Summary
The Western Pacific has one of the fastest-growing older adult populations globally, and tuberculosis (TB) remains one of the foremost infectious causes of disease and death in the region. Older adults are at higher risk of TB due to immunosenescence, comorbidities, and increased institutionalisation. Atypical symptoms and reduced access to health services may delay care-seeking and TB diagnosis, while co-morbidity and increased risk of adverse drug reactions complicate TB treatment. Post-TB sequelae and socioeconomic challenges may decrease the quality of life after TB treatment completion. Despite their high disease burden and special challenges, there is a lack of regionally coordinated policies and guidelines to manage TB among older adults. Routine TB screening at aged-care facilities, age-friendly infrastructure and services, awareness of atypical TB features, integration of TB and non-communicable diseases services, and person-centred approaches to treatment support could improve TB management among older adults. Addressing these challenges and adopting the best practices identified should inform policy formulation and implementation.
Funding
This project was funded by 1) the World Health Organization Regional Office for the Western Pacific, with financial contributions from the Government of the Republic of Korea through the Korean Disease Control and Prevention Agency and the Government of Japan through the Ministry of Health, Labour and Welfare, and 2) NUS Start-up Grant. The funders had no role in the paper design, collection, analysis, and interpretation of data and in writing of the paper.
Keywords: Tuberculosis, Aged, Policy
Background
The Western Pacific Region, home to 1.9 billion people in 37 countries and areas, has one of the largest and fastest-growing populations of older adults globally. The average life expectancy of people in the region was 77.7 years in 2019, 4 years above the global estimate (73.3 years).1,2 The increased TB risk of this ageing population poses a major public health challenge. While TB deaths have gradually declined across all age groups since the 1990s, TB remains one of the leading infectious causes of death worldwide, with increasing age recognised as a major risk factor for TB-related death.3,4 In 2021, the estimated incidence in the Western Pacific Region was the highest among persons aged ≥65 years (men—255,000 and women—116,000), accounting for 19.5% of the total TB incidence.5 Between 2020 and 2021, people with TB aged ≥65 years made up a large proportion of the notified TB cases in countries and areas such as Japan (69%), the Republic of Korea (51%), and Hong Kong Special Administrative Region, China (hereinafter Hong Kong SAR) (45%).5
Failure to control and manage TB among older adults places them at risk and could also perpetuate transmission to other age groups. Given the absence of regional guidance on how best to deal with this challenge, a careful assessment of the situation is required to assist countries in meeting ambitious End TB Strategy targets.6 In this article, we describe the risk factors for TB disease and transmission and the clinical disease manifestations of TB in older adults, with a focus on the Western Pacific Region. We also highlight key challenges, relevant strategies, and associated research priorities related to TB transmission, detection, diagnosis, treatment, and post-TB health (See on-line Supplementary Material for a detailed description of the literature search methods and limitations).
Contextual heterogeneity
The Western Pacific Region includes a diverse range of countries and areas with different population structures, cultural beliefs and social norms, economic resources, and healthcare systems. This heterogeneity reflects differences in TB transmission risk, as well as risk factors for disease development, which contributes to variable disease burden observed among older adults in different countries and states (Table 1).
Table 1.
Epidemiological profiles and variable TB incidence among older adults in countries and areas in the Western Pacific Region (2021).
| Countries and areas | Country income classification7,f | UHC indices of service coverage 20198,d | TB incidence rate per 100,000 population per year5,e, f | Epidemiological classification9 for TB based on incidence rate5,e, f | Estimated TB case load5,e, f | Estimated TB incidence in older adults5,e, f | % of incident TB cases in older adults5,f | BCG coverage10,c |
|---|---|---|---|---|---|---|---|---|
| Western Pacific Region | 8011 | 98 | 1,900,000 | 371,000 | 19.5% | 89.0% | ||
| Philippinesa | Lower-middle | 55 | 650 | Severely endemic | 741,000 | 78,000 | 10.5% | 47.0% |
| Marshall Islands | Upper-middle | N/A | 483 | Highly endemic | 200 | 10 | 5.0% | 83.0% |
| Mongoliaa | Lower-middle | 63 | 428 | Highly endemic | 14,000 | 1080 | 7.7% | 99.4% |
| Papua New Guineaa | Lower-middle | 33 | 424 | Highly endemic | 42,000 | 920 | 2.2% | 42.0% |
| Kiribati | Lower-middle | 51 | 424 | Highly endemic | 550 | 35 | 6.4% | 96.0% |
| Tuvalu | Upper-middle | N/A | 296 | Endemic | 33 | 6 | 18.2% | 100.0% |
| Cambodiab | Upper-middle | 61 | 288 | Endemic | 48,000 | 8900 | 18.5% | 92.0% |
| Nauru | High | N/A | 193 | Endemic | 24 | 2 | 8.3% | 100.0% |
| Viet Nama | Lower-middle | 70 | 173 | Endemic | 169,000 | 41,000 | 24.3% | 87.9% |
| Lao People's Democratic Republic | Lower-middle | 50 | 143 | Endemic | 11,000 | 2010 | 18.3% | 81.4% |
| Malaysia | Upper-middle | 76 | 97 | Upper moderate | 33,000 | 5400 | 16.4% | 99.0% |
| Northern Mariana Islands | High | N/A | 81 | Upper moderate | 40 | 7 | 17.5% | 13.0% (2010) |
| Micronesia (Federated States of) | Lower-middle | 48 | 80 | Upper moderate | 90 | 0 | 0.0% | 59.2% |
| Fiji | Upper-middle | 61 | 66 | Upper moderate | 610 | 49 | 8.0% | 99.6% (2020) |
| Solomon Islands | Lower-middle | 50 | 65 | Upper moderate | 460 | 40 | 8.7% | 83.5% |
| Brunei Darussalam | High | 77 | 61 | Upper moderate | 270 | 58 | 21.5% | 99.9% |
| China, Macao SAR | High | N/A | 57 | Upper moderate | 390 | 143 | 36.7% | 99.7% |
| China, Hong Kong SAR | High | N/A | 57 | Upper moderate | 4300 | 1920 | 44.7% | 95.0% |
| Chinaa | Upper-middle | 82 | 55 | Upper moderate | 780,000 | 210,000 | 26.9% | 99.7% |
| Palau | Upper-middle | N/A | 51 | Upper moderate | 9 | 0 | 0.0% | N/A |
| Niue | N/A | N/A | 48 | Lower moderate | 0 | 0 | 0.0% | 88.0% |
| Singapore | High | 86 | 48 | Lower moderate | 2800 | 720 | 25.7% | 98.0% (2018) |
| Republic of Korea | High | 87 | 44 | Lower moderate | 23,000 | 11,700 | 50.9% | 98.0% (2019) |
| Guam | High | N/A | 39 | Lower moderate | 67 | 10 | 14.9% | N/A |
| Vanuatu | Lower-middle | 52 | 34 | Lower moderate | 110 | 9 | 8.2% | 76.0% |
| Tokelau | N/A | N/A | 19 | Upper moderate | 0 | 0 | 0.0% | 100.0% |
| French Polynesia | High | N/A | 13 | Lower moderate | 39 | 8 | 20.5% | 96.0% (2019) |
| Cook Islands | N/A | N/A | 13 | Lower moderate | 2 | 0 | 0.0% | 100.0% |
| Japan | High | 85 | 11 | Lower moderate | 13,000 | 9200 | 70.8% | 95.0% (2020) |
| New Caledonia | High | N/A | 10 | Lower moderate | 29 | 14 | 48.3% | 95.0% (2018) |
| Tonga | Upper-middle | 56 | 7.6 | Low incidence | 8 | 2 | 25.0% | 100.0% |
| New Zealand | High | 86 | 6.8 | Low incidence | 350 | 54 | 15.4% | 9.9% |
| Samoa | Lower-middle | 53 | 6.8 | Low incidence | 15 | 3 | 20.0% | 92.0% |
| Australia | High | 87 | 6.5 | Low incidence | 1700 | 290 | 17.1% | N/A |
| American Samoa | Upper-middle | N/A | 4.1 | Low incidence | 2 | 1 | 50.0% | 91.0% (1998) |
| Wallis and Futuna Islands | N/A | N/A | 1.9 | Low incidence | 0 | 0 | 0.0% | 97.0% (2016) |
| Pitcairn Islands | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
BCG; Bacillus Calmette–Guérin, N/A; data not available, SAR; special administration region, TB; tuberculosis, UHC; Universal health coverage.
WHO high TB burden country.
Recently removed from the WHO high TB burden list and was included on a global TB watchlist.
Latest data (2021) is presented unless otherwise stated. If official, administrative, and WHO/UNICEF estimates are provided for the same year, official data is presented.
Average coverage of essential services based on tracer interventions that include reproductive, maternal, newborn and child health, infectious diseases, non-communicable diseases and service capacity and access, among the general and the most disadvantaged population. The indicator is an index reported on a unitless scale of 0–100, with 0 being the worst, and 100 the best.
These estimates (including those used for grouping) have uncertainty ranges but only best estimates are provided in the table and were used for calculating % of incidence TB cases (age ≥65 years).
All data for 2021 (as stated in heading, unless otherwise specified).
In settings with a lower TB burden and limited community transmissions, such as Hong Kong SAR, Japan, and Singapore, TB incidence among older adults is mainly driven by increased rates of reactivation disease.12, 13, 14 In high-burden settings such as Cambodia and Viet Nam with evidence of ongoing community transmission, older adults are at risk of developing TB disease through both reactivations of past TB infection and disease progression following recent primary or re-infection.15,16 China demonstrates a country in transition where enhanced TB management has reduced TB transmission within communities, leading to a relative increase in reactivation disease among older people in the coming decades.17 Given various TB disease burdens in the Western Pacific Region,18 both reactivation pathway and exogenous infection/re-infection pathway should be considered, especially in households and congregate settings such as aged care facilities and hospitals.13,16,19,20 Institutional transmission among residents and staff within and between aged-care and health facilities has been reported,13,20,21 posing a major infection control challenge, particularly if disease detection is delayed.
Increasing immunosenescence in older individuals, a process of age-associated immune dysfunction, increases the risk of TB reactivation.19,22,23 In addition, the presence of other comorbidities that increase with age, such as diabetes, could experience ≥1.5-folds increased risk of developing TB.24, 25, 26 The prevalence and severity of chronic respiratory disease, including chronic obstructive pulmonary disease (COPD) and bronchiectasis, increase as a person ages.27 The relationship between chronic respiratory disease and TB, both as a cause and a consequence, is more significant in high TB incidence settings.28 Another condition prevalent among older adults is undernutrition,29 especially among those living in aged care facilities.30 Undernutrition leads to impaired immune function, thereby increasing the risk of TB reactivation and the risk that a new infection will rapidly progress to TB disease.31,32 The detrimental synergistic effect of ageing, diabetes, and undernutrition on the immune system elevates the vulnerability of older adults to develop TB, including from drug-resistant strains.24,33 With improved health care, people living with HIV are also ageing and therefore are at risk of similar age-related comorbidities.34
Clinical disease manifestations
Typical pulmonary TB symptoms such as prolonged cough, haemoptysis, night sweats, chills, fatigue, and loss of appetite are less prominent in older adults with TB,35,36 while those with extrapulmonary TB usually experience symptoms localised to the site of disease. Disseminated TB, which more frequently occurs in immunocompromised individuals or people with immunosenescence, is associated with non-specific symptoms such as unexplained fever and weight loss.35 The presentation of TB-associated symptoms may also be confounded by concurrent comorbidities, making TB more challenging to diagnose in older adults. Radiological features also differ between younger and older adults,37 with older adults less likely to have lung nodules or consolidation on computed tomography (CT)38 or typical apical lung cavities on the chest radiograph.39, 40, 41 The presence of lung nodules and soft tissue masses is also more likely to indicate malignancies in older adults, which may co-exist with TB disease.
Key challenges in addressing TB among older adults
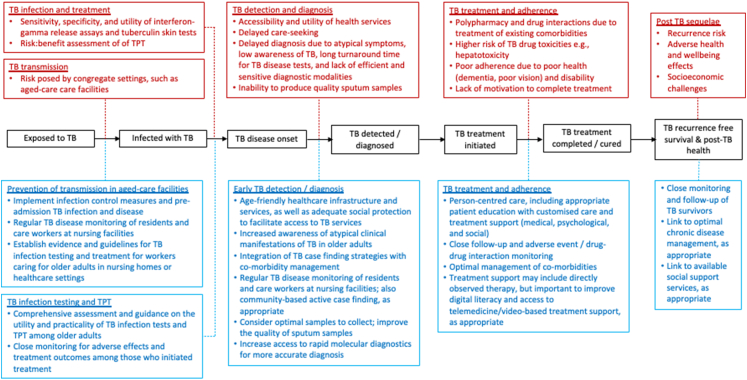
Fig. 1 and Supplementary Table S1 provide an overview of key challenges along the TB care cascade with a brief description of the best practices identified to overcome them.
Fig. 1.
Key challenges faced by older adults along the TB care pathway and the corresponding best practices identified to overcome these barriers. Red boxes represent key challenges, and blue boxes reflect best practices to address them. TB; tuberculosis, TPT; TB preventive treatment.
TB infection management
Aged-care facilities where TB infection is common in older people represent congregate settings with a high risk of TB transmission. In general, the WHO recommends the traditional tuberculin skin test (TST) and newer interferon-gamma-releasing assays (IGRAs) to test for TB infection. Nonetheless, age-related immunosenescence may reduce overall sensitivity,42, 43, 44, 45 although newer generation IGRAs have reported better performance in detecting TB infection among older adults.46 The utility of TST for TB infection testing might also be affected by Bacillus Calmette–Guérin (BCG) vaccination status (BCG coverage by countries and areas is provided in Table 1),47 and nutritional status.48 The systematic screening of older adults for TB infection using TST or IGRA is not currently recommended by the WHO.
A recent study among aged-care residents favoured IGRA over TST in predicting future TB disease.38,49 In practice, the preferred test will depend on local availability, accessibility, and cost,50,51 especially in low-income settings. The adoption of TB infection screening strategies among older adults in the national policies also relies on the availability of evidence, including risk-benefit assessments, which are currently lacking.
Apart from the residents, the caretakers and staff at these facilities are also at risk of TB infection and disease, contributing to TB transmission.52, 53, 54, 55, 56 In China, TB preventive treatment (TPT) among older adults was projected to be the single most impactful intervention to reduce TB incidence and mortality.17 The WHO now conditionally recommends expanded TPT for children ≥5 and adults (in addition to children <5 years) in household contact with someone who has bacteriologically confirmed pulmonary TB who does not have active TB disease. However, the greater risk of drug-related toxicity in older adults is acknowledged with uncertain risk-benefit ratios.57 TPT has important short- and long-term benefits in preventing TB disease among those with TB infection or re-infection.57 TB infection testing and TPT were also recommended for other household contacts of people with bacteriologically confirmed TB and other at-risk populations, such as those receiving tumour necrosis factor inhibitors, dialysis, and organ/haematological transplants. Consideration should also be given to incarcerated people, health workers, homeless people, and people who use drugs.57 In general, however, systematic treatment of older adults for TB infection is not recommended by WHO at present. The adverse effects of the TPT regimen, such as the hepatic adverse effects of isoniazid, the most commonly used TPT drug, in older adults, remain a concern.58,59 Moreover, in a survey among national TB programs of high-burden countries (including lower-middle-income economies in the region: Philippines, Papua New Guinea, Cambodia, and Viet Nam), the lack of time and funding for procurement and implementation of the safer and shorter TPT regimen was reported as barriers to their inclusion in the national policies.60
TB diagnosis and detection
Delayed TB care-seeking
Older adults may face significant challenges in accessing TB services, which can be attributed to various socio-economic and health system challenges. Challenges include a lack of comprehensive support systems to help manage the complex health issues that disproportionately affect this population, as well as physical and financial barriers that impede healthcare access.61, 62, 63, 64, 65, 66 In situations where social security and protection schemes are inadequate, older adults with limited financial means or independence may be reluctant to impose on others to initiate the care-seeking process.66 This could potentially result in more advanced disease at the time of diagnosis, making treatment more difficult and expensive, and incurring a greater cost burden on the person and household affected by TB. For instance, in China, the median time from symptom onset to TB disease diagnosis was >90 days among older adults,67 and this population has 4 times the odds of experiencing catastrophic costs due to TB compared to younger adults.68
A systematic review that assessed healthcare access among older females living in rural settings reported long waiting times (reported by studies in Nigeria, India, the United States of America, and China), limited medical resources (reported by studies in Ghana and South Africa), and poor attitude of healthcare staff towards older adults (reported by studies in South Africa, India, and China), as barriers to health service utilisation.69 In Cambodia, private healthcare-seeking was common upon falling ill with TB, as it was perceived to provide better care and higher quality medicines.70 However, access to TB diagnostics in the Cambodian private sector was reported to be limited.
Older adults are more likely to dismiss TB symptoms due to concurrent medical conditions, such as existing cough due to smoking-induced chronic lung disease, misconceptions of TB disease risk, and fear of discrimination, leading to delayed care-seeking and TB diagnosis.62,64,71 Inadequate TB awareness and knowledge were also documented among older adults who reported lower TB knowledge, attitude, and practice scores in China.72 However, few studies explored care-seeking behaviours and other predictors of delayed TB diagnosis among older adults in the Western Pacific Region. In general, TB care-seeking behaviour is influenced by local cultural practices, beliefs, and social norms,73 as well as universal challenges related to healthcare cost, accessibility and perceived levels of care.
Delayed diagnosis
On presentation to health facilities, TB is often misdiagnosed due to atypical presentation in older adults.55,74,75 TB diagnosis in older adults is further complicated by challenges related to advanced age, such as cognitive impairment, hearing loss, and communication difficulties.71 Non-specific TB symptoms and low awareness among health practitioners,76 especially in lower TB incidence settings, also contribute to delayed diagnosis. Between 2012 and 2016, data from the national health insurance system in the Republic of Korea showed that 7186 people diagnosed with TB were not recognised on initial hospitalisation; the majority (64%) of those with initial missed diagnosis were aged >60 years.77 Clinicians’ lack of responsiveness to health concerns expressed by older adults has also been perceived as a barrier to health care delivery.78
A critical factor in determining the validity of any sputum-based test is the quality of the sputum specimen provided, and older adults may struggle to produce an adequate expectorated sputum sample.15 An assessment of sputum smear results in four countries (including Mongolia) reported higher proportions of low-grade positive smears reported in populations at the extremes of ages.79 Samples with low bacillary load reduce the sensitivity of microscopic examination, resulting in missed cases of TB.79,80 Delays in TB diagnosis and treatment initiation, as well as inappropriate isolation facilitates nosocomial TB transmission.31 There is a wide spectrum of TB disease risk and presentation across finer age groups,81 with the extent of delay in care-seeking, diagnosis and treatment and the outcomes of TB treatment generally worse in the older age groups.41 This may have implications for aged-based surveillance and tailored treatment.
TB treatment and adherence
Drug–drug interactions due to the treatment of existing comorbidities
Polypharmacy, often defined as the simultaneous use of ≥5 medications, is common among older adults. A recent systematic review reported a pooled prevalence of 37% (36% among studies conducted in Asia).82 A separate systematic review conducted among residents of aged-care facilities reported the prevalence of polypharmacy ranging from 38% to 91%.83 The pooled prevalence of polypharmacy among older adults in China was estimated to be 48%, with three-quarters occurring in in-patient settings.84
For older adults with TB, the co-administration of TB treatment with drugs used to manage co-morbid conditions frequently lead to drug–drug interactions that may adversely affect clinical outcomes. For instance, rifampicin could reduce the therapeutic levels of some drugs, such as the antiarrhythmic drug amiodarone, the anticoagulant warfarin and other novel oral anticoagulants, and anti-diabetic drug rosiglitazone.85 Isoniazid may cause increased hepatotoxicity if used with paracetamol, alcohol, or valproic acid.85 The use of common over-the-counter medications, such as antacids, could also reduce the concentrations of isoniazid and rifampicin.85
Higher risk of TB drug toxicities
In general, older adults are at higher risk of adverse drug events than younger people. Increasing age is a risk factor for hepatotoxicity associated with isoniazid and rifampicin.31 The risk of pyrazinamide-related adverse events also increases with age, with liver toxicity and gastrointestinal intolerance being the most commonly reported side effects.86,87 For older adults who are unlikely to tolerate pyrazinamide, a treatment regimen of 9 months without pyrazinamide (2 months of ethambutol, rifampicin, and isoniazid; and 7 months of rifampicin and isoniazid) has been used.88 However, studies in Japan reported that pyrazinamide-containing TB regimens did not lead to significantly higher treatment discontinuation rates, liver toxicity, or death than regimens without pyrazinamide among older adults.89, 90, 91 Nevertheless, even in the absence of pyrazinamide, the frequency of treatment-related adverse events was generally higher in older adults with TB.92 Ethambutol, for instance, has been reported to be the most common drug responsible for major adverse events (e.g., dermatologic, gastrointestinal, arthralgia, liver injuries, and visual changes) among older adults in the Republic of Korea.93 Its potential effect on visual acuity should be considered in the presence of other age-related eye problems such as retinopathy and cataracts.31 Among second-line TB drugs,94 fluoroquinolone use among older adults has been associated with a higher risk of hepatoxicity, tendinopathy, neuropsychiatric reactions, and QT prolongation.95 There is a lack of safety and efficacy data on bedaquiline use in older adults,96 and the use of linezolid (the third group A drug) has been associated with a higher risk of serious adverse events, particularly if used for longer periods.94 In general, there is a lack of safety data on linezolid among older adults.97,98 Most group B and C second-line TB drugs94 have also been reported to be either less tolerable among older adults or to lack age-specific data.74,99
Traditional, complementary, and herbal medicines are ubiquitously used in this region.100 A meta-analysis of randomised controlled trials in 2020 reported that combination therapy of various traditional Chinese medicines and standard TB treatment regimens had higher pulmonary lesion absorption and cavity closure rates than the control group (standard anti-TB regimen).101 However, the evaluated studies lacked rigour and were poorly standardised. The use of concurrent herbal medicines is generally discouraged, given their association with drug-induced liver injury.100 Ironically, its use as a ‘liver protector’ is pervasive among people on TB treatment, especially in China, despite a lack of evidence to support the practice.102 Further systematic evaluation is required.
Poor adherence due to health issues and inadequate support
TB treatment adherence is vital in ensuring a favourable outcome. However, loss to follow-up103 and treatment adherence are often an issue among older adults due to poor health understanding and general disability, as well as more frequent adverse events and drug–drug interactions.31,104, 105, 106 Cognitive impairment, mostly dementia, has also been associated with poor adherence to TB treatment.107 All these factors justify additional and tailored support to facilitate safe treatment completion.31
Post-TB health and rehabilitation
While TB is curable, TB survivors often experience long-term health sequelae, including the risk of future TB recurrence and ongoing socioeconomic challenges even after being cured. Studies conducted among older adults reported decreased lung functions and higher severity of COPD among TB survivors in the Republic of Korea.108,109 The risk of TB-related chronic lung disease is highest in high TB burden countries and among cigarette smokers.28 TB survivors also experience a significantly increased risk of death compared to the general population post-treatment, with most deaths attributed to cardiovascular disease.110, 111, 112, 113 TB and disabilities associated with it may have multiple negative impacts on a survivor's quality of life114, 115, 116 and mental health, compounded by TB-associated stigma.117 Apart from direct health impacts, the socioeconomic implications of TB may also endure post-treatment completion. TB-affected households report ongoing income loss and lack of employment beyond TB treatment completion, which also affects older adults in these households.118
Key interventions in addressing TB among older adults
TB transmission and infection management
Testing for TB infection, disease monitoring, and infection control
Pre-admission screening of risk factors for TB could facilitate early TB case finding.119 In Hong Kong SAR, systematic screening of new admissions to the aged-care facilities for TB infection using IGRA and for TB disease using chest X-rays, was highly cost-effective at US$19,712 and US$29,951 per quality-adjusted life-year (QALY) gained; compared to TB screening using GeneXpert® MTB/RIF and no screening.120 Further to the screening of residents, environmental control measures such as improved ventilation and selective use of ultraviolet germicidal irradiation in aged-care facilities could reduce TB transmission risk.121 Given the risk of infection among residents and staff in aged-care facilities, it is critical to generate evidence and establish clear guidelines for TB infection and disease screening, as well as ongoing TB disease monitoring in settings with high rates of TB infection. Minimum infection control criteria require consideration, with regular reporting and review of practices to limit TB disease and transmission risk.
TB preventive treatment (TPT)
Compared to isoniazid-monotherapy for 6–9 months, rifamycin-based combination TPT for 3–4 months has similar efficacy, lower risk of liver injury, and better treatment adherence.122 Given that the risk of hepatotoxicity associated with isoniazid use in older adults ≥65 years remains a concern,59 4-month rifampicin monotherapy, endorsed by the WHO for HIV-negative individuals,57 could provide a safer alternative for older adults.123 While there is empirical evidence on TPT administration, tolerability, and a TPT completion rate (isoniazid and rifampin-based) of >80% among older adults in the Republic of Korea,124 there remains a paucity of data and specific guidance on TPT use in this population. The risk of adverse events associated with TPT remains a concern (including the shorter regimen).125 Therefore, further assessments on approaches to systematically manage TB infection and TPT in this population are necessary.
Older adults who might be eligible (e.g., at-risk groups such as immunocompromised or documented TB contact) should be carefully assessed, tested for TB infection, and carefully monitored for adverse effects if TPT is initiated. Monitoring should extend beyond TPT administration, and a TPT register should provide an overview of the complete care cascade.126 If thorough risk-benefit assessments and emerging evidence recommend TPT to eligible older adults, timely and effective referrals and the integration of TB infection testing and treatment with other health services for chronic diseases should be explored as a pathway to administer TPT effectively.127,128 It is important that the design of relevant policies and programs should be evidence-based and adhere to the clinical standards for managing TB infection.126
TB diagnosis and detection
Timely diagnosis
WHO-approved NAATs are recommended as the initial test of choice to diagnose TB given improved sensitivity (compared to smear microscopy) and rapid turnaround time (∼2 h to perform the test) with simultaneous drug resistance prediction.129 Therefore, increasing the availability and accessibility of NAATs could facilitate timely diagnosis and treatment initiation.
The incorporation of NAATs in the field, particularly for ACF, was feasible yet resource intensive. Their efficiency hinges on the availability of a comprehensive laboratory and healthcare ecosystem such as workforce capacity, sample transportation, test results communications and follow-up.130 A lack thereof, especially in under-resourced settings, could pose a challenge in timely TB diagnosis and treatment.80
Interventions should also be implemented to improve the quality of sputum samples collected from older adults, especially those who are frail. More invasive approaches to obtain specimens from the respiratory system and early morning gastric aspirates could be used, but the potential benefit should be weighed against the risks of more invasive procedures.35,131 Among people living with HIV, lateral flow urine lipoarabinomannan assay (LF-LAM) could be considered a complementary tool to assist TB disease diagnosis, especially when sputum collection is impossible or disseminated disease is considered.132 LF-LAM's potential value in older adults with immune senescence or other forms of immunocompromise has not been evaluated and requires more formal exploration.
Social protection
Social protection in the form of income replacement and financial grants may reduce TB incidence and mortality in older adults.133 In Cambodia, the provision of health equity funds to support the poor improved access to healthcare services and reduced the overall medical financial burden.134 More specifically, the provision of free TB services, prioritisation of the needs of high-TB risk groups, and reduction of out-of-pocket payments on healthcare under the universal health coverage framework are critical to ensure those affected by the disease have access to the required TB care and rehabilitation.135 Interventions are also required to reduce the risk of falling into poverty after a TB diagnosis.136 It is noteworthy that progress toward UHC differs by country and area in the region (UHC indices range from 33 in Papua New Guinea to 87 in Australia). Therefore, intensified efforts are required by individual countries to develop a strong and more inclusive health systems for all, including those affected by TB.
Active case finding
Interventions to actively seek and diagnose TB disease among older adults are more effective than traditional passive case-finding (PCF).137 Targeted screening of specific risk groups (e.g., people with diabetes or a history of TB) using chest radiography as a screening tool helps to detect previously undiagnosed patients among older adults.138, 139, 140 In Cambodia, the use of computer aided detection (CAD) has resulted in an increased yield of TB detection among older adults.141 Incorporating novel technology combining ultra-mobile digital chest radiographs and CAD is a promising area for further research and scale-up to improve TB detection. Community-based active case-finding models that target older adults can be effective in settings with variable TB burden.141, 142, 143 The experience in the Republic of Korea and Cambodia demonstrated an increased TB yield among older adults compared to expected case notification rates.141,143,144 Beyond older adults-specific approaches, community-wide screening for TB using NAAT in high-incidence settings such as Viet Nam was also shown to lower the prevalence and transmission of TB in the population as a whole.145 Contact investigation in settings like the Philippines, where TB disease and infection are highly prevalent among TB-exposed household members, demonstrated the value of contact investigation for active case finding and prevention of future disease among individuals and affected families.146 However, its impact on community transmission has not been demonstrated. Furthermore, aged-care facilities could consider monitoring and screening healthcare staff and institutionalised older adults for TB disease at regular intervals. Apart from the major benefits of active case finding in high-risk groups, negative aspects related to potential stigma and misdiagnosis should be considered as well.147
TB and healthcare services organisation and governance
To improve the quality of healthcare for older adults, the WHO has introduced a set of age-friendly healthcare principles that seek to enhance the quality of life for the ageing population.61 The adoption of such a framework through training in the core competencies of managing geriatric conditions, enhancing the facility's physical environment, reducing waiting times, introducing an appointment system, and other service improvements should be considered to improve healthcare accessibility for older adults.61
The decentralisation of TB services to primary health care was also instrumental in increasing access to health services.62 In China, systematic screening of TB disease is done in tandem with the annual health screening offered to older adults at their local hospital free of charge, increasing the efficiency of active TB case-finding.17,138,148,149 It has been estimated that the inclusion of active TB case finding during annual health checks in China would result in a 48% and 58% decline in TB disease incidence and mortality, respectively.17 Beyond TB case finding, the integration of services also facilitates screening for other diseases and risk factors, particularly co-morbid conditions like undernutrition, cigarette smoking, diabetes, and excessive alcohol use.150 Such linkages encourage person-centred care and should improve clinical management for TB and other diseases, with better overall health outcomes.150
TB treatment and adherence
Person-centred approaches to treatment monitoring, and support
Person-centred TB care models should include tailored interventions and comprehensive care plans that improve adherence and limit loss to follow-up. These may include customised treatment support that extends beyond directly observed therapy (DOT), as well as education, social and psychological support for people with TB and their close family members.151 Facility-based treatment support requires TB patients to visit a health facility daily to administer medications, which can be challenging for older adults.152 Use of family or community treatment support has been associated with lower default rates,153 higher treatment success rates, and reduced death compared with facility-based treatment support.154,155 Better health services integration would facilitate the monitoring of TB treatment and the management of relevant co-morbidities, improving both the quality and efficiency of care.156
While social protection seeks to address the socioeconomic risk factors for TB and facilitates access to health care, a strong social support system is important to assist recognition of TB symptoms, early diagnosis and treatment completion. Studies have shown that social support improves knowledge, attitude, and beliefs regarding TB, positively impacting TB treatment adherence and outcomes.157,158 In China, a comprehensive system that included health education and peer support groups improved the outcomes of older adults treated for TB.159
Improve digital literacy and access to virtual support
A more person-centred approach could also benefit from the innovative use of new technology. The use of telemedicine, both synchronous (observed live through a video camera) and asynchronous (recorded and sent to treatment observers or health workers for documentation and verification), has been shown to be cost-effective and user-provider friendly in treatment observation.160, 161, 162, 163 As smartphone and mobile internet connectivity becomes more accessible than before, the feasibility of technology-enabled solutions increases. This was demonstrated by the COVID-19-induced health crisis, where the adoption of digital innovations allowed TB care to continue with minimal disruptions.164,165 Beyond direct observation and supervision, technological solutions such as electronic medication monitoring devices could be implemented to support and monitor treatment adherence.166,167 However, uptake of these technology-dependent approaches is likely less favourable among older adults.166 As technology adoption differs widely across the region, it is vital to understand the contextual determinants of acceptability and access to new technology,168 as well as its uptake and utility among older adults.
Post-TB health and rehabilitation
Recognition of the long-term physical and mental health impacts of TB identifies a need to identify, manage and prevent these ramifications.116 Post-TB rehabilitation should be guided by a careful assessment of ongoing physical and psychological needs at the end of TB treatment.
Better linkages between health services to assist in monitoring and follow-up
Considering the growing population of people who have survived TB, better linkages between TB, non-communicable diseases, and social and psychological support programs are essential.169 If required, post-TB care should include follow-up visits with appropriate healthcare professionals to manage ongoing TB-related complications. Ideally, all older adults should have a regular medical follow-up to assess their general health after TB treatment completion, including the possibility of TB recurrence and appropriate management of all co-morbidities.
Data on post-TB outcomes and care needs in older adults
A better understanding of the severity, frequency, and risk factors of adverse post-TB health outcomes113,170 is needed to inform potential interventions. The feasibility of conducting post-TB assessment under routine programmatic conditions has been demonstrated in China, and the experience could be adapted to other settings.171
Future research
Given the rapid growth of the ageing population in the Western Pacific Region and the vulnerability of older adults to TB, more research is required to generate the evidence needed to better prevent and manage TB in this population. Table 2 provides an overview of key knowledge gaps. As aged-care facilities are high-risk environments for TB transmission, the feasibility, effectiveness, and cost-effectiveness of TB screening strategies among institutionalised older adults and their caregivers must be investigated. Beyond aged-care facilities, understanding the access barriers and facilitators of TB service utilisation among older adults is important to assist case finding, treatment adherence and post-treatment follow-up. Understanding all risk factors associated with TB diagnostic delay and poor treatment outcome is essential for improving care. For TB diagnosis, a key challenge is developing an accurate non-sputum-based test. For TB treatment, shorter and safer regimens and more user-friendly approaches to treatment delivery and monitoring are needed. Further down the care cascade, the full range of post-TB sequelae requires better description to enable tailored interventions and care plans for TB survivors. It is also important to consider broader issues such as social protection and relevant ethical principles, with a particular need for more research in low-and-middle-income countries that are also experiencing a demographic transition.
Table 2.
TB research domains and priorities in older adults.
| Domains | Priorities |
|---|---|
| Transmission and infection management |
|
| Diagnosis and case-finding |
|
| Treatment |
|
| Post-TB health and rehabilitation |
|
TB; tuberculosis, TPT; TB preventive treatment.
Conclusion
The TB burden is generally reduced in populations with increased life expectancy, mainly through improved socioeconomic conditions. However, the risk of TB disease in these settings may be increased among older adults with past infection, given that age-associated immune dysfunction increases the risk of TB reactivation. Older adults exhibit atypical features of TB and often present with multiple comorbidities, which may delay TB diagnosis, while their care-seeking behaviour may be influenced by reduced mobility and more difficult interaction with the health care system. Existing comorbidities also complicate TB treatment, due to the higher risk of adverse drug reactions and drug–drug interactions. Post-TB sequelae and ongoing socioeconomic hardship may decrease the quality of life after TB treatment completion.
Age-friendly healthcare infrastructure and services, increased awareness of atypical TB manifestations, and integration of TB case-finding strategies with comorbidity management may assist earlier case detection. Treatment adherence and adverse event monitoring requires increased vigilance and careful consideration of age-considerate technology. Effective infection control measures and routine screening may reduce TB transmission risk, particularly in aged care facilities or other congregate settings.
Further research and innovation, as well as current best practices, should inform the formulation of policies and programmatic guidance to improve TB prevention, detection, and care practices among older adults. Considering the Western Pacific Region's rapidly ageing population and the increasing TB burden observed among older adults, it seems pertinent and timely to develop a roadmap for TB care focussed on this population.
Contributors
T.I., B.J.M., K.R., F.M., K.H.O., K.V., C.W.M.O., S.K., H.K., Y.L., S.Y., H.T.G.T. conceptualised and designed the study. A.K.J.T. conducted the literature search. B.J.M., C.W.M.O., L.K., F.M., K.V., K.R., M.Y. provided additional references. A.K.J.T. and F.M. drafted the manuscript. B.J.M., K.V., K.H.O., T.I., K.R., S.Y., C.W.M.O., S.K., T.Y., A.O., L.K., H.J.K., Y.L., M.Y., K.P., H.T.G.T. critically revised the manuscript. All authors contributed to the final version of the manuscript, reviewed, and approved the manuscript.
Data sharing statement
All data included in this paper are available from the reference list.
Declaration of interests
The authors declare that they have no competing interests.
Acknowledgements
The authors wish to thank Dr Philippe Glaziou and Dr Ernesto Jaramillo for their critical review of the research priorities. This project was funded by 1) the World Health Organization Regional Office for the Western Pacific, with financial contributions from the Government of the Republic of Korea through the Korean Disease Control and Prevention Agency and the Government of Japan through the Ministry of Health, Labour and Welfare, and 2) NUS Start-up Grant. F.M., T.I., K.V., K.H.O., K.R., M.Y., and H.T.G.T. are staff members of WHO. The authors alone are responsible for the views expressed in this publication and they do not necessarily represent the decisions or policies of WHO. The funders did not play any role in the paper design, data collection, data analysis, interpretation, and writing of the paper.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.lanwpc.2023.100770.
Contributor Information
Alvin Kuo Jing Teo, Email: alvin.teo@sydney.edu.au.
Fukushi Morishita, Email: morishitaf@who.int.
Tauhid Islam, Email: islamt@who.int.
Kerri Viney, Email: vineyk@who.int.
Catherine W.M. Ong, Email: catherine.ong@nus.edu.sg.
Seiya Kato, Email: kato@jata.or.jp.
HeeJin Kim, Email: hatchingbird@yahoo.co.kr.
Yuhong Liu, Email: liuyuhong0516@126.com.
Kyung Hyun Oh, Email: ohk@who.int.
Takashi Yoshiyama, Email: yoshiyama1962@yahoo.co.jp.
Akihiro Ohkado, Email: rit.epi.9305@jata.or.jp.
Kalpeshsinh Rahevar, Email: rahevark@who.int.
Lisa Kawatsu, Email: kawatsu@jata.or.jp.
Manami Yanagawa, Email: yanagawam@who.int.
Kiesha Prem, Email: kiesha.prem@nus.edu.sg.
Siyan Yi, Email: siyan@nus.edu.sg.
Huong Thi Giang Tran, Email: tranh@who.int.
Ben J. Marais, Email: ben.marais@sydney.edu.au.
Appendix A. Supplementary data
References
- 1.The Global Health Observatory . 2020. Life expectancy at birth (years)https://www.who.int/data/gho/data/indicators/indicator-details/GHO/life-expectancy-at-birth-(years) [Google Scholar]
- 2.The Global Health Observatory . 2019. Global health estimates: life expectancy and leading causes of death and disability.https://www.who.int/data/gho/data/themes/mortality-and-global-health-estimates/ [Google Scholar]
- 3.Institute for Health Metrics and Evaluation (IHME) IHME, University of Washington; Seattle, Washington: 2021. GBD compare data visualizations.http://vizhub.healthdata.org/gbd-compare [Google Scholar]
- 4.World Health Organization . 2021. Global tuberculosis report 2021. Geneva.https://www.who.int/publications/i/item/9789240037021 [Google Scholar]
- 5.World Health Organization . World Health Organization; Geneva: 2022. Global tuberculosis report 2022. [Google Scholar]
- 6.Li J., Chung P.-H., Leung C.L.K., Nishikiori N., Chan E.Y.Y., Yeoh E.-K. The strategic framework of tuberculosis control and prevention in the elderly: a scoping review towards End TB targets. Infect Dis Poverty. 2017;6:70. doi: 10.1186/s40249-017-0284-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.World Bank . 2022. World Bank country and lending groups.https://datahelpdesk.worldbank.org/knowledgebase/articles/906519 [Google Scholar]
- 8.Sachs J., Lafortune G., Kroll C., Fuller G., Woelm F. Cambridge University Press; Cambridge: 2022. Sustainable development report 2022. From crisis to sustainable developments: the SDGs as roadmap to 2030 and beyond.https://dashboards.sdgindex.org/ [Google Scholar]
- 9.World Health Organization Regional Office for the Western Pacific . World Health Organization Regional Office for the Western Pacific; Manila: 2022. Western Pacific regional framework to end TB 2021-2030.https://apps.who.int/iris/handle/10665/352278 [Google Scholar]
- 10.World Health Organization . 2022. Bacillus Calmette–Guérin (BCG) vaccination coverage.https://immunizationdata.who.int/pages/coverage/BCG.html?YEAR=&CODE= [Google Scholar]
- 11.World Health Organization . 2022. UHC service coverage index (SDG 3.8.1)https://www.who.int/data/gho/data/indicators/indicator-details/GHO/uhc-index-of-service-coverage [Google Scholar]
- 12.Chong K.C., Leung C.C., Yew W.W., et al. Mathematical modelling of the impact of treating latent tuberculosis infection in the elderly in a city with intermediate tuberculosis burden. Sci Rep. 2019;9:4869. doi: 10.1038/s41598-019-41256-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seto J., Wada T., Suzuki Y., et al. Mycobacterium tuberculosis transmission among elderly persons, Yamagata Prefecture, Japan, 2009–2015. Emerg Infect Dis. 2017;23:448–455. doi: 10.3201/eid2303.161571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chee C.B.E., James L. The Singapore Tuberculosis Elimination Programme: the first five years. Bull World Health Organ. 2003;81(3):217–221. [PMC free article] [PubMed] [Google Scholar]
- 15.Negin J., Abimbola S., Marais B.J. Tuberculosis among older adults--time to take notice. Int J Infect Dis. 2015;32:135–137. doi: 10.1016/j.ijid.2014.11.018. [DOI] [PubMed] [Google Scholar]
- 16.Cruz-Hervert L.P., García-García L., Ferreyra-Reyes L., et al. Tuberculosis in ageing: high rates, complex diagnosis and poor clinical outcomes. Age Ageing. 2012;41:488–495. doi: 10.1093/ageing/afs028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huynh G.H., Klein D.J., Chin D.P., et al. Tuberculosis control strategies to reach the 2035 global targets in China: the role of changing demographics and reactivation disease. BMC Med. 2015;13:88. doi: 10.1186/s12916-015-0341-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morishita F., Viney K., Lowbridge C., et al. Epidemiology of tuberculosis in the Western Pacific Region: progress towards the 2020 milestones of the end TB strategy. Western Pac Surveill Response J. 2020;11:10–23. doi: 10.5365/wpsar.2020.11.3.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mori T., Leung C.C. Tuberculosis in the global aging population. Infect Dis Clin North Am. 2010;24:751–768. doi: 10.1016/j.idc.2010.04.011. [DOI] [PubMed] [Google Scholar]
- 20.Kondo A., Oketani N., Kuwabara K., et al. An outbreak of pulmonary tuberculosis probably due to exogenous reinfection at a nursing home for the elderly. Kekkaku. 2002;77:401–408. [PubMed] [Google Scholar]
- 21.Forssman B., Gupta L., Mills K. A tuberculosis contact investigation involving two private nursing homes in inner western Sydney in 2004. NSW Public Health Bull. 2006;17:44–47. doi: 10.1071/nb06011. [DOI] [PubMed] [Google Scholar]
- 22.Shaw A.C., Joshi S., Greenwood H., Panda A., Lord J.M. Aging of the innate immune system. Curr Opin Immunol. 2010;22:507–513. doi: 10.1016/j.coi.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Woo J., Chan H.S., Hazlett C.B., et al. Tuberculosis among elderly Chinese in residential homes: tuberculin reactivity and estimated prevalence. Gerontology. 1996;42:155–162. doi: 10.1159/000213787. [DOI] [PubMed] [Google Scholar]
- 24.Menon S., Rossi R., Nshimyumukiza L., Wusiman A., Zdraveska N., Eldin M.S. Convergence of a diabetes mellitus, protein energy malnutrition, and TB epidemic: the neglected elderly population. BMC Infect Dis. 2016;16:361. doi: 10.1186/s12879-016-1718-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hayashi S., Chandramohan D. Risk of active tuberculosis among people with diabetes mellitus: systematic review and meta-analysis. Trop Med Int Health. 2018;23:1058–1070. doi: 10.1111/tmi.13133. [DOI] [PubMed] [Google Scholar]
- 26.Leung C.C., Lam T.H., Chan W.M., et al. Diabetic control and risk of tuberculosis: a cohort study. Am J Epidemiol. 2008;167:1486–1494. doi: 10.1093/aje/kwn075. [DOI] [PubMed] [Google Scholar]
- 27.Chandrasekaran R., Mac Aogáin M., Chalmers J.D., Elborn S.J., Chotirmall S.H. Geographic variation in the aetiology, epidemiology and microbiology of bronchiectasis. BMC Pulm Med. 2018;18:83. doi: 10.1186/s12890-018-0638-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Byrne A.L., Marais B.J., Mitnick C.D., Lecca L., Marks G.B. Tuberculosis and chronic respiratory disease: a systematic review. Int J Infect Dis. 2015;32:138–146. doi: 10.1016/j.ijid.2014.12.016. [DOI] [PubMed] [Google Scholar]
- 29.Donini L.M., ScarDeLLa P., PioMbo L. Malnutrition in elderly: social and economic determinants. J Nutr Health Aging. 2013;17:7. doi: 10.1007/s12603-012-0374-8. [DOI] [PubMed] [Google Scholar]
- 30.Izawa S., Kuzuya M., Okada K., et al. The nutritional status of frail elderly with care needs according to the mini-nutritional assessment. Clin Nutr. 2006;25:962–967. doi: 10.1016/j.clnu.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 31.Schaaf H.S., Collins A., Bekker A., Davies P.D.O. Tuberculosis at extremes of age. Respirology. 2010;15:747–763. doi: 10.1111/j.1440-1843.2010.01784.x. [DOI] [PubMed] [Google Scholar]
- 32.Macallan D.C. Malnutrition in tuberculosis. Diagn Microbiol Infect Dis. 1999;34:153–157. doi: 10.1016/s0732-8893(99)00007-3. [DOI] [PubMed] [Google Scholar]
- 33.Pradipta I.S., Forsman L.D., Bruchfeld J., Hak E., Alffenaar J.-W. Risk factors of multidrug-resistant tuberculosis: a global systematic review and meta-analysis. J Infect. 2018;77:469–478. doi: 10.1016/j.jinf.2018.10.004. [DOI] [PubMed] [Google Scholar]
- 34.Wing E.J. HIV and aging. Int J Infect Dis. 2016;53:61–68. doi: 10.1016/j.ijid.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 35.Rajagopalan S. Tuberculosis and aging: a global health problem. Clin Infect Dis. 2001;33:1034–1039. doi: 10.1086/322671. [DOI] [PubMed] [Google Scholar]
- 36.Hoheisel G., Hagert-Winkler A., Winkler J., et al. Tuberkulose der Lunge und Pleura im Alter∗. Med Klin. 2009;104:772. doi: 10.1007/s00063-009-1163-y. [DOI] [PubMed] [Google Scholar]
- 37.Lee J.H., Han D.H., Song J.W., Chung H.S. Diagnostic and therapeutic problems of pulmonary tuberculosis in elderly patients. J Korean Med Sci. 2005;20:784–789. doi: 10.3346/jkms.2005.20.5.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tsou P.-H., Huang W.-C., Huang C.-C., et al. Quantiferon TB-Gold conversion can predict active tuberculosis development in elderly nursing home residents. Geriatr Gerontol Int. 2015;15:1179–1184. doi: 10.1111/ggi.12416. [DOI] [PubMed] [Google Scholar]
- 39.Kwon Y.-S., Chi S.Y., Oh I.J., et al. Clinical characteristics and treatment outcomes of tuberculosis in the elderly: a case control study. BMC Infect Dis. 2013;13:121. doi: 10.1186/1471-2334-13-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Byng-Maddick R. Does tuberculosis threaten our ageing populations? BMC Infect Dis. 2016;16:119. doi: 10.1186/s12879-016-1451-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yano S., Kobayashi K., Kato K., Morita M., Tatsukawa T., Ikeda T. The clinical features of ultra-old tuberculosis patients in our hospital. Kekkaku. 2004;79:297–300. [PubMed] [Google Scholar]
- 42.Cho K., Cho E., Kwon S., et al. Factors associated with indeterminate and false negative results of QuantiFERON-TB gold in-tube test in active tuberculosis. Tuberc Respir Dis (Seoul) 2012;72:416–425. doi: 10.4046/trd.2012.72.5.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hang N.T.L., Lien L.T., Kobayashi N., et al. Analysis of factors lowering sensitivity of interferon-γ release assay for tuberculosis. PLoS One. 2011;6 doi: 10.1371/journal.pone.0023806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kamiya H., Ikushima S., Kondo K., et al. Diagnostic performance of interferon-gamma release assays in elderly populations in comparison with younger populations. J Infect Chemother. 2013;19:217–222. doi: 10.1007/s10156-012-0480-x. [DOI] [PubMed] [Google Scholar]
- 45.Kwon Y.S., Kim Y.H., Jeon K., et al. Factors that predict negative results of QuantiFERON-TB gold in-tube test in patients with culture-confirmed tuberculosis: a multicenter retrospective cohort study. PLoS One. 2015;10 doi: 10.1371/journal.pone.0129792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fukushima K., Kubo T., Akagi K., et al. Clinical evaluation of QuantiFERON®-TB Gold Plus directly compared with QuantiFERON®-TB Gold In-Tube and T-Spot®.TB for active pulmonary tuberculosis in the elderly. J Infect Chemother. 2021;27:1716–1722. doi: 10.1016/j.jiac.2021.08.016. [DOI] [PubMed] [Google Scholar]
- 47.Gao L., Lu W., Bai L., et al. Latent tuberculosis infection in rural China: baseline results of a population-based, multicentre, prospective cohort study. Lancet Infect Dis. 2015;15:310–319. doi: 10.1016/S1473-3099(14)71085-0. [DOI] [PubMed] [Google Scholar]
- 48.Chan-Yeung M., Dai D.L.K., Cheung A.H.K., et al. Tuberculin skin test reaction and body mass index in old age home residents in Hong Kong. J Am Geriatr Soc. 2007;55:1592–1597. doi: 10.1111/j.1532-5415.2007.01316.x. [DOI] [PubMed] [Google Scholar]
- 49.Kobashi Y., Mouri K., Yagi S., et al. Clinical utility of the QuantiFERON TB-2G test for elderly patients with active tuberculosis. Chest. 2008;133:1196–1202. doi: 10.1378/chest.07-1995. [DOI] [PubMed] [Google Scholar]
- 50.Rangaka M.X., Wilkinson K.A., Glynn J.R., et al. Predictive value of interferon-γ release assays for incident active tuberculosis: a systematic review and meta-analysis. Lancet Infect Dis. 2012;12:45–55. doi: 10.1016/S1473-3099(11)70210-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Trajman A., Steffen R.E., Menzies D. Interferon-gamma release assays versus tuberculin skin testing for the diagnosis of latent tuberculosis infection: an overview of the evidence. Pulm Med. 2013;2013 doi: 10.1155/2013/601737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yanagihara H. Risk of tuberculosis infection among care workers during an outbreak of tuberculosis at a care facility for the elderly. Kekkaku. 2014;89:631–636. [PubMed] [Google Scholar]
- 53.Moyo N., Trauer J., Trevan P., et al. Tuberculosis screening in an aged care residential facility in a low-incidence setting. Commun Dis Intell Q Rep. 2017;41:E209–E211. [PubMed] [Google Scholar]
- 54.Lin S.-Y., Chien J.-Y., Chiang H.-T., et al. Ambulatory independence is associated with higher incidence of latent tuberculosis infection in long-term care facilities in Taiwan. J Microbiol Immunol Infect. 2021;54:319–326. doi: 10.1016/j.jmii.2019.07.008. [DOI] [PubMed] [Google Scholar]
- 55.Suzuki N. Preventing tuberculosis infection in healthcare settings. Kekkaku. 2019;94:569–573. [Google Scholar]
- 56.Suzuki Y., Sone T. A study on preventive measures against tuberculosis in care facilities for the elderly in a Tokyo metropolitan district. Kekkaku. 2011;86:437–444. [PubMed] [Google Scholar]
- 57.World Health Organization . World Health Organization; Geneva: 2020. WHO consolidated guidelines on tuberculosis. Module 1: prevention: tuberculosis preventive treatment.https://www.who.int/publications/i/item/9789240001503 [PubMed] [Google Scholar]
- 58.Sterling T.R., Villarino M.E., Borisov A.S., et al. Three months of rifapentine and isoniazid for latent tuberculosis infection. N Engl J Med. 2011;365:2155–2166. doi: 10.1056/NEJMoa1104875. [DOI] [PubMed] [Google Scholar]
- 59.Smith B.M., Schwartzman K., Bartlett G., Menzies D. Adverse events associated with treatment of latent tuberculosis in the general population. CMAJ. 2011;183:E173–E179. doi: 10.1503/cmaj.091824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Médecins Sans Frontières . Médecins Sans Frontières (MSF) International; 2020. Step up for TB report 2020.https://www.msf.org/step-tb-report-2020 [Google Scholar]
- 61.World Health Organization . World Health Organization; Geneva: 2004. Active ageing: towards age-friendly primary health care.https://apps.who.int/iris/bitstream/handle/10665/43030/9241592184.pdf?sequence=1&isAllowed=y [Google Scholar]
- 62.Onozaki I., Law I., Sismanidis C., Zignol M., Glaziou P., Floyd K. National tuberculosis prevalence surveys in Asia, 1990–2012: an overview of results and lessons learned. Trop Med Int Health. 2015;20:1128–1145. doi: 10.1111/tmi.12534. [DOI] [PubMed] [Google Scholar]
- 63.Yi S., Teo A.K.J., Sok S., et al. Barriers in access to services and information gaps by genders and key populations in the national tuberculosis programme in Cambodia. Glob Public Health. 2021;17:1–14. doi: 10.1080/17441692.2021.1954226. [DOI] [PubMed] [Google Scholar]
- 64.Wang Y., Feng J., Zhang J., et al. Willingness to seek medical care for tuberculosis and associated factors among the elderly population in Shenzhen: a cross-sectional study. BMJ Open. 2021;11 doi: 10.1136/bmjopen-2021-051291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang X.J., Fu Q., Zhang Z.B., et al. Delay on care-seeking and related influencing factors among tuberculosis patients in Wuhan, 2008-2017. Zhonghua Liu Xing Bing Xue Za Zhi. 2019;40:643–647. doi: 10.3760/cma.j.issn.0254-6450.2019.06.008. [DOI] [PubMed] [Google Scholar]
- 66.Yan F., Thomson R., Tang S., et al. Multiple perspectives on diagnosis delay for tuberculosis from key stakeholders in poor rural China: case study in four provinces. Health Policy. 2007;82:186–199. doi: 10.1016/j.healthpol.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 67.Lin Y., Enarson D.A., Chiang C.-Y., et al. Patient delay in the diagnosis and treatment of tuberculosis in China: findings of case detection projects. Public Health Action. 2015;5:65–69. doi: 10.5588/pha.14.0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yang T., Chen T., Che Y., Chen Q., Bo D. Factors associated with catastrophic total costs due to tuberculosis under a designated hospital service model: a cross-sectional study in China. BMC Public Health. 2020;20:1009. doi: 10.1186/s12889-020-09136-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hamiduzzaman M., De Bellis A., Abigail W., Kalaitzidis E. The social determinants of healthcare access for rural elderly women - a systematic review of quantitative studies. Open Public Health J. 2017;10 doi: 10.2174/1874944501710010244. [DOI] [Google Scholar]
- 70.Teo A.K.J., Ork C., Eng S., et al. Determinants of delayed diagnosis and treatment of tuberculosis in Cambodia: a mixed-methods study. Infect Dis Poverty. 2020;9:49. doi: 10.1186/s40249-020-00665-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sharma M., Onozaki I., Nunn P. TB in older people in Asia: why it is important. Int J Tuberc Lung Dis. 2021;25:521–524. doi: 10.5588/ijtld.21.0191. [DOI] [PubMed] [Google Scholar]
- 72.Wang Y., Gan Y., Zhang J., et al. Analysis of the current status and associated factors of tuberculosis knowledge, attitudes, and practices among elderly people in Shenzhen: a cross-sectional study. BMC Public Health. 2021;21:1163. doi: 10.1186/s12889-021-11240-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Teo A.K.J., Singh S.R., Prem K., Hsu L.Y., Yi S. Duration and determinants of delayed tuberculosis diagnosis and treatment in high-burden countries: a mixed-methods systematic review and meta-analysis. Respir Res. 2021;22:251. doi: 10.1186/s12931-021-01841-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Van den Brande P. Revised guidelines for the diagnosis and control of tuberculosis: impact on management in the elderly. Drugs Aging. 2005;22:663–686. doi: 10.2165/00002512-200522080-00004. [DOI] [PubMed] [Google Scholar]
- 75.Toyota E., Machida K., Nagayama N., et al. Clinical investigation among elderly patients with tuberculosis. Kekkaku. 2010;85:655–660. [PubMed] [Google Scholar]
- 76.Nakao M., Sone K., Kagawa Y., et al. Diagnostic delay of pulmonary tuberculosis in patients with acute respiratory distress syndrome associated with aspiration pneumonia: two case reports and a mini-review from Japan. Exp Ther Med. 2016;12:835–839. doi: 10.3892/etm.2016.3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kim H.W., Myong J.-P., Kim J.S. Estimating the burden of nosocomial exposure to tuberculosis in South Korea, a nationwide population based cross-sectional study. Korean J Intern Med. 2021;36:1134–1145. doi: 10.3904/kjim.2020.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fitzpatrick A.L., Powe N.R., Cooper L.S., Ives D.G., Robbins J.A. Barriers to health care access among the elderly and who perceives them. Am J Public Health. 2004;94:1788–1794. doi: 10.2105/ajph.94.10.1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rieder H.L., Lauritsen J.M., Naranbat N., Katamba A., Laticevschi D., Mabaera B. Quantitative differences in sputum smear microscopy results for acid-fast bacilli by age and sex in four countries. Int J Tuberc Lung Dis. 2009;13:1393–1398. [PubMed] [Google Scholar]
- 80.Parsons L.M., Somoskövi Á., Gutierrez C., et al. Laboratory diagnosis of tuberculosis in resource-poor countries: challenges and opportunities. Clin Microbiol Rev. 2011;24:314–350. doi: 10.1128/CMR.00059-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.The Research Institute of Tuberculosis, Japan Anti-Tuberculosis Association . The Research Institute of Tuberculosis, Japan Anti-Tuberculosis Association; Tokyo: 2020. Tuberculosis annual report: tuberculosis in children and the elderly.https://jata-ekigaku.jp/wp-content/uploads/2022/04/2020_2.pdf [Google Scholar]
- 82.Delara M., Murray L., Jafari B., et al. Prevalence and factors associated with polypharmacy: a systematic review and Meta-analysis. BMC Geriatr. 2022;22:601. doi: 10.1186/s12877-022-03279-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jokanovic N., Tan E.C.K., Dooley M.J., Kirkpatrick C.M., Bell J.S. Prevalence and factors associated with polypharmacy in long-term care facilities: a systematic review. J Am Med Dir Assoc. 2015;16:535.e1–535.e12. doi: 10.1016/j.jamda.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 84.Tian F., Chen Z., Wu J. Prevalence of polypharmacy and potentially inappropriate medications use in elderly Chinese patients: a systematic review and meta-analysis. Front Pharmacol. 2022;13 doi: 10.3389/fphar.2022.862561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Riccardi N., Canetti D., Rodari P., et al. Tuberculosis and pharmacological interactions: a narrative review. Curr Res Pharmacol Drug Discov. 2021;2 doi: 10.1016/j.crphar.2020.100007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kwon B.S., Kim Y., Lee S.H., et al. The high incidence of severe adverse events due to pyrazinamide in elderly patients with tuberculosis. PLoS One. 2020;15 doi: 10.1371/journal.pone.0236109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wada M. Effectiveness and problems of PZA-containing 6-month regimen for the treatment of new pulmonary tuberculosis patients. Kekkaku. 2001;76:33–43. [PubMed] [Google Scholar]
- 88.Wang Y., Chee C., Hsu L., et al. Ministry of health clinical practice guidelines: prevention, diagnosis and management of tuberculosis. Singapore Med J. 2016;57:118–125. doi: 10.11622/smedj.2016051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hagiwara E., Suido Y., Asaoka M., et al. Safety of pyrazinamide-including regimen in late elderly patients with pulmonary tuberculosis: a prospective randomized open-label study. J Infect Chemother. 2019;25:1026–1030. doi: 10.1016/j.jiac.2019.05.030. [DOI] [PubMed] [Google Scholar]
- 90.Takaku T., Saito T., Nemoto K., Oisihi S., Hayashihara K. Comparison of adverse effects in tuberculosis patients over 80 years of age with and without pyrazinamide treatment. Eur Respir J. 2017;50 doi: 10.1183/1393003.congress-2017.PA3045. [DOI] [Google Scholar]
- 91.Miyazawa N., Horita N., Tomaru K., et al. Comparison of drug-induced hepatitis occurring in elderly and younger patients during anti-tuberculosis treatment with a regimen including pyrazinamide. Kekkaku. 2013;88:297–300. [PubMed] [Google Scholar]
- 92.Hase I., Toren K.G., Hirano H., et al. Pulmonary tuberculosis in older adults: increased mortality related to tuberculosis within two months of treatment initiation. Drugs Aging. 2021;38:807–815. doi: 10.1007/s40266-021-00880-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jeong J.I., Jung B.H., Kim M.H., et al. The influence of adverse drug reactions on first-line anti-tuberculosis chemotherapy in the elderly patients. Tuberc Respir Dis. 2009;67:325. [Google Scholar]
- 94.World Health Organization . World Health Organization; Geneva: 2019. WHO consolidated guidelines on drug-resistant tuberculosis treatment.http://www.ncbi.nlm.nih.gov/books/NBK539517/ [PubMed] [Google Scholar]
- 95.Stahlmann R., Lode H.M. Risks associated with the therapeutic use of fluoroquinolones. Expert Opin Drug Saf. 2013;12:497–505. doi: 10.1517/14740338.2013.796362. [DOI] [PubMed] [Google Scholar]
- 96.Fox G.J., Menzies D. A review of the evidence for using bedaquiline (TMC207) to treat multi-drug resistant tuberculosis. Infect Dis Ther. 2013;2:123–144. doi: 10.1007/s40121-013-0009-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lan Z., Ahmad N., Baghaei P., et al. Drug-associated adverse events in the treatment of multidrug-resistant tuberculosis: an individual patient data meta-analysis. Lancet Respir Med. 2020;8:383–394. doi: 10.1016/S2213-2600(20)30047-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Singh B., Cocker D., Ryan H., Sloan D.J. Linezolid for drug-resistant pulmonary tuberculosis. Cochrane Database Syst Rev. 2019;3(3) doi: 10.1002/14651858.CD012836.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lauzardo M., Peloquin C.A. Tuberculosis therapy for 2016 and beyond. Expert Opin Pharmacother. 2016;17:1859–1872. doi: 10.1080/14656566.2016.1215428. [DOI] [PubMed] [Google Scholar]
- 100.Devarbhavi H., Aithal G., Treeprasertsuk S., et al. Drug-induced liver injury: Asia pacific association of study of liver consensus guidelines. Hepatol Int. 2021;15:258–282. doi: 10.1007/s12072-021-10144-3. [DOI] [PubMed] [Google Scholar]
- 101.Li X., Li X., Liu Q., et al. Traditional Chinese medicine combined with western medicine for the treatment of secondary pulmonary tuberculosis: a PRISMA-compliant meta-analysis. Medicine (Baltimore) 2020;99 doi: 10.1097/MD.0000000000019567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Liu Q., Garner P., Wang Y., Huang B., Smith H. Drugs and herbs given to prevent hepatotoxicity of tuberculosis therapy: systematic review of ingredients and evaluation studies. BMC Public Health. 2008;8:365. doi: 10.1186/1471-2458-8-365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Son H., Mok J., Lee M., et al. Status and determinants of treatment outcomes among new tuberculosis patients in South Korea: a retrospective cohort study. Asia Pac J Public Health. 2021;33:907–913. doi: 10.1177/10105395211000529. [DOI] [PubMed] [Google Scholar]
- 104.Mallet L., Spinewine A., Huang A. The challenge of managing drug interactions in elderly people. Lancet. 2007;370:185–191. doi: 10.1016/S0140-6736(07)61092-7. [DOI] [PubMed] [Google Scholar]
- 105.Leung C.C., Yew W.W., Chan C.K., et al. Tuberculosis in older people: a retrospective and comparative study from Hong Kong. J Am Geriatr Soc. 2002;50:1219–1226. doi: 10.1046/j.1532-5415.2002.50308.x. [DOI] [PubMed] [Google Scholar]
- 106.Bele S., Jiang W., Lu H., et al. Population aging and migrant workers: bottlenecks in tuberculosis control in rural China. PLoS One. 2014;9 doi: 10.1371/journal.pone.0088290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bea S., Lee H., Kim J.H., et al. Adherence and associated factors of treatment regimen in drug-susceptible tuberculosis patients. Front Pharmacol. 2021;12 doi: 10.3389/fphar.2021.625078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Rhee C.K., Yoo K.H., Lee J.H., et al. Clinical characteristics of patients with tuberculosis-destroyed lung. Int J Tuberc Lung Dis. 2013;17:67–75. doi: 10.5588/ijtld.12.0351. [DOI] [PubMed] [Google Scholar]
- 109.Park H.J., Byun M.K., Kim H.J., et al. History of pulmonary tuberculosis affects the severity and clinical outcomes of COPD. Respirology. 2018;23:100–106. doi: 10.1111/resp.13147. [DOI] [PubMed] [Google Scholar]
- 110.Romanowski K., Baumann B., Basham C.A., Ahmad Khan F., Fox G.J., Johnston J.C. Long-term all-cause mortality in people treated for tuberculosis: a systematic review and meta-analysis. Lancet Infect Dis. 2019;19:1129–1137. doi: 10.1016/S1473-3099(19)30309-3. [DOI] [PubMed] [Google Scholar]
- 111.Fox G.J., Nguyen V.N., Dinh N.S., et al. Post-treatment mortality among patients with tuberculosis: a prospective cohort study of 10 964 patients in Vietnam. Clin Infect Dis. 2019;68:1359–1366. doi: 10.1093/cid/ciy665. [DOI] [PubMed] [Google Scholar]
- 112.Harries A.D., Ade S., Burney P., Hoa N.B., Schluger N.W., Castro J.L. Successfully treated but not fit for purpose: paying attention to chronic lung impairment after TB treatment. Int J Tuberc Lung Dis. 2016;20:1010–1014. doi: 10.5588/ijtld.16.0277. [DOI] [PubMed] [Google Scholar]
- 113.Marais B.J., Chakaya J., Swaminathan S., et al. Tackling long-term morbidity and mortality after successful tuberculosis treatment. Lancet Infect Dis. 2020;20:641–642. doi: 10.1016/S1473-3099(20)30167-5. [DOI] [PubMed] [Google Scholar]
- 114.Pasipanodya J.G., Miller T.L., Vecino M., et al. Using the St. George respiratory questionnaire to ascertain health quality in persons with treated pulmonary tuberculosis. Chest. 2007;132:1591–1598. doi: 10.1378/chest.07-0755. [DOI] [PubMed] [Google Scholar]
- 115.Daniels K.J., Irusen E., Pharaoh H., Hanekom S. Post-tuberculosis health-related quality of life, lung function and exercise capacity in a cured pulmonary tuberculosis population in the Breede Valley District, South Africa. S Afr J Physiother. 2019;75:1319. doi: 10.4102/sajp.v75i1.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Alene K.A., Wangdi K., Colquhoun S., et al. Tuberculosis related disability: a systematic review and meta-analysis. BMC Med. 2021;19:203. doi: 10.1186/s12916-021-02063-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Basham C.A., Romanowski K., Johnston J.C. Life after tuberculosis: planning for health. Lancet Respir Med. 2019;7:1004–1006. doi: 10.1016/S2213-2600(19)30371-6. [DOI] [PubMed] [Google Scholar]
- 118.Meghji J., Gregorius S., Madan J., et al. The long term effect of pulmonary tuberculosis on income and employment in a low income, urban setting. Thorax. 2021;76:387–395. doi: 10.1136/thoraxjnl-2020-215338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ohmori M., Wada M., Mitarai S., et al. Tuberculosis control in health care facilities for the elderly, from the viewpoint of risk management. Kekkaku. 2006;81:71–77. [PubMed] [Google Scholar]
- 120.Li J., Yip B.H.K., Leung C., et al. Screening for latent and active tuberculosis infection in the elderly at admission to residential care homes: a cost-effectiveness analysis in an intermediate disease burden area. PLoS One. 2018;13 doi: 10.1371/journal.pone.0189531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Furuya H. Estimation of environmental control measures for tuberculosis transmission in care facilities for the elderly. Tokai J Exp Clin Med. 2013;38:135–141. [PubMed] [Google Scholar]
- 122.Sterling T.R., Njie G., Zenner D., et al. Guidelines for the treatment of latent tuberculosis infection: recommendations from the national tuberculosis controllers association and CDC, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:16. doi: 10.15585/mmwr.rr6901a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Campbell J.R., Trajman A., Cook V.J., et al. Adverse events in adults with latent tuberculosis infection receiving daily rifampicin or isoniazid: post-hoc safety analysis of two randomised controlled trials. Lancet Infect Dis. 2020;20:318–329. doi: 10.1016/S1473-3099(19)30575-4. [DOI] [PubMed] [Google Scholar]
- 124.Noh C.S., Kim H.I., Choi H., et al. Completion rate of latent tuberculosis infection treatment in patients aged 65 years and older. Respir Med. 2019;157:52–58. doi: 10.1016/j.rmed.2019.09.004. [DOI] [PubMed] [Google Scholar]
- 125.Gao L., Zhang H., Xin H., et al. Short-course regimens of rifapentine plus isoniazid to treat latent tuberculosis infection in older Chinese patients: a randomised controlled study. Eur Respir J. 2018;52 doi: 10.1183/13993003.01470-2018. [DOI] [PubMed] [Google Scholar]
- 126.Migliori G.B., Wu S.J., Matteelli A., et al. Clinical standards for the diagnosis, treatment and prevention of TB infection. Int J Tuberc Lung Dis. 2022;26:190–205. doi: 10.5588/ijtld.21.0753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Tam G., Lai S.W. Is Singapore on track to eliminate tuberculosis by 2030? A policy case study. SAGE Open Med. 2019;7 doi: 10.1177/2050312119851331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Huang H.-L., Huang W.-C., Lin K.-D., et al. Completion rate and safety of programmatic screening and treatment for latent tuberculosis infection in elderly patients with poorly controlled diabetic mellitus: a prospective multicenter study. Clin Infect Dis. 2021;73:e1252–e1260. doi: 10.1093/cid/ciab209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.World Health Organization . World Health Organization; Geneva: 2021. WHO consolidated guidelines on tuberculosos. Module 3: diagnosis - rapid diagnostics for tuberculosis detection. 2021 update.https://www.who.int/publications/i/item/9789240029415 [Google Scholar]
- 130.Camelique O., Scholtissen S., Dousset J.-P., Bonnet M., Bastard M., Hewison C. Mobile community-based active case-finding for tuberculosis among older populations in rural Cambodia. Int J Tuberc Lung Dis. 2019;23:1107–1114. doi: 10.5588/ijtld.18.0611. [DOI] [PubMed] [Google Scholar]
- 131.Pérez-Guzmán C., Vargas M.H., Torres-Cruz A., Villarreal-Velarde H. Does aging modify pulmonary tuberculosis?: a meta-analytical review. Chest. 1999;116:961–967. doi: 10.1378/chest.116.4.961. [DOI] [PubMed] [Google Scholar]
- 132.Bjerrum S., Schiller I., Dendukuri N., et al. Lateral flow urine lipoarabinomannan assay for detecting active tuberculosis in people living with HIV. Cochrane Database Syst Rev. 2019;10 doi: 10.1002/14651858.CD011420.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Reeves A., Basu S., McKee M., Stuckler D., Sandgren A., Semenza J. Social protection and tuberculosis control in 21 European countries, 1995–2012: a cross-national statistical modelling analysis. Lancet Infect Dis. 2014;14:1105–1112. doi: 10.1016/S1473-3099(14)70927-2. [DOI] [PubMed] [Google Scholar]
- 134.Jacobs B., Bajracharya A., Saha J., et al. Making free public healthcare attractive: optimizing health equity funds in Cambodia. Int J Equity Health. 2018;17:88. doi: 10.1186/s12939-018-0803-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ban K. Building a tuberculosis-free world on a foundation of universal health coverage. Lancet. 2019;393:1268–1270. doi: 10.1016/S0140-6736(19)30433-7. [DOI] [PubMed] [Google Scholar]
- 136.Carter D.J., Glaziou P., Lönnroth K., et al. The impact of social protection and poverty elimination on global tuberculosis incidence: a statistical modelling analysis of sustainable development goal 1. Lancet Glob Health. 2018;6:e514–e522. doi: 10.1016/S2214-109X(18)30195-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Liu K., Peng Y., Zhou Q., et al. Assessment of active tuberculosis findings in the eastern area of China: a 3-year sequential screening study. Int J Infect Dis. 2019;88:34–40. doi: 10.1016/j.ijid.2019.07.029. [DOI] [PubMed] [Google Scholar]
- 138.Zhang C., Xia L., Rainey J.J., et al. Findings from a pilot project to assess the feasibility of active tuberculosis case finding among seniors in rural Sichuan Province, China, 2017. PLoS One. 2019;14 doi: 10.1371/journal.pone.0214761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Cheng J., Sun Y.-N., Zhang C.Y., et al. Incidence and risk factors of tuberculosis among the elderly population in China: a prospective cohort study. Infect Dis Poverty. 2020;9:13. doi: 10.1186/s40249-019-0614-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Cheng J., Wang L., Zhang H., Xia Y. Diagnostic value of symptom screening for pulmonary tuberculosis in China. PLoS One. 2015;10 doi: 10.1371/journal.pone.0127725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Codlin A.J., Monyrath C., Ky M., Gerstel L., Creswell J., Eang M.T. Results from a roving, active case finding initiative to improve tuberculosis detection among older people in rural Cambodia using the Xpert MTB/RIF assay and chest X-ray. J Clin Tuberc Other Mycobact Dis. 2018;13:22–27. doi: 10.1016/j.jctube.2018.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Chen C., Yang C.-G., Gao X., et al. Community-based active case finding for tuberculosis in rural western China: a cross-sectional study. Int J Tuberc Lung Dis. 2017;21:1134–1139. doi: 10.5588/ijtld.17.0123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kim H., Kim H.-J., Oh K.-H., Oh H.-W., Choi H. A pilot project of systematic tuberculosis screening in the elderly in a South Korean province. Tuberc Respir Dis (Seoul) 2019;82:194–200. doi: 10.4046/trd.2018.0063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Lee S.H. Active case finding in the elderly tuberculosis in South Korea. Tuberc Respir Dis (Seoul) 2019;82:261–263. doi: 10.4046/trd.2019.0043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Marks G.B., Nguyen N.V., Nguyen P.T.B., et al. Community-wide screening for tuberculosis in a high-prevalence setting. N Engl J Med. 2019;381:1347–1357. doi: 10.1056/NEJMoa1902129. [DOI] [PubMed] [Google Scholar]
- 146.Sia I.G., Orillaza R.B., St Sauver J.L., et al. Tuberculosis attributed to household contacts in the Philippines. Int J Tuberc Lung Dis. 2010;14:122–125. [PubMed] [Google Scholar]
- 147.Biermann O., Klüppelberg R., Lönnroth K., Viney K., Caws M., Atkins S. ‘A double-edged sword’: perceived benefits and harms of active case-finding for people with presumptive tuberculosis and communities—a qualitative study based on expert interviews. PLoS One. 2021;16 doi: 10.1371/journal.pone.0247568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Li J., Liu X.-Q., Jiang S.-W., et al. Improving tuberculosis case detection in underdeveloped multi-ethnic regions with high disease burden: a case study of integrated control program in China. Infect Dis Poverty. 2017;6:151. doi: 10.1186/s40249-017-0365-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Zhang X.-L., Li S.-G., Li H.-T., et al. Integrating tuberculosis screening into annual health examinations for the rural elderly improves case detection. Int J Tuberc Lung Dis. 2015;19:787–791. doi: 10.5588/ijtld.14.0617. [DOI] [PubMed] [Google Scholar]
- 150.Creswell J., Raviglione M., Ottmani S., et al. Tuberculosis and noncommunicable diseases: neglected links and missed opportunities. Eur Respir J. 2011;37:1269–1282. doi: 10.1183/09031936.00084310. [DOI] [PubMed] [Google Scholar]
- 151.World Health Organization Regional Office for Europe . 1st ed. World Health Organization Regional Office for Europe; Copenhagen: 2017. A people-centered model of tuberculosis care: a blueprint for eastern European and central Asian countries.https://www.euro.who.int/__data/assets/pdf_file/0004/342373/TB_Content_WHO_PRO_eng_final.pdf [Google Scholar]
- 152.Hou W.-L., Song F.-J., Zhang N.-X., et al. Implementation and community involvement in DOTS strategy: a systematic review of studies in China [review article] Int J Tuberc Lung Dis. 2012;16:1433–1440. doi: 10.5588/ijtld.12.0080. [DOI] [PubMed] [Google Scholar]
- 153.Hoshino H., Kobayashi N. Evaluation of effect of community DOTS on treatment outcomes by TB surveillance data. Kekkaku. 2006;81:591–602. [PubMed] [Google Scholar]
- 154.Zhang H., Ehiri J., Yang H., Tang S., Li Y. Impact of community-based DOT on tuberculosis treatment outcomes: a systematic review and meta-analysis. PLoS One. 2016;11 doi: 10.1371/journal.pone.0147744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Dobler C.C., Korver S., Batbayar O., et al. Success of community-based directly observed anti-tuberculosis treatment in Mongolia. Int J Tuberc Lung Dis. 2015;19:657–662. doi: 10.5588/ijtld.14.0927. [DOI] [PubMed] [Google Scholar]
- 156.Magee M., Ali M., Prabhakaran D., Ajay V., Narayan K. Cardiovascular, respiratory, and related disorders. 3rd ed. The International Bank for Reconstruction and Development/The World Bank; Washington: 2017. Integrated public health and health service delivery for noncommunicable diseases and comorbid infectious diseases and mental health. [PubMed] [Google Scholar]
- 157.van Hoorn R., Jaramillo E., Collins D., Gebhard A., van den Hof S. The effects of psycho-emotional and socio-economic support for tuberculosis patients on treatment adherence and treatment outcomes – a systematic review and meta-analysis. PLoS One. 2016;11 doi: 10.1371/journal.pone.0154095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Munro S.A., Lewin S.A., Smith H.J., Engel M.E., Fretheim A., Volmink J. Patient adherence to tuberculosis treatment: a systematic review of qualitative research. PLoS Med. 2007;4:e238. doi: 10.1371/journal.pmed.0040238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Li X., Wang B., Tan D., et al. Effectiveness of comprehensive social support interventions among elderly patients with tuberculosis in communities in China: a community-based trial. J Epidemiol Community Health. 2018;72:369–375. doi: 10.1136/jech-2017-209458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.DeMaio J., Schwartz L., Cooley P., Tice A. The application of telemedicine technology to a directly observed therapy program for tuberculosis: a pilot project. Clin Infect Dis. 2001;33:2082–2084. doi: 10.1086/324506. [DOI] [PubMed] [Google Scholar]
- 161.Perry A., Chitnis A., Chin A., et al. Real-world implementation of video-observed therapy in an urban TB program in the United States. Int J Tuberc Lung Dis. 2021;25:655–661. doi: 10.5588/ijtld.21.0170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Story A., Aldridge R.W., Smith C.M., et al. Smartphone-enabled video-observed versus directly observed treatment for tuberculosis: a multicentre, analyst-blinded, randomised, controlled superiority trial. Lancet. 2019;393:1216–1224. doi: 10.1016/S0140-6736(18)32993-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Nguyen T.A., Pham M.T., Nguyen T.L., et al. Video directly observed therapy to support adherence with treatment for tuberculosis in Vietnam: a prospective cohort study. Int J Infect Dis. 2017;65:85–89. doi: 10.1016/j.ijid.2017.09.029. [DOI] [PubMed] [Google Scholar]
- 164.Visca D., Tiberi S., Pontali E., Spanevello A., Migliori G.B. Tuberculosis in the time of COVID-19: quality of life and digital innovation. Eur Respir J. 2020;56(2) doi: 10.1183/13993003.01998-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Migliori G.B., Thong P.M., Akkerman O., et al. Worldwide effects of coronavirus disease pandemic on tuberculosis services, January-April 2020. Emerg Infect Dis. 2020;26:2709–2712. doi: 10.3201/eid2611.203163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Wang N., Shewade H.D., Thekkur P., et al. Electronic medication monitor for people with tuberculosis: implementation experience from thirty counties in China. PLoS One. 2020;15 doi: 10.1371/journal.pone.0232337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Wang N., Guo L., Shewade H.D., et al. Effect of using electronic medication monitors on tuberculosis treatment outcomes in China: a longitudinal ecological study. Infect Dis Poverty. 2021;10:29. doi: 10.1186/s40249-021-00818-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Mason P.H., Lyttleton C., Marks G.B., Fox G.J. The technological imperative in tuberculosis care and prevention in Vietnam. Glob Public Health. 2020;15:307–320. doi: 10.1080/17441692.2019.1650950. [DOI] [PubMed] [Google Scholar]
- 169.Marais B.J., Lönnroth K., Lawn S.D., et al. Tuberculosis comorbidity with communicable and non-communicable diseases: integrating health services and control efforts. Lancet Infect Dis. 2013;13:436–448. doi: 10.1016/S1473-3099(13)70015-X. [DOI] [PubMed] [Google Scholar]
- 170.Migliori G.B., Marx F.M., Ambrosino N., et al. Clinical standards for the assessment, management and rehabilitation of post-TB lung disease. Int J Tuberc Lung Dis. 2021;25:797–813. doi: 10.5588/ijtld.21.0425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Lin Y., Liu Y., Zhang G., et al. Is it feasible to conduct post-tuberculosis assessments at the end of tuberculosis treatment under routine programmatic conditions in China? Trop Med Infect Dis. 2021;6:164. doi: 10.3390/tropicalmed6030164. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.