Abstract
BACKGROUND:
In 2012 U.S. diabetes costs were estimated to be $245 billion, with $176 billion related to direct diabetes treatment and associated complications. Although a few studies have reported positive glycemic and economic benefits for diabetes patients treated under primary care physician (PCP)-pharmacist collaborative practice models, no studies have evaluated the cost-effectiveness of an endocrinologist-pharmacist collaborative practice model treating complex diabetes patients versus usual PCP care for similar patients.
OBJECTIVE:
To estimate the cost-effectiveness and cost benefit of a collaborative endocrinologist-pharmacist Diabetes Intense Medical Management (DIMM) “Tune-Up” clinic for complex diabetes patients versus usual PCP care from 3 perspectives (clinic, health system, payer) and time frames.
METHODS:
Data from a retrospective cohort study of adult patients with type 2 diabetes mellitus (T2DM) and glycosylated hemoglobin A1c (A1c) ≥ 8% who were referred to the DIMM clinic at the Veterans Affairs San Diego Health System were used for cost analyses against a comparator group of PCP patients meeting the same criteria. The DIMM clinic took more time with patients, compared with usual PCP visits. It provided personalized care in three 60-minute visits over 6 months, combining medication therapy management with patient-specific diabetes education, to achieve A1c treatment goals before discharge back to the PCP. Data for DIMM versus PCP patients were used to evaluate cost-effectiveness and cost benefit. Analyses included incremental cost-effectiveness ratios (ICERs) at 6 months, 3-year estimated total medical costs avoided and return on investment (ROI), absolute risk reduction of complications, resultant medical costs, and quality-adjusted life-years (QALYs) over 10 years.
RESULTS:
Base case ICER results indicated that from the clinic perspective, the DIMM clinic costs $21 per additional percentage point of A1c improvement and $115-$164 per additional patient at target A1c goal level compared with the PCP group. From the health system perspective, medical cost avoidance due to improved A1c was $8,793 per DIMM patient versus $3,506 per PCP patient (P = 0.009), resulting in an ROI of $9.01 per dollar spent. From the payer perspective, DIMM patients had estimated lower total medical costs, a greater number of QALYs gained, and appreciable risk reductions for diabetes-related complications over 2-, 5- and 10-year time frames, indicating that the DIMM clinic was dominant. Sensitivity analyses indicated results were robust, and overall conclusions did not change appreciably when key parameters (including DIMM clinic effectiveness and cost) were varied within plausible ranges.
CONCLUSIONS:
The DIMM clinic endocrinologist-pharmacist collaborative practice model, in which the pharmacist spent more time providing personalized care, improved glycemic control at a minimal cost per additional A1c benefit gained and produced greater cost avoidance, appreciable ROI, reduction in long-term complication risk, and lower cost for a greater gain in QALYs. Overall, the DIMM clinic represents an advanced pharmacy practice model with proven clinical and economic benefits from multiple perspectives for patients with T2DM and high medication and comorbidity complexity.
What is already known about this subject
In the United States, the indirect and direct costs of diabetes were estimated to be $245 billion in 2012, with $176 billion of that related to direct treatment of diabetes and associated complications.
Studies have shown that improving glycemic control can prevent or delay the development and progression of costly complications. Improved glycemic control has also been associated with reduced direct medical costs.
What this study adds
An endocrinologist-pharmacist collaborative practice model in which the pharmacist spent more time with patients, compared with usual PCP visits, was cost-effective from the clinic, health system, and payer perspectives.
The magnitude of cost savings was greater than has been reported for PCP-pharmacist collaborative practice models possibly because the Diabetes Intense Medical Management (DIMM) clinic is a specialty clinic focusing on complex diabetes patients.
The DIMM clinic “tune-up” model differs from previously studied advanced pharmacy practice models, since it employs a limited time frame of intense pharmacist intervention and then discharges back to PCPs for ongoing care, thus producing large changes in clinical parameters in a short amount of time at a limited cost.
Diabetes mellitus imposes a substantial economic burden on society. In the United States, the indirect and direct costs of diabetes were estimated to be $245 billion dollars in 2012, with $176 billion of that related to direct treatment of diabetes and associated complications.1 Over the past 2 decades, the cost to the patient has doubled, with prescription drug costs accounting for 55% of the increase.2 Previous studies have shown that improving glycemic control can prevent or delay the development and progression of costly complications.3-7 Improved glycemic control has also been associated with reduced direct medical costs.8-12
A cohort study using claims data from a large health maintenance organization reported that the patient group (predominately type 2 diabetes mellitus [T2DM] patients) whose glycosylated hemoglobin A1c (A1c) decreased 1% or more had lower total health care costs ($685 to $950 less per year) than those without A1c improvement.8 A retrospective analysis from a large U.S. health plan revealed that for every 1% increase in A1c, health care costs rose 7% over the next 3 years.9 In an updated analysis, the same primary author confirmed A1c level was an independent predictor of heath care costs along with the presence of coronary heart disease (CHD), hypertension (HTN), and depression, which are often comorbid conditions in diabetes patients.10
A retrospective analysis in a large managed care organization found that total diabetes-related costs for patients whose A1c exceeded the target level of 7% was $1,540 per patient during the 1-year follow-up period, 32% higher than the total diabetes-related costs ($1,171) for patients at or below the 7% level.11 Similarly, a longitudinal analysis using managed care claims data found direct medical costs attributable to T2DM were 16% lower ($1,505 vs. $1,801) for patients with good glycemic control (A1c ≤ 7%) compared with those with fair control (A1c > 7% and ≤ 9%), and 20% lower ($1,505 vs. $1,871) for those with good glycemic control compared with those with poor control (A1c > 9%).12
Collaborative practice interventions involving physicians, pharmacists, nurses, and other health care professionals have been encouraged by the U.S. Public Health Service to improve patient and health system outcomes.13 A systematic literature review regarding effectiveness of team-based care for blood pressure control found that care was most effective when pharmacists, as opposed to nurses or other team members, were added to a team to manage medication regimens. In addition, care results were more effective when pharmacists independently made changes in medications or with prescriber approval (vs. providing only adherence support and medication education).14
A few studies have examined the clinical and economic impact of a physician and clinical pharmacist collaborative practice model in which pharmacists initiated, adjusted, or discontinued medications for patients with diabetes.15-18 Franklin et al. (2013) found a pharmacist intervention to be cost saving in excess of the pharmacist salary from an academic medical institution perspective for 206 patients across 7 primary care practices for 12 months with no comparator group.15 Sease et al. (2013) estimated the cost savings from improved A1c control attributable to pharmacist intervention in a rural, primary care, free clinic setting to be $74,906 per year from a health system perspective (n = 95) with no comparator group.16
A study in Kaiser Permanente Northern California primary care clinics (2013) reported lowered risk of CHD and stroke over 10 years in the pharmacist group (n = 147), as well as lower cost incurred and greater quality-adjusted life-years (QALYs) gained from a third-party payer perspective as compared with a usual care group (n = 147).17 T2DM patients receiving care from university health system pharmacists, practicing under collaborative care protocols with community-based primary care providers (PCPs), had greater improvement in glycemic control and a less substantial increase in health care costs over 18 months compared with usual care patients.18 Although each study reported positive results, all were conducted in primary care clinics, the scope of economic analyses in each was limited to a single perspective and time frame, and 2 did not have a comparator group.
In a recent study, we reported positive clinical outcomes for complex diabetes patients at the Veterans Affairs San Diego Health System (VASDHS) who were obtaining care in an endocrinologist-pharmacist collaborative practice called the Diabetes Intense Medical Management (DIMM) “Tune-Up” Clinic, as compared with patients receiving usual care from their PCP. 19 The DIMM clinic, which took more time with patients compared with time spent during usual PCP visits, provided personalized care in three 60-minute visits over 6 months, resulted in significantly greater A1c reduction versus usual PCP care.
The objective of this study was to explore the cost per extra benefit gained in the DIMM clinic from 3 perspectives and time frames. Specifically, this study examined the cost per A1c benefit gained at 6 months from the clinic perspective, 3-year medical cost avoidance and return on investment (ROI) from the health system perspective, and 10-year complication risk reduction and cost per QALY gained from the payer perspective. Results can help inform decisions regarding expansion of similar practice models treating complex patients both within the Veterans Affairs (VA) health care system and other health care settings.
Methods
The DIMM clinic study was a retrospective cohort study of adult patients with T2DM and A1c ≥ 8% referred to the DIMM clinic versus a comparator group of PCP patients at the VASDHS between April 2009 and November 2013.19 PCP patients met the same inclusion criteria, were observed for the same period of treatment time, and received no pharmacist-led medication management clinical services. The DIMM clinic “tune-up” model, new within the VA system, combines pharmacist-provided medication therapy management (MTM) with patient-specific diabetes education during approximately three 60-minute visits over a 6-month period, to achieve treatment goals before the patient is discharged back to his or her PCP. Working in a collaborative care plan with an endocrinologist, the DIMM clinic pharmacist developed a personalized care plan using an MTM Spider Web model approach, which considers each patient’s comorbidities, complications, and clinical, socioeconomic, and behavioral issues.20 The pharmacist saw patients independently under a collaborative agreement that allowed initiation, adjustment, or discontinuation of medications related to diabetes and related conditions. In addition, the pharmacist ordered and interpreted laboratory tests.
In addition to overseeing the DIMM clinic, the endocrinologist oversaw multiple clinics, 2 nurse practitioners, 2 medical fellows and/or residents, diabetes educators, and a lipid pharmacist clinic. Specifically for the DIMM clinic, the endocrinologist reviewed care plans for new patients only and was available if needed by the pharmacist for acute symptom evaluation or a new diagnosis. In brief, patients in the DIMM and PCP groups were complex (average of 8 comorbidities, approximately one half with at least 1 mental health diagnosis, and taking 12 to 14 medications). There were no significant differences between the DIMM and PCP groups in baseline demographics; the majority was male (98% vs. 96%), non-Hispanic (84% vs. 84%), and white (62% vs. 59%), with a mean age of 62 years in each group. No significant differences in clinical parameters was observed, except that the DIMM group had higher mean (standard deviation [SD]) A1c than the PCP group (10.5 [1.6] vs. 9.7 [1.6], P = 0.002). The DIMM group (n = 99) experienced a significantly greater mean improvement in A1c than the PCP group (n = 56) at 6 months (-2.4 [2.1] vs. -0.8 [1.7], P < 0.001). In addition, a higher percentage of patients in the DIMM group met A1c goal levels of < 9%, < 8%, and < 7% at 6 months than in the PCP group (73.7%, 55.6%, 27.3% vs. 53.6%, 26.8%, 5.4%, respectively).
For the clinic perspective, the cost per A1c benefit gained was examined using incremental cost-effectiveness ratios (ICERs).
ICER calculations used published 6-month data for the 4 A1c clinical outcomes; difference in mean A1c point reduction, and percentage of patients at A1c < 9%, < 8%, and < 7%.19 Intervention costs per patient included cost of visits with the pharmacist (60 minutes per visit × 3 visits × $66/hour [includes 25% benefits] = $198) or PCP (30 minutes per visit × 3 visits × $110/hour [includes 25% benefits] = $165).21,22 Other costs were assumed to be equal in each group.
For the health system perspective, 3-year medical cost avoidance was calculated as the difference in estimated total medical costs based on patients’ baseline A1c versus estimated costs using the patients’ 6-month A1c values. A regression model, using charges derived from a nonprofit health maintenance organization, was used to estimate medical costs for each patient.9
The model estimated total medical cost (outpatient and inpatient) based on A1c, age, sex, and existence of specific comorbidities (HTN, hyperlipidemia, and heart disease) in each group.
ln (cost) = β0 (constant) + β1 (age) + β2 (age2) + β3 (age × female) + β4 (age × female)2 + β5 (A1c) + β6 (A1c2) + β7 (age × A1c) + β 8(HTN) + β9 (age × HTN) + β10 (female × HTN) + β11 (age × female × HTN) + β12 (age × heart disease) + β13 (female × heart disease) + β14 (age × female × heart disease) + β15 (age × lipid disorder) +β16 (female × lipid disorder) + β17 (age × female × lipid disorder) + ε.
Because only A1c varied within this study’s 6-month period, cost-avoidance estimates reflect change in A1c, while the magnitude of total medical cost estimates also considers other model variables. The base case estimate assumes A1c remained constant at the 6-month level over the remaining 2.5-year period. Monetary values were inflated from original model values to reflect 2014 values using U.S. medical cost inflation.23
ROI was calculated using the base case cost-avoidance gain per patient (i.e., difference in estimated 3-year cost avoidance in the DIMM vs. PCP groups), estimated cost of maintenance care associated with a DIMM patient over 3 years, and current annual patient capacity for the DIMM clinic (n = 60).
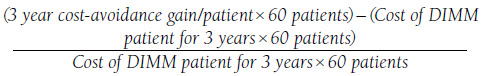 |
Maintenance cost of a DIMM patient over 3 years was assumed to include the cost of the DIMM clinic in year 1 and PCP follow-up in years 2 and 3 after discharge from the DIMM clinic. Year 1 costs included clinical pharmacists (including follow-up phone contact, nonpatient administrative cost, and patient no-show time, and recognizing nonscheduled weeks for the DIMM clinic), estimated at $308/patient (7 hours/week × $66/hour × 40 weeks [i.e., current clinic coverage] = $18,480/60 patients). Years 2 and 3 costs were estimated to be $220/patient, assuming two 30-minute PCP visits per patient annually to maintain 6-month A1c levels (2 PCP visits/year × $55/30-minute visit × 2 years = $220/patient). Therefore the 3-year cost of DIMM patients was $31,680 ($308/patient + $220/patient) × 60 patients = $31,680).
For the payer perspective, reductions in the 2-, 5-, and 10-year incidence of diabetic complications (macrovascular and microvascular), resulting medical costs, and QALYs were estimated using the Archimedes model.24 The Archimedes model is a trial-validated, individual-level simulation model of human physiology, disease progression, and health care utilization. The model represents the average level of U.S. health care delivery and includes care processes representative of current national treatment guidelines. The model has been used extensively for diabetes, and the diabetes portion of the model has been validated by simulating 18 different clinical trials.24
Simulated individuals have a unique physiology that is evolutionary over time and causes them to acquire diseases, have symptoms, and seek medical care. Specifically for diabetes, this evolution may result in health-related outcomes, such as a myocardial infarction (MI) or foot amputation. The model tracks events that could affect utilization (visits, admissions, tests, and procedures), costs, health outcomes, and quality of life. Costs are computed by multiplying all cost-generating events by the cost of the event. Categories of costs in the model include inpatient, outpatient, ambulatory, treatment, and other costs. The full details of the model and associated details have been described in detail elsewhere.24
QALYs are calculated by multiplying the time a patient spends with a particular symptom or health outcome by a factor representing the associated decrease in quality of life.24 Patient data used for the DIMM and PCP group simulations included age, sex, body mass index, weight, blood pressure, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, total cholesterol, triglycerides, fasting plasma glucose, A1c, and glomerular filtration rate. Data also included history of MI, congestive heart failure, coronary artery disease (CAD), dyslipidemia, HTN, T2DM, chronic kidney disease, or chronic obstructive pulmonary disease.19 The discount rate used to convert medical costs and QALYs over time to present value was 3%.25
Sensitivity analyses were conducted to determine how cost estimates could vary with changes in key assumptions made in initial base case analyses. For the clinic perspective A1c ICER analysis, 1-way, 2-way, and probabilistic sensitivity analyses were conducted using upper and lower limits of 95% confidence intervals (CIs) for clinical outcome parameters, 50% decrease in PCP visit time to 15 minutes, 20% increase in pharmacist salary assumptions, and 20% decrease in PCP salary assumptions. Probabilistic analyses simulated 1,000 replications simultaneously varying assumptions for clinical outcomes and cost components by drawing data from their respective distributions. Three-year medical costavoidance 1-way sensitivity analyses increased the primary variable of uncertainty in the model, A1c-level change after the observed 6-month level by 1 and 2 percentage points, and by applying minimum (0.17), average (0.36), and maximum (0.73) state-level hospital cost-to-charge ratios (CCRs) to reflect costs incurred (as opposed to charges used in the base case analysis).26 Since the medical cost-avoidance estimates included outpatient as well as hospital costs, the CCRs were applied to only one half of the cost-avoidance estimate.
Other variables in the model were not expected to vary appreciably in the short 3-year time period. For the Archimedes modeling, intervention costs for the DIMM group were increased by 25% and 50% to test the influence of geographic pharmacist salary differences or individual pharmacist effectiveness, requiring potentially more visits to achieve the same outcomes. The discount rate was doubled to 6% to ascertain the effect of change in discounting for medical costs and QALYs over time.
Analyses were conducted using Microsoft Excel software package 14.4.9; ARCHeS Outcomes Analyzer (Microsoft Corp., Redmond, WA); and @Risk software (Palisade Corp., Ithaca, NY).
Results
From the clinic perspective, the A1c benefit gained in the DIMM versus PCP group cost $21 per additional percentage point of A1c improvement, and the cost per additional patient treated to goal at 6 months ranged from $115 to $164 depending on the target goal (Table 1).
TABLE 1.
Cost-Effectiveness Base Case Results
| Cost per Additional A1c Benefit in DIMM Clinic | |||
|---|---|---|---|
| Value | Incremental | ICERa ($) | |
| Intervention cost per patient ($) | |||
| DIMM | 198 | 33 | N/A |
| PCP | 165 | ||
| A1c point reduction (%) | |||
| DIMM | 2.4 | 1.6 | 21 |
| PCP | 0.8 | ||
| Percentage of patients at A1c < 9% | |||
| DIMM | 73.7 | 20.1 | 164 |
| PCP | 53.6 | ||
| Percentage of patients at A1c < 8% | |||
| DIMM | 55.6 | 28.8 | 115 |
| PCP | 26.8 | ||
| Percentage of patients at A1c < 7% | |||
| DIMM | 27.3 | 21.9 | 151 |
| PCP | 5.4 | ||
| Three-Year Medical Cost Avoidance per Patient and Return on Investment ($) | |||
| DIMM | 8,793 | 5,287 | 9.01b |
| PCP | 3,506 | ||
| Cost per Additional QALY Benefit in DIMM Clinic | |||
| Cost ($) | QALYs | ICERc | |
| 2 years | |||
| DIMM | 899,371 | 97 | Dominant |
| PCP | 962,565 | 96 | |
| 5 years | |||
| DIMM | 2,137,659 | 222 | Dominant |
| PCP | 2,272,572 | 218 | |
| 10 years | |||
| DIMM | 3,879,964 | 385 | Dominant |
| PCP | 4,114,363 | 375 | |
aRounded to nearest dollar.
bReturn on investment.
cDominant = negative ICER, indicating the DIMM clinic group is less costly and more effective than the PCP group.
A1c = glycosylated hemoglobin; DIMM = Diabetes Intense Medical Management; ICER = incremental cost-effectiveness ratio; N/A = not applicable; PCP = primary care physician; QALYs = quality-adjusted life-years.
From the health system perspective, estimated 3-year cost per patient based on baseline and 6-month A1c values in each group were $44,733 and $35,940 (P < 0.001) for the DIMM group versus $42,367 and $38,861 (P = 0.01) for the PCP group. Thus, the estimated mean 3-year cost difference per patient due to improved A1c levels was significantly greater in the DIMM versus PCP group ($8,793 vs. $3,506, P = 0.009), a $5,287 cost-avoidance gain per DIMM patient. The ROI was $9.01 per dollar spent on the DIMM clinic based on a $317,220 estimated 3-year cost-avoidance gain for a 60-patient cohort and a $31,680 3-year maintenance cost of DIMM clinic patients ([$317,220-$31,680]/$31,680).
From the payer perspective, estimated medical costs (including intervention costs) were lower and QALYs gained were greater in the DIMM versus the PCP group over the 2-, 5-, and 10-year time frames (e.g., at 5 years medical costs incurred and QALYs gained respectively for the DIMM group: $2,137,659 and 222 QALYs vs. the PCP group; $2,272,572 and 218 QALYs). The resulting negative ICERs at each time point indicate that the DIMM clinic was dominant; that is, DIMM is more effective and has a lower total cost than PCP care (Table 1). Over a 10-year period, DIMM patients were projected to experience absolute risk reductions in the incidence of several diabetes-related complications and all-cause death when compared with the PCP group (Table 2).
TABLE 2.
Absolute Risk Reduction of Diabetes Complications in DIMM Versus PCP: Base Casea
| Event | 2 Years, % (95% CI) | 5 Years, % (95% CI) | 10 Years, % (95% CI) |
|---|---|---|---|
| Myocardial infarction | 0.32 (-0.02-0.66)a | 0.76 (0.21-1.32) | 1.41 (0.63-2.19) |
| Congestive heart failure | 0.55 (0.17-0.86) | 1.29 (0.75-1.83) | 2.76 (1.97-3.55) |
| Acute heart failure | 1.10 (0.56-1.60) | 1.94 (1.22-2.66) | 3.41 (2.48-4.34) |
| End-stage renal disease | 0.13 (-0.08-0.34) | 0.16 (-0.15-0.47) | 0.12 (-0.30-0.54) |
| Foot ulcer | 1.82 (1.27-2.37) | 3.13 (2.31-3.95) | 5.25 (4.18-6.32) |
| Foot amputation | 0.56 (0.24-0.89) | 1.30 (0.79-1.81) | 2.05 (1.35-2.75) |
| Major adverse cardiovascular events | 0.42 (-0.06-0.90) | 0.96 (0.21-1.71) | 1.80 (0.79-2.81) |
| Death | 0.43 (-0.20-1.00) | 1.17 (0.25-2.09) | 2.21 (0.99-3.43) |
aSource: Archimedes model results.
CI = confidence interval; DIMM = Diabetes Intense Medical Management; PCP = primary care physician.
Sensitivity Analyses
One- and 2-way sensitivity analyses of A1c benefit gained from the clinic perspective indicated that ICERs were most sensitive to the assumption regarding PCP visit time (30 minutes in the base case). When PCP visit time was reduced to 15 minutes, ICERs for the 4 A1c outcomes increased by a factor of approximately 3 (Table 3). Regarding other sensitivity analyses, only in 1 instance when extreme values (lower 95% CI for the DIMM group [65%] and higher 95% CI for the PCP group [66.7%]) were assumed for the percentage of patients achieving A1c < 9% did the PCP group become dominant (lower cost and greater effectiveness). ICER values were not changed appreciably in all other sensitivity analyses, with the highest reaching $458 per additional patient at A1c < 7% in the DIMM group, when extreme values were assumed (lower 95% CI for the DIMM group [18.5%] vs. higher 95% CI for the PCP group [11.3%]).
TABLE 3.
Extremes Sensitivity Analyses: 6-Month Cost-Effectiveness
| Base Case Parameter Value | Alternative Value Rationale | Alternative Parameter Value | Base Case ICER ($) | Alternative ICER ($) | |
|---|---|---|---|---|---|
| Effectiveness | |||||
| Mean A1c point reduction, % | |||||
| DIMM | 2.4 | Lower 95% CI | 2.0 | 21 | 41 |
| PCP | 0.8 | Higher 95% CI | 1.2 | ||
| Percentage of patients at A1c < 9% | |||||
| DIMM | 73.7 | Lower 95% CI | 65.0 | 164 | -1,941 |
| PCP | 53.6 | Higher 95% CI | 66.7 | ||
| Percentage of patients at A1c < 8% | |||||
| DIMM | 55.6 | Lower 95% CI | 45.8 | 115 | 446 |
| PCP | 26.8 | Higher 95% CI | 38.4 | ||
| Percentage of patients at A1c < 7% | |||||
| DIMM | 27.3 | Lower 95% CI | 18.5 | 151 | 458 |
| PCP | 5.4 | Higher 95% CI | 11.3 | ||
| Costs | |||||
| PCP time, minutes per visit | 30.0 | Reduced 50% | 15.0 | ||
| Mean A1c point reduction, % | 21 | 72 | |||
| Percentage of patients at A1c < 9% | 164 | 575 | |||
| Percentage of patients at A1c < 8% | 115 | 401 | |||
| Percentage of patients at A1c < 7% | 151 | 527 | |||
| PCP wage (per minute), $ | 1.83 | Reduced 20% | 1.46 | ||
| Mean A1c point reduction, % | 21 | 41 | |||
| Percentage of patients at A1c < 9% | 164 | 328 | |||
| Percentage of patients at A1c < 8% | 115 | 229 | |||
| Percentage of patients at A1c < 7% | 151 | 301 | |||
| Pharmacist wage (per minute), $ | 1.10 | Increased 20% | 1.38 | ||
| Mean A1c point reduction, % | 21 | 45 | |||
| Percentage of patients at A1c < 9% | 164 | 361 | |||
| Percentage of patients at A1c < 8% | 115 | 252 | |||
| Percentage of patients at A1c < 7% | 151 | 332 | |||
A1c = glycosylated hemoglobin; CI = confidence interval; DIMM = Diabetes Intense Medical Management; ICER = incremental cost-effectiveness ratio; PCP = primary care physician.
Probabilistic sensitivity analyses indicated that in 25% of the simulations, the DIMM group was dominant (negative ICER), with lower estimated cost and greater efficacy than the PCP group (Table 4). In the remainder of simulations, the ICER was estimated to be between $21.04 and $59.36 per additional percentage point of A1c improvement, $157.40 to $634.87 per additional patient at < 7%, $131.08 to $384.77 per additional patient at < 8%, and $189.45 to $588.17 per additional patient at < 9%.
TABLE 4.
Probabilistic Sensitivity Analyses: 6-Month A1c Cost-Effectiveness
| Percentile | Incremental Costs (DIMM –PCP) $ | Incremental Outcomes (DIMM – PCP) | ICER | ||||||
|---|---|---|---|---|---|---|---|---|---|
| A1c Point Reduction |
A1c < 7%
% |
A1c < 8%
% |
A1c < 9%
% |
$ per A1c Point Reduction | $ per Patient A1c < 7% | $ per Patient A1c < 8% | $ per Patient A1c < 9% | ||
| 5th | -75.35 | 1.22 | 15.7 | 17.9 | 8.0 | -45.28 | -445.79 | -261.92 | -298.05 |
| 10th | -34.12 | 1.33 | 16.5 | 21.0 | 10.6 | -18.55 | -151.89 | -106.30 | -224.20 |
| 25th | -1.50 | 1.49 | 18.2 | 24.6 | 15.8 | -1.11 | -5.22 | -4.16 | -8.43 |
| 50th (median) | 34.83 | 1.68 | 21.6 | 28.8 | 20.0 | 21.04 | 157.40 | 131.08 | 189.45 |
| 75th | 73.29 | 1.93 | 24.7 | 32.3 | 24.2 | 42.00 | 319.72 | 234.39 | 378.33 |
| 90th | 95.90 | 2.03 | 27.8 | 37.4 | 28.8 | 50.88 | 527.22 | 302.52 | 520.05 |
| 95th | 107.87 | 2.16 | 28.8 | 39.2 | 31.2 | 59.36 | 634.87 | 384.77 | 588.17 |
A1c = glycosylated hemoglobin; DIMM = Diabetes Intense Medical Management; ICER = incremental cost-effectiveness ratio; PCP = primary care physician.
From the health system perspective, gain in 3-year medical cost avoidance per DIMM patient and ROI remained positive and substantial throughout the sensitivity analyses. Even when A1c was assumed to have risen 2 percentage points for the DIMM group and only 1 percentage point for the PCP group over the 3-year period, 3-year medical cost-avoidance gain per patient was still positive ($1,611), as was the ROI ($2.05). When considering costs (instead of charges) using base case A1c levels, we found that 3-year cost avoidance per DIMM patient was reduced to $3,080, $3,599, and $4,585 (using minimum, average, and maximum CCRs) with corresponding ROIs of $4.83, $5.82, and $7.68.
From the payer perspective, the DIMM clinic group remained dominant (i.e., higher QALYs and lower cost than the PCP group) in all cases when the base case assumptions for DIMM clinic cost and discount rate for costs and QALYs were varied across their plausible range (Table 5). Similarly, there was little change in the absolute risk reduction of diabetes-related complications, with the incidence of end-stage renal disease proving to be the most sensitive to model assumptions (10-year absolute risk-reduction range: -0.30% to 0.54%; Table 2).
TABLE 5.
Sensitivity Analyses: Cost per QALY ICER
| 2 Years, $ | 5 Years, $ | 10 Years, $ | |
|---|---|---|---|
| Base case reference scenario | -63,194 | -33,728 | -23,440 |
| DIMM intervention costs | |||
| 25% increase | -60,224 | -32,986 | -23,143 |
| 50% increase | -57,254 | -32,243 | -22,846 |
| Discount rate for medical costs and QALYs | |||
| 6% | -62,288 | -31,927 | -23,269 |
DIMM = Diabetes Intense Medical Management; QALY = quality-adjusted life-year
Discussion
The minimal cost per additional A1c benefit gained illustrated the cost-effectiveness of the DIMM clinic “tune-up” model for complex patients with diabetes from multiple perspectives, time frames, and outcomes. From the clinic perspective, the A1c benefit gained in the DIMM versus PCP group cost $21 per additional percentage point of A1c improvement and ranged from $115 to $164 per additional patient at standard goal levels. These values are small in comparison to the $1,211 medical cost savings that has been estimated to result from a 1% A1c reduction.7
From the health system perspective, estimated mean 3-year cost avoidance per patient in the DIMM group due to improved A1c levels was 2.5 times greater than that in the PCP group and resulted in a substantial ROI of $9.01 per dollar invested in the DIMM clinic. Finally, from the payer perspective, the DIMM clinic group was dominant (i.e., lower total cost and greater number of QALYs than the PCP group) and produced appreciable reductions in diabetes-related complications early on at 2 years and as time progressed at 5 and 10 years.
Sensitivity analyses indicated the results were robust, and overall conclusions did not change appreciably when key parameters (including DIMM clinic effectiveness and cost) were varied within plausible ranges. These positive results were driven by the large difference in A1c outcomes achieved in the DIMM versus the PCP group, as well as the relatively low cost of the pharmacist intervention designed as a limited series of visits to treat patients to goal before being discharged back to the referring PCP.
Our results reveal positive findings, of greater magnitude, than similar studies of PCP-pharmacist collaborative practice models. Using comparable savings estimates (converting estimated 3-year savings in this study to reflect cost [assuming average CCR], rather than charges, and 2011 dollar values), annual savings (assuming an equal spread of 3-year savings) would be $970/patient in this study as compared with $421/patient, based on achieving both A1c and systolic blood pressure goals in the Franklin study and $788/patient in the Sease study ($74,906 annual/95 patients; data not shown).15,16
It is difficult to discern why our study resulted in a greater magnitude of cost savings than these studies. Reasons may include the DIMM clinic being a specialty clinic, with a limited time frame of intense pharmacist intervention and then discharge back to PCPs for ongoing care, thus producing large changes in clinical parameters in a short amount of time at a limited cost. From a longer-term perspective, our study results are similar to those reported for primary care patients in Kaiser Permanente Northern California.17 As in their study, our results revealed patients had greater QALYs gained and lower cost incurred as compared with usual care. The results in this study were achieved in more complex patients and in a much shorter time period of 6 months than the PCP studies with interventions running 1 to 2 years.
The comparative complexity of DIMM patients versus the PCP studies is exemplified by diabetes-related comorbidities, mental health status, diabetes duration, and medication regimen complexity. The DIMM patients had higher baseline macrovascular and microvascular comorbidities than the Sease study patients: more than 2 times higher CAD and more than 4 times higher retinopathy. Dyslipidemia and HTN were also higher in the DIMM patients compared with Sease study patients: 93% versus 81% and 92% versus 75%, respectively).16
While it is not possible to compare our study results with the PCP studies because these data were not reported, the DIMM patient complexity is further illustrated, as 71% of DIMM patients had diabetes with end organ damage, 65% were obese, and 57% had neuropathy. Sixty-four percent of DIMM patients had mental health issues, with depression and posttraumatic stress disorder highest among them. DIMM patients had a mean duration of diabetes of 9.5 (± 7.7) years and were managing a large number of medications (mean 13), and the magnitude of medication regimen complexity was in the highest range based on published values.27
Our study results demonstrate the value of the DIMM “tune-up” clinic personalized care model in which, compared with usual PCP visits, time was allowed to manage complex patients with T2DM, and those patients achieved significant glycemic control in only three 60-minute clinic visits over 6 months. The comparisons to usual care demonstrate that the more intensive, pharmacist-managed program yielded superior clinical results and was cost-effective.
A number of implications for this study warrant additional investigation, including the following:
The results of this study should be validated in a larger trial at multiple facilities to see if similar results can be achieved. If it is found that an intensive, specialty clinic consistently achieves results that are similarly cost-effective, then consideration should be given to widespread adoption within the VA.
Studies outside the VA setting should also be conducted to assess the feasibility and cost benefits of this type of program in the general population. The demographics of the VA population in this study are likely different from the general population of patients who may be enrolled in such a clinic, and the costs of providing care in different settings will vary.
This program could provide a great deal of insight into possible care structures involving clinical pharmacists under accountable care organizations (ACOs) and similar evolving health care systems. If these patients consistently achieve better outcomes at a lower cost, then this program might serve as a template for ACOs to engage health system and even community pharmacy partners to share risk in patient care in an economically beneficial manner.
Limitations
While positive significant study outcomes were realized, there are study limitations to consider. Clinical data used to populate models were from a study in which we were limited to implementing a nonrandomized study design, for a limited 6-month treatment period, to compare PCP outcomes with the single DIMM clinic setting in a veteran population health care system in patients with poorly controlled diabetes. Optimally, a larger, longer-term, randomized multicenter, multihealth system study of the new DIMM model would provide more robust clinical and economic data.
Medications for VA patients are also provided with low or no copays, which could positively affect patient adherence to medications, resulting in better outcomes than may be seen when patients are faced with considerable copays for each medication. We assumed equal costs in the DIMM and PCP models, except for the main differences of clinical pharmacist versus PCP time. A more detailed analysis could investigate if there were other appreciable differences in cost between groups such as laboratory monitoring, medication usage, or endocrinologist time.
Although assumptions were used for cost- and risk-reduction analyses, results remained positive throughout a range of sensitivity analyses. In sensitivity analyses for 3-year cost avoidance, we varied A1c (plus 1 and plus 2 percentage points) uniformly in each group; therefore, any possibility that DIMM patients would regress more over time than PCP patients was not considered in our analyses. The model used to predict 3-year cost avoidance was the original model proposed by Gilmer et al. (1997), which has been updated.9,10 We chose to use the original model because the new model maintained the variables in the original, and we did not have data for most of the variables that were added to the more recent model (e.g., income, education, drug insurance). The impact of this decision is not known.
Conclusions
Our study outcomes demonstrate the collaborative DIMM “tune-up” clinic approach that allowed more time, compared with usual PCP visits, for personalized care is a successful model. The essentials of this model are approximately three 60-minute pharmacist visits that combine patient-centered clinical care with real-time patient-specific diabetes education at the same clinic visit. Despite the complex nature of DIMM clinic patients, significant glycemic control was achieved in a 6-month period and, most importantly, minimal costs were incurred per additional clinical and economic benefit gained. A greater estimated 3-year cost avoidance, appreciable ROI, and relative reduction in risk of long-term complications, as well as lower cost for greater QALYs gained compared with usual PCP care, support the value of the unique DIMM model for complex patients. Overall, the DIMM clinic represents an advanced pharmacy practice model with proven clinical and economic benefits in patients with T2DM and high medication and comorbidity complexity.
ACKNOWLEDGMENTS
The authors acknowledge the following people for their support in clinic development and/or expertise: Victoria Aldridge, PharmD; Megan Chynoweth, PharmD; Patrick Chung, PharmD; Andrea Bechtold, PharmD; John Khoan, PharmD; and Sunder Mudaliar, MD. In addition, the authors acknowledge the contribution of Shraddha Chhaugule, who assisted in performing sensitivity analyses.
REFERENCES
- 1.American Diabetes Association.. Economic costs of diabetes in the U.S. in 2012. Diabetes Care. 2013;36(4):1033-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou X, Zhang P, Kahn HS, Bardenheier BH, Li R, Gregg EW.. Change in medical spending attributable to diabetes: national data from 1987 to 2011. Diabetes Care. 2015;38(4):581-87. [DOI] [PubMed] [Google Scholar]
- 3.Nathan DM;DCCT/EDIC Research Group.. The diabetes control and complications trial/epidemiology of diabetes interventions and complications study at 30 years: overview. Diabetes Care. 2014;37(1):9-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.ACCORD Study Group, ACCORD Eye Study Group, Chew EY, et al.. Effects of medical therapies on retinopathy progression in type 2 diabetes. N Engl J Med. 2010;363(3):233-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cugnet-Anceau C, Bauduceau B.. Glycaemic control and cardiovascular morbi-mortality: the contribution of the 2008 studies. Ann Endocrinol (Paris). 2009;70(1):48-54. [DOI] [PubMed] [Google Scholar]
- 6.Gore MO, McGuire DK.. The 10-year post-trial follow-up of the United Kingdom Prospective Diabetes Study (UKPDS): cardiovascular observations in context. Diab Vasc Dis Res. 2009;6(1):53-55. [DOI] [PubMed] [Google Scholar]
- 7.Baxter M, Hudson R, Mahon J, et al.. Estimating the impact of better management of glycaemic control in adults with Type 1 and Type 2 diabetes on the number of clinical complications and the associated financial benefit. Diabet Med. 2016;33(11):1575-81. [DOI] [PubMed] [Google Scholar]
- 8.Wagner EH, Sandhu N, Newton KM, McCulloch DK, Ramsey SD, Grothaus LC.. Effect of improved glycemic control on health care costs and utilization. JAMA. 2001;285(2):182-89. [DOI] [PubMed] [Google Scholar]
- 9.Gilmer TP, O’Connor PJ, Manning WG, Rush WA.. The cost to health plans of poor glycemic control. Diabetes Care. 1997;20(12):1847-53. [DOI] [PubMed] [Google Scholar]
- 10.Gilmer TP, O’Connor PJ, Rush WA, et al.. Predictors of health care costs in adults with diabetes. Diabetes Care. 2005;28(1):59-64. [DOI] [PubMed] [Google Scholar]
- 11.Shetty S, Secnik K, Oglesby AK.. Relationship of glycemic control to total diabetes-related costs for managed care health plan members with type 2 diabetes. J Manag Care Pharm. 2005;11(7):559-64. Available at: http://www.jmcp.org/doi/10.18553/jmcp.2005.11.7.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oglesby AK, Secnik K, Barron J, Al-Zakwani I, Lage MJ.. The association between diabetes related medical costs and glycemic control: a retrospective analysis. Cost Eff Resour Alloc. 2006;4:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Giberson S, Yoder S, Lee MP.. Improving patient and health system outcomes through advanced pharmacy practice. A report to the U.S. Surgeon General. Office of the Chief Pharmacist. U.S. Public Health Service. December 2011. Available at: http://www.accp.com/docs/positions/misc/improving_patient_and_health_system_outcomes.pdf. Accessed February 4, 2017.
- 14.Proia KK, Thota AB, Njie GJ, et al.. Team-based care and improved blood pressure control: a community guide systematic review. Am J Prev Med. 2014;47(1):86-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Franklin BE, Farland MZ, Thomas J, McFarland MS, Ray SM, Byrd DC.. Pharmacoeconomic analysis of the diabetes initiative program: a pharmacist-physician collaborative care model. Ann Pharmacother. 2013:47(12);1627-34. [DOI] [PubMed] [Google Scholar]
- 16.Sease JM, Franklin MA, Gerrald KR.. Pharmacist management of patients with diabetes mellitus enrolled in a rural free clinic. Am J Health Syst Pharm. 2013:70(1);43-47. [DOI] [PubMed] [Google Scholar]
- 17.Yu J, Shah BM, Ip EJ, Chan J.. A Markov model of the cost-effectiveness of pharmacist care for diabetes in prevention of cardiovascular diseases: evidence from Kaiser Permanente Northern California. J Manag Care Pharm. 2013;19(2);102-14. Available at: http://www.jmcp.org/doi/10.18553/jmcp.2013.19.2.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McAdam-Marx C, Dahal A, Jennings B, Singhal M, Gunning K.. The effect of a diabetes collaborative care management program on clinical and economic outcomes in patients with type 2 diabetes. J Manag Care Spec Pharm. 2015;21(6):452-68. Available at: http://www.jmcp.org/doi/10.18553/jmcp.2015.21.6.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morello CM, Christopher ML, Ortega L, et al.. Clinical outcomes associated with a collaborative pharmacist-endocrinologist Diabetes Intense Medical Management “Tune-Up” Clinic in complex patients. Ann Pharmacother. 2016;50(1):8-16. [DOI] [PubMed] [Google Scholar]
- 20.Morello CM, Hirsch JD, Lee KC.. Navigating complex patients using an innovative tool: the MTM Spider Web. J Am Pharm Assoc. 2013;53(5):530-38. [DOI] [PubMed] [Google Scholar]
- 21.Salary.com. Physician-family practice salaries. Available at: http://www1.salary.com/family-physician-salary.html. Accessed February 4, 2017.
- 22.Salary.com. Clinical pharmacist salaries. Available at: http://www1.salary.com/Clinical-Pharmacist-Salary.html. Accessed February 4, 2017.
- 23.Halfhill.com. Tom’s inflation calculator. Available at: http://www.half-hill.com/inflation_js.html. Accessed February 4, 2017.
- 24.Eddy DM, Schlessinger L.. Archimedes: a trial-validated model of diabetes. Diabetes Care. 2003:26(11);3093-101. [DOI] [PubMed] [Google Scholar]
- 25.Rascati KL. Essentials of Pharmacoeconomics. 2nd ed. Philadelphia: Lippincott Williams & Wilkins; 2014. [Google Scholar]
- 26.Centers for Medicare & Medicaid Services. FY 2013 final rule tables. Table 8A. Available at: https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/AcuteInpatientPPS/FY-2013-IPPS-Final-Rule-Home-Page-Items/FY2013-Final-Rule-Tables.html. Accessed February 4, 2017.
- 27.Libby AM, Fish DN, Hosokawa PW, et al.. Patient-level medication regimen complexity across populations with chronic disease. Clin Ther. 2013;35(4):385-98. [DOI] [PubMed] [Google Scholar]


