Abstract
BACKGROUND:
Medication nonadherence is a prevalent public health issue, particularly among patients with diabetes mellitus (DM), and negatively affects health outcomes. Because of the prevalence of DM among U.S. veterans, it is crucial to understand how well these patients adhere to oral antidiabetic (OAD) medication and whether certain subgroups are more likely to be nonadherent.
OBJECTIVE:
To assess initial OAD medication use among veterans with uncomplicated DM and determine factors associated with adherence in the first 2 years of treatment.
METHODS:
This was a retrospective cohort study using data from the Veterans Affairs (VA) Corporate Data Warehouse from 2002 through 2014. The first diagnosis for uncomplicated DM was determined, and then medication use was assessed following OAD initiation. OAD use was assessed by proportion of days covered (PDC) for the first 2 years of therapy using outpatient VA pharmacy records. Adherence was determined both continuously and categorically, with a PDC of ≥ 80% used to indicate adherence. Logistic regression was used to determine if certain patient characteristics were associated with being adherent to OADs.
RESULTS:
A total of 148,544 veterans with uncomplicated DM were assessed, most of whom were white, aged ≥ 55 years, and initiated OAD therapy on metformin. A large portion resided in the southern part of the United States. In the first year, PDC averaged 79.2% (SD = 25.9), and 63.2% were adherent to OAD therapy; however, these numbers declined in the second year, when the average PDC was 71.3% (SD = 35.8), and only 59.1% were adherent. Over the course of both years, PDC averaged 75.3% (SD = 28.4), and 50.9% were adherent. The odds of being adherent were higher among older adults and significantly lower among veterans self-identifying as either African American (OR = 0.61; 95% CI = 0.59-0.63), Native American (OR = 0.67; 95% CI = 0.61-0.75), or Hawaiian/Pacific Islander (OR = 0.84; 95% CI = 0.76-0.92) when compared with whites. Veterans who were either divorced/separated (OR = 0.86; 95% CI = 0.83-0.88) or never married (OR = 0.89; 95% CI = 0.86-0.93) also had lower odds of being initially adherent to OAD therapy compared with those who reported being married. Being nonadherent in year 1 was highly predictive of remaining nonadherent in year 2 (OR = 12.8; 95% CI = 12.23-12.94), with only 22.2% nonadherent in the first year (8.2% overall) becoming adherent in the second year of therapy. Across both years, all minorities were less likely to be adherent (compared with whites), and average adherence differed among all geographic regions of the country.
CONCLUSIONS:
Within the first year of OAD therapy, medication adherence was suboptimal among veterans with DM, and second-year results indicate that adherence is likely to decline over time. Future studies should consider deeper regional and subgroup analysis to determine what contributes to variation in medication use in communities across the country.
What is already known about this subject
Medication adherence among patients with diabetes mellitus (DM) is generally suboptimal and contributes to poorer health outcomes in this patient population.
A variety of factors have been identified that may influence DM medication use at a given point in the therapeutic process.
What this study adds
This analysis used a nationwide database to identify patients from their first uncomplicated DM diagnosis and followed them once treatment was initiated, an approach that is relatively limited among claims-based analyses using U.S. patients.
Suboptimal antidiabetic medication use begins as early as the first year of therapy and declines significantly in the second year of treatment.
Significant differences in initial antidiabetic medication adherence were observed across races and between veterans residing in different regions of the United States.
Medication nonadherence is a pervasive public health issue. Approximately 50% of all patients with chronic disease deviate from their prescribed treatment regimen, and such deviation can have dire consequences, including hospitalization and premature death.1-3 Among patients with diabetes mellitus (DM), medication nonadherence is a particularly extensive issue. While some studies involving patients with DM have reported high adherence rates (up to 98%), estimates may be as low as 31% and vary considerably depending on the metric.4-8 Importantly, reviews of these studies concluded that overall levels have not improved significantly over time, suggesting the need for increased attention to improving this behavior.7
Numerous reasons for variation in medication use exist, the majority of which are generally categorized as either system, patient, treatment, condition, or social and economic factors.9 Among factors tied to social influences, a growing number of studies have investigated whether variation in adherence can be tied to race or location of residence in the United States. Studies involving patients with DM have suggested that minorities are more likely to have lower rates of medication use.10-20 Moreover, some analyses have demonstrated worse health outcomes among minorities who are nonadherent to therapy.12,21,22 Similarly, regions of the United States, such as portions of the South, have been associated with significantly lower adherence among DM patients, suggesting potentially local influencers on medication use.23-25 While race and place of residence are only 2 factors that may influence adherence, they represent a cadre of social factors that can be potentially tied to suboptimal medication use and, ultimately, health outcomes.
Challenging our ability to address disparities in adherence is identifying the point at which variation in medication use emerges. While numerous adherence studies have involved patients with DM, the majority have focused on established patients. These studies provide insight on those having disease for multiple years, but lack the ability to observe early influencers on medication use.
Published results on patients initiating antidiabetic medications shortly after their initial diabetes diagnosis are limited, perhaps due to the challenge in collecting accurate longitudinal patient information from the actual initial diagnosis through multiple years of treatment. The limited number of studies investigating initial adherence to DM medications indicates that differences by patient characteristics, such as race and ethnicity, are evident and that early nonadherence may increase the odds of hospitalization within the first years of therapy.18,19,26-28 Such findings aid in targeting particular patients with DM who may be more likely to be nonadherent from the beginning of therapy, but a pressing need remains to better understand and address the social determinants of health among these patients.
Consequently, using data able to identify the first DM diagnosis, this study focused on identifying factors related to nonadherence to OAD therapy and how patients’ adherence changed over the first 2 years of treatment, particularly the extent to which medication use varied by patient race and region of residence.
Methods
Study Design and Data Source
This was a retrospective observational study using the Veterans Affairs (VA) Corporate Data Warehouse from 2002 through 2014. These data included nationwide extracts from the VA Decision Support System National Data Extracts, Inpatient and Outpatient Medical SAS Datasets, and Vital Status Files.29 This study was approved by institutional review boards at the University of Tennessee Health Science Center and Memphis VA Medical Center.
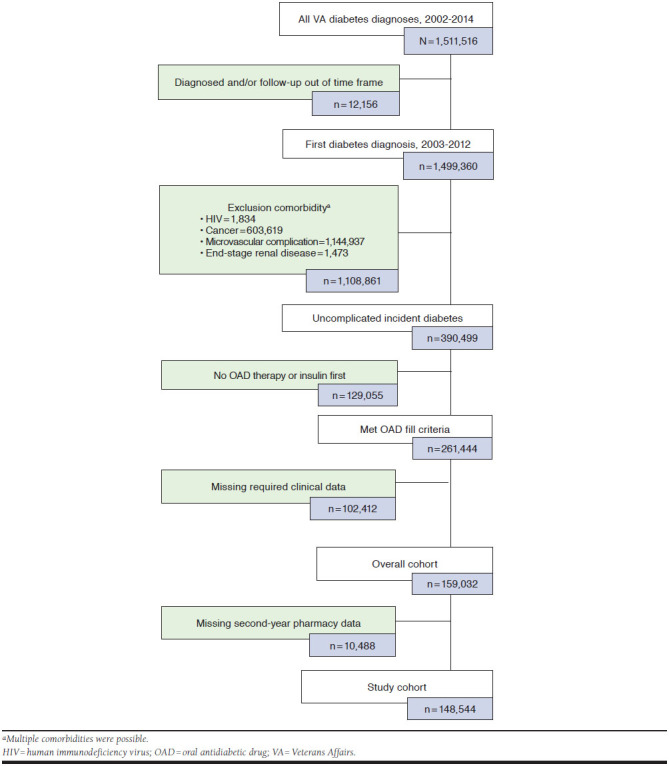
The analysis focuses on incident cases of uncomplicated DM among veterans diagnosed between January 1, 2003, and December 31, 2012. To be eligible, patients must have been aged ≥ 18 years, been diagnosed with DM (International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CD] codes 250.00 or 250.02) for the first time (i.e., no DM diagnostic codes in the year before the initial DM diagnosis); been prescribed an oral antidiabetic drug (OAD) as their first-line therapy for the first time (i.e., no OAD fills in the year before the first DM diagnosis code); and had at least 1 year of data before and 2 years of data after their initial OAD fill. Patients were excluded if they were insulin dependent or had been diagnosed with a DM-related microvascular complication (nephropathy, retinopathy, or neuropathy) before or in conjunction with their DM diagnosis (Appendix A, available in online article); with HIV at any point in their record; or with malignant cancer before their initial DM diagnosis.
Identification of eligible patients started with determining the first DM diagnosis in outpatient and inpatient records over the study period. Determination of the initial diagnosis was made by identifying all encounters in which DM was listed as the primary or secondary diagnosis code, and then isolating the encounter before which no such codes were listed in a visit within the previous 365 days. The initial DM diagnosis was then confirmed by a second visit with DM listed in the primary or secondary position followed by the first OAD fill from a VA outpatient pharmacy. This initial OAD fill served as the index date, after which each patient was followed for 2 years (Appendix B, available in online article).
Patient Characteristics and Outcomes
Most patient characteristics used were sourced from VA electronic health records (EHR). These included race; ethnicity; age; gender; marital status; residential region (Northeast, South, Midwest, West); Veterans Integrated Service Network (VISN); and residential type (urban, rural, highly rural). Median income (by zip code) was sourced from the U.S. Census Bureau.30 A range of clinical measures and diagnoses were also available, including hemoglobin A1c (A1c; ± 90 days of both the initial OAD fill and 365 days later), existing conditions, prior events, weight, and height. Events and conditions recorded in the EHR were used to calculate the Charlson Comorbidity Index before DM diagnosis, and select incident microvascular complications and macrovascular events were determined from outpatient and inpatient records during the first year of treatment.31 Outpatient VA pharmacy records were queried in the years following the first DM diagnosis to determine medication use from the first OAD fill through the subsequent 730 days.
The main outcome was adherence to OADs in the first 2 years following the initial fill from a VA outpatient pharmacy. Proportion of days covered (PDC) was used to determine adherence in each year and then over the entire 2-year observation period.32 To calculate PDC, we determined whether medication was on hand (coverage for at least 1 medication for those on multiple therapies) for each day of the observation periods (365 days [individual years] and 730 days [both years]), and the sum of those days constituted the numerator. The denominator was the number of days in the observation period that started with the date of the first OAD fill (day 1 [first year] or day 366 [second year]) and ran either 365 days (individual years) or 730 days (both years). Overlapping fills for the same medication were accounted for in the calculation code used, shifting the start date for that fill to the end of the previous fill’s supply.
Only approved oral medications were included: biguanides, meglitinides, sulfonylureas, alpha-glucosidase inhibitors, thiazolidinediones (TZD), dipeptidyl peptidase-4 inhibitors, selective sodium-glucose transporter-2 inhibitors, and available combination products. A PDC of 80% dichotomized patients into adherent and nonadherent status, as this value has been validated in patients with DM as a threshold above which better outcomes are more likely.33 Categories of adherence (PDC binned into 5 groups of equally spaced intervals of 20% bands) were also calculated for all time periods to track the trajectory of patients from year to year.
Statistical Analyses
Characteristics of those adherent and nonadherent across years were assessed using t-tests and chi-square tests. Spearman rank correlation coefficient related adherence with continuous characteristics and clinical values. The odds of being adherent were determined by logistic regression, controlling for baseline patient characteristics, conditions, and clinical values. In the second year, first-year adherence status was added as a covariate among the study population, and a separate model was run that only included those adherent in the first year to identify characteristics of becoming nonadherent in year 2. Stepwise, backward selection was used, and variables with a P value of < 0.10 remained in the model at each step. Regression analysis was limited to veterans in the final cohort who had complete demographic information, and steps were not taken to impute missing values.
Following regression analysis, more specific geographic variation was assessed descriptively using geographic boundaries within each VISN—VA-specific regions that define the Veterans Health Administration organization across the country. For these purposes, VA sectors (subgroups of each VISN) were analyzed, allowing for assessment of patients within portions of the country most likely to have similar medical practice models.34,35 To compare across sectors, standard deviations (SDs) of PDC for each sector from that of the national average PDC were mapped. SAS Enterprise Guide 7.1 (SAS Institute, Cary, NC) was used for all statistical analyses, and ArcGIS software package 10.4 (Esri, Redlands, CA) mapped medication use across the country.
Results
Patient Demographics
A total of 148,544 patients had pharmacy data for the first 2 years of OAD use and were included in the final analysis. The average age was 62.2 years (SD = 10.98), with the vast majority of patients (76.7%) aged at least 55 years at OAD initiation. Most patients self-identified as white (78.4%), male (95.6%), and married (58.9%), and a significant proportion resided in the southern part of the United States and in urban areas (Table 1). Few significant comorbidities were evident, although chronic obstructive pulmonary disease was particularly prevalent (19.3%).
TABLE 1.
Study Population Characteristics
| Characteristic | Full Study Cohort | Adherent (Year 1) | Nonadherent (Year 1) |
|---|---|---|---|
| n (%) | n (%) | n (%) | |
| 148,544 | 93,928 | 54,616 | |
| Age, years, mean (SD)a | 62.2 (10.98) | 62.6 (10.5) | 61.5 (11.7) |
| <35 | 1,338 (0.9) | 531 (0.6) | 807 (1.5) |
| 35-44 | 7,631 (5.1) | 4,078 (4.3) | 3,553 (6.5) |
| 45-54 | 25,701 (17.3) | 15,197 (16.2) | 10,504 (19.2) |
| 55-64 | 60,583 (40.8) | 40,036 (42.6) | 20,547 (37.6) |
| 65-74 | 32,825 (22.1) | 21,296 (22.7) | 11,529 (21.1) |
| 75-84 | 17,992 (12.1) | 11,328 (12.1) | 6,664 (12.2) |
| 85+ | 2,474 (1.7) | 1,462 (1.6) | 1,012 (1.9) |
| Male | 142,054 (95.6) | 90,212 (96.0) | 51,842 (94.9) |
| Raceb | |||
| White | 104,369 (78.4) | 68,679 (81.4) | 35,690 (73.3) |
| African American | 24,216 (18.2) | 13,012 (15.4) | 11,204 (23.0) |
| Asian | 1,168 (0.9) | 700 (0.8) | 468 (1.0) |
| Native American | 1,480 (1.1) | 846 (1.0) | 634 (1.3) |
| Hawaiian/Pacific Islander | 1,841 (1.4) | 1,116 (1.3) | 725 (1.5) |
| Marital statusb | |||
| Married | 86,377 (58.9) | 55,714 (60.0) | 30,663 (56.9) |
| Divorced/separated | 37,455 (25.5) | 22,793 (24.6) | 14,662 (27.2) |
| Single/never married | 11,922 (8.1) | 7,119 (7.7) | 4,803 (8.9) |
| Widowed | 10,993 (7.5) | 7,185 (7.7) | 3,808 (7.1) |
| Regionb | |||
| Northeast | 18,311 (12.4) | 11,464 (12.3) | 6,847 (12.6) |
| Midwest | 33,974 (23.0) | 22,600 (24.2) | 11,374 (20.9) |
| South | 66,635 (45.1) | 41,550 (44.4) | 25,085 (46.1) |
| West | 28,950 (19.6) | 17,896 (19.1) | 11,054 (20.3) |
| Population densityb | |||
| Urban | 89,451 (60.5) | 54,744 (58.5) | 34,707 (63.8) |
| Rural | 56,348 (38.1) | 37,367 (39.9) | 18,981 (34.9) |
| Highly rural | 2,182 (1.5) | 1,449 (1.5) | 733 (1.3) |
| Initial oral medication | |||
| Metformin | 99,148 (66.8) | 60,678 (64.6) | 38,470 (70.4) |
| Sulfonylurea | 44,446 (29.9) | 29,856 (31.8) | 14,590 (26.7) |
| TZD | 2,863 (1.9) | 2,204 (2.3) | 659 (1.2) |
| Combination | 1,616 (1.1) | 940 (1.0) | 676 (1.2) |
| All othersc | 471 (0.3) | 250 (0.3) | 221 (0.4) |
| A1c, mean (SD)a | 7.3 (1.53) | 7.4 (1.6) | 7.2 (1.4) |
| Comorbiditiesd | |||
| Charlson Comorbidity Index, mean (SD) | 0.4 (0.63) | 0.4 (0.63) | 0.4 (0.62) |
| Cerebrovascular disease | 8,576 (5.8) | 5,575 (5.9) | 3,001 (5.5) |
| Previous myocardial infarction | 6,396 (4.3) | 4,145 (4.4) | 2,251 (4.1) |
| Congestive heart failure | 6,315 (4.3) | 4,194 (4.5) | 2,121 (3.9) |
| Chronic obstructive pulmonary disease | 28,632 (19.3) | 18,313 (19.5) | 10,319 (18.9) |
| Peripheral artery disease | 6,650 (4.5) | 4,307 (4.6) | 2,343 (4.3) |
aMeasured at/near treatment initiation.
bMissing values: Race = 15,470; Marital status = 1,797; Region = 674; Population density = 563.
cIncludes alpha-glucosidase inhibitors, meglitinides, DPP-4 inhibitors, SGLT-2 inhibitors.
dMeasured at diabetes diagnosis and not including the value applied to diabetes mellitus.
A1c = hemoglobin A1c; DPP-4 = dipeptidyl peptidase-4; SD = standard deviation; SGLT-2 = selective sodium-glucose transporter-2; TZD = thiazolidinediones.
Initial Medication Use
Most patients initiated OAD therapy on metformin (66.8%), and 22.5% either switched therapies or added another medication during the first year. Adherence to OADs averaged 79.2% (SD = 25.9) in the first year, with 63.2% achieving a PDC ≥ 80%. Adherence was weakly correlated with several characteristics: age (P = 0.03, P<0.0001), A1c (P = 0.07, P < 0.0001), and body mass index (P = 0.04, P < 0.0001). Between medication classes, those who initiated on a TZD had the highest average PDC (86.7%, SD = 22.6) and largest proportion of adherent patients (76.1%).
Controlling for patient characteristics and clinical values, we found several factors related to first-year adherence emerging (Figure 1). Compared with veterans aged < 35 years, all other age ranges were more likely to be adherent; those with higher baseline A1c levels were also more likely to be adherent (odds ratio [OR] = 1.12; 95% confidence interval [CI] = 1.113-1.131). With the exception of Asians, all other minorities were less likely to be adherent compared with whites; African Americans had the lowest adjusted odds (OR = 0.62; 95% CI = 0.605-0.645). Veterans indicating they were either single (OR = 0.93; 95% CI = 0.891-0.974) or divorced/separated (OR = 0.87; 95% CI = 0.850-0.899) were less likely to be adherent compared with married counterparts. Compared with veterans in the Northeast region, veterans residing in the Midwest had higher adjusted odds of being adherent (OR = 1.13; 95% CI = 1.082-1.173), while those in the West were less likely to achieve a PDC ≥ 80% (OR = 0.94; 95% CI = 0.902-0.982). Also, compared with veterans in urban areas, those residing in more rural areas had higher adjusted odds of being adherent, but no significant effect was observed when we controlled for median income.
FIGURE 1.
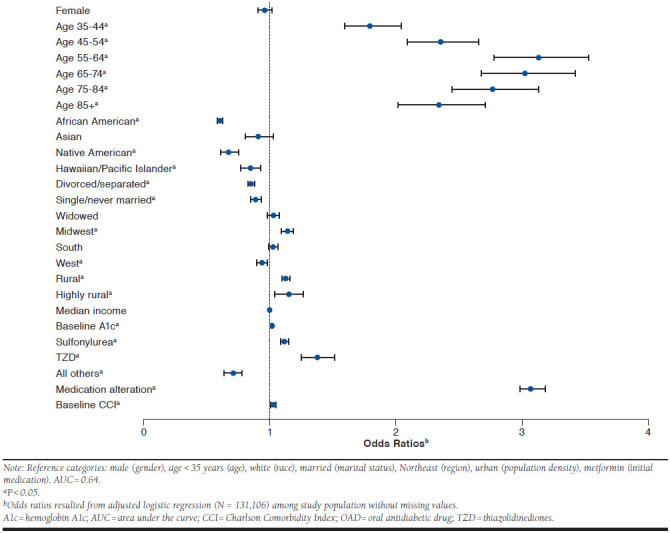
Odds of Being Adherent to OAD Therapy in the First Year
Continuing Medication Use
In the second year of OAD use, average adherence decreased to 71.3% (SD = 35.8) and the proportion of those adherent dipped slightly to 59.1%. Adjusted for baseline patient factors, characteristics related to second-year adherence were similar to those in year 1. However, differences observed between the models of adherence in years 1 and 2 included the significance of being Hawaiian/Pacific Islander, being a widower/widow, residing in a highly rural area, and the direction of the effect seen in patients initiating therapy on medications other than metformin, a sulfonylurea, or a TZD. Additionally, regional variation was no longer a significant covariate.
To account for changes in health status during year 1, several other covariates were added to the second year’s model. Of significance, those veterans whose A1c levels either worsened or remain unchanged during the first year were more likely to be adherent in year 2 (OR = 1.21; 95% CI = 1.002-1.103), as were those diagnosed with a microvascular complication (OR = 1.05; 95% CI = 1.002-1.103). However, experiencing a cardiovascular (myocardial infarction or angina) or cerebrovascular event (ischemic stroke or transient ischemic attack) was not associated with being adherent in year 2 (OR = 0.89; 95% CI = 0.746-1.067). The single largest predictor of adherence in year 2 was having been adherent in year 1 (OR = 12.2; 95% CI = 11.79-12.52).
Mean adherence over both years was 75.3% (SD = 28.5), and just over half of all patients were adherent in both years (50.9%). In terms of significance and direction, the adjusted odds of being adherent in both years were nearly identical to those observed in year 1. Notable exceptions included females (OR = 0.93; 95% CI = 0.880-0.987) and Asians (OR = 0.82; 95% CI = 0.721-0.929) now being less likely to be adherent, whereas these ORs, albeit in the same direction, did not reach significance in year 1.
Change in Medication Use
Mean adherence declined 7.9% (SD = 25.6) between years 1 and 2 and was consistently lower in year 2 across all characteristics (Table 2). However, declines were not statistically different between those adherent and nonadherent to therapy in year 1 (P = 0.459). In terms of percent adherent, proportionately larger decreases (≥ 10% change) were observed among the youngest (< 35 years) and oldest veterans (≥ 85 years), as well as Asians. As reflected in the year 2 model, those adherent in year 1 tended to remain so in year 2: 80.5% sustained a PDC ≥ 80% in the second year after doing so in the first year. A similar proportion (77.8%) remained nonadherent after being so in year 1.
TABLE 2.
Levels of OAD Adherence in the First 2 Years of Therapy
| Characteristic | Adherencea | P Valuec | Adherentb | P Valued | ||
|---|---|---|---|---|---|---|
| First Year | Second Year | First Year | Second Year | |||
| Mean (SD) | Mean (SD) | % | % | |||
| Age, years | ||||||
| < 35 | 66.0 (29.4) | 51.0 (39.1) | < 0.0001 | 39.7 | 33.6 | < 0.0001 |
| 35-44 | 74.3 (27.4) | 64.5 (37.3) | < 0.0001 | 53.4 | 48.8 | < 0.0001 |
| 45-54 | 77.5 (26.2) | 68.9 (35.9) | < 0.0001 | 59.1 | 54.2 | < 0.0001 |
| 55-64 | 81.1 (24.7) | 74.8 (33.8) | < 0.0001 | 66.1 | 62.9 | < 0.0001 |
| 65-74 | 80.0 (25.9) | 71.8 (36.2) | < 0.0001 | 64.9 | 60.9 | < 0.0001 |
| 75-84 | 78.1 (27.1) | 67.9 (38.3) | < 0.0001 | 63.0 | 57.0 | < 0.0001 |
| 85+ | 75.1 (28.9) | 62.7 (40.5) | < 0.0001 | 59.1 | 52.2 | < 0.0001 |
| Gender | ||||||
| Male | 79.4 (25.8) | 71.6 (35.7) | < 0.0001 | 63.5 | 59.4 | < 0.0001 |
| Female | 75.9 (27.5) | 66.0 (37.6) | < 0.0001 | 57.3 | 51.8 | < 0.0001 |
| Race | ||||||
| White | 80.5 (25.4) | 73.1 (35.4) | < 0.0001 | 65.8 | 61.9 | < 0.0001 |
| African American | 74.8 (26.6) | 66.1 (35.8) | < 0.0001 | 53.7 | 49.3 | < 0.0001 |
| Asian | 77.1 (26.8) | 67.2 (37.3) | < 0.0001 | 59.9 | 52.7 | < 0.0001 |
| Native American | 75.8 (27.7) | 65.9 (37.3) | < 0.0001 | 57.2 | 50.5 | < 0.0001 |
| Hawaiian/Pacific Islander | 77.8 (26.5) | 69.5 (36.5) | < 0.0001 | 60.6 | 57.0 | < 0.0001 |
| Marital status | ||||||
| Married | 79.9 (25.7) | 72.2 (35.7) | < 0.0001 | 64.5 | 60.6 | < 0.0001 |
| Divorced/separated | 78.1 (26.2) | 70.0 (35.7) | < 0.0001 | 60.9 | 56.3 | < 0.0001 |
| Single/never married | 77.3 (26.7) | 68.8 (36.5) | < 0.0001 | 59.7 | 55.3 | < 0.0001 |
| Widowed | 80.1 (25.7) | 72.0 (36.0) | < 0.0001 | 65.4 | 61.0 | < 0.0001 |
| Region | ||||||
| Northeast | 78.5 (26.4) | 70.7 (36.2) | < 0.0001 | 62.6 | 58.9 | < 0.0001 |
| Midwest | 81.0 (25.1) | 73.4 (35.1) | < 0.0001 | 66.5 | 62.2 | < 0.0001 |
| South | 78.9 (25.9) | 71.0 (35.8) | < 0.0001 | 62.4 | 58.2 | < 0.0001 |
| West | 78.3 (26.4) | 70.2 (36.2) | < 0.0001 | 61.8 | 57.4 | < 0.0001 |
| Population density | ||||||
| Urban | 78.2 (26.3) | 69.9 (36.2) | < 0.0001 | 61.2 | 57.0 | < 0.0001 |
| Rural | 80.9 (25.2) | 73.5 (35.0) | < 0.0001 | 66.3 | 62.2 | < 0.0001 |
| Highly rural | 80.2 (26.1) | 74.1 (34.7) | < 0.0001 | 66.4 | 63.3 | < 0.0001 |
Note: N = 148,544.
aValues listed are PDC in the specified year.
bPDC ≥ 80%.
cP values represent results of t-tests.
dP values represent results of chi-square tests.
OAD = oral antidiabetic drug; PDC = proportion of days covered; SD = standard deviation.
Several factors associated with nonadherence in year 2 emerged when we assessed the proportion of the population adherent in year 1 (Figure 2). Notably, the odds of becoming nonadherent were higher for African Americans (OR = 1.51; 95% CI = 1.43-1.59); Asians (OR = 1.30; 95% CI = 1.067-1.594); Native Americans (OR = 1.46; 95% CI = 1.223-1.739); those divorced/separated (OR = 1.13; 95% CI = 1.083-1.186); women (OR = 1.10; 95% CI = 1.003-1.205); and those who experienced a cardiovascular or cerebrovascular event in the first year of OAD therapy (OR = 1.30; 95% CI = 1.043-1.612).
FIGURE 2.
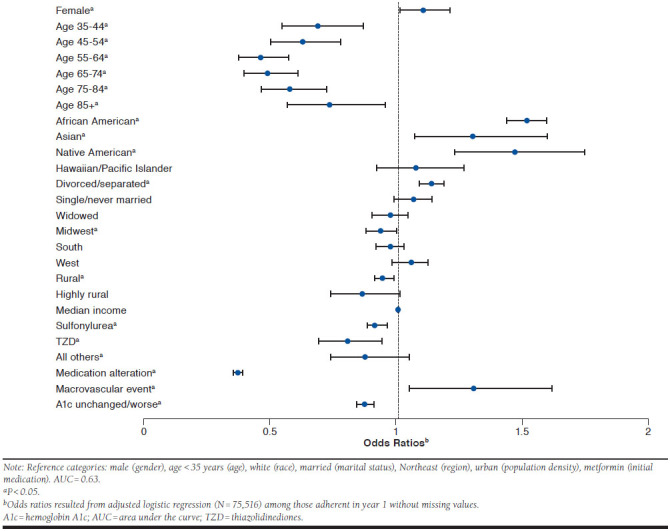
Characteristics of Becoming Nonadherent in Year 2
Regional Variation
Analysis of medication use across the country identified several pockets of particularly better and worse adherence. The highest average PDC was in a sector near St. Cloud, Minnesota, and in general, the upper Midwest, particularly North Dakota, Minnesota, and Michigan, had consistently better average adherence in the first year. A sector located in western Iowa (along the Nebraska border) achieved the highest proportion (76.8%) of adherent patients.
Conversely, the worst average PDC was in a sector within the New York City metropolitan area, while a sector in central Massachusetts had the lowest proportion of adherent patients in the first year (42%). The Southwest, upstate New York, western Massachusetts, southern Florida, and the coastal region of Virginia had comparatively worse OAD adherence (Figure 3). These areas remained problematic in the second year, with Florida and more areas of the western United States growing increasingly worse in terms of average adherence.
FIGURE 3.
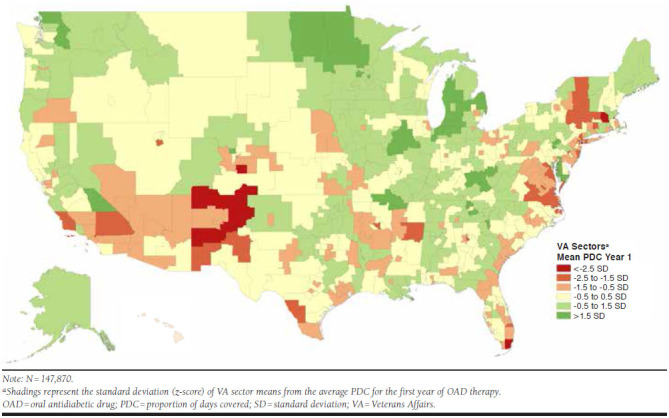
Regional Variation in Proportion Adherent to OAD Therapy by VA Sector in Year 1
Largely, as reflected in the multivariable regressions, the Midwest maintained the most consistently high levels of adherence in the second year. Similarly, there was propensity for lower mean PDCs to be located in urban areas, particularly New York/New Jersey, Northern Virginia, Chicago, Los Angeles, Miami, and Memphis. The lowest average PDC, and the fewest adherent in year 2, were located in the same central Massachusetts sector that had the fewest adherent in year 1.
Between years, the largest crude differences in adherence were observed in upstate New York, eastern Massachusetts, large portions of the Mid-South, Southeast, and Northern California. Comparatively less change was observed in patients residing in the plains (e.g., South Dakota, Nebraska, Kansas, and Oklahoma), Arkansas, and rural Texas. However, in general, areas of greatest comparative change were in either the Northeast or Southeast. Again, the same central Massachusetts sector had the greatest change in adherence between the first and second year of OAD therapy with a 17.0% drop in PDC.
Discussion
This study is one of the few to examine levels of and change in adherence to OADs in the initial years of treatment. Similar to previous findings, results reiterate that adherence to antidiabetic medications is suboptimal and significant diversions in therapy are manifest in the first year. Our first-year PDC of 79.2% is similar to other earlier studies, whose average adherence rates varied from 74% to 90.2%.15,25,26,28 While this value is marginally below the accepted adherence threshold for DM medications, a significant number of veterans with DM—slightly more than one third—were nonadherent in the first year of therapy, reinforcing earlier, similar estimates that demonstrated more than one third of patients with DM are nonadherent.25,36
However, implications of the current analysis are potentially more alarming as, unlike previous studies, our results focus on patients new to therapy. These findings suggest that patients may not be engaged with their care from the start of treatment, a behavior that may be difficult to reverse in subsequent years. This contention is supported by the relatively small change in adherence status from year 1 to year 2 among those initially nonadherent to their OAD, which supports an earlier observation across treatment years among adults with DM.36
Conversely, the largest predictor of adherence in year 2 was having been adherent in year 1, and the proportion of those remaining adherent in year 2 was nearly identical to an earlier study.36 Moreover, the proportion of our study cohort that achieved a PDC ≥ 80% was similar to what was observed by a recent analysis of veterans’ adherence in the first year of antihypertensive therapy.37 Such results are suggestive of the importance of stressing and supporting adherence to therapy from the first prescription to increase the odds of long-term adherence.
Results were also suggestive of patient race being a particularly important factor associated with nonadherence. In modeling adherence in the first year, both years of observation, and in the second year (after being adherent in year 1), African Americans had consistently worse adherence. These findings add to a growing body of evidence demonstrating lower adherence among African Americans in both veterans and the general population.15,16,18,19,26 More generally, that each minority, in nearly all regressions, was associated with reduced odds of adherence is evidence of a potential systemic issue, and one, in conjunction with other adherence studies, that may not be unique to DM.10,11,13 Given that evidence exists connecting poorer antidiabetic medication use by minorities with higher rates of mortality, efforts should be made to better understand the unique challenges that non-white patients experience.12
As demonstrated in both statistical analyses and geographic inference, OAD use varied across the country. While studies investigating geographic variation in DM medication use have often used state-level or regional information, our analysis provides a more granular assessment.16,24,25 By focusing on VA sectors, our estimates allow for inference at a level where practice models and access to care were likely to be more homogenous.
Similar to earlier studies examining geographic variation in DM medication use, adherence was higher among those residing in the Midwest.16,24,25 Our unadjusted findings also reiterate evidence of poorer medication use among patients with DM in western and southwestern states,16,24,25 while also being suggestive of better adherence among veterans residing in nonurban areas.16 Having been presented at the sector level, these results may provide impetus for the VA system to extract best practices from VISNs of higher adherence and apply them in consistently poorer-performing sectors. Moreover, in combination with results tied to race, efforts may be justified to look deeper into social determinants of health at the VA sector level to better understand how influencers beyond this health care system may be affecting veterans’ DM medication use.
Beyond race and geography, several other characteristics associated with adherence are noteworthy. Similar to most other studies of patients with DM, medication use tended to be better among older veterans, with those aged 55-64 years having the highest odds of adherence compared with veterans < 35 years.10,19,24,28,38 However, in spite of this increased propensity by age, most age groups still had suboptimal adherence, and that of the youngest subgroup was particularly poor—this youngest subgroup also experienced a dramatic year-over-year decline in OAD use.
These observations may indicate that younger patients may not be convinced of the value of chronic medications to address their DM and are quicker to neglect treatment shortly after initiation. To avoid such disengagement, prescribers may need to better reinforce the need for early and continued OAD use to better assist their younger patients in becoming accustomed to medically treating DM.
Our results also point to the potential role of support systems for patients managing DM. Consistently, unmarried veterans—those divorced, separated, single, or never married—had lower adjusted odds of adherence. While consistent with effects aggregated elsewhere, the current findings highlighting this effect among veterans may be more alarming.12,39 Given the high prevalence of depression and post-traumatic stress disorder (PTSD) among veterans, the need for social support is arguably higher than in the general population, with low levels of support increasing the odds of developing PTSD and depressive symptoms among veterans.40,41
While this study was not aimed at determining the role of social support in adherence among veterans, nor did it contain data to corroborate the existence or perception of social support, the results tied to marital status provide a signal worth investigating further. Combined with earlier findings, the propensity for unmarried veterans with DM to be nonadherent in the early stages of disease may indicate the need for other forms of support to avoid detrimental outcomes.12
Another observation worthy of future inquiry is the impact of a microvascular complication or macrovascular event in the first year of OAD therapy. Although not a significant effect, we observed that those diagnosed with a DM-related complication in the first year were more likely to be adherent in the second year, whereas the occurrence of a cardiovascular or cerebrovascular event had no effect. However, when examining the odds of nonadherence among those initially adherent, we found that the impact of having a microvascular complication was no longer relevant, but having a cardiovascular or cerebrovascular event then became a significant predictor of nonadherence in year 2.
This differential effect may suggest that patients react differently to the diagnosis of another condition compared with the occurrence of a potentially life-altering or threatening event. Considering the role that medication use plays in secondary prevention of cardiovascular and cerebrovascular events, we believe it is vital to address the needs of postdischarge patients to ensure that adherence to antidiabetic and other medications remains high. Future studies should consider investigating how detrimental health outcomes or events may affect future medication nonadherence among patients with DM.42
Limitations
This study had several limitations. First, only data from the VA’s EHR were used; therefore, results may not be generalizable to the general U.S. or other populations. Second, data were drawn from the EHR without adjudication; therefore, errors made in the system and those with significant missingness (e.g., race) could have affected our analyses. This would also include missing patients who were previously diagnosed with DM but who had a visit in the pre-index period without a DM-related code listed and unmeasured confounders that may have influenced the observed associations. Additionally, only medications filled within the VA system could be accounted for, but it is likely that some medication use was external to VA records (e.g., discount generic programs, Medicare Part D). Finally, while PDC is a widely used and accepted adherence metric, it is still an indirect method, and actual use cannot be accounted for using this method. Moreover, the method used did not account for the impact that being on multiple medications may have had on the calculated PDC.
Conclusions
Adherence to OAD medications was suboptimal among veterans with uncomplicated DM, even within the first year of therapy. Nonadherence among particular subgroups of the population is particularly problematic and may call for focused attention to decrease the odds of poor health outcomes. Efforts within the VA in certain areas of the country may be necessary to target particular veterans with DM at the outset of therapy.
APPENDIX A. Diagnosis and Procedure Codes
| Diagnosesa | Codes |
|---|---|
| HIV | 042-044.9, V08, 795.71 |
| Malignant cancer | 140-172.9, 174-195.8, 200-208.9 |
| Retinopathy | 250.5, 362.0x |
| Neuropathy | 250.6, 357.2 |
| Foot ulcer | 707.1x |
| Nephropathy | 250.4x |
| End-stage renal disease | 585.6 |
| Cerebrovascular disease | 430-438 |
| Ischemic stroke | 434.x, 436.x |
| Transient ischemic attack | 435.x |
| Myocardial infarction | 410-410.9, 412 |
| Peripheral arterial disease | 440.0-440.9, 443.x, 38.0, 38.1, 39.50, 39.22, 39.24-39.26, 29.28 |
| Chronic obstructive pulmonary disease | 490-496, 500-505, 506.4 |
| Congestive heart failure | 428-428.9 |
| Hypoglycemia | 251.2, 250.8 |
| Procedures | Codes |
| Coronary artery bypass graft | 36.10-36.17, 36.19b, 33510-33519, 33521-33523, 33533-33536C |
| Percutaneous transluminal coronary angioplasty | 36.03, 36.04, 36.06, 36.07, 36.09b, 92980-92982, 92984-92996c |
aCodes listed for this section are ICD-9-CM diagnosis codes.
bThese codes are ICD-9-CM procedure codes.
cThese codes are CPT codes.
CPT = Current Procedural Terminology; ICD-9-CM = International Classification of Diseases, Ninth Revision, Clinical Modification; HIV = human immunodeficiency virus.
APPENDIX B. Patient Selection
REFERENCES
- 1.National Council on Patient Information and Education. Accelerating progress in prescription medication adherence: the adherence action agenda. 2013. Available at: http://www.bemedwise.org/docs/resources/a3_report.pdf. Accessed February 17, 2018.
- 2.Sokol MC, McGuigan KA, Verbrugge RR, Epstein RS.. Impact of medication adherence on hospitalization risk and health care cost. Med Care. 2005;43(6):521-30. [DOI] [PubMed] [Google Scholar]
- 3.DiMatteo MR, Giordani PJ, Lepper HS, Croghan TW.. Patient adherence and medical treatment outcomes: a meta-analysis. Med Care. 2002;40(9):794-811. [DOI] [PubMed] [Google Scholar]
- 4.Paes AH, Bakker A, Soe-Agne CJ.. Impact of dosage frequency on patient compliance. Diabetes Care. 1997;20(10):1512-17. [DOI] [PubMed] [Google Scholar]
- 5.Donnan PT, MacDonald TM, Morris AD; for the DARTS/MEMO Collaboration. . Adherence to prescribed oral hypoglycemic medication in a population of patients with type 2 diabetes: a retrospective cohort study. Diabet Med. 2002;19:279-82. [DOI] [PubMed] [Google Scholar]
- 6.Odegard PS, Capoccia K.. Medication taking and diabetes: a systematic review of the literature. Diabetes Educ. 2007;33(6):1014-29. [DOI] [PubMed] [Google Scholar]
- 7.Capoccia K, Odegard PS, Letassy N.. Medication adherence with diabetes medication: a systematic review of the literature. Diabetes Educ. 2016;42(1):34-71. [DOI] [PubMed] [Google Scholar]
- 8.Cramer JA. A systematic review of adherence with medications for diabetes. Diabetes Care. 2004;27(5):1218-24. [DOI] [PubMed] [Google Scholar]
- 9.Sabaté E, ed.. Adherence to Long-Term Therapies: Evidence for Action. Geneva, Switzerland: World Health Organization; 2003. [Google Scholar]
- 10.Rolnick SJ, Pawloski PA, Hedblom BD, Asche SE, Bruzek RJ.. Patient characteristics associated with medication adherence. Clin Med Res. 2013;11(2):54-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marcum ZA, Zheng Y, Perera S, et al. Prevalence and correlates of self-reported medication nonadherence among older adults with coronary heart disease, diabetes mellitus, and/or hypertension. Res Social Adm Pharm. 2013;9(6):817-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Egede LE, Lynch CP, Gebregziabher M, et al. Differential impact of longitudinal medication nonadherence on mortality by race/ethnicity among veterans with diabetes. J Gen Intern Med. 2012;28(2):208-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhu VJ, Tu W, Marrero DG, Rosenman MB, Overhage JM.. Race and medication adherence and glycemic control: findings from and operational health information exchange. AMIA Annu Symp Proc. 2011;2011:1649-57. [PMC free article] [PubMed] [Google Scholar]
- 14.Osborn CY, Cavanaugh K, Wallston KA, et al. Health literacy explains racial disparities in diabetes medication adherence. J Health Commun. 2011;13(Supp 3):268-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gebregziabher M, Lynch CP, Mueller M, et al. Using quantile regression to investigate racial disparities in medication nonadherence. BMC Med Res Methodol. 2011;11:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Egede LE, Gebregziabher M, Hunt KJ, et al. Regional, geographic, and ethnic differences in medication adherence among adults with type 2 diabetes. Ann Pharmacother. 2011;45(2):169-78. [DOI] [PubMed] [Google Scholar]
- 17.Yang Y, Thumula V, Pace PF, Banahan BF 3rd, Wilkin NE, Lobb WB.. Predictors of medication nonadherence among patients with diabetes in Medicare Part D programs: a retrospective cohort study. Clin Ther. 2009;31(10):2178-88. [DOI] [PubMed] [Google Scholar]
- 18.Trinacty CM, Adams AS, Soumerai SB, et al. Racial differences in long-term adherence to oral antidiabetic drug therapy: a longitudinal cohort study. BMC Health Serv Res. 2009;9:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shenolikar RA, Balkrishnan R, Camacho FT, Whitmire JT, Anderson RT.. Race and medication adherence in Medicaid enrollees with type 2 diabetes. J Natl Med Assoc. 2006;98(7):1071-77. [PMC free article] [PubMed] [Google Scholar]
- 20.Juarez DT, Tan C, Davis JW, Mau MM.. Using quantile regression to assess disparities in medication adherence. Am J Health Behav. 2014;38(1):53-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hunt KJ, Gebregziabher M, Lynch CP, Echols C, Mauldin PD, Egede LE.. Impact of diabetes control on mortality by race in a national cohort of veterans. Ann Epidemiol. 2013;23(2):74-79. [DOI] [PubMed] [Google Scholar]
- 22.Egede LE, Gebregziabher M, Hunt KJ, et al. Regional, geographic, and racial/ethnic variation in glycemic control in a national sample of veterans with diabetes. Diabetes Care. 2011;34:938-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Desai V, Nau D, Conklin M, Heaton PC.. Impact of environmental factors on differences in quality of medication use: an insight for the Medicare Star Rating System. J Manag Care Spec Pharm. 2016;22(7):779-86. Available at: https://www.jmcp.org/doi/10.18553/jmcp.2016.22.7.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Couto JE, Panchal JM, Lal LS, et al. Geographic variation in medication adherence in commercial and Medicare Part D populations. J Manag Care Spec Pharm. 2014;20(8):834-42. Available at: https://www.jmcp.org/doi/abs/10.18553/jmcp.2014.20.8.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tan E, Yang W, Pang B, Dai M, Loh FE, Hogan P.. Geographic variation in antidiabetic agent adherence and glycemic control among patients with type 2 diabetes. J Manag Care Spec Pharm. 2015;21(12):1195-202. Available at: https://www.jmcp.org/doi/full/10.18553/jmcp.2015.21.12.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Adams AS, Trinacty CM, Zhang F, et al. Medication adherence and racial differences in HbA1c control. Diabetes Care. 2008;31(5):916-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fernandez A, Quan J, Moffet H, Parker MM, Schillinger D, Karter AJ.. Adherence to newly prescribed diabetes medications among insured Latino and White patients with diabetes. JAMA Intern Med. 2017;177(3):371-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sun P, Lian J.. Treatment adherence in newly diagnosed type 2 diabetes: patient characteristics and long-term impact of adherence on inpatient care utilization. Postgrad Med. 2016;128(4):338-45. [DOI] [PubMed] [Google Scholar]
- 29.U.S. Department of Veterans Affairs. Veterans Health Administration Decision Support System Clinical National Data Extracts. 2nd ed. VIReC Research User Guide. 2009. [Google Scholar]
- 30.U.S. Census Bureau. American FactFinder 2010 census. December 27, 2016. Available at: http://factfinder2.census.gov. Accessed February 17, 2018.
- 31.Deyo RA, Cherkin DC, Ciol MA.. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613-19. [DOI] [PubMed] [Google Scholar]
- 32.Peterson AM, Nau DP, Cramer JA, Benner J, Gwadry-Sridhar F, Nichol M.. A checklist for medication compliance and persistence studies using retrospective databases. Value Health. 2007;10(1):3-12. [DOI] [PubMed] [Google Scholar]
- 33.Pharmacy Quality Alliance. PQA performance measures. Available at: http://pqaalliance.org/measures/default.asp. Accessed February 17, 2018.
- 34.USVA VHA Healthcare Services Geographies. FY2011 Q4 Markets, Geodatabase Feature Class. Washington, DC: Veterans Health Administration; 2013. [Google Scholar]
- 35.U.S. Government Accountability Office. VA Health Care. Overview of VA’s capital asset management. GAO-09-686T. 2009. Available at: https://www.gao.gov/products/GAO-09-686T. Accessed February 17, 2018.
- 36.Saundankar V, Peng X, Fu H, et al. Predictors of change in adherence status from 1 year to the next among patients with type 2 diabetes mellitus on oral antidiabetes drugs. J Manag Care Spec Pharm. 2016;22(5):467-82. Available at: https://www.jmcp.org/doi/10.18553/jmcp.2016.22.5.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gosmanova EO, Lu JL, Streja E, Cushman WC, Kalantar-Zadeh K, Kovesdy CP.. Association of medical treatment nonadherence with all-cause mortality in newly treated hypertensive U.S. veterans. Hypertension. 2014;64:951-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Curkendall SM, Thomas N, Bell KF, Juneau PL, Weiss AJ.. Predictors of medication adherence in patients with type 2 diabetes mellitus. Curr Med Res Opin. 2013;29(10):1275-86. [DOI] [PubMed] [Google Scholar]
- 39.DiMatteo MR. Social support and patient adherence to medical treatment: a meta-analysis. Health Psych. 2004;23(2):207-18. [DOI] [PubMed] [Google Scholar]
- 40.Boscarino JA. Post-traumatic stress and associated disorders among Vietnam veterans: the significance of combat exposure and social support. J Trauma Stress. 1995;8(2):317-36. [DOI] [PubMed] [Google Scholar]
- 41.Pietrzak RH, Johnson DC, Goldstein MB, et al. Psychosocial buffers of traumatic stress, depressive symptoms, and psychosocial difficulties in veterans of Operations Enduring Freedom and Iraqi Freedom: the role of resilience, unit support, and postdeployment social support. J Affec Disord. 2010;120(1-3):188-92. [DOI] [PubMed] [Google Scholar]
- 42.Molnar MZ, Gosmanova EO, Sumida K, et al. Predialysis cardiovascular disease medication adherence and mortality after transition to dialysis. Am J Kidney Dis. 2016;68:609-18. [DOI] [PMC free article] [PubMed] [Google Scholar]


