Abstract
Zingiber officinale and Citrus limon, well known as ginger and lemon, are two vegetals widely used in traditional medicine and the culinary field. The juices of the two vegetals were evaluated based on their inflammation, both in vivo and in vitro. High-performance liquid chromatography (HPLC) was used to characterize different juices from Zingiber officinale Roscoe and Citrus limon. After the application of the HPLC method, different compounds were identified, such as 6-gingerol and 6-gingediol from the ginger juice and isorhamnetin and hesperidin from the lemon juice. In addition, the two juices and their formulation were assessed for their anti-inflammatory activity, in vitro by utilizing the BSA denaturation test, in vivo using the carrageenan-induced inflammation test, and the vascular permeability test. Important and statistically significant anti-inflammatory activities were observed for all juices, especially the formulation. The results of our work showed clearly that the Zingiber officinale and Citrus limon juices protect in vivo the development of the rat paw edema, especially the formulation F composed of the Zingiber officinale and Citrus limon juices, which shows an anti-inflammatory activity equal to −35.95% and −44.05% using 10 and 20 mg/kg of the dose, respectively. Our work also showed that the formulation was the most effective tested extract since it inhibits the vascular permeability by −37% and −44% at the doses of 200 and 400 mg/kg, respectively, and in vitro via the inhibition of the denaturation of BSA by giving a synergetic effect with the highest IC50 equal to 684.61 ± 7.62 μg/mL corresponding to the formulation F. This work aims to develop nutraceutical preparations in the future and furnishes the support for a new investigation into the activities of the various compounds found in Zingiber officinale Roscoe and Citrus limon.
1. Introduction
Inflammation is a complicated physiological and pathological process. Inflammation is generally an adaptive response resulting from dangerous stimuli and conditions (which include contamination and tissue damage) to maintain the framework of homeostasis. Inflammation may be divided into acute infection and persistent infection. The acute infection is best short-lived and is usually beneficial to the host. However, if the infection persists for an extended period, it becomes persistent. It might contribute to many persistent diseases, including arthritis; diabetes obesity; pancreatitis; neurodegenerative, cardiovascular, and metabolic diseases; and some cancers.1
Numerous pathogenic factors, along with tissue injury, cardiac infarction, or infection, can result in infection inflicting tissue damage. The origin of infection may be infectious such as viruses, bacteria, or other microorganisms or non-infectious such as frostbite, physical injury, burn, ionizing radiation, toxins, alcohol, chemical irritants, or excitement. In reply to tissue injury, the framework initiates a chemical signaling cascade that stimulates responses toward recovering touched tissues. These alerts set off leukocyte chemotaxis from the overall flow to damaged locations. These operated leukocytes fabricate cytokines that result in inflammatory responses.2
Over the past decades, pharmaceutical research has deciphered the chemical composition of the properties of many medicinal plants. The pharmaceutical industry has succeeded in chemically reproducing many of their components and in discovering new combinations for the benefit of patients and the protection of natural resources.3
Potent anti-inflammatory molecules could represent treatments for many health problems.4 Thus, polyphenols of botanical sources have demonstrated an anti-inflammatory power in vitro and in vivo, featuring their beneficial role as therapeutic mechanisms in numerous acute and chronic maladies.5 Consequently, numerous epidemiological and experimental studies have investigated dietary polyphenols’ anti-inflammatory and immune-modulating activities.6,7
Ginger is a herbal compound with the first-rate ability for sickness treatment. A massive range of research has proved that ginger has many organic capacities, including anti-inflammatory impact, which is a notable characteristic. Inflammation is a complicated and extensive physiological and pathological process.8 The chemical composition of ginger has been widely studied since9−11 all of the studies were able to determine the chemical composition of Zingiber officinale, and all found that Zingiber officinale was composed of a wide range of molecules, where 6-gingerol was the main compound, accompanied by several other bioactive compounds such as 4-gingerol and 8-gingerol.
Citrus limon fruits carry high proportions of molecules that have health benefits, including polyphenols, tocopherols, carotenoids, and ascorbic acid.12 They have a significant value in traditional medicine and making edible products13 as they present several biological effects, such as antioxidant, anti-cancer, antimicrobial, and anti-inflammatory activities.14 Consumption of fresh citrus fruits or their juices seems to be correlated with ameliorated blood lipid profiles, survival in the elderly, lower risk of cancers, decreased blood pressure, diminished risk of heart maladies’ occurrence, and treatment of obesity.15Citrus limon fruits also have anti-allergic properties due to their richness in hesperidin and quercetin, which are inhibitors of histamine, a neurotransmitter implicated in allergic and inflammatory reactions.16 The anti-cancer activity of flavonoids can occur through two effects according to ref (17).
This article aims to study Zingiber officinale and Citrus limon juices, especially the formulation composed of the ginger and lemon juices for their anti-inflammatory capacity, because of their biological agents and phenolic molecules, which were recognized by the HPLC method.
2. Materials and Methods
2.1. Plant Material
2.1.1. Ginger (Zingiber officinale Roscoe)
The Zingiber officinale rhizomes were acquired from a herbalist from the town of Oujda, washed with distilled water to eliminate any impurity, and then authenticated by Pr. Mohammed Fennane of the Scientific Institute of Rabat. A reference voucher specimen was placed in the Sciences Faculty’s herbarium of the Mohamed First University (Oujda, Morocco) with the reference number (HUMPOM-352).
2.1.2. Lemon (Citrus limon L.)
The Citrus limon culture was obtained from the market of the metropolis of Oujda, nicely washed, and then authenticated by Pr. Mohammed Fennane of the medical institute of Rabat. A voucher specimen wide variety was placed in the Sciences Faculty’s herbarium of the Mohamed First University (Oujda, Morocco) with the reference wide variety (HUMPOM-450).
2.2. Juice Extraction and Formulation Preparation
2.2.1. Ginger Juice Extraction
The ginger rhizomes (500 g) were minced into minor pieces and then ground in a blender to extract maximum juice from the ginger, all under ambient conditions. After this, the resulting juice (G.J.) was filtered, concentrated using a rotary vacuum evaporator, and then dried and stored at −20 °C until needed. The extraction yield was 2.3% (w/w).
2.2.2. Lemon Juice Extraction
500 g of the lemon was washed and then cut into smaller pieces and cold ground for 1–2 min until lemon juice was obtained. After filtering through a filter paper, the lemon juice (L.J.) was concentrated through a rotary evaporator and stored at −20 °C until needed. The extraction yield was 2% (w/w).
2.2.3. Formulation of Ginger and Lemon Juices
The formulation of Zingiber officinale juice and Citrus limon juice (F) tested was composed of two juices: ginger and lemon juices. The formulation consisted of 50% ginger juice (G.J.) and 50% lemon juice (L.J.). This exact formulation was due to the mixture of ginger and lemon juices and also due to studies previously done on the formulation containing Zingiber officinale and Citrus limon with different biological effects.18−21
2.3. Chemicals
Methanol, acetonitrile, formic acid, Folin–Ciocalteu, gallic acid, quercetin, phosphate (Na3PO4), sodium carbonate (Na2CO3), aluminum chloride (AlCl3), sodium hydroxide (NaOH), sodium nitrate (NaNO3), sodium phosphate dibasic (Na2HPO4), sodium phosphate monobasic (NaH2PO4), carrageenan, indomethacin, B.S.A., Evans blue, and acetic acid were all bought from Sigma Chemical Co. (Taufkirchen, Germany).
2.4. Determination of Bioactive Compounds in Zingiber officinale and Citrus limon Juices
2.4.1. Determination of Total Phenols
The total polyphenol amounts of Zingiber officinale and Citrus limon juices were determined using the marginally changed Folin–Ciocalteu colorimetric method.22 1 mL of Folin–Ciocalteu reagent was mixed with 0.2 mL of every extract concentration. After 5 min of incubation at room temperature, 0.8 mL of the aqueous sodium carbonate solution (7.5%) was added to the mixture. Then, the samples were vortexed and incubated in the dark for 1 h. The absorbances were recorded afterward at 760 nm against a blank solution containing 0.2 mL of distilled water, 1 mL of Folin–Ciocalteu reagent, and 0.8 mL of the aqueous sodium carbonate solution (7.5%). Gallic acid was used to acquire a calibration line. The number of general polyphenols was expressed as mg gallic acid/g plant extract of the gallic acid equivalents. All measurements were performed in triplicate.
2.4.2. Determination of Flavonoids
The flavonoid content was determined spectrophotometrically in line with that of Chen et al.23 with a few modifications and using a technique primarily based on forming a flavonoid-aluminum complex with an absorption maximum at 430 nm. To 0.2 mL of every extract (0.5 mg/mL), 50 μL of sodium nitrate (NaNO3, 5% w/v) and 1 mL of bidistilled water were added, and after 6 min, 120 μL of aluminum chloride (AlCl3, 10% w/v) was added. After 5 min, the reaction mixture was basified with 400 μL of NaOH (1 M). The absorbances were recorded at 430 nm toward a blank solution containing 50 μL of NaNO3 (5%), 0.2 mL of bidistilled water, and 120 μL of AlCl3 (10%)]; quercetin was used to determine a calibration line, and the flavonoid content material was expressed as mg quercetin/g plant extract of the quercetin equivalents. Three replicates were taken for every pattern tested.
2.4.3. HPLC Chromatography of Zingiber officinale and Citrus limon Juices
The qualitative evaluation of phenolic compounds found in Zingiber officinale and Citrus limon juices was performed using a high-overall performance liquid chromatography (HPLC) system (Waters Alliance 2695 system, Milford, MA, USA) using standards, such as 4-gingerol, 6-gingerol, 6-gingediol, eriodictyol, rutin, hesperidin, and isorhamnetin.
HPLC of the two juices was done in previous work.21
Indeed, the chromatographic separation was done on a reversed section C18 column (250 × 4.6 mm, 5 μm pore size).
The mobile phase was composed of solvent A: water-formic acid (90:10 (v/v)) and solvent B: water, methanol, and acetonitrile (40:50:10 (v/v)).
The phenolic molecules were eluted with the use of the subsequent gradient:
0 min: 88% A + 12% B;
20 min: 70% A + 30% B;
30 min: 0% A + 100% B;
45 min: 88% A + 12% B.
The eluent flow was 1 mL/min with the drift charge turned into same to at least 1 mL/min with the administered quantity at 20 μL. All studies were carried out at room temperature. Standard and extract solutions ofZingiber officinaleandCitrus limon(G.J., L.J., and F) were liquefied in methanol and filtered via a millipore membrane (0.45 μm).
2.5. Study of the Anti-Inflammatory Activity of Ginger and Lemon Juices
2.5.1. Effects of Ginger and Lemon Juices on the Inhibition of Heat-Induced Denaturation of Bovine Serum Albumin (BSA)
The effect of ginger and lemon juices on the denaturation of BSA induced by high temperature was studied utilizing the method described by24 with some modifications. BSA (1% w/v) was prepared in phosphate buffer solution (PBS, pH = 6.4), and PBS was utilized as a reference. The reaction mixes were stored at 37 °C for 20 min; the temperature was intensified to keep the test tubes at 70 °C for 5 min. After refrigerating, turbidity was quantified at 660 nm utilizing a UV–vis spectrophotometer (Shimadzu Double Beam UV-2600, Japan). 100% of protein denaturation was designated as the reference. The percent of stopping the BSA denaturation was calculated using the following relationship:
where A1 represents the optical density of the reference and A2 is the optical density of the sample.
2.5.2. Effects of Ginger and Lemon Juices on the Inhibition of Carrageenan-Induced Edema of the Rat Paw
2.5.2.1. Animal Grouping and Treatment
The effect of ginger and lemon juices on carrageenan-induced inflammation was determined following the method reported by Winter et al. with slight adjustments.25
Wistar rats (200 to 250 g) raised at the Faculty of Sciences of Oujda’s animal house were utilized. The anti-inflammatory potential was evaluated utilizing the carrageenan-induced rat paw edema test. Animals were fractionated into 12 groups of 6 rats each.
Each extract is liquefied in sterile bidistilled water and administered intraperitoneally at numerous doses, and then the right hind paw was put in carrageenan solution (1%, w/v).
Group I: The control group was given carrageenan (1%, w/v) in saline solution under the right hind paw;
Groups II and III: Rats received the juice of Zingiber officinale at two doses: 10 and 20 mg/kg, respectively, and then administered carrageenan (1%, w/v) in saline solution under the right hind paw;
Groups IV and V: Rats received the Citrus limon juice at two doses: 10 and 20 mg/kg, respectively, and were then administered carrageenan;
Groups VI and VII: Rats received the formulation at two doses: 10 mg/kg (50% of Zingiber officinale juice and 50% of Citrus limon juice) and 20 mg/kg (50% of Zingiber officinale juice and 50% of Citrus limon juice), respectively, and then administered carrageenan;
Group VIII: Rats received indomethacin at 10 mg/kg and were then administered carrageenan;
Linear paw circumference is computed every hour for 3 h.
Paw circumference was quantified using calipers. Calculations were taken at 0, 1, 2, and 3 h after carrageenan administration.
2.5.2.2. Ethical Approval
The anti-inflammatory studies were done based on the American National Institutes of Health and accredited with the aid of the Vice Dean of the Scientific Research of the Faculty of Sciences, University Mohammed First, Oujda. Indeed, the Vice Dean of Scientific Research at the Faculty of Sciences, University Mohammed First of Oujda attested to the overall admiration of the requirements of animal experimentation through a signed and stamped certificate, approving that each of the animal tests has been performed following the internationally accepted Guide for the care and use of laboratory animals.
2.5.3. Effects of Ginger and Lemon Juices on Acetic Acid-Induced Vascular Permeability in Mice
According to Kou et al.,26 vascular permeability in mice was studied. Eight groups of 10 mice were used, with treated mice receiving a 0.2 mL volume of 200 and 400 mg/kg of ginger and lemon juices or 50 mg/kg of indomethacin orally.
Animals in the control group were given 0.2 mL of a 0.9% NaCl solution, while mice in other treated groups received 0.2 mL of the different extracts (ginger juice, lemon juice, formulation, and indomethacin) orally at different doses (200 and 400 mg/kg for ginger, lemon, and formulation and 50 mg/kg for indomethacin). After 1 h, the mice received an intravenous injection of 10 mL/kg of a 1% Evans blue solution, followed by an intraperitoneal injection of 10 mL/kg of 0.7% acetic acid. Thirty minutes later, the animals were anesthetized by ether after washing the peritoneal cavity with 3 mL of a saline solution of NaCl (9 ‰). The exudate was assembled and centrifuged. The optical density of the supernatant was calculated at 610 nm besides a 0.9% NaCl solution as a blank. The percent of inhibition of vascular permeability is calculated based on the formula:
3. Results
3.1. Determination of Biomolecules in Zingiber officinale and Citrus limon Juices
3.1.1. Determination of Total Phenols
The whole polyphenol content material of the juices of Zingiber officinale and Citrus limon was determined using the Folin–Ciocalteu method; this method confirmed that Zingiber officinale and Citrus limon juices contain an important quantity of polyphenols. Thus, the G.J. includes 18.48 ± 1.14 mg gallic acid equivalent/g extract, and the L.J. includes 25.23 ± 1.54 mg gallic acid equivalent/g extract (Table 1).
Table 1. Polyphenol and Flavonoid Amounts of Ginger and Lemon Juicesa.
| total phenols (mg GAE/g DW) | flavonoids (mg QE/g of DW) | |
|---|---|---|
| G.J. | 18.48 ± 1.14 | 7.26 ± 2.05 |
| L.J. | 25.23 ± 1.54 | 12.75 ± 2.10 |
Values are expressed as “mean ± S.E.M.”; G.J.: ginger juice; L.J.: lemon juice; GAE/g DW: gallic acid equivalent/gram of dry weight; QE/g DW: quercetin equivalent/gram of dry weight.
3.1.2. Determination of Flavonoids
The determination of the flavonoids revealed numerous flavonoids since the contents obtained were 7.26 ± 2.05 and 12.75 ± 2.10 mg eq of quercetin/g of extract for G.J. and L.J., respectively (Table 1). These values indicate that G.J. and L.J. contain a large number of flavonoids.
3.1.3. HPLC Analysis of Zingiber officinale and Citrus limon Juices
The HPLC characterization was carried out on the Zingiber officinale and Citrus limon juices, measured according to the different standards; we could show that the G.J. contains 6-gingerol as the main compound, 4-gingerol, and 6-gingediol (Figure 1A; Table 2).
Figure 1.
HPLC chromatographic profiles of Zingiber officinale (A) and Citrus limon (B) juices.21 Ginger (Zingiber officinale Roscoe), lemon (Citrus limon L.) juices as preventive agents from chronic liver damage induced by CCl4: a biochemical and histological study. Antioxidants. 2022;11(2)/Copyright [2022/Copyright Oussama Bekkouch][MDPI/Copyright Oussama Bekkouch].
Table 2. Peaks’ Characterization ofZingiber officinaleJuice (A)*21.
| peak number | compound | retention time (min) | % of area |
|---|---|---|---|
| 1 | 4-gingerol | 3.97 | 0.81 |
| 2 | 6-gingediol | 6.41 | 0.19 |
| 3 | 6-gingerol | 21.60 | 15.22 |
The HPLC analysis of L.J. showed the existence of several molecules since this analysis allowed us to prove the existence of hesperidin, rutin, isorhamnetin, and eriodictyol (Figure 1B; Table 3).
Table 3. Peaks’ Characterization of Citrus limon Juice (B)a21.
| peak number | compound | retention time (min) | % of area |
|---|---|---|---|
| 1 | eriodictyol | 9.17 | 3.12 |
| 2 | rutin | 13.25 | 5.69 |
| 3 | hesperidin | 16.31 | 13.88 |
| 4 | isorhamnetin | 18.23 | 18.43 |
Bekkouch Oussama et al., ginger (Zingiber officinale Roscoe), lemon (Citrus limon L.) juices as preventive agents from chronic liver damage induced by CCl4: a biochemical and histological study. Antioxidants. 2022;11(2)/Copyright [2022/Copyright Oussama Bekkouch][MDPI/Copyright Oussama Bekkouch].
3.2. Study of the Anti-Inflammatory Activity of Ginger and Lemon Juices
3.2.1. Effects of Ginger and Lemon Juices on the Inhibition of Heat-Induced Denaturation of BSA
The data in Table 4 show that concentrations of 250 to 1000 μg/mL of Zingiber officinale, Citrus limon juices, and diclofenac sodium stopped the denaturation of BSA induced by high temperature in a concentration-dependent way.
Table 4. Effect of Ginger and Lemon Juices on Heat-Induced Denaturation of BSAa.
| extract | concentration (μg/mL) | inhibition (%) | IC50 (μg/mL) |
|---|---|---|---|
| G.J. | 250 | 12.23 ± 0.89 | 838.86 ± 9.81a |
| 500 | 27.91 ± 1.31 | ||
| 1000 | 59.82 ± 2.07 | ||
| L.J. | 250 | 11.1 ± 0.60 | 831.09 ± 9.66a |
| 500 | 29.63 ± 1.41 | ||
| 1000 | 60.09 ± 1.96 | ||
| F | 250 | 17.71 ± 0.92 | 684.61 ± 7.62a |
| 500 | 32.76 ± 1.01 | ||
| 1000 | 74.5 ± 1.99 | ||
| diclofenac sodium | 250 | 35.43 ± 1.61 | 449.32 ± 5.97a |
| 500 | 52.19 ± 1.17 | ||
| 1000 | 91.58 ± 1.45 |
Values are expressed as “mean ± S.E.M.”; G.J.: ginger juice; L.J.: lemon juice; F: formulation of ginger and lemon juices; a: p < 0.001.
The formulation F showed the highest inhibition of BSA denaturation (IC50 = 684.61 ± 7.62 μg/mL), followed by L.J. (IC50 = 831.09 ± 9.66 μg/mL). Then, G.J. (IC50 = 838.86 ± 9, 81 μg/mL), which showed the lowest inhibition of BSA denaturation, is resulted compared to diclofenac sodium, one of the most internationally used anti-inflammatory cures worldwide, which gave an IC50 equal to 449.32 ± 5.97 μg/mL.
3.2.2. Effects of Ginger and Lemon Juices on Acetic Acid-Induced Vascular Permeability in Mice
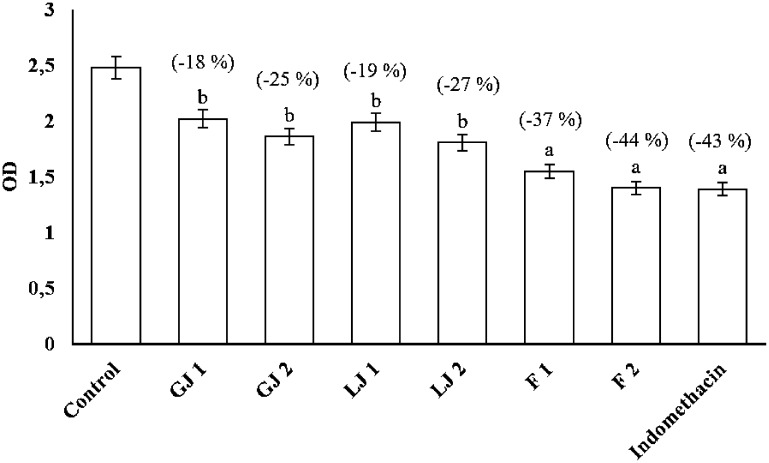
The results obtained show that the groups treated with Zingiber officinale juice and Citrus limon juice or indomethacin 1 h before induction of inflammation by acetic acid significantly (p < 0.001 and p < 0.01) and in a dose-dependent manner reduced their vascular permeability at the peritoneal level (Figure 2). Treatment with 50 mg/kg indomethacin-induced a 44% inhibition of vascular permeability. At the same time, ginger juice decreased vascular permeability by −18 and −25% at the 200 and 400 mg/kg doses, respectively.
Figure 2.
Effects of Zingiber officinale and Citrus limon juices on acetic acid-induced vascular permeability in mice; values are expressed as “mean ± S.E.M.”; G.J. 1: ginger juice at the dose of 200 mg/kg; G.J. 2: ginger juice at the dose of 400 mg/kg; L.J. 1: lemon juice at the dose of 200 mg/kg; L.J. 2: lemon juice at the dose of 400 mg/kg; F 1: formulation of ginger and lemon juices at the dose of 200 mg/kg; F 2: formulation of ginger and lemon juices at the dose of 400 mg/kg a: p < 0.001; b: p < 0.01.
Treating 200 and 400 mg/kg of Citrus limon juice orally induced a – 19 and −27% decrease in vascular permeability. However, the formulation of both juices gave an equivalent inhibition rate of −37 and −44% at 200 and 400 mg/kg, respectively, whose results are quite comparable to those obtained with indomethacin (−43%) (Figure 2).
3.3. Effects of Ginger and Lemon Extracts on the Inhibition of Carrageenan-Induced Edema of the Rat Paw
Table 5 shows that carrageenan increases paw size in rats by 2.16 mm after 3 h, corresponding to +220.67%. However, the extracts of Zingiber officinale and Citrus limon all had anti-inflammatory effects and dose-dependently prevented inflammation of the mouse paws. Thus, the Zingiber officinale juice also showed an anti-inflammatory effect of −27.09 and −34.18%, corresponding to 10 and 20 mg/kg doses, respectively. In addition, the Citrus limon juice revealed an inhibition rate of inflammation in rat paws equal to −26.08 and −38.99%, corresponding to the doses of 10 and 20 mg/kg, respectively. Similarly, the formulation of Zingiber officinale and Citrus limon juices also showed a synergistic effect and gave inhibition rates of −35.95 and −44.05%. Finally, all these extracts were compared to the positive control group treated with indomethacin at a dose of 10 mg/kg and gave an inhibition rate of −44.81% of the installation of edema.
Table 5. Effect of Zingiber officinale and Citrus limon Juices for 3 h on Inflammation of the Paw in Ratsa.
| initial size | 1 h | 2 h | 3 h | difference after 3 h (cm) | inhibition (%) | |
|---|---|---|---|---|---|---|
| control | 1.79 ± 0.05 | 3.31 ± 0.06 | 3.87 ± 0.07 | 3.95 ± 0.06 | 2.16 | - |
| G.J. 1 | 1.83 ± 0.09 | 3.29 ± 0.04 | 3.03 ± 0.05 | 2.88 ± 0.05 | 1.05 | 27.09a |
| G.J. 2 | 1.80 ± 0.06 | 3.15 ± 0.05 | 2.98 ± 0.06 | 2.60 ± 0.05 | 0.80 | 34.18a |
| L.J. 1 | 1.81 ± 0.04 | 3.24 ± 0.06 | 3.08 ± 0.05 | 2.92 ± 0.04 | 1.11 | 26.08a |
| L.J. 2 | 1.78 ± 0.07 | 3.10 ± 0.05 | 2.85 ± 0.05 | 2.41 ± 0.06 | 0.63 | 38.99a |
| F 1 | 1.81 ± 0.05 | 3.17 ± 0.06 | 2.90 ± 0.07 | 2.53 ± 0.07 | 0.72 | 35.95a |
| F 2 | 1.80 ± 0.04 | 2.95 ± 0.05 | 2.76 ± 0.04 | 2.21 ± 0.06 | 0.41 | 44.05a |
| indomethacin | 1.81 ± 0.06 | 2.98 ± 0.07 | 2.61 ± 0.06 | 2.18 ± 0.05 | 0.37 | 44.81a |
Sizes were expressed in millimeters; inhibition of the inflammation was expressed in ≪ % ≫; G.J. 1: group treated with ginger juice at the dose of 10 mg/kg; G.J. 2: group treated with ginger juice at the dose of 20 mg/kg; L.J. 1: group treated with lemon juice at the dose of 10 mg/kg; L.J. 2: group treated with lemon juice at the dose of 20 mg/kg; F 1: group treated with the formulation at the dose of 10 mg/kg; F 2: group treated with the formulation at the dose of 20 mg/kg. Values are expressed as “mean ± S.E.M.”; the control group was compared with the G.J. 1, G.J. 2, L.J. 1, L.J. 2, F 1, and F 2 groups and the indometacin group. a: p < 0.001.
4. Discussion
Inflammation is a defense process of the body whose purpose is to neutralize, fight, or eliminate the pathogen (endogenous or exogenous) and to prepare for tissue repair. Acute inflammation is marked by signs including fever, redness, swelling, and pain.27 Inflammation is usually a helpful operation: its goal is to remove the microbe and restore tissue injury. At times, it could be painful because of the aggression of the microbe, its perseverance, the site of the inflammation, the anomaly of regulation of the inflammatory operation, or by a quantitative or qualitative anomaly of the cells implicated in the inflammation.28
Medicinal plants are widely used in conventional therapy to relieve inflammatory maladies like rheumatoid bronchitis, asthma, arthritis, eczema, osteoarthritis, gout, allergic rhinitis, and gastric and duodenal ulcers.29,30
Plant molecules with an anti-inflammatory effect are mainly polyphenols, sterols, and terpenes.31 Landolfi et al. showed that some polyphenols, as an anti-inflammatory substance, can modify the metabolism of arachidonic acid in platelets.32 For example, the effects of quercetin and myricetin are dose-dependent: at high concentrations, they inhibit cyclooxygenase and lipoxygenase. However, at low concentrations, only lipoxygenase is affected. In contrast, other flavonoids such as apigenin and chrysin act primarily on cycloxygenase activity.
The denaturation of the protein is a natural biochemical reaction that happens throughout a chronic inflammatory replay that can lead to loss of tissue function.33,34 Furthermore, the disintegration of lysosomal membranes throughout chronic inflammation has been shown to liberate pro-inflammatory molecules, counting proteases, histamines, and operated neutrophils, at the localized spot of tissue injury.35,36 Therefore, a medicinal plant that inhibits the denaturation of the protein and stabilizes the cell membrane besides disintegration could serve as a potential source of anti-inflammatory drug candidates.
The results demonstrate that Zingiber officinale and Citrus limon juices stopped the denaturation of BSA induced by heat in a manner dependent on the concentration compared with diclofenac sodium.
Therefore, edema measurement is an excellent tool for quantifying derm inflammation caused by phlogistic molecules such as carrageenan. Carrageenan-induced pate edema is a widely utilized method to examine the skin’s inflammatory process, in addition to identifying anti-inflammatory agents that can be useful in treating skin diseases and in the search for anti-inflammatory extracts and compounds that act on different levels.37
Carrageenan-induced paw edema is one of the famous and widely used assessments searching for natural substances with anti-inflammatory activities.25 It is a susceptible and duplicatable assessment for non-steroidal anti-inflammatory medicaments and has long been confirmed as a valid model for studying new anti-inflammatory medicaments. In addition, carrageenan-induced inflammation helps detect orally active anti-inflammatory molecules; therefore, it has a notable predictive value for anti-inflammatory molecules performing via mediators of acute inflammation.38 It is prominent that the progression of carrageenan-induced edema is a three-step procedure: in the first step (the first 90 min), serotonin and histamine are liberated. The second step (90–150 min) is characterized by kinin, and the third step (after 180 min) is prostaglandin moderated.39 Our results indicate that Zingiber officinale and Citrus limon juices act effectively throughout the third phase of the inflammatory procedure and consequently may act by preventing prostaglandin release and/or action.
The molecular and cellular mechanism through which carrageenan induces the inflammatory procedure is well known. It activates the deliverance of serotonin and histamine from mast cells, thereby beginning a succession of incidents that fabricate other mediators that produce the acute inflammatory response.40 In fact, through the early phase (1–2 h) of the inflammatory reaction, carrageenan induces the fabrication of pro-inflammatory factors such as histamine, serotonin, leukotrienes, P.A.F., and prostanoids. These factors provoke vascular modifications that lead to plasma ejection. Throughout the final phase of this inflammatory process (4–12 h), these chemoattractants provoke neutrophil recruitment by chemotaxis to the inflammatory spot. They release their cytotoxic arsenal and other inflammatory mediators.41 Two populations of inflammatory cells are involved during carrageenan-induced inflammation. Neutrophils predominate during the first 12 h. They are then replaced by monocytes that differentiate into tissue macrophages. These mononuclear cells control the inflammatory reaction till its resolution after 48 h.42 The infiltration of P.M.N.s within the pleural cavity of rats through the primary 3 h after injection of λ-carrageenan was utilized in the present assessment to estimate the in vivo anti-inflammatory activity of Zingiber officinale and Citrus limon juices. We observed that providing oral dose of these extracts to the rats notably diminished the progression of pleurisy. The size of the exudate and the quantity of P.M.N.s migration within the pleural cavity of these rats were significantly reduced. In addition to inhibiting the fabrication of pro-inflammatory mediators, biomolecules of Zingiber officinal and Citrus limon juices inhibit the recruitment of neutrophils to the pleural cavity by inhibiting the expression of adhesion molecules on the wall of the endothelial cells.43
The major characteristics of acute inflammation are the dilatation of vessels, the efflux of plasma, the growth of permeability of vessels, and cell migration (generally neutrophils) within the location of inflammation.44 Enhanced vascular permeability happens because of the contraction and separation of endothelial cells at their boundaries to expose the basement membrane, freely permeable to plasma proteins and fluid.45 Acetic acid brought about vascular permeability is a standard capillary permeability assay in mouse models.46
Zingiber officinale and Citrus limon juices substantially inhibited the increase of vascular permeability, proving the overpowering vascular reaction in the extreme irritation process. Indeed, Zingiber officinale and Citrus limon juices displayed an inhibitory movement in opposition to peritoneal capillary permeability added through acetic acid provocation withinside the mice model.
Alike to those found by Klimek et al., Haidari et al., Tag et al., and De Freitas et al. have all proved an anti-inflammatory power of various extracts of Citrus limon,47−50 effects undoubtedly due to the bioactive compounds of the two vegetals.
The results we found were quite comparable to those found by Mohammed et al., Nile and Park, Lakhan et al., and Li et al., which showed an anti-inflammatory effect of various extracts of Zingiber officinale.51−54 In addition, Habib et al. demonstrated that the ginger extract could diminish the high expression of NFκB and TNF-α in rats with liver cancer.55 NF-κB stimulation is associated with various inflammatory maladies. In experiments with rats, quercetin has been proven to be an essential contributor to ulcer reduction and gastric cell protection. Furthermore, it has been suggested that quercetin exerts its activity via a complex mechanism involving mucus production, inhibiting leukotriene production.56
The HPLC analysis of Zingiber officinale and Citrus limon juices showed that ginger juice is composed of 6-gingerol as the main compound along with 4-gingerol and 6-gingediol.
Flavonoids stop leukocyte migration by inhibiting their adherence to the vascular membrane. This activity would be because of the stopping of the fabrication of IL-1 and TNF-α, the main inciters of the expression of adhering molecules on the vascular wall.57
Indeed, gingerol, shogaol, and several other molecules inhibited prostaglandin biosynthesis by suppressing 5-lipoxygenase or prostaglandin synthetase. In addition, they are also able to stop the fabrication of pro-inflammatory molecules such as IL-1, TNF-α, and IL-8.58
6-Gingerol has been proven to inhibit IL6, IL8, and SAA1 expression in cytokine-inspired HuH7 cells, suppressing COX2 expression. We have additionally proven that the repressing of COX2 is accomplished through the obstruction of the NFκB signaling pathway. Finally, we have proven that S-[6]-gingerol blocks the NFκB/COX2 path by repressing the cytokine-caused oxidative stress.59
In other work by Liang et al., gingerol had an anti-inflammatory power related to the NF-κB pathway by inhibiting the expression of NO, TNF-α, IL-1β, IL-6, and PGE2 and decreasing the expression of iNOS and COX2, by the downregulation of p-IκB and p-p65.60
Jung et al. (2009) reported that the hexane extract of the Zingiber officinale rhizome inhibited the uncontrolled fabrication of NO, P.G.E., TNF-alpha, and IL-1beta.61 The powerful biomolecules of the ginger rhizome to inhibit allergic responses can help treat and prevent allergic diseases.62
Lantz et al. proved that gingerols could block LPS-induced COX-2 expression, showing that essential compounds in ginger can inhibit P.G.E. production.63 Rutin has also shown anti-inflammatory effects because of its NO and TNF-α inhibitory activities and inactivated human neutrophils.64
The HPLC analysis of the Citrus limon juice showed the existence of several molecules as this analysis allowed us to prove the existence of hesperidin, rutin, isorhamnetin, and eriodictyol.
Rutin, one of the compounds found in Citrus limon juice, has been validated for its anti-inflammatory effects by effectively reducing arthritis problems.65
Also, rutin was found to inhibit HMGB1 liberation, downregulate HMGB1-dependent inflammatory reactions in human endothelial cells, and stop HMGB1-mediated hyperpermeability and leukocyte migration in mice.66
Patel and Patel have mentioned in their work the pharmacological activities of rutin regarding its medicinal utilizations and pharmacological effects in distinct biological systems, including the anti-inflammatory activity.67
Another essential molecule in Citrus limon juice, hesperidin, exerted significant anti-inflammatory effects through the Opuntia ficus-indica extract. Tejada et al. and Parhiz et al. demonstrated that hesperidin, another bioactive compound in Citrus limon juice, possessed considerable anti-inflammatory activity.68,69
Hesperidin increased self-renewal capability and chondrogenesis of mesenchymal stem cells, blocked the secretion of pro-inflammatory molecules: IFN-γ, IL-2, IL-4, and IL-10, and repressed the expression of p65, inhibited the secretion of pro-inflammatory cytokines, and stopped the improving impact of hesperidin on the chondrogenesis of mesenchymal stem cells.70
Guazelli et al. (2021) demonstrated the anti-inflammatory consequences of hesperidin methyl chalcone in acetic acid-caused colitis. Hesperidin methyl chalcone blocked colitis-caused tissue oxidative stress, inflammatory molecular infiltration, and pro-inflammatory cytokine manufacturing by blocking NF-kB stimulation.71 In addition, hesperidin methyl chalcone notably diminishes colon edema, macroscopic injuries, colon shortening, and histological harm, displaying an extensive development in colon inflammation.
One of the bioactive compounds found in Citrus limon juice through HPLC is isorhamnetin. It was also found that isorhamnetin possesses extensive pharmacological activities, including anti-inflammatory activity, involving the regulation of PI3K/AKT/PKB, NF-κB, MAPK, and other signaling paths as the expression of associated cytokines and kinases.72
Isorhamnetin extracted from Opuntia ficus-indica significantly decreased the manufacturing of nitric oxide in RAW 264.7 macrophage cells without drastically influencing their viability, while, in vivo, confirmed effectiveness near to indomethacin to decrease rat ear edema caused by croton oil, the effects were moderated by the suppression of COX-2 activity and block on the fabrication of pro-inflammatory cytokines such as TNF-α and IL-6.73
Another discovered molecule by HPLC in Citrus limon juice is eriodictyol. Indeed, pretreatment with eriodictyol notably diminished pulmonary infection and lung damage in mice with LPS-brought-on acute lung lesions, and the protecting impact of eriodictyol may correspond to its capabilities to relieve the immoderate oxidative damage and stop the manufacturing of inflammatory cytokines, which include TNF-α, IL-6, IL-1β, and MIP-2, in macrophages. Additionally, the protecting impact of eriodictyol in acute lung injury can be related to the blocking of NF-κB signaling and the stimulation of the Nrf2 path, which finally causes a significant decrease in inflammatory reactions to oxidative damage in the lung tissue.74
Based on the results of Mokdad-Bzeouich et al., we conclude that eriodictyol has immunomodulating effects on splenocytes, NK cells, and macrophages.75 Furthermore, another study by Wang et al. proved that eriodictyol diminishes the IL-1β-induced inflammatory activity in the chondrocytes of human origin.76 More research on possible mechanisms demonstrated that the protective activity of eriodictyol was performed by stopping NF-κB stimulation via amplifying the Nrf2/HO-1 reaction path.
Taking these data together, the juices of Zingiber officinale and Citrus limon, as well as their formulation, would exert their anti-energetic effect by diminishing the production of inflammatory intermediaries implicated in the course of the steps of the acute inflammatory reaction caused by λ-carrageenan, as well as by stopping the employment of leukocytes to the pleural cavity by exerting antichemoattractant effects on the latter.73
Based on our findings, it is observed that Zingiber officinale and Citrus limon juices possess anti-inflammatory power in vivo and in vitro, either by decreasing or inhibiting prostaglandin synthesis and/or NO production and/or TNF-α synthesis or by decreasing or inhibiting pro-inflammatory cytokine production or by suppressing the pronouncement of genes implicated in the inflammatory process.
5. Conclusions
The different juices of ginger and lemon showed anti-inflammatory activities in vivo by protecting the progress of the rat paw edema and inhibiting the vascular permeability and in vitro via the inhibition of the denaturation of BSA, of which the most effective extract is the formulation F that has shown a considerable synergistic effect, followed by lemon juice and ginger juice; these numerous activities are indeed due to the different biomolecules recognized utilizing HPLC, such as gingediol, gingerol, hesperidin, isorhamnetin, rutin, and eriodyctiol. Additional research studies need to be performed for more utilizations of these vegetals as substitute tools to prevent the treatment of immune and inflammatory maladies, either by stopping the liberation of some pro-inflammatory molecules or by regulating the transcription factors of genes coding for pro-inflammatory substances.
Acknowledgments
M.H.A. author thanks Deanship of Scientific research Taif University for its support for this manuscript.
Author Contributions
Conceptualization: O.B.; Methodology, O.B, S.A., M.H.; Validation: S.A.; Formal Analysis: O.B., I.T., and E.S.; Resources: M.H.A.; Data curation: O.B. and A.K.; Writing and original draft preparation: O.B. and G.Z.; Reviewing and editing: O.B., M.H.A., and S.A.; Supervision: S.A., H.H.; Funding acquisition: M.H.A. All authors have interpreted and admitted to the published version of the manuscript.
The manuscript was fully funded by Taif University, under the reference: ≪TURSP2020/91, Taif University, Taif, K.S.A.≫.
The authors declare no competing financial interest.
References
- Peng Y.; Ao M.; Dong B.; Jiang Y.; Yu L.; Chen Z.; Hu C.; Xu R. Anti-inflammatory effects of curcumin in the inflammatory diseases: Status, limitations and countermeasures. Drug Des., Dev. Ther. 2021, 15, 4503–4525. 10.2147/DDDT.S327378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L.; Deng H.; Cui H.; Fang J.; Zuo Z.; Deng J.; Li Y.; Wang X.; Zhao L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2018, 9, 7204–7218. 10.18632/oncotarget.23208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkele U.; Lobmeyer T. R.. Plantes Médicinales, Identification, Récolte, Propriétés et Emplois. Edition Pa.; 2007.
- Fredman G.; Tabas I. Boosting Inflammation Resolution in Atherosclerosis: The Next Frontier for Therapy. Am. J. Pathol. 2017, 187, 1211–1221. 10.1016/j.ajpath.2017.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scalbert A.; Manach C.; Morand C.; Rémésy C.; Jiménez L. Dietary polyphenols and the prevention of diseases. Crit. Rev. Food Sci. Nutr. 2005, 45, 287–306. 10.1080/1040869059096. [DOI] [PubMed] [Google Scholar]
- Bengmark S. Acute and “chronic” phase reaction – A mother of disease. Clin. Nutr. 2004, 23, 1256–1266. 10.1016/j.clnu.2004.07.016. [DOI] [PubMed] [Google Scholar]
- Yoon J. H.; Baek S. J. Molecular targets of dietary polyphenols with anti-inflammatory properties. Yonsei Med. J. 2005, 46, 585–596. 10.3349/ymj.2005.46.5.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahrajabian M. H.; Sun W.; Cheng Q. Clinical aspects and health benefits of ginger (Zingiber officinale) in both traditional Chinese medicine and modern industry. Acta Agric. Scand., Sect. B 2019, 69, 546–556. 10.1080/09064710.2019.1606930. [DOI] [Google Scholar]
- Asamenew G.; Kim H. W.; Lee M. K.; Lee S. H.; Kim Y. J.; Cha Y. S.; Yoo S. M.; Kim J. B. Characterization of phenolic compounds from normal ginger (Zingiber officinale Rosc.) and black ginger (Kaempferia parviflora Wall.) using UPLC–DAD–QToF–MS. Eur. Food Res. Technol. 2019, 245, 653–665. 10.1007/s00217-018-3188-z. [DOI] [Google Scholar]
- Aly U.; Abbas M.; Taha H.; Gaber E. S. Characterization of 6-Gingerol for In Vivo and In Vitro Ginger (Zingiber officinale) Using High Performance Liquid Chromatography. Global J. Bot. Sci. 2013, 1, 9–17. 10.12974/2311-858x.2013.01.01.2. [DOI] [Google Scholar]
- Tao Y. I.; Li W.; Liang W.; Van Breemen R. B. Identification and quantification of gingerols and related compounds in ginger dietary supplements using high-performance liquid chromatography-tandem mass spectrometry. J. Agric. Food Chem. 2009, 57, 10014–10021. 10.1021/jf9020224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bursal E.; Gülçin I. Polyphenol contents and in vitro antioxidant activities of lyophilised aqueous extract of kiwifruit (Actinidia deliciosa). Food Res. Int. 2011, 44, 1482–1489. 10.1016/j.foodres.2011.03.031. [DOI] [Google Scholar]
- Hoi S. Y.; Ko H. C.; Ko S. Y.; Hwang J. H.; Park J. G.; Kang S. H.; Han S. H.; Yun S. H.; Kim S. J. Correlation between flavonoid content and the NO production inhibitory activity of peel extracts from various citrus fruits. Biol. Pharm. Bull. 2007, 30, 772–778. 10.1248/bpb.30.772. [DOI] [PubMed] [Google Scholar]
- Del Río J. A.; Fuster M. D.; Gómez P.; Porras I.; García-Lidón A.; Ortuño A. Citrus limon: A source of flavonoids of pharmaceutical interest. Food Chem. 2004, 84, 457–461. 10.1016/S0308-8146(03)00272-3. [DOI] [Google Scholar]
- Ramful D.; Tarnus E.; Aruoma O. I.; Bourdon E.; Bahorun T. Polyphenol composition, vitamin C content and antioxidant capacity of Mauritian citrus fruit pulps. Food Res. Int. 2011, 44, 2088–2099. 10.1016/j.foodres.2011.03.056. [DOI] [Google Scholar]
- González-Molina E.; Domínguez-Perles R.; Moreno D. A.; García-Viguera C. Natural bioactive compounds of Citrus limon for food and health. J. Pharm. Biomed. Anal. 2010, 51, 327–345. 10.1016/j.jpba.2009.07.027. [DOI] [PubMed] [Google Scholar]
- Tripoli E.; La G. M.; Giammanco S.; Di M. D.; Giammanco M. Citrus flavonoids: Molecular structure, biological activity and nutritional properties: A review. Food Chem. 2007, 104, 466–479. 10.1016/j.foodchem.2006.11.054. [DOI] [Google Scholar]
- Derrick K.Effect of Zingiber Officinale and Citrus Limon Extracts on Aspergillus Flavus, 2017.
- Chitindingu K.; Marume A.; Pharmaceuticals G. An anti-diabetic poly-herbal medicine prepared from extracts of Annona stenophylla , Citrus limon and Zingiber officinales An Anti-Diabetic Poly-Herbal Medicine Prepared From Extracts Of Annona Stenophylla, Citrus Limon And Zingiber Officinale and Tafadz. Int. J. Pharm. Sci. Res. 2019, 8, 1048. 10.13040/IJPSR.0975-8232.8(3).1048-55. [DOI] [Google Scholar]
- Tiencheu B.; Nji D.; Achidi A. U.; Egbe A. C.; Tenyang N.; Tiepma N.; Eurydice F. D.; Fabrice T. F.; Bertrand T. Nutritional, sensory, physico-chemical, phytochemical, microbiological and shelf-life studies of natural fruit juice formulated from orange (Citrus sinensis), lemon (Citrus limon), Honey and Ginger (Zingiber officinale). Heliyon 2021, 7, e07177 10.1016/j.heliyon.2021.e07177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekkouch O.; Dalli M.; Harnafi M.; Touiss I.; Mokhtari I.; El Assri S.; Harnafi H.; Choukri M.; Ko S. J.; Kim B.; Amrani S. Ginger (Zingiber officinale Roscoe), Lemon (Citrus limon L.) Juices as Preventive Agents from Chronic Liver Damage Induced by CCl4: A Biochemical and Histological Study. Antioxidants 2022, 11, 390. 10.3390/antiox11020390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ainsworth E. A.; Gillespie K. M. Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin – Ciocalteu reagent. Nat. Protoc. 2007, 2, 875–877. 10.1038/nprot.2007.102. [DOI] [PubMed] [Google Scholar]
- Chen G. L.; Chen S. G.; Xie Y. Q.; Chen F.; Zhao Y. Y.; Luo C. X.; Gao Y. Q. Total phenolic, flavonoid and antioxidant activity of 23 edible flowers subjected to in vitro digestion. J. Funct. Foods 2015, 17, 243–259. 10.1016/j.jff.2015.05.028. [DOI] [Google Scholar]
- Chandra S.; Chatterjee P.; Dey P.; Bhattacharya S. Evaluation of in vitro anti-inflammatory activity of coffee against the denaturation of protein. Asian Pac. J. Trop Biomed. 2012, 2, S178–S180. 10.1016/S2221-1691(12)60154-3. [DOI] [Google Scholar]
- Winter C. A.; Risley E. A.; Nuss G. W. Carrageenin-induced edema in hind paw. Exp. Biol. Med. 1962, 3, 544–547. 10.3181/00379727-111-27849. [DOI] [PubMed] [Google Scholar]
- Kou J.; Si M.; Dai G.; Lin Y.; Zhu D. Antiinflammatory activity of Polygala japonica extract. Fitoterapia 2006, 77, 411–415. 10.1016/j.fitote.2006.04.011. [DOI] [PubMed] [Google Scholar]
- Joris M. G.; Joris I.. Cellules, tissus et maladies; Oxford University Press, 2004. [Google Scholar]
- Medzhitov Origin and physiological roles of inflammation. Nature 2008, 454, 428–435. 10.1038/nature07201. [DOI] [PubMed] [Google Scholar]
- Setty A. R.; Sigal L. H. Herbal medications commonly used in the practice of rheumatology: mechanisms of action, efficacy, and side effects. Semin. Arthritis Rheum. 2005, 34, 773–784. 10.1016/j.semarthrit.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Wiart C.Medicinal Plants of Asia and the Pacific, 2006.
- Adedapo A. A.; Jimoh F. O.; Koduru S.; Masika P. J.; Afolayan A. J. Assessment of the medicinal potentials of the methanol extracts of the leaves and stems of Buddleja saligna. BMC Complementary Altern. Med. 2009, 9, 21. 10.1186/1472-6882-9-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landolfi R.; Mower R. L.; Steiner M. Modification of platelet function and arachidonic acid metabolism by bioflavonoids: Structure-activity relations. Biochem. Pharmacol. 1984, 33, 1525. 10.1016/0006-2952(84)90423-4. [DOI] [PubMed] [Google Scholar]
- Sangeetha G.; Vidhya R. In vitro anti-inflammatory activity of different parts of Pedalium murex (L.). Int. J. Herb. Med. 2016, 4, 31–36. [Google Scholar]
- Ikwegbue P. C.; Masamba P.; Oyinloye B. E.; Kappo A. P. Roles of heat shock proteins in apoptosis, oxidative stress, human inflammatory diseases, and cancer. Pharmaceuticals 2017, 11, 2. 10.3390/ph11010002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labu Z. K.; Laboni F. R.; Tarafdar M.; Howlader M. S. I.; Rashid M. H. Membrane stabilization as a mechanism of anti-inflammatory and thrombolytic activities of ethanolic extract of arial parts of Spondiasis pinanata (Family: Anacardiaceae). Pharmacologyonline 2015, 2, 44–51. [Google Scholar]
- Ranasinghe P.; Ranasinghe P.; Kaushalya W. P.; Sirimal P. G. A.; Perera Yashasvi S. G.; Padmalal G.; Saman B. In vitro erythrocyte membrane stabilization properties of Carica papaya L. leaf extracts. Pharm. Res. 2012, 4, 196–202. 10.4103/0974-8490.102261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel Cabrini D. A.; Moresco H. H.; Imazu P.; Silva D. P.; Evelise F. M.; Daniel Augusto G. B.; Prudente A. D. S.; Pizzolatti M. G. B.; Ins M. C. Analysis of the potential topical anti-inflammatory activity of Averrhoa carambola L. in mice. J. Evidence-Based Complementary Altern. Med. 2011, 2011, 908059. 10.1093/ecam/neq026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansouri M. T.; Hemmati A. A.; Naghizadeh B.; Mard S. A.; Rezaie A.; Ghorbanzadeh B. A study of the mechanisms underlying the anti-inflammatory effect of ellagic acid in carrageenan-induced paw edema in rats. Indian J. Pharmacol. 2015, 47, 292–298. 10.4103/0253-7613.157127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock E. M.; Limebeer C. L.; Parker L. A. Effect of cannabidiolic acid and Δ9-tetrahydrocannabinol on carrageenan-induced hyperalgesia and edema in a rodent model of inflammatory pain. Psychopharmacology 2018, 235, 3259–3271. 10.1007/s00213-018-5034-1. [DOI] [PubMed] [Google Scholar]
- Myers M. J.; Deaver C. M.; Lewandowski A. J. Molecular mechanism of action responsible for carrageenan-induced inflammatory response. Mol. Immunol. 2019, 109, 38–42. 10.1016/j.molimm.2019.02.020. [DOI] [PubMed] [Google Scholar]
- Cuzzocrea S.; Mazzon E.; Calabro G.; Dugo L.; De Sarro A.; Van de Loo F. A. J.; Caputi A. P. Inducible nitric oxide synthase - Knockout mice exhibit resistance to pleurisy and lung injury caused by carrageenan. Am. J. Respir. Crit. Care Med. 2000, 162, 1859–1866. 10.1164/ajrccm.162.5.9912125. [DOI] [PubMed] [Google Scholar]
- Gilroy D. W.; Colville-Nash P. R.; Willis D.; Chivers J.; Paul-Clark M. J.; Willoughby D. A. Inducible cyclooxygenase may have anti-inflammatory properties. Nat. Med. 1999, 5, 698–701. 10.1038/9550. [DOI] [PubMed] [Google Scholar]
- Ginwala R.; Bhavsar R.; Chigbu D. G. I.; Jain P.; Khan Z. K. Potential role of flavonoids in treating chronic inflammatory diseases with a special focus on the anti-inflammatory activity of apigenin. Antioxidants 2019, 8, 35. 10.3390/antiox8020035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwood E. R.; Toliver-Kinsky T. Mechanisms of the inflammatory response. Best Pract. Res., Clin. Anaesthesiol. 2004, 18, 385–405. 10.1016/j.bpa.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Goodman L. S.Goodman and Gilman’s the pharmacological basis of therapeutics; McGraw-Hill: New York, 1996; Vol. 1549, pp 1361 −1373. [Google Scholar]
- Antonisamy P.; Dhanasekaran M.; Kim H. R.; Jo S. G.; Agastian P.; Kwon K. B. Anti-inflammatory and analgesic activity of ononitol monohydrate isolated from Cassia tora L. in animal models. Saudi. J. Biol. Sci. 2017, 24, 1933–1938. 10.1016/j.sjbs.2017.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimek-szczykutowicz M.; Szopa A.; Ekiert H. Citrus limon (Lemon) phenomenon—a review of the chemistry, pharmacological properties, applications in the modern pharmaceutical, food, and cosmetics industries, and biotechnological studies. Plants 2020, 9, 119. 10.3390/plants9010119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haidari F.; Mohammadshahi M.; Zarei M.; Fathi M. Protective effect of citrus lemon on inflammation and adipokine levels in acrylamide-induced oxidative stress in rats. Braz. J. Pharm. Sci. 2019, 55, e18285 10.1590/s2175-97902019000218285. [DOI] [Google Scholar]
- Tag H. M.; Kelany O. E.; Tantawy H. M.; Fahmy A. A. Potential anti-inflammatory effect of lemon and hot pepper extracts on adjuvant-induced arthritis in mice. J. Basic Appl. Zool. 2014, 67, 149–157. 10.1016/j.jobaz.2014.01.003. [DOI] [Google Scholar]
- De Freitas R. M.; Campêlo L. M. L.; De Almeida A. A. C.; Antonia A. C.; De Freitas R.; Mendes C.; Gilberto Santos D. S.; Geane F. S.; Gláucio B.; Chistiane M. Antioxidant and antinociceptive effects of Citrus limon essential oil in mice. J. Biomed. Biotechnol. 2011, 2011, 678673 10.1155/2011/678673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammed A.; Ali A.; Owais M.; Yagi S. In vitro anti-inflammatory activity of ginger (Zingiber officinale Rosc.) rhizome, callus and callus treated with some elicitors. J. Med. Plants Res. 2019, 13, 227–235. 10.5897/JMPR2019.6758. [DOI] [Google Scholar]
- Nile S. H.; Park S. W. Chromatographic analysis, antioxidant, anti-inflammatory, and xanthine oxidase inhibitory activities of ginger extracts and its reference compounds. Ind. Crops Prod. 2015, 70, 238–244. 10.1016/j.indcrop.2015.03.033. [DOI] [Google Scholar]
- Lakhan S. E.; Ford C. T.; Tepper D. Zingiberaceae extracts for pain: A systematic review and meta-analysis. Nutr. J. 2015, 14, 50. 10.1186/s12937-015-0038-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X. H.; McGrath K. C. Y.; Nammi S.; Heather A. K.; Roufogalis B. D. Attenuation of Liver Pro-Inflammatory Responses by Zingiber officinale via Inhibition of NF-kappa B Activation in High-Fat Diet-Fed Rats. Basic Clin. Pharmacol. Toxicol. 2012, 110, 238–244. 10.1111/j.1742-7843.2011.00791.x. [DOI] [PubMed] [Google Scholar]
- Habib S. H. M.; Makpol S.; Hamid N. A. A.; Das S.; Ngah W. Z. W.; Yusof Y. A. M. Ginger extract (Zingiber officinale) has anti-cancer and anti-inflammatory effects on ethionine-induced hepatoma rats. Clinics 2008, 63, 807–813. 10.1590/S1807-59322008000600017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Carlo N.; Mascolo A. A.; Izzo F. C. Flavonoids: old and new aspects of a class of natural therapeutic drugs. Life Sci. 1999, 65, 337. 10.1016/S0024-3205(99)00120-4. [DOI] [PubMed] [Google Scholar]
- Maleki S. J.; Crespo J. F.; Cabanillas B. Anti-inflammatory effects of flavonoids. Food Chem. 2019, 299, 125124 10.1016/j.foodchem.2019.125124. [DOI] [PubMed] [Google Scholar]
- Verma S. K.; Singh M.; Jain P.; Bordia A. Protective effect of ginger, Zingiber officinale Rosc on experimental atherosclerosis in rabbits. Indian J. Exp. Biol. 2004, 42, 736–738. [PubMed] [Google Scholar]
- Li X. H.; McGrath K. C. Y.; Tran V. H.; Nammi S.; Heather A. K.; Roufogalis B. D. Attenuation of proinflammatory responses by S -[6]-Gingerol via inhibition of ROS/NF-Kappa B/COX2 activation in HuH7 cells. J. Evidence-Based Complementary Altern. Med. 2013, 2013, 146142 10.1155/2013/146142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang N.; Sang Y.; Liu W.; Yu W.; Wang X. Anti-Inflammatory Effects of Gingerol on Lipopolysaccharide-Stimulated RAW 264.7 Cells by Inhibiting NF-κB Signaling Pathway. Inflammation 2018, 41, 835–845. 10.1007/s10753-018-0737-3. [DOI] [PubMed] [Google Scholar]
- Jung H. W.; Yoon C. H.; Park K. M.; Han H. S.; Park Y. K. Hexane fraction of Zingiberis Rhizoma Crudus extract inhibits the production of nitric oxide and proinflammatory cytokines in LPS-stimulated BV2 microglial cells via the NF-kappaB pathway. Food Chem. Toxicol. 2009, 47, 1190–1197. 10.1016/j.fct.2009.02.012. [DOI] [PubMed] [Google Scholar]
- Chen B. H.; Wu P. Y.; Chen K. M.; Fu T. F.; Wang H. M.; Chen C. Y. Antiallergic potential on RBL-2H3 cells of some phenolic constituents of Zingiber officinale (ginger). J. Nat. Prod. 2009, 72, 950–953. 10.1021/np800555y. [DOI] [PubMed] [Google Scholar]
- Lantz R. C.; Chen G. J.; Sarihan M.; Sólyom A. M.; Jolad S. D.; Timmermann B. N. The effect of extracts from ginger rhizome on inflammatory mediator production. Phytomedicine 2007, 14, 123–128. 10.1016/j.phymed.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Nikfarjam B. A.; Adineh M.; Hajiali F.; Nassiri-Asl M. Treatment with rutin - A therapeutic strategy for neutrophil-mediated inflammatory and autoimmune diseases: Anti-inflammatory effects of rutin on neutrophils. J Pharmacopuncture 2017, 20, 52–56. 10.3831/KPI.2017.20.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guardia T.; Rotelli A. E.; Juarez A. O.; Pelzer L. E. Anti-inflammatory properties of plant flavonoids. Effects of rutin, quercetin and hesperidin on adjuvant arthritis in rat. Farmaco 2001, 56, 683–687. 10.1016/S0014-827X(01)01111-9. [DOI] [PubMed] [Google Scholar]
- Yoo H.; Ku S. K.; Baek Y. D.; Bae J. S. Anti-inflammatory effects of rutin on HMGB1-induced inflammatory responses in vitro and in vivo. Inflammation Res. 2014, 63, 197–206. 10.1007/s00011-013-0689-x. [DOI] [PubMed] [Google Scholar]
- Patel K.; Patel D. K.. The Beneficial Role of Rutin, A Naturally Occurring Flavonoid in Health Promotion and Disease Prevention: A Systematic Review and Update. In Bioactive Food as Dietary Interventions for Arthritis and Related Inflammatory Diseases, 2nd ed.; Elsevier, 2019; pp 457–479. [Google Scholar]
- Tejada S.; Pinya S.; Martorell M.; Capó X.; Tur J. A.; Pons A.; Sureda A. Potential Anti-inflammatory Effects of Hesperidin from the Genus Citrus. Curr. Med. Chem. 2019, 25, 4929–4945. 10.2174/0929867324666170718104412. [DOI] [PubMed] [Google Scholar]
- Parhiz H.; Roohbakhsh A.; Soltani F.; Rezaee R.; Iranshahi M. Antioxidant and anti-inflammatory properties of the citrus flavonoids hesperidin and hesperetin: An updated review of their molecular mechanisms and experimental models. Phyther. Res. 2015, 29, 323–331. 10.1002/ptr.5256. [DOI] [PubMed] [Google Scholar]
- Xiao S.; Liu W.; Bi J.; Liu S.; Zhao H.; Gong N.; Xing D.; Gao H.; Gong M. Anti-inflammatory effect of hesperidin enhances chondrogenesis of human mesenchymal stem cells for cartilage tissue repair. J. Inflamm. 2018, 15, 14. 10.1186/s12950-018-0190-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guazelli C. F. S.; Fattori V.; Ferraz C. R.; Borghi S. M.; Casagrande R.; Baracat M. M.; Verri W. A. Antioxidant and anti-inflammatory effects of hesperidin methyl chalcone in experimental ulcerative colitis. Chem.-Biol. Interact. 2021, 333, 109315 10.1016/j.cbi.2020.109315. [DOI] [PubMed] [Google Scholar]
- Gong G.; Guan Y. Y.; Zhang Z. L.; Rahman K.; Wang S. J.; Zhou S.; Luan X.; Zhang H. Isorhamnetin: A review of pharmacological effects. Biomed. Pharmacother. 2020, 128, 110301 10.1016/j.biopha.2020.110301. [DOI] [PubMed] [Google Scholar]
- Antunes-Ricardo M.; Gutiérrez-Uribe J. A.; López-Pacheco F.; Alvarez M. M.; Serna-Saldívar S. O. In vivo anti-inflammatory effects of isorhamnetin glycosides isolated from Opuntia ficus-indica (L.) Mill cladodes. Ind. Crops Prod. 2015, 76, 803–808. 10.1016/j.indcrop.2015.05.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu G. F.; Guo H. J.; Huang Y.; Wu C. T.; Zhang X. F. Eriodictyol, a plant flavonoid, attenuates LPS-induced acute lung injury through its antioxidative and anti-inflammatory activity. Exp. Ther. Med. 2015, 10, 2259–2266. 10.3892/etm.2015.2827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mokdad-Bzeouich I.; Mustapha N.; Sassi A.; Bedoui A.; Ghoul M.; Ghedira K.; Chekir-Ghedira L. Investigation of immunomodulatory and anti-inflammatory effects of eriodictyol through its cellular anti-oxidant activity. Cell Stress Chaperones 2016, 21, 773–781. 10.1007/s12192-016-0702-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y.; Chen Y.; Chen Y.; Zhou B.; Shan X.; Yang G. Eriodictyol inhibits IL-1β-induced inflammatory response in human osteoarthritis chondrocytes. Biomed. Pharmacother. 2018, 107, 1128–1134. 10.1016/j.biopha.2018.08.103. [DOI] [PubMed] [Google Scholar]