Key Points
Question
Can patient data be analyzed to systematically identify copositivity groups in standard series patch testing?
Findings
In this retrospective analysis, 5943 patients tested to the 80 allergen Mayo Clinic Standard Series from 2012 to 2021 were analyzed, comprising 9545 positive reactions. Background correction coupled with hierarchical clustering confirmed many known prior copositivity groups and revealed novel allergen associations.
Meaning
Copositivity groups can be identified in an unbiased and systematic manner using background correction with hierarchical clustering to aid contact avoidance for patients with allergic contact dermatitis.
This retrospective analysis uses patient data to systematically determine allergen copositivity groups in the Mayo Clinic Standard Series to provide guidance on contact avoidance protocols.
Abstract
Importance
Patients are frequently copositive for multiple allergens simultaneously, either due to chemical similarity or simultaneous sensitization. A better understanding of copositivity groups would help guide contact avoidance.
Objective
To use patient data to systematically determine copositivity groups in the Mayo Clinic Standard Series.
Design, Setting, and Participants
In this retrospective cross-sectional analysis, the Mayo Clinic patch test database was queried for pairwise copositivity rates in the 80 allergen Mayo Clinic Standard Series between 2012 and 2021. Data were collected from 3 tertiary care sites of the Mayo Clinic Contact Dermatitis Group and a total of 5943 patients were included, comprising all patients undergoing patch testing to the Mayo Clinic Standard Series allergens.
Main Outcomes and Measures
Copositivity rates between every 2 allergens in the 80-allergen Mayo Clinic Standard Series were estimated. After background correction, copositivity rates were analyzed using unsupervised hierarchical clustering to systematically identify copositivity groups in an unbiased manner.
Results
Overall, 394 921 total patches were applied to 5943 patients (4164 [70.1%] women, 1776 [29.9%] men, with a mean [SD] age of 52.3 [18.8] years ), comprising 9545 positive reactions. After background correction based on overall positivity rates, hierarchical clustering revealed distinct copositivity groups. Many were supported by prior literature, including formaldehyde releasers, cobalt-nickel-potassium dichromate, acrylates, 3-dimethylaminopropylamine-amidoamine-oleamidopropyl dimethylamine, alkyl glucosides, budesonide-hydrocortisone-17-butyrate, certain fragrances, compositae-sesquiterpene lactone mix, mercapto mix-mercaptobenzothiazole, carba mix-thiuram mix, and disperse orange-p-phenylenediamine. However, novel associations were also found, including glutaraldehyde-sorbitan sesquioleate, benzalkonium chloride-neomycin-bacitracin, bronopol-methylchloroisothiazolinone-methylisothiazolinone, and benzoic acid-iodopropynyl butylcarbamate.
Conclusions and Relevance
This retrospective cross-sectional analysis found that copositivity rates varied between allergens; allergens with extremely high positivity rates demonstrated nonspecific copositivity to multiple other allergens. Background correction based on positivity rates followed by hierarchical clustering confirmed prior known copositivity groups, contaminants and/or excipients leading to copositivity, and novel associations to guide contact avoidance.
Introduction
In patch testing, patients are exposed to multiple allergens to elicit an immune response to find the culprit for allergic contact dermatitis. In some instances, patients may be allergic to 1 clinically relevant allergen; however, many times, patients are allergic to multiple allergens simultaneously.1,2 The simultaneously positive allergens may be unrelated to each other but may often be closely related.3,4 These allergens could be concurrently positive at the cellular and/or molecular level, whereby the same reactive T cell recognizes 2 distinct but structurally similar allergens (termed cross-reactivity). Alternatively, simultaneous exposure to 2 allergens can trigger 2 different T cells to react concomitantly (termed coreactivity). To broadly include both coreactivity and cross-reactivity, we will use the term copositivity for the purposes of this study, as before.5
Regardless of the underlying cause, there are well-documented copositivity patterns, which define allergen groups to help patients avoid related haptens.3 However, previous research has focused on specific allergens of clinical interest, potentially introducing selection bias. Here, we systematically evaluated copositivity patterns in the entire 80 allergen Mayo Clinic Standard Series using hierarchical clustering to identify copositivity groups.
Methods
Study Population and Definitions
After Mayo Clinic institutional review board approval, we retrospectively analyzed Mayo Clinic Standard Series patch testing from 2012 to 2021 conducted by the Mayo Clinic Contact Dermatitis Group (Arizona, Florida, and Minnesota). The study was deemed exempt from written informed consent because all data used were deidentified. Patch testing was conducted using published protocols.1 Positive reactions were defined as weak, strong, or extreme; negative reactions included no reaction, macular erythema, and irritant reactions.
Pairwise copositivity rates were then determined as previously published.5 Herein, copositivity is determined between any 2 allergens (A and B) tested simultaneously on the same patient (eTable 1 in Supplement 1). For example, 63 patients showed positive reaction to methyl methacrylate (allergen A; overall positivity rate 1.17%); of these patients, 21 were positive to nickel as well (allergen B; overall positivity rate 17.5%), resulting in a copositivity rate of 21/63 (33.33%) (eFigure 1 in Supplement 2). The Mayo Clinic Standard Series is frequently modified to account for new emerging allergens and optimize test sensitivity. Hence, not all patients were tested to the exact same allergens. However, as published previously,5 the copositivity rates were determined in a strict pairwise comparison as a percentage/ratio, accounting for differences in testing frequency.
The most common contact allergens resulted in extremely high copositivity rates (eg, myroxylon pereirae, methylisothiazolinone, nickel, etc.). To prevent overinflated copositivity rates due to high background positivity, a correction factor was used, which we call the Contact Allergy Background Correction Factor (CAB-CF). If allergen A had a higher background positivity rate than allergen B, CAB-CF was defined as the ratio between the 2 allergen overall positivity rates, and 1 otherwise. To determine background corrected copositivity (henceforth called CAB-CF copositivity), the copositivity rate was divided by the CAB-CF. This approach corrects for the fact that 1 allergen may be much higher in reactivity rate than another, thereby preventing skewing of the results. For the previous example (eFigure 1 in Supplement 2), nickel’s positivity rate was much higher at 17.5% than methyl methacrylate at 1.17%; hence, CAB-CF was 17.5/1.17 = 14.96. In other words, nickel had a 14.96-fold higher likelihood to be positive, given the greater baseline positivity rate. The resulting CAB-CF copositivity for methyl methacrylate and nickel is computed as 33.33 (uncorrected copositivity rate)/14.96 (correction factor CAB-CF), or 2.23%.
We found that background correction (CAB-CF copositivity) was crucial in understanding copositivity data. To use a theoretical example, as much as 75% of the population may be allergic to poison ivy (urushiol).6 If urushiol were patch tested, then every other allergen (eg, HEMA) would be expected to demonstrate at least a 75% copositivity rate to urushiol; eg, when positive to HEMA, at least 75% of patients would be copositive to urushiol. Notably, this is solely owing to the high background copositivity rate for urushiol, and has no bearing on coreactivity or cross-reactivity. By incorporating background correction with CAB-CF copositivity, we have adjusted for variable general positivity rates.
Statistical Analysis
Background correction using CAB-CF was conducted for each pairwise ratio, and CAB-CF copositivity rates were then visualized on a heat map and analyzed through Morpheus (https://software.broadinstitute.org/morpheus/), a matrix analyzer for unsupervised hierarchical clustering of genomic data sets. Here, darker colors indicate higher CAB-CF copositivity. Allergens with less than 5 positive total reactions were excluded due to lack of generalizability. Then each allergen was compared in a paired fashion against the entire series, and clustered based on similarity using the default 1 minus Pearson correlation. Sequential clustering resulted in a tree-like dendrogram, where closely related allergens are depicted with shorter heights, and analyzed descriptively. Notably, this is different than traditional hierarchical clustering used in gene expression analysis, which examines various samples against the same set of genes to determine patterns.
To determine a copositivity group, we used the dendrogram height, namely, the relatedness of a cluster of allergens via branching trees, seen on the x- and y-axes. Smaller height measurements indicate stronger associations. The dendrogram height of the formaldehyde group was selected as 1 benchmark to identify a copositivity group because these are well-established cross-reactors. The combined (x- and y-axes) dendrogram heights for formaldehyde/formaldehyde releasers was low at 1.4. As another benchmark, the metals (nickel, cobalt, and potassium dichromate) are also a well-established copositivity group, with a low dendrogram height of 1.45. We used a slightly more permissive dendrogram height of 1.5 to identify copositivity groups in this study. To reduce small number bias, at least 3 copositive reactions (positive to both allergen A and B) were required to identify a copositivity group.
Results
Copositivity Analysis of the Patient Population
Between 2012 and 2021, 5943 patients were tested for 394 921 allergens applied, resulting in 9545 positive reactions (eTable 2 in Supplement 2). Overall positivity rates were highest with nickel (17.5%), methylisothiazolinone (12.3%), and myroxylon pereirae (10.1%), similar to previous reports.1,2 The lowest positivity rates were with cetyl alcohol (0.06%), triamcinolone (0.07%), and phenoxyethanol (0.11%). The CAB-CF copositivity ranged from 0 (multiple pairwise allergens) to 58.1% (amidoamine to 3-[dimethylamino]-1-propylamine [DMAPA], a known association7), with a mean (SD) CAB-CF copositivity rate of 3.4% (4.8%).
The CAB-CF copositivite results were then color coded, where darker green corresponded to higher rates, and analyzed via hierarchical clustering in Morpheus (Figure, for a more detailed view see eFigure 2 in Supplement 2). From hierarchical clustering, several copositivity groups were then identified and discussed in detail below, both known (Table 1) and novel (Table 2).
Figure. Mayo Clinic Standard Series CAB-CF Copositivity Heat Map and Dendrogram.
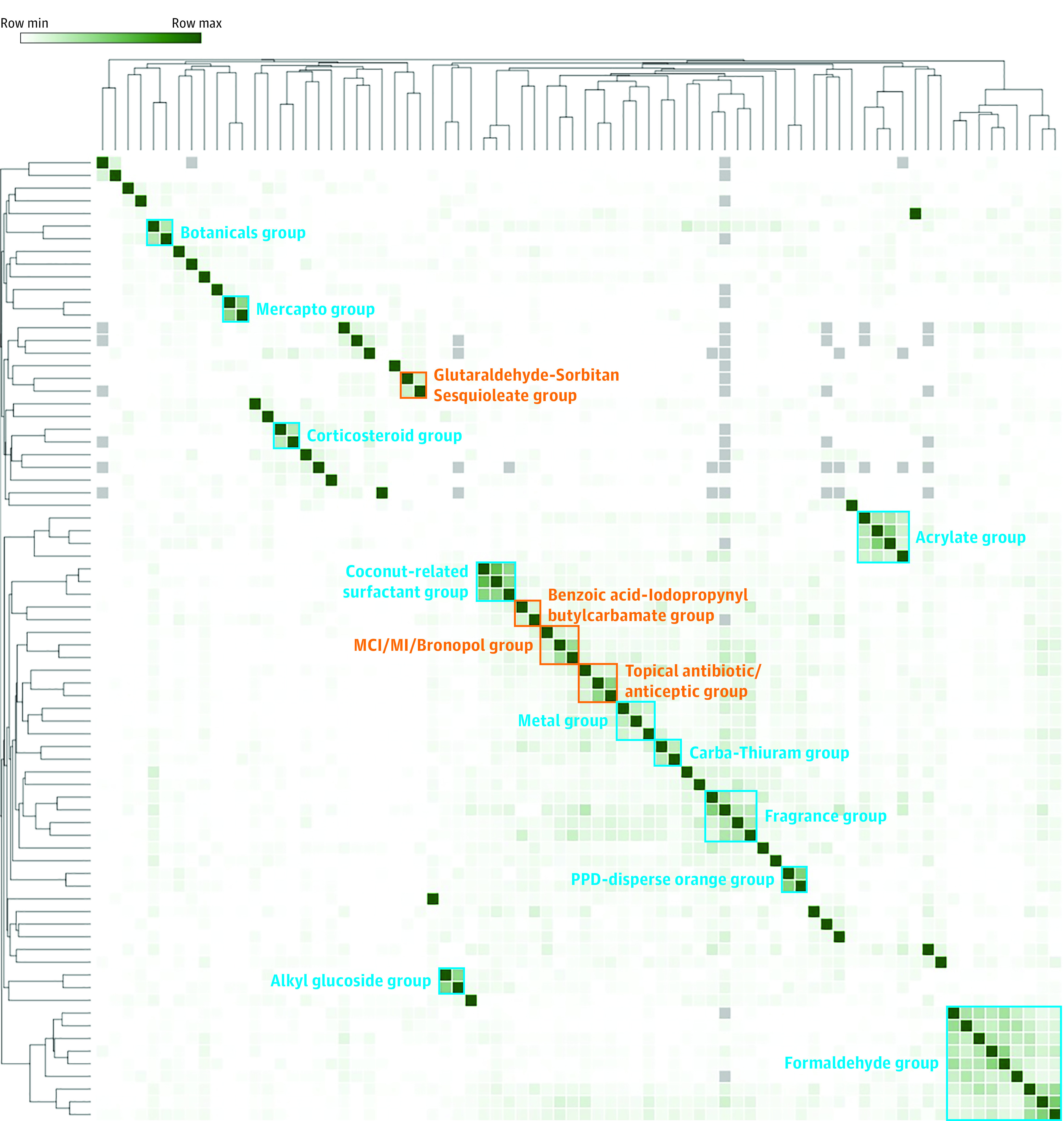
CAB-CF indicates contact allergy background correction factor
Table 1. Known Copositivity Groups.
| Formaldehyde group | Metal group |
| Diazolidinyl urea, 1% | Cobalt (II) chloride hexahydrate, 1% |
| DMDM hydantoin, 1% | Nickel (II) sulfate hexahydrate, 2.5% |
| DMDM hydantoin, 2% in aq | Potassium dichromate, 0.25% |
| Ethyleneurea, melamine formaldehyde mix, 5% | Fragrance group |
| Formaldehyde, 1% Aq | Fragrance mix 1 8% |
| Hexahydro-1, 3, 5-tris (2-hydroxyethyl) triazine, 1% | Hydroperoxides of limonene, 0.3% |
| Imidazolidinyl urea, 2% | Hydroperoxides of linalool, 0.5% |
| Quaternium 15, 1% | Myroxylon pereirae resin, 25% |
| Toluenesulphonamide formaldehyde resin, 10% | Botanicals group |
| Acrylate group | Compositae mix 2 5% |
| 2-Hydroxyethyl methacrylate, 2% (HEMA) | Sesquiterpene lactone mix, 0.1% |
| Ethyl acrylate, 0.1% | Mercapto group |
| Ethyl cyanoacrylate, 10% | Mercapto mix, 1% |
| Methyl methacrylate, 2% | Mercaptobenzothiazole, 1% |
| Coconut-related surfactant group | Carba-thiuram group |
| 3-(dimethylamino)-1-propylamine (DMAPA), 1% Aq | Carba mix, 3% |
| Amidoamine, 0.1% aq | Thiuram mix, 1% |
| Oleamidopropyl dimethylamine, 0.1% aq | PPD-disperse orange group |
| Alkyl glucoside surfactant group | Disperse orange 3, 1% |
| Decyl glucoside, 5% | p-Phenylenediamine, 1% |
| Lauryl polyglucose, 3% pet | |
| Corticosteroid group | |
| Budesonide, 0.01% | |
| Hydrocortisone 17-butyrate, 1% alc |
Abbreviations: aq, aqueous; pet, in petrolatum; alc, in alcohol.
Table 2. Novel Copositivity Groups.
| Glutaraldehyde-sorbitan sesquioleate group (contaminant) | MCI/MI/bronopol group |
| Glutaral (glutaraldehyde), 0.2%a | 2-Bromo-2-nitropropane-1,3-diol, 0.5%a |
| Sorbitan sesquioleate, 20%a | MCI/MI, 100 ppm aq |
| Topical antibiotic/antiseptic group | Methylisothiazolinone, 0.2% aq |
| Bacitracin, 20% | Benzoic acid-iodopropynyl butylcarbamate group |
| Benzalkonium chloride, 0.1% aqa | Benzoic acid, 5%a |
| Neomycin sulfate, 20% | Iodopropynyl butylcarbamate, 0.2%a |
Abbreviations: aq, aqueous; pet, in petrolatum; alc, in alcohol.
Novel associations.
Known Copositivity Groups
Formaldehyde Group
Formaldehyde and formaldehyde-releasing preservatives and/or resins are united in causing exposure to formaldehyde and clustered with a low dendrogram height of 1.4. Given their well-established copositivity rates caused by a shared chemical, the formaldehyde group was used to benchmark the threshold to identify other groups as having meaningful copositivity rates. We found that the formaldehyde group was comprised of 2 more closely associated copositivity subgroups. The first subgroup includes formaldehyde, quaternium-15, and hexahydro-1,3,5-tris(2-hydroxyethyl)triazine; the known strong association between formaldehyde and quaternium-15 has been noted previously.8,9,10,11
The second subgroup includes DMDM hydantoin, ethyleneurea melamine formaldehyde mix, diazolidinyl urea, imidazolidinyl urea, and toluene sulfonamide formaldehyde resin. In the second subgroup, there was a particularly notable close association between the structurally similar molecules, diazolidinyl urea and imidazolidinyl urea, and has been reported.8,10,11,12
Importantly, DMDM hydantoin and ethyleneurea melamine formaldehyde mix had a moderate copositivity association. Ethyleneurea melamine formaldehyde mix is comprised of dimethylol dihydroxyethylene urea and melamine formaldehyde. Of them, dimethylol dihydroxyethylene urea (a weak formaldehyde releaser) is tested in the Mayo Clinic Standard series and does not cluster with the formaldehydes. This suggests that the melamine formaldehyde component, which is a strong formaldehyde releaser, merits the inclusion of the ethyleneurea melamine formaldehyde mix in the formaldehyde group.13
Finally, similar to prior reports, 2-bromo-2-nitropropane-1, 3-diol (bronopol),4,8,10,11,12 and phenol formaldehyde resin did not group with the with the formaldehyde group.14,15
Metal Group
Copositive reactions between nickel, cobalt, and chromium have been well established,16,17 and clustered with a low dendrogram height of 1.45. Thus, these metals were also used to help benchmark meaningful copositivity thresholds. In this study, we found that nickel and cobalt clustered more strongly together than either to potassium dichromate, which has been seen.16 However, this stronger association between nickel and cobalt in the group may be influenced by factors including occupation, local exposures, and ongoing metal mixture trends.
Acrylate Group
All 4 acrylates tested in the Mayo Clinic Standard Series clustered together with a low dendrogram height of 1.32; cross reactions between acrylates have not been previously well described.18 We found that ethyl acrylate and methylmethacrylate clustered more closely, which is understandable due to their chemical similarity (same molecular formula/mass, but differing carbon structure), as opposed to HEMA and ethyl cyanoacrylate.
Coconut-Related Surfactant Group and Alkyl Glucoside Surfactant Group
Surfactants clustered separately as anticipated, into the coconut-related and alkyl glucoside groups. Coconut-related surfactants involved in manufacturing (DMAPA, oleamidopropyl dimethylamine, and amidoamine) strongly clustered with a very low dendrogram height of 0.56, but did not cluster with cocamidopropyl betaine.7 There have been suggestions that clinical cocamidopropyl betaine contact dermatitis may stem from hypersensitivity to these contaminants from manufacturing. Coconut diethanolamide notably did not cluster with the coconut-related surfactants as previously described.19
The alkyl glucosides tested also strongly clustered as a group with a very low dendrogram height of 0.64, similar to recent reports.20,21,22
Corticosteroid Group
In the Mayo Clinic Standard Series, we routinely test to 5 corticosteroids—budesonide, hydrocortisone-17-butyrate, tixocortol-21-pivalate, desoximetasone, and triamcinolone. The rates of positivity for desoximetasone and triamcinolone were insufficient for analysis. Of the remaining corticosteroids, budesonide and hydrocortisone-17-butyrate clustered with a dendrogram height of a moderately low 1.06. In our previous work, we notably observed similar findings that budesonide had copositive results to hydrocortisone-17 butyrate, but not specifically enough to define a corticosteroid class. Similarly, tixocortol-21-pivalate was did not have copositive results with the other corticosteroids.5
Fragrance Group and Botanicals Group
Fragrance and botanicals typically show high levels of copositivity based on shared chemicals and exposure.23 In this study, 4 fragrances comprised the fragrance group with a low dendrogram height of 1.29, with previously known preferential associations: limonene with linalool,24,25 and fragrance mix 1 with myroxylon pereirae.23,26 As noted, we established the dendrogram threshold height for this study (1.5) using the formaldehyde group (dendrogram height, 1.4) and the metal group (dendrogram height, 1.45). Some fragrances clustered just outside this threshold, including fragrance mix 2 and colophonium (higher dendrogram height, 1.59), Tea tree oil (dendrogram height, 1.68), and propolis (dendrogram height, 1.81), indicating a looser association.
Notably, cinnamic aldehyde, benzyl alcohol, and benzoic acid did not cluster with the fragrances, even though myroxylon pereirae is a complex mixture that contains the above as components.26 We postulate that the fragrance-relevant positive patch test in myroxylon pereirae may not be one of these allergens.
As would be expected given their shared origin, compositae mix and sesquiterpene lactone mix (alantolactone, costunolide, and dehydrocostus lactone) clustered closely to identify the botanical group with a dendrogram height of 1.1.23,27,28 Compositae mix alone showed a weaker association with the fragrances listed above; however, sesquiterpene lactone mix did not show as strong copositivity result with the fragrances listed, a trend seen in prior data.23 Thus, patients allergic to sesquiterpene lactone alone (but not fragrances) may consider using fragrances.
For comparison, the North American Contact Dermatitis Group (NACDG) published their data from 2007 to 2016, which showed similar positivity rates for fragrance mix 1, myroxylon pereirae, propolis, colophonium, compositae mix, tea tree oil, sesquiterpene lactone mix, and benzyl alcohol.23 However, compared with our data, the NACDG found an approximately 3- to 4-fold higher positivity rate for fragrance mix 2 and cinnamic aldehyde; these were 2 of the 4 highest likely positive allergens. Because patients in the present study were tested to the entire standard series, all patch tests were read at 96 to 120 hours. However, fragrance patch tests have been reported to peak possibly earlier, which may have resulted in lower positivity rates.29 In addition, the NACDG population may have generally higher positive fragrance allergy than in our Mayo Clinic series.
Rubber-Related Groups (Mercapto Group and Carba-Thiuram Group)
Here, we found that the rubber-related groups are very highly copositive, based on structure. Carba mix and thiuram mix components are closely related structurally, can convert to the other based on reduction and/or oxidation, and are known to be frequently copositive.30,31 Likewise, mercaptobenzothiazole is found in mercapto mix, resulting in high copositivity rates.32 In contrast to prior reports,32 we found that the mercapto group and the carba-thiuram group were distinct and separate in terms of copositivity rates. The mercapto group clustered strongly, with a very low dendrogram height of 0.62. In contrast, carba mix and thiuram mix clustered weaker with a dendrogram height of 1.16. Thus, patients allergic to mercapto mix may consider using carba mix or thiuram mix, and vice versa.
Para-Phenylenediamine (PPD)-Disperse Orange Group
Of the 3 dyes, PPD clustered strongly with disperse orange 3 with a dendrogram height of a very low 0.58, but neither grouped with disperse blue mix 106/12433,34,35,36 or parabens,37 similar to prior reports. Likewise, we found that PPD did not cluster with black rubber mix.38
Novel Copositivity Groups
Through our analysis, we further identified novel copositivity groups and new associations (Table 2).
Glutaraldehyde-Sorbitan Sesquioleate Group (Contaminant)
In this study, glutaraldehyde (0.27% positivity rate) and sorbitan sesquioleate (0.30% positivity rate) were rarely positive. Still, the CAB-CF copositivity rates between the 2 allergens was 18% to 20%, with a dendrogram height of 1.32, which was a stronger association than the formaldehyde group or metal group. These 2 molecules are structurally dissimilar and thus unlikely to cause cross reactivity. We therefore propose that this high rate of copositivity is owing to the fact that our glutaraldehyde is mixed with the emulsifier sorbitan sesquioleate 5% (sourced from Chemotechnique Diagnostics), and have since switched to testing to glutaraldehyde alone.
Sorbitan sesquioleate may be a concerning emulsifier used in other allergens from a recent report.39 However, we did not find clustering of sorbitan sesquioleate with other allergens that contain this excipient, such as fragrance mix 1, myroxylon pereirae, DMDM hydantoin, formaldehyde, HEMA, and ethylene melamine formaldehyde mix. Our identification of the glutaraldehyde/sorbitan sesquioleate copositivity is likely owing to the very low reactivity rate of sorbitan sesquioleate, compared with the other allergens in question (roughly 2.2-33 fold difference).
Topical Antibiotic/Antiseptic Group
Bacitracin and neomycin have been consistently in the top 15 allergens noted by both Mayo Clinic and the NACDG.1,2 Copositivity between the 2 is also common, which is likely caused by concomitant exposure in antibiotic preparations given that the 2 allergens are structurally distinct.40,41 Here we found that neomycin and bacitracin had a high CAB-CF copositivity rate as expected (40.7%-42.1%).
Surprisingly, benzalkonium chloride was also commonly copositive with the topical antibiotics, with a CAB-CF copositivity rate of 15.4% to 18.1%. The dendrogram height with these antibiotics was low at 1.39, similar in strength to the formaldehyde group (dendrogram height, 1.4) and more closely associated than the metal group (dendrogram height, 1.45). When testing positive to bacitracin or neomycin, 18.6% or 16.4% of patients also hadpositive results to benzalkonium chloride, respectively. Thus, one of almost 5 to 6 patients who had positive results for either antibiotic may also have positive results for benzalkonium chloride. For patients not tested to benzalkonium chloride who are allergic to bacitracin or neomycin, awareness of the potential association with benzalkonium chloride may aid contact avoidance, if their contact dermatitis persists despite topical antibiotic avoidance.
Whereas benzalkonium chloride and similar quaternary ammonium compounds are ubiquitous in personal care products, benzalkonium chloride itself is notably used over the counter in first aid products as a topical antiseptic (liquids, sprays, and towelettes), and antibacterial bandages.42 Patients using topical over the counter antibiotics may likely also be using antibacterial bandages and antiseptics for wounds as well, resulting in cosensitization.
Methylchloroisothiazolinone/Methylisothiazolinone (MCI/MI) and Bronopol Group
MCI and MI are preservatives with elevated positivity rates in North America, but recently decreasing in Europe.1,43 Notably, MCI/MI contains MI, resulting in a known strong association.44,45,46 Here, we found that MCI/MI and MI were strongly copositive (35.0-36.9)%. Interestingly, both clustered weakly with Bronopol, with CAB-CF copositivity of 11.6%-16.8%, and a dendrogram height of 1.48, which is just above the metal group (dendrogram height of 1.45). As noted above, bronopol has been historically classified as a formaldehyde releaser, but does not cluster with the Formaldehyde Group. As structurally bronopol has little in common with MCI/MI and MI, this grouping may be caused by cosensitization. Still, patients allergic to bronopol may consider avoiding MCI and MI, and vice versa.
Benzoic Acid and Iodopropynyl Butylcarbamate Group
Both benzoic acid and iodopropynyl butylcarbamate are preservatives. Our study found a weak association, with CAB-CF copositivity rates of 15.6% to 16.8%, and a low dendrogram height of 1.44, better than that of the metal group (dendrogram height, 1.45). Because molecularly the 2 appear distinct, we suspect this also may be caused by cosensitization (simultaneous exposure). Patients allergic to benzoic acid may consider avoiding iodopropynyl butylcarbamate, and vice versa.
Discussion
Herein we used background correction with unsupervised hierarchical clustering to systematically identify copositivity groups in the Mayo Clinic Standard Series. In contrast to prior studies, copositivity groups were determined algorithmically across the whole series, without any bias from prior clinical knowledge or allergen selection based on structure. Still, we found these data were largely consistent with findings reported by others, finding 11 established copositivity groups. Based on the thresholds determined, we further discovered 4 new associations, which may aid allergen avoidance.
Novel here, we applied background correction (termed CAB-CF) to account for differing general allergen positivity rates (as in the previous urushiol example). In biostatistics, similar adjustments are made when comparing nominal rates of 2 differing populations.47 For example, area A may have a much higher crude death rate than area B. However, if area A has an older population (eg, a retirement community), the age-adjusted death rates may be similar.48 Similarly, the crude copositivity rate between 2 allergens may appear very high; however, high background positivity of 1 allergen would also skew the results. By dividing the uncorrected copositivity values by CAB-CF, we factored in the variation in background positivity, allowing copositivity comparisons.
A better understanding of copositivity rates has substantial clinical implications. Instead of needing to avoid broad groups of allergens, patients may be able to have a more focused copositivity-directed avoidance protocol. For example, individuals allergic solely to phenol formaldehyde resin likely do not need to avoid formaldehyde or formaldehyde releasers. Similarly, if allergic to sesquiterpene lactone mix alone, patients may not need to avoid fragrances in their personal care products. In addition, novel groups reveal new and emerging associations; for example, if a patient is allergic to bacitracin and neomycin, they may likely be allergic to benzalkonium chloride as well.
Limitations
This study has limitations. The data were collected from 3 tertiary care centers in the US, which may not represent other populations. Ideally, all patients would be tested to the same allergens. However, in this study, we used a strict pairwise analysis, reducing potential bias as noted.5 In addition, certain allergens may demonstrate secondary copositivity outside their primary group (eg, colophonium with fragrance group). Further research may explore such secondary associations. Because copositivity is a continuous variable, threshold heights to defined copositivity groups can be changed; this may also help patients in their stringency of contact avoidance. Finally, our methods used traditional algorithms used in genomic data sets. Other algorithmic approaches to clustering and determining statistical significance may provide further insights, particularly if additional external data sets can be used to validate algorithm functionality.
Conclusions
The results of this cross-sectional analysis suggest that background correction coupled with hierarchical clustering may emphasize known copositivity groups and reveal novel associations. Future studies of copositivity patterns should take into account differing background general positivity rates using CAB-CF. A stronger understanding of copositivity trends may aid patients in contact allergen avoidance.
eTable 1. Uncorrected Copositivity Table, Mayo Clinic Standard Series Patch Test Results 2012-2021
eFigure 1. Sample Calculations for Uncorrected Copositivity Rate and CAB-CF Copositivity
eFigure 2. Detailed Mayo Clinic Standard Series CAB-CF Copositivity Heat Map and Dendrogram
eTable 2. Mayo Clinic Standard Series Patch Test Results 2012-2021
Data Sharing Statement
References
- 1.Veverka KK, Hall MR, Yiannias JA, et al. Trends in patch testing with the Mayo Clinic Standard Series, 2011-2015. Dermatitis. 2018;29(6):310-315. doi: 10.1097/DER.0000000000000411 [DOI] [PubMed] [Google Scholar]
- 2.DeKoven JG, Silverberg JI, Warshaw EM, et al. North American Contact Dermatitis Group patch test results: 2017-2018. Dermatitis. 2021;32(2):111-123. doi: 10.1097/DER.0000000000000729 [DOI] [PubMed] [Google Scholar]
- 3.Scheman A, Ruggiero JL, Kerchinsky L, et al. Contact allergy cross-reactions and thresholds: a review. Dermatitis. 2022;33(2):106-109. doi: 10.1097/DER.0000000000000798 [DOI] [PubMed] [Google Scholar]
- 4.Scheman A, Hipolito R, Severson D, Youkhanis N. Contact allergy cross-reactions: retrospective clinical data and review of the literature. Dermatitis. 2017;28(2):128-140. doi: 10.1097/DER.0000000000000254 [DOI] [PubMed] [Google Scholar]
- 5.Chen JY, Yiannias JA, Hall MR, et al. Reevaluating corticosteroid classification models in patient patch testing. JAMA Dermatol. 2022;158(11):1279-1286. doi: 10.1001/jamadermatol.2022.3774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gladman AC. Toxicodendron dermatitis: poison ivy, oak, and sumac. Wilderness Environ Med. 2006;17(2):120-128. doi: 10.1580/PR31-05.1 [DOI] [PubMed] [Google Scholar]
- 7.Presley CL, Militello M, Barber C, et al. The history of surfactants and review of their allergic and irritant properties. Dermatitis. 2021;32(5):289-297. doi: 10.1097/DER.0000000000000730 [DOI] [PubMed] [Google Scholar]
- 8.Whitehouse H, Uter W, Geier J, et al. Formaldehyde 2% is not a useful means of detecting allergy to formaldehyde releasers- results of the ESSCA network, 2015-2018. Contact Dermatitis. 2021;84(2):95-102. doi: 10.1111/cod.13691 [DOI] [PubMed] [Google Scholar]
- 9.Boonchai W, Pruksaeakanan C, Wongdama S, Bunyavaree M, Kumpangsin T, Chaiyabutr C. Trends in formaldehyde and formaldehyde-releaser contact allergies as compared with market exposure in Thailand. Contact Dermatitis. 2023;88(1):18-26. doi: 10.1111/cod.14190 [DOI] [PubMed] [Google Scholar]
- 10.Latorre N, Borrego L, Fernández-Redondo V, et al. Patch testing with formaldehyde and formaldehyde-releasers: multicentre study in Spain (2005-2009). Contact Dermatitis. 2011;65(5):286-292. doi: 10.1111/j.1600-0536.2011.01953.x [DOI] [PubMed] [Google Scholar]
- 11.Lundov MD, Johansen JD, Carlsen BC, Engkilde K, Menné T, Thyssen JP. Formaldehyde exposure and patterns of concomitant contact allergy to formaldehyde and formaldehyde-releasers. Contact Dermatitis. 2010;63(1):31-36. doi: 10.1111/j.1600-0536.2010.01745.x [DOI] [PubMed] [Google Scholar]
- 12.Sanz-Sánchez T, García PM, Silvestre Salvador JF, et al. Contact allergy to formaldehyde releasers. prospective multicenter study. Contact Dermatitis. 2020;82(3):173-175. doi: 10.1111/cod.13417 [DOI] [PubMed] [Google Scholar]
- 13.Fowler JF Jr, Skinner SM, Belsito DV. Allergic contact dermatitis from formaldehyde resins in permanent press clothing: an underdiagnosed cause of generalized dermatitis. J Am Acad Dermatol. 1992;27(6 Pt 1):962-968. doi: 10.1016/0190-9622(92)70295-Q [DOI] [PubMed] [Google Scholar]
- 14.Dear K, Palmer A, Nixon RL. Allergic contact dermatitis to phenol-formaldehyde resin at a single tertiary dermatology centre. Contact Dermatitis. 2021;85(1):26-31. doi: 10.1111/cod.13771 [DOI] [PubMed] [Google Scholar]
- 15.Owen CM, Beck MH. Occupational allergic contact dermatitis from phenol-formaldehyde resins. Contact Dermatitis. 2001;45(5):294-295. doi: 10.1034/j.1600-0536.2001.450509.x [DOI] [PubMed] [Google Scholar]
- 16.Hegewald J, Uter W, Pfahlberg A, Geier J, Schnuch A; IVDK . A multifactorial analysis of concurrent patch-test reactions to nickel, cobalt, and chromate. Allergy. 2005;60(3):372-378. doi: 10.1111/j.1398-9995.2005.00693.x [DOI] [PubMed] [Google Scholar]
- 17.Uter W, Larese Filon F, Rui F, et al. ESSCA results with nickel, cobalt and chromium, 2009-2012. Contact Dermatitis. 2016;75(2):117-121. doi: 10.1111/cod.12582 [DOI] [PubMed] [Google Scholar]
- 18.Voller LM, Warshaw EM. Acrylates: new sources and new allergens. Clin Exp Dermatol. 2020;45(3):277-283. doi: 10.1111/ced.14093 [DOI] [PubMed] [Google Scholar]
- 19.Grey KR, Hanson JL, Hagen SL, Hylwa SA, Warshaw EM. Epidemiology and co-reactivity of novel surfactant allergens: a double-blind randomized controlled study. Dermatitis. 2016;27(6):348-354. doi: 10.1097/DER.0000000000000226 [DOI] [PubMed] [Google Scholar]
- 20.Warshaw EM, Xiong M, DeKoven JG, et al. Co-reactivity of glucosides: retrospective analysis of North American Contact Dermatitis Group data 2019-2020. Contact Dermatitis. 2023;88(2):153-156. doi: 10.1111/cod.14237 [DOI] [PubMed] [Google Scholar]
- 21.Severin RK, Belsito DV. Patch testing with decyl and lauryl glucoside: how well does one screen for contact allergic reactions to the other? Dermatitis. 2017;28(6):342-345. doi: 10.1097/DER.0000000000000327 [DOI] [PubMed] [Google Scholar]
- 22.Tous-Romero F, Giménez-Arnau AM, Sanz-Sánchez T, et al. Allergic contact dermatitis to alkyl glucosides: epidemiological situation in Spain. J Eur Acad Dermatol Venereol. 2023;37(3):e334-e337. doi: 10.1111/jdv.18595 [DOI] [PubMed] [Google Scholar]
- 23.Atwater AR, Ward JM, Liu B, et al. Fragrance- and botanical-related allergy and associated concomitant reactions: a retrospective analysis of the North American Contact Dermatitis Group data 2007-2016. Dermatitis. 2021;32(1):42-52. doi: 10.1097/DER.0000000000000661 [DOI] [PubMed] [Google Scholar]
- 24.Sukakul T, Bruze M, Mowitz M, et al. Contact allergy to oxidized linalool and oxidized limonene: patch testing in consecutive patients with dermatitis. Contact Dermatitis. 2022;86(1):15-24. doi: 10.1111/cod.13980 [DOI] [PubMed] [Google Scholar]
- 25.Bråred Christensson J, Karlberg AT, Andersen KE, et al. Oxidized limonene and oxidized linalool - concomitant contact allergy to common fragrance terpenes. Contact Dermatitis. 2016;74(5):273-280. doi: 10.1111/cod.12545 [DOI] [PubMed] [Google Scholar]
- 26.de Groot AC. Myroxylon pereirae resin (balsam of Peru) - a critical review of the literature and assessment of the significance of positive patch test reactions and the usefulness of restrictive diets. Contact Dermatitis. 2019;80(6):335-353. doi: 10.1111/cod.13263 [DOI] [PubMed] [Google Scholar]
- 27.Bauer A, Geier J, Schreiber S, Schubert S; IVDK . Contact sensitization to plants of the Compositae family: data of the Information Network of Departments of Dermatology (IVDK) from 2007 to 2016. Contact Dermatitis. 2019;80(4):222-227. doi: 10.1111/cod.13169 [DOI] [PubMed] [Google Scholar]
- 28.Bizjak M, Adamič K, Bajrovič N, et al. Patch testing with the European baseline series and 10 added allergens: Single-centre study of 748 patients. Contact Dermatitis. 2022;87(5):439-446. doi: 10.1111/cod.14178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Davis MD, Bhate K, Rohlinger AL, Farmer SA, Richardson DM, Weaver AL. Delayed patch test reading after 5 days: the Mayo Clinic experience. J Am Acad Dermatol. 2008;59(2):225-233. doi: 10.1016/j.jaad.2008.04.022 [DOI] [PubMed] [Google Scholar]
- 30.Warshaw EM, Gupta R, Silverberg JI, et al. Positive patch test reactions to carba mix and thiuram mix: the North American Contact Dermatitis Group experience (1994-2016). Dermatitis. 2021;32(3):173-184. doi: 10.1097/DER.0000000000000648 [DOI] [PubMed] [Google Scholar]
- 31.Aalto-Korte K, Pesonen M. Patterns of simultaneous patch test reactions to thiurams and dithiocarbamates in 164 patients. Contact Dermatitis. 2016;75(6):353-357. doi: 10.1111/cod.12687 [DOI] [PubMed] [Google Scholar]
- 32.Warshaw EM, Gupta R, DeKoven JG, et al. Patch testing of mercaptobenzothiazole and mercapto mix: the North American Contact Dermatitis Group experience, 1994-2016. Dermatitis. 2021;32(4):232-244. doi: 10.1097/DER.0000000000000675 [DOI] [PubMed] [Google Scholar]
- 33.Heratizadeh A, Geier J, Molin S, Werfel T. Contact sensitization in patients with suspected textile allergy. Data of the Information Network of Departments of Dermatology (IVDK) 2007-2014. Contact Dermatitis. 2017;77(3):143-150. doi: 10.1111/cod.12760 [DOI] [PubMed] [Google Scholar]
- 34.Ryberg K, Agner T, Andersen KE, et al. Patch testing with a textile dye mix–a multicentre study. Contact Dermatitis. 2014;71(4):215-223. doi: 10.1111/cod.12244 [DOI] [PubMed] [Google Scholar]
- 35.Goon AT, Gilmour NJ, Basketter DA, White IR, Rycroft RJ, McFadden JP. High frequency of simultaneous sensitivity to disperse orange 3 in patients with positive patch tests to para-phenylenediamine. Contact Dermatitis. 2003;48(5):248-250. doi: 10.1034/j.1600-0536.2003.00049.x [DOI] [PubMed] [Google Scholar]
- 36.Isaksson M, Ale I, Andersen KE, et al. Patch testing to a textile dye mix by the international contact dermatitis research group. Dermatitis. 2015;26(4):170-176. doi: 10.1097/DER.0000000000000125 [DOI] [PubMed] [Google Scholar]
- 37.Turchin I, Moreau L, Warshaw E, Sasseville D. Cross-reactions among parabens, para-phenylenediamine, and benzocaine: a retrospective analysis of patch testing. Dermatitis. 2006;17(4):192-195. doi: 10.2310/6620.2006.06026 [DOI] [PubMed] [Google Scholar]
- 38.Dhadwal G, de Gannes G. Most common co/cross-reactants identified in p-phenylenediamine-allergic patients and impact on available alternative hair dyes. Dermatitis. 2014;25(5):265-267. doi: 10.1097/DER.0000000000000060 [DOI] [Google Scholar]
- 39.Sukakul T, Bruze M, Mowitz M, Svedman C. Use of sorbitan sesquioleate in patch test preparations and patch testing with the substance-What do our results mean? Contact Dermatitis. 2023;88(2):134-138. doi: 10.1111/cod.14239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sood A, Taylor JS. Bacitracin: allergen of the year. Am J Contact Dermat. 2003;14(1):3-4. doi: 10.2310/6620.2003.38621 [DOI] [PubMed] [Google Scholar]
- 41.Sasseville D. Dermatitis. 2010;21(1):3-7. doi: 10.2310/6620.2009.09073 [DOI] [PubMed] [Google Scholar]
- 42.Merchel Piovesan Pereira B, Tagkopoulos I. Benzalkonium chlorides: uses, regulatory status, and microbial resistance. Appl Environ Microbiol. 2019;85(13):e00377-19. doi: 10.1128/AEM.00377-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reeder MJ, Warshaw E, Aravamuthan S, et al. Trends in the prevalence of methylchloroisothiazolinone/methylisothiazolinone contact allergy in North America and Europe. JAMA Dermatol. 2023;159(3):267-274. doi: 10.1001/jamadermatol.2022.5991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Herman A, Aerts O, de Montjoye L, Tromme I, Goossens A, Baeck M. Isothiazolinone derivatives and allergic contact dermatitis: a review and update. J Eur Acad Dermatol Venereol. 2019;33(2):267-276. doi: 10.1111/jdv.15267 [DOI] [PubMed] [Google Scholar]
- 45.Özkaya E, Kılıç Sayar S, Babuna Kobaner G, Pehlivan G. Methylchloroisothiazolinone/methylisothiazolinone and methylisothiazolinone contact allergy: a 24-year, single-center, retrospective cohort study from Turkey. Contact Dermatitis. 2021;84(1):24-33. doi: 10.1111/cod.13656 [DOI] [PubMed] [Google Scholar]
- 46.Herman A, Aerts O, Jacobs MC, et al. Evolution of methylisothiazolinone sensitization: a Belgian multicentric study from 2014 to 2019. Contact Dermatitis. 2021;85(6):643-649. doi: 10.1111/cod.13956 [DOI] [PubMed] [Google Scholar]
- 47.White S. Basic & Clinical Biostatistics. McGraw Hill Professional; 2019. [Google Scholar]
- 48.United States Cancer Statistics (USCS) CfDCaP. Incidence and death rates. Accessed March 24, 2023. https://www.cdc.gov/cancer/uscs/technical_notes/stat_methods/rates.htm
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Uncorrected Copositivity Table, Mayo Clinic Standard Series Patch Test Results 2012-2021
eFigure 1. Sample Calculations for Uncorrected Copositivity Rate and CAB-CF Copositivity
eFigure 2. Detailed Mayo Clinic Standard Series CAB-CF Copositivity Heat Map and Dendrogram
eTable 2. Mayo Clinic Standard Series Patch Test Results 2012-2021
Data Sharing Statement


