Summary
Background
Evidence on the associations between long-term exposure to multiple air pollutants and cardiopulmonary mortality is limited, especially for developing regions with higher pollutant levels. We aimed to characterise the individual and joint (multi-pollutant) associations of long-term exposure to air pollutants with cardiopulmonary mortality, and to identify air pollutant that primarily contributes to the mortality risk.
Methods
We followed 37,442 participants with a mean age of 43.5 years in four cities in northern China (Tianjin, Shenyang, Taiyuan, and Rizhao) from January 1998 to December 2019. Annual particulate matter (PM) with diameters ≤2.5 μm (PM2.5), ≤10 μm (PM10), sulfur dioxide (SO2) and nitrogen dioxide (NO2) were estimated using daily average values from satellite-derived machine learning models and monitoring stations. Time-varying Cox proportional hazards model was used to evaluate the individual association between air pollutants and mortality from non-accidental causes, cardiovascular diseases (CVDs), non-malignant respiratory diseases (RDs) and lung cancer, accounting for demographic and socioeconomic factors. Effect modifications by age, sex, income and education level were also examined. Quantile-based g-Computation integrated with time-to-event data was additionally applied to evaluate the co-effects and the relative weight of contributions for air pollutants.
Findings
During 785,807 person-years of follow-up, 5812 (15.5%) died from non-accidental causes, among which 2932 (7.8%) were from all CVDs, 479 (1.3%) from non-malignant RDs, and 552 (1.4%) from lung cancer. Long-term exposure to PM10 (mean [baseline]: 136.5 μg/m3), PM2.5 (mean [baseline]: 70.2 μg/m3), SO2 (mean [baseline]: 113.0 μg/m3) and NO2 (mean [baseline]: 39.2 μg/m3) were adversely and consistently associated with all mortality outcomes. A 10 μg/m3 increase in PM2.5 was associated with higher mortality from non-accidental causes (hazard ratio 1.20; 95% confidence interval 1.17–1.23), CVDs (1.23; 1.19–1.28), non-malignant RDs (1.37; 1.25–1.49) and lung cancer (1.14; 1.05–1.23). A monotonically increasing curve with linear or supra-linear shape with no evidence of a threshold was observed for the exposure-response relationship of mortality with individual or joint exposure to air pollutants. PM2.5 consistently contributed most to the elevated mortality risks related to air pollutant mixture, followed by SO2 or PM10.
Interpretation
There was a strong and positive association of long-term individual and joint exposure to PM10, PM2.5, SO2, and NO2 with mortalities from non-accidental causes, CVDs, non-malignant RDs and lung cancer in high-exposure settings, with PM2.5 potentially being the main contributor. The shapes of associations were consistent with a linear or supra-linear exposure-response relationship, with no lower threshold observed within the range of concentrations in this study.
Funding
National Key Research and Development Program of China, the China Scholarship Council, the National Natural Science Foundation of China, Natural Science Foundation of Guangdong Province.
Keywords: Air pollution, Mortality, Cohort study, Cardiorespiratory disease, Lung cancer, Joint association
Research in context.
Evidence before this study
We searched Medline and PubMed without language restrictions for studies published up to November 2022, using the search terms “air pollution”, “long-term”, “cohort”, “mortality” and “cardiovascular” or “respiratory” or “lung cancer”. Abstracts and full texts were then screened for relevant studies. We found that epidemiological evidence from relevant cohort studies is mostly from developed regions with low air pollution levels (e.g., Western Europe and North America), especially for cause-specific mortalities, while the evidence from low- and middle-income countries (LMICs) with much higher air pollution levels is still relatively limited. An increasing number of cohort studies have been conducted in China in the past 2 years, while these studies generally focused on either single pollutant or specific mortality outcome. Additionally, the results (e.g., exposure-response relationship) had a high degree of heterogeneity, and uncertainty from limited consideration for co-exposures of air pollutants.
Added value of this study
In this large population-based Chinese cohort with a follow-up of 22 years (785,807 person-years), we characterised the mortality pattern of non-accidental causes, cardiovascular diseases, non-malignant respiratory diseases and lung cancer associated with long-term individual and joint exposure to PM2.5, PM10, SO2, and NO2, accounting for the time-varying exposures and individual demographic and socioeconomic factors. Additionally, we applied Quantile-based g-Computation integrated with time-to-event data to identify the potential air pollutant that primarily contributes to the elevated mortality risk. We found a consistently elevated risk of cardiopulmonary mortality associated with both single or combined long-term exposure to PM2.5, PM10, SO2, and NO2, with monotonically increasing linear or supra-linear non-threshold curves observed for the exposure-response relationships. PM2.5 consistently contributed most to the elevated mortality risks related to air pollutant mixture, followed by SO2 or PM10.
Implications of all the available evidence
The study supports a strong and robust adverse association that may be underestimated previously between long-term exposure to air pollutants and cardiopulmonary mortality in LMICs with high-exposure settings. The shapes of associations were consistent with a linear or supra-linear exposure-response relationship, with no lower threshold observed within the range of concentrations in high-exposure settings. The findings from this study contribute to very limited evidence on the pattern of cardiopulmonary mortality due to long-term individual and joint exposure to air pollutants for high exposure settings typical in developing countries.
Introduction
Outdoor air pollution is the leading environmental contributor to the global disease burden. It is estimated that more than 4.2 million premature deaths were attributable to ambient air pollution worldwide per year, with about 91% occurring in low- and middle-income countries (LMICs), especially those in South-East Asia and Western Pacific regions, including China.1 Though the LMICs bear a disproportionately high disease burden due to air pollution, the majority of the evidence on long-term exposure to outdoor air pollution and mortality, especially for cause-specific mortalities, was from Western Europe and North America.2 A positive association and non-threshold exposure-response relationship has been well established in these regions, especially for particulate matters (PM).2,3 However, these regions have comparatively low exposure settings around or below the air quality guidelines for the European Union or the United States (US).2,3 Evidence from LMICs with much higher air pollution levels and different sources of pollution is still relatively limited,4,5 leading to gaps in the knowledge about the shape of the exposure-response (ER) relationships between air pollutants and mortality at higher exposure levels, which is important for informing future policy in countries where the greatest health burden exists.3
As the largest LMIC, China has experienced rapid changes of air pollution exposure levels with a broad range over the past decades.6, 7, 8 An increasing number of cohort studies have been conducted in China to characterise the ER relationships between chronic exposure to air pollutants and mortality in the past 2 years.9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19 These studies suggested that long-term exposure to ambient air pollution, particularly PM2.5, was associated with increased risks of various adverse health outcomes, including lung cancer, chronic obstructive pulmonary disease, cardiovascular mortality, and all-cause mortality. However, the studies generally focused on either single pollutant or specific mortality outcome (e.g., all-cause/non-accidental mortality), possibly due to the data unavailability, limited number of cases and/or the strong collinearity between air pollutants. To our knowledge, there are no published studies on the joint effects of chronic exposure to air pollutants on individual causes of death.
Because people are exposed to complex and varying pollutant mixtures throughout their life course, multi-pollutant models, which consider the potential synergistic linear or non-linear effects among air pollutants, may be more appropriate for estimating ER functions.20, 21, 22 Further, previous studies have been generally based on air pollution at baseline, which did not allow for changes in air pollution exposure levels and participant characteristics throughout the follow-up period. While empirical knowledge on the shape of the ER relationship at higher exposures has grown appreciably in the last 5 years, the extent to which findings to date resemble those using alternative model specifications to account for these issues is unknown. Investigation into these research gaps will help develop policies specific to such settings.
In the context of a Chinese cohort with 22 years of follow-up, we aimed to utilize an integrated multi-pollutant model with time-varying Cox analysis to investigate the associations of long-term exposure to PM with an aerodynamic diameter of equal to or less 10 μm (PM10), equal to or less 2.5 μm (PM2.5), sulfur dioxide (SO2), nitrogen dioxide (NO2) with mortalities from non-accidental causes, cardiovascular diseases (CVDs), non-malignant respiratory diseases (RDs) and lung cancer, and to identify potential vulnerable subpopulations and main contributor of air pollutant.
Methods
Study setting and population
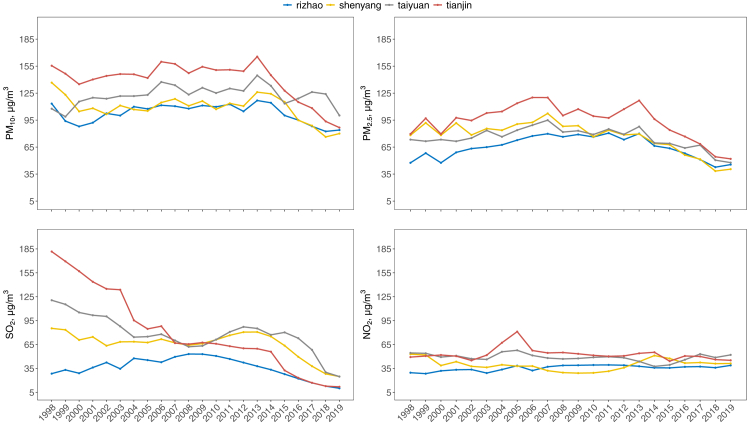
The study was based on data collected between January 1998 and December 2019 in four cities selected to be typical of those in northern China: Tianjin, Shenyang, Taiyuan, and Rizhao (Fig. S1). These cities represent a wide range of air pollution sources and concentrations (Fig. 1). Tianjin is one of the national central cities and was one of the first, mainly coal-consuming, heavy industrial cities in China. Tianjin had a population of 15.6 million and an area of 11,917 km2 in 2019. Shenyang is the capital of Liaoning province and the largest city, economic centre in Northeast China with a total area of 12,860 km2 and a population of 7.6 million in 2019. Taiyuan is the capital of Shanxi province and produces the most coal in China. It had a population of 4.5 million and an area of 6988 km2 in 2019. Rizhao is an emerging port city with a population of 2.9 million and an area of 5359 km2 in 2019.
Fig. 1.
Annual average exposure (μg/m3) of PM10, PM2.5, SO2, and NO2 of the cohort participants in four cities from 1998 to 2009.
For each city, we randomly selected participants from small neighbourhoods (e.g., street blocks or apartment buildings) in residential and commercial areas. Individuals born prior to January 1, 1975, and lived in the defined area since January 1, 1998 were included as study participants. Socio-demographic information, residential addresses, smoking or drinking status, occupational exposures, and exercise level for leisure were collected for these participants at baseline (1998) and at follow-up (2009) from local neighbourhood offices and via standard questionnaires by trained interviewers. From the initial 39,054 participants who completed the study, 1576 (4.0%) were excluded due to missing data on covariates, resulting in a total of 37,478 participants in the study. The residence for the majority of the cohort (81.2%) did not change from January 1998 to the follow-up, whereas a move within the city was observed for the rest of the residents. The ethical committee of the coordinating centre of Tianjin Medical University approved the study. Written informed consents were obtained from all participants.
Mortality outcomes definition
The mortality outcomes for each participant were followed up until 31 December 2019 or their date of death. Detailed information on death (e.g., time, place, and cause of death) was obtained from the questionnaires completed by the family members of deceased subjects and the Centre for Disease Control and Prevention (CDC). The mortality outcome was coded and defined based on the International Classification of Disease 10th revision (ICD-10). Four mortality outcomes were selected: non-accidental causes (ICD-10: A00‒R99), CVDs (ICD-10: I10‒I70), non-malignant RDs (ICD-10: J00‒J99), and lung cancer (ICD-10: C34).23 To investigate the long-term (>1 year) effects of air pollutants, individuals who died within 1 year of the baseline were excluded (N = 36), yielding a final of 37,442 participants for the analysis.
Ambient air pollutants assessment
Ambient exposures of PM10 and PM2.5 from 2000 to 2019, and SO2 and NO2 from 2013 to 2019 were assessed using gridded (∼10 km) air pollution datasets for China, which were derived from machine learning models with predictors of ground measurements, satellite retrievals, emissions, chemical transport model simulations, and other sources.24, 25, 26, 27 These datasets have been shown high quality (cross-validation coefficients of determination 0.80–0.93; root-mean-square error 4.89–24.28 μg/m3) in mainland China and were widely used in previous studies on health impact assessment of air pollution.8,28, 29, 30, 31, 32, 33, 34 As these datasets did not include PM before 2000, as well as SO2 and NO2 before 2013, we estimated PM10 between 1998 and 1999, and SO2 and NO2 between 1998 and 2009 using the concentrations from the monitor stations (within 10 km of home) during these years calibrated with a ratio calibration factor (the ratio of the concentrations of model-derived air pollutants to those of the monitoring stations during the year when both of them were available). For years that monitoring and modelled air pollutants were both unavailable (PM2.5 between 1998 and 1999, SO2 and NO2 between 2010 and 2012), we imputed them with a natural spline function of the available data for the other years.35 A 10-km buffer around the geocoded residential addresses was used as the primary buffer to quantify the annual ambient exposures of air pollutants for each participant between 1998 and 2019. Annual average ambient air pollution before the death date or end of follow-up was defined as the primary long-term exposure for each individual.36, 37, 38
Statistical analysis
For the descriptive analysis, we characterised distributions for all variables and used the mean ± standard deviation (SD) to describe normally distributed continuous variables and the t-test to compare between-group differences. Categorical variables were compared between groups using a chi-square test. Spearman rank tests were used to test the pairwise correlations among air pollutants.
In the main analyses, we applied a time-varying Cox proportional hazards model adjusted for age at baseline (in years), sex (female vs. male), body mass index (BMI, kg/m2), level of education (<High school vs. ≥High school), monthly income level (<1500 Yuan vs. ≥1500 Yuan), occupational exposure (self-reported exposure to dust and fumes in the workplace, yes vs. no), physical exercise (active vs. non-active), marital status (married vs. single/separated/divorced), and current smoking (yes vs. no) and alcohol consumption (yes vs. no) to investigate the associations between mortality and air pollutants.39 A random intercept of city was also included in the model to accommodate any residual spatial clustering. Considering the long-term time trend (e.g., changes in medical treatment level and death reporting accuracy over the long study period), we used calendar year as the time scale in the Cox model.40 Air pollutant exposures, income, BMI, physical exercise and current status of smoking and drinking were included in the models as time-dependent variables. The follow-up period began on 1 January 1998 and ended on 31 December 2019 or at the date of death for each participant, whichever came first. The results are presented as hazard ratios (HRs) for mortality from non-accidental causes, CVDs, non-malignant RDs and lung cancer, and their corresponding 95% confidence intervals (CIs), corresponding to a 10 μg/m3 increase in air pollutants (PM10, PM2.5, SO2, NO2).
A set of sensitivity analyses were conducted to check the robustness of the results. Firstly, as a common practice in cohort studies, we repeated the analysis using a baseline model, in which a Cox proportional hazards model was fitted with baseline air pollutants and other covariates. Secondly, to examine the influence of selected confounders on our results, we re-fitted the main model with different sets of covariates: Model 1 (Crude), adjusted for age and sex; Model 2, additionally adjusted for individual socioeconomic status (SES) including level of education, income, and marital status; and Model 3, additionally adjusted for BMI and physical exercise. Thirdly, we repeated the analysis with city-level air pollutions to investigate the possible bias due to the potential move within the city of the study participants. Fourthly, we used different imputation functions (linear, Stineman, or periodic) for missing data on PM2.5 and SO2 and re-conducted the main analysis.41,42 Fifthly, to evaluate the possible bias from excluding participants due to missing covariates at follow-up, we conducted an inverse probability weighting analysis.43 We included the inverse probabilities of being included in the main analyses, which were estimated by using baseline covariates as predictors, as a weight in the Cox model. Sixthly, we utilized multivariate imputation by chained equations for missing covariates at baseline and follow-up with 10 multiple imputations and 50 iterations.44, 45, 46 Estimates from the main analyses re-conducted with the 10 imputed complete datasets were then pooled according to Rubin’s rules.47 Seventhly, we obtained annual ozone estimates from 1998 to 2019 which were used in the Global Burden of Disease (GBD) 2019 study and additionally adjust ozone in the main model to examine the influence of ozone on the results.48 Finally, we considered multiple exposure time windows, including average exposure in the 1–5 years prior to death or end of follow-up.
To visualize the ER curve of the associations between PM10, PM2.5, SO2, NO2, and mortality outcomes, we further re-fitted the main models with a penalized spline, a variation of basis spline that is robust against the number of knots and knot placements, for the pollutant variable instead of a linear term.49
We also examined the potential effect modification of the associations between long-term exposure to PM10, PM2.5, SO2, NO2 and mortality by introducing an interaction term of each air pollutant and effect modifier in the model and testing with the Wald test. Pre-specified modifiers include age categories at baseline classified by Chinese retirement age (<60 years or ≥60 years), sex, education (<High school or ≥High school), and income level (<1500 Yuan/month or ≥1500 Yuan/month).
To assess the joint associations between air pollutants and mortality and the relative contribution of each air pollutant, we performed multi-pollutant analysis using Quantile-based g-Computation.50 Compared to the traditional multi-pollutant model of weighted quantile sum (WQS) regression, this approach further integrates its estimation procedure with g-Computation in a marginal structural model that overcomes the assumption of uni-direction and can incorporate non-linearities for the joint exposures.50 Additionally, it allows including Cox proportional hazards models as the underlying model for time-to-event analysis. Air pollutants (PM10, PM2.5, SO2, NO2) were included in the multi-pollutant analysis with a quantile number of 20 in an underlying model of time-varying Cox proportional hazards, with the adjustment of the same confounders as in the main analysis, to assess their relative contribution weights. The air pollutants with positive weight were further modelled as air pollutant mixture for the associations with mortality. We used a quantile number of 20 to create quantile indicators representing the air pollutants exposures. A total of 1000 Monte Carlo simulations were adopted to obtain accurate point estimates and 500 bootstraps to calculate the 95% CIs.51
All statistical analyses were conducted in R v4.0.3 (R Development Core Team). A two-tailed P-value less than 0.05 was considered statistically significant.
Role of the funding source
The funders had no role in study design, data collection, data analysis and interpretation, or report writing.
Results
Study population and exposure
Table 1 shows the baseline characteristics of the included participants in the cohort of four northern Chinese cities by outcome (alive or dead). A total of 37,442 participants with a mean age of 43.5 years (SD: 13.4) at the baseline were included in the final analyses. The average BMI at baseline was 22.6 kg/m2 (SD: 2.95). Among the included participants, 50.7% were female, and 24.9% and 20.2% were current smokers and alcohol drinkers at baseline, respectively. During a mean follow-up time of 21.0 (SD: 3.12) years or 785,807 person-years, 5812 (15.5%) died from non-accidental causes. Among these deaths, 2932 were from all CVDs, 479 from non-malignant RDs, and 552 from lung cancer. Compared to individuals who were alive at the end of follow-up, participants who had died were more likely to be male, older, single/separated/divorced, current smokers or drinkers, have occupational exposures, lower education level and higher BMI or income level (Table 1).
Table 1.
Baseline characteristics of the participants from the four-city cohort in Northern China by the status (alive or dead) from the baseline (1998) to 2019.
| Characteristics | Total (N = 37,442) | Censored (N = 31,630) | Deada (N = 5812) |
|---|---|---|---|
| Age at baseline (years), mean (SD)b | 43.5 (13.4) | 43.0 (13.2) | 46.2 (14.1) |
| BMI, mean (SD)b | 22.6 (2.95) | 22.6 (2.96) | 22.9 (2.92) |
| Sex, n (%)b | |||
| Male | 18,456 (49.3) | 15,250 (48.2) | 3206 (55.2) |
| Female | 18,986 (50.7) | 16,380 (51.8) | 2606 (44.8) |
| Education level, n (%)b | |||
| <High school | 21,476 (57.4) | 17,801 (56.3) | 3675 (63.2) |
| ≥High school | 15,966 (42.6) | 13,829 (43.7) | 2137 (36.8) |
| Income level per month, n (%)b | |||
| <1500 Yuan | 35,115 (93.8%) | 29,770 (94.1%) | 5345 (92.0%) |
| ≥1500 Yuan | 2327 (6.21%) | 1860 (5.88%) | 467 (8.04%) |
| Occupational exposure, n (%)b | 2705 (7.22) | 2197 (6.95) | 508 (8.74) |
| Marital status, n (%)b | |||
| Married | 34,455 (92.0) | 29,187 (92.3) | 5268 (90.6) |
| Single/separated/divorced | 2987 (7.98) | 2443 (7.72) | 544 (9.36) |
| Smoking status, n (%)b | |||
| Never | 27,124 (72.4) | 23,133 (73.1) | 3991 (68.7) |
| Former | 989 (2.64) | 815 (2.58) | 174 (2.99) |
| Current | 9329 (24.9) | 7682 (24.3) | 1647 (28.3) |
| Alcohol intake, n (%)b | 7558 (20.2) | 6266 (19.8) | 1292 (22.2) |
| Exercise, n (%) | 18,880 (50.4) | 15,918 (50.3) | 2962 (51.0) |
Abbreviations: BMI, body mass index; SD, standard deviation.
Mortality from all non-accidental causes.
P < 0.05 for difference between alive and dead groups.
Fig. 1 shows the annual average exposure of four air pollutants (PM10, PM2.5, SO2, and NO2) for the participants in four study cities from 1998 to 2019. For each air pollutant, there were generally high but similar decreasing trends of exposure levels across the four cities, especially for PM10, PM2.5, and SO2. From 1998 to 2019, the overall annual average concentration changed from 136.47 μg/m3 (SD: 27.1) to 87.66 μg/m3 (SD: 7.7) for PM10, 70.16 μg/m3 (SD: 13.2) to 46.55 μg/m3 (SD: 4.1) for PM2.5, 113.03 μg/m3 (SD: 88.6) to 18.0 μg/m3 (SD: 6.9) for SO2, and 39.20 μg/m3 (SD: 16.1) to 44.34 μg/m3 (SD: 5.1) for NO2. The greatest decline was observed for SO2, followed by PM10 and PM2.5. There were generally mild to modest positive correlations between air pollutants (correlation coefficient ranged from 0.03 to 0.55, Table S1).
Associations between air pollution and mortality
Single pollutant model
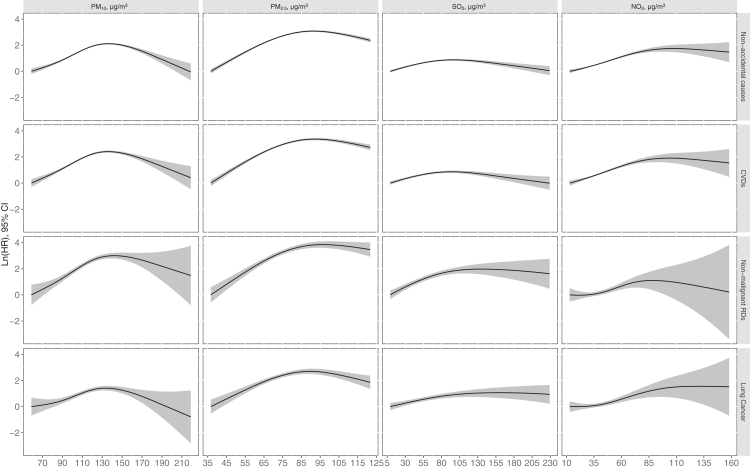
Table 2 shows the results from the time-varying Cox proportional hazards models for the associations between air pollutants and mortality. We observed generally consistent and positive associations between PM10, PM2.5, SO2, and NO2 and non-accidental mortality, CVD mortality, as well as non-malignant RDs and lung cancer mortality. HRs for non-accidental mortality were 1.19 (95% CI: 1.17–1.21), 1.20 (1.17–1.23), 1.04 (1.03–1.05), and 1.24 (1.21–1.27) per 10 μg/m3 increment in PM10, PM2.5, SO2, and NO2, respectively. Similar HRs were found for CVD mortality. In comparison, stronger associations were observed for PM10, PM2.5, SO2, and non-malignant RDs mortality. When additionally adjusted for ozone, the HRs of PM10 and SO2 did not change apparently, while the HRs of NO2 and PM2.5 increased slightly (Table S2). The results were generally robust in models with inverse probability weighting, and to different sets of adjusted covariates, different calibration approaches, different interpolation functions and exposure time windows (Tables S3‒S7). The models using the full cohort with multivariate imputation also presented almost identical results to those in the main analysis (Table S8). However, the effect estimates changed materially and generally tend to be smaller in models using city-level or baseline air pollutants (Tables S9‒S10). Fig. 2 further depicts the ER curve of mortality risks associated with four air pollutants. The associations between PM10, PM2.5, SO2, and NO2 and mortality from non-accidental causes, CVDs, non-malignant RDs and lung cancer generally showed a non-threshold linear or supra-linear shape.
Table 2.
Associations between per 10 μg/m3 increase in PM10, PM2.5, SO2, NO2 and mortality from non-accidental causes, CVDs, non-malignant RDs and lung cancer (N = 37,442).
| Air pollutants | HR (95% CI)a |
|||
|---|---|---|---|---|
| Non-accidental causes | CVDs | Non-malignant RDs | Lung cancer | |
| PM10 | 1.19 (1.17, 1.21) | 1.20 (1.17, 1.23) | 1.30 (1.23, 1.39) | 1.11 (1.05, 1.17) |
| PM2.5 | 1.20 (1.17, 1.23) | 1.23 (1.19, 1.28) | 1.37 (1.25, 1.49) | 1.14 (1.05, 1.23) |
| SO2 | 1.04 (1.03, 1.05) | 1.04 (1.03, 1.06) | 1.11 (1.07, 1.16) | 1.05 (1.02, 1.08) |
| NO2 | 1.24 (1.21, 1.27) | 1.26 (1.22, 1.31) | 1.17 (1.06, 1.28) | 1.16 (1.08, 1.26) |
Abbreviations: CVDs, cardiovascular diseases; CI, confidence interval; HR, hazard ratio; NO2, nitrogen dioxide; PM10, particulate matter with aerodynamic diameter ≤10 μm; PM2.5, particulate matter with aerodynamic diameter ≤2.5 μm; RDs, respiratory diseases; SO2, sulfur dioxide.
Adjusted for age, sex, BMI, level of education, income, occupational exposure, physical exercise and status of marital, smoking and drinking.
Fig. 2.
Exposure-response curve for the association of PM10, PM2.5, SO2, and NO2 with mortality from non-accidental causes, CVDs, non-malignant RDs and lung cancer (N = 37,442). Models were adjusted for age, sex, BMI, level of education, income, occupational exposure, physical exercise and status of marital, smoking and drinking. Abbreviations: CVDs, cardiovascular diseases; CI, confidence interval; HR, hazard ratio; NO2, nitrogen dioxide; PM10, particulate matter with aerodynamic diameter ≤10 μm; PM2.5, particulate matter with aerodynamic diameter ≤2.5 μm; RDs, respiratory diseases; SO2, sulfur dioxide.
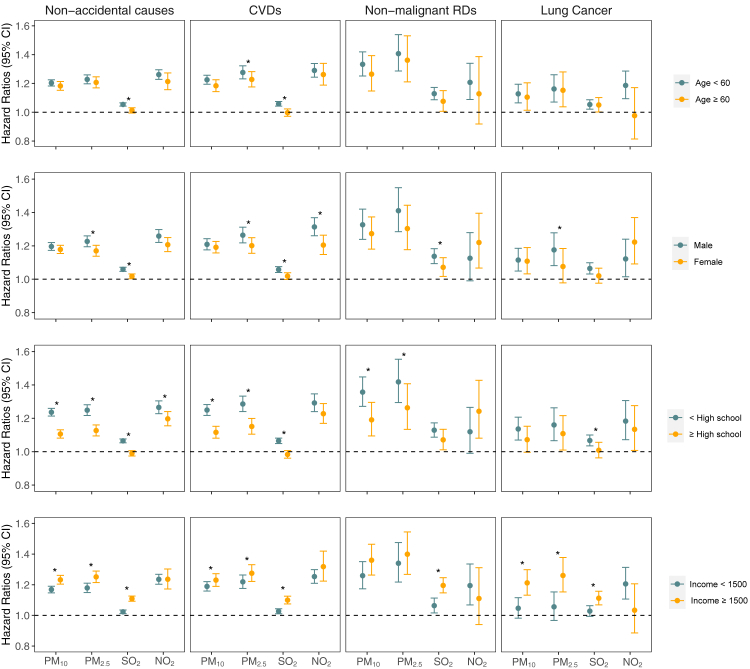
Fig. 3 exhibits the results of stratification analysis by age, sex, education, and income level. In stratified analysis by age, we found consistent and similar elevated risks of mortality from non-accidental causes, CVDs, non-malignant RDs and lung cancer associated with PM10, and PM2.5 among the different age subgroups. Generally, no modification effect of age on the associations was observed. Regarding the effect modification by sex, we found generally stronger associations for PM2.5 and SO2 with mortality outcomes among males compared with female participants, but without heterogeneous effects for PM10 and NO2. Level of education modified associations of mortality with PM10, PM2.5, and SO2 where associations were adverse and generally stronger for participants with lower education level. Significant interactions were also generally found for income with PM10, PM2.5, and SO2 on mortality outcomes, with stronger and adverse associations among those with higher income compared to those with lower income. No heterogeneous effects were observed for NO2 with mortality among subgroups of different income levels.
Fig. 3.
Association of per 10 μg/m3 increase in PM10, PM2.5, SO2, and NO2 with mortality from non-accidental causes, CVDs, non-malignant RDs and lung cancer, stratified by age, sex, education, and income level (N = 37,442). Abbreviations: CVDs, cardiovascular diseases; CI, confidence interval; HR, hazard ratio; NO2, nitrogen dioxide; PM10, particulate matter with aerodynamic diameter ≤10 μm; PM2.5, particulate matter with aerodynamic diameter ≤2.5 μm; RDs, respiratory diseases; SO2, sulfur dioxide. ∗Interaction is statistically significant (P < 0.05).
Multi-pollutant model
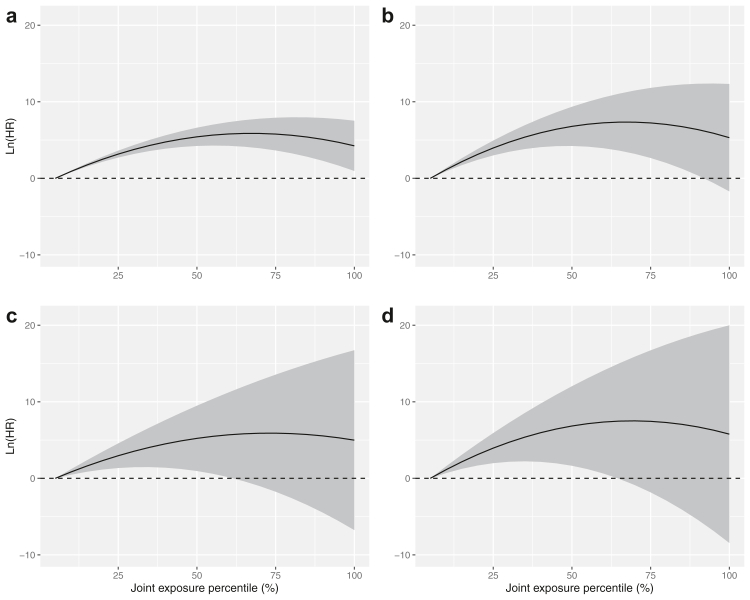
In multi-pollutant analysis, we found that PM2.5 consistently contributed most to the adverse associations between air pollutants (PM10, PM2.5, SO2, and NO2) and mortality, followed by SO2 for mortality from non-malignant RDs and lung cancer or PM10 for mortality from non-accidental causes and CVDs, while the adverse effects of NO2 were largely attenuated and covered by other air pollutants (Fig. S2). We found non-threshold monotonically increasing curves for non-accidental mortality, CVDs mortality, non-malignant RDs and lung cancer mortality, where HRs increased with respect to percentiles of air pollutant mixture and levelling out at around median level (i.e., 65% of the maximum concentration of each air pollutant: 124.1 μg/m3 for PM10, 82.7 μg/m3 for PM2.5, 75.2 μg/m3 for SO2, and 43.5 μg/m3 for NO2) (Fig. 4 and Table S11). However, large uncertainties could be seen at a high level of air pollutant mixtures as reflected by wide CI, especially for non-malignant RDs and lung cancer mortality.
Fig. 4.
Associations of mortality from non-accidental causes (a), CVDs (b), non-malignant RDs (c), and lung cancer (d) with percentiles of joint exposure of PM10, PM2.5, SO2, and NO2. The shaded regions are 95% CIs (N = 37,442). Models were adjusted for age, sex, BMI, level of education, income, occupational exposure, physical exercise and status of marital, smoking and drinking. Abbreviations: CVDs, cardiovascular diseases; CI, confidence interval; HR, hazard ratio; NO2, nitrogen dioxide; PM10, particulate matter with aerodynamic diameter ≤10 μm; PM2.5, particulate matter with aerodynamic diameter ≤2.5 μm; RDs, respiratory diseases; SO2, sulfur dioxide.
Discussion
Our large cohort study characterised the independent and joint associations between long-term exposure to air pollutants and mortality and identified vulnerable populations. Long-term exposure to a higher level of air pollutants, including PM10, PM2.5, SO2, and NO2, was consistently associated with increased risks of mortality from non-accidental causes, CVDs, non-malignant RDs and lung cancer. A generally non-threshold linear or supra-linear ER function without safety level was observed for the mortality pattern associated with individual exposure to air pollutants. For the elevated mortality risk associated with joint exposure to air pollutants, PM2.5 consistently contributed most to it, followed by SO2 or PM10. Inverted J-shaped ER relationships were observed for the joint associations of air pollutant mixtures with all mortality outcomes, with a HR levelling out at around the median level of the overall exposure. Stronger adverse associations were found for PM2.5 and SO2 with all mortality outcomes among participants being male, with lower education or higher income level compared to their counterparts, while no heterogeneous effects were observed for other air pollutants among subgroups.
To the best of our knowledge, this is one of the longest cohort studies with the most comprehensive evaluation of the associations between air pollutants and mortality from an LMIC. Previous relevant studies generally focused on the risks of single mortality outcome, mostly non-accidental or all-cause, related to individual air pollutant (mostly PM2.5).9,10,52 For example, three cohort studies with a mean follow-up duration of 15 years in Mainland China investigated the mortality risks related to ambient PM in the general population and found HRs varying from 1.08 to 1.11 for all-cause/non-accidental mortality per 10-μg/m3 increase in PM2.5,7,8,53 which is relatively lower than our fully adjusted HR of 1.20 (95% CI: 1.17–1.21). Similar patterns but generally higher HRs were observed in our current study by comparing limited studies on other air pollutants (i.e., PM10, SO2, NO2) and causes of death (i.e., CVDs, non-malignant RDs and lung cancer) in developing regions.7,8,44,54, 55, 56 This could be attributable to the relatively higher level of air pollution exposure and the potentially different sources and chemical compositions in our study.57,58 For example, higher risk estimates of PM2.5 and SO2 in this study may be due to their primary source from coal power plant emissions and the heavy metals (e.g., mercury) in them.59,60 Besides, the time-varying Cox model adopted in this study accounts for the cumulative and generally decreasing level of air pollutant exposures over the study period.61 Previous studies generally linked health endpoints with a relatively high level of air pollution at baseline, which could overestimate the actual exposure levels given the substantial downward trend in pollution levels and may lead to an underestimation of the risks, as suggested by our sensitive analysis (Table S9).
In our study, we observed generally linear or supra-linear and monotonically increasing ER curves with no evidence of a threshold and safety level for the mortality pattern related to air pollutants exposures, which is generally consistent with the existing evidence. A linear or supra-linear ER relationship of mortality with long-term exposure to air pollution has been well established in studies from developed countries or regions of relatively low levels of exposure, with a generally steeper concentration response at the lowest levels.2,3,52,62, 63, 64, 65, 66, 67 For example, a very recent study that involved seven cohorts with 28 million participants in Europe suggested non-threshold and monotonically increasing concentration-response functions for risks of non-accidental mortality related to long-term exposures of ambient PM2.5 and NO2, with the steepest part of the curves occurring at low exposures (5–10 μg/m3 for PM2.5, 0–20 μg/m3 for NO2).23 We identified a similar pattern for the less established ER relationships of PM10, PM2.5, SO2, and NO2 with mortality from non-accidental causes, CVDs, non-malignant RDs and lung cancer in high air pollution concentration settings. Further, we observed similar but significantly strong ER relationships of mortality with long-term exposure to air pollutant mixtures. However, few studies have been conducted to investigate the joint effect of long-term exposure to ambient air pollutant mixtures on health and more research needs to be conducted to further characterise it. Given the potentially strong additive or synergic effects detected in our study, the ER relationships of heath outcomes with air pollutant mixtures instead of only a single air pollutant should be considered in future air quality standards/guidelines or policy development.
Our study shows higher risks of mortality due to PM2.5 and SO2 among participants being male, with lower education or higher income level. However, previous relevant evidence considered subgroups depending on sex, education, or income level was generally limited or mixed.10,68,69 For example, four studies from developed countries with relatively low air pollution levels (the US, the United Kingdom, Japan, and Sweden) found a stronger adverse association between SO2 and mortality in the female population.70, 71, 72, 73 By comparison, one study from China suggested no between-sex difference and the other study from Italy observed higher risk in men.74,75 Consistent with our findings, Carey et al. found higher mortality risks due to SO2 for the subgroup with higher income level,71 while Dong et al. did not observe significant differences across subgroups of different income levels.75 The difference could be attributed to the difference in population characteristics, air pollution levels and modelling strategy. Overall, the stratified results have varied in previous studies and no clear explanation for such differences has yet been proposed.9,52,68,76 Current epidemiological evidence on the effect modifiers on the associations between air pollutions and health is still inconclusive. More studies are required to better elucidate this issue and the underlying mechanisms.
In the multi-pollutant and time-varying analysis, we found that PM2.5 consistently contributed most to the joint adverse associations between air pollutant mixtures (PM10, PM2.5, SO2, and NO2) and mortality, followed by SO2 or PM10, while the adverse effects of NO2 were largely attenuated and covered by other pollutants. Evidence had been well-established to suggest that the health risks of PM10 could be dominated by the PM2.5 fraction, with finer particulate matter being more harmful to humans.77,78 Similar to our findings, Ji et al. assessed the associations of PM2.5 and NO2 with mortality in a national Chinese elderly cohort from 2008 to 2018 and found an insignificant HR for NO2 after adjusting PM2.5.79 However, little published evidence is available on the joint associations of mortality with long-term exposure to air pollutants and their relative contributions.80,81 A cohort study of 7600 middle-aged and elderly participants in Canada assessed the combined effects of air pollutants on rheumatoid arthritis (RA) based on WQS regression models.82 Similarly, they found that PM2.5 was the major contributor to the detrimental effects of air pollutants on RA, followed by SO2 and NO2. The difference in the pattern of relative contributions of each air pollutant may be explained by the difference in health outcomes, modelling strategy and exposure levels and chemical compositions. Besides, the original WQS method relies on the uni-directional assumption (the effects of exposures on the outcome were either all positive or negative), which is a critical restriction and could lead to the non-convergence of models or biased estimates.83,84 By comparison, Quantile-based g-Computation builds up on WQS regression by integrating its estimation procedure with g-Computation, which could overcome the assumption of uni-direction.50 Overall, the relative contribution of each air pollutant to the adverse health risks associated with long-term exposure to air pollutions remains highly unclear due to the very limited evidence. Our findings of the potentially strongest long-term associations of mortality with PM2.5 among air pollutants highlight the great significance of reducing PM2.5 to a relatively low level in China over the past decade.85
This study has several strengths. First, we included a relatively large sample size (∼37 thousand) of the general population with a long-follow up of more than two decades (22 years) in Mainland China, which provided new evidence on the mortality pattern related to long-term exposure to ambient air pollutions in a rapidly developing setting with a wide range and high level of exposures. Second, we conducted a comprehensive analysis by including four air pollutants (PM10, PM2.5, SO2, and NO2) and four main mortality outcomes (non-accidental mortality, CVDs mortality, non-malignant RDs and lung cancer mortality), as well as a series of individual-level risk factors that may confound the associations (e.g., BMI, level of education, income, occupational exposure, physical exercise and status of marital, smoking, and drinking). Furthermore, we used a time-varying (accounting for the changes of exposures and confounders) multi-pollutant model (accounting for the collinearity and potential additive or synergic effects among air pollutants) to characterise the joint associations of mortality with air pollutant mixtures and assess the contribution of each air pollutant, which are of great importance for understanding the effects of air pollution and for delivering targeted strategies to minimize the adverse health impacts of primary air pollutants.
However, several limitations of this study should be acknowledged. First, though many potential confounders were adjusted in our main model, the possibility of residual confounding cannot be excluded. For example, though the long-term time trend was controlled in the model, the potential confounding effects of the covariates that changed over the long study period like the medical treatment level and death reporting accuracy were not adequately accounted for due to the lack of these data for the study locations. The significant improvement in medical treatment level along with the substantial decrease in air pollution level between 1998 and 2019 in mainland China may lead us to overestimate the adverse effects of air pollution. Second, we only had data from four cities in Northern China with a typical setting of rapid industrialization and urbanization. The findings of our study may therefore not be generalizable to other northern areas (e.g., rural areas with a relatively stable air pollution level). Third, due to the lack of satellite-derived air pollution data early in the cohort, we estimated PM10 (1998–1999), PM2.5 (1998–1999), SO2 (1998–2012), and NO2 (1998–2012) using calibrated monitoring air pollution data and/or imputation to ensure consistent yearly satellite-derived air pollution data from 1998 to 2019 for the time-varying multi-pollutant analysis. This could increase the uncertainty of our results. However, considering the almost identical results in the sensitivity analysis of different calibration approaches or imputation functions, we believed the impacts could be modest. Fourth, there were potential recall and self-reported bias when collecting the death information or covariates (e.g., smoking or drinking status), which could result in misclassification of both covariates and outcomes. However, the observed adverse associations were robust to a series of sensitivity analyses including different adjustments of covariates. Therefore, we supposed that recall and self-reported bias did not exert a great influence on our findings. Finally, we were not able to account for ozone due to a lack of regional ozone data as it has only been monitored and available since 2013. Therefore, we used ozone data from GBD 2019 that should correlate well with regional ozone as a proxy.48 Elevated risks were observed in the main models with additional adjustment of ozone, suggesting that the observed long-term adverse associations between PM10, PM2.5, SO2, and NO2 and mortality may not be attenuated by ozone. However, due to increasing evidence of the adverse effects of long-term exposure to ozone, further explorations with a more comprehensive inclusion of air pollutants are warranted in the future.86,87
In summary, the results of this study support a strong and robust adverse association of long-term exposure to air pollutants, including PM10, PM2.5, SO2, and NO2, with risks of mortality from non-accidental causes, CVDs, non-malignant RDs and lung cancer. PM2.5 consistently contributed most to the elevated mortality risk among air pollutants, followed by SO2 or PM10. A generally non-threshold linear or supra-linear exposure-response curve without safety level was observed for the mortality risks associated with both individual and joint exposure to air pollutants.
Contributors
GD, NT, and YY set up the collaborative network. GD, LZ, AS, JC, TW, and NT designed the study. YG and WH developed the statistical methods. WH took the lead in manuscript drafting and results interpreting. All authors provided the data, and contributed to interpreting the results and revising the manuscript. GD and NT accessed and verified the data.
Data sharing statement
The cohort data could not be shared externally due to the data sharing agreement by collaborators.
Editor note
The Lancet Group takes a neutral position with respect to territorial claims in published maps and institutional affiliations.
Declaration of interests
The authors declare no conflicts of interest.
Acknowledgements
This work was supported by the Guangxi Key Research and Development Plan (GUIKEAB18050024), National Key Research and Development Program of China (2018YFC1004300, 2018YFC1004301, 2018YFE0106900), the China Scholarship Council (202006380055), the National Natural Science Foundation of China (M-0420, 82103823, 81872582), and Natural Science Foundation of Guangdong Province (2021B1515020015, 2021A1515011754, 2020A1515011131, 2018B030312005, 2017A090905042).
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.lanwpc.2023.100776.
Contributor Information
Yunjiang Yu, Email: yuyunjiang@scies.org.
Naijun Tang, Email: tangnaijun@tmu.edu.cn.
Guanghui Dong, Email: donggh5@mail.sysu.edu.cn, donggh512@hotmail.com.
Appendix A. Supplementary data
References
- 1.WHO . 2021. Fact sheet: ambient (outdoor) air pollution.https://www.who.int/en/news-room/fact-sheets/detail/ambient-(outdoor)-air-quality-and-health Available from: [Google Scholar]
- 2.Chen J., Hoek G. Long-term exposure to PM and all-cause and cause-specific mortality: a systematic review and meta-analysis. Environ Int. 2020;143 doi: 10.1016/j.envint.2020.105974. [DOI] [PubMed] [Google Scholar]
- 3.So R., Jorgensen J.T., Lim Y.H., et al. Long-term exposure to low levels of air pollution and mortality adjusting for road traffic noise: a Danish Nurse Cohort study. Environ Int. 2020;143 doi: 10.1016/j.envint.2020.105983. [DOI] [PubMed] [Google Scholar]
- 4.Philip S., Martin R.V., van Donkelaar A., et al. Global chemical composition of ambient fine particulate matter for exposure assessment. Environ Sci Technol. 2014;48(22):13060–13068. doi: 10.1021/es502965b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lelieveld J., Evans J.S., Fnais M., Giannadaki D., Pozzer A. The contribution of outdoor air pollution sources to premature mortality on a global scale. Nature. 2015;525(7569):367–371. doi: 10.1038/nature15371. [DOI] [PubMed] [Google Scholar]
- 6.Brauer M., Freedman G., Frostad J., et al. Ambient air pollution exposure estimation for the Global Burden of Disease 2013. Environ Sci Technol. 2016;50(1):79–88. doi: 10.1021/acs.est.5b03709. [DOI] [PubMed] [Google Scholar]
- 7.Yang X., Liang F., Li J., et al. Associations of long-term exposure to ambient PM2.5 with mortality in Chinese adults: a pooled analysis of cohorts in the China-PAR project. Environ Int. 2020;138 doi: 10.1016/j.envint.2020.105589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yin P., Brauer M., Cohen A., et al. Long-term fine particulate matter exposure and nonaccidental and cause-specific mortality in a large national cohort of Chinese men. Environ Health Perspect. 2017;125(11) doi: 10.1289/EHP1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chung C.Y., Yang J., Yang X.G., He J. Long-term effects of ambient air pollution on lung cancer and COPD mortalities in China: a systematic review and meta-analysis of cohort studies. Environ Impact Assess Rev. 2022;97 [Google Scholar]
- 10.Yang Z., Mahendran R., Yu P., et al. Health effects of long-term exposure to ambient PM2.5 in Asia-Pacific: a systematic review of cohort studies. Curr Environ Health Rep. 2022;9(2):130–151. doi: 10.1007/s40572-022-00344-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen Y., Chen R., Chen Y., et al. The prospective effects of long-term exposure to ambient PM2.5 and constituents on mortality in rural East China. Chemosphere. 2021;280 doi: 10.1016/j.chemosphere.2021.130740. [DOI] [PubMed] [Google Scholar]
- 12.Xu D., Zhang Y., Sun Q., Wang X., Li T. Long-term PM2.5 exposure and survival among cardiovascular disease patients in Beijing, China. Environ Sci Pollut Res Int. 2021;28(34):47367–47374. doi: 10.1007/s11356-021-14043-w. [DOI] [PubMed] [Google Scholar]
- 13.Zhang Y. All-cause mortality risk and attributable deaths associated with long-term exposure to ambient PM2.5 in Chinese adults. Environ Sci Technol. 2021;55(9):6116–6127. doi: 10.1021/acs.est.0c08527. [DOI] [PubMed] [Google Scholar]
- 14.Yao Y., Liu L., Guo G., Zeng Y., Ji J.S. Interaction of Sirtuin 1 (SIRT1) candidate longevity gene and particulate matter (PM2.5) on all-cause mortality: a longitudinal cohort study in China. Environ Health. 2021;20(1):25. doi: 10.1186/s12940-021-00718-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang Y., Li Z., Wei J., et al. Long-term exposure to ambient NO(2) and adult mortality: a nationwide cohort study in China. J Adv Res. 2022;41:13–22. doi: 10.1016/j.jare.2022.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu S., Zhang Y., Ma R., et al. Long-term exposure to ozone and cardiovascular mortality in a large Chinese cohort. Environ Int. 2022;165 doi: 10.1016/j.envint.2022.107280. [DOI] [PubMed] [Google Scholar]
- 17.Liang R., Chen R., Yin P., et al. Associations of long-term exposure to fine particulate matter and its constituents with cardiovascular mortality: a prospective cohort study in China. Environ Int. 2022;162 doi: 10.1016/j.envint.2022.107156. [DOI] [PubMed] [Google Scholar]
- 18.Guo C., Yu T., Bo Y., et al. Long-term exposure to fine particulate matter and mortality a longitudinal cohort study of 400,459 adults. Epidemiology. 2022;33(3):309–317. doi: 10.1097/EDE.0000000000001464. [DOI] [PubMed] [Google Scholar]
- 19.Wang Y., Du Z., Zhang Y., et al. Long-term exposure to particulate matter and COPD mortality: insights from causal inference methods based on a large population cohort in Southern China. Sci Total Environ. 2023;863 doi: 10.1016/j.scitotenv.2022.160808. [DOI] [PubMed] [Google Scholar]
- 20.Billionnet C., Sherrill D., Annesi-Maesano I., study G. Estimating the health effects of exposure to multi-pollutant mixture. Ann Epidemiol. 2012;22(2):126–141. doi: 10.1016/j.annepidem.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 21.Huang W.Z., He W.Y., Knibbs L.D., et al. Improved morbidity-based air quality health index development using Bayesian multi-pollutant weighted model. Environ Res. 2022;204 doi: 10.1016/j.envres.2021.112397. [DOI] [PubMed] [Google Scholar]
- 22.Hamra G.B., Buckley J.P. Environmental exposure mixtures: questions and methods to address them. Curr Epidemiol Rep. 2018;5(2):160–165. doi: 10.1007/s40471-018-0145-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stafoggia M., Oftedal B., Chen J., et al. Long-term exposure to low ambient air pollution concentrations and mortality among 28 million people: results from seven large European cohorts within the ELAPSE project. Lancet Planet Health. 2022;6(1):E9–E18. doi: 10.1016/S2542-5196(21)00277-1. [DOI] [PubMed] [Google Scholar]
- 24.Wei J., Li Z., Xue W., et al. The ChinaHighPM10 dataset: generation, validation, and spatiotemporal variations from 2015 to 2019 across China. Environ Int. 2021;146 doi: 10.1016/j.envint.2020.106290. [DOI] [PubMed] [Google Scholar]
- 25.Wei J., Liu S., Li Z., et al. Ground-level NO2 surveillance from space across China for high resolution using interpretable spatiotemporally weighted artificial intelligence. Environ Sci Technol. 2022;56(14):9988–9998. doi: 10.1021/acs.est.2c03834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Geng G., Xiao Q., Liu S., et al. Tracking air pollution in China: near real-time PM2.5 retrievals from multisource data fusion. Environ Sci Technol. 2021;55(17):12106–12115. doi: 10.1021/acs.est.1c01863. [DOI] [PubMed] [Google Scholar]
- 27.Wei J., Li Z. 2021. ChinaHighSO2: big data seamless 10 Km ground-level SO2 dataset for China (Version 1) [Data Set] [Google Scholar]
- 28.Guo Q., Zhao Y., Zhao J., et al. Identifying the threshold of outdoor PM2.5 reversing the beneficial association between physical activity and lung function: a national longitudinal study in China. Sci Total Environ. 2022;839 doi: 10.1016/j.scitotenv.2022.156138. [DOI] [PubMed] [Google Scholar]
- 29.Liu M., Tang W., Zhang Y., et al. Urban-rural differences in the association between long-term exposure to ambient air pollution and obesity in China. Environ Res. 2021;201 doi: 10.1016/j.envres.2021.111597. [DOI] [PubMed] [Google Scholar]
- 30.Wang L., Chen G., Pan Y., et al. Association of long-term exposure to ambient air pollutants with blood lipids in Chinese adults: the China Multi-Ethnic Cohort study. Environ Res. 2021;197 doi: 10.1016/j.envres.2021.111174. [DOI] [PubMed] [Google Scholar]
- 31.Wang S., Zhou Q., Tian Y., Hu X. The lung microbiota affects pulmonary inflammation and oxidative stress induced by PM2.5 exposure. Environ Sci Technol. 2022;56(17):12368–12379. doi: 10.1021/acs.est.1c08888. [DOI] [PubMed] [Google Scholar]
- 32.Xu H., Guo B., Qian W., et al. Dietary pattern and long-term effects of particulate matter on blood pressure: a large cross-sectional study in Chinese adults. Hypertension. 2021;78(1):184–194. doi: 10.1161/HYPERTENSIONAHA.121.17205. [DOI] [PubMed] [Google Scholar]
- 33.Zhang Y., Wei J., Shi Y., et al. Early-life exposure to submicron particulate air pollution in relation to asthma development in Chinese preschool children. J Allergy Clin Immunol. 2021;148(3):771–782.e12. doi: 10.1016/j.jaci.2021.02.030. [DOI] [PubMed] [Google Scholar]
- 34.Wu H., Zhang B., Wei J., et al. Short-term effects of exposure to ambient PM1, PM2.5, and PM10 on ischemic and hemorrhagic stroke incidence in Shandong Province, China. Environ Res. 2022;212(Pt C) doi: 10.1016/j.envres.2022.113350. [DOI] [PubMed] [Google Scholar]
- 35.Moritz S., Bartz-Beielstein T. imputeTS: time series missing value imputation in R. R J. 2017;9(1):207–218. [Google Scholar]
- 36.Lipsett M.J., Ostro B.D., Reynolds P., et al. Long-term exposure to air pollution and cardiorespiratory disease in the California teachers study cohort. Am J Respir Crit Care Med. 2011;184(7):828–835. doi: 10.1164/rccm.201012-2082OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gandini M., Scarinzi C., Bande S., et al. Long term effect of air pollution on incident hospital admissions: results from the Italian longitudinal study within LIFE MED HISS project. Environ Int. 2018;121(Pt 2):1087–1097. doi: 10.1016/j.envint.2018.10.020. [DOI] [PubMed] [Google Scholar]
- 38.Cheng I., Tseng C., Wu J., et al. Association between ambient air pollution and breast cancer risk: the multiethnic cohort study. Int J Cancer. 2020;146(3):699–711. doi: 10.1002/ijc.32308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Therneau T., Crowson C., Atkinson E. 2022. Using time dependent covariates and time dependent coefficients in the Cox model.https://cran.r-project.org/web/packages/survival/vignettes/timedep.pdf Available from: [Google Scholar]
- 40.Griffin B.A., Anderson G.L., Shih R.A., Whitsel E.A. Use of alternative time scales in Cox proportional hazard models: implications for time-varying environmental exposures. Stat Med. 2012;31(27):3320–3327. doi: 10.1002/sim.5347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stineman R.W. A consistently well-behaved method of interpolation. Creat Comput. 1980;6(7):54–57. [Google Scholar]
- 42.Piegl L., Tiller W. Springer Science & Business Media; 1996. The NURBS book. [Google Scholar]
- 43.Hernan M.A., Hernandez-Diaz S., Robins J.M. A structural approach to selection bias. Epidemiology. 2004;15(5):615–625. doi: 10.1097/01.ede.0000135174.63482.43. [DOI] [PubMed] [Google Scholar]
- 44.Chen G., Wang A., Li S., et al. Long-term exposure to air pollution and survival after ischemic stroke. Stroke. 2019;50(3):563–570. doi: 10.1161/STROKEAHA.118.023264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Klompmaker J.O., Hoek G., Bloemsma L.D., et al. Surrounding green, air pollution, traffic noise exposure and non-accidental and cause-specific mortality. Environ Int. 2020;134 doi: 10.1016/j.envint.2019.105341. [DOI] [PubMed] [Google Scholar]
- 46.Van Buuren S., Groothuis-Oudshoorn K. mice: multivariate imputation by chained equations in R. J Stat Softw. 2011;45:1–67. [Google Scholar]
- 47.Rubin D.B. John Wiley & Sons; 2004. Multiple imputation for nonresponse in surveys. [Google Scholar]
- 48.Chang K.L., Cooper O.R., West J.J., et al. A new method (M 3 Fusion v1) for combining observations and multiple model output for an improved estimate of the global surface ozone distribution. Geosci Model Dev. 2019;12(3):955–978. [Google Scholar]
- 49.Govindarajulu U.S., Malloy E.J., Ganguli B., Spiegelman D., Eisen E.A. The comparison of alternative smoothing methods for fitting non-linear exposure-response relationships with Cox models in a simulation study. Int J Biostat. 2009;5(1) doi: 10.2202/1557-4679.1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Keil A.P., Buckley J.P., O'Brien K.M., Ferguson K.K., Zhao S., White A.J. A Quantile-based g-Computation approach to addressing the effects of exposure mixtures. Environ Health Perspect. 2020;128(4) doi: 10.1289/EHP5838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ding N., Karvonen-Gutierrez C.A., Mukherjee B., Calafat A.M., Harlow S.D., Park S.K. Per- and polyfluoroalkyl substances and incident hypertension in multi-racial/ethnic women: the study of women's health across the nation. Hypertension. 2022;79(8):1876–1886. doi: 10.1161/HYPERTENSIONAHA.121.18809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pope C.A., 3rd, Coleman N., Pond Z.A., Burnett R.T. Fine particulate air pollution and human mortality: 25+ years of cohort studies. Environ Res. 2020;183 doi: 10.1016/j.envres.2019.108924. [DOI] [PubMed] [Google Scholar]
- 53.Li T., Zhang Y., Wang J., et al. All-cause mortality risk associated with long-term exposure to ambient PM2.5 in China: a cohort study. Lancet Public Health. 2018;3(10):e470–e477. doi: 10.1016/S2468-2667(18)30144-0. [DOI] [PubMed] [Google Scholar]
- 54.Liang F., Liu F., Huang K., et al. Long-term exposure to fine particulate matter and cardiovascular disease in China. J Am Coll Cardiol. 2020;75(7):707–717. doi: 10.1016/j.jacc.2019.12.031. [DOI] [PubMed] [Google Scholar]
- 55.Cao J., Yang C., Li J., et al. Association between long-term exposure to outdoor air pollution and mortality in China: a cohort study. J Hazard Mater. 2011;186(2–3):1594–1600. doi: 10.1016/j.jhazmat.2010.12.036. [DOI] [PubMed] [Google Scholar]
- 56.Chen X., Wang X., Huang J.J., et al. Nonmalignant respiratory mortality and long-term exposure to PM10 and SO2: a 12-year cohort study in northern China. Environ Pollut. 2017;231(Pt 1):761–767. doi: 10.1016/j.envpol.2017.08.085. [DOI] [PubMed] [Google Scholar]
- 57.Zhang L., Yao M. Ambient particle composition and toxicity in 31 major cities in China. Fundam Res. 2022 doi: 10.1016/j.fmre.2022.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu Z., Gao W., Yu Y., et al. Characteristics of PM2.5 mass concentrations and chemical species in urban and background areas of China: emerging results from the CARE-China network. Atmos Chem Phys. 2018;18(12):8849–8871. [Google Scholar]
- 59.Zhang Y., Ji X., Ku T., Li G., Sang N. Heavy metals bound to fine particulate matter from northern China induce season-dependent health risks: a study based on myocardial toxicity. Environ Pollut. 2016;216:380–390. doi: 10.1016/j.envpol.2016.05.072. [DOI] [PubMed] [Google Scholar]
- 60.Tang S., Wang L., Feng X., et al. Actual mercury speciation and mercury discharges from coal-fired power plants in Inner Mongolia, Northern China. Fuel. 2016;180:194–204. [Google Scholar]
- 61.Platt R.W., Joseph K.S., Ananth C.V., Grondines J., Abrahamowicz M., Kramer M.S. A proportional hazards model with time-dependent covariates and time-varying effects for analysis of fetal and infant death. Am J Epidemiol. 2004;160(3):199–206. doi: 10.1093/aje/kwh201. [DOI] [PubMed] [Google Scholar]
- 62.Strak M., Weinmayr G., Rodopoulou S., et al. Long term exposure to low level air pollution and mortality in eight European cohorts within the ELAPSE project: pooled analysis. BMJ. 2021;374 doi: 10.1136/bmj.n1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Burnett R., Chen H., Szyszkowicz M., et al. Global estimates of mortality associated with long-term exposure to outdoor fine particulate matter. Proc Natl Acad Sci U S A. 2018;115(38):9592–9597. doi: 10.1073/pnas.1803222115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang Z., Wang J., Kwong J.C., et al. Long-term exposure to air pollution and mortality in a prospective cohort: the Ontario Health Study. Environ Int. 2021;154 doi: 10.1016/j.envint.2021.106570. [DOI] [PubMed] [Google Scholar]
- 65.Di Q., Wang Y., Zanobetti A., et al. Air pollution and mortality in the medicare population. N Engl J Med. 2017;376(26):2513–2522. doi: 10.1056/NEJMoa1702747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cesaroni G., Badaloni C., Gariazzo C., et al. Long-term exposure to urban air pollution and mortality in a cohort of more than a million adults in Rome. Environ Health Perspect. 2013;121(3):324–331. doi: 10.1289/ehp.1205862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pope C.A., 3rd, Burnett R.T., Thun M.J., et al. Lung cancer, cardiopulmonary mortality, and long-term exposure to fine particulate air pollution. JAMA. 2002;287(9):1132–1141. doi: 10.1001/jama.287.9.1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Atkinson R.W., Butland B.K., Anderson H.R., Maynard R.L. Long-term concentrations of nitrogen dioxide and mortality: a meta-analysis of cohort studies. Epidemiology. 2018;29(4):460–472. doi: 10.1097/EDE.0000000000000847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Newell K., Kartsonaki C., Lam K.B.H., Kurmi O. Cardiorespiratory health effects of gaseous ambient air pollution exposure in low and middle income countries: a systematic review and meta-analysis. Environ Health. 2018;17(1):41. doi: 10.1186/s12940-018-0380-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Abbey D.E., Nishino N., McDonnell W.F., et al. Long-term inhalable particles and other air pollutants related to mortality in nonsmokers. Am J Respir Crit Care Med. 1999;159(2):373–382. doi: 10.1164/ajrccm.159.2.9806020. [DOI] [PubMed] [Google Scholar]
- 71.Carey I.M., Atkinson R.W., Kent A.J., van Staa T., Cook D.G., Anderson H.R. Mortality associations with long-term exposure to outdoor air pollution in a national English cohort. Am J Respir Crit Care Med. 2013;187(11):1226–1233. doi: 10.1164/rccm.201210-1758OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Katanoda K., Sobue T., Satoh H., et al. An association between long-term exposure to ambient air pollution and mortality from lung cancer and respiratory diseases in Japan. J Epidemiol. 2011;21(2):132–143. doi: 10.2188/jea.JE20100098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Andersson E., Persson B., Bryngelsson I.L., Magnuson A., Westberg H. Cancer mortality in a Swedish cohort of pulp and paper mill workers. Int Arch Occup Environ Health. 2010;83(2):123–132. doi: 10.1007/s00420-009-0446-1. [DOI] [PubMed] [Google Scholar]
- 74.Minichilli F., Gorini F., Bustaffa E., Cori L., Bianchi F. Mortality and hospitalization associated to emissions of a coal power plant: a population-based cohort study. Sci Total Environ. 2019;694 doi: 10.1016/j.scitotenv.2019.133757. [DOI] [PubMed] [Google Scholar]
- 75.Dong G.H., Zhang P., Sun B., et al. Long-term exposure to ambient air pollution and respiratory disease mortality in Shenyang, China: a 12-year population-based retrospective cohort study. Respiration. 2012;84(5):360–368. doi: 10.1159/000332930. [DOI] [PubMed] [Google Scholar]
- 76.Stieb D.M., Berjawi R., Emode M., et al. Systematic review and meta-analysis of cohort studies of long term outdoor nitrogen dioxide exposure and mortality. PLoS One. 2021;16(2) doi: 10.1371/journal.pone.0246451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lin H., Tao J., Du Y., et al. Particle size and chemical constituents of ambient particulate pollution associated with cardiovascular mortality in Guangzhou, China. Environ Pollut. 2016;208(Pt B):758–766. doi: 10.1016/j.envpol.2015.10.056. [DOI] [PubMed] [Google Scholar]
- 78.Liu J., Yin H., Tang X., et al. Transition in air pollution, disease burden and health cost in China: a comparative study of long-term and short-term exposure. Environ Pollut. 2021;277 doi: 10.1016/j.envpol.2021.116770. [DOI] [PubMed] [Google Scholar]
- 79.Ji J.S., Liu L., Zhang J.J., et al. NO2 and PM2.5 air pollution co-exposure and temperature effect modification on pre-mature mortality in advanced age: a longitudinal cohort study in China. Environ Health. 2022;21(1):97. doi: 10.1186/s12940-022-00901-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Huangfu P., Atkinson R. Long-term exposure to NO2 and O3 and all-cause and respiratory mortality: a systematic review and meta-analysis. Environ Int. 2020;144 doi: 10.1016/j.envint.2020.105998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Davalos A.D., Luben T.J., Herring A.H., Sacks J.D. Current approaches used in epidemiologic studies to examine short-term multipollutant air pollution exposures. Ann Epidemiol. 2017;27(2):145–153. doi: 10.1016/j.annepidem.2016.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhao N., Smargiassi A., Hatzopoulou M., et al. Long-term exposure to a mixture of industrial SO2, NO2, and PM2.5 and anti-citrullinated protein antibody positivity. Environ Health. 2020;19(1):86. doi: 10.1186/s12940-020-00637-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Carrico C., Gennings C., Wheeler D.C., Factor-Litvak P. Characterization of weighted quantile sum regression for highly correlated data in a risk analysis setting. J Agric Biol Environ Stat. 2015;20(1):100–120. doi: 10.1007/s13253-014-0180-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gennings C., Curtin P., Bello G., Wright R., Arora M., Austin C. Lagged WQS regression for mixtures with many components. Environ Res. 2020;186 doi: 10.1016/j.envres.2020.109529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Xiao Q., Liang F., Ning M., et al. The long-term trend of PM2.5-related mortality in China: the effects of source data selection. Chemosphere. 2021;263 doi: 10.1016/j.chemosphere.2020.127894. [DOI] [PubMed] [Google Scholar]
- 86.Niu Y., Zhou Y., Chen R., et al. Long-term exposure to ozone and cardiovascular mortality in China: a nationwide cohort study. Lancet Planet Health. 2022;6(6):e496–e503. doi: 10.1016/S2542-5196(22)00093-6. [DOI] [PubMed] [Google Scholar]
- 87.Lim C.C., Hayes R.B., Ahn J., et al. Long-term exposure to ozone and cause-specific mortality risk in the United States. Am J Respir Crit Care Med. 2019;200(8):1022–1031. doi: 10.1164/rccm.201806-1161OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.