Summary
Background
A delay in reaching HbA1c targets in patients with newly-diagnosed type 2 diabetes (T2D) is associated with an increased long-term risk of developing cardiovascular diseases (CVD), a phenomenon referred to as legacy effect. Whether an early introduction of glucose-lowering drugs with proven benefit on CVD can attenuate this phenomenon is unknown.
Methods
Using data derived from a large Italian clinical registry, i.e. the AMD Annals, we identified 251,339 subjects with newly-diagnosed T2D and without CVD at baseline. Through Cox regressions adjusted for multiple risk factors, we examined the association between having a mean HbA1c between 7.1 and 8% or >8%, compared with ≤7%, for various periods of early exposure (0–1, 0–2, 0–3 years) and the development of later (mean subsequent follow-up 4.6 ± 2.9 years) CVD, evaluated as a composite of myocardial infarction, stroke, coronary or peripheral revascularization, and coronary or peripheral bypass. We performed this analysis in the overall cohort and then splitting the population in two groups of patients: those that introduced sodium-glucose transport protein 2 inhibitors (SGLT-2i) during the exposure phase and those not treated with these drugs.
Findings
Considering the whole cohort, subjects with both a mean HbA1c between 7.1 and 8% and >8%, compared with patients attaining a mean HbA1c ≤ 7%, showed an increased risk of developing the outcome in all the three early exposure periods assessed, with the highest risk observed in patients with mean HbA1c > 8% in the 3 years exposure period (hazard ratio [HR]1.33; 95% confidence interval [CI] 1.063–1.365). The introduction of SGLT-2i during the exposure periods of 0–1 and 0–2 years eliminated the association between poor glycemic control and the outcome (p for interaction 0.006 and 0.003, respectively, vs. patients with the same degree of glycemic control but not treated with these drugs).
Interpretation
Among patients with newly diagnosed T2D and free of CVD at baseline, a poor glycemic control in the first three years after diagnosis is associated with an increased subsequent risk of CVD. This association is no longer evident when SGLT-2i are introduced in the first two years, suggesting that these drugs attenuate the phenomenon of legacy effect. An early treatment with these drugs might thus promote a long-lasting benefit in patients not attaining proper glycemic control after T2D diagnosis.
Funding
This work was supported, in part, by the Italian Ministry of Health (Ricerca Corrente) to IRCCS MultiMedica.
Keywords: AMD Annals initiative, Type 2 diabetes, Metabolic memory, Legacy effect, Cardiovascular diseases, Sodium-glucose cotransporter 2 inhibitors
Research in context.
Evidence before this study
Data from historic clinical trials and the subsequent observational follow-ups such as the UKPDS and the DCCT/EDIC, as well as large observational studies such as the Diabetes & Aging study, suggest that a poor glycemic control in patients with early-stage diabetes increases the long-term risk of macrovascular complications, a phenomenon known as the legacy effect. However, such studies were conducted when glucose-lowering drugs with proven cardiovascular benefit, e.g., the SGLT-2i, were not available.
Added value of this study
With this study we substantiate the evidence that poor glycemic control after the diagnosis of type 2 diabetes is associated with an increased cardiovascular risk during the subsequent follow-up. However, we show also that the introduction of an SGLT-2i during the first two years after diabetes diagnosis eliminated the association between poor glycemic control and the later development of cardiovascular events, measured as a composite of myocardial infarction, stroke, coronary or peripheral revascularization, and coronary or peripheral bypass.
Implications of all the available evidence
While the results of this study need confirmation in independent and prospective cohorts, they might suggest that SGLT-2i could attenuate the deleterious long-term damage promoted by poor glycemic control in the first years after diabetes diagnosis. Thus, while reaching the HbA1c target as soon as possible remains the main therapeutic goal in early diabetes management, the introduction of SGLT-2i might be considered an option for those patients unable to attain rapidly the recommended HbA1c target.
Introduction
Patients with type 2 diabetes (T2D) have an increased risk of cardiovascular diseases (CVD) and poor glycemic control is a key risk factor in this population.1,2 An early and intensive glycemic control has been associated with a long-term benefit on the development of CVD, a phenomenon referred to as legacy effect.3 Indeed, findings from the follow-up of the UKPDS trial suggested that patients with a recent T2D diagnosis benefit from an intensive glycemic control even after the intensive therapy is discontinued.4 Albeit selected, subsequent studies enrolling patients with more advanced stages of T2D did not confirm these results.5, 6, 7 On the contrary, two large observational studies and additional, recent, follow-up data of the same UKPDS cohort provided consistent evidence that newly diagnosed T2D patients with various degrees of poor glycemic control in the years following diagnosis have an increased risk of late CVD and death, supporting the existence of a long-lasting damage promoted by hyperglycemia.8, 9, 10 However, all the studies exploring the phenomenon of legacy effect were conducted when glucose-lowering drugs with proven cardiovascular benefit, e.g., sodium-glucose transport protein 2 inhibitors (SGLT-2i), were not available. Thus, whether an early introduction of these drugs blunt the deleterious consequence of poor glycemic control after T2D diagnosis is unknown.
SGLT-2i have a demonstrated ability to lower the incidence of cardiovascular and other outcomes. In particular, multiple trials have demonstrated that SGLT-2i reduce the incidence of cardiovascular mortality, heart failure, and kidney-related events including the development of albuminuria in patients with T2D,11, 12, 13, 14 while large cohort studies suggest also a benefit on atherosclerotic endpoints in this population.15 Several mediation analyses indicate that the effect of these drugs on multiple, canonical risk factors, including attained HbA1c levels, unlikely explain the observed effect on hard outcomes,16,17 thus suggesting that the benefit might derive from peculiar mechanisms attributable to this class. Among other frameworks, it was hypothesized that these drugs are able to antagonize the major pathological imbalances of T2D, thus potentially changing the trajectory of the disease.18, 19, 20 As a corollary of this postulate, an early introduction of SGLT-2i should thus promote a long-term beneficial effect on the vasculature independently of the attained HbA1c targets.
To explore this hypothesis, we took advantage of a large Italian clinical registry of people with T2D, the AMD Annals Initiative, to extrapolate data from newly-diagnosed patients and free of CVD at baseline, in order to examine the association between attained HbA1c targets in the years following T2D diagnosis and later CVD in the whole cohort and then in two populations of patients: those introducing early an SGLT-2i and patients not treated with these drugs during the same period.
Methods
Study design and population
Data derived from the registry of the Italian Association of Clinical Diabetologists [Associazione Medici Diabetologi (AMD)] Annals initiative, which was established in 2004 to monitor quality of diabetes care in Italy.21 The database includes information on all patients with T2DM receiving care at 230 diabetes clinics in Italy from January 1st 2004 to December 31st 2021. All diabetes clinics adhering to AMD Annals initiative, a third of those existing throughout the country, used a common electronic clinical record system for the everyday management of outpatients and a software was specifically developed to extract information from these clinical databases. Anonymized data from all participating clinics were collected and centrally analyzed. Available data included demographic, clinical, and biochemical information, including values of glycated haemoglobin (HbA1c), blood pressure, total-cholesterol, low-density lipoprotein cholesterol (LDL-C) or high-density lipoprotein cholesterol (HDL-C), and triglycerides. The use of specific classes of drugs (glucose lowering, lipid lowering and antihypertensive agents), based on ATC codes, was available. Information on the presence of diabetic complications was based on ICD-9 CM codes.
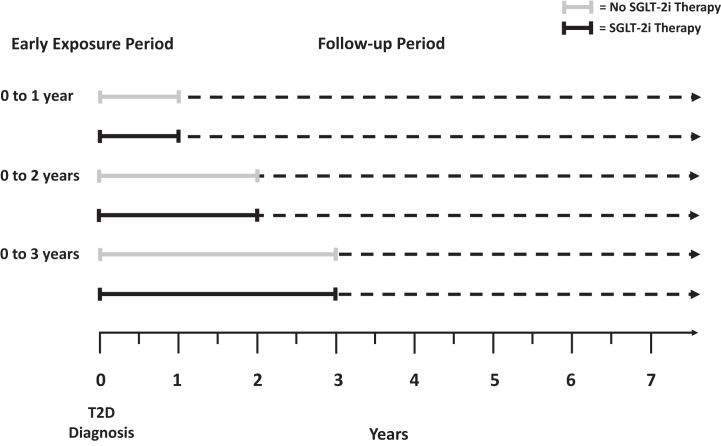
The experimental design is summarized in Fig. 1. To explore the effects of various periods of early glycemic exposure, we defined three definition of early exposure periods (0–1, 0–2 and 0–3 years). The mean HbA1c value was calculated for each early exposure period by using all HbA1c results except the value at diagnosis. The value at diagnosis was excluded because it reflects control before treatment was initiated, and the glycemic legacy effect has been demonstrated only in populations receiving diabetes treatment. To assess also the effect of various degrees of glycemic control, the mean HbA1c value for each of the three early exposure periods was categorized into either HbA1c ≤ 7.0% (≤53 mmol/mol), >7.0%–≤8.0% (>53–≤64 mmol/mol), >8.0% (>64 mmol/mol). The exposure period starts at the diagnosis date and end after first, second or third year from diagnosis. The follow-up period starts after first, second or third year from diagnosis (baseline/t0) and was ended after the first occurrence of the outcome of interest or censored at last visit. The outcome of interest was the composite of myocardial infarction, stroke, coronary or peripheral revascularization, and coronary or peripheral bypass. Patients with prevalent CVD at baseline were excluded. The risk factors used to adjust the analysis derived from the last observed values for each of the three early exposure periods (baseline/t0). When a variable was not available at baseline, it was carried backward in the previous years. In case of missing data relative to covariates, a category of missing data was added for each covariate in the multivariate analysis. This design was adopted for both the analysis of the whole cohort and for the analyses stratified according to the use of SGLT-2i during the exposure phase vs. non-user (Fig. 1). Patients could have introduced the drug at any moment during the exposure phase considered, thus the subgroup using SGLT-2i in the 0–3 years exposure period include also those subjects introducing the drugs in the 0–1 year and 0–2 years’ exposure periods, while those of the 0–2 years exposure phase include also patients introducing the drugs in the first year after diagnosis. This design was selected to maximize the chances of observing an effect of the drug against the legacy effect.
Fig. 1.
Schematic representation of the experimental design.
Statistical analysis
We summarized data for patient characteristics using means and standard deviations (SDs) for continuous variables and counts and percentages for categorical variables stratified by the three classes of HbA1c mean in early exposure period. The characteristics were compared by the t-test and χ2 test respectively for continuous and categorical variables.
The Cox proportional hazards models were used to examine associations between glycemic control and the risk of CVD. The Cox model were adjusted for potentially confounding variables: sex (male vs. female), age (by 5 years), total-cholesterol (by 10 mmol/l), HDL-cholesterol (by 10 mmol/l) and LDL-cholesterol (by 10 mmol/l), triglycerides (by 10 mmol/l), BMI, systolic blood pressure (by 5 mmHg), smoking status (Yes vs. No), eGFR (ml/min/1.73m2 <60 vs. ≥60), microalbuminuria (Yes vs. No), the use of different classes of glucose-lowering drugs (Yes vs. No for each one), statin (Yes vs. No), antihypertensive medication (Yes vs. No), HbA1c, and the number of HbA1c measurements during the exposure period. A backward selection was introduced in the Cox models in order to exclude the confounders without a significant association with the outcome. The descriptive and multivariate analysis were performed three cohorts. Then, the population was stratified for SGLT2 use during the exposure phase or no-use and the Cox models were run separately for these populations. p-values for treatment by subgroup interaction were obtained from tests of homogeneity of treatment group differences among subgroups. For each model, patient follow-up was censored after the first occurrence of the outcome of interest or last visit. A two-sided p < 0.05 was considered statistically significant for all analyses. Analyses were performed using SAS 9.4 statistical software (SAS Institute, Cary, NC).
Role of the funding source
The funders had no role in study design, data collection, data analysis, interpretation or writing of the report.
Results
Early poor glycemic control is associated with later risk of cardiovascular diseases
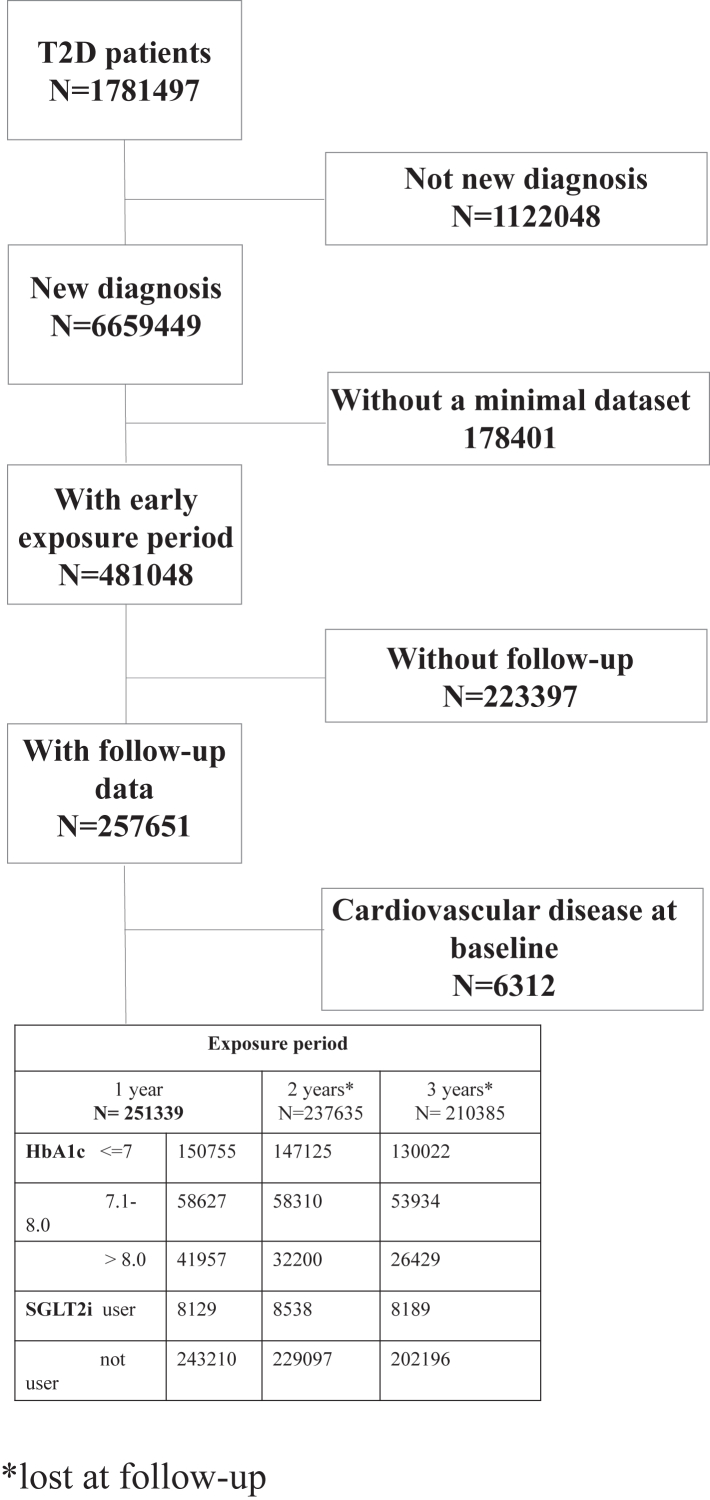
From the AMD Annals database, 251,339 patients with newly diagnosed T2D and free of CVD at baseline were identified and included in the study (Fig. 2). Clinical characteristics of the groups categorized according to the degree of glycemic control attained in the three different exposure periods are summarized in Supplementary Table S1A–C. The groups showed significant differences for almost all the cardiovascular risk factor assessed. Thus, all these variables were added as covariates to adjust the subsequent analyses.
Fig. 2.
Flow-diagram showing included and excluded patients.
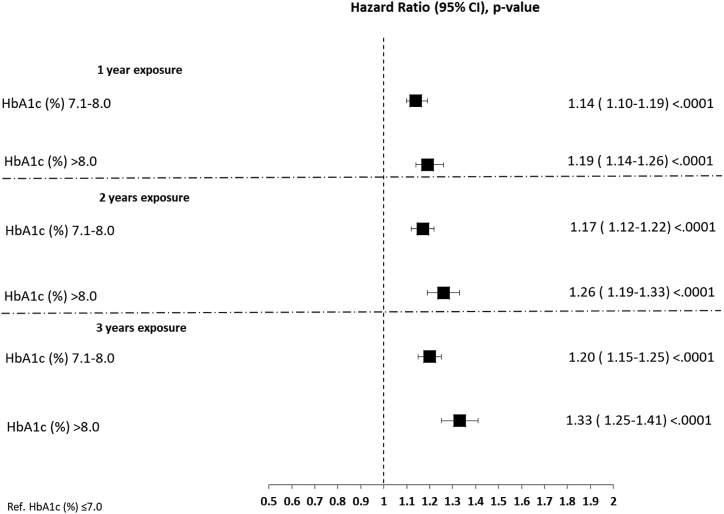
Cox regression analysis, adjusted for sex, age, total-cholesterol, HDL-cholesterol, LDL-cholesterol, triglycerides, BMI, systolic blood pressure, smoking status, eGFR, microalbuminuria, the use of different classes of glucose-lowering drugs, statin, antihypertensive medication, HbA1c, and the number of HbA1c measurements, showed that, compared with patients with a mean HbA1c ≤ 7%, those above this range had in increased risk of CVD at follow-up for all the three early exposure periods considered and for both strata of poor glycemic control considered (Fig. 3). In detail, compared with HbA1c ≤ 7%, patients with mean HbA1c > 7 and <8% had a hazard ratio [HR] of 1.14, 95% confidence interval [CI] 1.10–1.19 for the 0–1-year exposure period, a HR of 1.17; 95%, CI 1.12–1.22 for the 0–2 years exposure period, and a HR 1.20, 95% CI 1.15–1.25 for the 0–3 years exposure period (p < 0.0001 for all). Similar data were obtained for patients with mean HbA1c > 8% (HR 1.19; 95%, CI 1.14–1.26 for the 0–1-year exposure period, HR 1.26; 95%, CI 1.19–1.33 for the 0–2 years exposure period, and HR 1.33, 95% CI 1.25–1.41 for the 0–3 years exposure period, p < 0.0001 for all) (Fig. 3). There was a significant trend toward an increasing risk of CVD with progressively higher levels of mean HbA1c in each of the exposure periods assessed (p for trend <0.0001 for all).
Fig. 3.
Poor, early glycemic control and the subsequent risk of cardiovascular diseases. Pseudo-forest plot showing the adjusted hazard ratios (HR) with the relative 95% confidence interval (CI) and the p value, derived from the Cox regression analyses exploring the associations between glycemic control and the risk of the CVD at follow-up in the whole cohort according to the degree of glycemic control in the three exposure periods assessed. HbA1c ≤ 7% is the reference.
Introduction of SGLT-2i in the first two years after diagnosis ameliorates the legacy effect
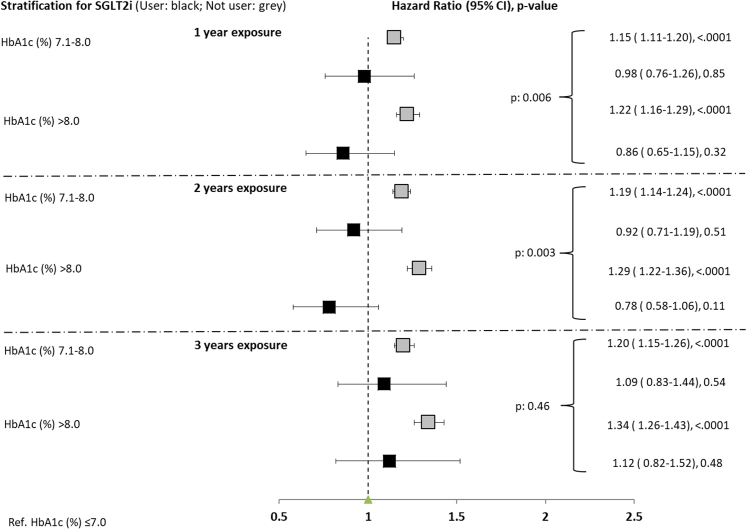
We then split the cohort in two populations: those introducing SGLT-2i during any time of the exposure phase considered and non-users. Clinical characteristics of the groups in the three exposure periods are presented in Table 1. All the significantly different risk factors were used to adjust the Cox models. As shown in Fig. 4, the introduction of SGLT-2i in the 0–1 year and 0–2 years exposure phases blunted the association between poor glycemic control and later CVD (HR 0.98; 95%, CI 0.76–1.26 in users vs. HR 1.15; 95%, CI 1.11–1.20 in non-users for the 7% < HbA1c mean ≤8% strata and HR 0.86; 95%, CI 0.65–1.15 in users vs. HR 1.22; 95%, CI 1.16–1.29 in non-users for the mean HbA1c > 8% strata in the 0–1-year exposure period, p for interaction 0.006; HR 0.92; 95%, CI 0.71–1.19 in users vs. HR 1.19; 95%, CI 1.14–1.24 in non-users for the 7% < HbA1c mean ≤8% strata and HR 0.78; 95%, CI 0.58–1.06 in users vs. HR 1.29; 95%, CI 1.22–1.36 in non-users for the mean HbA1c > 8% strata in the 0–2 years exposure period, p for interaction 0.003). This phenomenon was not observed when SGLT-2i were introduced in the 0–3 years exposure phase (p for interaction = 0.46).
Table 1.
Characteristics of the study population by SGLT2i use in three exposure periods.
| Variable | 1 year exposure |
2 years exposure |
3 years exposure |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Not user | User | p-value | Not user | User | p-value | Not user | User | p-value | |
| No. of patients | 243,210 | 8129 | 229,097 | 8538 | 202,196 | 8189 | |||
| Age at baseline (year) | 63.8 ± 12.5 | 58.9 ± 10.2 | <0.0001 | 64.5 ± 12.3 | 58.9 ± 10.1 | <0.0001 | 65.1 ± 12.2 | 58.9 ± 10.1 | <0.0001 |
| Gender (% males) | 137,996 (56.7) | 5170 (63.6) | <0.0001 | 129,167 (56.4) | 5335 (62.5) | <0.0001 | 113,652 (56.2) | 5061 (61.8) | <0.0001 |
| Microalbuminuria | 47,728 (31.3) | 2117 (35.1) | <0.0001 | 58,231 (35.4) | 2867 (40.4) | <0.0001 | 61,181 (39.2) | 3190 (45.0) | <0.0001 |
| Antihypertensive medication | 115,342 (47.4) | 4500 (55.4) | <0.0001 | 118,426 (51.7) | 5020 (58.8) | <0.0001 | 110,785 (54.8) | 4917 (60.0) | <0.0001 |
| BMI | 29.8 ± 5.6 | 31.4 ± 6.0 | <0.0001 | 29.8 ± 5.6 | 31.8 ± 6.1 | <0.0001 | 29.9 ± 5.5 | 32.2 ± 6.2 | <0.0001 |
| Total cholesterol (mmol/l) | 187.7 ± 40.7 | 181.5 ± 42.4 | <0.0001 | 184.7 ± 39.4 | 179.5 ± 40.9 | <0.0001 | 182.6 ± 38.9 | 177.9 ± 40.2 | <0.0001 |
| eGFR (ml/min/1.73m2) <60 | 37,587 (18.1) | 757 (9.8) | <0.0001 | 41,170 (20.2) | 932 (11.2) | <0.0001 | 40,672 (22.1) | 990 (12.3) | <0.0001 |
| Follow-up (years) | 4.7 ± 2.9 | 2.1 ± 1.3 | <0.0001 | 4.6 ± 2.7 | 2.0 ± 1.2 | <0.0001 | 4.4 ± 2.4 | 2.0 ± 1.2 | <0.0001 |
| HbA1c at baseline (%) | 6.7 ± 1.1 | 7.0 ± 1.3 | <0.0001 | 6.8 ± 1.1 | 7.1 ± 1.2 | <0.0001 | 6.8 ± 1.1 | 7.3 ± 1.2 | <0.0001 |
| HbA1c at diagnosis (%) 3.0–6.9 | 45,985 (18.9) | 552 (6.8) | <0.0001 | 46,679 (20.4) | 632 (7.4) | <0.0001 | 42,287 (20.9) | 697 (8.5) | <0.0001 |
| HbA1c at diagnosis (%) 7.0–8.0 | 34,370 (14.1) | 894 (11.0) | 32,418 (14.2) | 976 (11.4) | 28,219 (14.0) | 937 (11.4) | |||
| HbA1c at diagnosis (%) 8.1–9.0 | 15,349 (6.3) | 701 (8.6) | 13,798 (6.0) | 696 (8.2) | 11,905 (5.9) | 651 (7.9) | |||
| HbA1c at diagnosis (%) >9.0 | 38,624 (15.9) | 2785 (34.3) | 33,398 (14.6) | 2706 (31.7) | 28,032 (13.9) | 2408 (29.4) | |||
| HbA1c at diagnosis (%) NA | 8.3 ± 2.3 | 9.7 ± 2.3 | <0.0001 | 8.2 ± 2.2 | 9.6 ± 2.4 | <0.0001 | 8.1 ± 2.2 | 9.5 ± 2.4 | <0.0001 |
| HbA1c at diagnosis (%) | 49.1 ± 13.1 | 47.7 ± 12.3 | <0.0001 | 49.5 ± 13.3 | 47.6 ± 12.5 | <0.0001 | 49.6 ± 13.4 | 47.2 ± 12.3 | <0.0001 |
| HDL cholesterol (mmol/l) | 110.9 ± 35.0 | 104.6 ± 35.9 | <0.0001 | 107.6 ± 34.0 | 101.9 ± 34.8 | <0.0001 | 105.5 ± 33.6 | 100.1 ± 33.8 | <0.0001 |
| LDL cholesterol (mmol/l) | 147,507 (60.7) | 3248 (40.0) | <0.0001 | 143,846 (62.8) | 3279 (38.4) | <0.0001 | 127,113 (62.9) | 2909 (35.5) | <0.0001 |
| HbA1c mean in exposure period ≤7.0 | 56,211 (23.1) | 2416 (29.7) | 55,277 (24.1) | 3033 (35.5) | 50,759 (25.1) | 3175 (38.8) | |||
| HbA1c mean in exposure period 7.1–8.0 | 39,492 (16.2) | 2465 (30.3) | 29,974 (13.1) | 2226 (26.1) | 24,324 (12.0) | 2105 (25.7) | |||
| HbA1c mean in exposure period >8.0 | 78.8 ± 9.9 | 79.8 ± 10.2 | <0.0001 | 78.8 ± 9.9 | 79.8 ± 9.8 | <0.0001 | 78.7 ± 9.7 | 79.8 ± 9.9 | <0.0001 |
| Diastolic blood pressure (mmHg) | 133.9 ± 18.1 | 133.5 ± 18.2 | 0.005 | 134.2 ± 17.9 | 133.1 ± 17.3 | <0.0001 | 134.4 ± 17.8 | 133.6 ± 17.6 | <0.0001 |
| Systolic blood pressure (mmHg) | 18,874 (7.8) | 376 (4.6) | <0.0001 | 22,818 (10.0) | 740 (8.7) | <0.0001 | 23,928 (11.8) | 1098 (13.4) | <0.0001 |
| Smoking | 30,907 (20.1) | 1367 (24.8) | <0.0001 | 29,680 (19.8) | 1532 (25.3) | <0.0001 | 26,554 (19.6) | 1481 (24.8) | <0.0001 |
| Statins | 82,775 (34.0) | 3893 (47.9) | <0.0001 | 92,140 (40.2) | 4683 (54.8) | <0.0001 | 90,106 (44.6) | 4797 (58.6) | <0.0001 |
| Composite CV Outcome | 13,485 (5.5) | 337 (4.1) | <0.0001 | 12,596 (5.5) | 302 (3.5) | <0.0001 | 10,864 (5.4) | 282 (3.4) | <0.0001 |
| Exposure time (year) | 0.7 ± 0.3 | 0.8 ± 0.2 | <0.0001 | 1.6 ± 0.5 | 1.7 ± 0.3 | <0.0001 | 2.4 ± 0.7 | 2.7 ± 0.4 | <0.0001 |
| Triglycerides (mmol/l) | 143.4 ± 89.0 | 154.7 ± 102.5 | <0.0001 | 142.4 ± 86.9 | 159.3 ± 104.1 | <0.0001 | 142.3 ± 85.8 | 163.6 ± 106.3 | <0.0001 |
| DPP4i | 7843 (3.2) | 65 (0.8) | <0.0001 | 8878 (3.9) | 109 (1.3) | <0.0001 | 9093 (4.5) | 155 (1.9) | <0.0001 |
| Glinides | 5008 (2.1) | 140 (1.7) | 0.0349 | 6121 (2.7) | 238 (2.8) | 0.5152 | 6639 (3.3) | 358 (4.4) | <0.0001 |
| GLP1-RAs | 5113 (2.1) | 328 (4.0) | <0.0001 | 6023 (2.6) | 669 (7.8) | <0.0001 | 6337 (3.1) | 980 (12.0) | <0.0001 |
| Acarbose | 3643 (1.5) | 58 (0.7) | <0.0001 | 4404 (1.9) | 94 (1.1) | <0.0001 | 4661 (2.3) | 154 (1.9) | 0.0118 |
| Insulin | 42,306 (17.4) | 2641 (32.5) | <0.0001 | 39,855 (17.4) | 2966 (34.7) | <0.0001 | 36,113 (17.9) | 3005 (36.7) | <0.0001 |
| Metformin | 140,715 (57.9) | 7409 (91.1) | <0.0001 | 140,670 (61.4) | 7934 (92.9) | <0.0001 | 129,717 (64.2) | 7701 (94.0) | <0.0001 |
| Sulphonylureas | 23,452 (9.6) | 497 (6.1) | <0.0001 | 26,166 (11.4) | 861 (10.1) | 0.0001 | 26,560 (13.1) | 1130 (13.8) | 0.0818 |
Fig. 4.
Early introduction of SGLT-2i attenuate metabolic memory. Pseudo-forest plot showing the adjusted hazard ratios (HR) with the relative 95% confidence interval (CI) and the p value, derived from the Cox regression analyses exploring the associations between glycemic control and the risk of the CVD at follow-up in patients stratified according to use of SGLT-2i during the exposure phase or not users, in the three exposure periods assessed. HbA1c ≤ 7% is the reference.
To corroborate the latter findings, we extended the exposure phase adding a 0–4 years exposure period. Patients’ characteristics are shown in Supplementary Table S1D. Even in this case, we did not observe a significant interaction between patients introducing or not introducing SGLT-2i during the exposure phase (Supplementary Table S2, p for interaction = 0.14), possibly suggesting that the time window to attenuate the legacy effect might be limited.
Finally, we checked the persistence rate of SGLT-2i prescription during the follow-up. Data are shown in Supplementary Table S3 and suggest that discontinuation rate ranged from 12.6% to 13.7% in the four exposure periods considered.
Discussion
The burden of evidence showing the ability of SGLT-2i to halt the progression of CVD and renal failure in T2D, coupled by mechanistic data highlighting their effects on major pathophysiological abnormalities of T2D, suggest them as potential disease-modifying drugs.18, 19, 20 If true, the benefit of an early introduction of these drugs should be long-lasting, independently of the degree of glycemic control. Legacy effect is a well-recognized phenomenon clearly emerged in cohort studies and in selected clinical trials, which envisages that poor glycemic control after T2D diagnosis promote an enduring damage on the vasculature.3 Here we show for the first time that an early introduction of SGLT-2i is able to eliminate the association between poor glycemic control in the first two years after T2D diagnosis and the later development of CVD, independently of the glycemic control attained. If confirmed, these data might sustain the argument that these drugs actually work as disease-modifying drugs, an observation with obvious clinical implications.18,22,23
The results relative to the overall cohort are similar to those observed previously.8, 9, 10 For instance, in the Diabetes and Aging study, patients with HbA1c levels ≥6.5% for the 0-to-1-year early exposure period had a higher risk for late macrovascular events, with this risk being progressively higher for longer periods of exposure to very poor glycemic control, i.e. HbA1c levels ≥9.0%.9 Our results are also compatible with a framework where the damage promoted by poor glycemic control after T2D diagnosis has a progressive nature in terms of both years of exposure and HbA1c levels, albeit further studies are required to clarify these issues. On the other hand, the introduction of SGLT-2i in the first two years after diagnosis, but not in the 0–3 years and 0–4 years periods, blunted this association, possibly suggesting that the time window to change the pathological trajectory induced by poor glycemic control might be limited. While these results need to be substantiated and expanded, our observation might sustain the argument that the beneficial effects of SGLT-2i on CVD might be mediated by early direct effects of these drugs on T2D pathogenetic mechanisms, with long-term consequences on the vasculature.
SGLT-2i are the only glucose lowering drugs not needing the action of insulin to induce glucose clearance, promoting also a net elimination of calories and fluids. This action fosters a metabolic shift at the systemic level, lowering insulinemia and increasing the glucagon/insulin ratio, finally promoting ketones and fatty acids utilization as alternative substrates.24,25 This metabolic, hormonal, and hemodynamic reshaping is accompanied by the modulation of a number of major pathways and phenomena mainly underlying the typical pathological imbalances of T2D or the most relevant risk factors for CVD, such as hypertension, obesity, liver dysfunction, kidney disease, beta-cell dysfunction, low-grade inflammation, endothelial dysfunction, and insulin resistance in multiple tissues.19,20,26, 27, 28 In addition, SGLT-2i have molecular data supporting their ability to counteract the activation of a large range of detrimental mechanisms held to underlie the legacy effect, e.g., long-lasting oxidative stress, non-enzymatic glycation of proteins, epigenetic modifications, senescent cells accumulation, and the enduring activation of inflammatory pathways.20,29, 30, 31, 32, 33, 34, 35, 36 Whether these phenomena explain the data presented here and whether they underly the observation that the effect of SGLT-2i against the legacy effect might be confined to the first two years after diagnosis warrants further investigation. Of note, these same mechanisms have been proposed to mediate part of the deleterious effects of hyperglycemia on the development of microvascular complications, which also suffer the legacy effect.3, 4, 5, 6, 7, 8, 9, 10 Future work is warranted to explore whether the introduction of SGLT-2i also ameliorate the legacy effect of poor glycemic control on the development of microvascular diseases, i.e. kidney failure, retinopathy, and neuropathy.
Despite our effort to adjust for all known risk factors, residual unmeasured confounders are inherently linked to all registry-based studies. For instance, the outcome is represented by a composite of hard outcomes with no details on the severity of each event. In addition, patients treated with SGLT-2i were younger, had a better mean renal function, and were more often on metformin and less often on sulphonylureas as background therapy, all factors that might have influenced the observed results. Also, differences in baseline disease severity are likely intertwined with the inability of reaching HbA1c targets early in the course of the disease. Another limitation might be represented by the impossibility to comment whether the results are effectively causal and the lack of a possible mechanism for such evidence. In addition, we did not perform subgroup analyses to explore eventual heterogeneity of the effect among men and women or according to age strata.37 Finally, risk factors levels could have changed during the follow-up phase, thus impacting the results. Thus, the results presented here should be considered as hypothesis generating and further work needs to be done to achieve more definitive answers. A prospective study with fully matched groups would help in obtaining more consistent observations. Furthermore, given the study design, we did not explore the effect of SGLT-2i in people with prevalent CVD nor in those with good glycemic control (i.e. with HbA1c < 7%). Of note, while the most definitive evidence for cardioprotection with SGLT-2i comes from people with established CVD,11, 12, 13 more work should be done to explore whether prescribing an SGLT-2i is beneficial in subjects with newly-diagnosed diabetes, good glycemic control, and free of CVD.
In summary, among patients with newly-diagnosed T2D and without CVD at baseline, we evidenced that mean HbA1c levels >7% or >8% during the 0–1, 0–2, or 0–3 years after diagnosis are associated with a greater risk of subsequent CVD compared with an HbA1c ≤ 7%. These associations are no longer visible when patients are treated with SGLT-2i in the 0–1- and 0–2-years’ time ranges. These results suggest that the legacy effect phenomenon is still observable in contemporary cohorts and that an early introduction of SGLT-2i might be able to ameliorate or even suppress the noxious long-term consequence of early, poor glycemic control on the vasculature. If confirmed in prospective studies with fully-matched populations, these findings might suggest that SGLT-2i act as disease-modifying drugs, thus advocating a wider and earlier usage for them.
Contributors
AC and FP: conceived the idea and wrote the manuscript; GL and AN: contributed to study design, made the statistical analysis, wrote, and discussed the manuscript; RLG and CF: contributed to data analysis and reviewed the manuscript; SDC, PDB, GDC, PF, CG, RP, GR, FV: collected data, verified the underlying data, and reviewed the manuscript for intellectual content. All authors approve the final version of the manuscript.
Data sharing statement
The datasets generated during or analyzed during the current study are available from the corresponding author on reasonable request.
Declaration of interests
AC is on the advisory board and does consultancy and lectures for AstraZeneca, BERLIN-CHEMIE, Eli Lilly, Novo Nordisk, Mitsubishi, Roche Diagnostics, and Theras Lifetech. FP is a lecturer for BERLIN-CHEMIE. AN has received honoraria from AstraZeneca, Eli Lilly, Novo Nordisk, and research support from Alfasigma, Novo Nordisk, Sanofi, Shionogi, SOBI. GR is on the advisory board and does consultancy and lectures for Novo Nordisk, Astra Zeneca, Sanofi, Boehringer, Lilly, Mundipharma, and Sanchio. SDC received honoraria for lectures from Eli Lilly, Boehringer, Astra-Zeneca, MundiPharma, MSD, Sanofi, Novo-Nordisk, Daiichi Sankyo, and Bayer. RP received honoraria for lectures from Lilly, Boehringer, AstraZeneca, Novartis, Menarini, MSD, Sanofi, Novo-Nordisk, Vifor, Alfa-Sigma, and Bayer. P.F. reports receiving personal fees from Astra Zeneca, Lilly, Boehringer Ingelheim, Bayer, and Novo Nordisk. The remaining authors declare no conflict of interests.
Acknowledgements
Funding: This work was supported, in part, by the Italian Ministry of Health (Ricerca Corrente) to IRCCS MultiMedica.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.lanepe.2023.100666.
Contributor Information
Antonio Ceriello, Email: antonio.ceriello@multimedica.it.
Francesco Prattichizzo, Email: francesco.prattichizzo@multimedica.it.
Appendix A. Supplementary data
References
- 1.Wright A.K., Suarez-Ortegon M.F., Read S.H., et al. Risk factor control and cardiovascular event risk in people with type 2 diabetes in primary and secondary prevention settings. Circulation. 2020;142(20):1925–1936. doi: 10.1161/CIRCULATIONAHA.120.046783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rawshani A., Rawshani A., Franzen S., et al. Risk factors, mortality, and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2018;379(7):633–644. doi: 10.1056/NEJMoa1800256. [DOI] [PubMed] [Google Scholar]
- 3.Chalmers J., Cooper M.E. UKPDS and the legacy effect. N Engl J Med. 2008;359(15):1618–1620. doi: 10.1056/NEJMe0807625. [DOI] [PubMed] [Google Scholar]
- 4.Holman R.R., Paul S.K., Bethel M.A., Matthews D.R., Neil H.A. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359(15):1577–1589. doi: 10.1056/NEJMoa0806470. [DOI] [PubMed] [Google Scholar]
- 5.Group A.S. Nine-year effects of 3.7 Years of intensive glycemic control on cardiovascular outcomes. Diabetes Care. 2016;39(5):701–708. doi: 10.2337/dc15-2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zoungas S., Chalmers J., Neal B., et al. Follow-up of blood-pressure lowering and glucose control in type 2 diabetes. N Engl J Med. 2014;371(15):1392–1406. doi: 10.1056/NEJMoa1407963. [DOI] [PubMed] [Google Scholar]
- 7.Prattichizzo F., de Candia P., De Nigris V., Nicolucci A., Ceriello A. Legacy effect of intensive glucose control on major adverse cardiovascular outcome: systematic review and meta-analyses of trials according to different scenarios. Metabolism. 2020;110 doi: 10.1016/j.metabol.2020.154308. [DOI] [PubMed] [Google Scholar]
- 8.Paul S.K., Klein K., Thorsted B.L., Wolden M.L., Khunti K. Delay in treatment intensification increases the risks of cardiovascular events in patients with type 2 diabetes. Cardiovasc Diabetol. 2015;14:100. doi: 10.1186/s12933-015-0260-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Laiteerapong N., Ham S.A., Gao Y., et al. The legacy effect in type 2 diabetes: impact of early glycemic control on future complications (the diabetes & aging study) Diabetes Care. 2019;42(3):416–426. doi: 10.2337/dc17-1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lind M., Imberg H., Coleman R.L., Nerman O., Holman R.R. Historical HbA(1c) values may explain the type 2 diabetes legacy effect: UKPDS 88. Diabetes Care. 2021;44(10):2231–2237. doi: 10.2337/dc20-2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McGuire D.K., Shih W.J., Cosentino F., et al. Association of SGLT2 inhibitors with cardiovascular and kidney outcomes in patients with type 2 diabetes: a meta-analysis. JAMA Cardiol. 2021;6(2):148–158. doi: 10.1001/jamacardio.2020.4511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zelniker T.A., Wiviott S.D., Raz I., et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet. 2019;393(10166):31–39. doi: 10.1016/S0140-6736(18)32590-X. [DOI] [PubMed] [Google Scholar]
- 13.Staplin N., Roddick A.J., Emberson J., et al. Net effects of sodium-glucose co-transporter-2 inhibition in different patient groups: a meta-analysis of large placebo-controlled randomized trials. eClinicalMedicine. 2021;41 doi: 10.1016/j.eclinm.2021.101163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lazzaroni E., Lunati M.E., Montefusco L., et al. Dapagliflozin acutely improves kidney function in type 2 diabetes mellitus. The PRECARE study. Pharmacol Res. 2022 Sep;183 doi: 10.1016/j.phrs.2022.106374. [DOI] [PubMed] [Google Scholar]
- 15.Li C.X., Liang S., Gao L., Liu H. Cardiovascular outcomes associated with SGLT-2 inhibitors versus other glucose-lowering drugs in patients with type 2 diabetes: a real-world systematic review and meta-analysis. PLoS One. 2021;16(2) doi: 10.1371/journal.pone.0244689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Inzucchi S.E., Zinman B., Fitchett D., et al. How does empagliflozin reduce cardiovascular mortality? Insights from a mediation analysis of the EMPA-REG OUTCOME trial. Diabetes Care. 2018;41(2):356–363. doi: 10.2337/dc17-1096. [DOI] [PubMed] [Google Scholar]
- 17.Tye S.C., de Vries S.T., Wanner C., Denig P., Heerspink H.J.L. Prediction of the effects of empagliflozin on cardiovascular and kidney outcomes based on short-term changes in multiple risk markers. Front Pharmacol. 2021;12 doi: 10.3389/fphar.2021.786706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mosenzon O., Del Prato S., Schechter M., et al. From glucose lowering agents to disease/diabetes modifying drugs: a "SIMPLE" approach for the treatment of type 2 diabetes. Cardiovasc Diabetol. 2021;20(1):92. doi: 10.1186/s12933-021-01281-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sen T., Heerspink H.J.L. A kidney perspective on the mechanism of action of sodium glucose co-transporter 2 inhibitors. Cell Metab. 2021;33(4):732–739. doi: 10.1016/j.cmet.2021.02.016. [DOI] [PubMed] [Google Scholar]
- 20.Packer M. Critical reanalysis of the mechanisms underlying the cardiorenal benefits of SGLT2 inhibitors and reaffirmation of the nutrient deprivation signaling/autophagy hypothesis. Circulation. 2022;146(18):1383–1405. doi: 10.1161/CIRCULATIONAHA.122.061732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rossi M.C., Nicolucci A., Arcangeli A., et al. Baseline quality-of-care data from a quality-improvement program implemented by a network of diabetes outpatient clinics. Diabetes Care. 2008;31(11):2166–2168. doi: 10.2337/dc08-0469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Prattichizzo F., La Sala L., Ceriello A. Two drugs are better than one to start T2DM therapy. Nat Rev Endocrinol. 2020;16(1):15–16. doi: 10.1038/s41574-019-0294-3. [DOI] [PubMed] [Google Scholar]
- 23.Russo G., Monami M., Perseghin G., et al. The "early treatment" approach reducing cardiovascular risk in patients with type 2 diabetes: a consensus from an expert panel using the delphi technique. Diabetes Ther. 2021;12(5):1445–1461. doi: 10.1007/s13300-021-01045-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ferrannini E., Muscelli E., Frascerra S., et al. Metabolic response to sodium-glucose cotransporter 2 inhibition in type 2 diabetic patients. J Clin Invest. 2014;124(2):499–508. doi: 10.1172/JCI72227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ferrannini E., Baldi S., Frascerra S., et al. Renal handling of ketones in response to sodium-glucose cotransporter 2 inhibition in patients with type 2 diabetes. Diabetes Care. 2017;40(6):771–776. doi: 10.2337/dc16-2724. [DOI] [PubMed] [Google Scholar]
- 26.Liu Z., Ma X., Ilyas I., et al. Impact of sodium glucose cotransporter 2 (SGLT2) inhibitors on atherosclerosis: from pharmacology to pre-clinical and clinical therapeutics. Theranostics. 2021;11(9):4502–4515. doi: 10.7150/thno.54498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Prattichizzo F., de Candia P., Ceriello A. Diabetes and kidney disease: emphasis on treatment with SGLT-2 inhibitors and GLP-1 receptor agonists. Metabolism. 2021;120 doi: 10.1016/j.metabol.2021.154799. [DOI] [PubMed] [Google Scholar]
- 28.Leoncini G., Russo E., Bussalino E., Barnini C., Viazzi F., Pontremoli R. SGLT2is and renal protection: from biological mechanisms to real-world clinical benefits. Int J Mol Sci. 2021;22(9):4441. doi: 10.3390/ijms22094441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.La Grotta R., Frige C., Matacchione G., et al. Repurposing SGLT-2 inhibitors to target aging: available evidence and molecular mechanisms. Int J Mol Sci. 2022;23(20):12325. doi: 10.3390/ijms232012325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.La Grotta R., de Candia P., Olivieri F., et al. Anti-inflammatory effect of SGLT-2 inhibitors via uric acid and insulin. Cell Mol Life Sci. 2022;79(5):273. doi: 10.1007/s00018-022-04289-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim M.N., Moon J.H., Cho Y.M. Sodium-glucose cotransporter-2 inhibition reduces cellular senescence in the diabetic kidney by promoting ketone body-induced NRF2 activation. Diabetes Obes Metab. 2021;23(11):2561–2571. doi: 10.1111/dom.14503. [DOI] [PubMed] [Google Scholar]
- 32.Kim S.R., Lee S.G., Kim S.H., et al. SGLT2 inhibition modulates NLRP3 inflammasome activity via ketones and insulin in diabetes with cardiovascular disease. Nat Commun. 2020;11(1):2127. doi: 10.1038/s41467-020-15983-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Steven S., Oelze M., Hanf A., et al. The SGLT2 inhibitor empagliflozin improves the primary diabetic complications in ZDF rats. Redox Biol. 2017;13:370–385. doi: 10.1016/j.redox.2017.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Scisciola L., Taktaz F., Fontanella R.A., et al. Targeting high glucose-induced epigenetic modifications at cardiac level: the role of SGLT2 and SGLT2 inhibitors. Cardiovasc Diabetol. 2023;22(1):24. doi: 10.1186/s12933-023-01754-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Iannantuoni F., M de Marañon A., Diaz-Morales N., et al. The SGLT2 inhibitor empagliflozin ameliorates the inflammatory profile in type 2 diabetic patients and promotes an antioxidant response in leukocytes. J Clin Med. 2019;8(11):1814. doi: 10.3390/jcm8111814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Natarajan R. Epigenetic mechanisms in diabetic vascular complications and metabolic memory: the 2020 edwin bierman award lecture. Diabetes. 2021;70(2):328–337. doi: 10.2337/dbi20-0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lunati M.E., Cimino V., Gandolfi A., et al. SGLT2-inhibitors are effective and safe in the elderly: the SOLD study. Pharmacol Res. 2022 Sep;183 doi: 10.1016/j.phrs.2022.106396. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.