Abstract
BACKGROUND:
The use of non-vitamin K oral anticoagulants (NOACs) has increased steadily following marketing approval; however, their relative safety in nonvalvular atrial fibrillation (NVAF) patients in real-world clinical practice remains unclear.
OBJECTIVE:
To compare the risk of major bleeding during anticoagulation therapy between warfarin and NOACs.
METHODS:
This retrospective cohort study analyzed administrative claims data on new NVAF users of warfarin, dabigatran, apixaban, or rivaroxaban in routine clinical care from November 2010 to February 2015 in a commercially insured population in the United States. The primary outcome was time to first major bleeding event requiring hospitalization. Patients were followed until discontinuation or switch of anticoagulants, health plan disenrollment, death, or end of study. All patient characteristics were balanced after propensity score inverse probability of treatment (IPT) weighting. Event rates by type of anticoagulant exposure were compared using IPT-weighted Cox proportional hazards models.
RESULTS:
The study cohort comprised 44,057 patients who used warfarin (n = 23,431), dabigatran (n = 8,539), apixaban (n = 3,689), and rivaroxaban (n = 8,398). Overall mean (SD) age was 70 (12) years, and 41% of the patients were women. A total of 2,337 major bleeding events occurred during 36,636.2 person-years of follow-up. The unadjusted rate of major bleeding with warfarin was 6.0 per 100 person-years versus 2.8 with dabigatran, 3.3 with apixban, and 5.0 with rivaroxaban. Relative to warfarin, major bleeding risk was lower with dabigatran (HR = 0.67, 95% CI = 0.60-0.76) and apixaban (HR = 0.52, 95% CI = 0.41-0.67). Compared with rivaroxaban, major bleeding risk was also lower with dabigatran (HR = 0.67, 95% CI = 0.58-0.78) and apixaban (HR = 0.52, 95% CI = 0.40-0.68). Major bleeding risk was similar for rivaroxaban and warfarin. Relative to apixaban, dabigatran was associated with a significantly higher risk of major gastrointestinal bleeding (HR = 1.43, 95% CI = 1.09-1.88).
CONCLUSIONS:
Study results were consistent with safety findings from pivotal clinical trials comparing NOACs with warfarin and added the perspective of a large real-world observational study that compared bleeding risks associated with NOACs during anticoagulation therapy. Apixaban and dabigatran were associated with lower major bleeding risk compared with warfarin or rivaroxaban; however, apixaban had a lower risk of major gastrointestinal bleeding than dabigatran. These findings can help inform the choice of an optimal agent, which must balance effectiveness and bleeding risk in complex patients.
What is already known about this subject
Clinical trial results have demonstrated the efficacy and safety of non-vitamin K oral anticoagulants (NOACs) relative to warfarin for stroke prevention in nonvalvular atrial fibrillation (NVAF) patients.
Concerns persist regarding the real-world safety of NOACs.
What this study adds
This study examined major bleeding risk among NVAF patients using NOACs during anticoagulation therapy.
Relative to warfarin, dabigatran and apixaban were associated with a 33% lower major bleeding risk, while dabigatran and apixaban were associated with a 48% lower risk of major bleeding compared with rivaroxaban.
Apixaban was associated with a lower risk of major gastrointestinal bleeding than dabigatran.
Atrial fibrillation is estimated to be responsible for approximately 15%-20% of all strokes.1 Long-term anticoagulation with vitamin K antagonists (e.g., warfarin) is effective for stroke prevention, but patients face a significant risk of major bleeding, and therapeutic effectiveness is complicated by dosing adjustments and dietary compliance.2-4 Patients who bleed often discontinue anticoagulation so are at a higher risk of thromboembolism.2-4
In recent years, the approval of several non-vitamin K oral anticoagulants (NOACs) expanded the therapeutic options for long-term anticoagulation in atrial fibrillation. NOAC agents (i.e., dabigatran, rivaroxaban, and apixaban) have compared favorably with warfarin in phase 3 trials with patients with nonvalvular atrial fibrillation (NVAF)5-7 and in a few observational studies.8-12 In addition, fewer drug-food or drug-drug interactions make NOACs appealing warfarin alternatives for stroke prophylaxis in atrial fibrillation.2-4
NOACs accounted for about 62% of new prescriptions of oral anticoagulants in a 2014 report.13 Nonetheless, concerns persist regarding the lack of long-term experience with NOAC anticoagulation and effective management strategies in the event of major bleeding. The limited number of studies on the real-world safety of NOACs have reported conflicting findings. Whereas an analysis of U.S. Medicare data—covering beneficiaries who are typically aged 65 years and older—found a significantly higher risk of major bleeding and gastrointestinal bleeding with dabigatran compared with warfarin,14 another study reported no difference in the risk of gastrointestinal bleeding with dabigatran or rivaroxaban compared with warfarin in younger, commercially insured NVAF patients.15
In a large retrospective study of Medicare patients, Graham et al. (2016) reported that patients receiving rivaroxaban (20 mg once daily) were associated with statistically significant increases in major gastrointestinal bleeding, compared with patients who received dabigatran (150 mg twice daily).10 Noseworthy et al. (2016) reported lower bleeding risk among apixaban compared with dabigatran and rivaroxaban users, while rivaroxaban was associated with a higher bleeding risk compared with dabigatran.11 In a related study that compared NOACs with warfarin, Yao et al. (2016) reported that while apixaban and dabigatran were associated with lower risks of bleeding relative to warfarin, rivaroxaban and warfain appeared to have similar risks.12 In another large observational study, Deitelzweig et al. (2016) reported a higher risk of bleeding-related hospital readmissions among patients using rivaroxaban compared with those on apixaban, based on data from 2 large claims databases.9 Furthermore, Sussman et al. (2016) reported that patients treated with dabigatran in integrated networks had lower bleed-related health care utilization than those on warfarin.8
Meanwhile, a specific reversal agent has been approved for dabigatran,16 and factor Xa antidotes for apixaban- and rivaroxaban-related bleeding are in development,17,18 although experience with these antidotes in the management of NOAC-related major bleeding is limited.
Data on the relative safety of long-term anticoagulation with the newer agents in NVAF patients in routine care could inform the choice of anticoagulant. In this retrospective cohort study, we compared major bleeding outcomes for NVAF patients treated with warfarin, dabigatran, apixaban, or rivaroxaban.
Methods
Data Source
We used medical and pharmacy claims data from the HealthCore Integrated Research Environment (HIRE) from November 1, 2009, through January 31, 2016. HIRE includes medical and pharmacy claims data for nearly 40 million members with commercial insurance across 14 U.S. regional health plans. All study data were kept anonymous to safeguard patient confidentiality; researchers only accessed a limited dataset, which was devoid of individual patient identifiers, in compliance with relevant provisions of the Health Insurance Portability and Accountability Act (HIPAA). This observational study, conducted under the research exception provisions of the HIPAA Privacy Rule 45 CFR 164.514(e), was granted an exemption from institutional review board review.
Study Population
New users of warfarin, dabigatran, apixaban, or rivaroxaban were identified from pharmacy claims. The index date was defined as the earliest prescription fill date of a comparator anticoagulant during the intake period (November 1, 2010, through February 28, 2015). Continuous health plan eligibility for 6 months was required before the index date. Patients were considered to be new users if they had no prescriptions for any anticoagulant in the 6-month period preceding their index dates. We identified patients with NVAF by the presence of ≥ 2 medical claims (inpatient, emergency department, and outpatient) with International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) code 427.31 in the 6-month period before the index date. Patients with a diagnosis of cardiac valve disorders or valve replacement, hyperthyroidism, deep vein thrombosis, pulmonary embolism, kidney transplant, dialysis, or hyperthrombotic conditions were excluded (Figure 1). We followed each patient from the index date until a switch to a comparator anticoagulant, discontinuation of index anticoagulant (more than 45 days after 30-day prescription fill), health plan disenrollment, death, or end of study period, whichever occurred first.
FIGURE 1.
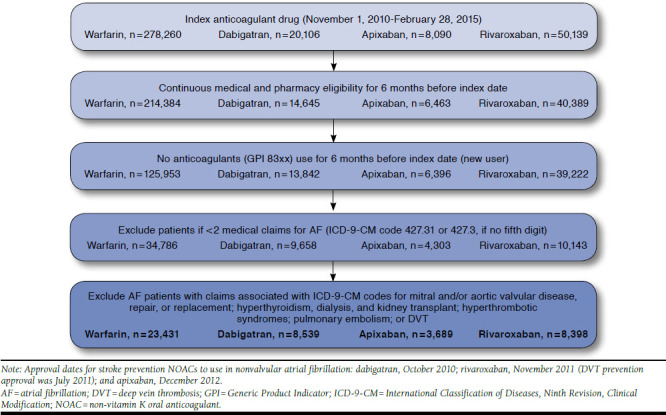
Cohort Definition Flow Diagram
Outcomes and Covariates
Outcomes.
The primary outcome was time to the first major bleeding event leading to hospitalization. We identified major bleeding by an inpatient stay with a primary diagnosis of extracranial hemorrhage or any diagnosis of intracranial hemorrhage using previously validated algorithms.19-22 The use of inpatient ICD 9-CM codes to identify major bleeding has been shown to have a positive predictive value between 89%-98%.21 In addition, we examined the risk of major gastrointestinal and intracranial bleeding separately.
Covariates.
The type of anticoagulant exposure was our predictor variable of interest. We controlled for confounding due to age; sex; geographic region of residence; comorbidity burden by Deyo-Charlson Comorbidity Index23; stroke risk by CHA2DS2-VASc score (1 point each, except where noted, for history of congestive heart failure, hypertension, diabetes mellitus, previous stroke or transient ischemic attack or throm-boembolism [2 points], vascular disease, sex category, and age 65 to 74 years (age ≥ 75 is 2 points)24; bleeding risk by modified HAS-BLED concomitant score (1 point each for hypertension, abnormal liver function, abnormal renal function, stroke, bleed history, elderly [> 65 years], drugs, e.g., antiplatelet or nonsteroidal anti-inflammatory drugs [NSAIDs] and alcohol use/abuse, if documented as diagnosis on medical claim, labile international normalized ratios [INR] not included, since these data were not available for all patients)25; history of chronic kidney disease; coronary artery disease; peripheral vascular disease; cancer; dyslipidemia; pericarditis; dementia; gait abnormalities; dizziness; diabetic and alcoholic neuropathy; esophageal varices; major trauma; coagulation defect factors; bleeding; and hospitalization before treatment initiation. We also assessed the use of antiplatelet agents, amiodarone, dronedarone, and any other antiarrhythmics, diuretics, vaso-pressors, steroids, progestin, estrogen, proton pump inhibitors (PPIs), NSAIDs, and COX-2 inhibitors in the baseline period (Appendix A, available in online article).
Statistical Analysis
Cox proportional hazards regression models with propensity score weighting were used to compare event rates by the type of anticoagulant exposure.26,27 In survival models, propensity score weighting has the advantage of greater bias reduction relative to stratification on propensity scores or covariate adjustment with propensity scores and outperforms matching on the propensity scores in terms of precision (provides estimates with lower mean squared error when estimating the effect of treatment in the treated).26
The analysis was conducted in 2 stages. First, we estimated the probabilities of receiving each anticoagulant, including the previously mentioned covariates as predictors using a generalized logit model.27-29 Each patient received 4 predicted probabilities for each anticoagulant. We used the inverse of the probability of the treatment a patient actually received as propensity score weights. To reduce the variance of the resulting weights, we multiplied the weights by the marginal probability of being treated with the anticoagulant received. To assess the degree of overlap (i.e., whether each patient had a positive probability of receiving each anticoagulant under comparison), we compared the distribution of estimated propensity scores and weights of each treatment regardless of the treatment actually received, using separate box plots. We assessed balance in the distribution of baseline characteristics using a maximum absolute standardized difference (MASD) threshold of 0.10 for all possible pairs of treatment groups (i.e., 6 in all). An MASD of 0.10 or less indicates negligible difference between measured covariates, although some methodologists would consider 0.20 or less adequate.27
Second, event rates were compared between treatment groups using Cox models with inverse probability of treatment weighting (i.e., 6 pairwise comparisons for the 3 NOACs vs. warfarin [reference] and among the 3 NOACs). Proportional hazard assumptions were visually assessed and tested using the product term of drug exposure and time.
After propensity score weighting, we achieved balance on all the measured covariates, as evidenced by MASD < 0.1, with the exception of PPI use (MASD = 0.23). Therefore, we included this covariate in all Cox models.
Sensitivity Analysis
We examined treatment effects in prespecified subgroups and for the secondary outcome by repeating the analytic steps previously described (Figures 2 and 3). The robustness of our findings was assessed using several sensitivity analyses. First, to account for the potential bias from differential censoring, we conducted an intention-to-treat analysis in which patients were censored only at end of enrollment or death, compared with the “as-treated” approach used in the primary analysis. Second, because multiple comorbid illnesses adversely affect anticoagulation control, we excluded individuals with 7 or more comorbid illnesses.
FIGURE 2.
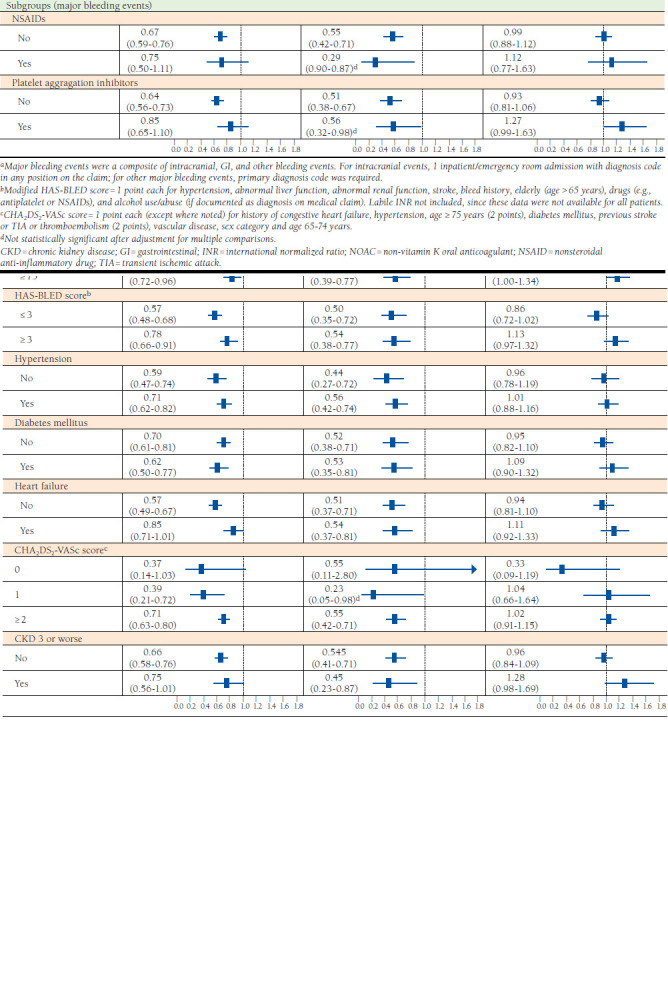
Bleeding Outcomes, Entire Cohort and Subgroups, and NOACs Versus Warfarin, After Propensity Score Weighting
FIGURE 3.
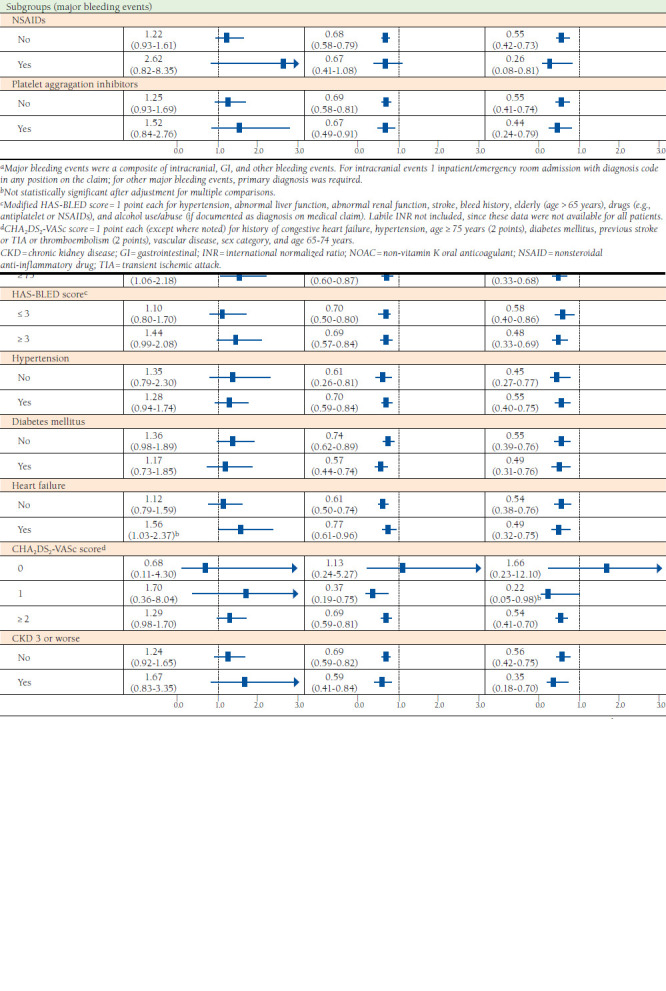
Bleeding Events, Entire Cohort and Subgroups, Among NOACs, After Propensity Score Weighting
We controlled for multiplicity by adjusting the P values of the hazard ratios (HRs) for the primary outcome and subgroups using Benjamini-Hochberg false discovery rates. Statistical analyses were conducted with SAS software version 9.4 (SAS Institute, Cary, NC).
Results
Cohort Characteristics
A total of 44,057 NVAF patients initiated anticoagulation with warfarin (n = 23,431), dabigatran (n = 8,539), apixaban (n = 3,689), or rivaroxaban (n = 8,398; Figure 1). Before propensity score weighting, warfarin users were older than dabigatran, apixaban, and rivaroxaban users (mean age: 73 vs. 66 vs. 69 vs. 67 years). The warfarin cohort had the highest proportion of women (warfarin vs. dabigatran vs. apixaban vs. rivaroxaban: 43.5% vs. 34.5% vs. 40.9% vs. 38.9%). Mean CHA2DS2-VASc and HAS-BLED scores were significantly higher in warfarin users at baseline (warfarin vs. dabigatran vs. apixaban vs. rivaroxaban: 3.7 vs. 2.7 vs. 3.2 vs. 2.9 and 2.3 vs. 1.8 vs. 2.1 vs. 1.9, respectively). Balance was achieved on all baseline characteristics across the treatment groups except the use of PPIs (Table 1).
TABLE 1.
Baseline Characteristics of Treatment Groups, After Propensity Score Weighting
| Characteristic | Warfarin (n = 23,431) | Dabigatran (n = 8,539) | Apixaban (n = 3,689) | Rivaroxaban (n = 8,398) | Maximum ASDa |
|---|---|---|---|---|---|
| Demographic, % | |||||
| Age, years, mean (SD) | 70 (12.2) | 70 (12.3) | 70 (12.6) | 70 (12.3) | 0.01 |
| < 65 | 33.4 | 33.9 | 33.3 | 33.9 | 0.01 |
| 65-74 | 27.0 | 25.9 | 26.5 | 26.5 | 0.02 |
| ≥ 75 | 39.6 | 40.2 | 40.2 | 39.6 | 0.01 |
| Female | 40.9 | 41.1 | 40.5 | 41.3 | 0.01 |
| Medicare Advantage | 33.4 | 33.3 | 33.0 | 33.7 | 0.01 |
| Residence, region | |||||
| Missing | 1.8 | 1.9 | 2.1 | 1.8 | 0.02 |
| Northeast | 18.8 | 18.7 | 18.9 | 18.7 | 0.01 |
| Midwest | 34.5 | 34.6 | 34.4 | 34.4 | 0.00 |
| West | 20.4 | 20.5 | 19.8 | 20.4 | 0.02 |
| South | 24.6 | 24.3 | 24.8 | 24.8 | 0.01 |
| Clinical indicators/medication use at baseline, % | |||||
| Deyo-Charlson comorbidity score, mean (SD) | 4.4 (2.3) | 4.4 (2.3) | 4.5 (2.3) | 4.4 (2.3) | 0.01 |
| HAS-BLED score,b mean (SD) | 2.1 (1.4) | 2.1 (1.4) | 2.1 (1.4) | 2.1 (1.4) | 0.01 |
| CHA2DS2-VASc score,c mean (SD) | 3.3 (1.8) | 3.3 (1.9) | 3.3 (1.9) | 3.3 (1.9) | 0.01 |
| Presence of 7 or more comorbiditiesd | 9.5 | 10.0 | 9.6 | 9.9 | 0.01 |
| Diabetes mellitus | 28.4 | 28.5 | 29.4 | 28.3 | 0.02 |
| Hypertension | 59.8 | 60.5 | 60.0 | 60.0 | 0.01 |
| Liver disease | 4.7 | 4.5 | 4.6 | 4.7 | 0.01 |
| Congestive heart failure | 27.7 | 28.0 | 28.2 | 28.1 | 0.00 |
| Chronic kidney disease | 10.1 | 10.3 | 10.1 | 10.4 | 0.01 |
| Cerebrovascular disease | 15.9 | 16.2 | 15.6 | 16.2 | 0.02 |
| Coronary heart disease | 36.2 | 36.5 | 36.6 | 36.3 | 0.01 |
| Peripheral vascular disease | 19.9 | 20.1 | 19.8 | 20.1 | 0.01 |
| History of bleeding | 14.9 | 14.5 | 14.3 | 14.2 | 0.02 |
| Helicobacter pylori infection | 0.3 | 0.3 | 0.2 | 0.2 | 0.02 |
| NSAID | 9.8 | 9.5 | 9.7 | 9.9 | 0.01 |
| Platelet aggregation inhibitors | 10.4 | 10.4 | 10.5 | 10.6 | 0.00 |
| Amiodarone | 18.1 | 17.5 | 18.9 | 18.1 | 0.02 |
| Proton pump inhibitors | 34.0 | 35.0 | 24.6 | 28.7 | 0.23 |
aMaximum absolute standardized difference considering all pairwise treatment groups.
bModified HAS-BLED score = 1 point each for hypertension, abnormal liver/renal function, stroke, bleed history, labile INR, elderly (aged > 65 years), drugs (e.g., antiplatelet or NSAIDs), or alcohol excess/abuse. Labile INR was set to missing for all patients, since these data were not available for all patients.
cCHA2DS2-VASc score = 1 point each (except where noted) for history of congestive heart failure, hypertension, aged ≥ 75 years (2 points), diabetes mellitus, previous stroke or TIA or thromboembolism (2 points), vascular disease, sex category, and aged 65-74 years.
dThe number of comorbidities was calculated as the sum of previous history of hypertension, diabetes mellitus, pericarditis, dizziness, Parkinson’s disease, liver disease, dementia, gait abnormality, major trauma including fractures, anemia, coronary artery disease, cerebrovascular disease, congestive heart failure, peripheral vascular disease, dyslipidemia, osteoporosis, osteoarthritis or rheumatoid arthritis, and any cancer.
INR = international normalized ratio; NSAID = nonsteroidal anti-inflammatory drug; SD = standard deviation; TIA = transient ischemic attack.
Bleeding Risk
Major bleeding events numbered 2,337 during 36,636.2 person-years of follow-up (1,729 warfarin vs. 245 apixaban vs. 62 dabigatran vs. 301 rivaroxaban; Table 2). The number of events, person-years at risk, and crude event rates for all the subgroups are shown in Appendix B (available in online article).
TABLE 2.
Number of Events, Person-Years at Risk, and Crude Event Rates by Drug Exposure
| Warfarin (n = 23,431) | Dabigatran (n = 8,539) | Apixaban (n = 3,689) | Rivaroxaban (n = 8,398) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | PY | Rate | N | PY | Rate | N | PY | Rate | N | PY | Rate | |
| Major bleeding | 1,729 | 28,739.6 | 6.0 | 245 | 8,775.3 | 2.8 | 62 | 1,893.5 | 3.3 | 301 | 6,003.10 | 5.00 |
| Median follow-up, days (IQR) | 285 (80-714) | 212 (68-572) | 139 (62-271) | 169 (63-382) | ||||||||
| Major GI bleeding | 649 | 29,623.4 | 2.2 | 124 | 8,848.0 | 1.4 | 29 | 1,905.6 | 0.9 | 92 | 6,083.30 | 1.50 |
| Intracranial bleeding | 338 | 29,959.0 | 1.1 | 37 | 8,878.1 | 0.4 | 14 | 1,907.0 | 0.7 | 46 | 6,104.61 | 0.75 |
GI = gastrointestinal; IQR = interquartile range; PY = person-years.
Comparing the crude HRs for NOACs versus warfarin and within NOAC comparisons after propensity score weighting, dabigatran (HR = 0.67, 95% confidence interval [CI] = 0.60-0.76) and apixaban users (HR = 0.52, 95% CI = 0.41-0.67) experienced fewer major bleeding events compared with warfarin users. Major bleeding risk was similar between rivaroxaban (HR = 1.00, 95% CI = 0.89-1.12) and warfarin users. While dabigatran users had a significantly higher risk of major gastrointestinal bleeding (HR = 1.17, 95% CI = 1.04-1.32), they experienced significantly fewer intracranial bleeding events (HR = 0.47, 95% CI = 0.35-0.65), compared with warfarin.
Compared with rivaroxaban users, major bleeding risk was 33% and 48% lower in dabigatran and apixaban users, respectively (HR = 0.67, 95% CI = 0.58-0.78 and HR = 0.52, 95% CI = 0.40-0.68; Figure 3). Relative to apixaban, dabigatran was associated with a significantly higher risk of major gastrointestinal bleeding (HR = 1.43, 95% CI = 1.09-1.88) but no difference in overall major bleeding risk. Dabigatran and apixaban users experienced fewer major bleeding events compared with warfarin in almost all of the subgroups examined. No meaningful differences in major bleeding risk exist between warfarin and rivaroxaban users across the subgroups examined (Figure 2).
Across most subgroups of NOAC users that we examined, there was no difference in major bleeding risk with dabigatran and apixaban (Figure 3). However, rivaroxaban users were more likely to experience major bleeding events compared with dabigatran and apixaban (Figure 3).
We conducted sensitivity analyses and found that major bleeding risk among the treatment groups did not considerably change in magnitude or direction.
Discussion
In this retrospective cohort study of major bleeding risk with anticoagulants for NVAF, we found that, compared with warfarin, dabigatran and apixaban were associated with fewer major bleeding events, which was consistent with the pivotal trials.5-7 The rates of major bleeding in this study (2.8%-6% per 100 patient-years), while significantly higher than the rates in the pivotal trials, are consistent with recent reports from NOAC registries and likely reflect the difference between a clinical trial population and real-world patients.
The findings in this study are directionally consistent with several recent observational studies that compared bleeding rates associated with NOACs and warfarin and assessed bleeding rates among the newer anticoaulants relative to each other.8,9,11,12,30 In their comparison of major bleeding rates between 3 NOACs and warfarin, Yao et al. reported lower risk for apixaban and dabigatran (apixaban: HR = 0.45, 95% CI = 0.34-0.59, P < 0.001; dabigatran: HR = 0.79, 95% CI = 0.67-0.94, P < 0.01); however, rivaroxaban appeared to have similar risk (HR = 1.04, 95% CI = 0.90-1.20, P = 0.60) per 100 patient-years.12
In an associated study that compared bleeding rates across the newer NOACs, Noseworthy et al. reported that apixaban was associated with less bleeding risk than dabigatran (HR = 0.50, 95% CI = 0.36-0.70, P < 0.001) and rivaroxaban (HR = 0.39, 95% CI = 0.28-0.54, P < 0.001).11 Relative to dabigatran, rivaroxaban was associated with increased bleeding risk (HR = 1.30; 95% CI = 1.10-1.53, P < 0.01).11 The study populations evaluated in the Yao et al. and Noseworthy et al. studies most closely approximate the cohorts in our study and were similarly derived from large databases of commercially insured NVAF patients initating NOACs.11,12 Our findings regarding apixaban (i.e., it was associated with lower major bleeding risk compared with warfarin, and among the NOACs evaluated, it appeared to be associated with the lowest risk of major bleeding) are consistent with the findings of these comparable and representative studies.
Also consistent with the results of these studies was our finding that dabigatran appeared to present lower bleeding risks than rivaroxaban and warfarin. We found that the risk of major bleeding was significantly lower across all age categories with dabigatran relative to warfarin, differing from the RE-LY trial,31 which reported a higher major bleeding risk rate in patients aged 75 years or older, although the risk was not significant.5 While the overall risk of major bleeding, including intracranial bleeding, was lower with dabigatran compared with warfarin, this safety benefit was attenuated by a higher risk of major gastrointestinal bleeding, a pattern of bleeding first reported in the RE-LY trial and in recent observational studies.5,14,30,31
Apixaban was associated with fewer major bleeds compared with warfarin across the subgroups examined, which is consistent with findings from the ARISTOLE trial. Although not examined in this study, a recent reanalysis of ARISTOTLE data suggested that apixaban has a lower risk of major bleeding regardless of dosage adjustment in higher risk groups such as the elderly and individuals with renal impairment.6
There were no differences in major bleeding risk between rivaroxaban and warfarin in the entire cohort and subgroups, which is consistent with the results of the ROCKET-AF trial and several other recent observational studies.7,15,32,33 The finding of a persistently higher major bleeding risk with rivaroxaban compared with apixaban across subgroups has important implications for patients, providers, and payers and warrants further investigation in randomized clinical trials.
A strength of this study lies in the assembling of a large new user cohort, which allowed us to examine important subgroups that had limited enrollment in the pivotal trials (e.g., elderly individuals, aged ≥ 75 years, and patients with renal dysfunction). Also, the use of propensity score weighting enabled excellent control of measured baseline differences, and several sensitivity analyses were used to examine the robustness of the findings.
Limitations
This study has some important limitations to consider. First, although we systematically identified potential confounders in estimating the propensity score weights, our assessment of balance achieved between the treatment groups was limited to the covariates measured. For example, other risk-modifying exposures, such as over-the-counter products (e.g., aspirin, NSAIDs, and PPIs), are not fully captured in administrative claims data. Although there is no reason to believe such exposure misclassification is differentially distributed between the cohorts, residual and unmeasured confounding cannot be excluded. It is also possible that residual confounding arising from baseline differences such as age and other potential confounders may persist despite excellent statistical adjustments.
Second, because the risk of major bleeding is higher in the early phase of anticoagulant therapy, the longer follow-up in the warfarin cohort may disproportionately reflect the anticoagulation experience of patients who continue treatment beyond the early phase of therapy.
Finally, we could not assess the quality of anticoagulation in the warfarin arm or measure the magnitude of this possible confounder. It is possible that patients who are likely to be noncompliant are not selected for anticoagulation with warfarin. Although this limitation is an important one to note, the purpose of this study was to evaluate “real-world” outcomes, so—given that real-world anticoagulation control may be sub-optimal—the results of this study need not be misconstrued as nonrepresentative.
Conclusions
The approval of NOAC medications in recent years provided a promise of less complicated management of anticoagulation compared with warfarin. However, many patients are not treated because of concerns of major bleeding and limited experience with NOACs. In patients treated with warfarin, optimal anticoagulation control is often problematic. In part because of these challenges, anticoagulation for stroke prophylaxis is underused in patients with NVAF who would otherwise benefit.34,35 The significantly lower risk of overall major bleeding and major gastrointestinal bleeding observed with apixaban in this study provides evidence of its safety as a suitable warfarin alternative in NVAF patients in a real-world setting, especially for those who have difficulty achieving and maintaining optimal anticoagulation.
APPENDIX A. Covariate Definitions
| Covariate | Definition | Coding |
|---|---|---|
| Patient characteristics | ||
| Age | Age in years | Continuous |
| Male | Male sex | Dichotomous (yes/no) |
| Medicare supplemental | Enrollment in a private health insurance in addition to Medicare | Dichotomous (yes/no) |
| Medicare Advantage | Managed health care – PPO or HMO | Dichotomous (yes/no) |
| Region of residence | Region of residence at index date | Categorical (Northeast, Midwest, West, South, missing) |
| Indexes | ||
| Comorbidity index | Deyo-Charlson Comorbidity Index | Interval (0-33) |
| CHA2DS2-VASc score | Congestive heart failure, hypertension, age ≥ 75 years, diabetes mellitus, previous stroke or TIA or thromboembolism, vascular disease, age 65-74 years, sex category (female gender) | (1 point each, 2 points for age ≥ 75 years and stroke for a maximum of 9) |
| HAS-BLED score | Hypertension, abnormal liver/renal function, stroke, bleeding history, labile INR, elderly (age > 65), drugs (e.g., antiplatelet or NSAIDs) or alcohol concomitantly (1 point each). Labile INR set to missing for all patients | (1 point each for a maximum of 8) |
| Comorbid illnesses | ||
| Ischemic stroke | ICD-9-CM codes 433.01, 433.10, 433.11, 433.21, 433.31, 433.81, 433.91, 434.00, 434.01, 434.11, 434.91, 436 | Dichotomous (yes/no) |
| Hemorrhagic stroke | ICD 9-CM codes 430.x-432.x | Dichotomous (yes/no) |
| Transient ischemic attack | ICD-9-CM code 435.xx | Dichotomous (yes/no) |
| Renal insufficiency | ICD-9-CM codes 582.xx, 585.xx, 588.xx | Dichotomous (yes/no) |
| Myocardial infarction | ICD-9-CM code 410.xx | Dichotomous (yes/no) |
| Congestive heart failure | ICD-9-CM codes 402.01, 402.11, 402.91, 404.01, 404.03, 404.11, 404.13, 404.91, 404.93, 428.xx | Dichotomous (yes/no) |
| Cerebrovascular disease | ICD-9-CM codes 430.x-435.x | |
| Coronary artery disease | ICD-9-CM codes 410.xx, 411.1x, 411.8x, 412.xx, 413.xx, 414.0 x, 414.2x, 414.3x | Dichotomous (yes/no) |
| Peripheral vascular disease | ICD-9-CM codes 433.xx, 437.0x, 437.1x, 440.xx, 443.xx | Dichotomous (yes/no) |
| Cancer | ICD-9-CM codes 140.xx-239.xx, V10.xx | Dichotomous (yes/no) |
| Osteoarthritis | ICD-9-CM codes 715.xx, 721.x | Dichotomous (yes/no) |
| Diabetes mellitus | ICD-9-CM codes 250.xx AND/OR use of oral glucose-lowering medications (GPI starts with 27, except 2710 and 2730) | Dichotomous (yes/no) |
| Hypertension | ICD-9-CM codes 401.xx, 402.xx, 403.xx, 404.xx | Dichotomous (yes/no) |
| Dyslipidemia | ICD-9-CM code 272.xx OR GPI starts with 39 | Dichotomous (yes/no) |
| Pericarditis | ICD-9-CM code 420.xx | Dichotomous (yes/no) |
| Hyperthyroidism | ICD-9-CM codes 242.0x-242.9x | Dichotomous (yes/no) |
| Coagulation defect factors | ICD-9-CM codes 286.1x, 286.3x, 286.5x, 285.5x, 286.5x, 286.4x, 286.4x, 286.3x, 286.5x, 286.5x, 287.4x, 287.5x, 287.8x, 270.4x | Dichotomous (yes/no) |
| Dementia | ICD-9-CM codes 290.xx, 294.xx, 330.xx, 331.xx | Dichotomous (yes/no) |
| Parkinson’s disease | ICD-9-CM code 332.xx | Dichotomous (yes/no) |
| Gait abnormality | ICD-9-CM codes 334.xx, 781.2x | Dichotomous (yes/no) |
| Dizziness | ICD-9-CM codes 458.0 x, 780.4 x | Dichotomous (yes/no) |
| Diabetic and alcoholic neuropathy | ICD-9-CM codes 357.2x and/or 357.5x | Dichotomous (yes/no) |
| Esophageal varices | ICD-9-CM codes 456.0x-456.2x | Dichotomous (yes/no) |
| Major trauma | ICD-9-CM codes 806.xx, 808.xx, 809.xx, 820.xx, 821.xx, 822.xx, 823.xx, 824.xx, 825.xx, 826.xx, 827.xx, 828.xx, 829.xx, 835.xx, 843.xx, 901.1x, 901.2x, 901.3x, 901.83, 902.0x, 902.1x, 902.5x, 902.51, 902.52, 902.53, 902.54, 902.59, 902.87, 903.0x, 903.1x, 904.0x, 904.1x, 904.2x, 904.4x, 904.5x, 924.xx, 928.xx, 942.xx, 943.xx, 945.xx, 952.xx, 959.6x, 959.7x, 897.xx, 820.xx-929.xx | Dichotomous (yes/no) |
| Pre-index medications | ||
| Antiarrhymics | Any pharmacy claim | Dichotomous (yes/no) |
| Amiodarone | Any pharmacy claim | Dichotomous (yes/no) |
| Diuretics | Any pharmacy claim | Dichotomous (yes/no) |
| Pre-index medications | ||
| Vasopressors | Any pharmacy claim | Dichotomous (yes/no) |
| Antihyperlipidemics | Any pharmacy claim | Dichotomous (yes/no) |
| NSAIDs | Any pharmacy claim | Dichotomous (yes/no) |
| COX-2 inhibitors | Any pharmacy claim | Dichotomous (yes/no) |
| Platelet aggregation inhibitors | Any pharmacy claim | Dichotomous (yes/no) |
| Anti-inflammatory agents | Any pharmacy claim | Dichotomous (yes/no) |
| Proton pump inhibitor | Any pharmacy claim | Dichotomous (yes/no) |
| Stroke | ICD-9-CM codes for ischemic (433.01, 433.10, 433.11, 433.21, 433.31, 433.81, 433.91, 434.00, 434.01, 434.11, 434.91, 436) or hemorrhagic stroke (430.x-432.x) | Dichotomous (yes/no) |
| Arterial embolism and thrombosis | ICD-9-CM codes for 444.xx | Dichotomous (yes/no) |
| Venous thromboembolism (VTE) | Inpatient or emergency department ICD-9-CM codes for deep venous thromboembolism (451.1x, 451.2x, 451.81, 451.83, 451.84, 451.9x, 453.1x, 453.2x, 453.4x, 453.5x, 453.6x, 453.7x, 453.8x, 453.9x, 997.2x) or pulmonary embolism (415.1x) | Dichotomous (yes/no) |
| Major bleeding | ICD-9-CM codes 423.0x, 430.xx, 431xx, 432xx, 852.0x, 852.2x, 852.4x, 853.0x, 455.2, 455.5, 455.8, 456.0, 456.20, 459.0x, 530.7x, 530.82, 531.00, 531.01, 531.20, 531.21, 531.40, 531.41, 531.60, 531.61, 533.01, 533.20, 533.21, 533.40, 533.41, 533.60, 533.61, 534.00, 534.01, 534.20, 534.21, 534.40, 534.41, 534.60, 534.61, 535.11, 535.21, 535.31, 535.41, 535.51, 535.61, 537.83, 562.02, 562.03, 562.12, 562.13, 568.81, 569.3, 569.85, 578, 578.0, 578.1, 578.9, 593.81, 599.7, 719.10, 719.11, 719.12, 719.13, 719.14, 719.15, 719.16, 719.17, 719.18, 719.19, 784.7, 784.8x, 786.3x 1 inpatient claim with ICD-9-CM diagnosis codes 430.xx, 431.xx, 432.xx, 852.0x, 852.2x, 852.4x, 853.0x in any position of the claim and the remaining ICD-9-CM codes in the primary position (primary diagnosis) of the claim |
Dichotomous (yes/no) |
| Exclusions | ICD-9-CM codes 394.0x, 394.1x, 394.2x, 394.9x, 396.xx, V43.3x, Z95.2x, V42.2x, 238.4x, 238.71, 283.2x, 289.81, 289.91, 5855, 5856, | Dichotomous (yes/no) |
| Kidney transplant | ICD-9-CM codes V42.0x, 996.81 or ICD-9-CM procedure codes 55.6x or CPT codes 50340, 50360, 50365, 50370, 50380 | |
| Chronic dialysis | ICD-9-CM codes 792.5x, V56.2x or ICD-9-CM procedure codes 39.95, 54.98 or CPT codes 90935, 90937, 90945, 90947, 99512, 99601, 99602 | |
CPT = Current Procedural Terminology; GPI = Generic Product Identifier; ICD-9-CM = International Classification of Diseases, Ninth Revision, Clinical Modification; INR = international normalized ratio; NSAID = nonsteroidal anti-inflammatory drug; TIA = transient ischemic attack.
APPENDIX B. Number of Events, Person-Years at Risk, and Crude Event Rates of Major Bleeding Events
| Major Bleeding Subgroups | Warfarin | Dabigatran | Apixaban | Rivaroxaban | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | PYs | Rate | N | PYs | Rate | N | PYs | Rate | N | PYs | Rate | |
| History of bleeding | ||||||||||||
| No | 1,328 | 24,111.3 | 5.51 | 195 | 7,817.1 | 2.49 | 51 | 1,635.3 | 3.12 | 243 | 5,315.1 | 4.57 |
| Yes | 401 | 4,628.3 | 8.66 | 50 | 958.2 | 5.22 | 11 | 258.2 | 4.26 | 11 | 258.2 | 4.26 |
| Age categories, years | ||||||||||||
| < 65 | 195 | 5,273.5 | 3.70 | 44 | 3,659.2 | 1.20 | 11 | 716.2 | 1.54 | 62 | 2,423.3 | 2.56 |
| 65-74 | 433 | 8,477.4 | 5.11 | 59 | 2,421.8 | 2.44 | 18 | 485.3 | 3.71 | 80 | 1,678.5 | 4.77 |
| ≥ 75 | 1,101 | 14,988.7 | 7.35 | 142 | 2,694.3 | 5.27 | 33 | 692.0 | 4.77 | 159 | 1,901.3 | 8.36 |
| Hypertension | ||||||||||||
| No | 550 | 11,334.2 | 4.85 | 73 | 3,665.8 | 1.99 | 14 | 652.3 | 2.15 | 85 | 2,338.3 | 3.64 |
| Yes | 1,179 | 17,405.4 | 6.77 | 172 | 5,109.6 | 3.37 | 48 | 1,241.2 | 3.87 | 216 | 3,664.8 | 5.89 |
| Diabetes mellitus | ||||||||||||
| No | 1,133 | 19,764.9 | 5.73 | 181 | 6,538.3 | 2.77 | 42 | 1,413.5 | 2.97 | 204 | 4,436.6 | 4.60 |
| Yes | 596 | 8,974.7 | 6.64 | 64 | 2,237.0 | 2.86 | 20 | 480.0 | 4.17 | 97 | 1,566.5 | 6.19 |
| Heart failure | ||||||||||||
| No | 1,041 | 20,172.2 | 5.16 | 154 | 6,866.3 | 2.24 | 40 | 1,455.4 | 2.75 | 196 | 4,691.4 | 4.18 |
| Yes | 688 | 8,567.4 | 8.03 | 91 | 1,909.0 | 4.77 | 22 | 438.1 | 5.02 | 105 | 1,311.6 | 8.01 |
| CHA2DS2-VASc scorea | ||||||||||||
| 0 | 19 | 799.1 | 2.38 | 7 | 734.9 | 0.95 | 2 | 109.8 | 1.82 | 4 | 404.2 | 0.99 |
| 1 | 67 | 2,042.0 | 3.28 | 16 | 1,370.3 | 1.17 | 3 | 271.0 | 1.11 | 29 | 931.1 | 3.11 |
| ≥ 2 | 1,643 | 25,897.5 | 6.34 | 222 | 6,670.2 | 3.33 | 57 | 1,512.6 | 3.77 | 268 | 4,667.7 | 5.74 |
| Previous stroke or TIA | ||||||||||||
| No | 1,443 | 25,308.7 | 5.70 | 208 | 7,933.0 | 2.62 | 48 | 1,680.1 | 2.86 | 250 | 5,375.5 | 4.65 |
| Yes | 286 | 3,431.0 | 8.34 | 37 | 842.3 | 4.39 | 14 | 213.4 | 6.56 | 51 | 627.6 | 8.13 |
| CKD 3 or worse | ||||||||||||
| No | 1,420 | 25,717.5 | 5.52 | 222 | 8,348.5 | 2.66 | 53 | 1,728.2 | 3.07 | 259 | 5,685.3 | 4.56 |
| Yes | 309 | 3,022.1 | 10.2 | 23 | 426.8 | 5.39 | 9 | 165.2 | 5.45 | 42 | 317.8 | 13.2 |
| HAS-BLED scoreb | ||||||||||||
| < 3 | 840 | 17,993.0 | 4.67 | 124 | 6,384.9 | 1.94 | 29 | 1,262.0 | 2.30 | 145 | 4,212.5 | 3.44 |
| ≥ 3 | 889 | 10,746.6 | 8.27 | 121 | 2,390.4 | 5.06 | 33 | 631.4 | 5.23 | 156 | 1,790.6 | 8.71 |
| NSAID | ||||||||||||
| No | 1,601 | 26,416.9 | 6.06 | 218 | 7,846.6 | 2.78 | 58 | 1,687.6 | 3.44 | 266 | 5,325.5 | 4.99 |
| Yes | 128 | 2,322.7 | 55.1 | 27 | 928.8 | 2.91 | 4 | 205.8 | 1.94 | 35 | 677.6 | 5.17 |
| Platelet aggregation inhibitors | ||||||||||||
| No | 1,467 | 26,066.5 | 5.63 | 197 | 7,914.2 | 2.49 | 49 | 1,662.2 | 2.95 | 240 | 5,374.0 | 4.47 |
| Yes | 262 | 2673.1 | 9.80 | 48 | 861.1 | 5.57 | 13 | 231.3 | 5.62 | 61 | 629.1 | 9.70 |
aCHA2DS2-VASc score = 1 point each (except where noted) for history of congestive heart failure, hypertension, age ≥ 75 years (2 points), diabetes mellitus, previous stroke or TIA or thromboembolism (2 points), vascular disease, sex category, and age 65-74 years.
bModified HAS-BLED = 1 point each for hypertension, abnormal liver function, abnormal renal function, stroke, bleed history, elderly (age > 65 years), drugs (e.g., antiplatelet or NSAIDs), and alcohol use/abuse (if documented as diagnosis on medical claim). Labile INR not included, since these data were not available for all patients.
CKD = chronic kidney disease; INR = international normalized ratio; NSAID = nonsteroidal anti-inflammatory drug; PY = person-years; TIA = transient ischemic attack.
REFERENCES
- 1.Hart RG, Pearce LA, Rothbart RM, McAnulty JH, Asinger RW, Halperin JL. Stroke with intermittent atrial fibrillation: incidence and predictors during aspirin therapy. Stroke Prevention in Atrial Fibrillation Investigators. J Am Coll Cardiol. 2000;35(1):183-87. [DOI] [PubMed] [Google Scholar]
- 2.Birman-Deych E, Radford MJ, Nilasena DS, Gage BF. Use and effectiveness of warfarin in Medicare beneficiaries with atrial fibrillation. Stroke. 2006;37(4):1070-74. [DOI] [PubMed] [Google Scholar]
- 3.Hanley CM, Kowey PR. Are the novel anticoagulants better than warfarin for patients with atrial fibrillation? J Thorac Dis. 2015;7(2):165-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hylek EM, Evans-Molina C, Shea C, Henault LE, Regan S. Major hemorrhage and tolerability of warfarin in the first year of therapy among elderly patients with atrial fibrillation. Circulation. 2007;115(21):2689-96. [DOI] [PubMed] [Google Scholar]
- 5.Connolly SJ, Ezekowitz MD, Yusuf S, et al. . Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361(12):1139-51. [DOI] [PubMed] [Google Scholar]
- 6.Granger CB, Alexander JH, McMurray JJ, et al. . Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365(11):981-92. [DOI] [PubMed] [Google Scholar]
- 7.Patel MR, Mahaffey KW, Garg J, et al. . Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365(10):883-91. [DOI] [PubMed] [Google Scholar]
- 8.Sussman M, Ghate S, Sutherland S, et al. . Resource use among nonvalvular atrial fibrillation patients. Am J Pharm Benefits. 2016;8(5):84-92. [Google Scholar]
- 9.Deitelzweig S, Bruno A, Trocio J, et al. . An early evaluation of bleeding-related hospital readmissions among hospitalized patients with nonvalvular atrial fibrillation treated with direct oral anticoagulants. Curr Med Res Opin. 2016;32(3):573-82. [DOI] [PubMed] [Google Scholar]
- 10.Graham DJ, Reichman ME, Wernecke M, et al. . Stroke, bleeding, and mortality risks in elderly medicare beneficiaries treated with dabigatran or rivaroxaban for nonvalvular atrial fibrillation. JAMA Intern Med. 2016;176(11):1662-71. [DOI] [PubMed] [Google Scholar]
- 11.Noseworthy PA, Yao X, Abraham NS, Sangaralingham LR, McBane RD, Shah ND. Direct comparison of dabigatran, rivaroxaban, and apixaban for effectiveness and safety in nonvalvular atrial fibrillation. Chest. 2016;150(6):1302-12. [DOI] [PubMed] [Google Scholar]
- 12.Yao X, Abraham NS, Sangaralingham LR, et al. . Effectiveness and safety of dabigatran, rivaroxaban, and apixaban versus warfarin in nonvalvular atrial fibrillation. J Am Heart Assoc. 2016;5(6):e003725. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4937291/. Accessed July 6, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Desai NR, Krumme AA, Schneeweiss S, et al. . Patterns of initiation of oral anticoagulants in patients with atrial fibrillation—quality and cost implications. Am J Med. 2014;127(11):1075-82.e1. [DOI] [PubMed] [Google Scholar]
- 14.Hernandez I, Baik SH, Pinera A, Zhang Y. Risk of bleeding with dabigatran in atrial fibrillation. JAMA Intern Med. 2015;175(1):18-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chang HY, Zhou M, Tang W, Alexander GC, Singh S. Risk of gastrointestinal bleeding associated with oral anticoagulants: population based retrospective cohort study. BMJ. 2015;350:h1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pollack CV, Jr., Reilly PA, Eikelboom J, et al. . Idarucizumab for dabigatran reversal. N Engl J Med. 2015;373(6):511-20. [DOI] [PubMed] [Google Scholar]
- 17.Ansell JE, Bakhru SH, Laulicht BE, et al. . Use of PER977 to reverse the anticoagulant effect of edoxaban. N Engl J Med. 2014;371(22):2141-42. [DOI] [PubMed] [Google Scholar]
- 18.Siegal DM, Curnutte JT, Connolly SJ, et al. . Andexanet alfa for the reversal of factor Xa inhibitor activity. N Engl J Med. 2015;373(25):2413-24. [DOI] [PubMed] [Google Scholar]
- 19.Abraham NS, Hartman C, Richardson P, Castillo D, Street RL Jr, Naik AD. Risk of lower and upper gastrointestinal bleeding, transfusions, and hospitalizations with complex antithrombotic therapy in elderly patients. Circulation. 2013;128(17):1869-77. [DOI] [PubMed] [Google Scholar]
- 20.Arnason T, Wells PS, van Walraven C, Forster AJ. Accuracy of coding for possible warfarin complications in hospital discharge abstracts. Thromb Res. 2006;118(2):253-62. [DOI] [PubMed] [Google Scholar]
- 21.Cunningham A, Stein CM, Chung CP, Daugherty JR, Smalley WE, Ray WA. An automated database case definition for serious bleeding related to oral anticoagulant use. Pharmacoepidemiol Drug Saf. 2011;20(6):560-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wahl PM, Rodgers K, Schneeweiss S, et al. . Validation of claims-based diagnostic and procedure codes for cardiovascular and gastrointestinal serious adverse events in a commercially-insured population. Pharmacoepidemiol Drug Saf. 2010;19(6):596-603. [DOI] [PubMed] [Google Scholar]
- 23.Quan H, Li B, Couris CM, et al. . Updating and validating the Charlson Comorbidity Index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol. 2011;173(6):676-82. [DOI] [PubMed] [Google Scholar]
- 24.Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the Euro Heart Survey on Atrial Fibrillation. Chest. 2010;137(2):263-72. [DOI] [PubMed] [Google Scholar]
- 25.Apostolakis S, Lane DA, Guo Y, Buller H, Lip GY. Performance of the HEMORR(2)HAGES, ATRIA, and HAS-BLED bleeding risk-prediction scores in patients with atrial fibrillation undergoing anticoagulation: the AMADEUS (evaluating the use of SR34006 compared to warfarin or acenocoumarol in patients with atrial fibrillation) study. J Am Coll Cardiol. 2012;60(9):861-67. [DOI] [PubMed] [Google Scholar]
- 26.Austin PC. The performance of different propensity score methods for estimating marginal hazard ratios. Stat Med. 2013;32(16):2837-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McCaffrey DF, Griffin BA, Almirall D, Slaughter ME, Ramchand R, Burgette LF. A tutorial on propensity score estimation for multiple treatments using generalized boosted models. Stat Med. 2013;32(19):3388-414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Feng P, Zhou XH, Zou QM, Fan MY, Li XS. Generalized propensity score for estimating the average treatment effect of multiple treatments. Stat Med. 2012;31(7):681-97. [DOI] [PubMed] [Google Scholar]
- 29.Spreeuwenberg MD, Bartak A, Croon MA, et al. . The multiple propensity score as control for bias in the comparison of more than two treatment arms: an introduction from a case study in mental health. Med Care. 2010;48(2):166-74. [DOI] [PubMed] [Google Scholar]
- 30.Graham DJ, Reichman ME, Wernecke M, et al. . Cardiovascular, bleeding, and mortality risks in elderly Medicare patients treated with dabigatran or warfarin for nonvalvular atrial fibrillation. Circulation. 2015;131(2):157-64. [DOI] [PubMed] [Google Scholar]
- 31.Eikelboom JW, Wallentin L, Connolly SJ, et al. . Risk of bleeding with 2 doses of dabigatran compared with warfarin in older and younger patients with atrial fibrillation: an analysis of the randomized evaluation of long-term anticoagulant therapy (RE-LY) trial. Circulation. 2011;123(21):2363-72. [DOI] [PubMed] [Google Scholar]
- 32.Maura G, Blotiere PO, Bouillon K, et al. . Comparison of the short-term risk of bleeding and arterial thromboembolic events in nonvalvular atrial fibrillation patients newly treated with dabigatran or rivaroxaban versus vitamin K antagonists: a French nationwide propensity-matched cohort study. Circulation. 2015;132(13):1252-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sherid M, Sifuentes H, Sulaiman S, et al. . Risk of gastrointestinal bleeding with dabigatran: a head-to-head comparative study with rivaroxaban. Digestion. 2014;90(2):137-46. [DOI] [PubMed] [Google Scholar]
- 34.Deitelzweig S. Practical considerations in the use of novel oral anticoagulants for stroke prevention in nonvalvular atrial fibrillation. Cardiovasc Ther. 2014;32(2):74-81. [DOI] [PubMed] [Google Scholar]
- 35.Merli G, Weitz HH. The decision to anticoagulate: assessing whether benefits outweigh the risks for patients with atrial fibrillation. Clin Cardiol. 2004;27(6):313-20. [DOI] [PMC free article] [PubMed] [Google Scholar]


