Plain language summary
Sepsis is the result of a dysregulated host response to an infection and causes high morbidity and mortality at the intensive care units worldwide. Despite intensive research, the current management of sepsis is supportive rather than curative. Therefore, new therapeutic interventions for sepsis and septic shock patients are urgently needed. In this issue of EMBO Reports, Fang et al have used rat sepsis models to show that macrophage‐expressed SPNS2, a major transporter of S1P, is a crucial mediator of metabolic reprogramming of macrophages during sepsis which regulates inflammation via the lactate‐ROS axis.
Subject Categories: Immunology, Metabolism, Signal Transduction
Sepsis is the result of a dysregulated host response to infection. A study in this issue shows that SPNS2, a major transporter of S1P, is a crucial mediator of metabolic reprogramming in macrophages during sepsis.
Sepsis is defined as a life‐threatening organ dysfunction caused by a dysregulated host response to an infection (Singer, 2016). With 49 million cases and 11 million deaths per year worldwide, sepsis remains a huge unmet medical need and challenge for science and the healthcare system (Rudd et al, 2020). The current management of sepsis relies on antibiotic treatment, hemodynamic stabilization, and support of failing organs. One of the key aspects herein is early recognition of sepsis so that (specific) antibiotics and other interventions can be started rapidly before organ dysfunction sets in or aggravates. In general, the improvements made in sepsis management are generally attributed to earlier recognition and rapid intervention, rather than finding new mechanistic insights which may lead to new therapies.
Based on the positive results obtained in preclinical sepsis animal models, a lot of clinical trials have been performed with anti‐inflammatory agents such as glucocorticoids (GCs), cytokine antagonists such as anti‐TNF or coagulation modulating therapies; however, none of these therapies have demonstrated a significant survival benefit in sepsis patients (Cohen et al, 2015). There are several reasons behind the big gap between preclinical animal research and the translation to the bedside. On the one hand, the choice of animal species and sepsis model applied is crucial (Libert et al, 2019). On the other hand, the mechanism under investigation is also of great importance. Current novel immunotherapy approaches in sepsis research are focusing on restoring or enhancing the normal functioning of the immune system to improve the outcome of sepsis patients rather than merely suppressing the immune system (Steinhagen et al, 2020).
Immune cell activation during inflammation is associated with rapid and fundamental changes in their metabolism. Switching from oxidative phosphorylation (OXPHOS) to glycolysis provides energy and building blocks swiftly to meet their rapid division and bioenergetic demands during inflammation. It has become clear that metabolic reprogramming in activated immune cells is not only important for their increased energy demands but can also be directly linked to their immune cell functions (Vandewalle & Libert, 2022).
In this issue of EMBO Reports, Fang et al (2023) have found that Spinster homolog 2 (Spns2) is playing a key role in macrophage metabolic reprogramming (Fig 1). Spns2 is a major transporter of sphingosine‐1‐phosphate (S1P), a C12 small molecule mainly found in endothelial cells, but also found in other cells such as macrophages. The authors found that Spns2 deficiency (Spns2 −/−) in peritoneal macrophages (PMs) is associated with compromised malate–aspartate shuttle (MAS) activity. This shuttle is essential for transferring the electrons from NADH (generated in the cytoplasm) to the electron transport chain supporting OXPHOS in the mitochondria. This process regenerates cytosolic NAD+ for the glycolysis to proceed and electrons in the mitochondria to generate ATP. Compromised MAS function could contribute to the mitochondrial reorganization as well as increased lactate dehydrogenase (LDH) activity observed in Spns2 −/− PMs. These changes support the metabolic switch from OXPHOS toward glycolysis. Indeed, Spns2 −/− PMs are characterized by a reduced respiration rate and increased glucose consumption, increased NAD+/NADH ratio, and increased lactate production in Spns2 −/− PMs under basal conditions. Supplementing Spns2 −/− PMs with S1P is able to restore OXPHOS, implicating an essential role for Spns2 via S1P in managing OXPHOS. The concomitant increase in intracellular lactate in turn promotes the inflammatory response by inducing reactive oxygen species (ROS). ROS generated by macrophages is essential for activating the immune response on the one hand and for bacterial killing on the other hand. Indeed, Spns2 −/− PMs were characterized by hyperinflammation upon LPS stimulation and challenging PMs with Salmonella typhimurium (S. typhimurium) revealed reduced intracellular replication in the KO cells. Taken together, Spns2/S1P signaling in macrophages is important to prevent uncontrolled inflammatory response. Importantly, it remains to be evaluated whether the observations in the Spns2 −/− PMs could be confirmed using a drug targeting Spns2 (e.g., SLF1081851).
Figure 1. Spns2 as an essential immunomodulator of macrophage function during bacterial infection.
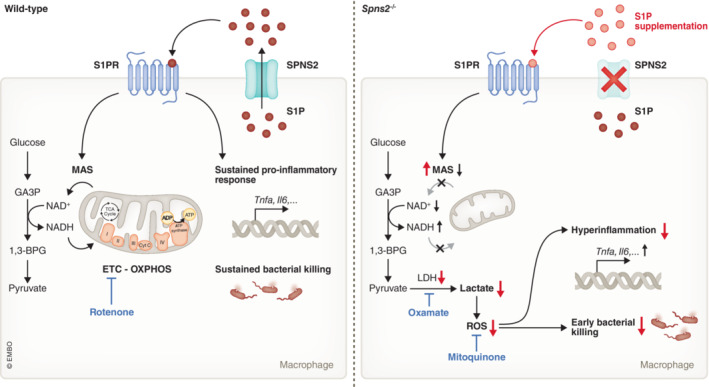
In wild‐type macrophages, S1P will be transported via SPNS2 and will induce autocrine (or paracrine) signaling via its receptor. S1P signaling supports MAS function and promotes mitochondrial dynamics to support OXPHOS. Furthermore, it sustains the pro‐inflammatory cytokine production and bacterial killing of macrophages during inflammation. Spns2 deficiency in macrophages causes more glycolysis due to a reduced functionality of MAS, which leads to lactate accumulation and ROS production. The overactivity of the lactate‐ROS axis drives hyperinflammation and impairs bacterial killing during bacterial infection in a later phase. These effects can be reverted by S1P supplementation (indicated in red), inhibition of LDH activity via oxamate or reducing ROS levels via mitoquinone. OXPHOS, oxidative phosphorylation; MAS, malate–aspartate shuttle; GA3P, Glyceraldehyde‐3‐phosphate; 1,3‐BPG, 1,3‐bisphosphoglycerate.
The authors studied the implications of these findings in vivo on sepsis models using transgenic rats with global deletion of Spns2 (Spns2 −/− rats). In three different sepsis models (cecal ligation and puncture [CLP]‐induced peritoneal sepsis, and Escherichia coli and S. typhimurium infection), Spns2 deficiency led to a severe innate immune response during early stages of sepsis characterized by exacerbated inflammation and high mortality. This hyperinflammatory response was quickly suppressed in the Spns2 −/− rats leading to early innate immunosuppression. The quickly suppressed innate immune response concurred with higher levels of unresolved infection in a later stage in Spns2 −/− rats. These findings reveal a dual role of Spns2 in sepsis, whereby first Spns2 limits the exaggerated inflammatory response in the early phase of sepsis, and in a later phase Spns2 enhances the production of pro‐inflammatory cytokines and bacterial killing.
Targeting Spns2 might thus be a valuable strategy to combat sepsis. In contrast to immunomodulatory molecules targeting the hyperinflammatory (e.g., GCs and anti‐TNF) or hypo‐inflammatory phase, Spns2 covers both phases limiting the risk of treating the patient in the wrong window. Moreover, targeting the glycolysis pathway (via mTOR inhibition, or through pyruvate kinase isoenzyme M2 inhibition) to inhibit cytokine release has been shown to undermine bacterial clearance (Van Wyngene et al, 2018). The hyperinflammatory phase could be prevented by using oxamate or mitoquinone; however, induction of the immunosuppression phase seemed to be independent of lactate. In contrast, S1P injection in Spns2 −/− rats was able to restore cytokine expression in the late phase and enhanced survival upon CLP surgery in these mice (Fang et al, 2023). These observations indicate that Spns2/S1P signaling is necessary to prevent innate immunosuppression. It remains to be studied whether S1P is also able to reduce mortality in WT septic subjects. Plasma S1P levels inversely correlate with SOFA score in sepsis patients (Winkler et al, 2019) supporting the potential of S1P to reduce sepsis severity. Whether Spns2 levels on macrophages are reduced upon a septic insult has not yet been described. Of note, Liu et al (2020) have shown that suppression of Spns2 expression in alveolar macrophages via LncRNA‐5657 silencing alleviated sepsis‐induced lung injury by inhibiting the inflammatory response, indicating that the role of Spns2 might be organ‐dependent (Liu et al, 2020). Furthermore, to further pinpoint the specific role of Spns2 in macrophages in vivo, the authors might want to consider working with a macrophage‐specific knockout line for Spns2.
To date, no clinical trials have been performed targeting the Spns2/S1P signaling. To really translate these findings to human sepsis patients, additional experiments in animal models with increased relevance for human sepsis, for example, pig sepsis models should be considered to close the big gap between the positive preclinical data obtained and before these results can be translated to the bedside.
Disclosure and competing interests statement
The author declare that they have no conflict of interest.
Acknowledgements
JV holds an FWO postdoctoral fellowship (1220924N‐7028), and TV is funded by an FWO‐SBO‐funded project (S003122N).
EMBO reports (2023) 24: e57615
See also: C Fang et al (August 2023)
References
- Cohen J, Vincent JL, Adhikari NK, Machado FR, Angus DC, Calandra T, Jaton K, Giulieri S, Delaloye J, Opal S et al (2015) Sepsis: a roadmap for future research. Lancet Infect Dis 15: 581–614 [DOI] [PubMed] [Google Scholar]
- Fang C, Ren P, Bian G, Wang J, Bai J, Huang J, Ding Y, Li X, Li M, Hou Z (2023) Enhancing Spns2/S1P in macrophages alleviates hyperinflammation and prevents immunosuppression in sepsis. EMBO Rep 24: e56635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libert C, Ayala A, Bauer M, Cavaillon JM, Deutschman C, Frostell C, Knapp S, Kozlov AV, Wang P, Osuchowski MF et al (2019) Part II: minimum quality threshold in preclinical sepsis studies (MQTiPSS) for types of infections and organ dysfunction endpoints. Shock 51: 23–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Hu S, Zhao N, Shao Q, Li Y, Jiang R, Chen J, Peng W, Qian K (2020) LncRNA‐5657 silencing alleviates sepsis‐induced lung injury by suppressing the expression of spinster homology protein 2. Int Immunopharmacol 88: 106875 [DOI] [PubMed] [Google Scholar]
- Rudd KE, Johnson SC, Agesa KM, Shackelford KA, Tsoi D, Kievlan DR, Colombara DV, Ikuta KS, Kissoon N, Finfer S et al (2020) Global, regional, and national sepsis incidence and mortality, 1990–2017: analysis for the Global Burden of Disease Study. Lancet 395: 200–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer M (2016) The new sepsis consensus definitions (Sepsis‐3): the good, the not‐so‐bad, and the actually‐quite‐pretty. Intensive Care Med 42: 2027–2029 [DOI] [PubMed] [Google Scholar]
- Steinhagen F, Schmidt SV, Schewe JC, Peukert K, Klinman DM, Bode C (2020) Immunotherapy in sepsis ‐ brake or accelerate? Pharmacol Ther 208: 107476 [DOI] [PubMed] [Google Scholar]
- Van Wyngene L, Vandewalle J, Libert C (2018) Reprogramming of basic metabolic pathways in microbial sepsis: therapeutic targets at last? EMBO Mol Med 10: e8712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandewalle J, Libert C (2022) Sepsis: a failing starvation response. Trends Endocrinol Metab 33: 292–304 [DOI] [PubMed] [Google Scholar]
- Winkler MS, Märtz KB, Nierhaus A, Daum G, Schwedhelm E, Kluge S, Gräler MH (2019) Loss of sphingosine 1‐phosphate (S1P) in septic shock is predominantly caused by decreased levels of high‐density lipoproteins (HDL). J Intensive Care 7: 1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]


