Abstract
INTRODUCTION: As clinical trials test efficacy rather than effectiveness of medications, real-world effectiveness data often vary from clinical trial data. Given the recent market entry of multiple biologics and biosimilars, a dedicated assessment of these diverse agents is needed to build the evidence base regarding efficacy and safety of innovator biologics and biosimilars.
PROGRAM DESCRIPTION: The Academy of Managed Care Pharmacy’s Biologics and Biosimilars Collective Intelligence Consortium (BBCIC) was convened to address the lack of real-world, postmarket outcome evidence generation for innovator biologics and corresponding biosimilars. The BBCIC is a multistakeholder scientific research consortium whose participants prioritize topics and collaboratively conduct research studies. The BBCIC conducts a wide range of analyses, including population characterization, epidemiologic studies, and active observational studies, and develops best practices for conducting large-scale studies to provide real-world evidence.
OBSERVATIONS: Over the past 3 years, we undertook multiple descriptive analyses with the goal of characterizing data availability and demonstrating the feasibility and efficacy of using the BBCIC distributed research network (DRN), which includes commercial claims data from 2008-2018 covering approximately 100 million lives, with approximately 20 million active members in 2017 from 2 major U.S. health plans and 3 regional integrated delivery networks. We analyzed 4 medication classes of particular interest to biologics and biosimilars development: insulins, granulocyte colony-stimulating factors, erythropoietic-stimulating agents, and anti-inflammatories. We were able to identify exposures and user characteristics in all 4 categories. Herein we describe the successes and challenges of conducting some of our analyses, specifically among insulin users with type 1 diabetes mellitus.
IMPLICATIONS: Our results demonstrate the BBCIC DRN’s ability to identify and characterize exposures, cohorts, and outcomes that can contribute to more sophisticated comparative surveillance of biosimilars and innovator biologics in the future. Additional linkages to laboratory data and a wider range of insurance carriers will further strengthen the BBCIC DRN.
What is already known about this subject
Large administrative databases such as Sentinel and Mini-Sentinel have proven useful in conducting pharmacovigilance studies.
There is a need to evaluate biologics, biosimilars, and follow-on products in real-world settings to build the evidence base with respect to safety and effectiveness.
What this study adds
The Biologics and Biosimilars Collective Intelligence Consortium (BBCIC) distributed research network (DRN) allows investigators to identify and describe a cohort of patients using biologics or biosimilars, along with relevant safety and effectiveness outcomes.
Future studies using the BBCIC DRN will move beyond descriptive analyses to examine the comparative effectiveness of biologics and biosimilars, real-world patterns of use, and associated health care utilization.
The Biologics and Biosimilars Collective Intelligence Consortium (BBCIC) is a nonprofit public service initiative established in 2015 by the Academy of Managed Care Pharmacy in response to the need for evidence generation on the safety and effectiveness of newly launched biosimilar products and innovator biologics following market entry.1 The BBCIC is a neutral platform for investigators, biopharmaceutical companies, managed care organizations, integrated delivery networks (IDNs), pharmacy benefit managers, physicians, and patient advocates to collaborate in conducting robust and relevant scientific research and cooperate to make meaningful contributions to the scientific record.
The BBCIC was established in part as a response to the U.S. Food and Drug Administration’s (FDA) accelerated approval process for biosimilars, which requires only comparative safety and efficacy data to the originator product, reducing lengthy clinical trial time frames before approval. Patients and providers are interested in assurances regarding safety and effectiveness of biosimilar products when used in clinical practice.2-5 The primary requirements for establishing biosimilarity for FDA approval rely heavily on analytical and preclinical studies to show that the product is “highly similar” to the reference product, including structural analyses, and to ensure there is no unexpected toxicity.6 Additional priority is placed on clinical pharmacology to assess pharmacokinetics and pharmacodynamics and on immunogenicity to demonstrate safety, purity, and potency in at least 1 relevant indication.6 Additional clinical trials are only conducted if necessary.
Clinical studies are often criticized for lack of generalizability to a real-world, diverse patient population, and when the study requirements are abbreviated, there is concern that relevant questions are not adequately answered.7,8 Augmenting the scientific requirements for registration with real-world evidence is gaining traction as a valuable and necessary source of information for prescribers, patients, and other stakeholders.7,8
Program Description
The BBCIC’s mission statement is to provide research services to support the following value propositions: (a) address important questions about the use, impact, safety, and clinical effectiveness of innovator biologics and biosimilars on human health; (b) increase the rigor and credibility of real-world evidence; (c) provide access to a large population for epidemiologic studies and health services research; (d) improve the efficiency and cost-effectiveness of postmarket observational studies; and (e) develop standard approaches to common data needs and address gaps in tools and methods.9 Until now, there has not been a system in the United States that has conducted proactive, science-driven, postapproval studies of biosimilar products and their reference biologics. The BBCIC conducts a wide range of analyses including population characterization, epidemiologic studies, and active observational studies and develops best practices for conducting large-scale studies with real-world data.
A transparent, consortium-based governance structure is a key part of the BBCIC; this governance structure defines research topic identification, guides study implementation, and stipulates results dissemination (Figure 1). There is a formal hierarchy, including an independent board of managing directors, a planning board that acts as the oversight and steering committee for the organization overall, a science committee to oversee the rigor and scientific integrity of the organization, a communications committee to oversee press releases and other public-facing information, and research teams or methodologic workgroups for each project. BBCIC participant organizations each have 1 seat on the planning board and science and communications committees and may participate on any research team or workgroup of interest. Public representatives also participate, such as patient advocacy organizations, and the FDA has appointed a liaison to serve on the planning board.
FIGURE 1.
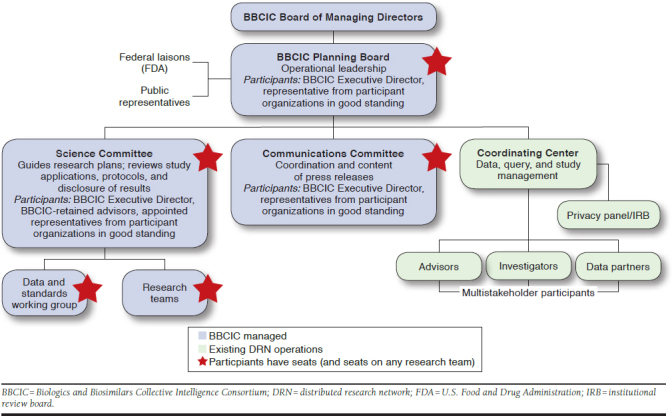
BBCIC Governance Structure
TABLE 1.
Baseline Characteristics, Insulin Episode and Use, and Outcomes of LAI and NPH Insulin Users with T1DM
| LAIa | LAI + RAI | NPHa | NPH + RAI | |
|---|---|---|---|---|
| Insulin episodes | ||||
| Unique patients with at least 1 episode | N = 4,591 | |||
| Total number of episodesa | N = 12,828 | |||
| Insulin episodes, n | 4,870 | 6,933 | 391 | 634 |
| Episodes per user, mean (SD) | 1.58 (1.1) | 1.81 (1.4) | 1.55 (1.1) | 1.87 (1.5) |
| Months per episode, mean (SD) | 1.32 (2.2) | 5.04 (5.0) | 1.44 (2.5) | 4.80 (5.0) |
| Months of follow-up, mean (SD) | 2.04 (3.2) | 9.12 (9.5) | 2.16 (3.60) | 9.00 (9.7) |
| Dispensing per user, mean (SD) | 2.06 (2.0) | 4.25 (4.8) | 2.21 (2.4) | 4.56 (5.3) |
| Dispensing per episode, mean (SD) | 1.30 (1.1) | 2.35 (2.8) | 1.42 (1.7) | 2.44 (3.3) |
| Days supply per user, mean (SD) | 40.7 (67.6) | 143.4 (178.0) | 48.2 (86.4) | 151.2 (191.1) |
| Days supply per episode, mean (SD) | 25.7 (47.5) | 79.2 (111.6) | 31.1 (63.8) | 80.8 (116.6) |
| Patient characteristics by insulin episode | ||||
| Females, n (%) | 1,786 (36.7) | 2,465 (35.6) | 152 (38.9) | 187 (29.5) |
| Age groups, n (%) | ||||
| 18-49 years | 4,198 (86.2) | 5,947 (85.8) | 287 (73.4) | 476 (75.1) |
| 50-64 years | 641 (13.2) | 949 (13.7) | 94 (24.0) | 149 (23.5) |
| 65-79 years | 29 (0.6) | 36 (0.5) | NC | NC |
| All LAI | All NPH | |||
| Clinical characteristics by insulin episode | ||||
| Combined CCI, mean (SD) | 0.1 (0.5) | 0 (0.6) | ||
| Hyperlipidemia, n (%) | 685 (14.0) | 74 (15.7) | ||
| Hypertension, n (%) | 459 (9.4) | 73 (15.5) | ||
| Metformin, n (%) | 137 (2.8) | 15 (3.2) | ||
| Peripheral neuropathy, n (%) | 68 (1.4) | 10 (2.1) | ||
| Retinopathy, n (%) | 195 (4.0) | 19 (4.0) | ||
| Hypoglycemia | MACE | |||
| Outcomes by insulin episode for all exposure cohorts | ||||
| Users with events, n | 13 | 15 | ||
| Episodes with events, n | 21 | 23 | ||
| Episodes per user with events, mean (SD) | 2.08 (1.7) | 2.00 (1.8) | ||
| Users with events per 10,000 years at risk, n | 35 | 40 | ||
| Episodes with events per 10,000 years at risk, n | 56.3 | 61.7 | ||
aDoes not include episodes in LAI + sulfonylurea (26 users, 34 events) or NPH + sulfonylurea (< 10 users, events not calculated).
CCI = Charlson Comorbidity Index; LAI = long-acting insulin; MACE = major adverse cardiac events; NPH = neutral protamine Hagedorn insulin; NC = not calculated; RAI = rapid-acting insulin; SD = standard deviation; T1DM = type 1 diabetes mellitus.
The structure of the BBCIC is based on a distributed research network (DRN) that leverages the FDA’s Sentinel infrastructure and the Sentinel Common Data Model version 6.0 (SCDM). Data include commercial and Medicare Advantage administrative claims data from 2 national health insurance providers and 3 regional health IDNs, providing access to data from 2008-2018 that cover approximately 100 million lives, with approximately 20 million active members in 2017.1,10 Currently, data from Medicaid and Medicare fee-for-service recipients are not included.
Each participating data site maintains data locally. After the establishment of a research query, the BBCIC Coordinating Center at the Harvard Pilgrim Health Care Institute (HPHCI) distributes analytic code for execution by each research partner site. After reviewing and running the query, research partner sites return the summarized results for aggregation and report via a secure distributed querying network based on the PopMedNet software, which is also used by FDA Sentinel, PCORnet, and others.10-12
Here, we describe the use of the BBCIC DRN to identify real-world patterns of medication use and patient outcomes. Analyses have been conducted for patients in 4 separate cohorts: those taking erythropoietic-stimulating agents (ESAs), anti-inflammatories (AIs), insulin, or granulocyte colony-stimulating factors (G-CSFs). We focus on patients with diabetes to describe our experience.
The 5 DRN research partners used the approved 6.0 version of the FDA SCDM database for the analyses. The publicly available Sentinel Cohort Identification and Descriptive Analysis tool (CIDA version 3.0.3) was used to conduct the distributed analyses.13,14 BBCIC analyses used a distributed approach in which only aggregate data were shared outside the study sites for reporting; no individual patient-level data were shared. The institutional review board (IRB) at HPHCI for BBCIC DRN and each research partner site IRB reviewed the protocol and provided a determination of not meeting the definition for research on human subjects.
Observations
For the insulin query, we determined insulin use, laboratory data availability (i.e., hemoglobin A1c values), incidence of insulin-related outcomes such as serious hypoglycemic events and major adverse cardiac events (MACE), and their consistency with previous studies. We also conducted queries for G-CSFs to determine the safety outcomes of anaphylaxis along with the primary outcome of febrile neutropenia. We evaluated the number of biologic users and incidence of serious infections among patients with rheumatoid arthritis, inflammatory bowel disease, psoriasis, psoriatic arthritis, or ankylosing spondylitis taking AIs. Finally, we looked at ESA use and laboratory data availability among dialysis patients with end-stage renal disease (ESRD). We offer brief observations, overall results, and challenges encountered, with insulin use among type 1 diabetes mellitus (T1DM) patients being our primary example.
In all analyses, we included adults aged 18 years or older with medical and pharmacy insurance and at least 1 pharmacy dispensing for the medication of interest. The final date of observation was September 30, 2015, across all 4 queries, with varying start dates for each query. For insulins, the observation window began on January 1, 2011. Patients had to have at least 183 days of continuous medical and pharmacy coverage before the first day that the medication of interest was dispensed, thus marking the start of a medication exposure episode. We considered a diagnosis of disease of interest to be at least 1 medical claim with an International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) diagnosis code in the BBCIC DRN available any time in the patient’s claims history.
To evaluate the ability of the BBCIC DRN to identify safety and effectiveness outcomes for biologics and biosimilars, specifically insulins in this example, we looked for the following outcomes: medically attended hypoglycemic events or MACE, such as cardiovascular death, nonfatal myocardial infarction, or nonfatal stroke.15,16 The secondary exploratory outcome was availability of A1c data, with baseline A1c value defined as those occurring in the 183-day look-back period before the index insulin exposure.
We determined medication exposure using days supply reported from pharmacy claims. Following the FDA Sentinel analytic standard, we used the days supply for each prescription plus a grace period of 60 days after the end of the prescription days supply for each episode exposure to allow for variability in insulin dosing. For patients using 2 insulins (e.g., a long-acting insulin [LAI] and a rapid-acting insulin [RAI]), the treatment episode and days supply were based on periods of concomitant use of both medications, and the 60-day episode gap was applied at the end of the concomitant episode. We truncated an exposure episode if a patient did not refill their medication within 60 days following the end of days supply or if an outcome of interest was observed.
Patients could contribute more than 1 episode with different index dates to a study if they met all inclusion criteria for each medication exposure. If a patient contributed more than 1 episode, we reset the index date and evaluated a new 183-day baseline period. For example, in our G-CSF descriptive analysis, a patient taking filgrastim who switched to pegfilgrastim could contribute 2 episodes to the G-CSF query as long as the patient met all other inclusion criteria. Similarly, as insulin use is often variable and prescription refills do not necessarily follow a regular cadence as with other chronic oral medications, a patient may contribute exposure time to multiple cohorts over time as long as all inclusion criteria are met. For example, a patient may fill a prescription for an LAI alone during 1 episode and thus be assigned to the LAI cohort; then the same patient may fill a prescription for an RAI at a later date and be reassigned to the LAI + RAI cohort after that point. This allowed us to capture all available insulin exposure time and not just those episodes when LAIs and RAIs were dispensed simultaneously.
Patients and Exposures
We identified 4,591 patients with T1DM for our insulin analysis. Of 4,591 patients with T1DM, 1,780 (39%) patients had only 1 insulin episode, and 2,811 (61%) patients had more than 1 episode. Patients who were categorized in only 1 insulin exposure cohort (e.g., LAI + RAI) had a mean (standard deviation [SD]) 1.5 (1.1) episodes per unique user, a mean (SD) 3.9 (4.6) insulins dispensed, 119 (154) days supply of insulin, a mean (SD) episode length of 4.9 (5.2) months, and a mean (SD) 7.3 (8.2) months of follow-up. Patients who were categorized in multiple exposure cohorts had 3.6 (2.5) episodes per user, 6.4 (5.6) mean insulins dispensed, 188 (204) days supply of insulin, a mean (SD) episode length of 3.1 (4.2) months, and a mean (SD) 11.3 (10.7) months of follow-up. These observations of episodes per use reflect the inherent nonlinearity in insulin treatment, since individual insulin dispensations often result in different numbers of treated days, allowing patients to move between and contribute to different cohorts as they use different types of insulin.
Outcomes
We observed approximately 21 hypoglycemic events and approximately 23 MACE events across all included T1DM patients. A total of 1,004 unique patients with T1DM and at least 1 insulin exposure contributed A1c values to the BBCIC DRN. Of these, 892 patients had baseline A1c values, and 314 patients had both baseline and at least 1 follow-up A1c value. After the index date, the mean time to the first A1c value was 2.9 months for patients with a baseline A1c value and 3.8 months for those without a baseline A1c. The mean number of study period A1c values was 1.6 for patients with a baseline A1c and 1.3 for patients without.
Implications
This proof-of-concept article describes the capacity of researchers to use the BBCIC DRN to identify patients with clinical conditions of interest, note medication exposures, describe outcomes such as medically attended hypoglycemic events and MACE, and determine the availability of laboratory data such as A1c values.
We have reported baseline characteristics that are similar to those reported in some retrospective observational studies.17-19 Our population was much younger and had fewer women than a study using the U.S. HMO Research Network; however, that study combined the results of patients with T1DM or type 2 diabetes mellitus.19 A recent systematic review and network meta-analysis found no significant difference between LAIs and neutral protamine Hagedorn (NPH) insulins with respect to severe hypoglycemic events among patients with T1DM, which echo our findings.18 Our calculated MACE incidence at 40.2 events per 10,000 person-years (10kPY) is lower than other studies using similar data, as a pooled analysis including 2,018 patients with diabetes and no history of cardiovascular disease from 3 previous studies of diabetes outcomes calculated 61.4 events per 10kPY.20 That study had older patients than in our evaluation, and the definition of cardiovascular disease included coronary heart disease, stroke, and heart failure, which differs slightly from our MACE outcome definition.
Similar to the Mini-Sentinel project, the BBCIC DRN has limited laboratory results and characteristics.21 The IDN patients had a higher reporting rate of A1c results compared with our large national insurers.21 Of the unique patients in this research, only 314 patients with T1DM had an A1c value in the baseline and follow-up periods, and 892 T1DM patients had a baseline A1c.
In our other studies to date, as we found with AI products, we were unable to identify measures of disease severity using only insurance claims, which limited our ability to provide robust information on clinical outcomes without using surrogate measures. Similar to insulins, absolute neutrophil counts for patients receiving G-CSF prophylaxis were not available for all patients, suggesting a need for data enrichment through linkage with electronic health records or other external sources. Regarding data availability, we were unable to complete a descriptive analysis of ESA use in the BBCIC DRN. This was the result of our inclusion criteria whereby all patients had to be receiving hemodialysis or peritoneal dialysis. Since Medicare is the primary insurer of patients with ESRD, regardless of age, and the BBCIC DRN draws from commercially insured patients, we did not find our sample of interest to support a robust analysis of ESA use in the BBCIC DRN.
This article illustrates the capacity of the BBCIC DRN and our ability to describe user cohort demographics and outcomes within a defined disease cohort exposed to a variety of drug products of interest, focusing primarily on insulin use in T1DM patients for this proof of concept. These results demonstrate the BBCIC DRN’s ability to identify and characterize exposures and patient outcomes for a large insured population. Future studies to evaluate diabetes mellitus using the Sentinel Network or DRN will need to use other data sources to augment the availability of laboratory data, while studies of patients receiving hemodialysis must use Medicare data.
ACKNOWLEDGMENTS
Special thanks to Sarah Malek, project manager, and Katie King, research assistant, at Harvard Pilgrim Healthcare Institute (HPHCI) for their ongoing support, and Rohan Das, data analyst at Aetna. Thanks also to the BBCIC Insulins Research Team: Berhanu Alemayehu (Merck), Bernadette Eichelberger (BBCIC), Kevin Haynes (HealthCore), Daniel J. Kent (Kaiser Permanente of Washington), Catherine M. Lockhart (BBCIC), James Marshall (HPHCI), Dorothy McCabe (Boehringer-Ingelheim), Kevin McConnell (Momenta), Cara L. McDermott (BBCIC), Cheryl N. McMahill-Walraven (Aetna), Lisa Ortendahl (HPHCI), Catherine A. Panozzo (HPHCI), Pamala A. Pawloski (HealthPartners), Brie Purcell (HPHCI), Jessica Sturtevant (HPHCI), and Jessica Young (HPHCI).
REFERENCES
- 1.AMCP Task Force on Biosimilar Collective Intelligence Systems, Baldziki M, Brown J, et al. Utilizing data consortia to monitor safety and effectiveness of biosimilars and their innovator products. J Manag Care Spec Pharm. 2015;21(1):23-34. Available at: https://www.jmcp.org/doi/10.18553/jmcp.2015.21.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Teeple A, Ellis LA, Huff L, et al. Physician attitudes about non-medical switching to biosimilars: results from an online physician survey in the United States. Curr Med Res Opin. 2019;35(4):611-17. [DOI] [PubMed] [Google Scholar]
- 3.Teeple A, Ginsburg S, Howard L, et al. Patient attitudes about non-medical switching to biosimilars: results from an online patient survey in the United States. Curr Med Res Opin. 2019;35(4):603-09. [DOI] [PubMed] [Google Scholar]
- 4.Jacobs I, Singh E, Sewell KL, Al-Sabbagh A, Shane LG. Patient attitudes and understanding about biosimilars: an international cross-sectional survey. Patient Prefer Adherence. 2016;10:937-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leonard E, Wascovich M, Oskouei S, Gurz P, Carpenter D. Factors affecting health care provider knowledge and acceptance of biosimilar medicines: a systematic review. J Manag Care Spec Pharm. 2019. Jan;25(1):102-12. Available at: https://www.jmcp.org/doi/full/10.18553/jmcp.2019.25.1.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.U.S. Food and Drug Administration . Biosimilars. 2018. Available at: https://www.fda.gov/drugs/developmentapprovalprocess/howdrugsaredevelopedandapproved/approvalapplications/therapeuticbiologicapplications/biosimilars/default.htm. Accessed July 16, 2019.
- 7.Greenfield S. Making real-world evidence more useful for decision making. Value Health. 2017;20(8):1023-24. [DOI] [PubMed] [Google Scholar]
- 8.Jarow JP, LaVange L, Woodcock J. Multidimensional evidence generation and FDA regulatory decision making: defining and using “real-world” data. JAMA. 2017;318(8):703-04. [DOI] [PubMed] [Google Scholar]
- 9.Biologics and Biosimilars Collective Intelligence Consortium . Academy of Managed Care Pharmacy (AMCP) and Biologics and Biosimilars Collective Intelligence Consortium, LLC (BBCIC, LLC) governance. October 23, 2015. Available at: https://www.bbcic.org/Charter-PP.pdf. Accessed July 16, 2019. [DOI] [PMC free article] [PubMed]
- 10.Curtis LH, Weiner MG, Boudreau DM, et al. Design considerations, architecture, and use of the Mini-Sentinel distributed data system. Pharmacoepidemiol Drug Saf. 2012;21(Suppl 1):23-31. [DOI] [PubMed] [Google Scholar]
- 11.Brown JS, Holmes JH, Shah K, Hall K, Lazarus R, Platt R. Distributed health data networks: a practical and preferred approach to multi-institutional evaluations of comparative effectiveness, safety, and quality of care. Med Care. 2010;48(6 Suppl):S45-51. [DOI] [PubMed] [Google Scholar]
- 12.Davies M, Erickson K, Wyner Z, Malenfant J, Rosen R, Brown J. Software-enabled distributed network governance: the PopMedNet experience. EGEMS (Wash DC). 2016;4(2):1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rosofsky R, Rucker M. Sentinel distributed query tool summary table descriptions. U.S. Food and Drug Administration. 2016. Available at: https://www.sentinelinitiative.org/sentinel/routine-querying-tools/summary-table-queries. Accessed July 16, 2019. [Google Scholar]
- 14.Sentinel Operations Center . Sentinel modular programs. Querying tools: overview of functionality and technical documentation. Version 5.4.4. August 7, 2018. Available at: https://www.sentinelinitiative.org/sites/default/files/surveillance-tools/routine-querying/Sentinel_Routine_Querying_System-Documentation-5.4.4.pdf. Accessed July 17, 2019.
- 15.Ginde AA, Blanc PG, Lieberman RM, Camargo CA, Jr.. Validation of ICD-9-CM coding algorithm for improved identification of hypoglycemia visits. BMC Endocr Disord. 2008;8:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fireman B, Toh S, Butler MG, et al. A protocol for active surveillance of acute myocardial infarction in association with the use of a new antidiabetic pharmaceutical agent. Pharmacoepidemiol Drug Saf. 2012;21(Suppl 1):282-90. [DOI] [PubMed] [Google Scholar]
- 17.Idris I, Peng X, He X, et al. The trend of high-dose insulin usage among patients with diabetes in the UK: a retrospective study. Diabetes Ther. 2018;9(6):2245-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dawoud D, O’Mahony R, Wonderling D, Cobb J, Higgins B, Amiel SA. Basal insulin regimens for adults with type 1 diabetes mellitus: a systematic review and network meta-analysis. Value Health. 2018;21(2):176-84. [DOI] [PubMed] [Google Scholar]
- 19.Vazquez-Benitez G, Desai JR, Xu S, et al. Preventable major cardiovascular events associated with uncontrolled glucose, blood pressure, and lipids and active smoking in adults with diabetes with and without cardiovascular disease: a contemporary analysis. Diabetes Care. 2015;38(5):905-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wong ND, Zhao Y, Patel R, et al. Cardiovascular risk factor targets and cardiovascular disease event risk in diabetes: a pooling project of the atherosclerosis risk in communities study, multi-ethnic study of atherosclerosis, and Jackson heart study. Diabetes Care. 2016;39(5):668-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Raebel M, Shetterly S, Paolino A, et al. Mini-Sentinel methods. Analytic methods for using laboratory test results in active database surveillance: final report. U.S. Food and Drug Administration. May 16, 2016. Available at: http://www.sentinelsystem.org/sites/default/files/Methods/Analytic_Methods_for_Using_Laboratory_Test_Results_In_Active_Database_Surveillance_Final_Report.pdf. Accessed July 17, 2019. [Google Scholar]


