Summary
Background
The direct-acting antiviral agents (DAAs) have revolutionized the treatment of Hepatitis C Virus (HCV) infection. However, a simple and feasible treatment strategy with high efficacy and safety for HCV in patients coinfected with Human Immunodeficiency Virus (HIV) remains an unmet medical need, especially in areas with limited health resource. This study aims to assess the efficacy and safety of 12 weeks of treatment with sofosbuvir and velpatasvir in patients with chronic HCV/HIV-1 coinfection.
Methods
We conducted a multicenter, single-arm, open-label study in China, which involved chronic HCV/HIV-1 coinfected patients who are receiving an antiretroviral regimen of a combination tablet consisting of elvitegravir, cobicistat, emtricitabine, tenofovir alafenamide, (EVG/c/FTC/TAF) once daily. Patients with liver cirrhosis or experienced to DAAs treatment were excluded. All patients received combined sofosbuvir (400 mg) and velpatasvir (100 mg) tablet once daily for 12 weeks regardless of HCV genotype. The primary efficacy endpoint was sustained virologic response, defined as HCV RNA <15 IU/mL at 12 weeks after completion of treatment (SVR12). The primary safety endpoint was the proportion of patients who prematurely discontinued treatment because of adverse events. Safety and efficacy data were analyzed with an intention-to-treat (ITT) population (last observation carried forward) and per-protocol (PP) population. This trial is registered on ChiCTR.org.cn with number being ChiCTR1800020246.
Findings
Of the 243 patients enrolled, 78% were male, 9% had been previously treated for HCV with interferon, and none had pre-defined cirrhosis, although 8% had Fibrosis 4 score (FIB-4) >3.25. A total of 233 patients completed 12-week post-treatment follow-up. Overall, 227/233 patients (97%) achieved SVR12: 100% (63/63) in those with HCV genotype 1, 67% (2/3) in those with genotype 2, 95% (84/88) in those with genotype 3, 99% (78/79) in those with genotype 6. Rates of SVR12 were lower among those with baseline FIB-4 >3.25 than those without (78% [14/18] vs. 99% [211/212], P < 0.001). HIV-1 suppression was not compromised. The most common adverse events were upper respiratory tract infection (5%), cough (3%), abnormal renal function (2%), abnormal liver function (2%), constipation (2%), urinary tract infection (2%) and sleep disorders (2%). No participant discontinued treatment because of adverse events or death.
Interpretation
Twelve weeks of treatment with sofosbuvir/velpatasvir provide high rates of SVR and is well-tolerated in patients coinfected with HIV-1 and HCV regardless of HCV genotypes. Non-invasive liver fibrosis score may help to further distinguish patients at greater likelihood of a suboptimal response.
Funding
The 13th Five Year Plan of the Ministry of Science and Technology of China for the prevention and treatment of major infectious diseases such as AIDS and viral hepatitis, the National Key Research and Development Program of China, Medical Key Discipline Program of Guangzhou-Viral Infectious Diseases (2021–2023), Basic research program on people's Livelihood Science and technology of Guangzhou, and National Natural Science Foundation of China.
Keywords: Hepatitis C virus, Human immunodeficiency virus, Coinfection, Sofosbuvir/velpatasvir, Efficacy, Safety
Research in context.
Evidence before this study
HCV/HIV coinfected patients have higher risk of liver cirrhosis, hepatocellular carcinoma, hepatic decompensation and all-cause mortality than patients monoinfected with HCV. A simple and feasible treatment strategy which is pangenotypic and ribavirin free, regardless of treatment history and liver fibrosis stage, and with limited potential drug–drug interaction, remains an unmet medical need, especially in areas with limited health resource. Previous studies have demonstrated that sofosbuvir/velpatasvir treatment is well-tolerated and results in high rates of sustained virologic response in HCV monoinfected patients. However, there are very limited data on the safety and efficacy of sofosbuvir/velpatasvir in HCV/HIV coinfected patients, especially in Asia. We searched PubMed using the term “sofosbuvir”, “velpatasvir”, “Hepatitis C virus”, “Human immunodeficiency virus”, and “coinfection”, for clinical trials published before November 1, 2022, without language restrictions. We found one open-label phase 3 study in the United States, in which 101/106 (95%) HCV/HIV coinfected patients achieved a sustained virological response after 12 weeks of generic sofosbuvir/velpatasvir without ribavirin.
Added value of this study
In our multicenter, single-arm trial, patients from China with chronic HCV/HIV-1 coinfection with HCV genotypes 1, 2, 3 and 6, without liver cirrhosis or experienced to DAAs treatment, were treated with open-label sofosbuvir/velpatasvir for 12 weeks, and were assessed for efficacy and safety. Overall, 227/233 (97%) patients achieved SVR12, consistent with data from the study in the United States. Rates of SVR12 were lower among those with baseline FIB-4 > 3.25 than those without (78% [14/18] vs. 99% [211/212], P < 0.001). FIB-4 > 3.25 was the only independent factor associated with HCV relapse at Week 12 post-treatment.
Implications of all the available evidence
These overall results support the use of sofosbuvir/velpatasvir as a simple and feasible treatment strategy in patients coinfected with HIV-1 and HCV regardless of HCV genotypes, especially in areas with limited health resource. Non-invasive liver fibrosis score may help to further distinguish patients at greater likelihood of a suboptimal response.
Introduction
Globally, an estimated 4 million to 5 million people are chronically coinfected with Hepatitis C Virus (HCV) and Human Immunodeficiency Virus (HIV).1 HCV/HIV coinfected patients have higher risk of liver cirrhosis, hepatocellular carcinoma, hepatic decompensation and all-cause mortality than patients monoinfected with HCV.2, 3, 4, 5 Since the introduction of combination antiretroviral therapy (ART), HIV-related morbidity and mortality have decreased while liver related complications have become a leading cause of non-HIV-related morbidities and death in coinfected patients.6,7 The introduction of direct-acting antiviral agents (DAAs) has revolutionized the treatment of HCV. However, before choosing the optimal treatment strategy for HCV/HIV coinfected patients, many obstacles need to be overcome, including HCV genotype, previous history of HCV treatment, liver cirrhosis and drug–drug interaction with antiretroviral therapy regimens. Therefore, a simple and feasible treatment strategy which is pangenotypic and ribavirin free, regardless of treatment history and liver fibrosis stage, and with limited potential drug–drug interaction, remains an unmet medical need, especially in areas with limited health resource.
Sofosbuvir and velpatasvir, two DAAs for the treatment of HCV with all genotypes, are available as a fixed-dose combination tablet (sofosbuvir 400 mg and velpatasvir 100 mg). Global registrational clinical trials and real-world data have demonstrated that sofosbuvir/velpatasvir treatment is well-tolerated and results in high rates of sustained virologic response in patients infected with HCV genotypes 1–6, with or without liver cirrhosis, regardless of previous treatment experience.8, 9, 10, 11 Unlike HCV monoinfected patients, there are very limited data on the safety and efficacy of sofosbuvir/velpatasvir in HCV/HIV coinfected patients. An open-label phase 3 study including 106 HCV/HIV coinfected patients, demonstrated a high rate of SVR12 and well-tolerance after 12 weeks of sofosbuvir/velpatasvir treatment.12 Despite its promising results, this study was only conducted in the United States with a small sample size, and mainly included patients with HCV genotype 1 and a small number of genotypes 2, 3 and 4, but did not include other genotypes such as genotypes 5 and 6. Thus, well-conducted clinical trials with adequate sample size and full genotypes are urgently needed, especially from Asia, a region with diverse HCV genotypes, limited and unevenly distributed medical resources, and a high burden of infectious diseases.13,14
The objective of this study in patients with chronic HCV/HIV-1 coinfection was to assess the efficacy and safety of 12 weeks of treatment with sofosbuvir (400 mg) and velpatasvir (100 mg), a simple and feasible treatment strategy that neither distinguishes HCV genotypes in advance nor includes ribavirin to facilitate implementation in areas with limited health resource.
Methods
Study design
This was a multicenter, single-arm, open-label study in China investigating the efficacy and safety of sofosbuvir/velpatasvir in patients who had chronic HCV/HIV-1 coinfection. Eligible patients were provided with an antiretroviral regimen of a combination tablet consisting of elvitegravir, cobicistat, emtricitabine and tenofovir alafenamide (EVG/c/FTC/TAF) once daily for 32 weeks, thereafter received ART per standard of care and followed up for another 8 weeks. After receiving EVG/c/FTC/TAF treatment for 4 weeks, all patients will initiate combined sofosbuvir (400 mg) and velpatasvir (100 mg) tablet once daily for 12 weeks regardless of HCV genotypes and then followed up for another 24 weeks (Figure S1). During the 12 weeks of sofosbuvir/velpatasvir treatment period, participants needed to report adverse events, no laboratory testing such as HCV RNA or liver function was required.
Patients
Patients aged between 18 and 65 years old, bodyweight of at least 35 kg, had chronic HCV with detectable HCV RNA and conformed HIV-1 coinfection for more than 6 months were enrolled. All patients were naive to direct antiviral drugs (DAAs) treatment, on stable ART with evidence of HIV RNA below the lower limit of detection (LLOD) within 12 months. If patients previously treated with pegylated interferon plus ribavirin (PR), they need to provide HCV RNA test results at initiation, 12 weeks of treatment, the end of treatment, and 24 weeks post-treatment. Primary exclusion criteria included acute HCV or HIV infection, liver cirrhosis (based on liver biopsy, FibroScan >12.5 kPa or aspartate aminotransferase to platelets [APRI] score >2.0), HBsAg positive, current opportunistic infections, opportunistic infections within 3 months but the condition remained unstable within 2 weeks prior to inclusion, AIDS-related tumors, or currently use of antituberculosis or antifungal drugs. Patients were also excluded based on abnormal laboratory results, including alanine aspartate aminotransferase (AST) or alanine aminotransferase (ALT) >10 upper limit of normal (ULN), total bilirubin (TBIL) >2 UNL, estimated glomerular filtration rate (eGFR) <50 ml/min/1.73 m2. We also excluded pregnant or lactating women, and patients with severe mental illness or other unstable medical conditions, current alcohol or intravenous drug abuse, or allergic to research drugs. Written informed consent was obtained from all patients at screening. The full protocol is provided in the Supplementary Appendix.
Study oversight
Before initiation of the trial, the protocol was approved by the Ethics Committee of Guangzhou Eighth People's Hospital and all collaborating centers and registered on Chinese Clinical Trial Registry (ChiCTR.org.cn, ChiCTR1800020246). The trial was performed in accordance with the International Conference on Harmonization's Guideline for Good Clinical Practice. Written informed consent was obtained from all screened patients after they fully understood the meaning of the trial and the potential risks involved. The corresponding authors had full access to all the data in the study, wrote the manuscript, and had final responsibility for the decision to submit for publication. All authors had access to the study data and reviewed and approved the final manuscript.
Assessments
Screening and on-treatment assessments included measurement of serum HCV RNA level, serum HIV RNA level, and standard laboratory and clinical testing. Assessment of HCV RNA load, HCV genotypes and HIV RNA load were carried out at the central laboratory set up by the research group. HCV RNA was detected by COBAS automatic virus load analysis system (COBAS TapMan48, Roche), and the LLOD was 15 IU/mL. HIV-1 RNA was detected by COBAS automatic virus load analysis system (COBAS TapMan48, Roche), and the LLOD was 20 copies/mL. HCV genotyping was retrospectively performed at the central laboratory using a detection kit (PCR-fluorescent probe method, Taipu Biological Sciences Co., LTD). Absolute CD4+ T lymphocyte count, CD8+ T lymphocyte count and CD4/CD8 ratio were measured using a BD CantoII flow cytometer with CD3/CD4/CD8 trichrome fluorescence reagent from BD according to the instructions. All reagents are within the period of validity, strictly abide by the laboratory standard operating procedures and carry out experiments in accordance with the requirements of the kit and all instruments have been verified. Fibrosis 4 (FIB-4) score and APRI score were calculated to assess the liver fibrosis.15,16
Adverse events (AEs) were recorded at all study visits. AE severity was determined using the Common Terminology Criteria for Adverse Events (CTCAE, Version 5.0, November 27, 2017), publish by United States Department of Health and Human Services, grading on a scale of 1 (mild) to 5 (death). Adherence was assessed by patient reporting and confirmatory pill counts conducted at all on treatment study visits.
Endpoints
Efficacy and safety were assessed in all patients who received at least one dose of study drug. The primary efficacy endpoint was sustained virologic response, defined as HCV RNA <15 IU/mL at 12 weeks after end of treatment (SVR12). The primary safety endpoint was the proportion of patients who prematurely discontinued treatment because of adverse events. Secondary endpoints were the proportion of patients with HCV RNA <15 IU/mL at the end of treatment, sustained virologic response 24 weeks after end of treatment (SVR24). In addition, the proportions of patients who maintained undetectable HIV-1 RNA and changes in CD4+ T lymphocyte count, FIB-4 score and liver function during study period were observed. Lack of efficacy was defined as nonresponse (detectable HCV RNA at the end of treatment with HCV RNA ≥15 IU/mL throughout treatment); rebound (HCV RNA >1 log10 increase from nadir while on treatment); breakthrough (HCV RNA ≥15 IU/mL after previously being <15 IU/mL); and relapse (HCV RNA ≥15 IU/mL during follow-up after having undetectable HCV RNA at end of treatment).
Statistical analysis
We calculated the proportion of patients who had a sustained virologic response along with exact two-sided 95% confidence intervals using the Clopper-Pearson method. With approximately 300 patients, the two-sided 95% confidence interval for the primary end point was expected extend no more than 3.4% in both directions from the observed rate on the assumption that the response rate would be 90%.17 All statistical analyses were performed using SPSS (Statistical Package for SPSS, version 26.0). Safety and efficacy data were analyzed with an intention-to-treat (ITT) population (last observation carried forward) and per-protocol (PP) population. We presented continuous measurements as median (interquartile range, IQR) and categorical variables as count (%). Means for continuous variables were compared using one-way ANOVA when the data were normally distributed; otherwise, the Mann–Whitney test was used. Proportions for categorical variables were compared using the Chi-square test or Fisher's exact tests. Logistic-regression analysis was performed to identify baseline factors that were independently associated with relapse. A two-sided a of less than 0.05 was considered statistically significant.
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
Baseline characteristics
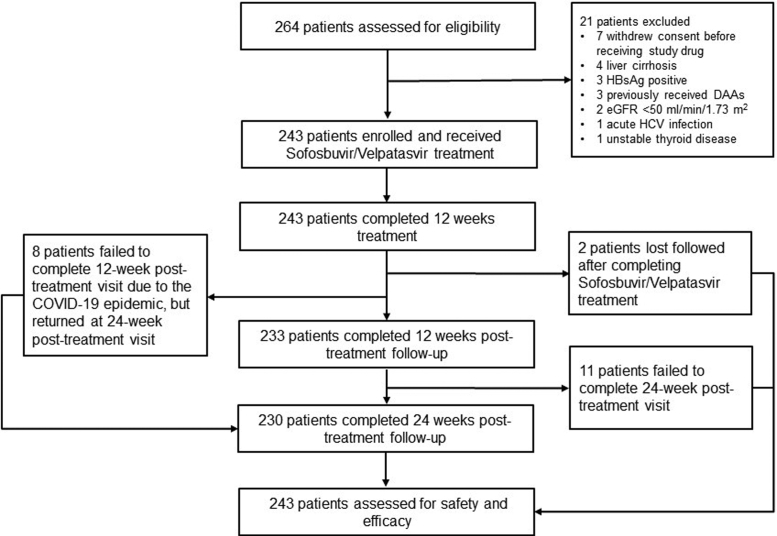
Between July 2019, and January 2021, of the 264 patients who were initially screened, 243 patients were enrolled in this study from 5 centers in southern China (Fig. 1, Table S1 in the Supplementary Appendix). The baseline characteristics of the enrolled patients are described in Table 1. The median age was 45 years, 78% were male. The median HCV RNA was 6.0 log10 IU/ml and genotype 3 were the most common followed by genotype 6, genotype 1 and genotype 2. Of the 243 enrolled patients, 220 (91%) were HCV treatment-naive, approximately three-quarters were undergoing stable antiretroviral therapy against HIV per standard of care over 5 years with 98% had undetectable HIV-1 RNA. None had pre-defined cirrhosis, although 8% had FIB-4 score >3.25.
Fig. 1.
Patient flow chart.
Table 1.
Demographic characteristics of study patients.
| Characteristics | Patients (n = 243) |
|---|---|
| Age, years; median (interquartile range, IQR) | 45 (41–48) |
| Gender | |
| Female, n (%) | 54 (22) |
| Male, n (%) | 189 (78) |
| Body mass index, kg/m2, median (IQR) | 20 (19–22) |
| HCV RNA, log10 IU/mL, median (IQR) | 6.0 (5.4–6.5) |
| HCV genotype | |
| 1a, n (%) | 10 (4) |
| 1b, n (%) | 60 (25) |
| 2a, n (%) | 4 (2) |
| 3a, n (%) | 36 (15) |
| 3b, n (%) | 53 (22) |
| 6a, n (%) | 65 (27) |
| 6e, n (%) | 2 (1) |
| 6n, n (%) | 11 (5) |
| 6v, n (%) | 2 (1) |
| Previous HCV treatment | |
| No, n (%) | 220 (91) |
| Yes, n (%) | 23 (9) |
| Interferon plus ribavirin, n (%) | 21/23 (91) |
| Pegylated interferon plus ribavirin, n (%) | 2/23 (9) |
| Response to previous HCV treatment | |
| Non-responder, n (%) | 5/23 (22) |
| Relapse or breakthrough, n (%) | 12/23 (52) |
| Early treatment discontinuation, n (%) | 3/23 (13) |
| Unknown, n (%) | 3/23 (13) |
| HCV/HIV transmission | |
| PWID, n (%) | 153 (63) |
| MSM, n (%) | 19 (8) |
| Heterosexuals, n (%) | 44 (18) |
| Unknown, n (%) | 27 (11) |
| Duration of ART | |
| ≤1 year | 5 (2) |
| 1–3 years | 24 (10) |
| 3–5 years | 34 (14) |
| >5 years | 180 (74) |
| Undetectable HIV RNA, n (%) | 239 (98) |
| CD4+ count, cells/mm3, median (IQR) | 452 (313–623) |
| CD4/CD8 ratio, median (IQR) | 0.6 (0.4–0.9) |
| Hemoglobin, g/L, median (IQR) | 150 (137–160) |
| Platelets, 109/L, median (IQR) | 198 (164–253) |
| ALT, IU/L, median (IQR) | 56 (39–72) |
| AST, IU/L, median (IQR) | 46 (34–65) |
| Albumen, g/L, median (IQR) | 46 (44–48) |
| Total bilirubin, μmol/L, median (IQR) | 10 (7–14) |
| eGFR, ml/min/1.73m2, median (IQR) | 112 (97–125) |
| FIB-4 score, median (IQR) | 1.43 (0.95–2.08) |
| FIB-4 score categories, n (%) | |
| <1.45 | 126 (52) |
| 1.45–3.25 | 97 (40) |
| >3.25 | 20 (8) |
Data are presented as No. (%) unless otherwise specified.
Abbreviations: IQR, interquartile range; HCV, hepatitis C virus; HIV, human immunodeficiency virus; PWID, people who inject drugs; MSM, men who have sex with men; ART, antiretroviral therapy; ALT, alanine aminotransferase; AST, aspartate aminotransferase; eGFR, estimated glomerular filtration rate; FIB-4, Fibrosis 4 marker.
Efficacy
Virologic response
All of the 243 enrolled patients completed 12 weeks of sofosbuvir/velpatasvir treatment and achieved serum HCV RNA <15 IU/mL at the end of treatment. Two patients lost follow-up since completion of sofosbuvir/velpatasvir treatment. Another 8 patients missed the 12-week post-treatment visit due to the coronavirus disease 2019 epidemic but returned at the 24-week post-treatment visit and all of them had a SVR24. A total of 233 patients completed 12-week and 230 completed 24-week post-treatment follow-up, respectively (Fig. 1).
In ITT population, 235 of 243 (97%; 95% CI, 94 to 99) patients achieved SVR12. In PP population, 227 of 233 (97%; 95% CI, 95 to 99) patients achieved SVR12 (Table 2). By genotype, SVR12 was achieved in 63 of 63 (100%) patients with genotype 1; 2 of 3 (67%) patients with genotype 2; 84 of 88 (95%) patients with genotype 3; and 78 of 79 (99%) patients with genotype 6. By HCV treatment history and fibrosis status, SVR12 was achieved at 206 of 211 (98%) patients who were treatment naïve and 21 of 22 (95%) patients who were interferon-based treatment experienced; 213 of 215 (99%) patients with FIB-4 ≤3.25 and 14 of 18 (78%) patients with FIB-4 >3.25.
Table 2.
Efficacy analysis.
| Characteristics | Patients (n = 243) | P values |
|---|---|---|
| Response | ||
| Treatment week 12 | 243/243 (100, 99–100) | – |
| Post-treatment week 12 | 227/233 (97, 95–99)a | – |
| By genotype | 0.024 | |
| Genotype 1 | 63/63 (100, 94–100) | |
| Genotype 2 | 2/3 (67, 9–99) | |
| Genotype 3 | 84/88 (95, 88–99) | |
| Genotype 6 | 78/79 (99, 93–100) | |
| By HCV treatment history | 0.452 | |
| Treatment naive | 206/211 (98, 95–99) | |
| Treatment experienced | 21/22 (95, 77–99) | |
| By FIB-4 score | <0.001 | |
| ≤3.25 | 213/215 (99, 97–100) | |
| >3.25 | 14/18 (78, 52–94) | |
| Post-treatment week 24 | 225/230 (98, 95–99) | |
| By genotype | 0.006 | |
| Genotype 1 | 65/65 (100, 94–100) | |
| Genotype 2 | 3/4 (75, 19–99) | |
| Genotype 3 | 82/86 (95, 88–99) | |
| Genotype 6 | 75/75 (100, 95–100) | |
| By HCV treatment history | 1.000 | |
| Treatment naive | 206/211 (98, 95–99) | |
| Treatment experienced | 19/19 (100, 82–100) | |
| By FIB-4 score | <0.001 | |
| ≤3.25 | 211/212 (99, 97–100) | |
| >3.25 | 14/18 (78, 52–94) | |
| Lost to follow-up | 2/243 (<1%) | – |
Data are no./No. (%, 95% CI) or no./No. (%). Response was defined as hepatitis C virus RNA <15 IU/mL.
Abbreviations: HCV, hepatitis C virus; FIB-4 Fibrosis 4 marker.
2 patients lost follow-up after completion of treatment, 8 patients missed 12 weeks post-treatment visit due to the COVID-19 epidemic but all completed 12 weeks post-treatment visit.
In ITT population, 255 of 243 (93%; 95% CI, 89 to 96) patients achieved SVR24. In PP population, 225 of 230 (98%; 95% CI, 95 to 99) patients achieved SVR24. Rates of SVR24 by genotypes, treatment experience and cirrhosis status are similar to that of SVR12.
During the study period, medication adherence was good and HIV-1 suppression was not compromised with over 98% of patients maintained undetectable HIV-1 RNA at visit points including baseline, week 16, week 28 and week 40 (Figure S2 in the Supplementary Appendix). Of the 230/243 patients who completed 32 weeks of EVG/c/FTC/TAF treatment and received ART per standard of care for another 8 weeks (week 40) according to the study protocol, 227 (99%) patients maintained undetectable HIV-1 RNA, and the rest 3 patients had HIV-1 levels of 870 copies/mL, 4390 copies/mL, and 7130 copies/mL, respectively.
Characteristics of HCV relapse
Of the 6 patients with HCV relapse at 12 weeks after completion of treatment, 4 were male and 2 were female, age range of 38–55 years (Table 3). At baseline, 4 patients were HCV genotype 3b, 1 was 6n, 1 was 2a. Only 1 of 6 relapsed patients had been treated with interferon in combination with ribavirin. At baseline, HCV RNA load ranged from 3.5 log10 IU/mL to 7.1 log10 IU/mL, with HIV RNA <20 copies/mL in all patients, FIB-4 score >3.25 in 4 patients, CD4 cell count <100 cells/mm3 in 1 patient, and CD4 cell count <350 cells/mm3 in 2 patients. At HCV relapse, HCV RNA load ranged from 1.9 log10 IU/mL to 6.8 log10 IU/mL. All 6 patients had good adherence and maintained HIV RNA <20 copies/mL during study period. To identify which baseline characteristics were associated with HCV relapse at week 12 post-treatment, exploratory analysis was performed, which found baseline FIB-4 score >3.25 was an independent predictor (Tables S2 in the Supplementary Appendix). After adjusting for potential confounders, patients with FIB-4 score >3.25 had an 18-fold risk of HCV relapse at week 12 post-treatment than those with FIB-4 score ≤3.25. In receiver operating characteristic (ROC) analysis, the area under the ROC curve (AUROC) of FIB-4 score for the prediction of HCV relapse was 0.74 (95% CI: 0.47–1.00) (Figure S3 in the Supplementary Appendix).
Table 3.
Detailed characteristics of patients not achieving SVR12.
| Characteristics | Study patients |
|||||
|---|---|---|---|---|---|---|
| 06K003 | 06K005 | 06K019 | 06K035 | 18C2027 | 18K024 | |
| SVR 12 | No | No | No | No | No | No |
| Age, years | 38 | 47 | 42 | 50 | 51 | 55 |
| Gender | Female | Female | Male | Male | Male | Male |
| HCV/HIV transmission | PWID | Unknown | PWID | PWID | PWID | Unknown |
| Adherence | 100% | 100% | 100% | 100% | 100% | 100% |
| Adverse event | No | Constipation | Urinary tract infection | No | No | No |
| At baseline | ||||||
| HCV genotype | 3b | 6n | 3b | 2a | 3b | 3b |
| Previous anti-HCV treatment | No | Interferon plus ribavirin | No | No | No | No |
| HCV RNA, log10 IU/mL | 3.5 | 6.7 | 3.7 | 6.6 | 7.0 | 6.9 |
| HIV RNA | <20 copies/ml | <20 copies/ml | <20 copies/ml | <20 copies/ml | <20 copies/ml | <20 copies/ml |
| FIB-4 score | 0.71 | 1.33 | 4.32 | 3.47 | 4.28 | 3.56 |
| CD4 count | 640 | 1486 | 127 | 713 | 244 | 295 |
| At relapse | ||||||
| HCV RNA, log10 IU/mL | 6.7 | 5.7 | 1.9 | 6.3 | 5.3 | 6.8 |
| FIB-4 score | 1.07 | 1.97 | 6.58 | 2.11 | 3.87 | 2.43 |
| CD4 count | 971 | 762 | 145 | Unavailable | 448 | 236 |
| HIV RNA | <20 copies/ml | <20 copies/ml | <20 copies/ml | <20 copies/ml | <20 copies/ml | <20 copies/ml |
Abbreviations: HCV, hepatitis C virus; HIV, human immunodeficiency virus; PWID, people who inject drugs; FIB-4 Fibrosis 4 marker.
Safety
Adverse events were reported for 70 of 243 (29%) patients, including 61 patients with mild adverse events, 4 patients with moderate adverse events, 5 patients with severe adverse events (Table 4). No patients discontinued or interrupted sofosbuvir/velpatasvir or EVG/c/FTC/TAF due to adverse events, no patients died. The most common adverse events were upper respiratory tract infection (5%), followed by cough (3%), abnormal renal function (2%), abnormal liver function (2%), constipation (2%), urinary tract infection (2%) and sleep disorders (2%).
Table 4.
Adverse events and laboratory abnormalities.
| Patient n/N (%) | |
|---|---|
| Any adverse event | 70/243 (29%) |
| Severity of adverse events | |
| Mild | 61/243(26%) |
| Moderate | 4/243 (2%) |
| Severe | 5/243 (2%) |
| Any adverse event leading to discontinuation of study drug | 0/243 (0%) |
| Death | 0/243 (0%) |
| Adverse events that occurred in ≥2% of patients | |
| Upper respiratory tract infection | 11/243 (5%) |
| Cough | 8/243 (3%) |
| Abnormal renal function | 6/243 (2%) |
| Abnormal liver function | 5/243 (2%) |
| Constipation | 4/243 (2%) |
| Urinary tract infection | 4/243 (2%) |
| Sleep disorders | 4/243 (2%) |
All the 5 patients who experienced severe adverse events had achieved SVR12 and SVR24 and maintained undetectable HIV-1 RNA during study period. None of severe adverse event was considered to be related to treatment with sofosbuvir/velpatasvir or EVG/c/FTC/TAF. The first patient was a 42-year-old male current smoker, presented femoral head necrosis after 54 days of EVG/c/FTC/TAF and 19 days of sofosbuvir/velpatasvir treatment. This patient underwent right hip replacement and continued EVG/c/FTC/TAF treatment. The second patient was a 52-year-old male current smoker with diabetes mellitus who received nateglinide treatment, was hospitalized because of recurrent bilateral lower extremity edema after 162 days of EVG/c/FTC/TAF and 84 days of sofosbuvir/velpatasvir treatment. The renal puncture biopsy pathology proved to be diabetic glomerulosclerosis. The third patient, a 47-year-old male, was hospitalized due to type 2 diabetes after 140 days of EVG/c/FTC/TAF and 84 days of sofosbuvir/velpatasvir treatment. The fourth patient, a 28-year-old female, discovered an unintended pregnancy after 96 days of EVG/c/FTC/TAF and 68 days of sofosbuvir/velpatasvir treatment. This patient had a medication abortion to end the pregnancy. The obstetrician and gynecologist and investigators confirmed that the pregnancy and the medication abortion had ended after several reviews of routine blood tests, blood biochemistry, routine urine tests, urine pregnancy tests, and abdominal ultrasound examination. The fifth patient was a was a 36-year-old female, experienced an acute attack of chronic appendicitis after 96 days of EVG/c/FTC/TAF and 68 days of sofosbuvir/velpatasvir treatment. This patient was subsequently treated with an appendectomy and was discharged in good condition.
Discussion
In this multicenter, single-arm, open-label study, 12 weeks of sofosbuvir/velpatasvir as a simple and feasible treatment strategy, resulted in high rates of sustained virologic response and was well-tolerated in patients coinfected with HIV-1 and HCV, regardless of HCV genotypes nor prior interferon-based treatment. We found pretreatment FIB-4 score >3.25 was an independent predictor of HCV relapse, providing a simple and reliable method of pretreatment stratification for optimizing and individualizing treatment and care.
Although current treatment guidelines recommend the same direct-acting antiviral regimens for HCV/HIV coinfected patients as for those with HCV monoinfection,18 it remains controversial whether these two populations can achieve the same efficacy and safety in real-world clinical practices because some studies show inconsistent results.19, 20, 21, 22, 23 Consistent with the clinical trial conducted in the United States, we also found a high rate of SVR with sofosbuvir/velpatasvir treatment.12 More importantly, this trial included 80 (33%) patients with HCV genotype 6, the largest sample size of this genotype reported to date, and found that the efficacy in these patients was comparable to that of genotype 1. In addition, we enrolled 89 (37%) patients with HCV genotype 3, despite a few treatment failures, we found rate of SVR12 (95%) was not significantly different from those of genotype 1 (100%) and 6 (99%). Even in patients with sub genotypes 3b, which is traditionally considered difficult to treat, treatment response is still satisfied (Tables S3 in the Supplementary Appendix). Although only 2 of 3 (67%) patients with HCV genotype 2 achieved SVR12 and 3 of 4 (75%) patients achieved SVR24, due to limited sample size, this study was not powered to compare treatment response between HCV genotype 2 and other genotypes, thus we need to interpret this result with caution and should not simply conclude that patients with HCV genotype 2 have a lower response rate. The study by Feld et al. in a large sample of HCV monoinfected population found no significant difference in SVR12 rates between HCV genotypes 1, 2, 4, 5 and 6, and the study by Wyles et al. in an HCV/HIV coinfected population also showed no significant difference in SVR12 rates between genotypes 1, 2, 3 and 4. We believed the main reason for treatment failure may not be differences in HCV genotype but other causes such as liver fibrosis or cirrhosis.8,11,12 In areas with limited health resource, pre-treatment HCV genotype and frequent on-treatment HCV RNA testing are often unavailable, and many patients are not even able to complete monthly follow-up. The novel finding of this study is that 12 weeks of treatment with sofosbuvir/velpatasvir can provide high rates of SVR in HCV/HIV-1 coinfected patients regardless of HCV genotypes. This simple and feasible treatment strategy would help facilitate implementation in areas with limited health resource. However, it should be noted that the subjects of this study are non-cirrhotic patients. For cirrhotic patients, especially decompensated ones, the treatment strategy of sofosbuvir/velpatasvir remains to be determined. Should such patients be first treated with sofosbuvir/velpatasvir for 12 weeks or all should have 24 weeks? Or should ribavirin be added to sofosbuvir/velpatasvir 12 weeks. This is an important issue that needs further clarification.
The other novel finding is that FIB-4 score >3.25 could serve as an independent predictor of HCV relapse with an AUC of 0.74. This result suggests that noninvasive liver fibrosis score can be used in HCV/HIV coinfected patients to distinguish the potentially suboptimal responders. Although Wyles et al. found that all the 19 HCV/HIV coinfected patients with compensated cirrhosis achieved SVR12, it has been well documented that cirrhosis is an independent risk factor for poor treatment outcome of DAAs.11 Current guidelines have clearly stated that there are differences in treatment and monitoring strategies between cirrhotic and non-cirrhotic populations.18,24 However, simple and reliable diagnose of liver fibrosis and cirrhosis remains a great challenge in clinical practice. Liver biopsy is certainly the universally accepted “gold-standard”, but its widespread use is limited due to its invasive nature. Tests such as ultrasound and Fibroscan, which require well-trained technicians and specialized equipment, remain insufficient in Asia and Africa, where health resources are limited and significantly unevenly distributed. FIB-4 score, a non-invasive liver fibrosis assessment tool, can be obtained with routine blood testing, is highly accessible and has been well validated for the assessment of liver fibrosis, making it a simple and reliable tool for pre-treatment assessment.16 We excluded cirrhosis based on liver biopsy, FibroScan and APRI score because of safety concerns, however, 8% of patients still have FIB-4 score >3.25 at baseline. Due to the setting of eligible criteria, the efficacy of the FIB-4 and APRI scores for the diagnosis of advanced fibrosis and cirrhosis could not be directly compared in this study. However, the present study emphasized that the FIB-4 score, a non-invasive fibrosis scoring system with good accessibility and reliability, could be an ideal pre-treatment assessment tool for DAAs in HCV patients and can help clinicians to select an appropriate anti-HCV treatment and monitoring strategy. Therefore, the application value of the FIB-4 score deserves further validation in further real-world studies with large samples.
Consistent with previous study, we also found treatment response was similar in patients with and without interferon-based treatment experience.12 We further analyzed 23 patients previously treated with interferon-based regimens, classified according to their responses to previous interferon treatment (non-responder vs. relapse or breakthrough vs. early treatment discontinuation vs. unknown), and compared their SVR12 rates. As expected, we did not find a significant difference among these groups (data no shown). At the end of 12 weeks of sofosbuvir/velpatasvir treatment, we observed a significant decrease in FIB-4 score and APRI score, and a significant increase in CD4 cell count compared to baseline, while these parameters remained stable without further decrease or increase during the subsequent 24 weeks follow-up (Figure S4 in the Supplementary Appendix). Nonetheless, whether and how far sofosbuvir/velpatasvir treatment could improve liver fibrosis and liver function and contributes to immune reconstitution in HCV/HIV-infected patients on long-term antiretroviral therapy for HIV require further investigation.
Previous phase 1 study have demonstrated that sofosbuvir/velpatasvir increase plasma tenofovir exposure when co-administered with TDF and current guideline suggests that patients on a regimen containing TDF will need to be monitored for renal adverse events.24,25 The safety profile in the current study was in line with that observed for HCV-monoinfected and HCV/HIV coinfected patients treated with sofosbuvir/velpatasvir for 12 weeks.8,9,12 All of enrolled patients in the current study received EVG/c/FTC/TAF, a cobicistat-boosted regimens as antiretroviral drug. Thirty-six patients (36/243, 15%) were treated with concomitant medication(s), most of which were used to control blood glucose, blood pressure or treatment of upper respiratory infections. None of the 6 patients with HCV relapses was treated with concomitant medication. Among the 70 patients with adverse events, 32 (46%) had concomitant medication(s), which was mainly used to treat adverse events. There is no evidence to support the drug–drug interaction between the combination medications in this study and sofosbuvir/velpatasvir or EVG/c/FTC/TAF. In general, treatment with sofosbuvir/velpatasvir for 12 weeks was safe and well tolerated without any adverse event leading to discontinuation of study drug or death. The 5 severe adverse events were independent and scattered. None of the 5 patients underwent drug dose adjustment or died, showing a well tolerance of this drug combination. We will continue to follow up and closely observe their long-term outcome.
There are a few limitations in the present study. First, the HCV genotypes included in this study were mainly genotypes 1, 3 and 6, while genotypes 2, 4 and 5 were insufficient. However, this HCV genotype distribution is consistent with the previously reported distribution in China.26 Second, the number of patients with advanced fibrosis or cirrhosis is insufficient to definitively confirm the efficacy and safety of sofosbuvir/velpatasvir in these coinfected patients. This study was designed in 2016 when the phase III clinical trial of SOF/VEL in HCV-infected patients in China had not yet been completed, and in HCV/HIV-1 co-infected patients, the data of sofosbuvir/velpatasvir was very limited. Third, drug resistance genetic testing and pharmacokinetic data are lacking in this study. In addition, due to the influence of COVID19, the number of patients in this study did not reach the preset number of 300 cases, which may potentially affect the statistical power. Therefore, studies with more HCV genotypes, more patients with liver fibrosis or cirrhosis, especially decompensated cirrhosis, and better detection of drug resistance genes and pharmacokinetics are needed before generalizing the result of this study to the coinfected population.
In conclusion, 12 weeks of treatment with sofosbuvir/velpatasvir provide high rates of sustained virologic response and is well-tolerated in patients coinfected with HIV-1 and HCV regardless of HCV genotypes. Non-invasive liver fibrosis score may help to further distinguish patients at greater likelihood of a suboptimal response.
Contributors
Linghua Li, Weiping Cai, Ruichao Lu, Xicheng Wang, Jianbo Zhang, and Wen Kang contributed to the study design. Weiyin Lin, Xicheng Wang, Jianbo Zhang, Chunyan Wen, Wen Kang, Lin Mao, Jie Yang, Yanyun Dou, Liying Shi, Bianli Dang, Yun Lan, Hong Li, Yonghong Li, Xiejie Chen, Haolan He, Min Xu, Yaozu He, and Fengyu Hu served as study investigators and collected data. Weiyin Lin, Linghua Li, and Weiping Cai analyzed and interpreted the data. All authors provided critical revision and approval of the manuscript.
Data sharing statement
Data collected for the study can be made available by request. Please contact the corresponding author with all requests.
Declaration of interests
We declare no competing interests.
Acknowledgments
This study was made possible by the generous support of the 13th Five Year Plan of the Ministry of Science and Technology of China for the prevention and treatment of major infectious diseases such as AIDS and viral hepatitis (No. 2017ZX10202101-003). Work toward this paper was also funded by the National Key Research and Development Program of China (No. 2022YFC2304800), Medical Key Discipline Program of Guangzhou-Viral Infectious Diseases (2021–2023), Basic research program on people's Livelihood Science and technology of Guangzhou (No. 202002020005), and National Natural Science Foundation of China (82072265). Professor Sun Jian from Nanfang Hospital, Southern Medical University, provides important suggestions for this manuscript. Gilead Sciences, Inc. provided the study drugs of sofosbuvir/velpatasvir and EVG/c/FTC/TAF, however, they had no role on the protocol and this manuscript.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.lanwpc.2023.100749.
Contributor Information
Ruichao Lu, Email: ruichaolu@163.com.
Weiping Cai, Email: gz8hcwp@126.com.
Linghua Li, Email: llheliza@126.com.
Appendix A. Supplementary data
Figure S1.
Study design.
Figure S2.

Rates of HIV-1 suppression during study period. HIV, Human Immunodeficiency Virus.
Figure S3.
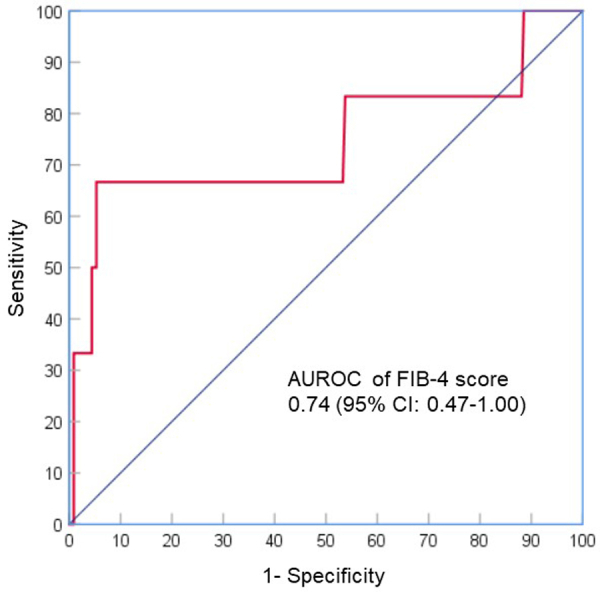
AUROC of FIB-4 score for the prediction of HCV relapse. AUROC, area under the receiver operator characteristic curve; FIB-4, Fibrosis 4; HCV, Hepatitis C Virus.
Figure S4.
Longitudinal changes of liver fibrosis, liver function and immune function during study period. Asterisk indicates a statistically significant difference (P values < 0.05). A, longitudinal changes of FIB-4 score. B, longitudinal changes of APRI score. C, longitudinal changes of ALT. D, longitudinal changes of CD4+ cell count. EOT, end of treatment; 12W POT, 12 weeks of post sofosbuvir/velpatasvir treatment; 24W POT, 24 weeks of post sofosbuvir/velpatasvir treatment; FIB-4, Fibrosis 4; APRI, aspartate aminotransferase to platelets; ALT, alanine aminotransferase; TBIL, total bilirubin; CD4, CD4+ cell count.
References
- 1.Platt L., Easterbrook P., Gower E., et al. Prevalence and burden of HCV co-infection in people living with HIV: a global systematic review and meta-analysis. Lancet Infect Dis. 2016;16(7):797–808. doi: 10.1016/S1473-3099(15)00485-5. [DOI] [PubMed] [Google Scholar]
- 2.Benhamou Y., Bochet M., Di Martino V., et al. Liver fibrosis progression in human immunodeficiency virus and hepatitis C virus coinfected patients. Hepatology. 1999;30(4):1054–1058. doi: 10.1002/hep.510300409. [DOI] [PubMed] [Google Scholar]
- 3.Graham C.S., Baden L.R., Yu E., et al. Influence of human immunodeficiency virus infection on the course of hepatitis C virus infection: a meta-analysis. Clin Infect Dis. 2001;33(4):562–569. doi: 10.1086/321909. [DOI] [PubMed] [Google Scholar]
- 4.Anderson K.B., Guest J.L., Rimland D. Hepatitis C virus coinfection increases mortality in HIV-infected patients in the highly active antiretroviral therapy era: data from the HIV Atlanta VA cohort study. Clin Infect Dis. 2004;39(10):1507–1513. doi: 10.1086/425360. [DOI] [PubMed] [Google Scholar]
- 5.Macías J., Berenguer J., Japón M.A., et al. Fast fibrosis progression between repeated liver biopsies in patients coinfected with human immunodeficiency virus/hepatitis C virus. Hepatology. 2009;50(4):1056–1063. doi: 10.1002/hep.23136. [DOI] [PubMed] [Google Scholar]
- 6.Price J.C., Thio C.L. Liver disease in the HIV–infected individual. Clin Gastroenterol Hepatol. 2010;8(12):1002–1012. doi: 10.1016/j.cgh.2010.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith C.J., Ryom L., Weber R., et al. Trends in underlying causes of death in people with HIV from 1999 to 2011 (D:A:D): a multicohort collaboration. Lancet. 2014;384(9939):241–248. doi: 10.1016/S0140-6736(14)60604-8. [DOI] [PubMed] [Google Scholar]
- 8.Feld J.J., Jacobson I.M., Hézode C., et al. Sofosbuvir and velpatasvir for HCV genotype 1, 2, 4, 5, and 6 infection. N Engl J Med. 2015;373(27):2599–2607. doi: 10.1056/NEJMoa1512610. [DOI] [PubMed] [Google Scholar]
- 9.Foster G.R., Afdhal N., Roberts S.K., et al. Sofosbuvir and velpatasvir for HCV genotype 2 and 3 infection. N Engl J Med. 2015;373(27):2608–2617. doi: 10.1056/NEJMoa1512612. [DOI] [PubMed] [Google Scholar]
- 10.Curry M.P., O Leary J.G., Bzowej N., et al. Sofosbuvir and velpatasvir for HCV in patients with decompensated cirrhosis. N Engl J Med. 2015;373(27):2618–2628. doi: 10.1056/NEJMoa1512614. [DOI] [PubMed] [Google Scholar]
- 11.Mangia A., Milligan S., Khalili M., et al. Global real-world evidence of sofosbuvir/velpatasvir as simple, effective HCV treatment: analysis of 5552 patients from 12 cohorts. Liver Int. 2020;40(8):1841–1852. doi: 10.1111/liv.14537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wyles D., Bräu N., Kottilil S., et al. Sofosbuvir and velpatasvir for the treatment of hepatitis C virus in patients coinfected with human immunodeficiency virus type 1: an open-label, phase 3 study. Clin Infect Dis. 2017;65(1):6–12. doi: 10.1093/cid/cix260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zaidi A.K.M., Awasthi S., DeSilva H.J. Burden of infectious diseases in South Asia. BMJ. 2004;328(7443):811–815. doi: 10.1136/bmj.328.7443.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wong M.C.S., Huang J.L.W., George J., et al. The changing epidemiology of liver diseases in the Asia-Pacific region. Nat Rev Gastroenterol Hepatol. 2019;16(1):57–73. doi: 10.1038/s41575-018-0055-0. [DOI] [PubMed] [Google Scholar]
- 15.Wai C., Greenson J.K., Fontana R.J., et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38(2):518–526. doi: 10.1053/jhep.2003.50346. [DOI] [PubMed] [Google Scholar]
- 16.Sterling R.K., Lissen E., Clumeck N., et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006;43(6):1317–1325. doi: 10.1002/hep.21178. [DOI] [PubMed] [Google Scholar]
- 17.Naggie S., Cooper C., Saag M., et al. Ledipasvir and sofosbuvir for HCV in patients coinfected with HIV-1. N Engl J Med. 2015;373(8):705–713. doi: 10.1056/NEJMoa1501315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ghany M.G., Morgan T.R. Hepatitis C guidance 2019 update: American Association for the Study of Liver Diseases–Infectious Diseases Society of America recommendations for testing, managing, and treating hepatitis C virus infection. Hepatology. 2019;71(2):686–721. doi: 10.1002/hep.31060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arias A., Aguilera A., Soriano V., et al. Rate and predictors of treatment failure to all-oral HCV regimens outside clinical trials. Antivir Ther. 2017;22(4):307–312. doi: 10.3851/IMP3061. [DOI] [PubMed] [Google Scholar]
- 20.Boesecke C., Ingiliz P., Berger F., et al. Liver cirrhosis as a risk factor for direct-acting antiviral therapy failure in real-Life hepatitis C virus/human immunodeficiency virus coinfection. Open Forum Infect Dis. 2017;4(3) doi: 10.1093/ofid/ofx158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gayam V., Hossain M.R., Khalid M., et al. Real-world clinical efficacy and tolerability of direct-acting antivirals in hepatitis C monoinfection compared to hepatitis C/human immunodeficiency virus coinfection in a community care setting. Gut Liver. 2018;12(6):694–703. doi: 10.5009/gnl18004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tapper E.B., Bacon B.R., Curry M.P., et al. Real-world effectiveness for 12 weeks of ledipasvir-sofosbuvir for genotype 1 hepatitis C: the Trio Health study. J Viral Hepat. 2017;24(1):22–27. doi: 10.1111/jvh.12611. [DOI] [PubMed] [Google Scholar]
- 23.Tang L., Parker A., Flores Y., et al. Treatment of hepatitis C with 8 weeks of ledipasvir/sofosbuvir: highly effective in a predominantly black male patient population. J Viral Hepat. 2018;25(2):205–208. doi: 10.1111/jvh.12796. [DOI] [PubMed] [Google Scholar]
- 24.Pawlotsky J., Negro F., Aghemo A., et al. EASL recommendations on treatment of hepatitis C 2018. J Hepatol. 2018;69(2):461–511. doi: 10.1016/j.jhep.2018.03.026. [DOI] [PubMed] [Google Scholar]
- 25.Mogalian E., Stamm L.M., Osinusi A., et al. Drug–drug interaction studies between hepatitis C virus antivirals sofosbuvir/velpatasvir and boosted and unboosted human immunodeficiency virus antiretroviral regimens in healthy volunteers. Clin Infect Dis. 2018;67(6):934–940. doi: 10.1093/cid/ciy201. [DOI] [PubMed] [Google Scholar]
- 26.Du G., Li X., Musa T.H., et al. The nationwide distribution and trends of hepatitis C virus genotypes in mainland China. J Med Virol. 2019;91(3):401–410. doi: 10.1002/jmv.25311. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.