 | 60.66 | 137.48 | 1.00 | 1,436.06 |
| Hematology | 57,284 | 22.47 | 22.86 | 1.00 | 143.40 |
| Clinical chemistry | 35,311 | 38.45 | 50.81 | 1.05 | 281.07 |
| Liver function test | 2,458 | 22.32 | 24.83 | 1.35 | 124.84 |
| Pancreatic (amylase and lipase) | 3,060 | 26.66 | 26.81 | 1.03 | 143.08 |
| Coagulation | 17,465 | 16.68 | 16.59 | 1.01 | 77.89 |
| Urinalysis | 1,754 | 5.96 | 3.51 | 1.00 | 30.00 |
| ECG | 2,093 | 31.23 | 11.12 | 1.06 | 79.44 |
| Thyroid function | 756 | 84.45 | 65.26 | 4.76 | 332.05 |
| Tumor marker | 4,775 | 284.92 | 276.04 | 1.09 | 1,304.93 |
| Radiology | |||||
| Oncology | 123,067 | 136.10 | 155.35 | 1.00 | 998.61 |
| Diagnostic | 3,733 | 146.39 | 260.60 | 1.01 | 1,452.01 |
| Nuclear | 3,733 | 146.39 | 260.60 | 1.01 | 1,452.01 |
| CT or MRI | 9,058 | 334.84 | 359.15 | 1.13 | 1,608.07 |
| DCE MRI | 1,223 | 598.32 | 567.46 | 5.43 | 2,234.96 |
| PET/CT scan | 456 | 1,376.36 | 812.23 | 57.20 | 3,700.28 |
| Echocardiogram | 104,703 | 340.00 | 231.38 | 1.05 | 1,104.75 |
| Multi-slice spiral CT | 6,149 | 223.03 | 258.11 | 1.68 | 1,156.00 |
| Nuclear scan | 53 | 190.95 | 198.26 | 9.63 | 855.44 |
| Stress echocardiogram | 85 | 258.41 | 225.40 | 31.06 | 909.30 |
| Total body X-ray | 146 | 155.91 | 188.31 | 23.92 | 650.04 |
| Mammography | 56 | 112.49 | 60.27 | 45.86 | 312.32 |
| Transvaginal ultrasound | 143 | 108.18 | 59.81 | 8.01 | 305.03 |
| Bone scan | 146 | 155.91 | 188.31 | 23.92 | 650.04 |
| Conventional CT scan | 6,145 | 248.21 | 287.45 | 1.89 | 1,289.40 |
| High-resolution CT scan | 9,058 | 334.84 | 359.15 | 1.13 | 1,608.07 |
| Spiral CT scan | 6,145 | 248.21 | 287.45 | 1.89 | 1,289.40 |
| MRI | 300 | 365.87 | 408.38 | 8.81 | 1,961.35 |
| Ultrasound | 448 | 112.16 | 92.05 | 9.36 | 489.86 |
| X-ray | 14,113 | 47.29 | 58.04 | 1.13 | 234.69 |
| Hospitalizations | |||||
| General ward (day) | 39,572 | 85.05 | 24.26 | 2.40 | 194.11 |
| Intensive care unit (day) | 3,169 | 6,346.05 | 7,572.10 | 9.01 | 43,579.65 |
| Emergency department visit | 23,671 | 238.50 | 316.54 | 1.01 | 2,154.60 |
| Procedures | |||||
| Endoscopy | 5,447 | 310.22 | 297.98 | 1.00 | 1,529.72 |
| Inpatient procedure | 22,064 | 873.39 | 941.61 | 1.48 | 5,078.44 |
| Surgery, colon and rectal | 332 | 147.68 | 169.41 | 1.05 | 979.18 |
| Surgery, hand | 6,154 | 136.51 | 118.14 | 1.57 | 720.43 |
| Surgery, vascular | 902 | 180.23 | 260.35 | 2.19 | 1,416.22 |
| Other | 47,302 | 120.63 | 43.32 | 1.21 | 355.02 |
| Outpatient procedure | 31,902 | 311.84 | 499.09 | 1.00 | 3,124.68 |
a All unit cost estimates were adjusted to 2013 U.S. dollars using the medical care component of the Consumer Price Index.
CT = computed tomography; DCE = dynamic contrast enhanced; ECG = electrocardiogram; MRI = magnetic resonance imaging; PET = positron emission tomography.
APPENDIX B. Grade 3 or Grade 4 Adverse Events (Not Including Laboratory Abnormalities) with ≥ 2% Incidence from the Clinical Trial, ICD-9-CM Claim Codes, and Unit Costs Estimated from the Truven MarketScan Databases
| Adverse Event | ICD-9-CM Codes | Number of Observations in Databases | Mean Unit Costs ($) a | Standard Deviation ($) | Minimum ($) | Maximum ($) |
|---|---|---|---|---|---|---|
| Hypertension | 362.11, 401, 402, 403, 404, 405, 437.2, 642.0, 642.1, 642.2 | 25,528 | 190.51 | 510.77 | 1.02 | 6,162.93 |
| Fatigue | 300.5, 780.7, 799.3, 780.72 | 8,361 | 131.14 | 225.95 | 1.00 | 1,904.16 |
| Diarrhea | 564.5, 787.91 | 1,376 | 174.29 | 417.61 | 1.01 | 3,826.26 |
| Palmer-plantar erythrodysesthesia syndrome (hand and foot syndrome) | 693.0 | 227 | 112.04 | 179.06 | 3.24 | 1,649.32 |
| Headache | 339.3, 346.0, 346.1, 346.8, 346.9, 784.0 | 2,492 | 250.61 | 451.52 | 1.05 | 3,685.62 |
| Nausea and vomiting | 536.2, 578.0, 787.0 | 14,304 | 174.55 | 326.92 | 1.00 | 2,448.50 |
| Arthralgia | 524.62, 719.4, 723.1, 724.1, 724.2, 724.5 | 16,388 | 127.16 | 209.70 | 1.01 | 1,631.30 |
| Dyspnea | 518.81, 518.82, 786.0 | 12,095 | 235.61 | 605.93 | 1.00 | 9,033.14 |
| Asthenia | 780.7, 799.3, 780.71, 780.72 | 8,336 | 131.60 | 227.13 | 1.00 | 2,007.30 |
| Anorexia | 783.0 | 158 | 138.45 | 212.39 | 1.22 | 1,790.61 |
| Mucosal inflammation (mucositis) | 538 | 711 | 171.42 | 460.08 | 1.47 | 4,499.54 |
| Dehydration | 276.5 | 9,999 | 195.79 | 604.29 | 1.00 | 7,597.94 |
| Syncope | 780.2 | 1,760 | 203.84 | 316.72 | 1.18 | 2,490.33 |
| Pleural effusion | 511.9 | 9,240 | 229.81 | 559.76 | 1.16 | 7,707.04 |
a All unit cost estimates were adjusted to 2013 U.S. dollars using the medical care component of the Consumer Price Index.
ICD-9-CM = International Classification of Diseases, Ninth Revision, Clinical Modification.
APPENDIX C. Mean (Standard Deviation) Monthly Health Care Resource Use from the Clinical Trial
| Pazopanib (n = 454) | Sunitinib (n = 452) | P Value | ||
|---|---|---|---|---|
| Mean follow-up time (months) | 10.4 | 10.8 | ||
| Providers | ||||
| Primary physician care visits | 0.293 (0.77) | 0.311 (0.84) | 0.75 | |
| Nurse practitioner/physician’s assistant/nurse visits | 0.069 (0.24) | 0.070 (0.18) | 0.96 | |
| Telephone consultations | 0.173 (0.54) | 0.161 (0.40) | 0.70 | |
| Medical/surgical specialist visits (all) | 0.218 (0.73) | 0.248 (0.82) | 0.55 | |
| Oncology, medical | 0.010 (0.05) | 0.013 (0.07) | 0.26 | |
| Hematology | 0.003 (0.05) | 0.000 (0.01) | 0.32 | |
| Nononcology | 0.155 (0.55) | 0.170 (0.63) | 0.69 | |
| Other | 0.051 (0.25) | 0.064 (0.29) | 0.46 | |
| Home health visits | 0.016 (0.12) | 0.065 (0.86) | 0.24 | |
| Diagnostics | ||||
| Laboratory visits | 0.134 (0.36) | 0.173 (0.44) | 0.14 | |
| Laboratory tests (all) | 0.238 (1.37) | 0.377 (2.25) | 0.26 | |
| Hematology | 0.213 (0.49) | 0.278 (0.68) | 0.37 | |
| Clinical chemistry | 0.226 (0.50) | 0.255 (0.69) | 0.69 | |
| Liver function tests | 0.101 (0.35) | 0.104 (0.32) | 0.95 | |
| Pancreatic (amylase and lipase) | 0.058 (0.44) | 0.081 (0.61) | 0.72 | |
| Coagulation | 0.086 (0.36) | 0.107 (0.53) | 0.69 | |
| Urinalysis | 0.067 (0.42) | 0.108 (0.58) | 0.50 | |
| ECG | 0.033 (0.10) | 0.022 (0.05) | 0.27 | |
| Thyroid function | 0.040 (0.20) | 0.094 (0.53) | 0.25 | |
| Other | 0.117 (0.23) | 0.089 (0.32) | 0.32 | |
| Radiological visits | 0.050 (0.13) | 0.072 (0.19) | 0.04 | |
| Radiological tests | ||||
| CT or MRI | 0.052 (0.07) | 0.058 (0.08) | 0.53 | |
| DCE MRI | 0.001 (0.01) | 0.000 (0.00) | 0.32 | |
| PET scan | 0.000 (0.00) | 0.001 (0.01) | 0.32 | |
| PET-CT | 0.001 (0.01) | 0.000 (0.00) | 0.32 | |
| Bone scan | 0.008 (0.03) | 0.008 (0.03) | 0.93 | |
| Other | 0.152 (0.17) | 0.204 (0.27) | 0.08 | |
| Hospitalizations | ||||
| Hospital days (hospitalization at least 24 hours) | ||||
| General ward | 0.200 (0.68) | 0.254 (0.71) | 0.24 | |
| Intensive care unit | 0.006 (0.07) | 0.017 (0.17) | 0.20 | |
| Emergency department visits | 0.022 (0.05) | 0.035 (0.07) | 0.003 | |
| Procedures | ||||
| At outpatient/physician clinic | 0.110 (0.65) | 0.154 (0.89) | 0.39 | |
| During any hospitalization | 0.031 (0.32) | 0.032 (0.16) | 0.95 | |
CT = computed tomography; DCE = dynamic contrast enhanced; ECG = electrocardiogram; MRI = magnetic resonance imaging; PET = positron emission tomography.
APPENDIX D. Adjusted Average Total Health Care Resource Use Costs (OLS); N = 898
| Parameter | North America Cost Estimate, $ (95% CI) a | Estimate | Standard Error | t Value | P Value |
|---|---|---|---|---|---|
| Interceptb | NA | 10.836 | 0.067 | 161.57 | < 0.001 |
| Pazopanib | 43,532 (33,137-57,189) | -0.155 | 0.072 | -2.15 | 0.032 |
| Sunitinib | 50,825 (44,557-57,975) | Reference | |||
| Europe | NA | 0.177 | 0.084 | 2.11 | 0.035 |
| Asia | NA | 0.218 | 0.098 | 2.22 | 0.026 |
| Australia | NA | 0.063 | 0.161 | 0.39 | 0.696 |
| North America | NA | Reference | |||
a Cost is the exponentiation of beta coefficient and is the geometric mean (straight transformation); estimate is the rate ratio of the cost.
b Intercept is the base cost for the United States.
CI = confidence interval; NA = not applicable; OLS = ordinary least-squares.
APPENDIX E. Adjusted Average Total Health Care Resource Use Costs (GLM); N = 898
| Parameter | North America Cost Estimate, $ (95% CI) | Estimate | Standard Error | t Value | P Value |
|---|---|---|---|---|---|
| Intercept | NA | 80,159 | 4,687 | 17.10 | < 0.001 |
| Pazopanib | 73,856 (54,786-92,927) | -6,302 | 5,029 | -1.25 | 0.211 |
| Sunitinib | 80,159 (70,959-89,359) | Reference | |||
| Europe | NA | 11,503 | 5,867 | 1.96 | 0.050 |
| Asia | NA | 12,820 | 6,836 | 1.88 | 0.061 |
| Australia | NA | 5,370 | 11,252 | 0.48 | 0.633 |
| North America | NA | Reference | |||
CI = confidence interval; GLM = generalized linear model; NA = not applicable.
APPENDIX F. Adjusted Average Total Health Care Resource Use Costs (GLM with Log Link); N = 898
| Parameter | North America Cost Estimate, $ (95% CI) a | Estimate | Standard Error | Wald 95% Confidence Limits | Wald Chi-Square | P Value | |
|---|---|---|---|---|---|---|---|
| Interceptb | NA | 11.2886 | 0.0563 | 11.1783 | 11.3989 | 40,214.70 | < 0.001 |
| Pazopanib | 73,984 (58,818-93,051) | -0.077 | 0.0607 | -0.1961 | 0.042 | 1.61 | 0.205 |
| Sunitinib | 79,906 (71,561-89,224) | Reference | |||||
| Europe | NA | 0.1404 | 0.0709 | 0.0015 | 0.2793 | 3.92 | 0.048 |
| Asia | NA | 0.1552 | 0.0826 | -0.0067 | 0.3170 | 3.53 | 0.060 |
| Australia | NA | 0.0707 | 0.1358 | -0.1955 | 0.3370 | 0.27 | 0.603 |
| North America | NA | Reference | |||||
| Scale | NA | 1.2095 | 0.0511 | 1.1134 | 1.3139 | NA | NA |
a Estimate is the rate ratio of the cost.
b Intercept is the base cost for the United States.
CI = confidence interval; GLM = generalized linear model; NA = not applicable.
Funding Statement
Funding for this study was provided by GlaxoSmithKline (GSK). All listed authors meet the criteria for authorship set forth by the International Committee for Medical Journal Editors. Hansen has nothing to declare. Sullivan and Ramsey are employees of VeriTech Corporation, which has received research funding from GSK for activities related to this study. Ramsey has also been a paid consultant on the Value Assessment Committee and has received grant funding from Premera Blue Cross unrelated to this study. Hackshaw, Nagar, Arondekar, and Deen are employees of and hold stock in GSK. The authors (funded by GSK) had a role in the conception and design of the study; collection, analysis, and interpretation of data; and final manuscript approval.
The authors wish to acknowledge individuals who contributed and provided critical review during the development of this manuscript. Nate Connors, PhD, and Nancy Price, PhD, of AOI Communications, L.P., provided medical writing and editorial assistance, which was funded by GSK.
REFERENCES
- 1.Siegel R, Ma J, Zou Z, Jemal A.. Cancer statistics, 2014. CA Cancer J Clin. 2014;64(1):9-29. Available at: http://onlinelibrary.wiley.com/doi/10.3322/caac.21208/pdf. Accessed November 17, 2014. [DOI] [PubMed] [Google Scholar]
- 2.American Cancer Society. Cancer facts and figures 2010. Atlanta, GA: American Cancer Society. 2010. Available at: http://www.cancer.org/acs/groups/content/@nho/documents/document/acspc-024113.pdf. Accessed November 17, 2014.
- 3.Sutent (sunitinib malate) capsules. Pfizer Inc. Revised June 2014. Available at: http://labeling.pfizer.com/ShowLabeling.aspx?id=607. Accessed November 17, 2014.
- 4.Votrient (pazopanib) tablets. GlaxoSmithKline. Revised June 2014. Available at: https://www.gsksource.com/gskprm/htdocs/documents/VOTRIENT-PI-MG.PDF. Accessed November 17, 2014.
- 5.National Comprehensive Cancer Network. Clinical practice guidelines in oncology. Kidney cancer, V2. 2014. Available at: http://www.nccn.org/professionals/physician_gls/pdf/kidney.pdf. Accessed November 17, 2014.
- 6.Motzer RJ, Hutson TE, Tomczak P, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356(2):115-24. Available at: http://www.nejm.org/doi/pdf/10.1056/NEJMoa065044. Accessed November 17, 2014. [DOI] [PubMed] [Google Scholar]
- 7.Motzer RJ, Hutson TE, Tomczak P, et al. Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinoma. J Clin Oncol. 2009;27(22):3584-90. Available at: http://jco.ascopubs.org/content/27/22/3584.full.pdf+html. Accessed November 17, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sternberg CN, Davis ID, Mardiak J, et al. Pazopanib in locally advanced or metastatic renal cell carcinoma: results of a randomized phase III trial. J Clin Oncol. 2010;28(6):1061-68. Available at: http://jco.ascopubs.org/content/28/6/1061.full.pdf+html. Accessed November 17, 2014. [DOI] [PubMed] [Google Scholar]
- 9.Sternberg CN, Hawkins RE, Wagstaff J, et al. A randomised, double-blind phase III study of pazopanib in patients with advanced and/or metastatic renal cell carcinoma: final overall survival results and safety update. Eur J Cancer. 2013;49(6):1287-96. [DOI] [PubMed] [Google Scholar]
- 10.Motzer RJ, Hutson TE, Cella D, et al. Pazopanib versus sunitinib in metastatic renal-cell carcinoma. N Engl J Med. 2013;369(8):722-31. [DOI] [PubMed] [Google Scholar]
- 11.Hansen LG, Chang S.. Health research data for the real world: the Thomson Reuters MarketScan databases. White Paper. Thomson Reuters. 2011. Available at: http://interest.truvenhealth.com/forms/HealthResearchWPRequest. Accessed December 8, 2014. [Google Scholar]
- 12.Lin DY. Linear regression analysis of censored medical costs. Biostatistics. 2000;1(1):35-47. Available at: http://biostatistics.oxfordjournals.org/content/1/1/35.long. Accessed November 17, 2014. [DOI] [PubMed] [Google Scholar]
- 13.Lin DY, Feuer EJ, Etzioni R, Wax Y.. Estimating medical costs from incomplete follow-up data. Biometrics. 1997;53(2):419-34. Available at: http://dlin.web.unc.edu/files/2013/04/LinEA97.pdf. Accessed November 17, 2014. [PubMed] [Google Scholar]
- 14.Willan AR, Lin DY, Manca A.. Regression methods for cost-effectiveness analysis with censored data. Stat Med. 2005;24(1):131-45. [DOI] [PubMed] [Google Scholar]
- 15.Manning WG, Mullahy J.. Estimating log models: to transform or not to transform? J Health Econ. 2001;20(4):461-94. [DOI] [PubMed] [Google Scholar]
- 16.Diehr P, Yanez D, Ash A, Hornbrook M, Lin DY.. Methods for analyzing health care utilization and costs. Annu Rev Public Health. 1999;20:125-44. [DOI] [PubMed] [Google Scholar]
- 17.Howlader N, Noone A, Krapcho M, et al. SEER Cancer Statistics Review, 1975-2011. SEER Stat Fact Sheets: kidney and renal pelvis cancer. National Cancer Institute. April 2014. Available at: http://seer.cancer.gov/statfacts/html/kidrp.html. Accessed November 17, 2014.
- 18.Weichle T, Hynes DM, Durazo-Arvizu R, Tarlov E, Zhang Q.. Impact of alternative approaches to assess outlying and influential observations on health care costs. Springerplus. 2013;2:614. Available at: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3843184/. Accessed November 17, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hagiwara M, Borker R, Oster G.. Economic burden of adverse events in patients with metastatic renal cell carcinoma. Clin Ther. 2013;35(12):1955-63. [DOI] [PubMed] [Google Scholar]
- 20.Hagiwara M, Hackshaw MD, Oster G.. Economic burden of selected adverse events in patients aged ≥ 65 years with metastatic renal cell carcinoma. J Med Econ. 2013;16(11):1300-06. [DOI] [PubMed] [Google Scholar]
- 21.Thompson Coon J, Hoyle M, Green C, et al. Bevacizumab, sorafenib tosylate, sunitinib and temsirolimus for renal cell carcinoma: a systematic review and economic evaluation. Health Technol Assess. 2010;14(2):1-184, iii-iv. Available at: http://www.ncbi.nlm.nih.gov/pubmedhealth/PMH0014943/pdf/summ1402.pdf. Accessed November 17, 2014. [DOI] [PubMed] [Google Scholar]
- 22.Mickisch G, Gore M, Escudier B, Procopio G, Walzer S, Nuijten M.. Costs of managing adverse events in the treatment of first-line metastatic renal cell carcinoma: bevacizumab in combination with interferon-alpha2a compared with sunitinib. Br J Cancer. 2010;102(1):80-86. Available at: http://www.nature.com/bjc/journal/v102/n1/pdf/6605417a.pdf. Accessed November 17, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Villa G, Hernández-Pastor LJ.. Budget impact analysis of first-line treatment with pazopanib for advanced renal cell carcinoma in Spain. BMC Cancer. 2013;13:399. Available at: http://www.biomedcentral.com/content/pdf/1471-2407-13-399.pdf. Accessed November 17, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]