Summary
Background
No randomized controlled trials have involved established HIV-diagnosed men who have sex with men (MSM) diagnosed for more than 6 months into the assisted partner service (aPS). We compared voluntary aPS involving community-based organizations (CBOs) and HIV self-testing (aPSST) with regular partner service (rPS) in HIV-diagnosed MSM irrespective of diagnosis time.
Methods
In this unblinded, multicentre trial, we enrolled HIV-diagnosed MSM irrespective of diagnosis time in three cities in northern China. Index patients were randomly assigned to aPSST or rPS. Index patients in the aPSST group were additionally provided a comprehensive intervention package including HIV self-testing and CBO-based aPS compared with rPS group. The primary outcome was the number of index patients whose any sexual partner tested for HIV during the 6-month study. Completion of HIV testing was defined as sexual partners taking a clinic-based HIV test or HIV self-testing. Safety was assessed preliminary at the end of the 6-month follow-up. This study has been registered at chictr.org.cn (ChiCTR2000038784).
Findings
From March to December 2021, 325 of HIV-diagnosed MSM were enrolled (90⋅2% were established HIV-diagnosed MSM) and randomly assigned to receive aPSST (n = 167) or rPS (n = 158). At 6 months, 110 (65⋅9%) index patients in the aPSST group had at least one sexual partner tested for HIV compared with 50 (31⋅6%) in the rPS group (hazard ratio 2⋅86; 95% confidence interval 2⋅03–4⋅03; p < 0⋅001). No significant difference was observed in effects of aPSST on HIV testing promotion between established and newly HIV-diagnosed MSM. Self-reported harms were infrequently observed in both groups (approximately 2⋅0%).
Interpretation
Among HIV-diagnosed MSM regardless of diagnosis time, voluntary aPS involving CBOs and HIV self-testing was effective and safe for promoting partner HIV testing.
Funding
This work was supported by the Mega-Projects of National Science Research, the National Natural Science Foundation of China and the Liaoning Revitalization Talents Program, China.
Keywords: Assisted partner service, Men who have sex with men, HIV self-testing, Community-based organization
Research in context.
Evidence before this study
We searched PubMed for all English publications issued between January 1, 1990, and July 13, 2022, with the search terms “HIV”, “MSM”, “men who have sex with men”, “partner”, “male couple” and “randomized trials”. We identified three randomized controlled trials in heterosexual couples in Kenya or Malawi, our previous trial in newly HIV-diagnosed MSM in Shenyang, and a trial with 76⋅0% of MSM in North Carolina, randomly assigned to assisted partner service (aPS) or regular partner service (rPS, i.e., patient referral). Previous studies rarely focused on established HIV-diagnosed individuals diagnosed for more than 6 months whose sexual partners are also at potential risk for HIV. Our previous work in Shenyang also failed to evaluate the safety of aPS. More evidence is needed for the effectiveness and safety of aPS in HIV-diagnosed MSM.
Added value of this study
This multicentre study was larger than our previous trial in Shenyang. It enhanced the evidence on applying aPS involving CBOs and HIV self-testing to MSM, especially in China. HIV-positive MSM are especially vulnerable to the stigma from HIV and their sexual preference. Providing aPS involving CBOs may be important in places where MSM is highly stigmatized. By combining with our previously published data, we provided evidence that aPS involving CBOs and HIV self-testing is an effective and safe public health intervention for newly or established HIV-diagnosed MSM and needs to be scaled up in low- and middle-income countries. Broad eligibility criteria for the diagnosis time of HIV-diagnosed MSM and diverse study settings could expand and enhance the adoption of aPS in Chinese MSM.
Implications of all the available evidence
HIV testing is the prerequisite for linkage to prevention and care services but remains suboptimal among MSM and their sexual partners in China. Additionally, rPS for HIV is still widely adopted by health providers in most countries. The consistency of our results with findings of previous work suggests that aPS involving CBOs and HIV self-testing is effective and safe for promoting partner HIV testing among HIV-diagnosed MSM irrespective of diagnosis time in China, which requires to be incorporated into pilot plans through policy action. The modest case-finding in study may argue for an effort to include practice like linking partners to PrEP to expand the yield of the intervention. Our findings can enrich approaches to promote HIV testing globally and accelerate the achievement of universal awareness of HIV status and HIV prevention.
Introduction
Globally, men who have sex with men (MSM) and their sexual partners are disproportionately affected by HIV1,2; they are more vulnerable to HIV than heterosexual men.3 HIV infections among MSM increased by 25% from 2010 to 2019 and are more serious than other key populations (from −7% to 5%).4 In China, the HIV infection rate was 6⋅0% among MSM in 2020,5 much higher than that among the general population (0⋅09%).6 Although antiretroviral therapy (ART) is an effective tool to break the chain of HIV transmission in the population, it failed to be applied in undiagnosed HIV-infected individuals. As of 2020, approximately 16% of HIV-infected individuals were unaware of their serostatus worldwide, while the proportion among MSM in China was 37⋅8%.5 The uptake of HIV testing, the prerequisite for initiating ART, remains suboptimal among MSM and their sexual partners in China.
HIV assisted partner service (aPS) is a voluntary process where trained health workers ask HIV-diagnosed individuals about their sexual partners, and with the consent of the HIV-diagnosed individuals, offer these partners voluntary HIV testing. Previous studies have revealed that aPS is more effective in notifying and increasing the uptake of HIV testing among exposed partners than regular partner service (rPS, i.e., patient referral).7,8 The aPS and HIV self-testing are recommended by World Health Organization to increase the access to HIV testing services for sexual partners of HIV-diagnosed individuals, and high proportions of HIV-positive individuals are diagnosed and linked to care.9 To improve the acceptability of aPS among HIV-diagnosed individuals, especially HIV-diagnosed MSM who are vulnerable to the stigma due to HIV and their sexual preference,10 community-based organizations (CBOs) rather than general health workers were involved in many studies.11,12 According to the 2025 targets and commitments of the Joint United Nations Programme on HIV/AIDS, 30% of testing and treatment services will be delivered by CBOs.13 Additionally, previous studies on aPS involving CBOs or HIV self-testing showed promising results on partner testing of HIV-diagnosed individuals.12,14,15 aPS has now been adopted in nations around the world, however, data on the contemporary effectiveness of aPS among MSM and how to implement aPS among MSM globally is lacking.16 More high-quality evidence in China is needed to test the effectiveness of aPS.
In addition, previous studies mainly focus on newly HIV-diagnosed heterosexual individuals diagnosed for less than 6 months.14,15,17,18 However, established HIV-diagnosed individuals diagnosed for more than 6 months may also not disclose their serostatus and encourage their sexual partners to test for HIV due to potential social discrimination.19,20 Compared with newly HIV-diagnosed individuals, adherence to ART during a long time in established HIV-diagnosed individuals is not always satisfactory,21, 22, 23 and unprotected sexual behaviours may exist more frequently for the concept of “undetectable equals untransmittable”.24 When HIV-diagnosed individuals are not in care or in care but viraemic, the sexual partners of these HIV-diagnosed individuals are also at potential risk for HIV acquisition and need HIV testing service. A secondary analysis of a randomized trial in Kenya explored that aPS may also promote HIV testing and case finding among sexual partners of established HIV-diagnosed individuals,25 which requires extra evidence.
Our previous work on newly HIV-diagnosed MSM did not explore the indicators of first-time HIV testing, linkage to HIV care, and potential harm from sexual partners,12 which are critical to assess the effectiveness of aPS on HIV partner testing and treatment. A randomized controlled trial (RCT) was conducted to evaluate the effectiveness of a comprehensive intervention (voluntary aPS involving CBOs and HIV self-testing, aPSST) on HIV partner testing among newly and established HIV-diagnosed MSM. Our secondary aim was to further evaluate the subsequent effectiveness of finding individuals who tested HIV for the first time, who tested positive, who newly tested positive, and who linked to HIV care, and preliminarily the safety of aPSST.
Methods
Study design and participant eligibility
An unblinded, multicentre RCT was conducted to compare the effectiveness of aPSST with rPS among newly and established HIV-diagnosed MSM in three cities (Shenyang, Beijing and Tianjin) in northern China. Newly HIV-diagnosed index patients refer to individuals diagnosed for less than 6 months whose virus load were not suppressed successfully; established HIV-diagnosed index patients refer to individuals diagnosed for more than 6 months who were virally suppressed in theory under treatment. The first participant was enrolled in the study in March 2021, and the last completed the study in April 2022. The trial protocol was approved by the ethics committee of the First Affiliated Hospital of China Medical University in Shenyang, China (Supplementary material S1). All participants provided written or digital informed consent. During the study, all personal data of participants were kept confidential, and they had the choice to withdraw from the study without any loss. To meet the needs of participants in the rPS group, we promised to provide HIV self-testing and CBOs-led aPS to participants in need after the study (data are not available). Study reporting follows CONSORT 2010 guidelines (Supplementary material S2).
We enrolled HIV-diagnosed (newly or established) MSM aged 18–60 years. Participants self-reported that have a sexual behaviour with their partners within the past 6 months (including oral, anal, or vaginal sex behaviour) and at least one contactable sexual partner (18 years or older with any contact information). We excluded participants who self-reported that all elicited partners were HIV-positive or had tested for HIV after their last sexual behaviour and those who refused to participate in the trial. Eligible HIV-diagnosed MSM were enrolled as index patients.
Randomization and masking
We simply randomized index patients to receive aPSST or rPS. Random assignment was performed based on a computer-generated random sequence using SPSS software version 26⋅0 (SPSS, IBM Inc) with a random seed of 400. The sequence (0–1) was divided into two groups by the Visual Binning function with a cut-off value of 0⋅5. Enrolment was ongoing during the trial. Due to the distinct treatments of the two groups, CBO staff and index patients were not blinded. To minimize the potential bias in results, the nurse responsible for blood sample collection, outcome assessors and adjudicators were blinded.
Procedures
After randomization, local CBO staff at each study site invited index patients to complete a self-administrated online baseline questionnaire on demographic and behavioural characteristics (questionnaire platform: https://jinshuju.net/home). The CBO staff interviewed index patients in both groups to elicit their contactable sexual partners and invited index patients in the aPSST group to provide their partners’ contact information (they could refuse). All index patients were assigned unique patient identification numbers, and each contactable partner was assigned a partner identification number linked to the index patient (i.e., 7001 and 7001 P1 for the index partner and his sexual partners, respectively).
Index patients assigned to the aPSST group received an additional comprehensive intervention package, including voluntary aPS based on CBOs and HIV self-testing. For the feasibility of HIV self-testing delivery, CBO staff demonstrated the usage of HIV self-testing kits to index patients and distributed the kits to them directly or provided quick response codes for online application of the kits. The HIV self-testing kits also provided instructions for HIV self-testing usage, result explanation and uploading. Voluntary aPS was provided according to the willingness of index patients during HIV testing promotion among their sexual partners. Index patients provided contact information of contactable sexual partners to CBO staff, and CBO staff contacted these partners in one week via phone and social media (i.e., WeChat and Blued). At least three contact attempts were made until they were contacted; otherwise, they were considered to refuse to engage in HIV testing services after the information verification from index patients. For credibility and a safe communication environment, CBO staff introduced themselves and their work and showed pictures of staff identification badges if necessary. When asked how information was obtained, staff responded that information from the MSM community is protected for confidentiality and the same to the current communication. Then, CBO staff inquired whether it was a suitable time for a conversation about health status. If so, CBO staff notified the partner of potential exposure to HIV; otherwise, another contact would be scheduled. During the conversation, CBO staff invited them to nearby clinics for HIV testing and provided quick response codes in case they failed to get tested at the clinic for any reason. Confidentiality was emphasized throughout the conversation. Index patients assigned to the rPS group were suggested to persuade their sexual partners by themselves to test for HIV through any methods including facility-based testing (recommended firstly) or self-testing. CBO staff informed them of potential harm during the process. We did not make it mandatory to disclose their HIV serostatus to their partners. They could disclose their serostatus to their partners according to the intimacy with partners and safety evaluation by themselves.
Confirmatory testing at clinics was suggested for sexual partners with HIV-positive results of HIV self-testing (rapid test results); those with confirmatory HIV-positive results were linked to HIV care; those with HIV-negative results were given information on regular HIV testing (once every 3–6 months) and protective sexual behaviours.
All index patients had a study visit at 3 and 6 months after randomization. To verify the self-reported results from index patients to some extent, after the completion of HIV testing, we tried to invite partners tested for HIV in both groups to complete a self-administrated online questionnaire to collect information about HIV testing. At the end of the 6-month follow-up, we collected a self-reported result of any physical or psychological harm from sexual partners among index patients. If appropriate, extra assistance would be provided to help index patients maintain their relationship with their partners.
Outcomes
The primary outcome was the number of index patients whose any sexual partners tested for HIV during the 6-month study. Secondary outcomes included the number of index patients whose any sexual partners tested HIV for the first time, the number of index patients whose any sexual partners had an HIV-positive result, the number of index patients whose any sexual partners had an HIV-positive result for the first time, the number of index patients whose any sexual partners linked to HIV care, and the number of index patients who reported any social harm from sexual partners within 6 months. Similar to other studies,26 to measure HIV testing outcomes of partners, we partly relied on the report from index patients and their partners (i.e., a report from the index patient that a partner tested for HIV defined to mean that the partner tested). Completion of HIV testing was defined as sexual partners receiving a clinic-based HIV test or HIV self-testing. A positive test result was defined as index patients or their sexual partners providing positive results by HIV self-testing or at clinics to staff after enrolling index patients. Linkage to HIV care was defined as newly HIV-diagnosed sexual partners being referred to clinics for ART.
Statistical analysis
Based on previous work,12 we assumed the primary outcome incidence of 35⋅1% in the aPSST group and 16⋅7% in the rPS group. We used PASS version 15⋅0 (NCSS, LLC. Kaysville, Utah, USA) to calculate the sample size. Each group of 118 index patients provided 90% power to detect a difference between groups in the incidence of any sexual partners tested for HIV (two-tailed α = 0⋅05). Assuming a 10% dropout rate, at least 131 patients per group were needed. Initially, we planned to enrol 400 patients for feasibility; however, the enrolment process was lower than anticipated due to the lockdown resulting from coronavirus disease 2019.
Index patients were analysed based on the intention-to-treat principle (all index patients after randomization). Sensitivity analysis was performed based on the per-protocol set population, which included all index patients without major protocol deviations. Index patients lost to follow-up were censored. No imputation was made during the analysis.
Different levels of HIV testing cascade (i.e., HIV testing, tested positive, newly tested positive, and linkage to HIV care) were compared between groups by the Fisher exact test.
All outcomes were binary events. For the primary outcome, Cox proportional hazards regression models were constructed to estimate hazard ratios (HRs) with their 95% confidence intervals (CIs) of aPSST versus rPS and adjusted for setting, age, education, ethnicity, marital status, diagnosis time, and the number of contactable sexual partners. The validity of the proportional hazard assumption was confirmed using parallel curves of the log-minus-log plots. We also plotted the event incidence over time using Kaplan–Meier curves and compared the two curves by the log-rank test. The absolute risk difference (RD) with 95% CI was calculated by the Newcombe/Wilson score with continuity correction.
Secondary outcomes with an event date were analysed similarly to the primary outcome. For secondary outcomes without an event date (i.e., the incidence of any sexual partners linkage to HIV care and any harm from sexual partners), a modified Poisson regression model was built to estimate the effect of aPSST versus rPS and adjusted for setting, age, education, ethnicity, marital status, diagnosis time, and the number of contactable sexual partners. Consequently, Poisson model estimates were given as a rate ratio (RR) with 95% CI.
The exploratory post-hoc subgroup analysis was conducted according to subgroups, including setting, age, education, and diagnosis time. The effect of aPSST versus rPS for each subgroup was evaluated using an unadjusted Cox proportional hazard model. The homogeneity of effects was measured among subgroups by adding interaction terms to the model.
A two-tailed p-value of 0⋅008 was considered significant (according to the Bonferroni method) for the primary outcome; other statistical tests were at a significance level of 0⋅05. The findings toward secondary outcomes were interpreted as exploratory since type I errors may result from multiple comparisons. All statistical analyses were conducted with SPSS version 26⋅0 (SPSS, IBM Inc), and Kaplan–Meier curves were plotted with MedCalc version 18⋅2⋅1 (MedCalc Software bvba, Ostend, Belgium). This trial has been registered at chictr.org.cn (ChiCTR2000038784).
Role of the funding source
The funders of the study had no role in the study design, data collection, analysis, interpretation, or writing of the paper.
Results
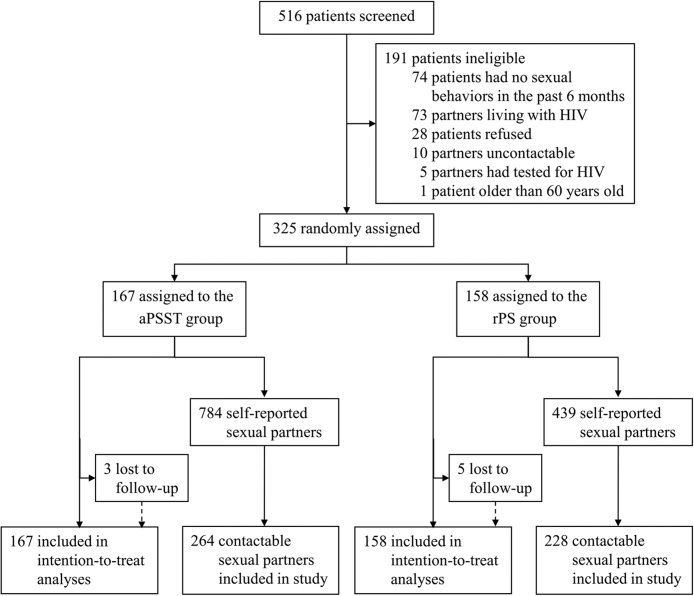
We recruited index patients in three cities in northern China from March 1, 2021, to December 31, 2021. Totally, 516 HIV-positive MSM were screened, of whom 325 were eligible and randomly assigned to the aPSST group (167 index patients) or the rPS group (158 index patients). Only 2% (8/325) of index patients were lost to follow-up after randomization (Fig. 1). Demographic and behavioural characteristics at baseline were similar between groups (Table 1). Most index patients had initiated ART (90⋅2%, 293/325), had not used HIV self-testing (54⋅2%, 176/325), and had disclosed serostatus to their sexual partners (59⋅7%, 194/325).
Fig. 1.
Trial profile.
Table 1.
Demographic and behavioural characteristics of between-groups at baseline.
| Group, No. (%) |
||
|---|---|---|
| aPSST (n = 167) | rPS (n = 158) | |
| Demographic characteristics | ||
| Settings | ||
| Shenyang | 87 (52⋅1) | 77 (48⋅7) |
| Beijing | 39 (23⋅4) | 42 (26⋅6) |
| Tianjin | 41 (24⋅6) | 39 (24⋅7) |
| Age, year | ||
| ≤29 | 46 (27⋅5) | 51 (32⋅3) |
| 30–39 | 86 (51⋅5) | 72 (45⋅6) |
| >39 | 35 (21⋅0) | 35 (22⋅2) |
| Median (IQR) | 33 (29–39) | 32 (27–39) |
| Ethnicity | ||
| Han | 44 (86⋅2) | 136 (86⋅1) |
| Other | 23 (13⋅8) | 22 (13⋅9) |
| Marital status | ||
| Single | 83 (49⋅7) | 92 (58⋅2) |
| Living with sexual partners | 51 (30⋅5) | 40 (25⋅3) |
| Married | 18 (10⋅8) | 16 (10⋅1) |
| Divorced or widowed | 15 (9⋅0) | 10 (6⋅3) |
| Education | ||
| Junior high school or below | 36 (21⋅6) | 31 (19⋅6) |
| Senior high school and technical secondary school | 47 (28⋅1) | 42 (26⋅6) |
| University or above | 84 (50⋅3) | 85 (53⋅8) |
| Behavioural characteristics | ||
| HIV diagnosis time (month) | ||
| ≤6 | 33 (19⋅8) | 25 (15⋅8) |
| >6 | 134 (80⋅2) | 133 (84⋅2) |
| ART | ||
| Yes | 149 (89⋅2) | 144 (91⋅1) |
| No | 18 (10⋅8) | 14 (8⋅9) |
| Previous use of HIV self-testing kits | ||
| Yes | 78 (46⋅7) | 71 (44⋅9) |
| No | 89 (53⋅3) | 87 (55⋅1) |
| Previously obtained HIV self-testing kits from others | ||
| Yes | 33 (19⋅8) | 25 (15⋅8) |
| No | 134 (80⋅2) | 133 (84⋅2) |
| Number of all self-reported sexual partners in the past 6 months | ||
| 1 | 57 (34⋅1) | 58 (36⋅7) |
| 2 | 32 (19⋅2) | 38 (24⋅1) |
| ≥3 | 78 (46⋅7) | 62 (39⋅2) |
| Number of contactable sexual partners in the past 6 months | ||
| 1 | 118 (70⋅7) | 119 (75⋅3) |
| 2 | 23 (13⋅8) | 26 (16⋅5) |
| ≥3 | 26 (15⋅6) | 13 (8⋅2) |
| Median (IQR) | 1⋅0 (1⋅0–2⋅0) | 1⋅0 (1⋅0–1⋅3) |
| HIV disclosure to anyone | ||
| Yes | 136 (81⋅4) | 121 (76⋅6) |
| No | 31 (18⋅6) | 37 (23⋅4) |
| HIV disclosure to sexual partners | ||
| Yes | 105 (62⋅9) | 89 (56⋅3) |
| No | 62 (37⋅1) | 69 (43⋅7) |
IQR, Interquartile range.
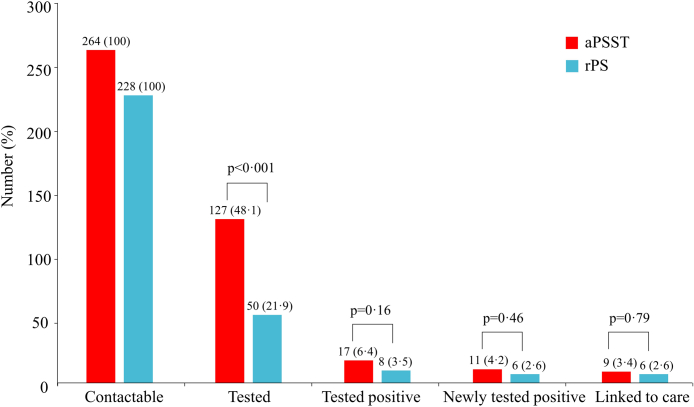
Among 177 sexual partners tested for HIV, 99 (55⋅9%) were tested through clinic, 52 (29⋅4%) were tested through HIV self-testing kits, and results of 26 partners (14⋅7%) were based on reports of index patients and their partners. We collected 144 questionnaires from partners about HIV testing within 6 months. Totally, 123 questionnaires had available HIV testing results, of which 119 (96⋅7%) of HIV testing results were consistent to results we obtained from clinic, index patients and their partners. In the aPSST group, 35 (21⋅0%) index patients accepted the assistance from CBO staff; CBO staff tried to contacted corresponding 50 sexual partners, in which 10 (20⋅0%) engaged in HIV testing.
Fig. 2 presents the different phases of the HIV testing cascade between groups. We found dramatic differences in the proportion of contactable sexual partners completing HIV testing between the aPSST and rPS groups (48⋅1% vs. 21⋅9%, p < 0⋅001). In contrast, there were no differences between the aPSST group and rPS groups on the number of partners tested HIV-positive, partners newly tested HIV-positive, and partners linked to HIV care (all p > 0⋅05).
Fig. 2.
HIV testing cascade among contactable sexual partners between groups. Fisher exact test was used. aPSST, Voluntary assisted partner service involving CBOs and HIV self-testing; rPS, Regular partner service.
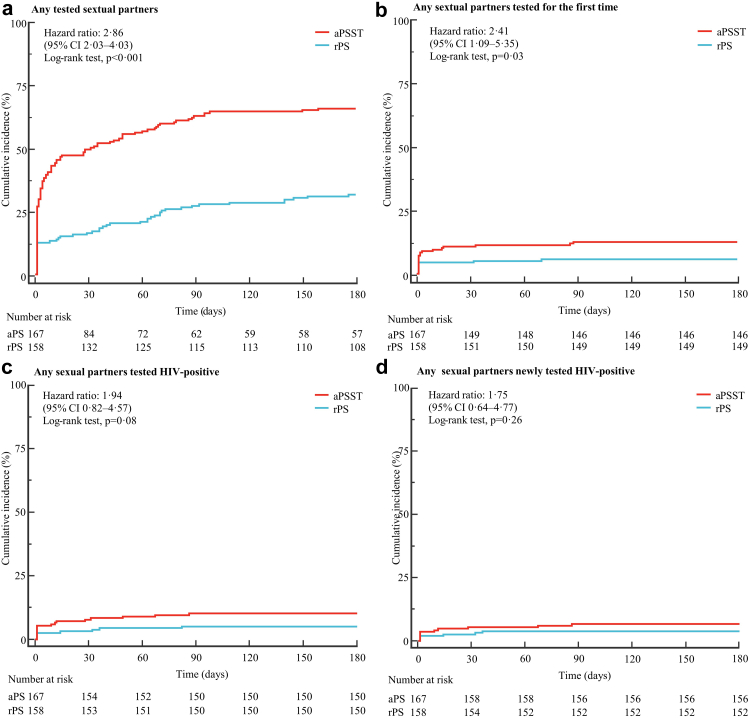
After a median follow-up of 4⋅7 months (interquartile range, IQR 0⋅1–6⋅0), 160 primary events were observed (110 in the aPSST group and 50 in the rPS group). The index patients whose any sexual partners were tested accounted for 65⋅9% (110/167) in the aPSST group, and 31⋅6% (50/158) in the rPS group, with an absolute RD of 34% (HR 2⋅86; 95% CI 2⋅03–4⋅03; p < 0⋅001). A total of 21 index patients (12⋅6%) in the aPSST group had at least one sexual partner tested for the first time, while 9 index patients (5⋅7%) in the rPS group, with an absolute RD of 7% (HR 2⋅41; 95% CI 1⋅09–5⋅35; p = 0⋅03). There was no significant difference among aPSST and rPS groups in the proportion of index patients whose any sexual partners tested HIV-positive, any sexual partners newly tested HIV-positive, any sexual partners linked to HIV care, and any harm from sexual partners (Table 2, Fig. 3).
Table 2.
Primary and secondary outcomes over 6 months.
| Group, No. (%) |
Effect size (95% CI)a | p-value | ||
|---|---|---|---|---|
| aPSST (n = 167) | rPS (n = 158) | |||
| Primary outcome | ||||
| Any tested sexual partners | 110 (65⋅9) | 50 (31⋅6) | 2⋅86 (2⋅03–4⋅03) | <0⋅001 |
| Secondary outcomes | ||||
| Any sexual partners tested for the first time | 21 (12⋅6) | 9 (5⋅7) | 2⋅41 (1⋅09–5⋅35) | 0⋅03 |
| Any sexual partners tested HIV-positive | 17 (10⋅2) | 8 (5⋅1) | 1⋅94 (0⋅82–4⋅57) | 0⋅13 |
| Any sexual partners newly tested HIV-positive | 11 (6⋅6) | 6 (3⋅8) | 1⋅75 (0⋅64–4⋅77) | 0⋅28 |
| Any sexual partners linked to HIV care | 10 (6⋅0) | 6 (3⋅8) | 1⋅49 (0⋅56–4⋅00) | 0⋅43 |
| Any harm from sexual partners | 3 (1⋅8) | 3 (1⋅9) | 1⋅38 (0⋅41–4⋅67) | 0⋅60 |
Estimates for aPSST versus rPS (i.e., rPS in the denominator) were adjusted for setting, age, education, ethnicity, marital status, diagnosis time, and the number of contactable sexual partners.
aPSST, Voluntary assisted partner service involving CBOs and HIV self-testing; rPS, Regular partner service.
For secondary events with event dates (i.e., the incidence of any sexual partners linked to HIV care and any harm from sexual partners), rate ratios are presented through the modified Poisson model; for all other outcomes, hazard ratios are presented through the Cox model.
Fig. 3.
Incidence of outcomes over 6 months. Kaplan–Meier estimates of the cumulative incidence of any tested sexual partners (a), any sexual partners tested for the first time (b), any sexual partners tested HIV-positive (c), and any sexual partners newly tested HIV-positive (d). Hazard ratios were adjusted for setting, age, education, ethnicity, marital status, diagnosis time, and the number of contactable sexual partners through the Cox proportional hazards model. aPSST, Voluntary assisted partner service involving CBOs and HIV self-testing; rPS, Regular partner service.
Adjusted results for the primary outcome was similar in the per-protocol population (16 index patients with major protocol violations were excluded). For the secondary outcome (i.e., the incidence of any sexual partners tested for the first time), the difference between groups was changed to be borderline statistically significant (p = 0⋅053; Supplementary material S3).
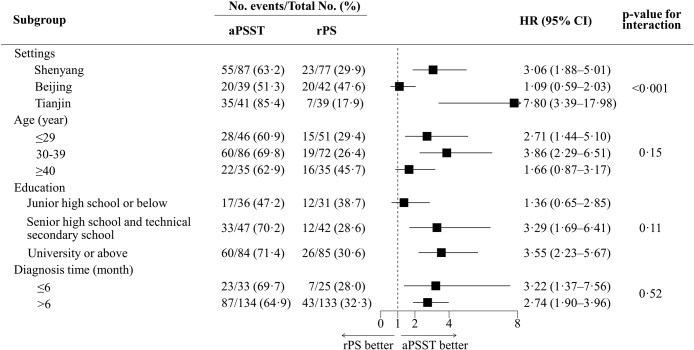
An exploratory subgroup analysis was conducted for the primary outcome across the setting, age, education, and diagnosis time. Compared with the rPS group, the effect of aPSST on promoting partner HIV testing was consistent across subgroups of age, education, and diagnosis time but differed in sites (Fig. 4). For index patients at different locations, the HR of promoting partner HIV testing was 3⋅06 (95% CI 1⋅88–5⋅01; p < 0⋅001) in Shenyang, 1⋅09 (95% CI 0⋅59–2⋅03; p = 0⋅78) in Beijing, and 7⋅80 (95% CI 3⋅39–17⋅98; p < 0⋅001) in Tianjin.
Fig. 4.
Subgroup results for the primary outcome. Using the unadjusted Cox proportional hazards model, exploratory subgroup analyses were conducted to estimate HRs with 95% CI and to test for interactions among subgroups using two-sided p-values. aPSST, Voluntary assisted partner service involving CBOs and HIV self-testing; rPS, Regular partner service; HR, Hazard ratio; CI, Confidence interval.
Social harms were infrequently observed in this study. At the end of follow-up, 3 (1⋅8%) index patients in the aPSST group and 3 (1⋅9%) in the rPS group suffered self-reported psychological harm from a current or former sexual partner (p > 0⋅99). Index patients did not associate these events with participation in the study or request extra assistance from CBOs and other staff.
Discussion
In this study, involving established HIV-diagnosed MSM diagnosed for more than 6 months, voluntary aPS involving CBOs and HIV self-testing had better effects on HIV testing promotion among sexual partners than rPS at 6 months with similar safety. This comprehensive intervention had the potential to identify more individuals having never tested for HIV, helping expand HIV testing coverage among MSM. No more harm from sexual partners was observed in the aPSST group.
This study concerned the first multicentre RCT comparing the effects of aPSST and rPS on HIV testing promotion involving established HIV-diagnosed MSM. Similar to previous studies among newly HIV-diagnosed individuals, index patients in this study treated with aPSST showed a higher probability of promoting partner HIV testing12,14,17 and finding first-time testers18 than the rPS group. Based on previous experience, the lockdown caused by coronavirus disease 2019 will hamper the process of in-person partner tracing and facility-based testing.27 However, the incidence of any sexual partners tested for HIV is two times higher in both groups than in our previous work among newly HIV-diagnosed MSM in Shenyang.12 On the one hand, the difference may be due to the availability of HIV self-testing application via quick response codes during the study, rather than an alternative option of HIV self-testing or aPS from baseline, which led to more attempts to test for their partners. On the other hand, established HIV-diagnosed MSM in this study may less vulnerable to partner testing promotion than new HIV-diagnosed MSM in previous work. We observed variation in the between-group difference in the incidence of finding any first-time testers in the per-protocol population. However, it is considered reasonable given the sample reduction in the per-protocol set; 16 (10⋅1%) index patients with major protocol violations in the rPS group were excluded. Increasing sample disparity and disturbed balance between the two groups may reduce the statistical power. Undiagnosed and late-diagnosed HIV individuals are the potential source of HIV transmission for a considerable period28 and are at high risk for clinical events and death.29 The aPSST involving established HIV-diagnosed MSM can also promote more frequent and earlier partner HIV testing to reduce these risks.
Previous studies have shown inconsistent results of two groups of partners who tested positive and newly tested positive among newly HIV-diagnosed adults.12,14,17,18 In this study, we did not observe more sexual partners tested HIV-positive or newly HIV-positive in the aPSST group than in the rPS group, which is consistent with findings of our previous work among MSM.12 However, the case-finding index for the aPSST group was 0⋅066 (new diagnoses in partners/index cases receiving aPSST). The incremental increase in case-finding was 0⋅03 (i.e., number needed to treat = 33⋅4). The index is lower than the result of a study in Kenya (0⋅20)18 and our previous study (0⋅10),12 but slightly higher than a result in the U.S. (0⋅05).30 The modest case-finding may argue for an effort in China to expand the yield of the intervention to include practice like linking partners to PrEP. Similarly, we detected no significant between-group difference in linkage to HIV care; however, two sexual partners who newly tested positive were not linked to HIV care (both in the aPSST group). The intervention involving HIV self-testing promoted partner testing but may reduce the proportion of partners seeking facility-based HIV testing.15 It may also weaken the linkage with other facility-based services on HIV prevention and treatment, which should be considered in comprehensive HIV programmes involving aPSST.
In the subgroup analysis, one group showed a statistically significant interaction p-value (i.e., for the primary outcome based on settings), which did not conform with our expectation (i.e., aPSST is effective in patients from different settings, whereas we failed to observe the effectiveness of aPSST in Beijing). In the per-protocol set, however, the RD for HIV testing promotion in Beijing increased from 3⋅7% to 20⋅5% (p from 0⋅78 to 0⋅14). The considerable p-value (0⋅14) may be explained by the limited samples (65) in the subgroup of the per-protocol set (16 index patients with protocol deviation in Beijing were excluded, which may explain the result above in Beijing). In addition, we observed that the effect of aPSST in Tianjin was much higher than other sites. Although aPSST is effective, different service providers among CBOs may influence the effect of aPSST; CBO staff hold different individual skills during aPS with HIV-diagnosed MSM. With the increasing role of CBOs in testing and treatment services,13 well-trained staff will improve the effects and quality of services, including aPSST. In addition, we did not observe a significant difference in the effect of aPSST on HIV testing promotion between established HIV-diagnosed MSM and newly diagnosed MSM, which indicates that established HIV-diagnosed MSM should also be considered by service providers,23 thus enlarging the population who can benefit from aPSST. Nevertheless, given the limitation on the explanation of subgroup analysis effect after randomization,31 results above should be interpreted with caution.
The potential social harm from sexual partners is a critical concern in aPS programmes. In this study, we did not observe a significant difference in social harm between aPSST and rPS, which is consistent with the results of previous studies.17,18 However, 6 index patients in both groups self-reported that they experienced social harm from their partners during the study. Future aPSST programmes should screen index patients to identify those at high risk of social harm, counsel them, and refer them for specialized social harm management.
This study involved the first multicentre RCT for the effects of aPSST compared with rPS among MSM. Differing from other studies, most HIV-diagnosed MSM in this study had been previously diagnosed for more than 6 months and had partners at potential risk for HIV. In addition, we preliminarily evaluated the safety of aPSST among HIV-diagnosed MSM who are at potential risk for social harm from their sexual partners, as other studies concerned. However, this study has several limitations. First, we relied partly on the self-reported HIV results from index patients, which may pose potential reporting bias. To learn the extent of bias, we invited partners tested for HIV to complete an online questionnaire to collect information about HIV testing results. Second, this study assigned the participants to the aPSST group and the rPS group by simple randomization, which inevitably resulted in bias due to various study sites. Due to the distinct measures to the two groups, CBO staff and index patients were unable to be blinded, which may result in the Hawthorne effect and diagnostic suspicion bias. Third, results for secondary outcomes may be impacted by the sample size. The sample size in this study was estimated based on the primary outcome, which may provide insufficient power to test secondary outcomes. Results based on these secondary outcomes should be exploratory. Fourth, the extrapolation of our results is constrained by geography. This study was conducted in northern China. Demographical and behavioural characteristics of MSM vary across regions of China, which may limit the extrapolation of the study findings. Finally, it may be feasible to link contactable sexual partners without HIV to pre-exposure prophylaxis services; however, we did not consider it, which should be included in the package of prevention and treatment services in this study.
In conclusion, voluntary aPS involving CBOs and HIV self-testing is effective and safe for promoting partner HIV testing among HIV-diagnosed MSM irrespective of diagnosis time for HIV. Established HIV-diagnosed MSM diagnosed for more than 6 months should also be considered by aPS providers. These data present an opportunity to boost HIV testing uptake among MSM in China.
Contributors
HS and QHH and JJX had the idea and designed the study. ZHY, ZXC, JYD, JY, JXZ, FL and HBD recruited patients and collected the data. ZHY analysed and interpreted the data. FL and XYB had a check for the analysis. ZHY wrote the first draft of the manuscript. QHH and JJX revised the manuscript. All authors had unrestricted access to the data and participated in data interpretation. FL and XYB accessed and verified the data, while HS, QHH and JJX were responsible for the decision to submit the manuscript. All the authors agreed to submit the article for publication.
Data sharing statement
The raw data supporting the conclusions of this article and additional, related documents will be made available by the corresponding authors, without undue reservation.
Declaration of interests
We declare no competing interests.
Acknowledgments
We are indebted to all staff in Shenyang, Beijing, and Tianjin for their contribution to the study, and to the MSM and their partners who participated in the study. This work was supported by the Mega-Projects of National Science Research (13th Five-Year Plan [No.2017ZX10201101]), the National Natural Science Fundation of China (82073620) and the Liaoning Revitalization Talents Program (XLYCYSZX1904).
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.lanwpc.2023.100772.
Contributor Information
Jun-Jie Xu, Email: jjxu@cmu.edu.cn.
Qing-Hai Hu, Email: qhhu@cmu.edu.cn.
Hong Shang, Email: hshang@cmu.edu.cn.
Appendix A. Supplementary data
References
- 1.Beyrer C., Baral S.D., van Griensven F., et al. Global epidemiology of HIV infection in men who have sex with men. Lancet. 2012;380(9839):367–377. doi: 10.1016/S0140-6736(12)60821-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.UNAIDS . 2021. Global AIDS update 2021.https://www.unaids.org/sites/default/files/media_asset/2021-global-aids-update_en.pdf [Google Scholar]
- 3.UNAIDS . 2021. Unaids data 2021.https://www.unaids.org/sites/default/files/media_asset/JC3032_AIDS_Data_book_2021_En.pdf [Google Scholar]
- 4.UNAIDS . 2020. World AIDS day report 2020.https://www.unaids.org/sites/default/files/media_asset/prevailing-against-pandemics_en.pdf [Google Scholar]
- 5.UNAIDS . 2022. Global data on HIV epidemiology and response.https://aidsinfo.unaids.org/ [Google Scholar]
- 6.Liu X.J., McGoogan J.M., Wu Z.Y. Human immunodeficiency virus/acquired immunodeficiency syndrome prevalence, incidence, and mortality in China, 1990 to 2017: a secondary analysis of the Global Burden of Disease Study 2017 data. Chin Med J. 2021;134(10):1175–1180. doi: 10.1097/CM9.0000000000001447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Landis S.E., Schoenbach V.J., Weber D.J., et al. Results of a randomized trial of partner notification in cases of HIV infection in North Carolina. N Engl J Med. 1992;326(2):101–106. doi: 10.1056/NEJM199201093260205. [DOI] [PubMed] [Google Scholar]
- 8.Dalal S., Johnson C., Fonner V., et al. Improving HIV test uptake and case finding with assisted partner notification services. AIDS. 2017;31(13):1867–1876. doi: 10.1097/QAD.0000000000001555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.WHO Guidelines Approved by the Guidelines Review Committee . World Health Organization. Copyright © World Health Organization 2016; Geneva: 2016. Guidelines on HIV self-testing and partner notification: supplement to consolidated guidelines on HIV testing services. [PubMed] [Google Scholar]
- 10.Yan X., Xu Y., Tucker J.D., Miller W.C., Tang W. Facilitators and barriers of HIV partner notification services among men who have sex with men in China: a qualitative analysis using a socioecological framework. Sex Transm Dis. 2022;49(8):541–545. doi: 10.1097/OLQ.0000000000001644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Onovo A., Kalaiwo A., Agweye A., Emmanuel G., Keiser O. Diagnosis and case finding according to key partner risk populations of people living with HIV in Nigeria: a retrospective analysis of community-led index partner testing services. eClinicalMedicine. 2022;43 doi: 10.1016/j.eclinm.2021.101265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hu Q.H., Qian H.Z., Li J.M., et al. Assisted partner notification and uptake of HIV testing among men who have sex with men: a randomized controlled trial in China. Lancet Reg Health West Pac. 2021;12 doi: 10.1016/j.lanwpc.2021.100171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.UNAIDS . End AIDS; 2021. Global AIDS strategy 2021-2026 — end inequalities.https://www.unaids.org/sites/default/files/media_asset/global-AIDS-strategy-2021-2026_en.pdf [Google Scholar]
- 14.Brown L.B., Miller W.C., Kamanga G., et al. HIV partner notification is effective and feasible in sub-Saharan Africa: opportunities for HIV treatment and prevention. J Acquir Immune Defic Syndr. 2011;56(5):437–442. doi: 10.1097/qai.0b013e318202bf7d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mutale W., Freeborn K., Graybill L.A., et al. Addition of HIV self-test kits to partner notification services to increase HIV testing of male partners of pregnant women in Zambia: two parallel randomised trials. Lancet Glob Health. 2021;9(12):e1719–e1729. doi: 10.1016/S2214-109X(21)00393-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Katz D.A., Wong V.J., Medley A.M., et al. The power of partners: positively engaging networks of people with HIV in testing, treatment and prevention. J Int AIDS Soc. 2019;22(Suppl 3) doi: 10.1002/jia2.25314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rosenberg N.E., Mtande T.K., Saidi F., et al. Recruiting male partners for couple HIV testing and counselling in Malawi's option B+ programme: an unblinded randomised controlled trial. Lancet HIV. 2015;2(11):e483–e491. doi: 10.1016/S2352-3018(15)00182-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cherutich P., Golden M.R., Wamuti B., et al. Assisted partner services for HIV in Kenya: a cluster randomised controlled trial. Lancet HIV. 2017;4(2):e74–e82. doi: 10.1016/S2352-3018(16)30214-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morkphrom E., Ratanasuwan W., Sittironnarit G., Rattanaumpawan P. Non-disclosure of HIV serostatus to sexual partners: prevalence, risk factors and clinical impact in patients with HIV. HIV Med. 2021;22(3):194–200. doi: 10.1111/hiv.13005. [DOI] [PubMed] [Google Scholar]
- 20.Yaya I., Saka B., Landoh D.E., et al. HIV status disclosure to sexual partners, among people living with HIV and AIDS on antiretroviral therapy at Sokodé regional hospital, Togo. PLoS One. 2015;10(2) doi: 10.1371/journal.pone.0118157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kalichman S.C., Kalichman M.O., Eaton L.A. Undisclosed HIV status to sex partners and its unintended consequences in the era of undetectable = untransmittable. J Acquir Immune Defic Syndr. 2021;88(2):149–156. doi: 10.1097/QAI.0000000000002762. [DOI] [PubMed] [Google Scholar]
- 22.Gunn J.K.L., Patterson W., Anderson B.J., Swain C.A. Understanding the risk of human immunodeficiency virus (HIV) virologic failure in the era of undetectable equals untransmittable. AIDS Behav. 2021;25(7):2259–2265. doi: 10.1007/s10461-020-03154-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Udeagu C.N., Shah S., Misra K., Xia Q. The usefulness of HIV partner services in the age of treatment as prevention: a registry-based study. Lancet HIV. 2020;7(7):e482–e490. doi: 10.1016/S2352-3018(20)30116-8. [DOI] [PubMed] [Google Scholar]
- 24.Edwards-Jackson N., Phanuphak N., Van Tieu H., et al. HIV serostatus disclosure is not associated with safer sexual behavior among HIV-positive men who have sex with men (MSM) and their partners at risk for infection in Bangkok, Thailand. AIDS Res Ther. 2012;9(1):38. doi: 10.1186/1742-6405-9-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Masyuko S.J., Cherutich P.K., Contesse M.G., et al. Index participant characteristics and HIV assisted partner services efficacy in Kenya: results of a cluster randomized trial. J Int AIDS Soc. 2019;22(Suppl 3) doi: 10.1002/jia2.25305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Masters S.H., Agot K., Obonyo B., Napierala Mavedzenge S., Maman S., Thirumurthy H. Promoting partner testing and couples testing through secondary distribution of HIV self-tests: a randomized clinical trial. PLoS Med. 2016;13(11) doi: 10.1371/journal.pmed.1002166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lagat H., Sharma M., Kariithi E., et al. Impact of the COVID-19 pandemic on HIV testing and assisted partner notification services, Western Kenya. AIDS Behav. 2020;24(11):3010–3013. doi: 10.1007/s10461-020-02938-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wong N.S., Wong K.H., Lee M.P., Tsang O.T., Chan D.P., Lee S.S. Estimation of the undiagnosed intervals of HIV-diagnosed individuals by a modified back-calculation method for reconstructing the epidemic curves. PLoS One. 2016;11(7) doi: 10.1371/journal.pone.0159021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Girardi E., Sabin C.A., Monforte A.D. Late diagnosis of HIV infection: epidemiological features, consequences and strategies to encourage earlier testing. J Acquir Immune Defic Syndr. 2007;46(Suppl 1):S3–S8. doi: 10.1097/01.qai.0000286597.57066.2b. [DOI] [PubMed] [Google Scholar]
- 30.Golden M.R., AugsJoost B., Bender M., et al. The organization, content, and case-finding effectiveness of HIV assisted partner services in high HIV morbidity areas of the United States. J Acquir Immune Defic Syndr. 2022;89(5):498–504. doi: 10.1097/QAI.0000000000002904. [DOI] [PubMed] [Google Scholar]
- 31.Sun X., Briel M., Walter S.D., Guyatt G.H. Is a subgroup effect believable? Updating criteria to evaluate the credibility of subgroup analyses. BMJ. 2010;340:c117. doi: 10.1136/bmj.c117. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.