Abstract
AF is a chronic and progressive heart rhythm disorder characterised by exacerbations and remissions. Contemporary guidelines recommend antiarrhythmic drugs (AADs) as the initial therapy for the maintenance of sinus rhythm. However, these medications have modest efficacy and are associated with significant adverse effects. Several recent trials have evaluated catheter ablation as an initial therapy for AF, demonstrating that cryoballoon catheter ablation significantly improves arrhythmia outcomes (e.g. atrial tachyarrhythmia recurrence and arrhythmia burden), produces clinically meaningful improvements in patient-reported outcomes (e.g. symptoms and quality of life), and significantly decreases healthcare resource usage (e.g. hospitalisation), without increasing the risk of serious adverse events. Moreover, in contrast to antiarrhythmic drugs, catheter ablation appears to be disease-modifying, significantly reducing the progression of disease. These findings are relevant to patients, providers, and healthcare systems, helping inform the initial choice of rhythm-control therapy in patients with treatment-naïve AF.
Keywords: Atrial fibrillation, antiarrhythmic drugs, ablation, catheter ablation, cryoballoon, cryotherapy
AF is the most common sustained cardiac dysrhythmia, affecting 2–3% of the population.[1] AF is associated with significant impairments in quality of life, as well as significantly increased risk of adverse cardiovascular outcomes (e.g. heart failure, thromboembolism and premature mortality). As such, AF management is focused on improving arrhythmia-related symptoms as well as reducing morbidity associated with AF by using strategies to meaningfully reduce AF-associated healthcare usage.[2]
While previous studies suggested that control of the ventricular rate was comparable to a strategy of sinus rhythm maintenance in patients with established AF, the recent EAST-AFNET 4 randomised trial demonstrated that early rhythm control (i.e. within the first year after diagnosis) provided significant benefit.[3] Specifically, early rhythm control was associated with significant reductions in the composite primary outcome of cardiovascular death, stroke and hospitalisation for worsening heart failure and acute coronary syndrome by 21% (from 5.0% per year to 3.9% per year) over a median follow-up of 5.1 years, which was driven by a significant reduction in cardiovascular death (1.0 versus 1.3% per year; HR 0.72; 95% CI [0.52–0.98]) as well as the incidence of stroke (0.6 versus 0.9% per year; HR 0.65; 95% CI [0.44–0.97]).[4] Consistent with major guidelines, rhythm control in the EAST-AFNET 4 trial was predominantly pharmacological, with the majority of patients receiving class Ic antiarrhythmic drug therapy.[5]
While antiarrhythmic drug therapy is relatively superior to placebo in the prevention of arrhythmia recurrence, these agents have only modest efficacy in maintaining sinus rhythm.[6,7] Moreover, antiarrhythmic drug therapy is associated with significant side effects, including, in the case of sotalol, an increase in all-cause mortality (RR 2.23 versus placebo; 95% CI [1.03–4.81]).[8]
This may explain the apparent lack of benefit observed with pharmacological rhythm control in previous studies. Highlighting this point was a time-dependent on-treatment analysis of the landmark AFFIRM trial, which demonstrated that the presence of sinus rhythm was associated with a lower risk of death (HR 0.53; 95% CI [0.42–0.70]; p<0.001). However, this benefit was offset by the harm associated with the use of pharmacologic rhythm control (HR 1.50; 95% CI [1.18–1.89]; p<0.001).[9]
Catheter ablation has been shown in multiple randomised controlled trials to be superior to antiarrhythmic drug therapy in maintaining sinus rhythm when antiarrhythmic drugs have been ineffective, are contraindicated, or cause intolerable adverse effects.[10] These percutaneous procedures are based on the electrical isolation of pulmonary veins, targeting the regions of the left atrium responsible for AF initiation and perpetuation. However, these second-line trials focused on patients who had already failed antiarrhythmic drug therapy. By design, these trials selected a population in whom antiarrhythmic drugs had already proven to be ineffectual, biasing the results towards a benefit for those patients randomised to catheter ablation. Until recently, it was not known whether early intervention (i.e. ablation performed prior to antiarrhythmic drug failure) would offer similar benefits in preventing arrhythmia, improving quality of life, and reducing healthcare utilisation.
Catheter Ablation as a First-line Therapy
Between 2001 and 2010, there were three randomised trials of first-line focal point-by-point radiofrequency ablation: RAAFT-1, MANTRA-PAF and RAAFT-2.[11–13] In aggregate, these three studies demonstrated that first-line ablation was more effective than antiarrhythmic drugs at preventing arrhythmia recurrence. However, the benefit was relatively limited (RR 0.81 for any arrhythmia; 95% CI [0.68–0.96]; p=0.01, Figure 1; and RR 0.62 for symptomatic arrhythmia; 95% CI [0.38–1.01]; p=0.06, Figure 2).[14] These results did not translate into clinically meaningful improvements in quality of life or healthcare usage. Moreover, these studies were individually limited by relatively small sample size, high rates of crossover from antiarrhythmic drugs to ablation, lack of procedural standardisation, and differing procedural and study endpoints. As such, these studies had only a modest impact on clinical practice and did not result in a significant change to the guidelines.
Figure 1: Recurrence of Any Atrial Tachyarrhythmia, Stratified by Ablation Energy.

M-H = Mantel–Haenszel. Source: Andrade et al. 2021.[14] Reproduced with permission from Elsevier.
Figure 2: Recurrence of Symptomatic Atrial Tachyarrhythmia, Stratified by Ablation Energy.
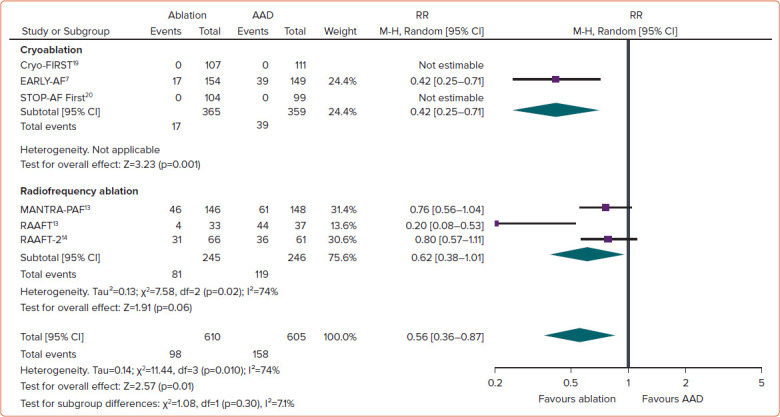
M-H = Mantel–Haenszel. Source: Andrade et al. 2021.[14] Reproduced with permission from Elsevier.
The emergence of dedicated ablation technologies meant there was renewed interest in the role of catheter ablation as the initial management of treatment-naïve AF. These novel ablation technologies are less reliant on operator dexterity, enabling procedural standardisation and ensuring consistent outcomes with relatively low rates of complications.[15–18]
Initiated between 2014 and 2017, three multicentre parallel-group, single-blinded randomised clinical trials examined the role of first-line cryoballoon ablation: EARLY-AF, STOP-AF First, and Cryo-FIRST.[7,19,20] The three trials included a total of 724 relatively young and relatively healthy patients with treatment-naïve paroxysmal AF in their intention-to-treat or modified intention-to-treat populations (Table 1).[7,19,20]
Table 1: Characteristics of the Three First-line Cryoballoon Ablation Trials.
| Cryo-FIRST[19] | Early-AF[7] | STOP-AF First[20] | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Ablation | AAD | Treatment Effect [95% CI] | Ablation | AAD | Treatment Effect [95% CI] | Ablation | AAD | Treatment Effect [95% CI] | |
| Setting (no. centres) | Australia, Europe, Latin America (20) | Canada (18) | US (24) | ||||||
| Randomised/included in analysis (n) | 107/107 | 111/111 | 154/154 | 149/149 | 108/104 | 102/99 | |||
| Baseline demographics | |||||||||
| Age (years) | 50.5 | 54.1 | 57.7 | 59.5 | 60.4 | 61.6 | |||
| Man, n (%) | 76 (71) | 72 (65) | 112 (73) | 102 (68) | 63 (61) | 57 (58) | |||
| Arrhythmia monitoring protocol | 7-day Holter every 3 months | Implantable cardiac monitor with daily transmissions | 24 h Holter every 6 months Weekly patient-activated trans-telephonic event recorder |
||||||
| Primary outcome | Any recurrence of atrial tachyarrhythmia (AF, AT, AFL) lasting longer than 30 s | ||||||||
|
19 (17.8) | 36 (32.4) | 0.48 [0.26– 0.86] | 66 (42.9) | 101 (67.8) | 0.48 [0.35–0.66] | 21 (20.2) | 35 (35.4) | 0.57 [0.36–0.91] |
| Absolute risk reduction | 14.6% | 24.9% | 15.2% | ||||||
| Key secondary outcomes | |||||||||
| AF burden (mean ± SD) | NR | NR | 0.6 ± 3.3 | 3.9 ± 12.4 | −3.3 ± 1.0* | NR | NR | ||
Symptoms:
|
NR 77 (86.5) |
NR 69 (70.4) |
1.23 (1.06, 1.43) | 17 (11.0) 131 (85.1) |
39 (26.2) 109 (73.2) |
0.39 (0.22, 0.68) 1.16 (1.03, 1.31) |
NR NR |
NR NR |
|
| Quality of life | |||||||||
|
88.9 ± 12.8 | 78.1 ± 19.8 | 9.9 [5.5–14.2] | 88.3 ± 19.1 | 80.3 ± 19.1 | 8.0 (3.69, 12.31) | 91.9 ± 15.4 | 84.9 ± 15.4 | 7.0 [2.6–11.4] |
|
25 | 39 | 0.66 [0.43–1.02] | 30 | 36 | 0.81 [0.53–1.24] | 31 | 43 | 0.69 [0.47–0.99] |
|
NR | NR | 10 | 14 | 0.69 [0.32–1.51] | 3 | 7 | 0.41 [0.11–1.53] | |
|
NR | NR | 28 | 30 | 0.90 [0.57–1.43] | 10 | 17 | 0.56 [0.27–1.16] | |
|
NR | NR | 5 | 13 | 0.37 [0.14–1.02] | 13 | 32 | 0.39 [0.22–0.69] | |
|
26 (24.3) 42 events |
37 (33.3) 56 events |
0.73 [0.48–1.12] | 14 (9.1) 15 events |
24 (16.1) 27 events |
0.56 [0.30–1.05] | 34 67 events |
45 82 events |
0.72 [0.51–1.02] |
± values are mean ± SE, except for AF burden, which is expressed as mean ± SD. Data in the second and third columns are observed data, and data in the fourth column are model-based effect estimates. AAD = antiarrhythmic drug; AFEQT = Atrial Fibrillation Effect on QualiTy-of-life; LVEF = left ventricular ejection fraction; NR = not reported; TIA = transient ischaemic attack.
Cryoballoon Ablation as a First-line Therapy: 1-year Outcomes
Atrial Tachyarrhythmia Recurrence and Burden
The primary outcome for each of these studies was the first recurrence of any atrial tachyarrhythmia (defined as AF, atrial flutter, or atrial tachycardia) lasting 30 seconds or longer between 91 and 365 days after treatment initiation.
Documented recurrence of any atrial tachyarrhythmia occurred in 17.2–42.9% of patients randomised to cryoballoon ablation and 32.4–67.8% of patients randomised to antiarrhythmic drugs (AADs), with the reduction in the absolute rates of atrial tachyarrhythmia recurrence ranging from 15% (Cryo-FIRST and STOP-AF First) to 25% (EARLY-AF) at 1-year after treatment initiation. Despite differences in the intensity of arrhythmia monitoring, the relative benefit of first-line cryoablation was consistent between studies (HR 0.50 [0.29–0.86] in Cryo-FIRST, 0.57 [0.36–0.91] in STOP-AF First, and 0.63 [0.51–0.78] in EARLY-AF; pooled RR 0.61, 95% CI [0.51–0.73]).[14,21]
In addition, the EARLY-AF study demonstrated that the recurrence of symptomatic atrial tachyarrhythmia and AF burden (percentage time in AF) were significantly reduced with first-line ablation (absolute difference in symptomatic AF of 15.2%; RR 0.42, 95% CI [0.25–0.71]; number needed to treat [NNT] 7, and mean difference in AF burden of 3.3 ± 1.0% between the ablation and antiarrhythmic groups, respectively).[7] In aggregate, 86% of patients randomised to ablation and 72% of patients randomised to antiarrhythmic drugs were free of AF-related symptoms at 1 year after treatment initiation (RR of being asymptomatic with ablation of 1.19; 95% CI [1.08–1.30]).[7,19]
Quality of Life
Patients enrolled in these studies had a significantly impaired quality of life at baseline (mean baseline Atrial Fibrillation Effect on QualiTy-of-Life [AFEQT] score of 60.1). At 1 year after treatment initiation, there was a significant improvement in health-related quality of life in both the ablation and the antiarrhythmic drug groups. However, patients randomised to catheter ablation achieved a significantly greater improvement in disease-specific and generic quality of life.[7,14,19]
Healthcare Usage
At 1 year after treatment initiation, significantly fewer patients randomised to first-line cryoballoon ablation experienced the composite healthcare usage outcome (RR 0.71; 95% CI [0.56–0.90]), with an absolute reduction of 9% (NNT 11).[14] Significant reductions in all-cause hospitalisation (RR 0.38; 95% CI [0.23–0.63]), and numerical reductions in emergency department visits (RR 0.78, 95% CI [0.50–1.20]) and cardioversions (RR 0.60; 95% CI [0.31–1.18]) were also observed.[14]
Safety and Adverse Events
At 1 year of follow-up, clinically significant serious adverse events were comparable between patients randomised to first-line cryoballoon catheter ablation and antiarrhythmic drug therapy (RR 0.74; 95% CI [0.35–1.56]).[14] However, with first-line cryoballoon ablation was associated with a slightly lower incidence of any adverse event at 1 year of follow-up (RR 0.70; 95% CI [0.54–0.89]).[14]
Cryoballoon Ablation as a First-line Therapy: Summary of 1-year Outcomes
Taken together, these three randomised studies demonstrated that an initial treatment strategy of cryoballoon catheter ablation for patients with treatment-naïve paroxysmal AF resulted in significant improvements in arrhythmia outcomes, produced clinically meaningful improvements in patient-reported outcomes, and significantly reduced healthcare resource usage, yet did not increase the risk of adverse events compared with initial antiarrhythmic drug therapy. This result was in contrast with contemporary guideline recommendations and strongly argued in favour of performing catheter ablation earlier.
The main limitation of these results was their relatively limited observation time. While the standard definition of ablation success involves an assessment of arrhythmia-free survival at 12 months post-ablation (a duration chosen based on the understanding that most recurrences transpire during this interval), this length of follow-up is inadequate when considering the impact of intervention on a relatively young population.[22] Specifically, longer-term follow-up is required to provide information regarding the natural history of AF (e.g. disease progression), the longer-term durability of intervention on arrhythmic and patient-reported outcomes, as well as the downstream healthcare usage. In other words, a comprehensive assessment of longer-term clinical effectiveness is important to inform decision-making and enable patient empowerment regarding the choice of initial treatment, as well as for the evaluation of the cost-effectiveness of invasive AF ablation procedures.
Cryoballoon Ablation as a First-line Therapy: Longer-term Outcomes
The EARLY-AF study program was designed as a pragmatic multiphase platform to evaluate the effect of initial rhythm control treatment in patients with symptomatic treatment-naïve paroxysmal AF.[23] The EARLY-AF trial intended to evaluate the effect of initial rhythm control treatment on atrial tachyarrhythmia recurrence and healthcare usage at 1 year of follow-up (the former being the guideline-recommended endpoint for AF ablation trials).[7,22] The second phase, designated PROGRESSIVE-AF, was designed to evaluate the effect of initial rhythm control treatment on disease progression at 36 months of follow-up as assessed by an implantable continuous rhythm monitor.[24]
Consistent with 1-year results, at 36 months of follow-up there was a significantly lower rate of atrial tachyarrhythmia recurrence (56.5 versus 77.2%; HR 0.51; 95% CI [0.38–0.67]), and a significantly lower AF burden (mean difference in absolute AF burden −1.9 ± 0.7) observed in patients randomised to first-line cryoballoon catheter ablation.[24]
In addition, patients randomised to initial cryoballoon catheter ablation achieved significantly greater improvement in disease-specific quality of life (mean between-group difference in AFEQT score 7.4 ± 2.2 at 36 months), generic quality of life (mean between-group difference in EQ-5D score 0.05 ± 0.02 at 36 months), and were significantly less likely to report symptoms of AF at 36 months of follow-up (RR 0.28; 95% CI [0.13–0.61], Table 2).
Table 2: Quality of Life and Symptoms Over Long-term Follow-up in the EARLY-AF Trial[7].
| Year 1, Mean ± SD | Year 2, Mean ± SD | Year 3, Mean ± SD | |
|---|---|---|---|
| AFEQT score Mean difference* |
8.0 ± 2.2 ABL 26.9 ± 1.9 versus AAD 22.9 ± 2.0 |
9.0 ± 2.3 ABL 29.7 ± 2.0 versus AAD 24.7 ± 2.0 |
7.4 ± 2.2 ABL 28.1 ± 2.0 vs AAD 24.8 ± 2.0 |
| EQ-5D score Mean difference† |
0.05 ± 0.02 ABL 0.06 ± 0.01 versus AAD 0.01 ± 0.01 |
0.03 ± 0.02 ABL 0.06 ± 0.02 versus AAD 0.04 ± 0.02 |
0.0 5 ± 0.02 ABL 0.06 ± 0.02 vs AAD 0.01 ± 0.02 |
| AF symptoms RR |
RR 0.56, 95% CI [0.35–0.88] ABL 14.9% versus AAD 26.8% |
RR 0.34, 95% CI [0.15–0.75] ABL 5.5% versus AAD 16.0% |
RR 0.28, 95% CI [0.13–0.61] ABL 4.8% versus AAD 17.1% |
*Clinically meaningful difference = 5 points; †clinically meaningful difference = 0.03. AAD = antiarrhythmic drugs; ABL = ablation; AFEQT = AF Effect on QualiTy-of-life.
Likewise, healthcare usage (Table 3) was significantly lower in patients randomised to initial cryoballoon catheter ablation. At 3 years, 5.2% of patients in the ablation group and 16.8% in the antiarrhythmic drug group had been hospitalised (RR 0.31; 95% CI [0.14–0.66]). Consistent with the 1-year outcomes, numerical reductions in emergency department visits and cardioversion were again observed in patients randomised to initial cryoballoon catheter ablation (RR 0.84; 95% CI [0.59–1.20] and RR 0.68; 95% CI [0.36–1.29], respectively).
Table 3: Healthcare Usage at 3 Years in the EARLY-AF Trial.
| ER Visit | Hospitalisation | Cardioversion | Non-protocol Ablation |
|---|---|---|---|
| RR 0.84, 95% CI [0.59–1.20] | RR 0.3, 95% CI 1 [0.14–0.66] | RR 0.68, 95% CI [0.36–1.29] | RR 0.41, 95% CI [0.28–0.61] |
| Ablation 40 (26.0%) versus AAD 46 (30.9%) |
Ablation 8 (5.2%) versus AAD 25 (16.8%) |
Ablation 14 (9.1%) versus AAD 20 (13.4%) |
Ablation 27 (17.5%) versus AAD 63 (42.3%) |
AAD = antiarrhythmic drugs.
In contrast to the 1-year data, at 3 years adverse events were less likely to have occurred in patients randomised to initial cryoballoon catheter ablation (Figure 3). Over 3 years of follow-up, serious adverse events occurred in seven patients (4.5%) in the ablation group and 15 (10.1%) in the antiarrhythmic drug group, and any safety endpoint occurred in 17 patients (11.0%) in the ablation group and 35 (23.5%) in the antiarrhythmic drug group.
Figure 3: Long-term Benefits of First-line Catheter Ablation for Paroxysmal AF, as Observed at 3-year Follow-up in the EARLY-AF Trial[7].

AAD = antiarrhythmic drugs; ABL = ablation; AFEQT = Atrial Fibrillation Effect on QualiTy-of-life.
Cryoballoon Ablation as a First-Line Therapy: Disease Progression
While often categorised as paroxysmal and persistent forms, it is important to recognise that AF is a dynamic and chronic progressive disease. Initially, AF manifests as an isolated electrical disorder; however, electrical and structural atrial remodelling result in progression to a more sustained disorder. In contrast to antiarrhythmic drug therapy, catheter ablation is a personalised procedure designed to modify the mechanism of AF initiation and perpetuation through pulmonary venous isolation (e.g. trigger eradication), autonomic nervous system modulation (e.g. vagal denervation) and left atrial substrate modification (predominantly at the pulmonary venous–left atrial junction). As such, it was hypothesised that early invasive intervention could alter the progressive pathoanatomical changes associated with AF and, therefore, positively alter the disease trajectory.
In effect, this was what was observed in the long-term follow-up from EARLY-AF, whereby patients randomised to an initial strategy of catheter cryoballoon ablation experienced a lower incidence of persistent AF compared to those randomised to antiarrhythmic drugs (HR 0.25; 95% CI [0.09–0.70]), as determined by implantable continuous cardiac rhythm monitoring.[24] Moreover, those who experienced an episode of persistent AF continued to experience progression in their disease, with the median duration of continuous AF episodes increasing from 15.8 days (8.0–88.2) to 54.4 days (11.4–163.8) by the end of study follow-up. Importantly, these findings were observed despite enrolling a relatively young population at objectively low risk of progression.
Unanswered Questions Regarding Ablation as First-line Therapy for AF
Can These Results be Extrapolated to Other Ablation Technologies?
As outlined above, previous studies evaluating the role of first-line focal point-by-point radiofrequency catheter ablation failed to demonstrate clinically meaningful improvements in arrhythmia outcomes, quality of life improvement, and healthcare usage.[11–13] However, these studies were performed using previous-generation ablation technology. Since the publication of these trials, there has been an evolution in radiofrequency catheter technology, integrating a real-time quantitative estimation of contact between the tip of the catheter and the target myocardium. This ensures that the operator can assess the adequacy of catheter ablation electrode–tissue contact, which is a critical determinant of lesion quality. A recent multicentre randomised clinical trial has demonstrated comparative effectiveness for patients with AAD-refractory AF treated with cryoballoon ablation and contact-force guided radiofrequency ablation.[25] However, an important observation is that the results observed with radiofrequency ablation are significantly associated with operator and centre experience, while cryoballoon ablation is associated with more consistent and reproducible procedural outcomes across a wider spectrum of operator and centre volumes.[16] As such, in the absence of comparative trials, it may be reasonable to extrapolate the results of these first-line cryoballoon studies to contact-force guided radiofrequency ablation performed by experienced operators in high-volume centres. However, further study is required to determine if the results are applicable in lower volume centres.
A novel addition to these modern-generation catheter ablation technologies is pulsed-field ablation. This non-thermal ablation energy creates tissue injury through the delivery of a sequence of short-duration high-intensity electrical pulses. This high-intensity electric field induces a charge across the lipid bilayer, resulting in the formation of cell membrane pores (electroporation) that induce apoptotic cellular death. In contrast to radiofrequency and cryoablation, lesion formation with pulsed-field ablation is non-thermal and tissue-selective (e.g. cells exposed to an electric field strength above the critical tissue-dependent electric field threshold undergo irreversible electroporation, as above, and those exposed to a field below the critical threshold undergo reversible electroporation, whereby the pores close in time to re-establish homeostasis and regain viability). As such, it has been postulated that pulsed-field ablation may offer an improved safety and efficacy profile relative to thermal ablation energies, potentially positioning it as the preferred ablation energy for first-line AF ablation procedures.
How Early is Early Ablation?
The exact characterisation of early AF remains undefined, likely owing to the heterogeneity of presentation and subsequent prognosis. First-detected AF has been associated with increased short-term rates of death, stroke/systemic thromboembolism and major bleeding, suggesting that the occurrence of AF may be a marker of severe underlying comorbidity, or a consequence of worsening baseline disease.[26]
Conversely, in patients without significant comorbidity a significant proportion with first-detected AF will remain free of arrhythmia over prolonged follow-up (e.g. up to 50% of patients being free of arrhythmia recurrence in the absence of preventative treatment up to 5 years of follow-up).[27–29] As such, a pragmatic definition may be to consider the definition employed in the aforementioned randomised clinical trials. EAST considered ‘early’ to be within the first year following diagnosis, which was similar to the median time from diagnosis to enrolment in the first-line cryoballoon catheter ablation trials.[4,14] However, an important distinction is that the ablation trials (e.g. EARLY-AF) focused on a population of symptomatic patients necessitating intervention for the management of their AF (either pharmacological or catheter-based), whereas EAST included asymptomatic patients.
Ensuring Timely Access to Ablation
Once the decision to proceed to ablation has been made, it makes sense to ensure that it is performed in a timely manner. Observational data suggests that increasing time from diagnosis to ablation is associated with worsening clinical outcomes following the ablation procedure, including higher rates of arrhythmia recurrence, hospitalisation, heart failure decompensation, stroke and mortality.[30–32] Unfortunately, many healthcare environments are constrained by lack of access to catheter ablation procedures, with procedural waitlists more than twice exceeding the recommended wait times.[33,34] These delays have been associated with a significant number of patients progressing to more advanced forms of AF, leading to longer and more complex ablation procedures, as well as higher rates of peri-procedural complication and subsequent hospitalisation.[35,36] As such, strategies to ensure timely access to intervention are essential when considering early intervention.
Conclusion
Ablation as a first-line therapy for AF is associated with significant reductions in arrhythmia recurrence, substantial improvements in arrhythmia-related symptoms and quality of life, and lower rates of adverse events. In addition, catheter ablation is associated with significantly lower rates of disease progression, suggesting that it is a disease-modifying intervention.
Clinical Perspective
Compared with initial antiarrhythmic drug therapy, a strategy of first-line cryoballoon ablation:
Significantly reduced atrial tachyarrhythmia recurrence and AF burden.
Significantly reduced the progression of AF.
Significantly improved quality of life.
Significantly reduced healthcare resource usage.
Did not increase the risk of adverse events.
References
- 1.Andrade J, Khairy P, Dobrev D, Nattel S. The clinical profile and pathophysiology of atrial fibrillation: relationships among clinical features, epidemiology, and mechanisms. Circ Res. 2014;114:1453–68. doi: 10.1161/CIRCRESAHA.114.303211. [DOI] [PubMed] [Google Scholar]
- 2.Andrade JG, Aguilar M, Atzema C et al. The 2020 Canadian Cardiovascular Society/Canadian Heart Rhythm Society comprehensive guidelines for the management of atrial fibrillation. Can J Cardiol. 2020;36:1847–948. doi: 10.1016/j.cjca.2020.09.001. [DOI] [PubMed] [Google Scholar]
- 3.de Denus S, Sanoski CA, Carlsson J et al. Rate vs rhythm control in patients with atrial fibrillation: a meta-analysis. Arch Intern Med. 2005;165:258–62. doi: 10.1001/archinte.165.3.258. [DOI] [PubMed] [Google Scholar]
- 4.Kirchhof P, Camm AJ, Goette A et al. Early rhythm-control therapy in patients with atrial fibrillation. N Engl J Med. 2020;383:1305–16. doi: 10.1056/NEJMoa2019422. [DOI] [PubMed] [Google Scholar]
- 5.Cheung CC, Nattel S, Macle L, Andrade JG. Management of atrial fibrillation in 2021: an updated comparison of the current CCS/CHRS, ESC, and AHA/ACC/HRS guidelines. Can J Cardiol. 2021;37:1607–18. doi: 10.1016/j.cjca.2021.06.011. [DOI] [PubMed] [Google Scholar]
- 6.Freemantle N, Lafuente-Lafuente C, Mitchell S et al. Mixed treatment comparison of dronedarone, amiodarone, sotalol, flecainide, and propafenone, for the management of atrial fibrillation. Europace. 2011;13:329–45. doi: 10.1093/europace/euq450. [DOI] [PubMed] [Google Scholar]
- 7.Andrade JG, Wells GA, Deyell MW et al. Cryoablation or drug therapy for initial treatment of atrial fibrillation. N Engl J Med. 2021;384:305–15. doi: 10.1056/NEJMoa2029980. [DOI] [PubMed] [Google Scholar]
- 8.Valembois L, Audureau E, Takeda A et al. Antiarrhythmics for maintaining sinus rhythm after cardioversion of atrial fibrillation. Cochrane Database Syst Rev. 2019;9 doi: 10.1002/14651858.CD005049.pub5. CD005049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Corley SD, Epstein AE, DiMarco JP et al. Relationships between sinus rhythm, treatment, and survival in the Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) study. Circulation. 2004;109:1509–13. doi: 10.1161/01.CIR.0000121736.16643.11. [DOI] [PubMed] [Google Scholar]
- 10.Calkins H, Reynolds MR, Spector P et al. Treatment of atrial fibrillation with antiarrhythmic drugs or radiofrequency ablation: two systematic literature reviews and meta-analyses. Circ Arrhythm Electrophysiol. 2009;2:349–61. doi: 10.1161/CIRCEP.108.824789. [DOI] [PubMed] [Google Scholar]
- 11.Cosedis Nielsen J, Johannessen A, Raatikainen P et al. Radiofrequency ablation as initial therapy in paroxysmal atrial fibrillation. N Engl J Med. 2012;367:1587–95. doi: 10.1056/NEJMoa1113566. [DOI] [PubMed] [Google Scholar]
- 12.Morillo CA, Verma A, Connolly SJ et al. Radiofrequency ablation vs antiarrhythmic drugs as first-line treatment of paroxysmal atrial fibrillation (RAAFT-2): a randomized trial. JAMA. 2014;311:692–700. doi: 10.1001/jama.2014.467. [DOI] [PubMed] [Google Scholar]
- 13.Wazni OM, Marrouche NF, Martin DO et al. Radiofrequency ablation vs antiarrhythmic drugs as first-line treatment of symptomatic atrial fibrillation: a randomized trial. JAMA. 2005;293:2634–40. doi: 10.1001/jama.293.21.2634. [DOI] [PubMed] [Google Scholar]
- 14.Andrade JG, Wazni OM, Kuniss M et al. Cryoballoon ablation as initial treatment for atrial fibrillation: JACC state-of-the-art review. J Am Coll Cardiol. 2021;78:914–30. doi: 10.1016/j.jacc.2021.06.038. [DOI] [PubMed] [Google Scholar]
- 15.Andrade JG. Cryoablation for atrial fibrillation. Heart Rhythm. 2020;1:44–58. doi: 10.1016/j.hroo.2020.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Providencia R, Defaye P, Lambiase PD et al. Results from a multicentre comparison of cryoballoon vs. radiofrequency ablation for paroxysmal atrial fibrillation: is cryoablation more reproducible? Europace. 2017;19:48–57. doi: 10.1093/europace/euw080. [DOI] [PubMed] [Google Scholar]
- 17.Andrade JG, Khairy P, Guerra PG et al. Efficacy and safety of cryoballoon ablation for atrial fibrillation: a systematic review of published studies. Heart Rhythm. 2011;8:1444–51. doi: 10.1016/j.hrthm.2011.03.050. [DOI] [PubMed] [Google Scholar]
- 18.Cardoso R, Mendirichaga R, Fernandes G et al. Cryoballoon versus radiofrequency catheter ablation in atrial fibrillation: a meta-analysis. J Cardiovasc Electrophysiol. 2016;27:1151–9. doi: 10.1111/jce.13047. [DOI] [PubMed] [Google Scholar]
- 19.Kuniss M, Pavlovic N, Velagic V et al. Cryoballoon ablation vs. antiarrhythmic drugs: first-line therapy for patients with paroxysmal atrial fibrillation. Europace. 2021;23:1033–41. doi: 10.1093/europace/euab029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wazni OM, Dandamudi G, Sood N et al. Cryoballoon ablation as initial therapy for atrial fibrillation. N Engl J Med. 2021;384:316–24. doi: 10.1056/NEJMoa2029554. [DOI] [PubMed] [Google Scholar]
- 21.Aguilar M, Macle L, Deyell MW et al. Influence of monitoring strategy on assessment of ablation success and postablation atrial fibrillation burden assessment: implications for practice and clinical trial design. Circulation. 2022;145:21–30. doi: 10.1161/CIRCULATIONAHA.121.056109. [DOI] [PubMed] [Google Scholar]
- 22.Calkins H, Hindricks G, Cappato R et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Heart Rhythm. 2017;14:e275–444. doi: 10.1016/j.hrthm.2017.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Andrade JG, Champagne J, Deyell MW et al. A randomized clinical trial of early invasive intervention for atrial fibrillation (EARLY-AF) – methods and rationale. Am Heart J. 2018;206:94–104. doi: 10.1016/j.ahj.2018.05.020. [DOI] [PubMed] [Google Scholar]
- 24.Andrade JG, Deyell MW, Macle L et al. Progression of atrial fibrillation after cryoablation or drug therapy. N Engl J Med. 2023;388:105–16. doi: 10.1056/NEJMoa2212540. [DOI] [PubMed] [Google Scholar]
- 25.Andrade JG, Champagne J, Dubuc M et al. Cryoballoon or radiofrequency ablation for atrial fibrillation assessed by continuous monitoring: a randomized clinical trial. Circulation. 2019;140:1779–88. doi: 10.1161/CIRCULATIONAHA.119.042622. [DOI] [PubMed] [Google Scholar]
- 26.Bassand JP, Accetta G, Camm AJ et al. Two-year outcomes of patients with newly diagnosed atrial fibrillation: results from GARFIELD-AF. Eur Heart J. 2016;37:2882–9. doi: 10.1093/eurheartj/ehw233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.De With RR, Erkuner Ö, Rienstra M et al. Temporal patterns and short-term progression of paroxysmal atrial fibrillation: data from RACE v. Europace. EP Europace. 2020;22:1162–72. doi: 10.1093/europace/euaa123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pappone C, Radinovic A, Manguso F et al. Atrial fibrillation progression and management: a 5-year prospective follow-up study. Heart Rhythm. 2008;5:1501–7. doi: 10.1016/j.hrthm.2008.08.011. [DOI] [PubMed] [Google Scholar]
- 29.Diederichsen SZ, Haugan KJ, Brandes A et al. Natural history of subclinical atrial fibrillation detected by implanted loop recorders. J Am Coll Cardiol. 2019;74:2771–81. doi: 10.1016/j.jacc.2019.09.050. [DOI] [PubMed] [Google Scholar]
- 30.Chew DS, Black-Maier E, Loring Z et al. Diagnosis-to-ablation time and recurrence of atrial fibrillation following catheter ablation: a systematic review and meta-analysis of observational studies. Circ Arrhythm Electrophysiol. 2020;13:350–7. doi: 10.1161/CIRCEP.119.008128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chew DS, Jones KA, Loring Z et al. Diagnosis-to-ablation time predicts recurrent atrial fibrillation and rehospitalization following catheter ablation. Heart Rhythm. 2022;3:23–31. doi: 10.1016/j.hroo.2021.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bunch TJ, May HT, Bair TL et al. Increasing time between first diagnosis of atrial fibrillation and catheter ablation adversely affects long-term outcomes. Heart Rhythm. 2013;10:1257–62. doi: 10.1016/j.hrthm.2013.05.013. [DOI] [PubMed] [Google Scholar]
- 33.Kaoutskaia A, Shurrab M, Amit G et al. Canadian national electrophysiology ablation registry report 2011–2016. BMC Health Serv Res. 2021;21:435. doi: 10.1186/s12913-021-06441-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Simpson CS, O’Neill BJ, Sholdice MM et al. Canadian Cardiovascular Society commentary on implantable cardioverter defibrillators in Canada: waiting times and access to care issues. Can J Cardiol. 2005;21((Suppl A)):19A–24A. [PubMed] [Google Scholar]
- 35.Kochhauser S, Dechering DG, Trought K et al. Predictors for progression of atrial fibrillation in patients awaiting atrial fibrillation ablation. Can J Cardiol. 2016;32:1348–54. doi: 10.1016/j.cjca.2016.02.031. [DOI] [PubMed] [Google Scholar]
- 36.Ha ACT, Wijeysundera HC, Qiu F et al. Differences in healthcare use between patients with persistent and paroxysmal atrial fibrillation undergoing catheter-based atrial fibrillation ablation: a population-based cohort study from Ontario, Canada. J Am Heart Assoc. 2021;10:1–29. doi: 10.1161/JAHA.120.016071. [DOI] [PMC free article] [PubMed] [Google Scholar]


