Dear Editor,
Collecting duct carcinoma (CDC) or Bellini carcinoma originates in the distal collecting ducts. CDC is a markedly aggressive subtype of renal cell carcinoma and accounts for less than 1% of all renal cell carcinomas [1]. We described a patient with CDC and psoriasis, who showed complete response (CR) after being treated with combination therapy of nivolumab and ipilimumab (NI). The study was conducted in accordance with and approved by the Ethics Committee of Yamagata University Faculty of Medicine (approval no. 2019-35). The need for patient consent was waived by the same institutional review board; all the patients provided written informed consents.
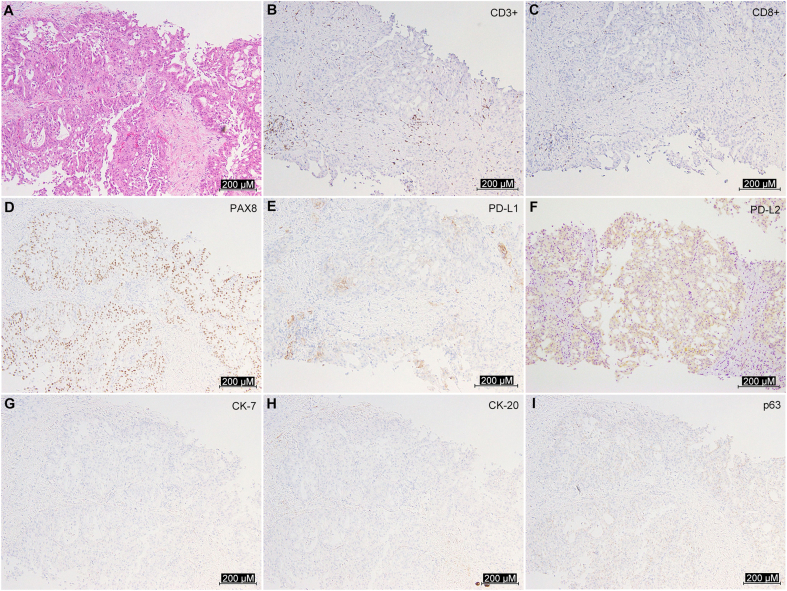
A 58-year-old man with a history of psoriasis and hypertension was admitted to our institution for the treatment of a metastatic renal tumor. Psoriasis was controlled with a topical ointment alone on admission; however, he had been treated with cyclosporine until a month before admission. Cyclosporine was started at 5 mg/kg, decreased by 1 mg/kg monthly and maintained at 3 mg/kg for a total of 1 year. It was discontinued 1 month before admission because the symptoms stabilized. A computed tomography scan revealed a 4.0-cm left renal tumor invading the renal vein with vertebral and hepatic metastases. The tumor was poorly enhanced by contrast medium and its boundary with normal kidney tissue was unclear, while the outline of the kidney was intact (Fig. 1A–C). He underwent a percutaneous biopsy that showed tubular growth and fibrous stroma with lymphocyte infiltration on hematoxylin and eosin staining. Furthermore, immunohistochemical staining of the specimen was positive for paired box 8, programmed death (PD) ligand 1 (PD-L1, positive rate of 5%), and PD-L2 (positive rate of 15%), whereas it was negative for cytokeratin-7 (CK-7), CK-20, or p63 (Supplementary Fig. 1). CDC is a poorly circumscribed tumor at the medullary pyramids and shows strong dysplasia, papillary or tubular growth, and fibrous stroma on hematoxylin and eosin staining [2]. Because of overlapping morphological and immunohistochemical characteristics between CDC and urothelial carcinoma, CDC is often misdiagnosed as urothelial carcinoma [2]. Recently, Albadine et al. [2] reported that in the differential diagnosis of them, an immunostaining pattern of paired box 8-positive and p63-negative supported the diagnosis of CDC with a sensitivity of 85.7% and specificity of 100.0% [2]. Hence, the patient was diagnosed with CDC.
Figure 1.
The radiographic images of this case. (A–C) Radiographic images at diagnosis. (A) Primary left renal lesion on CT; (B) Vertebral metastasis on CT; (C) Multiple hepatic metastases on MRI. (D–F) Radiographic images after two cycles of combination therapy with nivolumab and ipilimumab. (D) The primary lesion remarkably shrunk; (E and F) Vertebral and multiple hepatic metastases disappeared after 2 cycles of the therapy. (G–I) Radiographic images 4 months after the first diagnosis. CT and MRI showed complete response 4 months after the first diagnosis. (J) FDG-PET showed durable complete response 10 months after the initial diagnosis. CT, computed tomography; MRI, magnetic resonance imaging; FDG-PET, fluorodeoxyglucose-positron emission tomography.
NI was administered as first-line systemic therapy after three doses of radiation therapy with a total of 33 Gy for vertebral metastasis. After two cycles of nivolumab at 240 mg/body every 3 weeks and ipilimumab at 3 mg/kg every 3 weeks, the primary tumor and all the metastases shrunk remarkably (Fig. 1D–F). However, NI has to be stopped due to persistent Grade 1 interstitial pneumonia. Two months later, all lesions had disappeared (Fig. 1G–I), and the pneumonia was improved without any medication for pneumonia. Nine months after NI discontinuation, no lesions, including metastatic bone lesion, were detected on fluorodeoxyglucose-positron emission tomography (Fig. 1J). At 15 months after discontinuing systemic therapy, the patient maintained CR, and psoriasis has been controlled with topical ointment treatment alone.
The efficacy of systemic therapy is limited, and patients with metastatic CDC have an extremely poor prognosis. Although two previous case reports showed CR to gemcitabine and platinum salt with/without bevacizumab [3,4], a phase II trial of gemcitabine and platinum salt for metastatic CDC demonstrated no CR and the median progression-free survival and OS of 7.1 months and 10.5 months, respectively [5]. A retrospective study of seven patients with metastatic CDC treated with molecular-targeted drugs showed that five patients developed early disease progression with a very short 4-month survival, whereas two patients had a more prolonged survival of 49 months and 19 months [1]. In another retrospective study of five patients treated with a triple combination of gemcitabine and platinum salt and bevacizumab, the median progression-free survival and OS were 15.1 months and 27.8 months, respectively [6].
Although no clinical trial has been performed using immune-checkpoint inhibitors (ICIs), we found three case reports mentioned the effectiveness of ICIs in metastatic CDC, of which one patient was treated with NI and the remaining two patients were treated with nivolumab [[7], [8], [9]]. The patient treated with NI had small lymph node metastasis after nephrectomy and showed CR, whereas the two patients treated with nivolumab showed partial response. To the best of our knowledge, this is the second case of CR with NI and the first case of CR without nephrectomy for metastatic CDC. In addition, the present case had the largest volume of metastatic lesions among the reported patients with CR.
In accordance with the present case, a previous report demonstrated tumor-infiltrating lymphocytes in all the tumor specimens of CDC, and gene ontology analysis mentioned that genes overexpressed in CDC were highly enriched for the gene sets related to the positive regulation of T cells and leukocyte activation [10]. These findings supported the fact that ICIs are promising treatments for CDC. Furthermore, PD-L1 and PD-L2 were expressed in the present case, as also demonstrated by Mizutani et al. [9].
There are two major problems in using ICIs to treat patients with psoriasis: treatments for psoriasis may attenuate the effect of ICIs and ICIs may conversely exacerbate psoriasis. According to a retrospective study for non-small cell lung cancer, a baseline equivalent of corticosteroid use of greater than or equal to 10 mg prednisolone was associated with poorer outcome in patients treated with PD-L1 inhibitors than in patients treated with an equivalent of less than 10 mg prednisolone [11]. Nikolaou et al. [12] reported that drugs for psoriasis, acitretin, apremilast, and methotrexate did not regulate adaptive immunity. Furthermore, they mentioned that treatments for psoriasis did not interfere with ICI therapy because there was no significant difference in ICI discontinuation rates between topical and intravenous treatments for psoriasis.
Author contributions
Study concept and design: Keisuke Funajima, Sei Naito, Hayato Nishida, Tomoyuki Kato, Norihiko Tsuchiya.
Pathological works: Takanobu Kabasawa, Mitsuru Futakuchi.
Data acquisition: Keisuke Funajima, Sei Naito.
Drafting of manuscript: Keisuke Funajima, Sei Naito.
Critical revision of the manuscript: Hayato Nishida, Tomoyuki Kato, Norihiko Tsuchiya.
Conflicts of interest
Sei Naito has received honoraria from Ono, BMS, Takeda Pharm, Merk, Pfizer, and Eisai as their sponsored speaker. Tomoyuki Kato has received honoraria from Pfizer as their sponsored speaker. Norihiko Tsuchiya has received honoraria from Pfizer, Janssen, Novartis, Ono, Bayer, Sanofi, Takeda, BMS, and Astelas Pharma as their sponsored speaker, and research funds from Pfizer and Eisai. The other authors declare no conflict of interest.
Acknowledgments
We would like to thank Enago (www.enago.jp) for the English language editing.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ajur.2022.02.016.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
Supplementary Figure 1.
Pathological features. (A) H & E staining of tissue sections from the biopsy specimen showed tubular growth and fibrous stroma; (B and C) CD3+ and CD8+ cells infiltrate the specimens; (D–F) Immunohistochemical staining of the specimen was positive for PAX8, PD-L1, and PD-L2; (G–I) Immunohistochemical staining was negative for CK-7, CK-20, and p63. PD, programmed death; PAX8, paired box 8; PD-L, programmed death ligand; CK, cytokeratin-7; H & E, hematoxylin and eosin.
References
- 1.Procopio G., Verzoni E., Iacovelli R., Colecchia M., Torelli T., Mariani L. Is there a role for targeted therapies in the collecting ducts of Bellini carcinoma? Efficacy data from a retrospective analysis of 7 cases. Clin Exp Nephrol. 2012;16:464–467. doi: 10.1007/s10157-012-0589-3. [DOI] [PubMed] [Google Scholar]
- 2.Albadine R., Schultz L., Illei P., Ertoy D., Hicks J., Sharma R., et al. PAX8 (+)/p63 (−) immunostaining pattern in renal collecting duct carcinoma (CDC): a useful immunoprofile in the differential diagnosis of CDC versus urothelial carcinoma of upper urinary tract. Am J Surg Pathol. 2010;34:965–969. doi: 10.1097/PAS.0b013e3181dc5e8a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dano D., Delteil C., Boissier R., Delaporte V., Habert P., Salas S., et al. Rechallenge of carboplatin-gemcitabine based chemotherapy for rapidly progressing metastatic collecting duct carcinoma of the kidney leading to a delayed and durable complete response: a case report. Oncol Lett. 2019;17:3576–3580. doi: 10.3892/ol.2019.9991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barrascout E., Beuselinck B., Ayllon J., Bättig B., Moch H., Teghom C., et al. Complete remission of pulmonary metastases of Bellini duct carcinoma with cisplatin, gemcitabine and bevacizumab. Am J Case Rep. 2012;13:1–2. doi: 10.12659/AJCR.882234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oudard S., Banu E., Vieillefond A., Fournier L., Priou F., Medioni J., et al. Prospective multicenter phase II study of gemcitabine plus platinum salt for metastatic collecting duct carcinoma: results of a GETUG (Groupe d'Etudes des Tumeurs Uro-Génitales) study. J Urol. 2007;177:1698–1702. doi: 10.1016/j.juro.2007.01.063. [DOI] [PubMed] [Google Scholar]
- 6.Pécuchet N., Bigot F., Gachet J., Massard C., Albiges L., Teghom C., et al. Triple combination of bevacizumab, gemcitabine and platinum salt in metastatic collecting duct carcinoma. Ann Oncol. 2013;24:2963–2967. doi: 10.1093/annonc/mdt423. [DOI] [PubMed] [Google Scholar]
- 7.Watanabe K., Sugiyama T., Otsuka A., Miyake H. Complete response to combination therapy with nivolumab and ipilimumab for metastatic collecting duct carcinoma of the kidney. Int Cancer Conf J. 2020;9:32–35. doi: 10.1007/s13691-019-00389-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yasuoka S., Hamasaki T., Kuribayashi E., Nagasawa M., Kawaguchi T., Nagashima Y., et al. Nivolumab therapy for metastatic collecting duct carcinoma after nephrectomy: a case report. Medicine. 2018;97 doi: 10.1097/MD.0000000000013173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mizutani K., Horie K., Nagai S., Tsuchiya T., Saigo C., Kobayashi K., et al. Response to nivolumab in metastatic collecting duct carcinoma expressing PD-L1: a case report. Mol Clin Oncol. 2017;7:988–990. doi: 10.3892/mco.2017.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Malouf G.G., Compérat E., Yao H., Mouawad R., Lindner V., Rioux-leclercq N., et al. Unique transcriptomic profile of collecting duct carcinomas relative to upper tract urothelial carcinomas and other kidney carcinomas. Sci Rep. 2016;6 doi: 10.1038/srep30988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arbour K.C., Mezquita L., Long N., Rizvi H., Auclin E., Ni A., et al. Impact of Baseline steroids on efficacy of programmed cell death-1 and programmed death-ligand 1 blockade in patients with non-small-cell lung cancer. J Clin Oncol. 2018;36:2872–2878. doi: 10.1200/JCO.2018.79.0006. [DOI] [PubMed] [Google Scholar]
- 12.Nikolaou V., Sibaud V., Fattore D., Sollena P., Ortiz-Brugués A., Giacchero D., et al. Immune checkpoint-mediated psoriasis: a multicenter European study of 115 patients from the European Network for Cutaneous Adverse Event to Oncologic Drugs (ENCADO) group. J Am Acad Dermatol. 2021;84:1310–1320. doi: 10.1016/j.jaad.2020.08.137. [DOI] [PubMed] [Google Scholar]