Abstract
Background
Past research indicates a higher prevalence, incidence, and severe clinical manifestations of alpha-synucleinopathies in men, leading to a suggestion of neuroprotective properties of female sex hormones (especially estrogen). The potential pathomechanisms of any such effect on alpha-synucleinopathies, however, are far from understood. With that aim, we undertook to systematically review, and to critically assess, contemporary evidence on sex and gender differences in alpha-synucleinopathies using a bench-to-bedside approach.
Methods
In this systematic review, studies investigating sex and gender differences in alpha-synucleinopathies (Rapid Eye Movement (REM) Behavior Disorder (RBD), Parkinson’s Disease (PD), Dementia with Lewy Bodies (DLB), Multiple System Atrophy (MSA)) from 2012 to 2022 were identified using electronic database searches of PubMed, Embase and Ovid.
Results
One hundred sixty-two studies were included; 5 RBD, 6 MSA, 20 DLB and 131 PD studies. Overall, there is conclusive evidence to suggest sex-and gender-specific manifestation in demographics, biomarkers, genetics, clinical features, interventions, and quality of life in alpha-synucleinopathies. Only limited data exists on the effects of distinct sex hormones, with majority of studies concentrating on estrogen and its speculated neuroprotective effects.
Conclusion
Future studies disentangling the underlying sex-specific mechanisms of alpha-synucleinopathies are urgently needed in order to enable novel sex-specific therapeutics.
Keywords: alpha-synucleinopathies, sex differences, estrogen, Parkinson’s disease, Dementia with Lewy Bodies
Highlights
Key findings
There is conclusive evidence to suggest sex- and gender-specific differences in multiple aspects of alpha-synucleinopathies (i.e., genetics, demographics)
The alpha-synucleinopathy process has a distinct motor and non-motor symptoms phenotype in men, compared to women.
Gender, societal and lifestyle factors should be always considered when improving the quality of life and clinical management of patients suffering with alpha synucleinopathy.
What is known, and what is new?
Male sex has been implicated as a predisposing factor toward developing alpha synucleinopathy.
While there is evidence for the neuroprotective effects of female sex hormones, it is still unclear to what extent estrogen, or any other sex hormones, could be neuroprotective within the broad framework of alpha-synucleinopathies.
What is the implication, and what should change now?
Addressing sex and gender differences in clinical and research settings has significant implications in improving diagnosis and management, implementing prevention strategies, and developing novel sex-specific health therapeutics.
1. Introduction
It has been more than twenty years ago since the discovery of the essential role of α-synuclein in the pathogenesis of Parkinson’s disease (PD) (1, 2). Since then, abnormal aggregates of α-synuclein, such as Lewy bodies and Lewy neurites, and glial cell inclusions, have been similarly linked with several other sporadic neurodegenerative diseases termed alpha-synucleinopathies [also please refer to (3, 4)]. The alpha-synucleinopathies, including idiopathic PD, Dementia with Lewy Bodies (DLB), Multiple systems atrophy (MSA), pure autonomic failure and REM sleep behavior disorder (RBD), have been also associated with synaptopathy and inflammation, as of yet poorly understood α-synuclein-related mechanisms, that likely contribute to the initiation and propagation of the disease (3). A body of work suggests that abnormal forms of α-synuclein may trigger selective and progressive neuronal death and dopaminergic transmission through mitochondrial impairment, lysosomal dysfunction, and alteration of calcium homeostasis not just in PD, but also in RBD, DLB and MSA (3). Alpha-synuclein aggregates perturb dopaminergic transmission and induce presynaptic and postsynaptic dysfunctions (5). Similarly, the presence of early inflammation in experimental models and PD patients, known to occur before deposition and spreading of α-synuclein, further supports a mechanistic link between inflammation and synaptic dysfunction (5).
All alpha-synucleinopathies appear to share synuclein-related neuroinflammation and many clinical, neurochemical and morphological features (3). Nonetheless, multiple clinical phenotypes exist for each of the three main α-synucleinopathies (PD, DLB and MSA), and a diverse dynamic distribution of their underlying neuropathologies has been demonstrated [also see (4, 5)]. For instance, in both PD and DLB α-synuclein inclusions are thought to be predominantly present in neurons and neurites (3, 4). However, while in PD their occurrence is associated with the loss of dopaminergic neurons in the substantia nigra, resulting in the prevalent motor symptoms; in DLB, it predominates in the neocortex with most prevalent symptoms being fluctuating cognition, recurrent visual hallucinations and spontaneous extrapyramidal motor features (5). On the other hand, in MSA the predominant presence of α-synuclein inclusions is thought to occur in the cytoplasm of oligodendrocytes, with selective neurodegeneration of the multiple brain areas resulting in parkinsonism, cerebellar ataxia and autonomic failure (4, 5). The understanding of these mechanisms is of pivotal importance to support the research on reliable biomarkers to identify the disease and possible disease-modifying therapies (3, 5).
In a similar vein, sex and gender differences have been a focus of interest in alpha-synucleinopathies in recent years, due to their potential to disentangle sex-specific disease phenotypes, and translate them to develop novel sex-specific therapeutics – known as a ‘bench-to-bedside’ approach (6–8). According to the Institute of Medicine’s Committee on Sex and Gender Differences, sex and gender differences are biological, physiological, and clinical differences between males and females that arise due to environmental factors and biological effects due to sex chromosomes and gonadal hormones (9).
Cumulative evidence has reported higher prevalence, incidence, increased disease severity and susceptibility of men compared with women in alpha-synucleinopathies such as PD (10), MSA (11, 12) and DLB (13), and even in the prodromal stage of alpha-synucleinopathies such as REM Behavior Disorder (RBD) (14). To address this, animal and clinical studies have posited the notion of neuroprotective properties of the female sex hormone estrogen against alpha-synucleinopathies (15–18). However, asserting any causality to estrogen as a protective factor in alpha-synucleinopathies remains speculative without a thorough investigation into the observable sex-and gender-specific differences. Hence, this systematic review aims to critically review the literature on sex differences in alpha-synucleinopathies, broadening our scope to sex-lineated assessments of prevalence, demographics, biomarkers, genetic factors, clinical features, neuroinflammatory and neurochemical responses, interventions, and quality of life themes. A comprehensive assessment of sex and gender differences in alpha-synucleinopathies holds promise for improving clinical diagnosis and developing treatments with optimal efficacy in both men and women.
2. Methods
2.1. Search strategies
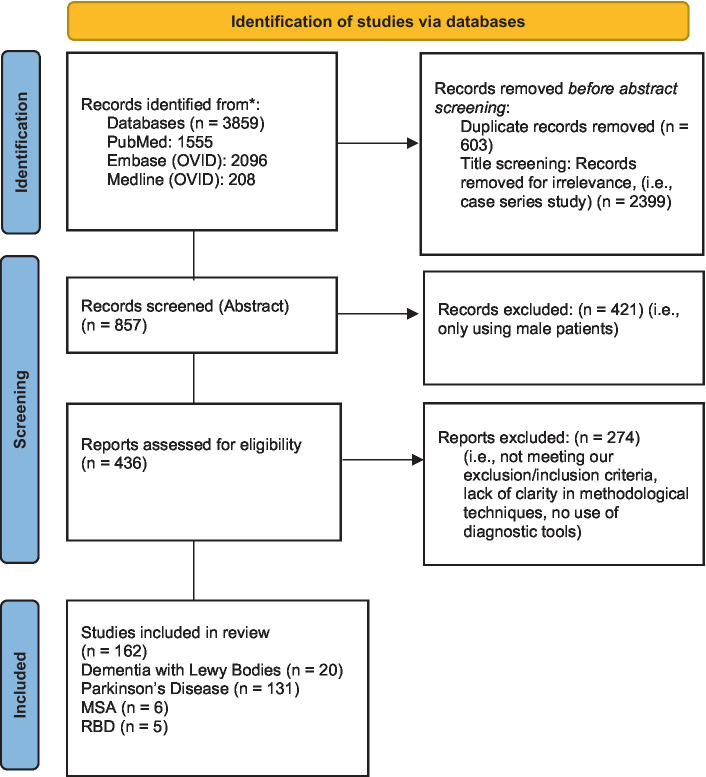
This systematic review was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyzes (PRISMA) guidelines (19) (see Figure 1). Relevant studies were identified by two reviewers using the electronic databases of PubMed, Embase (Ovid) and Medline (Ovid). The following keywords were used: (sex OR gender differences) AND (alpha-synucleinopathies OR REM Behavior Disorder OR Parkinson’s disease OR Dementia with Lewy Bodies (DLB) OR multiple system atrophy (MSA)) (see Table 1). Eligible papers were extracted from 2012 until October 2022. The references of the selected articles were also examined to retrieve documents missed by the literature search.
Figure 1.
PRISMA 2020 Flow Diagram of study selection process.
Table 1.
The search strategy and exclusion/inclusion criteria.
| Database | Search strategy | Limits |
|---|---|---|
| PubMed | (Gender differences OR sex differences) AND (alpha synucleinopathies OR Parkinson’s disease OR Dementia with Lewy Bodies (DLB) OR Parkinson’s disease dementia (PDD) OR multiple system atrophy) | Year: 2012–2022 Species: Human Age: > 18 Only in English |
| Embase (Ovid) | (Sex differences) AND (Parkinson’s disease OR diffuse Lewy body disease OR multiple system atrophy OR Shy Drager syndrome) | Year: 2012–2022 Species: Human |
| Medline (Ovid) | (Sex characteristics) AND (Parkinson’s disease, Multiple System Atrophy or shy Drager syndrome, or alpha synucleinopathies) | Year: 2012–2022 Species: Human |
2.2. Inclusion and exclusion criteria
Each article was first considered by title and abstract. This systematic review included: (1) original research articles; (2) only papers written in English; (3) observational, descriptive, longitudinal, retrospective, cross-sectional, or cohort studies; (4) meta-analyzes and systematic reviews that investigated sex differences in alpha-synucleinopathies; (5) human studies. Two reviewers (KR and GD) independently screened each eligible study, and disagreements were resolved through discussion after retrieving full text to determine whether inclusion and exclusion criteria were met (see Table 2). Please also refer to PICOS statement in Table 3.
Table 2.
Search criteria.
| Exclusion criteria | Inclusion criteria | |
|---|---|---|
| Manuscript characteristics |
|
|
| Patients’ diagnosis |
|
|
| Study design |
|
|
Table 3.
The PICOS statement.
| Component of question | Example |
|---|---|
| Patient population | Alpha-synucleinopathies: Parkinson’s Disease, Dementia with Lewy Bodies, Multiple System Atrophy, REM Behavior disorder |
| Intervention | Medications, Surgical interventions |
| Control | Male and Female patients and/or healthy controls |
| Outcomes | Sex differences in PD, RBD, MSA and DLB |
| Study design | Retrospective, longitudinal, cross-sectional, observational, cohort studies, case–control studies, meta-analyzes, systematic reviews, randomized, controlled trials |
2.3. Data extraction
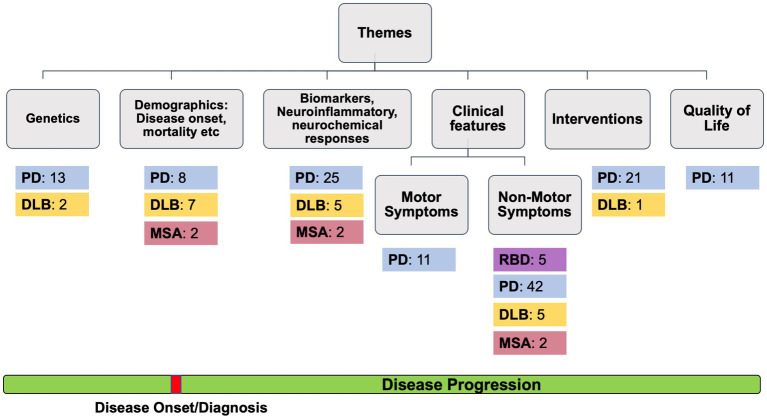
For each article, two reviewers (KR and GD) independently extracted the following data: study name and year, the country, type of study, study aim, the subtype of alpha-synucleinopathy, sample size and age of male and female patients, the methods used, main findings and critical evaluation of the study. Then, the articles were classified and grouped according to the theme of the study (i.e., genetics, demographics, clinical features, interventions, or quality of life) (see Figure 2).
Figure 2.
The number of studies found for REM Behavior Disorder (RBD), Parkinson’s Disease (PD), Dementia with Lewy Bodies (DLB) and Multiple System Atrophy (MSA).
2.4. Quality assessment
Two reviewers (KR and GD) independently evaluated the quality of studies that were included using the two quality assessment scales: (1) Quality Assessment Tool for Quantitative Studies, developed by the Effective Public Health Practice Project (EPHPP)1 for observational, descriptive, longitudinal, cross-sectional, or cohort studies original research articles (20) and (2) A Measurement Tool to Assess Systematic Reviews-2 (AMSTAR-2) for meta-analyzes and systematic reviews (21). Any disagreements were resolved by discussion or by consulting with a senior reviewer. For the EPHPP scale, the following criteria were rated for each study on a scale of strong, moderate, or weak: selection bias, study design, blinding, data collection methods, confounders, and withdrawals/attrition (if any). Subsequently, these ratings were compiled to form a global rating: studies were rated as strong if they had no weak ratings, moderate if they had one weak rating, and weak if they received two or more weak ratings. As for systematic reviews and meta-analyzes, the AMSTAR-2 is a comprehensive critical appraisal tool focusing on weaknesses in multiple domains. AMSTAR-2 assesses 16 questions, among which 7 are critical domains (21) (Questions 2, 4, 7, 9, 11, 13, and 15; See Supplementary section). Subsequent evaluation is conceptualized into three options, “Yes,” “Partial Yes,” and “No.”
3. Results
3.1. Rapid eye movement behavior disorder
Rapid Eye Movement (REM) behavior disorder (RBD) is a parasomnia characterized by abnormal behaviors during REM sleep, accompanied by the loss of REM sleep muscle atonia and dream enactment (22–24). RBD can be categorized as either idiopathic RBD (iRBD) when not ascribable to other conditions or secondary RBD (sRBD) when associated with other neurological conditions or the use of certain medications (e.g., antidepressants) (25). Importantly, iRBD has been recognized as a prodromal stage in the development of alpha-synucleinopathies such as Parkinson’s disease (PD), Dementia with Lewy Bodies (DLB) and Multiple System Atrophy (MSA) (26–28). Sex differences demonstrated in RBD studies from 2012 to 2022 are summarized in Table 4.
Table 4.
Sex differences in REM Behavior Disorder (RBD) studies from 2012 to 2022.
| Author/year country type of study | Subtype | Sample size (age at time of study unless stated otherwise) | Methods | Main findings | Critical evaluation |
|---|---|---|---|---|---|
| Clinical features: non-motor symptoms; cognition | |||||
| Takeuchi et al. (32) Tokyo, Japan Retrospective, cross-sectional study |
iRBD |
N = 220 M = 141 (66.7 ± 6.7) F = 43 (68.7 ± 7.3) |
Demographics and Clinical Assessments:
|
Clinical/Demographics:
|
|
| Castelnuovo et al. (33) Milan, Italy Retrospective, cross-sectional, clinical study |
iRBD |
N = 329 M = 280 (61.47 ± 6.66) F = 49 (64.88 ± 6.46) |
|
|
|
| Clinical features: non-motor symptoms; sleep | |||||
| Bugalho and Salavisa (38) Lisbon, Portugal Retrospective, cross-sectional, study |
iRBD sRBD |
M = 40 (71.13 ± 9.87) F = 17 (71.69 ± 10.62) IRBD: M = 18\u00B0F = 4 sRBD: PD = 23 DLB = 11 MSA = 1 M = 22, F = 13 |
REM Sleep Motor Event Assessment:
|
|
|
| Fernández-Arcos et al. (45) Barcelona, Spain Retrospective, cross-sectional, longitudinal study |
iRBD |
N = 203 M = 162 (age at diagnosis = 68.6 ± 6.1) F = 41 (age at diagnosis = 68.8 ± 6.7) |
Demographics and Clinical Assessments:
|
Clinical/Demographics:
|
|
| Zhou et al. (14) Sichuan, China Cross-sectional, clinical study |
iRBD sRBD |
N = 90 M = 63 (age at onset = 56.2 ± 14.1) F = 27 (age at onset = 45.3 ± 19.3) |
Demographics and Clinical Assessments:
|
Clinical/Demographics:
|
|
DLB, Dementia with Lewy Bodies; EMG, Electromyography; ESS, Epworth Sleepiness Scale; F, Female sample; iRBD, idiopathic RBD; M, Male sample; MMSE, Mini-Mental State Exam; MoCA-J, Japanese version of Montreal Cognitive Assessment; MSA, Multiple System Atrophy; N, Total number of sample; PD, Parkinson’s Disease; PLM index, Periodic Limb Movement index; PSG, Polysomnography, REM, Rapid Eye Movement; RBD, REM Sleep Behavior Disorder; RBDQ-JP, Japanese version of RBD Questionnaire; RBDQ-HK, Hong Kong version of RBD questionnaire; sRBD, secondary RBD; SST, Sniffin’ Sticks Test.
RBD has long been considered a male-dominant parasomnia, with more than 80% of patients being male (29–31). Additionally, women with RBD were reported to have a significantly later age onset of iRBD than men with RBD (32, 33). However, when sRBD patients were included, females make up a higher proportion of early-onset RBD patients than males (14). This latter result corroborates findings from previous studies that found a greater proportion of females in early onset RBD, as compared to the late-onset groups, predominantly due to secondary factors such as narcolepsy and antidepressant use (34–37).
Apparent sex differences in clinical presentation and polysomnography (PSG) findings have also been reported (32, 33, 38) (Table 4). In sleep architecture, sex differences in time spent in different sleep stages and electromyography (EMG) activity were found (14, 32, 33, 38). More specifically, sleep stage N1 percentage was significantly higher in males with RBD than in females with RBD (11.96 ± 7.32 vs. 9.60 ± 6.23, p = 0.047; 19.9 ± 13.1 vs. 12.1 ± 10.8, p = 0.028) [14, 33], while REM latency (132.03 ± 76.37 vs. 108.86 ± 69.99, p = 0.049) and slow wave sleep latency (9.3 ± 7.9 vs. 13.1 ± 6.0, p = 0.032) were significantly higher in females with RBD (14, 33). This could be due to the effects of female hormones on sleep architecture (39, 40), as female adults tend to engage in more deep sleep than males. It is also worth noting that slow wave sleep decreases with age, and in sRBD, younger age could explain the longer deep sleep in females with RBD (14, 40).
With regards to EMG activity, significantly higher phasic EMG activity was reported in females with RBD compared to males with RBD (p = 0.009), although no sex differences were found in the percentage of RBD patients with motor events (simple/complex) and vocalization (32). In contrast, Bugalho and Salavisa demonstrated a significantly higher phasic muscle activity index and relative number of myoclonic and trunk movements in males with RBD compared to females with RBD (p = 0.005) (38). This is supported by the fact that the periodic limb movements (PLM) index was significantly higher in males with RBD compared to females (p < 0.001) (33). However, Zhou et al. did not find any significant sex differences in phasic (p = 0.466) or tonic (p = 0.988) EMG quantification (14). These conflicting findings could be due to methodological discrepancies in stratifying for disease severity, stage of RBD and age onset.
Men with RBD were also more likely to exhibit violent and aggressive behavior (otherwise incongruous to their premorbid personality), while women with RBD experienced less dream-enacting behavior (14, 33, 45). Fernández-Arcos et al. reported that men with RBD displayed significantly more aggressive behavior [e.g., punching, assaulting bed partner, vocalizations (swearing)] and increased recall of violent, action-filled dreams, while females with RBD dreamt more about children in life-threatening situations (45). With the inclusion of sRBD patients, women with RBD also displayed significantly less dream-enacting behaviors, especially in movement-related dreams and falling out of bed (14).
Several biological and societal factors could explain the male predominance of RBD. Firstly, sex hormones (i.e., estrogen, androgens) may mediate the distinct phenotypical presentation of RBD (46, 47). Notwithstanding this, in a study conducted on men with RBD and healthy controls, no differences in serum sex hormone levels were found, suggesting that androgenic abnormalities may not account for this male predominance (46). More specifically, in this study, serum levels of total testosterone, calculated free testosterone, calculated bioavailable testosterone, luteinizing hormone, follicle stimulating hormone, estradiol-17 beta, sex-hormone binding globulin, and prolactin were not found different between male idiopathic RBD patients and healthy male controls (46). On the other hand, some evidence seems to point to the neuroprotective effect of estrogen against neurodegeneration in the nigrostriatal regions, although this remains obscure (48). Furthermore, on a more behavioral level, women with RBD tend to experience less disruptive behavior. This might make them less likely to seek medical consultation (49, 50). Additionally, RBD occurrence in females might also be underreported, predominantly due to the inadequacy of questionnaires for detecting female sleep behaviors (37).
3.2. Parkinson’s disease
Demographics, epidemiology, and prevalence: Parkinson’s Disease (PD) is the second most common neurodegenerative disorder associated with multiple neuropathological hallmarks, including neuronal loss in the substantia nigra (51). Consequently, patients with PD (PwP) typically display a range of motor and non-motor symptoms, including cognitive impairment, dementia, and motor dysfunction (52, 53) (please see Supplementary Table S1 for further details). Across prevalence, incidence, and mortality studies in PD, two trends emerged; (1) Higher incidence, prevalence, and mortality rate were consistently reported in male PwP, and (2) male-to-female incidence ratio across age groups were not constant; instead, it strikingly increases with age, and this was observed across different countries (54–61).
In a French nationwide study and meta-analysis, Moisan et al. reported that the prevalence and incidence of male-to-female ratio increased by 0.05 and 0.14 per decade, respectively, with incidence increasing over 1.6 (p < 0.001) times higher in male PwP, in age group over 80 years (59). When geographical locations are considered, Pringsheim et al. also showed a significantly higher prevalence of PD in males, particularly in Western countries and South America (60). However, when parsed by age groups, a significantly higher sex ratio PD prevalence was reported only in the younger age group 50 to 59 (PD prevalence of 41/100000 in females and 134/100000 in males; p < 0.05) (60). However, in a Norwegian study, Brakedal et al. did not observe an age-dependent change in male-to-female ratio of PD prevalence, which remained at approximately 1.5 across all age groups (54). Surprisingly, when adjusted for sex-specific mortality of the general population, mortality among female PwP was equal to or higher than mortality in male PwP (54). These findings also did not support previous mortality studies in which a higher mortality rate was consistently reported in male PwP (55, 58).
For example, an Italian mortality study conducted from 1980 to 2015 reported that male PwP have higher mortality, as compared to female PwP (Annual Mortality Rate (AMR)/100,000: 9.0 in males, 5.25 in females) (55). Similarly, PwP with dementia and male PwP had a higher mortality risk of 3.78-fold and 2.05-fold, respectively (58). Indeed, the male sex remains a significant predictor of mortality and survival predominantly due to increased disease severity in multiple domains, including cognition, postural instability, and a higher prevalence of dementia (56–58, 61).
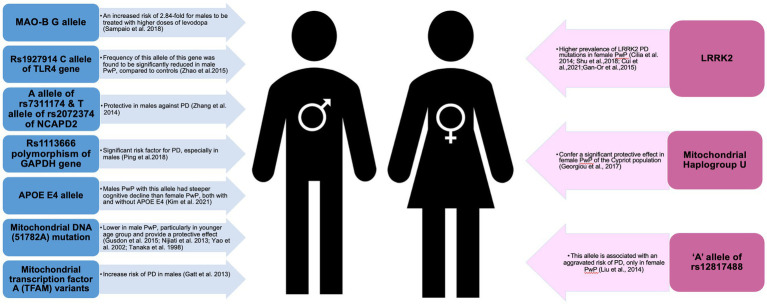
Genetics: Mutations in Leucine-Rich Repeat Kinase 2 (LRRK2) and Glucosidase Beta Acid (GBA) have often been considered the most common genetic cause of monogenic and sporadic forms of PD (62–66). Several studies have posited a higher prevalence of LRRK2 PD mutations in female PwP (67–69). In a meta-analysis that included 66 studies, Shu et al. parsed clinical heterogeneity among four LRRK2 variants in PD (G2019S, G2385R, R1628P and R1441G) and confirmed the association of female sex to G2019S. Interestingly, PwP with G2019S were more likely to have high University of Pennsylvania Smell Identification Test (UPSIT) scores (p = 0.01) and good response to levodopa (p < 0.0001) (68). Other variants of the LRRK2 mutation, such as G2385R, also displayed sex-related phenotypes differences, with male carriers of G2385R having a lower risk of cognitive impairments (p = 0.003) and female G2385R carriers displaying a lower risk of autonomic dysfunction (p = 0.04) (70). Crucially, these findings emphasize genetics’ key role in driving sex-specific phenotypical differences. Conversely, the GBA gene encodes for the lysosomal enzyme glucocerebrosidase known to maintain glycosphingolipid homeostasis (71). It has been suggested that up to 15% of PD patients may have mutations in the GBA gene, making it one of the most important genetic risk factor for PD (71). Clinically, GBA-associated PD may have an earlier age at onset, common cognitive impairment and more rapid progression (72, 73). Despite its importance, the relationship of sex and GBA mutation remains unclear to date.
Genes related to mitochondrial functions have also been identified to exhibit a sex-specific protective mechanism (74–76). For example, mitochondrial haplogroup U demonstrated a significant protective effect in female PwP of the Cypriot population (74); mutations on mitochondrial DNA (51782A) were lower in male PwP, particularly in younger age groups and provided a protective effect on longevity in Chinese Han, Uygur, and Japanese populations (76–78) while, variants of mitochondrial transcription factor A (TFAM) increase the risk of PD in males (75).
Other genes involved in immunological and inflammatory responses, estrogen regulation, dopamine modulation and chromosome condensation were similarly to affect (either direction) the pathogenesis of PD, especially in male PwP (79–82) (See Figure 3). For example, male PwP carriers of MAO-B G allele had a 2.84-fold increased risk of being treated with higher doses of levodopa (79); rs1113666 polymorphism of GAPDH gene was found to be a significant risk factor for PD, especially in male PwP (81); and A allele of rs7311174 and T allele of rs2072374 was reported to be protective in males (82). On top of this, male PwP with the APOE4 allele had steeper cognitive decline than female PwP groups, both with and without APOE4 (83), while association of rs12817488 with PD was reported only in females PwP (84), further reiterating the need to consider genes and sex differences in PD.
Figure 3.
Schematic presentation of genes implicated in male and female patients with Parkinson’s disease.
Biomarkers: Low uric acid (UA) levels have been consistently linked with an increased risk of PD and increased disease severity, particularly in male PwP (85–91). However, controversial findings were obtained when analyzes were stratified by age and estrogen levels (92). Notably, Cortese et al. showed a significant association between exposure to urate-lowering drugs in reducing PD risk in females in a higher age group (>70 years old) when there were higher UA levels premenopausally, but not in males (92). Based on these findings, there seems to be a sex-dependent predisposition of uric acid on nigrostriatal dopaminergic neurons and estrogen, which may confer beneficial neuroprotective properties in females, although further analyzes are warranted.
Another potential sex-specific biomarker for PD progression is serum homocysteine (93, 94). Elevated homocysteine levels displayed a sex-specific profiling of PD (93, 94). For instance, a positive association of elevated homocysteine with motor impairments (Unified PD Rating Scale (UPDRS)-III) in only male PwP (p < 0.001) and a negative association of elevated homocysteine with cognition only in female PwP (p = 0.021), further reiterating the distinct phenotypical sex-specific profiles of PD (93).
Metabolites and lipoproteins could also serve as sensitive biomarkers in identifying sex-specific profiles of PD (95–99). In lipid profiling studies, there is a mutual agreement on sex-specific lipid profiling and functioning in cognitive manifestations of PD (96, 97). For example, in female PwP, a positive association between hypertriglyceridemia and cognitive performance on the Frontal Assessment Battery (FAB) task was found (p = 0.013) and a negative correlation between triglyceride serum levels and cognitive performance on FAB task (p = 0.005) (96). However, in male PwP, a negative association was found between hypercholesterolemia and normal FAB performance and between high low-density lipoprotein cholesterol levels and FAB score (p = 0.027) (96), suggesting a differential functional role of lipids in sex-specific phenotype presentation of symptoms.
Biomarkers, such as alpha-synuclein, DJ-1 protein, and serum brain-derived neurotrophic factor (BDNF) levels, have also been expressed differently between sexes (100–102). Immunoenzymatic analyzes revealed lower plasma alpha-synuclein concentration levels in severe PD stages only in male PwP (100). This association is in line with more severe cognitive impairments, hallucinations, and sleep disorders, experienced by male PwP (100). Furthemore, DJ-1 protein levels was reported to be significantly higher by 1.7-fold in male PwP than male controls, suggesting a clear sex-specific biomarker of PD (101). In females, on the other hand, decreased BDNF levels were reported to be associated with females only among depressed PD patients, suggesting a sex-specific expression of biomarker and symptom profiling (102).
Sex differences in the expression of gut microbiome and immunological biomarkers have also been identified (103–105). In the first-ever metabolites profiling study using nuclear magnetic resonance (NMR), Baldini et al. analyzed 129 microbial metabolites through personalized metabolic modeling using microbiome data and genome-scale metabolic reconstructions of human gut microbes (103). The reported PD-associated microbial patterns were statistically dependent on sex, with Paraprevotella genera (a genus of bacteria) significantly influenced in female PwP (103). This was the first study to portray sex differences in the microbiome environment in PD, which supports the association of the gut-brain axis in immune response. Other analyzes of immune biomarkers in the stools of PD patients also reported a disease-related increase in numerous immune and angiogenesis mediators, only in stools of female PwP (106). This needs further research as monocyte response and phagocytic markers in PD have been reported to exhibit distinct sex-specific expression (104, 105, 107).
3.2.1. Clinical features
3.2.1.1. Motor symptoms
There is a general trend for severe motor impairment in male PwP than in females (108, 109). This is accompanied by an altered pattern of functional networks (e.g., sensorimotor networks), abnormal motor cortex measurements and lower dopaminergic binding in male PwP (110–112). In a recent study, Boccalini et al. investigated dopaminergic dysfunction according to PD-stratified clinical subtypes of motor function (i.e., mild, intermediate, or diffuse-malignant) in de novo PD patients using the Parkinson’s Progression Markers Initiative (PPMI) database (108). In mild motor and intermediate subtypes, they found that male PwP exhibited poorer cognitive performance than females, and those with motor impairments had lower dopamine binding in the putamen with more severe widespread connectivity alterations in the nigrostriatal dopaminergic neurons than female PwP (108). This dysfunction was also observed on a behavioral level (113, 114). For instance, in a 5-year longitudinal study, Picillo et al. reported that male PwP experienced a significantly higher longitudinal decline in self-reported motor symptoms, with a yearly increase in UPDRS-II by 0.57 relative to females (1.27 vs. 0.7, p < 0.001) (113).
Nonetheless, the findings of several studies suggest a more complex relationship between female hormones and motor symptoms in PD (115). For instance, in a study on female PwP, younger age of onset and higher Hoehn and Yahr (H&Y) stage were identified as risk factors of wearing off phenomenon, while younger onset age was associated with dyskinesia (115). Moreover, female PwP with wearing-off phenomenon and dyskinesia were shown to have higher levels of prolactin (115). It has been hypothesized that in some patients age onset and disease severity might override the neuroprotective benefits of female hormones.
Furthermore, motor symptoms tend to emerge later in female PwP and display a sex-specific phenotypical motor presentation (116, 117). Female PwP were more likely to experience reduced rigidity (116), tremor (117), and levodopa-induced dyskinesias (115, 118), while male PwP were reported to be more susceptible to later development of freezing of gait (119), and camptocormia (abnormal severe forward flexion of the trunk) (120).
3.2.1.2. Non-motor symptoms
Non-motor symptoms (NMS) consist of a wide range of symptomology spectrum and severity, such as cognitive deficits, sexual and urinary dysfunction, sleep, mood disorders and psychosis and odor discrimination (8, 53, 72, 113, 121–150). Despite methodological differences due to different screening tools being adopted, two trends emerged, (1) male PwP were more likely to experience severe non-motor symptoms, particularly in cognition, olfaction, sleep, speech problems, impulse control disorders (i.e., pathological gambling and hypersexuality), dementia, urinary and sexual dysfunction (113, 121, 125–127, 129, 131, 132, 136, 138–144, 151, 152) (2) female PwP were more likely to experience fatigue, higher pain levels, and psychosis and mood disorders (i.e., depression (153), anxiety) and impulse control disorders (i.e., binge eating and compulsive buying) (131–133, 139, 145–147, 151, 154–156).
The correlates of cognitive sex differences in healthy, neurotypical people remain poorly understood (157). It is thought that many biological and psychosocial factors act to modulate these cognitive abilities leading to mixed results in the scientific literature (157). Nonetheless, numerous studies have suggested that male sex may be a dominating risk factor for dementia and cognitive impairment (53, 121, 126, 142–144). In keeping, male PwP have been shown to develop a more rapid and severe cognitive decline by comparison to female PwP (53, 72, 113, 121). In a recent 5-year longitudinal study in de novo PD population, male PwP experienced a steeper decline in both motor (p = 0.009) and non-motor (p = 0.009) symptoms, with a yearly increase in self-assessed UPDRS I by a multiplicative factor of 0.98, as compared to 0.67 in female PwP (113). Sex differences were also noted in differential phenotypes of deficits in executive functioning (53, 121, 122, 126, 148, 149). Both healthy males and male PwP groups performed significantly worse than females in semantic verbal fluency and delayed recall, while healthy females and female PwP groups performed worse in visuospatial function (126).
Clear sex differences in sleep have also been reported (127, 128, 130). Male PwP were more likely to experience increased daytime sleepiness, higher motor impairment and lower mini-mental score in tandem with abnormal sleep-related motor-behavioral episodes (127, 128, 130). In line with this, RBD and PD studies have also shown that male PwP have a higher prevalence of RBD and display greater global cortical and subcortical gray matter atrophy even when compared with females in PD-RBD group (125, 126, 143, 158). This suggests distinct sex-specific heterogenous profiling of RBD and other sleep parameters in PD.
Across studies using different cohorts’ groups, male PwP consistently presented with more prominent sexual and urinary dysfunction than females (8, 131, 150). For instance, Martinez-Martin et al. reported a lower prevalence of sexual dysfunction in female PwP (~28%) as compared to males (~50%). This could be due to distinct biological features between sexes (8). The autonomic nervous system itself is sexually dimorphic with differences in urinary tracts (159, 160), brain anatomy (161, 162), and genital system (163).
Female PwP, on the other hand, have been reported to have a higher prevalence of mood disorders such as anxiety, depression and apathy, as well as to have a heightened experience of fatigue and pain (130–133, 139, 145–147, 154, 155). Zhu et al. reported higher scores on the Hamilton Rating Scale for Depression (HAMD) domains of anxiety/somatization, and hopelessness in female PwP (154), perhaps indicative of the functional role of estrogen in mood regulation (164). Specifically, affective regulation has been linked to neural structures rich in estrogen receptors and estrogenic regulation of neurotransmitters. Interestingly, even in healthy women, studies have reported a higher incidence of depression (165, 166) and anxiety (167) during peri/menopause – a period of drastic reduction in estrogen levels, which have been reported to coincide with the onset of PD (168, 169). Conversely, it has been shown that hormone therapy may prevent mood disorders during this period, and while the exact mechanism remains unknown, there is compelling evidence that supports neuromodulatory and neuroprotective effects of estrogen, which are directly relevant to mood symptomatology (164). In future, it would be important to elucidate the nature of postmenopausal exogenous hormone formulations in relation to premenopausal endogenous levels, as well as the ratio of estrone to estradiol, all of which warrants urgent consideration to address these debilitating non-motor symptoms in female PwP during the peri/menopause (164).
Moreover, impulse control disorders in PD, described as aberrant behaviors such as pathological gambling, hypersexuality, binge eating, and compulsive buying, which typically occur as a result of dopaminergic therapy, have all variably been shown to sport variable phenotypic sex-related expressions, e.g., with pathological gambling and hypersexuality more prevalent in men, whereas binge eating and compulsive buying occur more frequently in women (170). In that background, and given that specific impulse control disorders share clinical, phenomenological and biological features with obsessive–compulsive disorder (171), it is of note that sexually dimorphic pattern of genetic susceptibility to OCD’s clinical heterogeneity has been recently demonstrated, potentially requiring different specific therapeutic strategies (172). Further research is warranted to validate sex as one of the important determinants of the heterogeneity of impulse control disorders in PwP.
Pain is also more frequently reported in female PwP (133, 146, 173, 174). The mechanisms underlying this, and other mood phenomena, remain unclear. Arguably, however, they may reflect differential effect of the alpha-synucleinopathy process on distinct pain/mood centers in the female brain. For instance, one of the neuroanatomical candidates may be the dysfunction of the circuitry involving the posterior bed nucleus of the stria terminalis (BNST). The BNST is the center of the psychogenic circuit from the hippocampus to the paraventricular nucleus, this circuit is important in the stimulation of the hypothalamic–pituitary–adrenal axis, and its dysregulation may lead to mood, pain and anxiety disorders, social dysfunction and psychological trauma (175). It is known that oestradiol exerts its effects in the canonical pathway through the transcription factor estrogen receptor-α, the neuronal targets of which include the BNST for a review see (176). The BNST, is a sexually dimorphic structure, commonly approximately 1.5–2 times larger in men, compared to women (176). Of note, atrophy of the BNST has been demonstrated in de novo PD (177), possibly suggesting that in women, who have smaller BNST, any such neurodegenerative process may have proportionally larger negative impact on affective processing of pain.
3.2.2. Interventions
3.2.2.1. Pharmacological
One commonly used first-line PD treatment is levodopa (178). Several patterns were observed in levodopa pharmacokinetics and treatment outcome between sexes (79, 173, 178–180). Female PwP were more susceptible (“brittle response”) to levodopa-induced dyskinesia and wearing-off phenomenon (115, 118, 173, 181, 182). Studies into intra-and inter-individual variability in levodopa’s pharmacokinetics (PK) reported sex-specific treatment responses (178). Conti et al. measured plasma levodopa concentrations and pharmacokinetic parameters (Area under curve (AUC), Maximum plasma concentration (Cmax), time to reach Cmax (Tmax), half-life (t1/2)) in levodopa-naïve and levodopa-treated PD patients (178). Interestingly, AUC and Cmax were significantly higher in female PwP than in males, with body mass index (BMI) significantly predicting t1/2 only in female PwP (p = 0.027) (178). It is worth noting that in this study, female PwP had a longer duration of disease (59 ± 24.5 months) than male PwP (34 ± 28.5 months).
UA-level modification may also offer a tailored sex-specific PD treatment (183). Previous studies consistently reported the association of lower serum UA with higher disease severity, particularly in male PwP (85–88). This sex-specific profiling of UA also extends to urate-altering drugs (183). Schwarzschild et al. conducted a randomized, double-blinded clinical trial of the Safety Urate Elevation in PD (SURE-PD) trial and found that inosine elicited higher levels of serum urate that were 50% greater in female PwP (3.0 mg/dL) than in male PwP (2.0 mg/dL). CSF urate was also significantly higher on mild (+87%, p < 0.001) or moderate (+98%, p < 0.001) inosine than placebo, only in female PwP (183). Regarding motor severity, slower UPDRS progression was related to an increase in serum urate (p = 0.001) and plasma antioxidant capacity (p = 0.006). No relationship was found in male PwP, suggesting a protective effect of underlying female sex steroids interplay with urate (183).
Targeting a non-dopaminergic system may be effective in ameliorating motor and non-motor fluctuations that arise when on levodopa (184). One such treatment is safinamide (185). Safinamide acts on the reversible inhibition of the monoamine oxidase-B (MAO-B) enzyme and modulation of excessive glutamate release (186). In a recent study on the efficacy of safinamide on PwP, Pellechia et al. reported improvements in the total UPDRS score were 43.5% in males versus 39.1% in female PwP (185), further providing support for sex-specific treatment response in PD.
Surgical: Deep brain stimulation (DBS), a neurosurgical procedure that involves electrical stimulation of the global pallidus internus (GPi) or subthalamic nucleus (STN), is an alternative treatment for PD, particularly in advanced PD (187). In terms of sex disparities and treatment outcomes, three trends emerged: (1) sex disparities in DBS selection, particularly in the undertreatment, referral and follow-ups of female PwP, (2) similar surgical outcomes postoperatively after DBS between sexes, although males were more likely to display lasting improvements and (3) quality of life postoperatively depend on sex-specific symptoms phenotype (187–193).
Gender-specific disparities in treatment accessibility and patients’ behavioral approach to mitigating PD symptoms are a primary concern, particularly for healthcare professionals (187, 190). In a cross-sectional, pseudo-randomized study in the United Kingdom, female PwP were disproportionally underrepresented in referral compared to the general PD population (p = 0.002), although they were more likely to be approved for DBS than males (p = 0.029) (187). Furthermore, female PwP were less likely to undergo DBS due to their preference (p < 0.001), while male PwP were more likely to be lost to follow-up (p = 0.046) (190). In terms of behavioral approach, female PwP were more likely to express strong fear of complications and were more likely to consult with immediate family members prior to deciding on DBS (194).
Although there was no sex differences in postsurgical outcomes improvements right after DBS (187, 190, 195), in subsequent follow-ups, female PwP showed a trend toward worsening in bradykinesia after 1 year and a lower score in non-dopaminergic features after 10 years (196). Furthermore, a recent study has also identified male sex as a significant predictor of DBS-induced improvement in camptocormia and global postural angle (193). Despite that, interestingly, in a mortality study assessing PwP treated with DBS, only male sex and disease duration were significant predictors of mortality (197).
Another controversial aspect of gender disparities after DBS is the quality of life in PwP (198, 199). While the long-term effect and short-term effect of DBS are similar in cognitive function and depressive symptoms, at 5-year follow-up post-DBS, physical quality of life is significantly more improved only in male PwP (p < 0.001) but not in female PwP (p = 0.409) (198). Despite that, there are also reports that suggest that female PwP experience greater improvements in activities of daily life (ADL) and positive effects on mobility, stigma and cognition than males (199).
3.2.2.2. Quality of life
Despite the higher prevalence and disease severity in male PwP, there seems to be a trend of lower quality of life in multiple aspects of female PwP (156, 174, 200–206). This could be attributable to several gender, societal factors and the nature of clinical manifestation that contribute to lower quality of life in female PwP (174, 200, 207). In an Israeli study, lower quality of life in female PwP was attributable to the higher prevalence of depression and pain, while male PwP’s quality of life only worsened in advanced stages (174, 208). These findings align with studies conducted worldwide, in which severe anxiety, lower nutritional status, lower emotional well-being, higher stigma, and psychosocial functioning were the most robust features of poorer quality of life in female PwP (201–203, 205, 207). This suggests that societal expectations of gender role factors are crucial in disease management and interventions in PD.
Furthermore, other environmental factors such as living conditions and visitation/seeking-care behavior could also account for lower quality of life in female PwP (201, 209). Female PwP were more likely to live alone (18% had no caregivers, compared to 2.4% of males) (201). Even if they utilized care services, female PwP were more likely to use home health and nursing facility care more often. They had less outpatient physician contact than male PwP throughout PD (204). For effective delivery of treatment, these societal expectations and gender patterns of seeking help should be considered by clinicians.
3.3. Dementia with Lewy Bodies
Dementia with Lewy Bodies (DLB) is the second most common neurodegenerative dementia among the elderly (210). Core clinical features of DLB include neuropsychiatric symptoms (i.e., visual/auditory hallucinations), parkinsonism, and cognitive impairments (i.e., deficits in memory and executive functions) (211). On a pathological level, DLB is characterized by the presence of Lewy bodies (i.e., neuronal inclusions of alpha-synuclein) with differing degrees of co-existing Alzheimer’s disease (AD)-related pathology (i.e., amyloid plaques and neurofibrillary tangles (NFT)) (212, 213). In addition, it has been suggested that inflammation may also play an important role in DLB, for instance PET imaging and blood biomarkers support an increase in cerebral and peripheral inflammation in the early phases of DLB, while these features appear reduced with disease progression (214, 215). Numerous studies have reported a greater male predominance in the incidence, prevalence, and mortality, although these findings are inconsistent (216–221) (please see Table 5).
Table 5.
Sex differences in Dementia with Lewy Bodies (DLB) studies from 2012 to 2022.
| Author/year country type of study | Subtype | Sample size | Methods | Main findings | Critical evaluation |
|---|---|---|---|---|---|
| Disease diagnosis: epidemiology, prevalence, demographics, survival rate | |||||
| Mouton et al. (216) French National Alzheimer Database A repeated, cross-sectional study |
DLB AD PD PDD |
DLB: N = 10,309 (80.11 ± 7.84) M = 4,674\u00B0F = 5,635 AD: N = 135,664 (81.42 ± 7.98) M = 40,566\u00B0F = 95,098 PDD: N = 3,198 (79.45 ± 8.09) M = 1746\u00B0F = 1,452 PD: N = 8,744 (73.86 ± 10.79) M = 4,979\u00B0F = 3,765 |
Demographics and Clinical Assessments:
|
|
|
| Gan et al. (217) Beijing, Tianjin, China Retrospective, clinical study |
DLB PDD |
DLB & PDD: N = 455 M = 239 (age onset = 69.2 ± 8.1) F = 216 (age onset = 68 ± 8.8) |
Clinical Assessments:
|
|
|
| Savica et al. (218) Minnesota, USA Epidemiologic study |
DLB PDD |
DLB: N = 64 PDD: N = 46 |
|
|
|
| Price et al. (219) Cambridge, United Kingdom Retrospective study |
DLB AD |
DLB: N = 251 (age at diagnosis = 79.3 ± 7.6) M = 122\u00B0F = 129 AD: N = 222 (age at diagnosis = 80.2 ± 8.8) M = 83\u00B0F = 139 |
|
|
|
| Boot et al. (220) Rochester, USA Retrospective study |
DLB AD |
DLB: N = 147 (age at diagnosis = 72.5 ± 7.3) M = 113\u00B0F = 34 AD: N = 236 (age at diagnosis = 74.9 ± 10.1) M = 90\u00B0F = 146 Controls: N = 294 M = 226\u00B0F = 68 |
|
|
|
| Abdelnour et al. (234) European DLB (E-DLB) Consortium A multicentre, international study |
DLB |
N = 107 (68 ± 8.7) M = 77\u00B0F = 30 |
Clinical, neuroimaging and CSF assessments:
|
|
|
| Jones and O’Brian (221) Newcastle, Cambridge, United Kingdom Systematic review |
DLB | Total of 31 studies included in this review |
|
|
|
| Genetics | |||||
| Gámez-Valero et al. (228) Barcelona, Spain Post-mortem, clinical cohort study |
DLB | Post-mortem: DLB = 50 PD = 43 Controls = 34 Clinical cohort: DLB = 47 (75.8) Controls = 131 (72.3) |
|
|
|
| Liu et al. (229) Jilin, China Meta-analysis |
DLB | Total of 14 studies included in this review |
|
|
|
| Clinical Features: Non-motor Symptoms | |||||
| Utsumi et al. (13) Hokkaido, Japan Retrospective, clinical study |
Probable DLB |
N = 234 (age at diagnosis = 79 ± 7.5) M = 101 (age at diagnosis = 78.6 ± 6.7) F = 133 (age at diagnosis = 79.2 ± 8) |
Initial symptoms assessment by an interview with patients and caregivers in nine initial symptoms:
|
Initial symptoms findings:
|
|
| Chiu et al. (231) Taiwan, China Cross-sectional, longitudinal clinical study |
DLB |
N = 152 M = 87\u00B0F = 65 |
Demographics and Clinical Assessments:
|
|
|
| Tsunoda et al. (230) Kumamoto, Japan Cross-sectional, retrospective study |
DLB |
N = 124 (78.3 ± 5.6) M = 54\u00B0F = 70 |
Screening/Assessment:
|
|
|
| Bayram et al. (233) Data obtained from the NACC Neuropathology Data Set, Genetic Data, and Uniform Data Set (UDS) Case-controlled retrospective study |
Pathological confirmed DLB |
N = 211 M = 156 (age at last visit = 75.9 ± 8.4) F = 55 (age at last visit = 80 ± 8.7) |
Before death:
LB pathology staging
|
|
|
| Symptomology: non-motor symptoms; sleep | |||||
| Choudhury et al. (232) Minnesota, USA Longitudinal clinical study at the Mayo Clinic Alzheimer’s Disease Research Center (ADRC) |
DLB |
N = 488 (age at first visit = 73) M = 370 (age at first visit = 72) F = 118 (age at first visit = 75) |
Clinical assessments:
|
|
|
| Mechanisms: inflammatory responses, brain structures etc. | |||||
| Van de Beek et al. (236) Amsterdam, Netherlands; Amsterdam Dementia Cohort Retrospective, clinical study |
DLB |
N = 223 M = 184 (67.7 ± 7.3) F = 39 (70.1 ± 6) |
Clinical and cognitive features:
|
|
|
| Ferreira et al. (227) Multicentre cohort (Combination of E-DLB and the Mayo Clinic DLB Cohort) Prospective study |
DLB |
N = 417 M = 287 (70.2 ± 8.6) F = 129 (72.5 ± 8.2) |
Demographics and clinical assessments:
|
|
|
| Bayram et al. (226) NACC Uniform Data Set (UDS) Retrospective study |
DLB |
N = 691 M = 468 (Age at last visit = 76.4 ± 8.9) F = 223 (Age at last visit = 79.9 ± 10) |
Clinical and neuropathological assessments:
|
|
|
| Sarro et al. (237) NACC; Rochester, USA Retrospective study |
DLB AD Dementia |
DLB: N = 81 (Age at MRI = 72 ± 8) M = 67\u00B0F = 14 AD Dementia: N = 240 (Age at MRI = 75 ± 10) M = 135\u00B0F = 105 |
Clinical and neuropathological assessments:
|
|
|
| Wennström et al. (238) Malmö, Sweden Retrospective study |
DLB AD |
DLB: N = 18 (74 ± 7) AD: N = 26 (73 ± 6) Non-demented controls: N = 24 (72 ± 8) |
Clinical assessments and CSF profile:
|
|
|
| Interventions: pharmacological | |||||
| Agbomi et al. (235) South Carolina, USA: PRISMA Health Registry Retrospective study |
DLB PDD |
DLB: N = 608 M = 332 (75.93 ± 9.18) F = 276 (81.74 ± 9.24) PDD: N = 7,594 |
From PRISMA Health registry:
|
|
|
AD, Alzheimer’s Disease; CASI, Cognitive Abilities Screening Instrument; ChEIs, Central Acetylcholinesterase inhibitors; CDR, Clinical Dementia Rating; CSF, Cerebrospinal Fluid; DAT, Dopamine Active Transporter; DLB, Dementia with Lewy Bodies; DRS, Mattis Dementia Rating Scale; F, Females; GDS, Geriatric Depression Scale; GLDS, Global Deterioration Scale; LB, Lewy Body; M, Males; MMSE, Mini Mental State Examination; MoCA, Montreal Cognitive Assessment; MRI, Magnetic Reasonance Imaging; N, Total Sample Size; NPI, Neuropsychiatric Inventory; PET, Positron Emission Tomography; PD, Parkinson’s Disease; PDD, Parkinson’s Disease Dementia; PSG, Polysomnography; RBD, Rapid Eye Movement Behavior Disorder; RBDSQ, REM Behavior Sleep Disorder questionnaire; SGAs, Second Generation Antipsychotics; SSRIs, Selective Serotonin Reuptake Inhibitors; TMT, Trail Making Test; UPDRS, Unified Parkinson’s Disease Rating Scale; VHs, Visual Hallucinations.
In a retrospective study on Parkinson’s Disease Dementia (PDD) and DLB in China, DLB was found to be more common in women in the age group 60 to 69 years but more balanced in younger age groups (217). In contrast, for age groups older than 70 years, males have a greater prevalence of DLB than females (217). Further severity-stratified analyzes revealed that males were more likely to visit their physician when experiencing mild symptoms in both PDD (63.6%) and DLB (56.9%), while females were more likely to visit only when experiencing moderate to severe symptoms levels (217), reiterating the need for more focus on early stages of DLB in females.
Other studies on sex distribution in DLB show inconsistent findings of DLB incidence between sexes (216, 221). In a cross-sectional study of DLB, AD, PD, and PDD, Mouton et al. reported a slight predominance of females with DLB, particularly in those older than 75 years and the sex ratio with a preference for females increased with age (216). These inconsistencies in sex distribution findings of DLB could be due to three reasons. Firstly, most DLB diagnoses were made by clinical judgments rather than pathological results. Nelson et al. posited that clinically suspected DLB was more likely to be over-diagnosed in females, which might explain this variation in the prevalence of DLB in different studies (222). Secondly, DLB shares similarities in pathological and clinical characteristics with AD, which may result in a higher proportion of females being diagnosed as AD is predominantly associated with female sex (223–225). For instance, a recent study reported that females with DLB had a higher Braak tau staging and less nigrostriatal loss than males with DLB, despite having similar Lewy body staging with males with DLB (226). Thirdly, there is also a genetic component to DLB (227, 228). For example, a clinical cohort study reported the association of GBA mutations with early onset DLB and male sex, although these findings have been somewhat inconsistent (228, 229).
Sex differences have also been reported in the initial symptoms of DLB diagnosis (13). In the initial stage of clinical manifestations, females with DLB exhibited a significantly higher overall rate of psychiatric symptoms (p = 0.009), particularly in auditory hallucinations (AHs) (p = 0.012), while males with DLB had a higher incidence of RBD (p < 0.001) (13). These findings align with Tsunoda et al., in which AHs were significantly associated with female sex (p = 0.04) (230).
Visual hallucinations have also been reported in DLB, with different symptomatology profiling between sexes (231–233). Cumulative and 1-month frequency analyzes of visual hallucinations of DLB patients found that the contents of visual hallucinations frequencies of non-family people, passed families, and nonchildren families were significantly higher (231), and earlier in women with DLB than men (232). Additionally, both sexes had distinct predisposing factors associated with visual hallucinations (231). More specifically, older age (p = 0.003) and higher neuropsychiatric inventory (NPI) score (p = 0.009) were associated with women with DLB, while severe dementia stage (p = 0.008) and higher rates of antipsychotics (p < 0.047) were associated with men with DLB (231). Furthermore, in a factorial analysis using the European DLB consortium, Abdelnour et al. parsed DLB clinical presentations into four subtypes and reported a greater predominance of females with DLB with characteristics such as higher MMSE scores, cognitive fluctuations and cerebrovascular pathology (234). This could indicate a distinct phenotype of DLB between sexes and age groups, although this remains elusive (233, 234).
Understanding sex differences also have significant implications in identifying biomarkers, neuropathology and evaluating the efficacy of pharmacological interventions in DLB (226, 227, 235–238). Lower cerebrospinal fluid (CSF) alpha synuclein and CSF amyloid levels were reported in women with DLB, accompanied by distinct sex-specific characteristics, such as more frequent hallucination and lower scores on a cognitive task (236). This aligns with previous study by Wennstrom et al. who reported lower levels of CSF alpha synuclein and CSF orexin concentration, particularly in women with DLB, as compared to AD and controls (238). In other brain biomarkers, females with DLB have also been associated with greater white-matter hyperintensities (WMHs), further reiterating sex-specific biomarker profiles in DLB (237). Finally, in a recent study on medication use history, a differential preference of medications between sexes was reported, with second-generation antipsychotics such as risperidone associated with females with DLB, while olanzapine, escitalopram and tobacco use were associated with males with DLB (235). The pathomechanism behind this diversity is currently unclear.
3.4. Multiple system atrophy
Multiple system atrophy (MSA) is an uncommon progressive neurodegenerative disorder characterized by autonomic failure and motor involvement of parkinsonism (MSA-P) or cerebellar ataxia (MSA-C) (11, 239). Autonomic failure in MSA includes orthostatic hypotension, constipation, and sexual and urinary dysfunction (239). In MSA, an astrocytic and microglial activation, along with a significant change in the expression of a subset of inflammation-associated genes, have all been reported in the MSA brain, suggesting that targeting inflammation-related processes might limit the disease progression (240, 241). Sex differences in MSA have been reported in many studies focusing on gender distribution, survival, and clinical features studies (11, 242, 243) (as summarized in Table 6). MSA is known to be more prevalent in men (11, 244), however, other studies focusing on sex-differences report oppositional findings. For instance, some studies quote longer survival in women (11, 242), others report longer survival in men (243, 245, 246) or no differences between sexes (247–253).
Table 6.
Sex differences in Multiple System Atrophy (MSA) from 2012 to 2022.
| Author/year country type of study | Subtype | Sample size | Methods | Main findings | Critical evaluation |
|---|---|---|---|---|---|
| Demographics, clinical features, and mortality | |||||
| Coon et al. (11) Minnesota, USA Retrospective clinical study |
MSA-P MSA-C |
N = 685 M = 356 (age onset = 60.6 ± 9.8 F = 329 (61.3 ± 9.3) Alive = 100 M = 52\u00B0F = 48 |
|
There are sex and gender differences in MSA in terms of symptoms onset, presentation, and survival:
|
|
| Coon et al. (253) Minnesota, USA Retrospective, clinical study |
MSA-P MSA-C |
N = 685 (60.9 ± 9.6) M = 355\u00B0F = 330 |
Demographics and clinical assessments:
|
|
|
| Clinical Features: Non-motor Symptoms; Cognition | |||||
| Cuoco et al. (258) Salerno, Italy Case-controlled prospective, longitudinal clinical study |
MSA-P MSA-C |
Start: N = 55 M = 29 (61.79 ± 8.43) F = 26 (62.57 ± 7.51) After one year: N = 26/55 Attrition = 29/55 (10 died (4 M, 6F), 19 were unable to return due to worsening of disease (11 M, 8F) |
Neuropsychological and neuropsychiatry battery at the start and after one-year follow-up:
|
At baseline:
|
|
| Clinical Features: Non-motor symptoms; Others | |||||
| Yamamoto et al. (12) Chiba, Japan Retrospective, clinical study |
MSA-P MSA-C MSA-mixed |
N = 66 (62.2) M = 39\u00B0F = 27 |
Patients responded to a urinary symptoms questionnaire and underwent urodynamic examination twice:
|
|
|
| Mechanisms: Biomarkers, neurochemical or Inflammatory responses | |||||
| Chen et al. (259) Guangzhou, China Cross-sectional clinical study |
MSA-P | MSA: N = 47 (58.74 ± 10.18) M = 31\u00B0F = 16 Controls: N = 50 |
Clinical Assessments:
|
|
|
| Cao et al. (260) Sichuan, China Clinical, longitudinal study |
MSA-C MSA-P |
MSA: N = 234 M = 121\u00B0F = 113 Controls: N = 240 Follow-up (longitudinal): N = 107 M = 56\u00B0F = 51 |
|
|
|
ADL, Activities of Daily Living; BDI-II, Beck Depression Inventory-II; BJLO, Benton’s Judgment of Line Orientation; BMI, Body Mass Index; CRP, C-reactive protein; EMG, Electromyography; F, Females; Hcy, Homocysteine; H&Y, Hoehn and Yahr Scale; M, Males; MMSE, Mini Mental State Examination; MoCA, Montreal Cognitive Assessment; MRI, Magnetic Resonance Imaging; MSA, Multiple System Atrophy; N, Total Sample Size; NMS, Non-Motor Scale; PDSS, Parkinson’s Disease Sleep Scale; SVF, Semantic Verbal Fluency Test; TMT, Trail Making Test; UA, Uric Acid; UMSARS, Unified Multiple System Atrophy Rating Scale.
Several differences have been similarly reported regarding clinical presentation at disease onset (11). Women with MSA are more likely to have motor symptoms at onset, while men are more likely to experience severe autonomic symptoms (11). Men with MSA are also more likely to have orthostatic intolerance (p = 0.0156) and early catheterization (p = 0.0396), which may contribute to worse survival rates (11, 247, 251, 254). The distinct symptoms at onset may prompt women to seek an earlier referral to a neurologist, which could explain an earlier diagnosis of MSA in women (11). Women with MSA were also less likely to experience severe urinary and sexual dysfunction (11, 12, 255). However, there is also a possibility that these autonomic symptoms, such as urinary and sexual dysfunction, are underdiagnosed in women. For example, sexual dysfunction was addressed differently, with a significantly higher number of male patients with documented sexual dysfunction (p = 0.0001) than female patients (11). This could be due to a lack of appropriate scales for measuring sexual and urinary dysfunction in women (256, 257).
In line with other subtypes of alpha-synucleinopathies, sex differences in other non-motor symptoms and biomarkers in MSA also displayed a distinct sex-specific phenotype (258, 259). In cognitive abilities of MSA patients, Cuoco et al. demonstrated that at the start of the study, women with MSA had significantly lower performance on global cognitive abilities, language, visuospatial ability, and attention (258). Additionally, at follow-up, women with MSA deteriorated more than men with MSA, particularly in motor functions and their attention abilities, and they had higher prevalence of depression (258). Mirroring this, elevated serum homocysteine levels and lower UA levels have also been reported MSA patients, particularly in males (259, 260). Furthermore, these markers are positively correlated with the severity of MSA, such as movement dysfunction and declined cognition (259). This further corroborates the notion of sex-specific profiling in alpha-synucleinopathies.
4. Discussion
We have critically analyzed a body of work to date that investigated sex and gender differences in alpha-synucleinopathies. Our findings simultaneously demonstrate (1) a scarcity of studies that systematically focused on sex and gender differences, and (2) clear phenotypical differences in multiple aspects of alpha-synucleinopathies, solely driven by sex and gender differences. In addition, very little appears to be known about the specific interplay of various sex hormones in humans. Moreover, past clinical studies predominantly focus on the role of estrogen, and its potential protective role against the process of alpha-synucleinopathy, the argument for this is somewhat supported by higher incidence of PD in peri/and menopausal period (168, 169, 261). In preclinical studies, oestradiol and progesterone manipulation in ovariectomised, or gonadectomised mice, has demonstrated distinct sex differences in multiple aspects of alpha-synucleinopathy process (17, 262–267). Importantly, several of these animal models suggest that estrogen deprivation may results in dopaminergic neuron loss and lower dopaminergic binding (268).
Clinical studies on estrogen replacement therapy demonstrate a clear role for the estrogen in improving motor symptoms in post-menopausal women (269, 270). Nevertheless, many pieces of this pathomechanistic puzzle are missing; we are yet to clarify the importance of endogenous versus exogenous estrogen exposure, the causality of estrogen effects on multiple aspects of a disease (i.e., genetics) and the interplay between hormonal changes and the progression of alpha-synucleinopathies. Moreover, the threshold, the time-window (e.g., perimenopause versus postmenopause), and all other potentially modifying factors, to which estrogen confer a neuroprotective effect, remain unknown.
Similarly, there are several methodological caveats that should be considered while evaluating preclinical and clinical studies, all of which are rarely systematically considered in their translational importance. For example, in majority, if not in all, analyzed clinical and preclinical studies, there is a lack of focus on the synergistic and antagonistic effects of different sex hormones on various aspects of alpha-synucleinopathies. Most studies predominantly focus on one specific hormone (i.e., estrogen/progesterone), which makes it impossible to fully understand the pathomechanistic complexity. Additionally, even in clinical studies that included multiple hormone measures, women were frequently excluded (46, 47). Moreover, the stage of their menstrual cycle (e.g., follicular versus luteal stage, or and other endocrinology measures was rarely reported).
In conclusion, there is urgent need for future prospective multi-center studies that will account for a more integrated, representative account of sex differences in alpha-synucleinopathies. We suggest that the ideal research framework should systematically account for (1) a specific subtype and distinct phenotype of alpha-synucleinopathies (2) ethnicity and geographical location, (3) disease progression, rate and severity (i.e., early versus late onset), (4) monitoring menstrual cycle and endocrinology health in women, (5) direct quantification of sex hormones in both sexes, (6) medication history and responses (i.e., hormones replacement therapy) and (7) consideration of societal, cultural and gender factors that could impact treatment of PD.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
KR selected and reviewed. KR and GD assessed all the eligible studies. All authors contributed to the article and approved the submitted version.
Funding
This research was funded in whole, or in part, by the Wellcome Trust [103952/Z/14/Z]. For the purpose of open access, the author IR has applied a CC BY public copyright license to any Author Accepted Manuscript version arising from this submission.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2023.1204104/full#supplementary-material
References
- 1.Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M. Alpha-synuclein in Lewy bodies. Nature. (1997) 388:839–40. doi: 10.1038/42166, PMID: [DOI] [PubMed] [Google Scholar]
- 2.Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, et al. Mutation in the alpha-synuclein gene identified in families with Parkinson's disease. Science. (1997) 276:2045–7. doi: 10.1126/science.276.5321.2045, PMID: [DOI] [PubMed] [Google Scholar]
- 3.Calabresi P, Mechelli A, Natale G, Volpicelli-Daley L, Di Lazzaro G, Ghiglieri V. Alpha-synuclein in Parkinson's disease and other synucleinopathies: from overt neurodegeneration back to early synaptic dysfunction. Cell Death Dis. (2023) 14:176. doi: 10.1038/s41419-023-05672-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McCann H, Stevens CH, Cartwright H, Halliday GM. α-Synucleinopathy phenotypes. Parkinsonism Relat Disord. (2014) 20:S62–7. doi: 10.1016/S1353-8020(13)70017-8 [DOI] [PubMed] [Google Scholar]
- 5.Malfertheiner K, Stefanova N, Heras-Garvin A. The concept of alpha-Synuclein strains and how different conformations may explain distinct neurodegenerative disorders. Front Neurol. (2021) 12:737195. doi: 10.3389/fneur.2021.737195, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Voskuhl R, Itoh Y. The X factor in neurodegeneration. J Exp Med. (2022) 219:e20211488. doi: 10.1084/jem.20211488, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Voskuhl RR, Gold SM. Sex-related factors in multiple sclerosis susceptibility and progression. Nat Rev Neurol. (2012) 8:255–63. doi: 10.1038/nrneurol.2012.43, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martinez-Martin P, Falup Pecurariu C, Odin P, Van Hilten JJ, Antonini A, Rojo-Abuin JM, et al. Gender-related differences in the burden of non-motor symptoms in Parkinson’s disease. J Neurol. (2012) 259:1639–47. doi: 10.1007/s00415-011-6392-3, PMID: [DOI] [PubMed] [Google Scholar]
- 9.Pardue ML, Wizemann TM. Exploring the biological contributions to human health: Does sex matter? Washington, DC: National Academies Press; (2001). [PubMed] [Google Scholar]
- 10.Picillo M, Nicoletti A, Fetoni V, Garavaglia B, Barone P, Pellecchia MT. The relevance of gender in Parkinson’s disease: a review. J Neurol. (2017) 264:1583–607. doi: 10.1007/s00415-016-8384-9, PMID: [DOI] [PubMed] [Google Scholar]
- 11.Coon EA, Nelson RM, Sletten DM, Suarez MD, Ahlskog JE, Benarroch EE, et al. Sex and gender influence symptom manifestation and survival in multiple system atrophy. Auton Neurosci. (2019) 219:49–52. doi: 10.1016/j.autneu.2019.04.002, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yamamoto T, Sakakibara R, Uchiyama T, Yamaguchi C, Ohno S, Nomura F, et al. Time-dependent changes and gender differences in urinary dysfunction in patients with multiple system atrophy. Neurourol Urodyn. (2014) 33:516–23. doi: 10.1002/nau.22441, PMID: [DOI] [PubMed] [Google Scholar]
- 13.Utsumi K, Fukatsu R, Yamada R, Takamaru Y, Hara Y, Yasumura S. Characteristics of initial symptoms and symptoms at diagnosis in probable dementia with Lewy body disease: incidence of symptoms and gender differences. Psychogeriatrics. (2020) 20:737–45. doi: 10.1111/psyg.12586, PMID: [DOI] [PubMed] [Google Scholar]
- 14.Zhou J, Zhang J, Li Y, Du L, Li Z, Lei F, et al. Gender differences in REM sleep behavior disorder: a clinical and polysomnographic study in China. Sleep Med. (2015) 16:414–8. doi: 10.1016/j.sleep.2014.10.020, PMID: [DOI] [PubMed] [Google Scholar]
- 15.Vegeto E, Villa A, Della Torre S, Crippa V, Rusmini P, Cristofani R, et al. The role of sex and sex hormones in neurodegenerative diseases. Endocr Rev. (2020) 41:273–319. doi: 10.1210/endrev/bnz005, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gillies GE, McArthur S. Estrogen actions in the brain and the basis for differential action in men and women: a case for sex-specific medicines. Pharmacol Rev. (2010) 62:155–98. doi: 10.1124/pr.109.002071, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Poirier A-A, Côté M, Bourque M, Jarras H, Lamontagne-Proulx J, Morissette M, et al. Differential contribution of estrogen receptors to the intestinal therapeutic effects of 17β-estradiol in a murine model of Parkinson’s disease. Brain Res Bull. (2022) 187:85–97. doi: 10.1016/j.brainresbull.2022.06.019, PMID: [DOI] [PubMed] [Google Scholar]
- 18.Zhang M, Hu Z-F, Dong X-L, Chen W-F. Epimedin B exerts neuroprotective effect against MPTP-induced mouse model of Parkinson's disease: GPER as a potential target. Biomed Pharmacother. (2022) 156:113955. doi: 10.1016/j.biopha.2022.113955, PMID: [DOI] [PubMed] [Google Scholar]
- 19.Moher D, Liberati A, Tetzlaff J, Altman DG, P. Group* . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. (2009) 151:264–9. doi: 10.7326/0003-4819-151-4-200908180-00135, PMID: [DOI] [PubMed] [Google Scholar]
- 20.Thomas B, Ciliska D, Dobbins M, Micucci S. A process for systematically reviewing the literature: providing the research evidence for public health nursing interventions. Worldviews Evid-Based Nurs. (2004) 1:176–84. doi: 10.1111/j.1524-475X.2004.04006.x, PMID: [DOI] [PubMed] [Google Scholar]
- 21.Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. (2017) 358:j4008. doi: 10.1136/bmj.j4008, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.American Academy of Sleep Medicine . The international classification of sleep disorders:(ICSD-3). Darien, Illinois: American Academy of Sleep Medicine; (2014). [Google Scholar]
- 23.Wasserman D, Bindman D, Nesbitt AD, Cash D, Milosevic M, Francis PT, et al. Striatal dopaminergic deficit and sleep in idiopathic rapid eye movement behaviour disorder: An explorative study. Nat Sci Sleep. (2021) 13:1–9. doi: 10.2147/NSS.S267037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wasserman D, Gullone S, Duncan I, Veronese M, Gnoni V, Higgins S, et al. Restricted truncal sagittal movements of rapid eye movement behaviour disorder. NPJ Parkinsons Dis. (2022) 8:26. doi: 10.1038/s41531-022-00292-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Högl B, Stefani A, Videnovic A. Idiopathic REM sleep behaviour disorder and neurodegeneration–an update. Nat Rev Neurol. (2018) 14:40–55. doi: 10.1038/nrneurol.2017.157, PMID: [DOI] [PubMed] [Google Scholar]
- 26.Galbiati A, Verga L, Giora E, Zucconi M, Ferini-Strambi L. The risk of neurodegeneration in REM sleep behavior disorder: a systematic review and meta-analysis of longitudinal studies. Sleep Med Rev. (2019) 43:37–46. doi: 10.1016/j.smrv.2018.09.008, PMID: [DOI] [PubMed] [Google Scholar]
- 27.Boeve BF. Idiopathic REM sleep behaviour disorder in the development of Parkinson's disease. Lancet Neurol. (2013) 12:469–82. doi: 10.1016/S1474-4422(13)70054-1, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chan P-C, Lee H-H, Hong C-T, Hu C-J, Wu D. REM sleep behavior disorder (RBD) in dementia with Lewy bodies (DLB). Behav Neurol. (2018) 2018:9421098. doi: 10.1155/2018/9421098, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Olson EJ, Boeve BF, Silber MH. Rapid eye movement sleep behaviour disorder: demographic, clinical and laboratory findings in 93 cases. Brain. (2000) 123:331–9. doi: 10.1093/brain/123.2.331, PMID: [DOI] [PubMed] [Google Scholar]
- 30.Schenck CH, Hurwitz TD, Mahowald MW. REM sleep behaviour disorder: an update on a series of 96 patients and a review of the world literature. J Sleep Res. (1993) 2:224–31. doi: 10.1111/j.1365-2869.1993.tb00093.x, PMID: [DOI] [PubMed] [Google Scholar]
- 31.Wing Y, Lam S, Li S, Yu M, Fong S, Tsoh J, et al. REM sleep behaviour disorder in Hong Kong Chinese: clinical outcome and gender comparison. J Neurol Neurosurg Psychiatry. (2008) 79:1415–6. doi: 10.1136/jnnp.2008.155374 [DOI] [PubMed] [Google Scholar]
- 32.Takeuchi N, Sasai-Sakuma T, Inoue Y. Gender differences in clinical findings and α-synucleiopathy-related markers in patients with idiopathic REM sleep behavior disorder. Sleep Med. (2020) 66:216–9. doi: 10.1016/j.sleep.2019.11.1261, PMID: [DOI] [PubMed] [Google Scholar]
- 33.Castelnuovo A, Marelli S, Mombelli S, Salsone M, Ferini-Strambi L. Idiopathic RBD: the role of gender. J Neurol. (2020) 267:2157–8. doi: 10.1007/s00415-020-09968-0, PMID: [DOI] [PubMed] [Google Scholar]
- 34.Bonakis A, Howard RS, Ebrahim IO, Merritt S, Williams A. REM sleep behaviour disorder (RBD) and its associations in young patients. Sleep Med. (2009) 10:641–5. doi: 10.1016/j.sleep.2008.07.008, PMID: [DOI] [PubMed] [Google Scholar]
- 35.Ju Y-E, Larson-Prior L, Duntley S. Changing demographics in REM sleep behavior disorder: possible effect of autoimmunity and antidepressants. Sleep Med. (2011) 12:278–83. doi: 10.1016/j.sleep.2010.07.022, PMID: [DOI] [PubMed] [Google Scholar]
- 36.Teman PT, Tippmann-Peikert M, Silber MH, Slocumb NL, Auger RR. Idiopathic rapid-eye-movement sleep disorder: associations with antidepressants, psychiatric diagnoses, and other factors, in relation to age of onset. Sleep Med. (2009) 10:60–5. doi: 10.1016/j.sleep.2007.11.019, PMID: [DOI] [PubMed] [Google Scholar]
- 37.Bodkin CL, Schenck CH. Rapid eye movement sleep behavior disorder in women: relevance to general and specialty medical practice. J Women's Health. (2009) 18:1955–63. doi: 10.1089/jwh.2008.1348, PMID: [DOI] [PubMed] [Google Scholar]
- 38.Bugalho P, Salavisa M. Factors influencing the presentation of REM sleep behavior disorder: the relative importance of sex, associated neurological disorder, and context of referral to polysomnography. J Clin Sleep Med. (2019) 15:1789–98. doi: 10.5664/jcsm.8086, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Silva A, Andersen ML, De Mello M, Bittencourt LRA, Peruzzo D, Tufik S. Gender and age differences in polysomnography findings and sleep complaints of patients referred to a sleep laboratory. Braz J Med Biol Res. (2008) 41:1067–75. doi: 10.1590/S0100-879X2008001200005, PMID: [DOI] [PubMed] [Google Scholar]
- 40.Krishnan V, Collop NA. Gender differences in sleep disorders. Curr Opin Pulm Med. (2006) 12:383–9. doi: 10.1097/01.mcp.0000245705.69440.6a, PMID: [DOI] [PubMed] [Google Scholar]
- 41.Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease. A clinico-pathological study of 100 cases. JNNP. (1992) 55:181–84., PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McKeith IG, Boeve BF, Dickson DW, Halliday G, Taylor J-P, Weintraub D, et al. Diagnosis and management of dementia with Lewy bodies: fourth consensus report of the DLB consortium. Neurology. (2017) 89:88–100. doi: 10.1212/WNL.0000000000004058, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wenning GK, Colosimo C, Geser F, Poewe W. Multiple system atrophy. Lancet Neurol. (2004) 3:93–103. doi: 10.1016/S1474-4422(03)00662-8, PMID: [DOI] [PubMed] [Google Scholar]
- 44.Thorpy MJ. Classification of sleep disorders. Neurother. (2012) 9:687–701. doi: 10.1007/s13311-012-0145-6, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fernández-Arcos A, Iranzo A, Serradell M, Gaig C, Santamaria J. The clinical phenotype of idiopathic rapid eye movement sleep behavior disorder at presentation: a study in 203 consecutive patients. Sleep. (2016) 39:121–32. doi: 10.5665/sleep.5332, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Iranzo A, Santamaría J, Vilaseca I, de Osaba MJM. Absence of alterations in serum sex hormone levels in idiopathic REM sleep behavior disorder. Sleep. (2007) 30:803–6. doi: 10.1093/sleep/30.6.803, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chou KL, Moro-De-Casillas ML, Amick MM, Borek LL, Friedman JH. Testosterone not associated with violent dreams or REM sleep behavior disorder in men with Parkinson's. Mov Disord. (2007) 22:411–4. doi: 10.1002/mds.21339, PMID: [DOI] [PubMed] [Google Scholar]
- 48.Liu B, Dluzen DE. Oestrogen and nigrostriatal dopaminergic neurodegeneration: animal models and clinical reports of Parkinson's disease. Clin Exp Pharmacol Physiol. (2007) 34:555–65. doi: 10.1111/j.1440-1681.2007.04616.x, PMID: [DOI] [PubMed] [Google Scholar]
- 49.Schenck CH, Mahowald MW. REM sleep behavior disorder: clinical, developmental, and neuroscience perspectives 16 years after its formal identification in SLEEP. Sleep. (2002) 25:120–38. doi: 10.1093/sleep/25.2.120, PMID: [DOI] [PubMed] [Google Scholar]
- 50.Schenck CH, Bundlie SR, Mahowald MW. Delayed emergence of a parkinsonian disorder in 38% of 29 older men initially diagnosed with idiopathic rapid eye movement sleep behavior disorder. Neurology. (1996) 46:388–93. doi: 10.1212/WNL.46.2.388, PMID: [DOI] [PubMed] [Google Scholar]
- 51.Trist BG, Hare DJ, Double KL. Oxidative stress in the aging substantia nigra and the etiology of Parkinson's disease. Aging Cell. (2019) 18:e13031. doi: 10.1111/acel.13031, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tarolli CG, Zimmerman GA, Auinger P, McIntosh S, Horowitz RK, Kluger BM, et al. Symptom burden among individuals with Parkinson disease: a national survey. Neurology: clinical. Practice. (2020) 10:65–72. doi: 10.1212/CPJ.0000000000000746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bakeberg MC, Gorecki AM, Kenna JE, Jefferson A, Byrnes M, Ghosh S, et al. Differential effects of sex on longitudinal patterns of cognitive decline in Parkinson’s disease. J Neurol. (2021) 268:1903–12. doi: 10.1007/s00415-020-10367-8, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brakedal B, Toker L, Haugarvoll K, Tzoulis C. A nationwide study of the incidence, prevalence and mortality of Parkinson’s disease in the Norwegian population. NPJ Parkinsons Dis. (2022) 8:1–8. doi: 10.1038/s41531-022-00280-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ulivelli M, Bezzini D, Kundisova L, Grazi I, Battaglia MA, Nante N, et al. Mortality of Parkinson’s disease in Italy from 1980 to 2015. Neurol Sci. (2022) 43:3603–11. doi: 10.1007/s10072-021-05854-3, PMID: [DOI] [PubMed] [Google Scholar]
- 56.de Lau LM, Verbaan D, Marinus J, van Hilten JJ. Survival in Parkinson's disease. Relation with motor and non-motor features. Parkinsonism Relat Disord. (2014) 20:613–6. doi: 10.1016/j.parkreldis.2014.02.030, PMID: [DOI] [PubMed] [Google Scholar]
- 57.Pinter B, Diem-Zangerl A, Wenning GK, Scherfler C, Oberaigner W, Seppi K, et al. Mortality in Parkinson's disease: a 38-year follow-up study. Mov Disord. (2015) 30:266–9. doi: 10.1002/mds.26060, PMID: [DOI] [PubMed] [Google Scholar]
- 58.Xu J, Gong D, Man C, Fan Y. Parkinson's disease and risk of mortality: meta-analysis and systematic review. Acta Neurol Scand. (2014) 129:71–9. doi: 10.1111/ane.12201, PMID: [DOI] [PubMed] [Google Scholar]
- 59.Moisan F, Kab S, Mohamed F, Canonico M, Le Guern M, Quintin C, et al. Parkinson disease male-to-female ratios increase with age: French nationwide study and meta-analysis. J Neurol Neurosurg Psychiatry. (2016) 87:952–7. doi: 10.1136/jnnp-2015-312283, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pringsheim T, Jette N, Frolkis A, Steeves TD. The prevalence of Parkinson's disease: a systematic review and meta-analysis. Mov Disord. (2014) 29:1583–90. doi: 10.1002/mds.25945, PMID: [DOI] [PubMed] [Google Scholar]
- 61.Savica R, Grossardt BR, Rocca WA, Bower JH. Parkinson disease with and without dementia: a prevalence study and future projections. Mov Disord. (2018) 33:537–43. doi: 10.1002/mds.27277, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gelegen C, Cash D, Ilic K, Sander M, Kim E, Simmons C, et al. Relevance of sleep and associated structural changes in GBA1 mouse to human rapid eye movement behavior disorder. Sci Rep. (2022) 12:7973. doi: 10.1038/s41598-022-11516-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Menozzi E, Schapira AHV. Exploring the genotype-phenotype correlation in GBA-Parkinson disease: clinical aspects, biomarkers, and potential modifiers. Front Neurol. (2021) 12:694764. doi: 10.3389/fneur.2021.694764, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kluss JH, Mamais A, Cookson MR. LRRK2 links genetic and sporadic Parkinson's disease. Biochem Soc Trans. (2019) 47:651–61. doi: 10.1042/BST20180462, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Thaler A, Bregman N, Gurevich T, Shiner T, Dror Y, Zmira O, et al. Parkinson's disease phenotype is influenced by the severity of the mutations in the GBA gene. Parkinsonism Relat Disord. (2018) 55:45–9. doi: 10.1016/j.parkreldis.2018.05.009, PMID: [DOI] [PubMed] [Google Scholar]
- 66.Brockmann K, Berg D. The significance of GBA for Parkinson’s disease. J Inherit Metab Dis. (2014) 37:643–8. doi: 10.1007/s10545-014-9714-7, PMID: [DOI] [PubMed] [Google Scholar]
- 67.Cilia R, Siri C, Rusconi D, Allegra R, Ghiglietti A, Sacilotto G, et al. LRRK2 mutations in Parkinson's disease: confirmation of a gender effect in the Italian population. Parkinsonism Relat Disord. (2014) 20:911–4. doi: 10.1016/j.parkreldis.2014.04.016, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shu L, Zhang Y, Pan H, Xu Q, Guo J, Tang B, et al. Clinical heterogeneity among LRRK2 variants in Parkinson's disease: a meta-analysis. Front Aging Neurosci. (2018) 10:283. doi: 10.3389/fnagi.2018.00283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gan-Or Z, Leblond CS, Mallett V, Orr-Urtreger A, Dion PA, Rouleau GA. LRRK2 mutations in Parkinson disease; a sex effect or lack thereof? A meta-analysis. Parkinsonism Relat Disord. (2015) 21:778–82. doi: 10.1016/j.parkreldis.2015.05.002, PMID: [DOI] [PubMed] [Google Scholar]
- 70.Cui S-S, Fu R, Du J-J, Lin Y-Q, Huang P, Gao C, et al. Sex effects on clinical features in LRRK2 G2385R carriers and non-carriers in Parkinson's disease. BMC Neurosci. (2021) 22:22. doi: 10.1186/s12868-021-00623-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Smith L, Schapira AHV. GBA variants and Parkinson disease: mechanisms and treatments. Cells. (2022) 11:1261. doi: 10.3390/cells11081261, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cholerton B, Johnson CO, Fish B, Quinn JF, Chung KA, Peterson-Hiller AL, et al. Sex differences in progression to mild cognitive impairment and dementia in Parkinson's disease. Parkinsonism Relat Disord. (2018) 50:29–36. doi: 10.1016/j.parkreldis.2018.02.007, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gan-Or Z, Bar-Shira A, Mirelman A, Gurevich T, Kedmi M, Giladi N, et al. LRRK2 and GBA mutations differentially affect the initial presentation of Parkinson disease. Neurogenetics. (2010) 11:121–5. doi: 10.1007/s10048-009-0198-9, PMID: [DOI] [PubMed] [Google Scholar]
- 74.Georgiou A, Demetriou CA, Heraclides A, Christou YP, Leonidou E, Loukaides P, et al. Mitochondrial superclusters influence age of onset of Parkinson’s disease in a gender specific manner in the Cypriot population: a case-control study. PLoS One. (2017) 12:e0183444. doi: 10.1371/journal.pone.0183444, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gatt AP, Jones EL, Francis PT, Ballard C, Bateman JM. Association of a polymorphism in mitochondrial transcription factor a (TFAM) with Parkinson's disease dementia but not dementia with Lewy bodies. Neurosci Lett. (2013) 557:177–80. doi: 10.1016/j.neulet.2013.10.045, PMID: [DOI] [PubMed] [Google Scholar]
- 76.Gusdon AM, Fang F, Chen J, Mathews CE, Li W, Chu CT, et al. Association of the mt-ND2 5178A/C polymorphism with Parkinson’s disease. Neurosci Lett. (2015) 587:98–101. doi: 10.1016/j.neulet.2014.12.005, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nijiati M, Saidaming A, Qiao J, Cheng Z, Qiu C, Sun Y. GNB3, eNOS, and mitochondrial DNA polymorphisms correlate to natural longevity in a Xinjiang Uygur population. PLoS One. (2013) 8:e81806. doi: 10.1371/journal.pone.0081806, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tanaka M, Gong J-S, Zhang J, Yoneda M, Yagi K. Mitochondrial genotype associated with longevity. Lancet. (1998) 351:185–6. doi: 10.1016/S0140-6736(05)78211-8, PMID: [DOI] [PubMed] [Google Scholar]
- 79.Sampaio TF, Dos Santos EUD, de Lima GDC, Dos Anjos RSG, da Silva RC, Asano AGC, et al. MAO-B and COMT genetic variations associated with levodopa treatment response in patients with Parkinson's disease. J Clin Pharmacol. (2018) 58:920–6. doi: 10.1002/jcph.1096, PMID: [DOI] [PubMed] [Google Scholar]
- 80.Zhao J, Han X, Xue L, Zhu K, Liu H, Xie A. Association of TLR4 gene polymorphisms with sporadic Parkinson’s disease in a Han Chinese population. Neurol Sci. (2015) 36:1659–65. doi: 10.1007/s10072-015-2227-9, PMID: [DOI] [PubMed] [Google Scholar]
- 81.Ping Z, Xiaomu W, Xufang X, Wenfeng C, Liang S, Tao W. GAPDH rs1136666 SNP indicates a high risk of Parkinson’s disease. Neurosci Lett. (2018) 685:55–62. doi: 10.1016/j.neulet.2018.06.011, PMID: [DOI] [PubMed] [Google Scholar]
- 82.Zhang P, Liu L, Huang J, Shao L, Wang H, Xiong N, et al. Non-SMC condensin I complex, subunit D2 gene polymorphisms are associated with Parkinson’s disease: a Han Chinese study. Genome. (2014) 57:253–7. doi: 10.1139/gen-2014-0032, PMID: [DOI] [PubMed] [Google Scholar]
- 83.Kim R, Park S, Yoo D, Ju Suh Y, Jun J-S, Jeon B. Potential sex-specific effects of apolipoprotein E ɛ4 on cognitive decline in early Parkinson’s disease. J Parkinsons Dis. (2021) 11:497–505. doi: 10.3233/JPD-202288, PMID: [DOI] [PubMed] [Google Scholar]
- 84.Liu R-R, Zhou L-L, Cheng X, Sun M-X, Hu Y-B, Chen S-F, et al. CCDC62 variant rs12817488 is associated with the risk of Parkinson's disease in a Han Chinese population. Eur Neurol. (2014) 71:77–83. doi: 10.1159/000354333, PMID: [DOI] [PubMed] [Google Scholar]
- 85.Lee Y, Park Y-H, Lee JJ, Sohn YH, Lee J-M, Lee PH. Gender-specific effect of uric acid on resting-state functional networks in de novo Parkinson's disease. Parkinsonism Relat Disord. (2018) 52:49–54. doi: 10.1016/j.parkreldis.2018.03.023, PMID: [DOI] [PubMed] [Google Scholar]
- 86.Baik K, Chung S, Yoo H, Lee Y, Jung J, Sohn Y, et al. Sex-dependent association of urate on the patterns of striatal dopamine depletion in Parkinson’s disease. Eur J Neurol. (2020) 27:773–8. doi: 10.1111/ene.14152, PMID: [DOI] [PubMed] [Google Scholar]
- 87.Gao X, O'Reilly ÉJ, Schwarzschild MA, Ascherio A. Prospective study of plasma urate and risk of Parkinson disease in men and women. Neurology. (2016) 86:520–6. doi: 10.1212/WNL.0000000000002351, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jesus S, Perez I, Cáceres-Redondo M, Carrillo F, Carballo M, Gómez-Garre P, et al. Low serum uric acid concentration in Parkinson's disease in southern Spain. Eur J Neurol. (2013) 20:208–10. doi: 10.1111/j.1468-1331.2012.03745.x, PMID: [DOI] [PubMed] [Google Scholar]
- 89.McFarland NR, Burdett T, Desjardins CA, Frosch MP, Schwarzschild MA. Postmortem brain levels of urate and precursors in Parkinson's disease and related disorders. Neurodegener Dis. (2013) 12:189–98. doi: 10.1159/000346370, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhang H-N, Guo J-F, He D, Lei L-F, Wang Y-Q, Wang C-Y, et al. Lower serum UA levels in Parkinson's disease patients in the Chinese population. Neurosci Lett. (2012) 514:152–5. doi: 10.1016/j.neulet.2012.02.077, PMID: [DOI] [PubMed] [Google Scholar]
- 91.Shen C, Guo Y, Luo W, Lin C, Ding M. Serum urate and the risk of Parkinson's disease: results from a meta-analysis. Can J Neurol Sci. (2013) 40:73–9. doi: 10.1017/S0317167100012981, PMID: [DOI] [PubMed] [Google Scholar]
- 92.Cortese M, Riise T, Engeland A, Ascherio A, Bjørnevik K. Urate and the risk of Parkinson's disease in men and women. Parkinsonism Relat Disord. (2018) 52:76–82. doi: 10.1016/j.parkreldis.2018.03.026, PMID: [DOI] [PubMed] [Google Scholar]
- 93.Bakeberg MC, Jefferson A, Riley M, Byrnes M, Ghosh S, Mastaglia FL, et al. Elevated serum homocysteine levels have differential gender-specific associations with motor and cognitive states in Parkinson’s disease. Parkinsons Dis. (2019) 2019:3124295. doi: 10.1155/2019/3124295, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wu Q, Liu M, Yu M, Fu J. Sex differences in underweight and body mass index in Chinese early de novo patients with Parkinson's disease. Brain Behav. (2020) 10:e01893. doi: 10.1002/brb3.1893, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhang M, Chen H, Liu G, Wang X, Wang Z, Feng T, et al. Lower serum triglyceride levels linked to more severe motor performance in Parkinson’s disease. Neurol Sci. (2022) 43:5343–53. doi: 10.1007/s10072-022-06113-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Luca A, Monastero R, Cicero CE, Baschi R, Donzuso G, Mostile G, et al. Executive functioning and serum lipid fractions in Parkinson’s disease–a possible sex-effect: the PACOS study. J Neural Transm. (2022) 129:287–93. doi: 10.1007/s00702-022-02460-1, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bakeberg MC, Gorecki AM, Kenna JE, Jefferson A, Byrnes M, Ghosh S, et al. Elevated HDL levels linked to poorer cognitive ability in females with Parkinson’s disease. Front Aging Neurosci. (2021) 13:656623. doi: 10.3389/fnagi.2021.656623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Meoni G, Tenori L, Schade S, Licari C, Pirazzini C, Bacalini MG, et al. Metabolite and lipoprotein profiles reveal sex-related oxidative stress imbalance in de novo drug-naive Parkinson’s disease patients. NPJ Parkinsons Dis. (2022) 8:1–10. doi: 10.1038/s41531-021-00274-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hamid Z, Basit A, Pontis S, Piras F, Assogna F, Bossù P, et al. Gender specific decrease of a set of circulating N-acylphosphatidyl ethanolamines (NAPEs) in the plasma of Parkinson’s disease patients. Metabolomics. (2019) 15:1–9. doi: 10.1007/s11306-019-1536-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Caranci G, Piscopo P, Rivabene R, Traficante A, Riozzi B, Castellano AE, et al. Gender differences in Parkinson’s disease: focus on plasma alpha-synuclein. J Neural Transm. (2013) 120:1209–15. doi: 10.1007/s00702-013-0972-6, PMID: [DOI] [PubMed] [Google Scholar]
- 101.Ho DH, Yi S, Seo H, Son I, Seol W. Increased DJ-1 in urine exosome of Korean males with Parkinson’s disease. Biomed Res Int. (2014) 2014:704678. doi: 10.1155/2014/704678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Huang Y, Huang C, Zhang Q, Wu W, Sun J. Serum BDNF discriminates Parkinson’s disease patients with depression from without depression and reflect motor severity and gender differences. J Neurol. (2021) 268:1411–8. doi: 10.1007/s00415-020-10299-3, PMID: [DOI] [PubMed] [Google Scholar]
- 103.Baldini F, Hertel J, Sandt E, Thinnes CC, Neuberger-Castillo L, Pavelka L, et al. Parkinson’s disease-associated alterations of the gut microbiome predict disease-relevant changes in metabolic functions. BMC Biol. (2020) 18:62. doi: 10.1186/s12915-020-00775-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Nissen SK, Farmen K, Carstensen M, Schulte C, Goldeck D, Brockmann K, et al. Changes in CD163+, CD11b+, and CCR2+ peripheral monocytes relate to Parkinson’s disease and cognition. Brain Behav Immun. (2022) 101:182–93. doi: 10.1016/j.bbi.2022.01.005, PMID: [DOI] [PubMed] [Google Scholar]
- 105.Nissen SK, Ferreira SA, Nielsen MC, Schulte C, Shrivastava K, Hennig D, et al. Soluble CD163 changes indicate monocyte association with cognitive deficits in Parkinson's disease. Mov Disord. (2021) 36:963–76. doi: 10.1002/mds.28424, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Houser MC, Chang J, Factor SA, Molho ES, Zabetian CP, Hill-Burns EM, et al. Stool immune profiles evince gastrointestinal inflammation in Parkinson's disease. Mov Disord. (2018) 33:793–804. doi: 10.1002/mds.27326, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Carlisle SM, Qin H, Hendrickson RC, Muwanguzi JE, Lefkowitz EJ, Kennedy RE, et al. Sex-based differences in the activation of peripheral blood monocytes in early Parkinson disease. Npj. Parkinsons Dis. (2021) 7:1–10. doi: 10.1038/s41531-021-00180-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Boccalini C, Carli G, Pilotto A, Padovani A, Perani D. Gender differences in dopaminergic system dysfunction in de novo Parkinson's disease clinical subtypes. Neurobiol Dis. (2022) 167:105668. doi: 10.1016/j.nbd.2022.105668, PMID: [DOI] [PubMed] [Google Scholar]
- 109.Porta M, Pilloni G, Arippa F, Casula C, Cossu G, Pau M. Similarities and differences of gait patterns in women and men with Parkinson disease with mild disability. Arch Phys Med Rehabil. (2019) 100:2039–45. doi: 10.1016/j.apmr.2019.04.010, PMID: [DOI] [PubMed] [Google Scholar]
- 110.De Micco R, Esposito F, di Nardo F, Caiazzo G, Siciliano M, Russo A, et al. Sex-related pattern of intrinsic brain connectivity in drug-naïve Parkinson's disease patients. Mov Disord. (2019) 34:997–1005. doi: 10.1002/mds.27725, PMID: [DOI] [PubMed] [Google Scholar]
- 111.Kolmancic K, Perellón-Alfonso R, Pirtosek Z, Rothwell JC, Bhatia K, Kojovic M. Sex differences in Parkinson's disease: a transcranial magnetic stimulation study. Mov Disord. (2019) 34:1873–81. doi: 10.1002/mds.27870, PMID: [DOI] [PubMed] [Google Scholar]
- 112.Tremblay C, Abbasi N, Zeighami Y, Yau Y, Dadar M, Rahayel S, et al. Sex effects on brain structure in de novo Parkinson’s disease: a multimodal neuroimaging study. Brain. (2020) 143:3052–66. doi: 10.1093/brain/awaa234, PMID: [DOI] [PubMed] [Google Scholar]
- 113.Picillo M, LaFontant D-E, Bressman S, Caspell-Garcia C, Coffey C, Cho HR, et al. Sex-related longitudinal change of motor, non-motor, and biological features in early Parkinson’s disease. J Parkinsons Dis. (2022) 12:421–36. doi: 10.3233/JPD-212892, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lubomski M, Rushworth RL, Lee W, Bertram KL, Williams DR. Sex differences in Parkinson’s disease. J Clin Neurosci. (2014) 21:1503–6. doi: 10.1016/j.jocn.2013.12.016, PMID: [DOI] [PubMed] [Google Scholar]
- 115.Wan Z, Wang X, Ma H, Wang Z, Feng T. Risk factors for motor complications in female patients with Parkinson’s disease. Neurol Sci. (2022):4735–43. doi: 10.1007/s10072-022-05959-3 [DOI] [PubMed] [Google Scholar]
- 116.Baba Y, Putzke JD, Whaley NR, Wszolek ZK, Uitti RJ. Gender and the Parkinson’s disease phenotype. J Neurol. (2005) 252:1201–5. doi: 10.1007/s00415-005-0835-7, PMID: [DOI] [PubMed] [Google Scholar]
- 117.Haaxma CA, Bloem BR, Borm GF, Oyen WJ, Leenders KL, Eshuis S, et al. Gender differences in Parkinson’s disease. J Neurol Neurosurg Psychiatry. (2007) 78:819–24. doi: 10.1136/jnnp.2006.103788, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Colombo D, Abbruzzese G, Antonini A, Barone P, Bellia G, Franconi F, et al. The “gender factor” in wearing-off among patients with Parkinson’s disease: a post hoc analysis of DEEP study. Sci World J. (2015) 2015:787451. doi: 10.1155/2015/787451, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kim R, Lee J, Kim Y, Kim A, Jang M, Kim H-J, et al. Presynaptic striatal dopaminergic depletion predicts the later development of freezing of gait in de novo Parkinson's disease: an analysis of the PPMI cohort. Parkinsonism Relat Disord. (2018) 51:49–54. doi: 10.1016/j.parkreldis.2018.02.047, PMID: [DOI] [PubMed] [Google Scholar]
- 120.Ou R, Liu H, Hou Y, Song W, Cao B, Wei Q, et al. Predictors of camptocormia in patients with Parkinson's disease: a prospective study from Southwest China. Parkinsonism Relat Disord. (2018) 52:69–75. doi: 10.1016/j.parkreldis.2018.03.020, PMID: [DOI] [PubMed] [Google Scholar]
- 121.Reekes TH, Higginson CI, Ledbetter CR, Sathivadivel N, Zweig RM, Disbrow EA. Sex specific cognitive differences in Parkinson disease. Npj. Parkinsons Dis. (2020) 6:1–6. doi: 10.1038/s41531-020-0109-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Yang K, Shen B, Li D-K, Wang Y, Zhao J, Zhao J, et al. Cognitive characteristics in Chinese non-demented PD patients based on gender difference. Transl Neurodegener. (2018) 7:1–9. doi: 10.1186/s40035-018-0120-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Heller J, Mirzazade S, Romanzetti S, Habel U, Derntl B, Freitag NM, et al. Impact of gender and genetics on emotion processing in Parkinson's disease-a multimodal study. Neuroimage. (2018) 18:305–14. doi: 10.1016/j.nicl.2018.01.034, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bayram E, Banks SJ, Shan G, Kaplan N, Caldwell JZ. Sex differences in cognitive changes in de novo Parkinson’s disease. J Int Neuropsychol Soc. (2020) 26:241–9. doi: 10.1017/S1355617719001085, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Oltra J, Segura B, Uribe C, Monté-Rubio GC, Campabadal A, Inguanzo A, et al. Sex differences in brain atrophy and cognitive impairment in Parkinson’s disease patients with and without probable rapid eye movement sleep behavior disorder. J Neurol. (2022) 269:1591–9. doi: 10.1007/s00415-021-10728-x, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Oltra J, Uribe C, Campabadal A, Inguanzo A, Monté-Rubio GC, Martí MJ, et al. Sex differences in brain and cognition in de novo Parkinson's disease. Front Aging Neurosci. (2022) 13:791532. doi: 10.3389/fnagi.2021.791532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Liu M, Luo Y-J, Gu H-Y, Wang Y-M, Liu M-H, Li K, et al. Sex and onset-age-related features of excessive daytime sleepiness and night-time sleep in patients with Parkinson’s disease. BMC Neurol. (2021) 21:1–8. doi: 10.1186/s12883-021-02192-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ratti P-L, Terzaghi M, Minafra B, Repetto A, Pasotti C, Zangaglia R, et al. REM and NREM sleep enactment behaviors in Parkinson’s disease, Parkinson’s disease dementia, and dementia with Lewy bodies. Sleep Med. (2012) 13:926–32. doi: 10.1016/j.sleep.2012.04.015, PMID: [DOI] [PubMed] [Google Scholar]
- 129.Solla P, Masala C, Liscia A, Piras R, Ercoli T, Fadda L, et al. Sex-related differences in olfactory function and evaluation of possible confounding factors among patients with Parkinson’s disease. J Neurol. (2020) 267:57–63. doi: 10.1007/s00415-019-09551-2, PMID: [DOI] [PubMed] [Google Scholar]
- 130.Nicoletti A, Vasta R, Mostile G, Nicoletti G, Arabia G, Iliceto G, et al. Gender effect on non-motor symptoms in Parkinson's disease: are men more at risk? Parkinsonism Relat Disord. (2017) 35:69–74. doi: 10.1016/j.parkreldis.2016.12.008, PMID: [DOI] [PubMed] [Google Scholar]
- 131.Hu T, Ou R, Liu H, Hou Y, Wei Q, Song W, et al. Gender and onset age related-differences of non-motor symptoms and quality of life in drug-naïve Parkinson’s disease. Clin Neurol Neurosurg. (2018) 175:124–9. doi: 10.1016/j.clineuro.2018.11.001, PMID: [DOI] [PubMed] [Google Scholar]
- 132.Picillo M, Amboni M, Erro R, Longo K, Vitale C, Moccia M, et al. Gender differences in non-motor symptoms in early, drug naive Parkinson’s disease. J Neurol. (2013) 260:2849–55. doi: 10.1007/s00415-013-7085-x, PMID: [DOI] [PubMed] [Google Scholar]
- 133.Defazio G, Antonini A, Tinazzi M, Gigante A, Pietracupa S, Pellicciari R, et al. Relationship between pain and motor and non-motor symptoms in Parkinson's disease. Eur J Neurol. (2017) 24:974–80. doi: 10.1111/ene.13323, PMID: [DOI] [PubMed] [Google Scholar]
- 134.Wang S-M, Tickle-Degnen L. Emotional cues from expressive behavior of women and men with Parkinson’s disease. PLoS One. (2018) 13:e0199886. doi: 10.1371/journal.pone.0210200, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Picillo M, Palladino R, Erro R, Alfano R, Colosimo C, Marconi R, et al. The PRIAMO study: age-and sex-related relationship between prodromal constipation and disease phenotype in early Parkinson’s disease. J Neurol. (2021) 268:448–54. doi: 10.1007/s00415-020-10156-3, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Raciti L, De Cola MC, Ortelli P, Corallo F, Lo Buono V, Morini E, et al. Sexual dysfunction in Parkinson disease: a multicenter Italian cross-sectional study on a still overlooked problem. J Sex Med. (2020) 17:1914–25. doi: 10.1016/j.jsxm.2020.06.010, PMID: [DOI] [PubMed] [Google Scholar]
- 137.Wee N, Kandiah N, Acharyya S, Chander RJ, Ng A, Au WL, et al. Baseline predictors of worsening apathy in Parkinson's disease: a prospective longitudinal study. Parkinsonism Relat Disord. (2016) 23:95–8. doi: 10.1016/j.parkreldis.2015.12.004, PMID: [DOI] [PubMed] [Google Scholar]
- 138.Cereda E, Cilia R, Klersy C, Siri C, Pozzi B, Reali E, et al. Dementia in Parkinson's disease: is male gender a risk factor? Parkinsonism Relat Disord. (2016) 26:67–72. doi: 10.1016/j.parkreldis.2016.02.024, PMID: [DOI] [PubMed] [Google Scholar]
- 139.Liu R, Umbach DM, Peddada SD, Xu Z, Tröster AI, Huang X, et al. Potential sex differences in nonmotor symptoms in early drug-naive Parkinson disease. Neurology. (2015) 84:2107–15. doi: 10.1212/WNL.0000000000001609, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Anang JB, Gagnon J-F, Bertrand J-A, Romenets SR, Latreille V, Panisset M, et al. Predictors of dementia in Parkinson disease: a prospective cohort study. Neurology. (2014) 83:1253–60. doi: 10.1212/WNL.0000000000000842, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Picillo M, Palladino R, Erro R, Colosimo C, Marconi R, Antonini A, et al. The PRIAMO study: active sexual life is associated with better motor and non-motor outcomes in men with early Parkinson's disease. Eur J Neurol. (2019) 26:1327–33. doi: 10.1111/ene.13983, PMID: [DOI] [PubMed] [Google Scholar]
- 142.Pigott K, Rick J, Xie SX, Hurtig H, Chen-Plotkin A, Duda JE, et al. Longitudinal study of normal cognition in Parkinson disease. Neurology. (2015) 85:1276–82. doi: 10.1212/WNL.0000000000002001, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Szewczyk-Krolikowski K, Tomlinson P, Nithi K, Wade-Martins R, Talbot K, Ben-Shlomo Y, et al. The influence of age and gender on motor and non-motor features of early Parkinson's disease: initial findings from the Oxford Parkinson disease center (OPDC) discovery cohort. Parkinsonism Relat Disord. (2014) 20:99–105. doi: 10.1016/j.parkreldis.2013.09.025, PMID: [DOI] [PubMed] [Google Scholar]
- 144.Shin JY, Pohlig RT, Habermann B. Self-reported symptoms of Parkinson’s disease by sex and disease duration. West J Nurs Res. (2017) 39:1412–28. doi: 10.1177/0193945916670904, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Leentjens AF, Moonen AJ, Dujardin K, Marsh L, Martinez-Martin P, Richard IH, et al. Modeling depression in Parkinson disease: disease-specific and nonspecific risk factors. Neurology. (2013) 81:1036–43. doi: 10.1212/WNL.0b013e3182a4a503, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Guo X, Song W, Chen K, Chen X, Zheng Z, Cao B, et al. Gender and onset age-related features of non-motor symptoms of patients with Parkinson's disease–a study from Southwest China. Parkinsonism Relat Disord. (2013) 19:961–5. doi: 10.1016/j.parkreldis.2013.06.009, PMID: [DOI] [PubMed] [Google Scholar]
- 147.Perrin AJ, Nosova E, Co K, Book A, Iu O, Silva V, et al. Gender differences in Parkinson's disease depression. Parkinsonism Relat Disord. (2017) 36:93–7. doi: 10.1016/j.parkreldis.2016.12.026, PMID: [DOI] [PubMed] [Google Scholar]
- 148.Gao L, Nie K, Tang H, Wang L, Zhao J, Gan R, et al. Sex differences in cognition among Chinese people with Parkinson’s disease. J Clin Neurosci. (2015) 22:488–92. doi: 10.1016/j.jocn.2014.08.032, PMID: [DOI] [PubMed] [Google Scholar]
- 149.Fengler S, Roeske S, Heber I, Reetz K, Schulz JB, Riedel O, et al. Verbal memory declines more in female patients with Parkinson's disease: the importance of gender-corrected normative data. Psychol Med. (2016) 46:2275–86. doi: 10.1017/S0033291716000908, PMID: [DOI] [PubMed] [Google Scholar]
- 150.Kang KW, Choi S-M, Kim BC. Gender differences in motor and non-motor symptoms in early Parkinson disease. Medicine. (2022) 101:e28643. doi: 10.1097/MD.0000000000032579, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Weintraub D, Koester J, Potenza MN, Siderowf AD, Stacy M, Voon V, et al. Impulse control disorders in Parkinson disease: a cross-sectional study of 3090 patients. Arch Neurol. (2010) 67:589–95. doi: 10.1001/archneurol.2010.65, PMID: [DOI] [PubMed] [Google Scholar]
- 152.Kon T, Ueno T, Haga R, Tomiyama M. The factors associated with impulse control behaviors in Parkinson's disease: a 2-year longitudinal retrospective cohort study. Brain Behavior. (2018) 8:e01036. doi: 10.1002/brb3.1036, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Gyorfi O, Nagy H, Bokor M, Moustafa AA, Rosenzweig I, Kelemen O, et al. Reduced CA2-CA3 hippocampal subfield volume is related to depression and normalized by l-DOPA in newly diagnosed Parkinson's disease. Front Neurol. (2017) 8:84. doi: 10.3389/fneur.2017.00084, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Zhu J, Lu L, Pan Y, Shen B, Xu S, Hou Y, et al. Depression and associated factors in nondemented Chinese patients with Parkinson’s disease. Clin Neurol Neurosurg. (2017) 163:142–8. doi: 10.1016/j.clineuro.2017.10.031, PMID: [DOI] [PubMed] [Google Scholar]
- 155.Song Y, Gu Z, An J, Chan P. Gender differences on motor and non-motor symptoms of de novo patients with early Parkinson’s disease. Neurol Sci. (2014) 35:1991–6. doi: 10.1007/s10072-014-1879-1, PMID: [DOI] [PubMed] [Google Scholar]
- 156.Kovács M, Makkos A, Aschermann Z, Janszky J, Komoly S, Weintraut R, et al. Impact of sex on the nonmotor symptoms and the health-related quality of life in Parkinson’s disease. Parkinsons Dis. (2016) 2016:7951840. doi: 10.1155/2016/7951840, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Kheloui S, Brouillard A, Rossi M, Marin MF, Mendrek A, Paquette D, et al. Exploring the sex and gender correlates of cognitive sex differences. Acta Psychol. (2021) 221:103452. doi: 10.1016/j.actpsy.2021.103452, PMID: [DOI] [PubMed] [Google Scholar]
- 158.Bjørnarå KA, Dietrichs E, Toft M. REM sleep behavior disorder in Parkinson's disease–is there a gender difference? Parkinsonism Relat Disord. (2013) 19:120–2. doi: 10.1016/j.parkreldis.2012.05.027 [DOI] [PubMed] [Google Scholar]
- 159.Patra PB, Patra S. Sex differences in the physiology and pharmacology of the lower urinary tract. Current urology. (2012) 6:179–88. doi: 10.1159/000343536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Abelson B, Sun D, Que L, Nebel RA, Baker D, Popiel P, et al. Sex differences in lower urinary tract biology and physiology. Biol Sex Differ. (2018) 9:1–13. doi: 10.1186/s13293-018-0204-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Luders E, Toga AW. Sex differences in brain anatomy. Prog Brain Res. (2010) 186:2–12. doi: 10.1016/B978-0-444-53630-3.00001-4 [DOI] [PubMed] [Google Scholar]
- 162.Raznahan A, Disteche CM. X-chromosome regulation and sex differences in brain anatomy. Neurosci Biobehav Rev. (2021) 120:28–47. doi: 10.1016/j.neubiorev.2020.10.024, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Jost A. Hormonal factors in the development of the male genital system, the human testis: Proceedings of the workshop conference held at Positano, Italy, April 23–25. Berlin: Springer pp. 11–18. (1970).
- 164.Wharton W, Gleason CE, Sandra O, Carlsson CM, Asthana S. Neurobiological underpinnings of the estrogen-mood relationship. Curr Psychiatr Rev. (2012) 8:247–56. doi: 10.2174/157340012800792957, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Hardy R, Kuh D. Change in psychological and vasomotor symptom reporting during the menopause. Soc Sci Med. (2002) 55:1975–88. doi: 10.1016/S0277-9536(01)00326-4, PMID: [DOI] [PubMed] [Google Scholar]
- 166.Woods N, Mariella A, Mitchell E. Patterns of depressed mood across the menopausal transition: approaches to studying patterns in longitudinal data. Acta Obstet Gynecol Scand. (2002) 81:623–32. doi: 10.1034/j.1600-0412.2002.810708.x, PMID: [DOI] [PubMed] [Google Scholar]
- 167.Paoletti AM, Floris S, Mannias M, Orrù M, Crippa D, Orlandi R, et al. Evidence that cyproterone acetate improves psychological symptoms and enhances the activity of the dopaminergic system in postmenopause. J Clin Endocrinol Metabol. (2001) 86:608–12. doi: 10.1210/jcem.86.2.7179, PMID: [DOI] [PubMed] [Google Scholar]
- 168.Ragonese P, D'Amelio M, Callari G, Salemi G, Morgante L, Savettieri G. Age at menopause predicts age at onset of Parkinson's disease. Mov Disord. (2006) 21:2211–4. doi: 10.1002/mds.21127, PMID: [DOI] [PubMed] [Google Scholar]
- 169.Labandeira-Garcia JL, Rodriguez-Perez AI, Valenzuela R, Costa-Besada MA, Guerra MJ. Menopause and Parkinson’s disease. Interaction between estrogens and brain renin-angiotensin system in dopaminergic degeneration. Front Neuroendocrinol. (2016) 43:44–59. doi: 10.1016/j.yfrne.2016.09.003, PMID: [DOI] [PubMed] [Google Scholar]
- 170.Gatto EM, Aldinio V. Impulse control disorders in Parkinson's disease. A brief and comprehensive review. Front Neurol. (2019) 10:351. doi: 10.3389/fneur.2019.00351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Potenza MN, Koran LM, Pallanti S. The relationship between impulse-control disorders and obsessive-compulsive disorder: a current understanding and future research directions. Psychiatry Res. (2009) 170:22–31. doi: 10.1016/j.psychres.2008.06.036, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Benatti B, Girone N, Celebre L, Vismara M, Hollander E, Fineberg NA, et al. The role of gender in a large international OCD sample: a report from the International College of Obsessive-Compulsive Spectrum Disorders (ICOCS) network. Compr Psychiatry. (2022) 116:152315. doi: 10.1016/j.comppsych.2022.152315, PMID: [DOI] [PubMed] [Google Scholar]
- 173.Martinez-Ramirez D, Giugni J, Vedam-Mai V, Shukla AW, Malaty IA, McFarland NR, et al. The “brittle response” to Parkinson’s disease medications: characterization and response to deep brain stimulation. PLoS One. (2014) 9:e94856. doi: 10.1371/journal.pone.0094856, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Balash Y, Korczyn AD, Migirov AA, Gurevich T. Quality of life in Parkinson's disease: a gender-specific perspective. Acta Neurol Scand. (2019) 140:17–22. doi: 10.1111/ane.13095, PMID: [DOI] [PubMed] [Google Scholar]
- 175.Lebow MA, Chen A. Overshadowed by the amygdala: the bed nucleus of the stria terminalis emerges as key to psychiatric disorders. Mol Psychiatry. (2016) 21:450–63. doi: 10.1038/mp.2016.1, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Gegenhuber B, Wu MV, Bronstein R, Tollkuhn J. Gene regulation by gonadal hormone receptors underlies brain sex differences. Nature. (2022) 606:153–9. doi: 10.1038/s41586-022-04686-1, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Zeighami Y, Ulla M, Iturria-Medina Y, Dadar M, Zhang Y, Larcher KM, et al. Network structure of brain atrophy in de novo Parkinson's disease. Elife. (2015) 4:e08440. doi: 10.7554/eLife.08440, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Conti V, Izzo V, Russillo MC, Picillo M, Amboni M, Scaglione CL, et al. Gender differences in levodopa pharmacokinetics in levodopa-naïve patients with Parkinson’s disease. Front Med. (2022) 9:909936. doi: 10.3389/fmed.2022.909936, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Kumagai T, Nagayama H, Ota T, Nishiyama Y, Mishina M, Ueda M. Sex differences in the pharmacokinetics of levodopa in elderly patients with Parkinson disease. Clin Neuropharmacol. (2014) 37:173–6. doi: 10.1097/WNF.0000000000000051, PMID: [DOI] [PubMed] [Google Scholar]
- 180.Nishikawa N, Iwaki H, Shiraishi T, Mukai Y, Takahashi Y, Murata M. Female, aging, difference formulations of DCI, or lower body weight increases AUC4hr of levodopa in patients with Parkinson's disease. Parkinsonism Relat Disord. (2020) 76:16–20. doi: 10.1016/j.parkreldis.2020.05.020, PMID: [DOI] [PubMed] [Google Scholar]
- 181.Warren Olanow C, Kieburtz K, Rascol O, Poewe W, Schapira AH, Emre M, et al. Factors predictive of the development of levodopa-induced dyskinesia and wearing-off in Parkinson's disease. Mov Disord. (2013) 28:1064–71. doi: 10.1002/mds.25364, PMID: [DOI] [PubMed] [Google Scholar]
- 182.Bjornestad A, Forsaa EB, Pedersen KF, Tysnes O-B, Larsen JP, Alves G. Risk and course of motor complications in a population-based incident Parkinson's disease cohort. Parkinsonism Relat Disord. (2016) 22:48–53. doi: 10.1016/j.parkreldis.2015.11.007 [DOI] [PubMed] [Google Scholar]
- 183.Schwarzschild MA, Macklin EA, Bakshi R, Battacharyya S, Logan R, Espay AJ, et al. Sex differences by design and outcome in the safety of urate elevation in PD (SURE-PD) trial. Neurology. (2019) 93:e1328–38. doi: 10.1212/WNL.0000000000008194, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Fox SH. Non-dopaminergic treatments for motor control in Parkinson’s disease. Drugs. (2013) 73:1405–15. doi: 10.1007/s40265-013-0105-4, PMID: [DOI] [PubMed] [Google Scholar]
- 185.Pellecchia MT, Picillo M, Russillo MC, De Pandis MF, Bonizzoni E, Marjanovic I, et al. Efficacy of safinamide and gender differences during routine clinical practice. Front Neurol. (2021) 12:756304. doi: 10.3389/fneur.2021.756304, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Müller T, Foley P. Clinical pharmacokinetics and pharmacodynamics of safinamide. Clin Pharmacokinet. (2017) 56:251–61. doi: 10.1007/s40262-016-0449-5, PMID: [DOI] [PubMed] [Google Scholar]
- 187.Jost ST, Strobel L, Rizos A, Loehrer PA, Ashkan K, Evans J, et al. Gender gap in deep brain stimulation for Parkinson’s disease. NPJ Parkinsons Dis. (2022) 8:1–10. doi: 10.1038/s41531-022-00305-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Chandran S, Krishnan S, Rao RM, Sarma SG, Sarma PS, Kishore A. Gender influence on selection and outcome of deep brain stimulation for Parkinson's disease. Ann Indian Acad Neurol. (2014) 17:66. doi: 10.4103/0972-2327.128557, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Dalrymple WA, Pusso A, Sperling SA, Flanigan JL, Huss DS, Harrison MB, et al. Comparison of Parkinson’s disease patients’ characteristics by indication for deep brain stimulation: men are more likely to have dbs for tremor. Tremor Other Hyperkinet Mov. (2019) 9:676. doi: 10.7916/tohm.v0.676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Shpiner DS, Di Luca DG, Cajigas I, Diaz JS, Margolesky J, Moore H, et al. Gender disparities in deep brain stimulation for Parkinson's disease. Neuromodulation: Technology at the Neural. Interface. (2019) 22:484–8. doi: 10.1111/ner.12973 [DOI] [PubMed] [Google Scholar]
- 191.Chan AK, McGovern RA, Brown LT, Sheehy JP, Zacharia BE, Mikell CB, et al. Disparities in access to deep brain stimulation surgery for Parkinson disease: interaction between African American race and Medicaid use. JAMA Neurol. (2014) 71:291–9. doi: 10.1001/jamaneurol.2013.5798, PMID: [DOI] [PubMed] [Google Scholar]
- 192.Willis AW, Schootman M, Kung N, Wang X-Y, Perlmutter JS, Racette BA. Disparities in deep brain stimulation surgery among insured elders with Parkinson disease. Neurology. (2014) 82:163–71. doi: 10.1212/WNL.0000000000000017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Roediger J, Artusi CA, Romagnolo A, Boyne P, Zibetti M, Lopiano L, et al. Effect of subthalamic deep brain stimulation on posture in Parkinson's disease: a blind computerized analysis. Parkinsonism Relat Disord. (2019) 62:122–7. doi: 10.1016/j.parkreldis.2019.01.003, PMID: [DOI] [PubMed] [Google Scholar]
- 194.Hamberg K, Hariz G-M. The decision-making process leading to deep brain stimulation in men and women with Parkinson’s disease–an interview study. BMC Neurol. (2014) 14:1–10. doi: 10.1186/1471-2377-14-89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Chiou S-M. Sex-related prognostic predictors for Parkinson disease undergoing subthalamic stimulation. World Neurosurg. (2015) 84:906–12. doi: 10.1016/j.wneu.2015.05.023, PMID: [DOI] [PubMed] [Google Scholar]
- 196.Golfrè Andreasi N, Romito LM, Telese R, Cilia R, Elia AE, Novelli A, et al. Short-and long-term motor outcome of STN-DBS in Parkinson’s disease: focus on sex differences. Neurol Sci. (2022) 43:1769–81. doi: 10.1007/s10072-021-05564-w, PMID: [DOI] [PubMed] [Google Scholar]
- 197.Rocha AL, Oliveira A, Sousa C, Monteiro P, Rosas MJ, Vaz R. Long term mortality of patients with Parkinson’s disease treated with deep brain stimulation in a reference center. Clin Neurol Neurosurg. (2021) 202:106486. doi: 10.1016/j.clineuro.2021.106486, PMID: [DOI] [PubMed] [Google Scholar]
- 198.Kim R, Yoo D, Choi J-H, Shin JH, Park S, Kim H-J, et al. Sex differences in the short-term and long-term effects of subthalamic nucleus stimulation in Parkinson's disease. Parkinsonism Relat Disord. (2019) 68:73–8. doi: 10.1016/j.parkreldis.2019.09.027, PMID: [DOI] [PubMed] [Google Scholar]
- 199.Hariz GM, Limousin P, Zrinzo L, Tripoliti E, Aviles-Olmos I, Jahanshahi M, et al. Gender differences in quality of life following subthalamic stimulation for P arkinson's disease. Acta Neurol Scand. (2013) 128:281–5. doi: 10.1111/ane.12127, PMID: [DOI] [PubMed] [Google Scholar]
- 200.Sperens M, Georgiev D, Eriksson Domellöf M, Forsgren L, Hamberg K, Hariz GM. Activities of daily living in Parkinson's disease: time/gender perspective. Acta Neurol Scand. (2020) 141:168–76. doi: 10.1111/ane.13189 [DOI] [PubMed] [Google Scholar]
- 201.Nwabuobi L, Barbosa W, Sweeney M, Oyler S, Meisel T, Di Rocco A, et al. Sex-related differences in homebound advanced Parkinson’s disease patients. Clin Interv Aging. (2019) 14:1371. doi: 10.2147/CIA.S203690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Meng D, Jin Z, Gao L, Wang Y, Wang R, Fang J, et al. The quality of life in patients with Parkinson's disease: focus on gender difference. Brain Behav. (2022) 12:e2517. doi: 10.1002/brb3.2517, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Iwaki H, Blauwendraat C, Leonard HL, Makarious MB, Kim JJ, Liu G, et al. Differences in the presentation and progression of Parkinson's disease by sex. Mov Disord. (2021) 36:106–17. doi: 10.1002/mds.28312, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Fullard ME, Thibault DP, Todaro V, Foster S, Katz L, Morgan R, et al. Sex disparities in health and health care utilization after Parkinson diagnosis: rethinking PD associated disability. Parkinsonism Relat Disord. (2018) 48:45–50. doi: 10.1016/j.parkreldis.2017.12.012, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Abraham DS, Gruber-Baldini AL, Magder LS, McArdle PF, Tom SE, Barr E, et al. Sex differences in Parkinson's disease presentation and progression. Parkinsonism Relat Disord. (2019) 69:48–54. doi: 10.1016/j.parkreldis.2019.10.019, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Farhadi F, Vosoughi K, Shahidi GA, Delbari A, Lökk J, Fereshtehnejad S-M. Sexual dimorphism in Parkinson’s disease: differences in clinical manifestations, quality of life and psychosocial functioning between males and females. Neuropsychiatr Dis Treat. (2017) 13:329. doi: 10.2147/NDT.S124984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Dahodwala N, Pei Q, Schmidt P. Sex differences in the clinical progression of Parkinson's disease. J Obstet Gynecol Neonatal Nurs. (2016) 45:749–56. doi: 10.1016/j.jogn.2016.05.002, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Yoon J-E, Kim JS, Jang W, Park J, Oh E, Youn J, et al. Gender differences of nonmotor symptoms affecting quality of life in Parkinson disease. Neurodegener Dis. (2017) 17:276–80. doi: 10.1159/000479111, PMID: [DOI] [PubMed] [Google Scholar]
- 209.Vlaanderen FP, De Man Y, Krijthe JH, Tanke MA, Groenewoud A, Jeurissen PP, et al. Sex-specific patient journeys in early Parkinson's disease in the Netherlands. Front Neurol. (2019) 10:794. doi: 10.3389/fneur.2019.00794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Zupancic M, Mahajan A, Handa K. Dementia with Lewy bodies: diagnosis and management for primary care providers. Prim Care Companion CNS Disord. (2011) 13:26212. doi: 10.4088/PCC.11r01190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.McKeith IG, Dickson DW, Lowe J, Emre M, O’brien J, Feldman H, et al. Diagnosis and management of dementia with Lewy bodies: third report of the DLB consortium. Neurology. (2005) 65:1863–72. doi: 10.1212/01.wnl.0000187889.17253.b1, PMID: [DOI] [PubMed] [Google Scholar]
- 212.Vekrellis K, Xilouri M, Emmanouilidou E, Rideout HJ, Stefanis L. Pathological roles of α-synuclein in neurological disorders. Lancet Neurol. (2011) 10:1015–25. doi: 10.1016/S1474-4422(11)70213-7, PMID: [DOI] [PubMed] [Google Scholar]
- 213.Van Steenoven I, Aarsland D, Weintraub D, Londos E, Blanc F, Van der Flier WM, et al. Cerebrospinal fluid Alzheimer’s disease biomarkers across the spectrum of Lewy body diseases: results from a large multicenter cohort. J Alzheimers Dis. (2016) 54:287–95. doi: 10.3233/JAD-160322, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Amin J, Erskine D, Donaghy PC, Surendranathan A, Swann P, Kunicki AP, et al. Inflammation in dementia with Lewy bodies. Neurobiol Dis. (2022) 168:105698. doi: 10.1016/j.nbd.2022.105698, PMID: [DOI] [PubMed] [Google Scholar]
- 215.King E, O'Brien JT, Donaghy P, Morris C, Barnett N, Olsen K, et al. Peripheral inflammation in prodromal Alzheimer's and Lewy body dementias. J Neurol Neurosurg Psychiatry. (2018) 89:339–45. doi: 10.1136/jnnp-2017-317134, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Mouton A, Blanc F, Gros A, Manera V, Fabre R, Sauleau E, et al. Sex ratio in dementia with Lewy bodies balanced between Alzheimer’s disease and Parkinson’s disease dementia: a cross-sectional study. Alzheimers Res Ther. (2018) 10:1–10. doi: 10.1186/s13195-018-0417-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Gan J, Liu S, Wang X, Shi Z, Shen L, Li X, et al. Clinical characteristics of Lewy body dementia in Chinese memory clinics. BMC Neurol. (2021) 21:1–11. doi: 10.1186/s12883-021-02169-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Savica R, Grossardt BR, Bower JH, Boeve BF, Ahlskog JE, Rocca WA. Incidence of dementia with Lewy bodies and Parkinson disease dementia. JAMA Neurol. (2013) 70:1396–402. doi: 10.1001/jamaneurol.2013.3579, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Price A, Farooq R, Yuan J-M, Menon VB, Cardinal RN, O’Brien JT. Mortality in dementia with Lewy bodies compared with Alzheimer’s dementia: a retrospective naturalistic cohort study. BMJ Open. (2017) 7:e017504. doi: 10.1136/bmjopen-2017-017504, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Boot BP, Orr CF, Ahlskog JE, Ferman TJ, Roberts R, Pankratz VS, et al. Risk factors for dementia with Lewy bodies: a case-control study. Neurology. (2013) 81:833–40. doi: 10.1212/WNL.0b013e3182a2cbd1, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Jones SV, O’brien J. The prevalence and incidence of dementia with Lewy bodies: a systematic review of population and clinical studies. Psychol Med. (2014) 44:673–83. doi: 10.1017/S0033291713000494, PMID: [DOI] [PubMed] [Google Scholar]
- 222.Nelson PT, Jicha GA, Kryscio RJ, Abner EL, Schmitt FA, Cooper G, et al. Low sensitivity in clinical diagnoses of dementia with Lewy bodies. J Neurol. (2010) 257:359–66. doi: 10.1007/s00415-009-5324-y, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Podcasy JL, Epperson CN. Considering sex and gender in Alzheimer disease and other dementias. Dialogues Clin Neurosci. (2022) 18:437–46. doi: 10.31887/DCNS.2016.18.4/cepperson [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Zhao L, Mao Z, Woody SK, Brinton RD. Sex differences in metabolic aging of the brain: insights into female susceptibility to Alzheimer's disease. Neurobiol Aging. (2016) 42:69–79. doi: 10.1016/j.neurobiolaging.2016.02.011, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Noe E, Marder K, Bell KL, Jacobs DM, Manly JJ, Stern Y. Comparison of dementia with Lewy bodies to Alzheimer's disease and Parkinson's disease with dementia. Mov Disord. (2004) 19:60–7. doi: 10.1002/mds.10633, PMID: [DOI] [PubMed] [Google Scholar]
- 226.Bayram E, Coughlin DG, Litvan I. Sex differences for clinical correlates of alzheimer's pathology in people with lewy body pathology. Mov Disord. (2022) 37:1505–15. doi: 10.1002/mds.29044, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Ferreira D, Przybelski SA, Lesnick TG, Lemstra AW, Londos E, Blanc F, et al. β-Amyloid and tau biomarkers and clinical phenotype in dementia with Lewy bodies. Neurology. (2020) 95:e3257–68. doi: 10.1212/WNL.0000000000010943, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Gámez-Valero A, Prada-Dacasa P, Santos C, Adame-Castillo C, Campdelacreu J, Reñé R, et al. GBA mutations are associated with earlier onset and male sex in dementia with Lewy bodies. Mov Disord. (2016) 31:1066–70. doi: 10.1002/mds.26593, PMID: [DOI] [PubMed] [Google Scholar]
- 229.Liu L, Li J, Quan W, Qin Y, Zhang Q, Pei X, et al. Effect of GBA gene variants on clinical characteristics of dementia with Lewy bodies: a review and meta-analyses. Neurol Sci. (2022) 43:3541–50. doi: 10.1007/s10072-022-06031-w, PMID: [DOI] [PubMed] [Google Scholar]
- 230.Tsunoda N, Hashimoto M, Ishikawa T, Fukuhara R, Yuki S, Tanaka H, et al. Clinical features of auditory hallucinations in patients with dementia with Lewy bodies: a soundtrack of visual hallucinations. J Clin Psychiatry. (2018) 79:1673. doi: 10.4088/JCP.17m11623 [DOI] [PubMed] [Google Scholar]
- 231.Chiu PY, Teng PR, Wei CY, Wang CW, Tsai CT. Gender difference in the association and presentation of visual hallucinations in dementia with Lewy bodies: a cross-sectional study. Int J Geriatr Psychiatry. (2018) 33:193–9. doi: 10.1002/gps.4706, PMID: [DOI] [PubMed] [Google Scholar]
- 232.Choudhury P, Graff-Radford J, Aakre JA, Wurtz L, Knopman DS, Graff-Radford NR, et al. The temporal onset of the core features in dementia with Lewy bodies. Alzheimers Dement. (2022) 18:591–601. doi: 10.1002/alz.12411, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Bayram E, Coughlin DG, Banks SJ, Litvan I. Sex differences for phenotype in pathologically defined dementia with Lewy bodies. J Neurol Neurosurg Psychiatry. (2021) 92:745–50. doi: 10.1136/jnnp-2020-325668, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Abdelnour C, Ferreira D, van de Beek M, Cedres N, Oppedal K, Cavallin L, et al. Parsing heterogeneity within dementia with Lewy bodies using clustering of biological, clinical, and demographic data. Alzheimers Res Ther. (2022) 14:1–13. doi: 10.1186/s13195-021-00946-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Agbomi LL, Onuoha CP, Nathaniel SI, Coker-Ayo OO, Bailey-Taylor MJ, Roley LT, et al. Gender differences in Parkinson's disease with dementia and dementia with Lewy bodies. Aging Health Res. (2022) 2:100096. doi: 10.1016/j.ahr.2022.100096, PMID: 36826477 [DOI] [Google Scholar]
- 236.Van de Beek M, Babapour Mofrad R, van Steenoven I, Vanderstichele H, Scheltens P, Teunissen C, et al. Sex-specific associations with cerebrospinal fluid biomarkers in dementia with Lewy bodies. Alzheimers Res Ther. (2020) 12:1–8. doi: 10.1186/s13195-020-00610-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Sarro L, Tosakulwong N, Schwarz CG, Graff-Radford J, Przybelski SA, Lesnick TG, et al. An investigation of cerebrovascular lesions in dementia with Lewy bodies compared to Alzheimer's disease. Alzheimers Dement. (2017) 13:257–66. doi: 10.1016/j.jalz.2016.07.003, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Wennström M, Londos E, Minthon L, Nielsen HM. Altered CSF orexin and α-synuclein levels in dementia patients. J Alzheimers Dis. (2012) 29:125–32. doi: 10.3233/JAD-2012-111655, PMID: [DOI] [PubMed] [Google Scholar]
- 239.Fanciulli A, Wenning GK. Multiple-system atrophy. N Engl J Med. (2015) 372:249–63. doi: 10.1056/NEJMra1311488, PMID: [DOI] [PubMed] [Google Scholar]
- 240.Salvesen L, Winge K, Brudek T, Agander TK, Lokkegaard A, Pakkenberg B. Neocortical neuronal loss in patients with multiple system atrophy: a stereological study. Cereb Cortex. (2017) 27:400–10. doi: 10.1093/cercor/bhv228, PMID: [DOI] [PubMed] [Google Scholar]
- 241.Kiely AP, Murray CE, Foti SC, Benson BC, Courtney R, Strand C, et al. Immunohistochemical and molecular investigations show alteration in the inflammatory profile of multiple system atrophy brain. J Neuropathol Exp Neurol. (2018) 77:598–607. doi: 10.1093/jnen/nly035, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Kim HJ, Jeon BS, Lee JY, Yun JY. Survival of Korean patients with multiple system atrophy. Mov Disord. (2011) 26:909–12. doi: 10.1002/mds.23580, PMID: [DOI] [PubMed] [Google Scholar]
- 243.Schrag A, Wenning GK, Quinn N, Ben-Shlomo Y. Survival in multiple system atrophy. Mov Disord. (2008) 23:294–6. doi: 10.1002/mds.21839, PMID: [DOI] [PubMed] [Google Scholar]
- 244.Jamora R, Gupta A, Tan A, Tan L. Clinical characteristics of patients with multiple system atrophy in Singapore. Ann Acad Med Singap. (2005) 34:553. [PubMed] [Google Scholar]
- 245.Wenning G, Shlomo YB, Magalhaes M, Danie S, Quinn N. Clinical features and natural history of multiple system atrophy: an analysis of 100 cases. Brain. (1994) 117:835–45. doi: 10.1093/brain/117.4.835, PMID: [DOI] [PubMed] [Google Scholar]
- 246.O'Sullivan SS, Massey LA, Williams DR, Silveira-Moriyama L, Kempster PA, Holton JL, et al. Clinical outcomes of progressive supranuclear palsy and multiple system atrophy. Brain. (2008) 131:1362–72. doi: 10.1093/brain/awn065, PMID: [DOI] [PubMed] [Google Scholar]
- 247.Watanabe H, Saito Y, Terao S, Ando T, Kachi T, Mukai E, et al. Progression and prognosis in multiple system atrophy: an analysis of 230 Japanese patients. Brain. (2002) 125:1070–83. doi: 10.1093/brain/awf117, PMID: [DOI] [PubMed] [Google Scholar]
- 248.Ben-Shlomo Y, Wenning G, Tison F, Quinn N. Survival of patients with pathologically proven multiple system atrophy: a meta-analysis. Neurology. (1997) 48:384–93. doi: 10.1212/WNL.48.2.384, PMID: [DOI] [PubMed] [Google Scholar]
- 249.Wenning GK, Geser F, Krismer F, Seppi K, Duerr S, Boesch S, et al. The natural history of multiple system atrophy: a prospective European cohort study. Lancet Neurol. (2013) 12:264–74. doi: 10.1016/S1474-4422(12)70327-7, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Roncevic D, Palma J-A, Martinez J, Goulding N, Norcliffe-Kaufmann L, Kaufmann H. Cerebellar and parkinsonian phenotypes in multiple system atrophy: similarities, differences and survival. J Neural Transm. (2014) 121:507–12. doi: 10.1007/s00702-013-1133-7, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Figueroa JJ, Singer W, Parsaik A, Benarroch EE, Ahlskog JE, Fealey RD, et al. Multiple system atrophy: prognostic indicators of survival. Mov Disord. (2014) 29:1151–7. doi: 10.1002/mds.25927, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Low PA, Reich SG, Jankovic J, Shults CW, Stern MB, Novak P, et al. Natural history of multiple system atrophy in the USA: a prospective cohort study. Lancet Neurol. (2015) 14:710–9. doi: 10.1016/S1474-4422(15)00058-7, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Coon EA, Sletten DM, Suarez MD, Mandrekar JN, Ahlskog JE, Bower JH, et al. Clinical features and autonomic testing predict survival in multiple system atrophy. Brain. (2015) 138:3623–31. doi: 10.1093/brain/awv274, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Tada M, Onodera O, Tada M, Ozawa T, Piao Y-S, Kakita A, et al. Early development of autonomic dysfunction may predict poor prognosis in patients with multiple system atrophy. Arch Neurol. (2007) 64:256–60. doi: 10.1001/archneur.64.2.256, PMID: [DOI] [PubMed] [Google Scholar]
- 255.Papatsoris A, Papapetropoulos S, Singer C, Deliveliotis C. Urinary and erectile dysfunction in multiple system atrophy (MSA). Neurourol Urodyn. (2008) 27:22–7. doi: 10.1002/nau.20461, PMID: [DOI] [PubMed] [Google Scholar]
- 256.Raccagni C, Indelicato E, Sidoroff V, Daniaux M, Bader A, Toth B, et al. Female sexual dysfunction in multiple system atrophy: a prospective cohort study. Clin Auton Res. (2021) 31:713–7. doi: 10.1007/s10286-021-00825-2, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Oertel WH, Wächter T, Quinn NP, Ulm G, Brandstädter D. Reduced genital sensitivity in female patients with multiple system atrophy of parkinsonian type. Mov Disord. (2003) 18:430–2. doi: 10.1002/mds.10384, PMID: [DOI] [PubMed] [Google Scholar]
- 258.Cuoco S, Picillo M, Cappiello A, Carotenuto I, Erro R, Russillo MC, et al. Effects of gender on cognitive and behavioral manifestations in multiple system atrophy. J Neural Transm. (2020) 127:925–34. doi: 10.1007/s00702-020-02169-z, PMID: [DOI] [PubMed] [Google Scholar]
- 259.Chen D, Wei X, Zou J, Wang R, Liu X, Xu X, et al. Contra-directional expression of serum homocysteine and uric acid as important biomarkers of multiple system atrophy severity: a cross-sectional study. Front Cell Neurosci. (2015) 9:247. doi: 10.3389/fncel.2015.00247, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Cao B, Guo X, Chen K, Song W, Huang R, Wei Q-Q, et al. Uric acid is associated with the prevalence but not disease progression of multiple system atrophy in Chinese population. J Neurol. (2013) 260:2511–5. doi: 10.1007/s00415-013-7006-z, PMID: [DOI] [PubMed] [Google Scholar]
- 261.Cereda E, Barichella M, Cassani E, Caccialanza R, Pezzoli G. Reproductive factors and clinical features of Parkinson's disease. Parkinsonism Relat Disord. (2013) 19:1094–9. doi: 10.1016/j.parkreldis.2013.07.020, PMID: [DOI] [PubMed] [Google Scholar]
- 262.Pinizzotto CC, Patwardhan A, Aldarondo D, Kritzer MF. Task-specific effects of biological sex and sex hormones on object recognition memories in a 6-hydroxydopamine-lesion model of Parkinson's disease in adult male and female rats. Horm Behav. (2022) 144:105206. doi: 10.1016/j.yhbeh.2022.105206, PMID: [DOI] [PubMed] [Google Scholar]
- 263.Antzoulatos E, Jakowec MW, Petzinger GM, Wood RI. Sex differences in motor behavior in the MPTP mouse model of Parkinson's disease. Pharmacol Biochem Behav. (2010) 95:466–72. doi: 10.1016/j.pbb.2010.03.009, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Gillies GE, Murray HE, Dexter D, McArthur S. Sex dimorphisms in the neuroprotective effects of estrogen in an animal model of Parkinson's disease. Pharmacol Biochem Behav. (2004) 78:513–22. doi: 10.1016/j.pbb.2004.04.022, PMID: [DOI] [PubMed] [Google Scholar]
- 265.Bourque M, Soulet D, Di Paolo T. Androgens and Parkinson's disease: a review of human studies and animal models. Androgens. (2021) 2:294–303. doi: 10.1089/andro.2021.0011, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Bispo JM, Melo JE, Gois AM, Leal PC, Lins LC, Souza MF, et al. Sex differences in the progressive model of parkinsonism induced by reserpine in rats. Behav Brain Res. (2019) 363:23–9. doi: 10.1016/j.bbr.2019.01.041, PMID: [DOI] [PubMed] [Google Scholar]
- 267.Siani F, Greco R, Levandis G, Ghezzi C, Daviddi F, Demartini C, et al. Influence of estrogen modulation on glia activation in a murine model of Parkinson's disease. Front Neurosci. (2017) 11:306. doi: 10.3389/fnins.2017.00306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Smith KM, Dahodwala N. Sex differences in Parkinson's disease and other movement disorders. Exp Neurol. (2014) 259:44–56. doi: 10.1016/j.expneurol.2014.03.010, PMID: [DOI] [PubMed] [Google Scholar]
- 269.Investigators PSGP. A randomized pilot trial of estrogen replacement therapy in post-menopausal women with Parkinson’s disease. Parkinsonism Relat Disord. (2011) 17:757–60. doi: 10.1016/j.parkreldis.2011.07.007, PMID: [DOI] [PubMed] [Google Scholar]
- 270.Nicoletti A, Arabia G, Pugliese P, Nicoletti G, Torchia G, Condino F, et al. Hormonal replacement therapy in women with Parkinson disease and levodopa-induced dyskinesia: a crossover trial. Clin Neuropharmacol. (2007) 30:276–80. doi: 10.1097/wnf.0b013e318050c9f9, PMID: [DOI] [PubMed] [Google Scholar]
- 271.Rahkonen T, Eloniemi-Sulkava U, Rissanen S, Vatanen A, Viramo P, Sulkava R. Dementia with Lewy bodies according to the consensus criteria in a general population aged 75 years or older. J Neurol Neurosurg Psychiatry. (2003) 74:720–4. doi: 10.1136/jnnp.74.6.720, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Yokota O, Sasaki K, Fujisawa Y, Takahashi J, Terada S, Ishihara T, et al. Frequency of early and late-onset dementias in a Japanese memory disorders clinic. Eur J Neurol. (2005) 12:782–90. doi: 10.1111/j.1468-1331.2005.01072.x, PMID: [DOI] [PubMed] [Google Scholar]
- 273.Gascón-Bayarri J, Reñé R, Del Barrio J, De Pedro-Cuesta J, Ramón J, Manubens J, et al. Prevalence of dementia subtypes in El prat de Llobregat, Catalonia, Spain: the PRATICON study. Neuroepidemiology. (2007) 28:224–34. doi: 10.1159/000108597, PMID: [DOI] [PubMed] [Google Scholar]
- 274.Jhoo JH, Kim KW, Huh Y, Lee SB, Park JH, Lee JJ, et al. Prevalence of dementia and its subtypes in an elderly urban Korean population: results from the Korean longitudinal study on health and aging (KLoSHA). Dement Geriatr Cogn Disord. (2008) 26:270–6. doi: 10.1159/000160960, PMID: [DOI] [PubMed] [Google Scholar]
- 275.Yoshida H, Terada S, Honda H, Ata T, Takeda N, Kishimoto Y, et al. Validation of Addenbrooke's cognitive examination for detecting early dementia in a Japanese population. Psychiatry Res. (2011) 185:211–4. doi: 10.1016/j.psychres.2009.06.012, PMID: [DOI] [PubMed] [Google Scholar]
- 276.Yamada T, Hattori H, Miura A, Tanabe M, Yamori Y. Prevalence of Alzheimer's disease, vascular dementia and dementia with Lewy bodies in a Japanese population. Psychiatry Clin Neurosci. (2001) 55:21–5. doi: 10.1046/j.1440-1819.2001.00779.x [DOI] [PubMed] [Google Scholar]
- 277.Aarsland D, Rongve A, Nore SP, Skogseth R, Skulstad S, Ehrt U, et al. Frequency and case identification of dementia with Lewy bodies using the revised consensus criteria. Dement Geriatr Cogn Disord. (2008) 26:445–52. doi: 10.1159/000165917, PMID: [DOI] [PubMed] [Google Scholar]
- 278.Alladi S, Mekala S, Chadalawada SK, Jala S, Mridula R, Kaul S. Subtypes of dementia: a study from a memory clinic in India. Dement Geriatr Cogn Disord. (2011) 32:32–8. doi: 10.1159/000329862, PMID: [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.