This post hoc analysis of the Randomized Comparison of Efficacy and Safety of Lipid Lowering With Statin Monotherapy vs Statin/Ezetimibe Combination for High-Risk Cardiovascular Disease (RACING) randomized clinical trial investigates if a moderate-intensity statin plus ezetimibe combination therapy is a feasible therapeutic option for patients with very high-risk atherosclerotic cardiovascular disease.
Key Points
Question
Is moderate-intensity statin with ezetimibe combination therapy feasible for patients at very high risk of atherosclerotic cardiovascular disease (ASCVD)?
Findings
In this post hoc analysis of 1511 patients at very high risk of ASCVD from the Randomized Comparison of Efficacy and Safety of Lipid-Lowering With Statin Monotherapy vs Statin/Ezetimibe Combination for High-Risk Cardiovascular Disease (RACING) randomized clinical trial, moderate-intensity statin with ezetimibe combination therapy was comparable with high-intensity statin monotherapy in terms of 3-year primary end point and was associated with lower drug intolerance, greater low-density lipoprotein cholesterol (LDL-C) reduction, and achievement of LDL-C less than 70 mg/dL.
Meaning
Ezetimibe combination with moderate-intensity statin could be considered a feasible and effective therapeutic option for patients at very high risk of ASCVD.
Abstract
Importance
High-intensity statin is strongly recommended in patients at very high risk (VHR) of atherosclerotic cardiovascular disease (ASCVD). However, concerns about statin-associated adverse effects result in underuse of this strategy in practice.
Objective
To evaluate the outcomes of a moderate-intensity statin with ezetimibe combination in VHR and non-VHR patients with ASCVD.
Design, Setting, and Participants
This was a post hoc analysis of the Randomized Comparison of Efficacy and Safety of Lipid Lowering With Statin Monotherapy vs Statin/Ezetimibe Combination for High-Risk Cardiovascular Disease (RACING) open-label, multicenter, randomized clinical trial. The study was conducted from February 2017 to December 2018 at 26 centers in Korea. Study participants included patients with documented ASCVD. Data were analyzed from April to June 2022.
Interventions
Patients were randomly assigned to moderate-intensity statin with ezetimibe (rosuvastatin, 10 mg, with ezetimibe, 10 mg) or high-intensity statin monotherapy (rosuvastatin, 20 mg). Patients at VHR for ASCVD were defined according to the 2018 American Heart Association/American College of Cardiology guidelines.
Main Outcomes and Measures
The primary end point was the 3-year outcome of cardiovascular death, coronary or peripheral revascularization, hospitalization of cardiovascular events, or nonfatal stroke.
Results
A total of 3780 patients (mean [SD] age, 64 [10] years; 2826 male [75%]) in the RACING trial, 1511 (40.0%) were categorized as VHR, which was associated with a greater occurrence of the primary end point (hazard ratio [HR], 1.42; 95% CI, 1.15-1.75). There was no significant difference in the primary end point between those who received combination therapy and high-intensity statin monotherapy among patients with VHR disease (11.2% vs 11.7%; HR, 0.96; 95% CI, 0.71-1.30) and non-VHR disease (7.7% vs 8.7%; HR, 0.88; 95% CI, 0.66-1.18). The median low-density lipoprotein cholesterol (LDL-C) level was significantly lower in the combination therapy group than in the high-intensity statin group (VHR, 1 year: 57 [47-71] mg/dL vs 65 [53-78] mg/dL; non-VHR, 1 year: 58 mg/dL vs 68 mg/dL; P < .001). Furthermore, in both the VHR and non-VHR groups, combination therapy was associated with a significantly greater mean change in LDL-C level (VHR, 1 year: −19.1 mg/dL vs −10.1 mg/dL; 2 years: −22.3 mg/dL vs −13.0 mg/dL; 3 years: −18.8 mg/dL vs −9.7 mg/dL; non-VHR, 1 year: −23.7 mg/dL vs −12.5 mg/dL; 2 years: −25.2 mg/dL vs −15.1 mg/dL; 3 years: −23.5 mg/dL vs −12.6 mg/dL; all P < .001) and proportion of patients with LDL-C level less than 70 mg/dL (VHR, 1 year: 73% vs 58%; non-VHR, 1 year: 72% vs 53%; P < .001). Discontinuation or dose reduction of the lipid-lowering drug due to intolerance occurred less frequently in the combination therapy group (VHR, 4.6% vs 7.7%; P = .02; non-VHR, 5.0% vs 8.7%; P = .001).
Conclusions and Relevance
Results suggest that the outcomes of ezetimibe combination observed in the RACING trial were consistent among patients at VHR of ASCVD.
Trial Registration
ClinicalTrials.gov Identifier: NCT03044665
Introduction
The 2018 American Heart Association (AHA)/American College of Cardiology (ACC) guidelines on lipid management recommend the initial use of high-intensity statin in very high–risk (VHR) patients with atherosclerotic cardiovascular disease (ASCVD)1 because this population is associated with a greater risk of recurrent ASCVD events.2 However, despite the distinct and undoubtable benefit of high-intensity statins in VHR patients,3,4 concerns about drug-related adverse effects pose a clinical hurdle, inevitably leading to substantial underuse of the guideline-recommended therapy.5 Recently, the Randomized Comparison of Efficacy and Safety of Lipid-Lowering With Statin Monotherapy vs Statin/Ezetimibe Combination for High-Risk Cardiovascular Disease (RACING) trial demonstrated the noninferiority of a moderate-intensity statin with ezetimibe combination therapy compared with high-intensity statin monotherapy for the 3-year composite cardiovascular outcomes in patients with ASCVD6; however, whether the effect is preserved among VHR patients is not known. Therefore, in the present study, we sought to investigate the outcome of ezetimibe combination with moderate-intensity statin therapy in VHR patients with ASCVD.
Methods
This was a post hoc analysis of the multicenter, open-label, RACING randomized clinical trial, which was conducted from February 2017 to December 2018 at 26 centers in Korea. The trial protocol was approved by the institutional review board at each participating center, and every patient provided written informed consent. Race and ethnicity data were self-reported by participants in the RACING trial, which enrolled only Korean patients of East Asian ethnicity. Adults with documented ASCVD were randomly assigned (1:1) to either receive ezetimibe/moderate–intensity statin combination therapy (rosuvastatin, 10 mg plus ezetimibe, 10 mg) or high-intensity statin monotherapy (rosuvastatin, 20 mg). Detailed enrollment criteria are presented in eTable 1 in Supplement 1. VHR patients were defined as having a history of multiple major ASCVD events or 1 major ASCVD event in addition to various high-risk conditions in accordance with the 2018 AHA/ACC guidelines.1,2 The primary end point was the occurrence of cardiovascular death, coronary or peripheral revascularization, hospitalization for cardiovascular events, or nonfatal stroke within 3 years after randomization. Cardiovascular death was defined as death owing to myocardial infarction, heart failure, stroke, cardiovascular procedures, cardiovascular hemorrhage, sudden cardiac death, and any case of death in which a cardiovascular cause could not be excluded as adjudicated by a clinical end point committee. Myocardial infarction was defined based on symptoms, electrocardiographic changes, or abnormal imaging findings, combined with a creatine kinase MB fraction above the upper normal limits or a troponin T or troponin I level greater than the 99th percentile of the upper normal limit. Coronary or peripheral revascularization included percutaneous and surgical revascularization of the coronary, carotid, or lower-extremity arteries. Hospitalization for cardiovascular events included hospitalization for ischemic heart disease, heart failure, or peripheral artery disease management. Hospitalization for ischemic heart disease was defined as hospitalization due to the need for coronary revascularization based on typical symptoms and signs of myocardial ischemia documented by electrocardiography, exercise, or pharmacologic stress study; angiographic findings suggestive of new or worsening coronary artery disease; or hospitalization requiring at least an overnight stay due to substantial worsening of ischemic symptoms and signs. Nonfatal stroke was defined as an acute cerebrovascular event resulting in a neurologic deficit for longer than 24 hours or the presence of acute infarction on imaging studies. Secondary efficacy end points included individual components of the primary end point, serial changes in low-density lipoprotein cholesterol (LDL-C) level, and a proportion of participants with LDL-C level less than 70 mg/dL (to convert to millimoles per liter, multiply by 0.0259) at 1, 2, and 3 years. Safety end points included the discontinuation or dose reduction of the study drug due to intolerance or the occurrence of adverse events.
Statistical Analysis
Primary analyses were performed in an intention-to-treat manner. Categorical variables were described as counts and percentages and compared using the χ2 test or Fisher exact test. Continuous variables were reported as the mean and SD and compared using t test or Mann-Whitney U test. Event rates were plotted using Kaplan-Meier survival analysis and compared using the log-rank test. Hazard ratios (HRs) with 95% CIs were computed using Cox regression analysis. The consistency of treatment outcomes between the VHR vs non-VHR groups was evaluated using the interaction terms in a Cox proportional hazards model. Safety outcomes were evaluated in a safety population consisting of randomly assigned patients who received at least 1 dose of the assigned study medication. A 2-sided P value <.05 was considered significant. Statistical analyses were conducted from April to June 2022 using R, version 4.0.3 (R Foundation).
Results
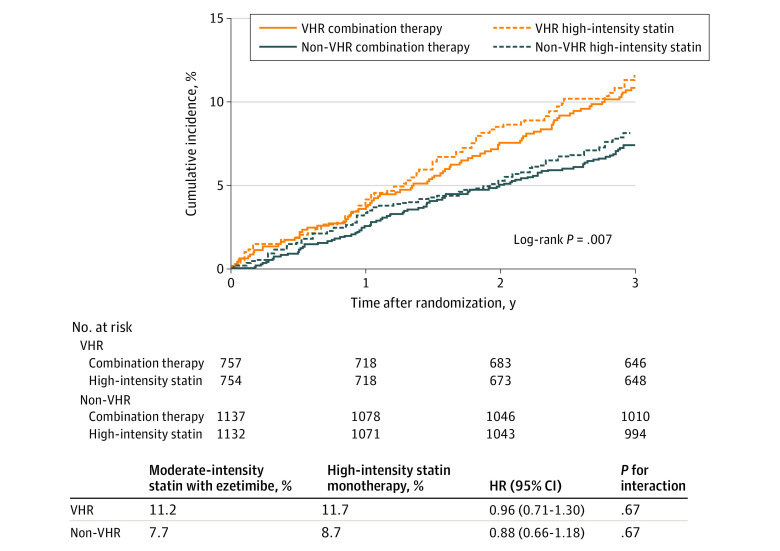
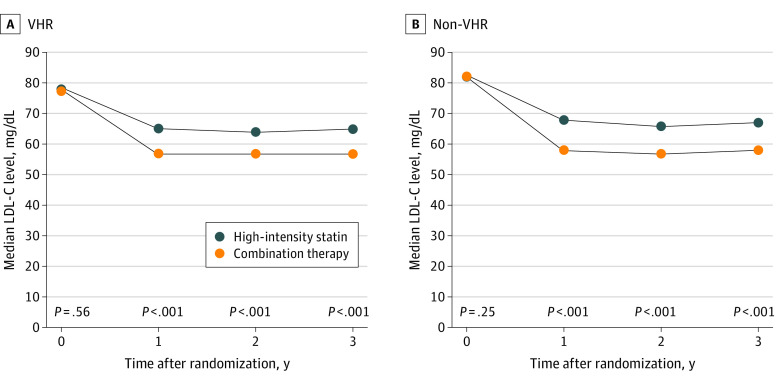
Among the 3780 patients (mean [SD] age, 64 [10] years; 2826 male [75%]; 954 female [25%]) enrolled in the RACING trial, 1511 patients (40.0%) in the VHR group (eTable 2 in Supplement 1) had a higher frequency of comorbidities and high-intensity statin medication before randomization (eTable 3 in Supplement 1). Of the 1511 VHR patients, 757 (50.1%) were allocated to moderate-intensity statin with ezetimibe combination therapy and 754 (49.9%) to high-intensity statin monotherapy, and the baseline characteristics were well-balanced between the groups (Table). Compared with non-VHR patients, VHR patients demonstrated a higher incidence of the primary end point (173 of 1511 [11.4%] vs 185 of 2269 [8.3%]; HR, 1.42; 95% CI, 1.15-1.75; P < .001) (eFigure 1 in Supplement 1). There was no significant difference in the primary end point between the combination therapy and high-intensity statin monotherapy groups for both VHR patients (85 of 757 [11.2%] vs 88 of 754 [11.7%]; HR, 0.96, 95% CI, 0.71-1.30) and non-VHR patients (87 of 1137 [7.7%] vs 98 of 1132 [8.7%]; HR, 0.88, 95% CI, 0.66-1.18) without statistical heterogeneity (P for interaction = .67) (Figure 1). Consistently, there was no significant difference in the occurrence of each clinical end point between the 2 treatment strategies in both VHR and non-VHR patients (eFigure 2 in Supplement 1). Before randomization, there was no significant difference between the groups receiving combination therapy and high-intensity statin therapy in the median (IQR) baseline LDL-C level (VHR, 78 [63-98] mg/dL vs 77 [62-97] mg/dL; non-VHR, 82 [65-102] mg/dL vs 82 [65-102] mg/dL) or proportion of patients with LDL-C level less than 70 mg/dL (Table). Compared with the high-intensity statin group, during follow-up, the median (IQR) LDL-C level was significantly lower in the combination therapy group (VHR, 1 year: 57 [47-71] mg/dL vs 65 [53-78] mg/dL; 2 years: 57 [45-69] mg/dL vs 64 [51-78] mg/dL; 3 years: 57 [46-72] mg/dL vs 65 [51-79] mg/dL; non-VHR, 1 year: 58 [47-71] mg/dL vs 68 [56-81] mg/dL; 2 years: 57 [46-70] mg/dL vs 66 [53-79] mg/dL; 3 years: 58 [47-70] mg/dL vs 67 [56-81] mg/dL; all P < .001) (Figure 2; and eTable 4 in Supplement 1). For both VHR and non-VHR patients, the mean (SD) change in LDL-C level from baseline was significantly greater in the combination group (VHR, 1 year: −19.1 [30.0] mg/dL vs −10.1 [31.4] mg/dL; 2 years: −22.3 [33.3] mg/dL vs −13.0 [33.8] mg/dL; 3 years: −18.8 [32.2] mg/dL vs −9.7 [34.5] mg/dL; non-VHR, 1 year: −23.7 [29.1] mg/dL vs −12.5 [33.6] mg/dL; 2 years: −25.2 [28.5] mg/dL vs −15.1 [35.4] mg/dL; 3 years: −23.5 [29.4] mg/dL vs −12.6 [31.9] mg/dL; all P < .001). Consequently, the proportion of patients with LDL-C level less than 70 mg/dL was significantly higher in combination group (VHR, 1 year: 492 of 673 [73%] vs 393 of 671 [58%]; 2 years: 467 of 617 [76%] vs 377 of 618 [61%]; 3 years: 380 of 530 [72%] vs 323 of 536 [60%]; non-VHR, 1 year: 725 of 1002 [72%] vs 530 of 1002 [53%]; 2 years: 701 of 941 [75%] vs 547 of 921 [59%]; 3 years: 598 of 819 [73%] vs 436 of 779 [56%]; all P < .001) (eFigure 3 in Supplement 1). Discontinuation or dose reduction of lipid-lowering drugs due to intolerance occurred less frequently in the combination group (VHR, 34 of 732 [4.6%] vs 56 of 731 [7.7%]; P = .02; non-VHR, 57 of 1114 [5.0%] vs 100 of 1105 [8.7%]; P = .001) (eFigure 4 in Supplement 1). The occurrence of other clinical adverse events associated with study drugs was comparable between the treatment groups in both VHR and non-VHR (eTable 5 in Supplement 1).
Table. Baseline Characteristics According to Treatment Assignment in Very High-Risk (VHR) and Non-VHR Patients With Atherosclerotic Cardiovascular Disease (ASCVD).
| Characteristics | VHR group (n = 1511) | Non-VHR group (n = 2269) | ||||
|---|---|---|---|---|---|---|
| Moderate-intensity statin with ezetimibe (n = 757) | High-intensity statin monotherapy (n = 754) | P value | Moderate-intensity statin with ezetimibe (n = 1137) | High-intensity statin monotherapy (n = 1132) | P value | |
| Age, mean (SD), y | 63.6 (9.9) | 64.3 (10.3) | .19 | 63.5 (9.3) | 63.9 (9.2) | .37 |
| Sex, No. (%) | ||||||
| Female | 141 (18.6) | 154 (20.4) | .41 | 333 (29.3) | 326 (28.8) | .83 |
| Male | 616 (81.4) | 600 (79.6) | 804 (70.7) | 806 (71.2) | ||
| Body mass index, mean (SD)a | 25.0 (3.2) | 25.0 (3.0) | .82 | 25.1 (3.1) | 25.1 (3.1) | .58 |
| Prior myocardial infarction, No. (%) | 650 (85.9) | 631 (83.7) | .57 | 94 (8.2) | 114 (10.0) | .18 |
| Prior percutaneous coronary intervention, No. (%) | 648 (85.6) | 632 (83.8) | .37 | 610 (53.6) | 607 (53.6) | <.99 |
| Prior coronary bypass graft surgery, No. (%) | 58 (7.7) | 47 (6.3) | .35 | 74 (6.5) | 68 (5.9) | .65 |
| History of ischemic stroke, No. (%) | 93 (12.3) | 101 (13.4) | .57 | 8 (0.7) | 11 (1.0) | .64 |
| Chronic kidney disease, No. (%)b | 106 (14.0) | 106 (14.1) | <.99 | 87 (7.7) | 93 (8.2) | .68 |
| End-stage kidney disease receiving hemodialysis, No. (%) | 11 (1.5) | 12 (1.6) | .97 | 2 (0.2) | 4 (0.3) | .69 |
| Hypertension, No. (%) | 569 (75.2) | 574 (76.1) | .71 | 677 (59.5) | 700 (61.8) | .28 |
| Peripheral artery disease, No. (%) | 54 (7.1) | 56 (7.4) | .90 | 12 (1.1) | 13 (1.1) | .99 |
| Diabetes, No. (%) | 334 (44.1) | 327 (43.4) | .81 | 367 (32.3) | 370 (32.7) | .87 |
| Insulin treatment | 26 (3.4) | 34 (4.5) | .35 | 24 (2.1) | 36 (3.2) | .15 |
| Current smoker, No. (%) | 172 (22.7) | 164 (21.8) | .70 | 156 (13.7) | 146 (12.9) | .61 |
| Dyslipidemia treatment before randomization, No. (%) | ||||||
| Drug naive | 48 (6.3) | 53 (7.0) | .78 | 112 (9.9) | 103 (9.1) | .42 |
| Low-intensity statin | 4 (0.5) | 3 (0.4) | 2 (0.2) | 2 (0.2) | ||
| Moderate-intensity statin | 243 (32.3) | 262 (34.7) | 438 (38.5) | 423 (37.4) | ||
| Moderate-intensity statin with ezetimibe | 101 (13.3) | 89 (11.8) | 150 (13.2) | 159 (14.0) | ||
| High-intensity statin | 324 (42.8) | 316 (41.9) | 387 (34.0) | 413 (36.5) | ||
| High-intensity statin with ezetimibe | 37 (4.9) | 31 (4.1) | 48 (4.2) | 32 (2.8) | ||
| Heart failure, No. (%) | 46 (6.1) | 45 (6.0) | <.99 | 25 (2.2) | 24 (2.1) | <.99 |
| Baseline serum LDL-C, median (IQR), mg/dL | 78 (63-98) | 77 (62-97) | .60 | 82 (65-102) | 82 (65-102) | .61 |
| No. of patients with LDL-C <70 mg/dL, No. (%) | 272 (35.9) | 278 (36.9) | .75 | 371 (32.6) | 338 (29.9) | .17 |
Abbreviation: LDL-C, low-density lipoprotein cholesterol.
SI conversion factor: To convert LDL-C level to millimoles per liter, multiply by 0.0259.
Calculated as weight in kilograms divided by height in meters squared.
Chronic kidney disease was defined as an estimated glomerular filtration rate of less than 60 mL per minute per 1.73 m2 of body surface area.
Figure 1. Primary End Point According to Assigned Treatment in Very High-Risk (VHR) and Non-VHR Patients With Atherosclerotic Cardiovascular Disease (ASCVD).
The cumulative incidences of the primary end point at 3 years after randomization (intention-to-treat population) comparing moderate-intensity statin with ezetimibe combination vs high-intensity statin monotherapy in VHR and non-VHR patients. The interaction P value shows no evidence of significant heterogeneity for the treatment outcomes of the primary endpoint among VHR and non-VHR. HR indicates hazard ratio.
Figure 2. Serial Changes of Low-Density Lipoprotein Cholesterol (LDL-C) Level According to Assigned Treatment in Very High-Risk (VHR) and Non-VHR Patients With Atherosclerotic Cardiovascular Disease.
Serial median values of LDL-C level among VHR patients (A) and non-VHR patients (B) with ASCVD. To convert LDL-C level to millimoles per liter, multiply by 0.0259.
Discussion
This post hoc analysis of the RACING randomized clinical trial investigated the outcome of ezetimibe combination with moderate-intensity statin vs high-intensity statin in VHR and non-VHR patients with ASCVD. Despite the guideline recommendation of high-intensity statin treatment in VHR,1,7 studies have reported substantial underuse of high-intensity statins in practice.5,8 In a cohort of 601 934 patients with ASCVD in the US, the prescription rate of a high-intensity statin was 22.5%, and strikingly, 49.9% of patients with prior ASCVD were not taking statin therapy.9 Further, in a Swedish national registry with 192 435 VHR patients who were initially treated with a moderate-intensity statin, uptitration to a high-intensity statin was observed in only 28%.10 Concerns about drug-associated adverse effects could be a plausible explanation for physicians’ reluctance to prescribe high-intensity statins.11 In this clinical dilemma, initial combination of ezetimibe, instead of uptitration of the statin until intolerance develops, could be a promising strategy. The RACING randomized clinical trial uniquely investigated the impact of early ezetimibe combination while simultaneously lowering statin intensity,6 in contrast to prior trials that investigated the additional benefit of nonstatin lipid-lowering therapy in conjunction with an equal dose of statin.12,13,14,15 By corroborating the results of the RACING trial, the current study results suggest that early ezetimibe combination could be a reasonable therapeutic approach for VHR patients with ASCVD.
Limitations
The present study has several limitations. First, this post hoc study was not sufficiently powered to draw definite conclusions on the heterogeneity of the treatment effect between VHR and non-VHR patients. Second, the current analysis was not prespecified because the definition of VHR was defined in the 2018 AHA/ACC dyslipidemia guidelines; conversely, the RACING trial randomly assigned participants from February 2017. Therefore, the results presented here should be considered as hypothesis generating. Lastly, the diagnosis of familial hypercholesterolemia was not collected during randomization in the RACING trial and thus was not accounted for in the definition of VHR patients with ASCVD in this study.
Conclusions
In this post hoc analysis of VHR patients with ASCVD from the RACING randomized clinical trial, results suggest that a moderate-intensity statin with ezetimibe combination therapy was comparable to high-intensity statin monotherapy in terms of 3-year primary end point and was associated with lower drug intolerance, greater LDL-C level reduction, and achievement of LDL-C level less than 70 mg/dL.
eTable 1. Inclusion and Exclusion Criteria
eTable 2. Number of VHR Patients Meeting Each of the Definition Criteria
eTable 3. Baseline Characteristics of VHR and Non-VHR Patients
eTable 4. Serial Changes of the Lipid Profile Among VHR and Non-VHR Patients
eTable 5. Safety End Points in VHR and non-VHR Patients (Safety Population)
eFigure 1. Clinical End Points in VHR and Non-VHR Patients
eFigure 2. Clinical End Points According to Statin Therapy in VHR and Non-VHR Patients
eFigure 3. Proportion of Patients With LDL-C <70 mg/dL Among VHR and Non-VHR ASCVD
eFigure 4. Kaplan-Meier Curves of Discontinuation or Dose Reduction of the Study Drugs by Intolerance in VHR Patients
Nonauthor Collaborators. RACING Investigators.
Data Sharing Statement
References
- 1.Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. J Am Coll Cardiol. 2019;73(24):e285-e350. doi: 10.1016/j.jacc.2018.11.003 [DOI] [PubMed] [Google Scholar]
- 2.Colantonio LD, Shannon ED, Orroth KK, et al. Ischemic event rates in very-high-risk adults. J Am Coll Cardiol. 2019;74(20):2496-2507. doi: 10.1016/j.jacc.2019.09.025 [DOI] [PubMed] [Google Scholar]
- 3.LaRosa JC, Grundy SM, Waters DD, et al. ; Treating to New Targets (TNT) Investigators . Intensive lipid lowering with atorvastatin in patients with stable coronary disease. N Engl J Med. 2005;352(14):1425-1435. doi: 10.1056/NEJMoa050461 [DOI] [PubMed] [Google Scholar]
- 4.Rodriguez F, Maron DJ, Knowles JW, Virani SS, Lin S, Heidenreich PA. Association between intensity of statin therapy and mortality in patients with atherosclerotic cardiovascular disease. JAMA Cardiol. 2017;2(1):47-54. doi: 10.1001/jamacardio.2016.4052 [DOI] [PubMed] [Google Scholar]
- 5.Nelson AJ, Haynes K, Shambhu S, et al. High-intensity statin use among patients with atherosclerosis in the US. J Am Coll Cardiol. 2022;79(18):1802-1813. doi: 10.1016/j.jacc.2022.02.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim BK, Hong SJ, Lee YJ, et al. ; RACING Investigators . Long-term efficacy and safety of moderate-intensity statin with ezetimibe combination therapy vs high-intensity statin monotherapy in patients with atherosclerotic cardiovascular disease (RACING): a randomised, open-label, noninferiority trial. Lancet. 2022;400(10349):380-390. doi: 10.1016/S0140-6736(22)00916-3 [DOI] [PubMed] [Google Scholar]
- 7.Mach F, Baigent C, Catapano AL, et al. ; ESC Scientific Document Group . 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020;41(1):111-188. doi: 10.1093/eurheartj/ehz455 [DOI] [PubMed] [Google Scholar]
- 8.Navar AM, Roe MT, White JA, et al. Medication discontinuation in the IMPROVE-IT trial. Circ Cardiovasc Qual Outcomes. 2019;12(1):e005041. doi: 10.1161/CIRCOUTCOMES.118.005041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schubert J, Lindahl B, Melhus H, et al. Low-density lipoprotein cholesterol reduction and statin intensity in myocardial infarction patients and major adverse outcomes: a Swedish nationwide cohort study. Eur Heart J. 2021;42(3):243-252. doi: 10.1093/eurheartj/ehaa1011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Banefelt J, Lindh M, Svensson MK, Eliasson B, Tai MH. Statin dose titration patterns and subsequent major cardiovascular events in very high-risk patients: estimates from Swedish population-based registry data. Eur Heart J Qual Care Clin Outcomes. 2020;6(4):323-331. doi: 10.1093/ehjqcco/qcaa023 [DOI] [PubMed] [Google Scholar]
- 11.Ray KK, Reeskamp LF, Laufs U, et al. Combination lipid-lowering therapy as first-line strategy in very high-risk patients. Eur Heart J. 2022;43(8):830-833. doi: 10.1093/eurheartj/ehab718 [DOI] [PubMed] [Google Scholar]
- 12.Cannon CP, Blazing MA, Giugliano RP, et al. ; IMPROVE-IT Investigators . Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372(25):2387-2397. doi: 10.1056/NEJMoa1410489 [DOI] [PubMed] [Google Scholar]
- 13.Sabatine MS, Giugliano RP, Keech AC, et al. ; FOURIER Steering Committee and Investigators . Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376(18):1713-1722. doi: 10.1056/NEJMoa1615664 [DOI] [PubMed] [Google Scholar]
- 14.Bhatt DL, Steg PG, Miller M, et al. ; REDUCE-IT Investigators . Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med. 2019;380(1):11-22. doi: 10.1056/NEJMoa1812792 [DOI] [PubMed] [Google Scholar]
- 15.Schwartz GG, Steg PG, Szarek M, et al. ; ODYSSEY OUTCOMES Committees and Investigators . Alirocumab and cardiovascular outcomes after acute coronary syndrome. N Engl J Med. 2018;379(22):2097-2107. doi: 10.1056/NEJMoa1801174 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Inclusion and Exclusion Criteria
eTable 2. Number of VHR Patients Meeting Each of the Definition Criteria
eTable 3. Baseline Characteristics of VHR and Non-VHR Patients
eTable 4. Serial Changes of the Lipid Profile Among VHR and Non-VHR Patients
eTable 5. Safety End Points in VHR and non-VHR Patients (Safety Population)
eFigure 1. Clinical End Points in VHR and Non-VHR Patients
eFigure 2. Clinical End Points According to Statin Therapy in VHR and Non-VHR Patients
eFigure 3. Proportion of Patients With LDL-C <70 mg/dL Among VHR and Non-VHR ASCVD
eFigure 4. Kaplan-Meier Curves of Discontinuation or Dose Reduction of the Study Drugs by Intolerance in VHR Patients
Nonauthor Collaborators. RACING Investigators.
Data Sharing Statement