Abstract
AIM
To evaluate whether latanoprost/timolol fixed combination (LTFC) dosed twice daily may provide further intraocular pressure (IOP) reduction and evaluate the safety profile at this dose.
METHODS
This is an open-labeled, randomized, prospective crossover study on fourty primary open angle glaucoma patients. Two weeks of washout period were followed by randomization to either once daily (OD, group A) or twice daily dosing (BD, group B) of LTFC for 4wk. After another 2-week washout period, the patients' treatment dose was crossed-over for another 4wk. IOP reduction alongside ocular and systemic side effects were evaluated.
RESULTS
Mean baseline IOP was 18.57±2.93 and 17.8±3.01 mm Hg before OD and BD dose respectively, (P=0.27). Mean IOP after BD dose was statistically lower (12.49±1.59 mm Hg) compared to OD (13.48±1.81 mm Hg, P=0.017). Although IOP reduction after BD dose was more (5.32±3.24 mm Hg, 29.89%) than after OD dosing (5.04 mm Hg, 27.14%), it did not reach statistical significance (P=0.68). Patients switched from OD to BD (group A) showed mean IOP reduction by 0.69 mm Hg [95% confidence interval (CI): -0.09 to 1.48 mm Hg, P=0.078]; but patients switched from BD to OD (group B) had significantly higher mean IOP by 1.25 mm Hg (95%CI: -2.04 to -0.46 mm Hg, P=0.006). BD dose had more ocular side effects albeit mild.
CONCLUSION
Mean IOP after LTFC dosed twice daily is statistically lower, with additional mild side effects.
Keywords: efficacy, fixed combination, latanoprost, timolol, primary open angle glaucoma, safety
INTRODUCTION
The incidence of glaucoma has more than doubled in the last century and as life expectancy increases, cases of glaucoma are more prevalent[1]. Since intraocular pressure (IOP) is the only modifiable risk factor, lowering the IOP is paramount in delaying glaucoma progression[2]. Adequate IOP reduction has been proven to decelerate optic nerve damage, and uncontrolled IOP is constantly associated with progressive visual field (VF) loss[3]–[4].
Treatment with topical IOP-lowering medications is still the mainstay of treatment, offering a non-invasive approach in earlier stages of glaucoma. Monotherapy is usually the first line of treatment and if IOP reduction is insufficient, treatment is either switched to an alternative monotherapy or supplemented with a second agent[5]. In the Ocular Hypertension Treatment Study, two or more topical medications were eventually required in 39.7% of patients in the medically treated arm in order to achieve a target IOP of 24 mm Hg or less; or 20% IOP reduction from baseline[3]. The Collaborative Initial Glaucoma Treatment Study reported that 75% of patients require more than one medication to achieve optimum IOP control[6]. Nevertheless, multi-drug therapy increases non-compliance to treatment[7], has a washout effect from consecutive drops instillation, and is associated with suboptimal medication absorption[8]. Additionally, there is a growing concern on the effects of preservatives on the ocular surface[9], especially with long-term anti-glaucoma therapy[10]–[12], which can lead to surgical failure in future glaucoma filtering surgeries[13]–[14].
Most fixed-combination therapies contain 2 ocular hypotensive agents in a single formulation; commonly a combination of prostaglandin analogue (PGA) and beta-blockers (BB). This simplifies treatment regimes, reduces instillation frequency and ocular exposure to preservatives, and improves adherence[15]. The latanoprost 0.005% and timolol maleate 0.5% fixed combination (Xalacom, Pfizer Inc, New York, NY, USA) was the first PGA/BB combination to be released commercially in the European Union in 2001 and is widely available. Latanoprost and timolol fixed combination (LTFC) provides a convenient alternative to the three daily instillations required with individual components. A single dose markedly reduces IOP for 48h after treatment[16].
Diestelhorst et al[17] and Quaranta et al[18] found that the unfixed combinations give better IOP reduction than LTFC in patients with open angle glaucoma and ocular hypertension. They postulate that the lower IOP seen with an unfixed combination may be attributed to the additional dose of timolol dosed twice daily[17]–[18]. Additionally, latanoprost in the unfixed combination group was instilled in the evening, the recommended time for PGA instillation[17]–[18]. However, a Meta-analysis found better IOP-lowering effect of LTFC compared to its monotherapy components[19].
Nevertheless, the recommended once-daily morning dose of LTFC means a reduced dose of timolol and a morning latanoprost dose instead of the recommended evening dose. It is quite possible that increasing the LTFC dose to twice daily may result in more IOP reduction within a tolerable window of side effects. Previous studies have shown that the side effects of latanoprost dosed four times daily are tolerable in a two months study[20].
We aim to evaluate whether further IOP reduction can be achieved if LTFC is dosed twice daily, to account for the underdosed timolol; and assess the side effects that come with it, in a group of patients with mild to moderate primary open angle glaucoma (POAG).
SUBJECTS AND METHODS
Ethical Approval
Ethical approval was obtained from the Medical Research and Ethical Committee, UKM Medical Centre (protocol number: FF-2019-058) and adhered to the Declaration of Helsinki and ICH guidelines for good clinical practice. The study protocol was reposited at clinicaltrial.org and the clinical trial number was NCT04098861. Written informed consent was obtained from all study participants and those who refused to participate in the study received their standard treatment as usual.
Participants
This was a cross-over, open-labeled, randomized clinical trial done from 2nd January 2019 to 1st April 2020. POAG patients on two IOP-lowering agents attending the Ophthalmology Clinic, Universiti Kebangsaan Malaysia Medical Center (UKMMC) that fulfilled the eligibility criteria were recruited.
The inclusion criteria included patients aged 50y and above, diagnosed with mild to moderate POAG, and treated with only two IOP-lowering agents. The severity of POAG was based on the VF changes assessed using the 24-2 Sita-standard strategy on Humphrey Field Analyzer (Carl Zeiss, USA) in three recent reliable VFs. Mild POAG was defined as mean deviation (MD) >-6 dB, a cluster of ≥3 points depressed below the 5% level on the pattern deviation plot, and at least one of which is depressed below the 1% level points; corrected pattern standard deviation (PSD)/PSD significant at P<0.05 and glaucoma Hemifield Test (GHT) reported as outside normal limits[20]. Moderate POAG was defined as MD ≤-6 dB to -12.00 dB, ≥25% but <50% of points on the pattern deviation plot depressed below the 5% level, and ≥15% but <25% of points depressed below 1% level, at least one point within the central 5° with a sensitivity of <15 dB but no points in the central 5° with a sensitivity of <0 dB and only one hemifield containing a point with sensitivity <15 dB within 5° of fixation[21]. If both eyes fulfilled the criteria, the right eye was selected for the study.
Exclusion criteria included a history of allergy to a PGA or BB, systemic co-morbidities that prevent the use of BB such as asthma or chronic obstructive pulmonary disease, congestive heart failure, bradycardia or second and third-degree atrioventricular block; history of ocular trauma, ocular surgery or argon trabeculoplasty within 6mo; corneal abnormalities or any condition preventing reliable measurement of applanation tonometry, ocular infection or inflammation, and patients with only one seeing eye.
Subjects were withdrawn from the study if they develop intolerable side effects, violate the study protocol, were not compliant to the treatment regime, or any other circumstances that could endanger the health of the subject if participation in the trial is continued. These patients were treated accordingly and their previous treatment regime before the study was resumed.
The sample size was calculated based on a study by Lindén and Alm[20]. With the sample size of 20 patients per group, this study was powered at 80% to detect a difference in IOP of 1.2 mm Hg [standard deviation (SD) 1.7] between the two treatment groups at a 5% significance level (PS Power and Sample Size Calculation version 3.0). The total sample size was therefore 40 patients.
Study Flow
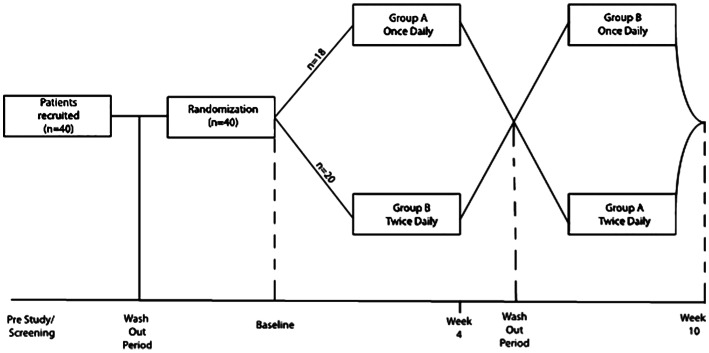
The subjects underwent 2wk wash-out period from their existing IOP-lowering medications (Figure 1). The patients were then randomized using block randomization (www.randomization.com) into Group A (started with LTFC once daily, OD) or Group B (started with LTFC twice daily, BD). Those receiving OD dose were instructed to instill the drops at 8 a.m. as recommended in the medication leaflet. The second dose in the group receiving BD dose is instilled at 8 p.m. After four weeks, the patients had a two-week washout period when all treatment was stopped, followed by a cross-over to the other dosing regime for another four weeks. Therefore, after the patients were randomized to either receive BD or OD dosing in the first phase of the study, they were switched to the other dosing in the second phase of the study. When analysis was done, the IOPs at baseline and after completion of each phase were combined regardless whether the patients were started with the first or second dosing. At the end of the study, the patients were given the option whether to continue with their medications prior to study entry or the new combination therapy.
Figure 1. Study flow of the participants.
At baseline visit, a thorough medical and ocular history were taken followed by a full ophthalmic examination including anterior segment examination using a slit lamp biomicroscopy (Slit lamp BP 900, Haag-Streit, Switzerland) and a dilated fundus examination for optic nerve evaluation using 90 D and 78 D lens.
The following assessments are done on every study visit. Blood pressure and heart rate were measured using an automated blood pressure monitor (Dinamap, Critikon Corp, Tampa, FL, USA). Three IOP measurements were taken by an operator (Hussein SH) and a reader (Azal AB), both blinded to the treatment protocol, and the mean IOP was included in the analysis. IOP was measured between 8–10 a.m. to eliminate diurnal fluctuation effects. Conjunctival hyperemia was measured based on the Cornea and Contact Lens Research Unit (CCLRU) grading scales[22]. Anterior chamber reaction was monitored using the Standardized Uveitis Nomenclature (SUN) Grading scales for anterior chamber cells[23], and compliance was assessed by asking the patient and making sure that the LTFC bottle was empty.
Statistical Analysis
Data was analyzed using Statistical Package for Social Science, version 22.0 (IBM Corp, USA). Normality was tested with Kolmogorov Smirnov test. Continuous data was presented as mean±SD, and categorical data was presented as frequency and percentages (%). Independent t-test was used to compare the change in IOP from baseline between the two-dosing regime. The cross-over effect of switching treatment was evaluated using paired t-test. Side effects were evaluated using Fisher's exact test.
RESULTS
Patients Demographic Characteristic
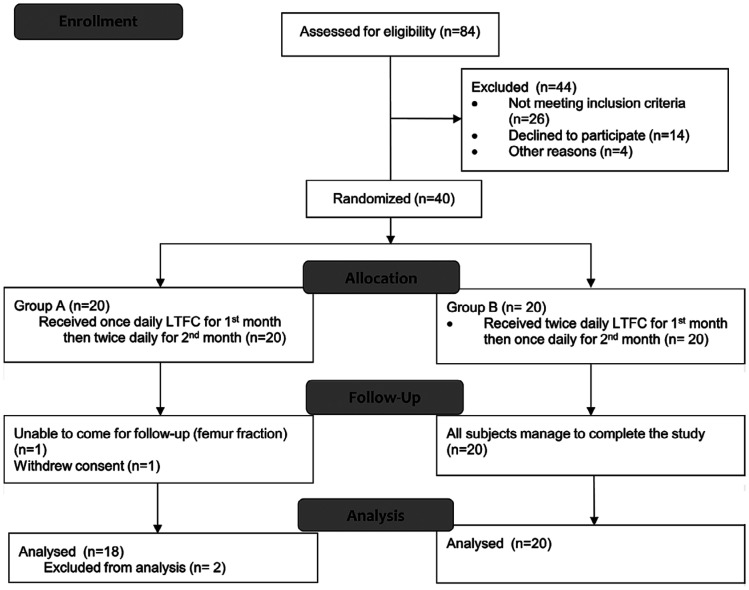
A total of 84 patients were screened but 44 patients have to be excluded as they did not fulfill the inclusion criteria (n=26), did not consent (n=14), and for other reasons (n=4). Only 40 patients consented and were recruited into this study. However, only 38 patients completed the treatment regime. Figure 2 shows the details in number of patients attending each study visit. The mean age of study participants was 62.31±7.88y. One patient was unable to complete the study due to a femur fracture within the study period and one withdrew consent. Demographic characteristics of participants are summarized in Table 1.
Figure 2. Consort flow diagram showing number of subjects through each stage of the randomized crossover clinical trial.
LTFC: Latanoprost timolol fixed combination.
Table 1. Demographic characteristics of study participants.
| Variables | n=40 (%) |
| Age (y) | |
| Mean±SD | 62.31±7.88 |
| Range | 56-72 |
| Gender | |
| Male | 20 (50.0) |
| Female | 20 (50.0) |
| Ethnicity | |
| Malay | 10 (25.0) |
| Chinese | 26 (65.0) |
| Indian | 4 (10.0) |
| Laterality | |
| Right eye | 37 (92.5) |
| Left eye | 3 (7.5) |
| Comorbidities | |
| Diabetes mellitus | 20 (50) |
| Hypertension | 23 (57.5) |
| Dyslipidaemia | 22 (55) |
| Ischaemic heart disease | 3 (7.5) |
| Cerebral vascular accident | 2 (5) |
| Nil | 5 (12.5) |
Efficacy
In general, the mean baseline IOP at study entry in the two groups combined was 18.2±0.7 mm Hg. There was no difference in mean baseline IOP before OD (18.57±2.93 mm Hg) and BD dosing (17.8±3.01 mm Hg, P=0.27). Mean IOP after BD dosing was statistically lower (12.49±1.59 mm Hg) compared to after OD dosing (13.48±1.81 mm Hg, P=0.017), although IOP reduction after BD dosing (5.32±3.24 mm Hg, 29.89%) was not statistically different than after OD dosing (5.04 mm Hg, 27.14%, P=0.68).
The cross-over effect of the 2 treatment arms was also analyzed. There was no significant difference in mean IOP and IOP reduction at all time points between the two groups, except for a borderline significance in group B after the second 4wk (Table 2). While IOP in eyes after receiving the BD dose (12.5 mm Hg in both groups) was 1 mm Hg lower than after the OD dose (13.2 mm Hg in group A and 13.7 mm Hg in group B), it did not reach statistical significance.
Table 2. Mean intraocular pressure among study subjects who completed the trial.
| Groups | IOP in first phase (4wk) |
IOP in second phase (4wk) |
IOP difference between end of first and second phase (95%CI), P | ||||||
| Baseline | After first phase | IOP reduction (95%CI) | P | Baseline | After second phase | IOP reduction (95%CI) | P | ||
| Group A | 18.3±3.3 | 13.2±1.5 | 5.0 (3.6, 6.4) | <0.001a | 17.2±3.2 | 12.5±1.5 | 4.2 (2.7, 5.7) | <0.001a | 0.7 (0.09, 1.5), 0.078b |
| Group B | 18.4±2.9 | 12.5±1.7 | 5.8 (4.7, 7.0) | <0.001a | 18.8±2.6 | 13.7±2.1 | 5.1 (3.9, 6.3) | <0.001a | 1.2 (-2.04, -0.46), 0.006b |
| P | 0.972b | 0.160b | 0.32b | - | 0.109b | 0.054b | 0.36b | - | |
IOP: Intraocular pressure; CI: Confidence interval; aIndependent t-test; bPaired t-test.
mean±SD, mm Hg
However, although patients switched from OD (13.2 mm Hg) to BD dose (12.5 mm Hg) in Group A showed no statistical difference in mean IOP change [0.7 mm Hg, 95% confidence interval (CI): -0.09 to 1.5, P=0.078], patients switched from BD (12.5 mm Hg) to OD dose (13.7 mm Hg) in Group B showed significantly higher IOP by a mean of 1.2 mm Hg (95%CI: -2.04 to -0.46, P=0.006).
To assess the period effect of treatment dosing (whether giving BD dose after OD dose will result in patients becoming less compliant and hence lesser IOP reduction), we compared the IOP after OD and BD dosing in both groups. We found no statistically significant difference in IOP after OD dosing in groups A (13.2±1.5 mm Hg) and B (13.7±2.1 mm Hg, P=0.43). Likewise, IOP after BD dosing in group B (12.5±1.7 mm Hg) was not statistically different from Group A (12.5±1.5 mmHg, P>0.05; Table 2), indicating no period effect of OD or BD dosing in both groups.
Side Effects
There were no reported severe adverse reactions and no patient withdraw from the study. All reported side effects were mild. Sixty-five eyes (81.3%) had no side effects, nine (11.3%, 8 eyes after BD dose and one eye after OD dose) had superficial punctate keratopathy (SPK), four eyes (5%, 3 in eyes after BD dosing and one eye after OD dosing) had occasional anterior chamber cells and resolves spontaneously; and two eyes (2.5%, all in eyes after BD dosing) had grade 2 conjunctival hyperemia. There was no iris pigmentation seen or hypertrichosis other than what was already present before study entry. There was a significant difference in the number of eyes with side effects between the two groups (P=0.006).
There was no difference in blood pressure and heart rate between the two groups at the end of the study (P=0.079).
DISCUSSION
Latanoprost 0.005% and timolol maleate 0.5% have distinct mechanisms of action and have been shown to have an addictive IOP-lowering effect when administered together[24]. They both aim at different targets or pathways to further lower the IOP. Latanoprost facilitates the uveoscleral outflow by remodelling the extracellular matrix and relaxation of the ciliary muscle[25]. Dose-response based studies on latanoprost demonstrated 24h duration of action and long-term studies confirmed an effective IOP control with such a dosing schedule. Therefore, these agents are typically dosed once daily[26].
Timolol, on the other hand, is a β-adrenergic receptor antagonist, which primarily acts by decreasing the rate of aqueous humor production by the ciliary epithelium[27]. The time to attain maximum concentration (Tmax) and half-lives of both LTFC and its separate components were comparable[28]. There was no effect of latanoprost on the ocular pharmacokinetics of timolol and vice versa. The bioavailability of LTFC in human aqueous humor was at least as strong as the component drugs administered separately[28]. While a Meta-analysis has demonstrated a better IOP-lowering effect of LTFC compared to its mono-therapy components, no such study has looked into increasing the dose of LTFC to twice daily to increase its efficacy[19].
We aimed to see whether giving LTFC twice daily to make up for the underdosed timolol, would result in more IOP lowering effect. Our study found that although IOP with BD dosing is 1 mm Hg lower than OD dosing, the net IOP reduction is not significantly different in our cohort of mild to moderate POAG patients.
The results of our study demonstrate that both dosing regimes effectively reduce the IOP in patients with POAG, with no significant difference in IOP reduction between the two dosings. We thus conclude that a BD dose of LTFC to account for timolol underdose does not give further IOP reduction as we postulated. The findings can be explained by a previous study by Lindén and Alm[29] who compared the efficacy of once vs twice daily dosing of latanoprost. They found that there is less IOP reduction when latanoprost is applied twice daily. The mean IOP difference between the two regimes was 1.2 mm Hg favoring the once-daily dosing. Lindén and Alm[20] in another study compared the effect of latanoprost one or four times daily on each eye of healthy individuals. They detected better IOP reduction in the 4-dosage daily group only in the first 2d. Thereafter, no significant IOP difference was found[20]. These 2 studies postulated that sub-sensitivity at the FP receptor level from either desensitization or downregulation of the FP receptor could account for the observed reduced efficacy with increased frequency of latanoprost administration.
The result of our study can be explained by the fact that overdosing latanoprost by giving twice daily LTFC may have resulted in desensitization of the receptors and masked the full effect of the BD dose of timolol[29]. Furthermore, increasing the dose of LTFC to BD may result in less patients' compliance due to inconvenience and more side effects. Some patients may have forgotten the second dose of LTFC after crossover.
While weighing the bottles and keeping a diary are alternative ways to assess compliance, there is no definite way to confirm compliance because, at the end of the day, the patients will be at home doing their thing. We chose to assess compliance based on the patients' reports and checking to see whether the bottle is empty or not. This issue reflects the real-world scenario and that given the natural environment in everyday practice, the efficacy and safety portrayed in the result of this study are to be expected.
In the present study, twice-daily dosing gives significantly more ocular side effects compared to once-daily dosing. The SPK and conjunctival hyperemia might be due to more exposure to preservatives as the dosing frequency increase. Lindén and Alm[20] showed that an increased dose of latanoprost can induce transient mild anterior chamber reaction in normal eyes. This occurred in 3 of our patients who received BD dose compared to only 1 patient who received OD dose. Fortunately, no systemic adverse event was seen in all patients. It is important to note, however, that the duration of the present study was too short to identify possible long-term side effects of LTFC dosed BD.
The strength of a crossover design in our study allows each case to be its own control thus abolishing any confounding factors. Also, we are able to assess, to a certain extent, the change in patients' behavior; translated to IOP reduction, when treatment dose was changed. However, we acknowledge the limitations in the lack of objective assessment for monitoring compliance such as weighing the eye drop bottles before and after completion of each treatment cycle. Additionally, being Asians, our patients' irides are mostly heavily pigmented, possibly reducing the efficacy of BB alongside increased pigmentation from prolonged prostaglandin analogue use. The long-term side effects of LTFC dosed BD should also be explored as patients are more likely to be on this treatment for a long period of time.
In conclusion, LTFC dosed twice daily results in statistically lower mean IOP compared to a once-daily dose. Albeit mild, twice-daily dosing gives more ocular side effects as compared to once-daily dosing.
Acknowledgments
Authors' contributions: Conceptualization: Anis Baidura Azal, Norshamsiah Md Din; Methodology: Anis Baidura Azal, Norshamsiah Md Din; Formal analysis and investigation: Anis Baidura Azal, Siti Husna Hussein; Writing-original draft preparation: Anis Baidura Azal; Writing-review and editing: Siti Husna Hussein, Seng Fai Tang, Othmaliza Othman, Norshamsiah Md Din; Funding acquisition: Norshamsiah Md Din; Project administration: Anis Baidura Azal, Siti Husna Hussein; Resources: Seng Fai Tang; Supervision: Norshamsiah Md Din; Visualization: Othmaliza Othman, Norshamsiah Md Din.
Foundations: Anis Baidura Azal holds a Masters scholarship funded by the Government of Malaysia; Norshamsiah Md Din receives funding from the UKMMC Fundamental Research Fund (No.FF-2019-058).
Conflicts of Interest: Azal AB, None; Hussein SH, None; Tang SF, None; Othman O, None; Din NM, None.
REFERENCES
- 1.Zhang N, Wang J, Li Y, Jiang B. Prevalence of primary open angle glaucoma in the last 20 years: a meta-analysis and systematic review. Sci Rep. 2021;11(1):13762. doi: 10.1038/s41598-021-92971-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jayaram H. Intraocular pressure reduction in glaucoma: does every mmHg count? Taiwan J Ophthalmol. 2020;10(4):255–258. doi: 10.4103/tjo.tjo_63_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kass MA, Heuer DK, Higginbotham EJ, et al. The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120(6):701–713; discussion 829-830. doi: 10.1001/archopht.120.6.701. [DOI] [PubMed] [Google Scholar]
- 4.Koenig SF, Montesano G, Fang CEH, Crabb DP, Jayaram H, Clarke J. Effect of trabeculectomy on the rate of progression of visual field damage. Eye (Lond) 2022 doi: 10.1038/s41433-022-02312-y. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.European Glaucoma Society Terminology and Guidelines for Glaucoma, 4th Edition - Chapter 2: Classification and terminology Supported by the EGS Foundation: Part 1: Foreword; Introduction; Glossary; Chapter 2 Classification and Terminology. Br J Ophthalmol. 2017;101(5):73–127. doi: 10.1136/bjophthalmol-2016-EGSguideline.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lichter PR, Musch DC, Gillespie BW, Guire KE, Janz NK, Wren PA, Mills RP, CIGTS Study Group Interim clinical outcomes in the Collaborative Initial Glaucoma Treatment Study comparing initial treatment randomized to medications or surgery. Ophthalmology. 2001;108(11):1943–1953. doi: 10.1016/s0161-6420(01)00873-9. [DOI] [PubMed] [Google Scholar]
- 7.Robin AL, Muir KW. Medication adherence in patients with ocular hypertension or glaucoma. Expert Rev Ophthalmol. 2019;14(4-5):199–210. [Google Scholar]
- 8.Urtti A. Challenges and obstacles of ocular pharmacokinetics and drug delivery. Adv Drug Deliv Rev. 2006;58(11):1131–1135. doi: 10.1016/j.addr.2006.07.027. [DOI] [PubMed] [Google Scholar]
- 9.Tokuda N, Kitaoka Y, Matsuzawa A, et al. Changes in ocular surface characteristics after switching from benzalkonium chloride-preserved latanoprost to preservative-free tafluprost or benzalkonium chloride-preserved tafluprost. J Ophthalmol. 2017;2017:3540749. doi: 10.1155/2017/3540749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Andole S, Senthil S. Ocular surface disease and anti-glaucoma medications: various features, diagnosis, and management guidelines. Semin Ophthalmol. 2023;38(2):158–166. doi: 10.1080/08820538.2022.2094714. [DOI] [PubMed] [Google Scholar]
- 11.Mylla Boso AL, Gasperi E, Fernandes L, Costa VP, Alves M. Impact of ocular surface disease treatment in patients with glaucoma. Clin Ophthalmol. 2020;14:103–111. doi: 10.2147/OPTH.S229815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Asiedu K, Abu SL. The impact of topical intraocular pressure lowering medications on the ocular surface of glaucoma patients: a review. J Curr Ophthalmol. 2018;31(1):8–15. doi: 10.1016/j.joco.2018.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mastropasqua R, Fasanella V, Brescia L, Oddone F, Mariotti C, Di Staso S, Agnifili L. In vivo confocal imaging of the conjunctiva as a predictive tool for the glaucoma filtration surgery outcome. Invest Ophthalmol Vis Sci. 2017;58(6):BIO114–BIO120. doi: 10.1167/iovs.17-21795. [DOI] [PubMed] [Google Scholar]
- 14.Boimer C, Birt CM. Preservative exposure and surgical outcomes in glaucoma patients: The PESO study. J Glaucoma. 2013;22(9):730–735. doi: 10.1097/IJG.0b013e31825af67d. [DOI] [PubMed] [Google Scholar]
- 15.Barnebey HS, Robin AL. Adherence to fixed-combination versus unfixed travoprost 0.004%/timolol 0.5% for glaucoma or ocular hypertension: a randomized trial. Am J Ophthalmol. 2017;176:61–69. doi: 10.1016/j.ajo.2016.12.002. [DOI] [PubMed] [Google Scholar]
- 16.Takmaz T, Aşik S, Kürkçüoğlu P, Gurdal C, Can I. Comparison of intraocular pressure lowering effect of once daily morning vs evening dosing of latanoprost/timolol maleate combination. Eur J Ophthalmol. 2008;18(1):60–65. doi: 10.1177/112067210801800110. [DOI] [PubMed] [Google Scholar]
- 17.Diestelhorst M, Larsson LI, European Latanoprost Fixed Combination Study Group A 12 week study comparing the fixed combination of latanoprost and timolol with the concomitant use of the individual components in patients with open angle glaucoma and ocular hypertension. Br J Ophthalmol. 2004;88(2):199–203. doi: 10.1136/bjo.2003.018234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Quaranta L, Biagioli E, Riva I, Rulli E, Poli D, Katsanos A, Floriani I. Prostaglandin analogs and timolol-fixed versus unfixed combinations or monotherapy for open-angle glaucoma: a systematic review and meta-analysis. J Ocul Pharmacol Ther. 2013;29(4):382–389. doi: 10.1089/jop.2012.0186. [DOI] [PubMed] [Google Scholar]
- 19.Xing Y, Jiang FG, Li T. Fixed combination of latanoprost and timolol vs the individual components for primary open angle glaucoma and ocular hypertension: a systematic review and meta-analysis. Int J Ophthalmol. 2014;7(5):879–890. doi: 10.3980/j.issn.2222-3959.2014.05.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lindén C, Alm A. The effect on intraocular pressure of latanoprost once or four times daily. Br J Ophthalmol. 2001;85(10):1163–1166. doi: 10.1136/bjo.85.10.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mills RP, Budenz DL, Lee PP, Noecker RJ, Walt JG, Siegartel LR, Evans SJ, Doyle JJ. Categorizing the stage of glaucoma from pre-diagnosis to end-stage disease. Am J Ophthalmol. 2006;141(1):24–30. doi: 10.1016/j.ajo.2005.07.044. [DOI] [PubMed] [Google Scholar]
- 22.Terry RL, Schnider CM, Holden BA, Cornish R, Grant T, Sweeney D, La Hood D, Back A. CCLRU standards for success of daily and extended wear contact lenses. Optom Vis Sci. 1993;70(3):234–243. doi: 10.1097/00006324-199303000-00011. [DOI] [PubMed] [Google Scholar]
- 23.Jabs DA, Nussenblatt RB, Rosenbaum JT, Standardization of Uveitis Nomenclature (SUN) Working Group Standardization of uveitis nomenclature for reporting clinical data. Results of the First International Workshop. Am J Ophthalmol. 2005;140(3):509–516. doi: 10.1016/j.ajo.2005.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xing Y, Zhu L, Zhang K, Huang S. The efficacy of the fixed combination of latanoprost and timolol versus other fixed combinations for primary open-angle glaucoma and ocular hypertension: a systematic review and meta-analysis. PLoS One. 2020;15(2):e0229682. doi: 10.1371/journal.pone.0229682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tripathy K, Geetha R. StatPearls. Treasure Island (FL): StatPearls Publishing; 2023. Jan, Latanoprost. 2023 May 3. [Google Scholar]
- 26.Subbulakshmi S, Kavitha S, Venkatesh R. Prostaglandin analogs in ophthalmology. Indian J Ophthalmol. 2023;71(5):1768–1776. doi: 10.4103/IJO.IJO_2706_22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barnes J, Moshirfar M. StatPearls. Treasure Island (FL): StatPearls Publishing; 2023. Jan, Timolol. 2022 Dec 11. [Google Scholar]
- 28.Calissendorff B, Sjöquist B, Högberg G, Grunge-Lowerud A. Bioavailability in the human eye of a fixed combination of latanoprost and timolol compared to monotherapy. J Ocul Pharmacol Ther. 2002;18(2):127–131. doi: 10.1089/108076802317373888. [DOI] [PubMed] [Google Scholar]
- 29.Lindén C, Alm A. Latanoprost twice daily is less effective than once daily: indication of receptor subsensitivity? Curr Eye Res. 1998;17(6):567–572. [PubMed] [Google Scholar]