Abstract
This article has been corrected. Please see J Manag Care Spec Pharm, 2020;26(5):682
BACKGROUND:
Clinical trials have shown that direct oral anticoagulants (DOACs)—including dabigatran, rivaroxaban, apixaban, and edoxaban—are at least as effective and safe as warfarin for the risk of stroke/systemic embolism (SE) and major bleeding (MB) in patients with atrial fibrillation (AF). However, few studies have compared oral anticoagulants (OACs) among elderly patients.
OBJECTIVE:
To compare hospitalization risks (all-cause, stroke/SE-related, and MB-related) and associated health care costs among elderly nonvalvular AF (NVAF) patients in the Medicare population who initiated warfarin, dabigatran, rivaroxaban, or apixaban.
METHODS:
Patients (aged ≥ 65 years) initiating warfarin or DOACs (apixaban, rivaroxaban, and dabigatran) were selected from the Centers for Medicare & Medicaid Services database from January 1, 2013, to December 31, 2014. Patients initiating each OAC were matched 1:1 to apixaban patients using propensity score matching to balance demographic and clinical characteristics. Cox proportional hazards models were used to estimate the risk of hospitalization of each OAC versus apixaban. Generalized linear models and two-part models with bootstrapping were used to compare all-cause health care costs and stroke/SE- and MB-related medical costs between matched cohorts.
RESULTS:
Of the 264,479 eligible patients, 77,480 warfarin-apixaban, 41,580 dabigatran-apixaban, and 77,640 rivaroxaban-apixaban patients were matched. The OACs were associated with a significantly higher risk of all-cause hospitalization compared with apixaban (warfarin: HR = 1.27, 95% CI = 1.23-1.31, P < 0.001; dabigatran: HR = 1.13, 95% CI = 1.08-1.18, P < 0.001; and rivaroxaban: HR = 1.22, 95% CI = 1.18-1.26, P < 0.001) and were associated with a significantly higher risk of hospitalization due to stroke/SE (warfarin: HR = 2.18, 95% CI = 1.80-2.64, P < 0.001; dabigatran: HR = 1.45, 95% CI = 1.12-1.88, P = 0.006; and rivaroxaban: HR = 1.40, 95% CI = 1.14-1.71, P = 0.001). Also, the OACs were associated with significantly higher risk of hospitalization due to MB-related conditions compared with apixaban (warfarin: HR = 1.76, 95% CI = 1.59-1.95, P < 0.001; dabigatran: HR = 1.44, 95% CI = 1.23-1.68, P < 0.001; and rivaroxaban: HR = 1.89, 95% CI = 1.71-2.09, P < 0.001). Compared with apixaban, warfarin ($3,577 vs. $3,183, P < 0.001); dabigatran ($3,217 vs. $3,060, P < 0.001); and rivaroxaban ($3,878 vs. $3,180, P < 0.001) had significantly higher all-cause total health care costs per patient per month. Patients initiating the OACs had significantly higher MB-related medical costs compared with apixaban: warfarin ($472 vs. $269; P < 0.001); dabigatran ($364 vs. $245, P < 0.001); and rivaroxaban ($493 vs. $270, P < 0.001). Warfarin was also associated with higher stroke/SE-related medical costs compared with apixaban ($124 vs. $62, P < 0.001).
CONCLUSIONS:
This real-world study showed that among elderly NVAF patients in the Medicare population, apixaban was associated with significantly lower risks of all-cause, stroke/SE-related, and MB-related hospitalizations compared with warfarin, dabigatran, and rivaroxaban. Accordingly, apixaban showed significantly lower all-cause health care costs and MB-related medical costs.
What is already known about this subject
Clinical trials have shown that direct oral anticoagulants (DOACs) are at least as effective as warfarin for stroke risk reduction and are associated with similar or lower rates of major bleeding (MB) in patients with atrial fibrillation.
Several real-world studies have compared the risks of stroke and MB between DOACs and warfarin in various databases; however, few real-world comparisons are available between DOACs.
What this study adds
In the elderly Medicare population, apixaban initiation was associated with significantly lower risks of all-cause, stroke/systemic embolism (SE)-related, and MB-related hospitalizations compared with warfarin, dabigatran, or rivaroxaban initiation.
The all-cause health care costs and MB-related medical costs were significantly higher for dabigatran, rivaroxaban, or warfarin initiators compared with apixaban initiators.
Atrial fibrillation (AF) is the most common sustained heart arrhythmia and is estimated to affect approximately 9% of the population aged ≥ 65 years in the United States.1,2 The presence of AF increases the relative risk of stroke by 5-fold, with attributable risk increasing from 4.6% among patients aged 50-59 years to over 20% among those aged 80-89 years.3 AF’s annual national incremental costs were estimated at $26 million compared with patients without AF, and hospitalizations were the primary cost driver.4 For Medicare beneficiaries, AF onset leads to an adjusted mean incremental treatment cost of $14,199 per patient per year.5
Warfarin, a vitamin K antagonist in use since the 1950s, has been proven to reduce ischemic and hemorrhagic stroke by 64% compared with placebo.6 However, the narrow therapeutic window managed by the international normalized ratio and increased risk of bleeding have hindered the proper use of warfarin, especially in the elderly population.2 Several new direct oral anticoagulants (DOACs) targeting key coagulation factors—including dabigatran, rivaroxaban, apixaban, and edoxaban—have been approved for stroke risk reduction in nonvalvular AF (NVAF) in recent years. Additionally, DOACs have demonstrated to be at least as effective as warfarin for the risk reduction of stroke and systemic embolism (SE) and are associated with similar or lower rates of major bleeding (MB).7-10
While there are NVAF trials of DOACs versus warfarin, there are no head-to-head clinical trials comparing DOACs to each other. A few real-world studies have examined the risk of hospitalizations due to stroke/SE and MB among OACs. However, there is a dearth of real-world data for all-cause hospitalizations and health care costs.11 Although warfarin has a lower pharmacy cost, using data from clinical trials and a Markov decision analysis model, apixaban, dabigatran, and rivaroxaban have shown to be more cost-effective than warfarin.12 Real-world studies comparing health care costs among NVAF patients have also shown that apixaban patients had lower hospitalization costs compared with warfarin patients.13,14
The objective of this study was to compare the risk of hospitalizations (all-cause, stroke/SE-related, and MB-related) and associated health care costs among elderly NVAF patients who initiated warfarin, dabigatran, rivaroxaban, or apixaban in the Medicare population.
Methods
Data Source
This real-world retrospective database analysis used data from the Centers for Medicare & Medicaid Services from January 1, 2012, to December 31, 2014. Medicare is the federal health insurance program for people aged ≥ 65 years, certain younger people with disabilities, and people with end-stage renal disease (permanent kidney failure requiring dialysis or a transplant). The database includes around 38 million fee-for-service beneficiaries.15 It contains medical and pharmacy claims from 100% national Medicare data, which includes hospital inpatient, outpatient, Medicare carrier, Part D pharmacy, skilled nursing facility, home health agency, and durable medical equipment files. Medical claims were obtained through the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) diagnosis and procedure codes, as well as Health Care Common Procedure Coding System and Current Procedural Terminology codes. Pharmacy claims were obtained through National Drug Code numbers. The comparative effectiveness research methods guidance documents aided researchers in designing the study.16-19
Patient Selection
OAC treatment-naive patients were included in the study if they had ≥ 1 prescription claim for apixaban, dabigatran, rivaroxaban, or warfarin during the identification period (January 1, 2013-December 31, 2014). Edoxaban was approved by the U.S. Food and Drug Administration in 2015; therefore, it was not included in our study. The first OAC pharmacy claim date was designated as the index date. Patients were required to be aged ≥ 65 years on the index date, have ≥ 1 AF medical claim (ICD-9-CM code 427.31), and have continuous health plan enrollment with medical and pharmacy benefits for 12 months before the index date (baseline period).20
Patients were excluded if they had evidence of rheumatic mitral valvular heart disease, mitral valve stenosis, heart valve replacement or surgery; transient AF (pericarditis, hyperthyroidism, and thyrotoxicity), venous thromboembolism, or an OAC pharmacy claim during the 12-month baseline period; pregnancy during the study period; or > 1 OAC prescription claim on the index date.
Patients were followed from the index date until the earliest of the OAC prescription discontinuation date, switch date from index drug to another OAC, date of death, date of health plan disenrollment, or December 31, 2014. Discontinuation was defined as no evidence of an index prescription for 30 days from the last day of the supply of the last filled prescription (discontinuation date). Switching was defined as having a prescription for an OAC other than the index drug within 30 days before or after the discontinuation date.21
Outcomes
The primary outcomes were likelihood of all-cause hospitalization, hospitalization due to stroke/SE, hospitalization due to MB-related conditions, and health care costs, including all-cause health care, all-cause medical, all-cause pharmacy, all-cause hospitalization, all-cause emergency room (ER)/outpatient, stroke/SE-related medical, and MB-related medical costs.
Stroke/SE and MB hospitalization events were identified using hospital claims that had a stroke/SE or MB code as the primary discharge diagnosis.22 The ICD-9-CM codes used for stroke and MB were based on a validated administrative claims-based algorithm as well as the clinical trial definition of stroke and MB.7,23,24 Stroke/SE was stratified by ischemic stroke, hemorrhagic stroke, and SE; MB was stratified by gastrointestinal bleeding, intracranial hemorrhage, and other MB.
Stroke/SE-related medical costs were defined as hospitalization costs associated with the first stroke/SE event plus all subsequent stroke/SE costs occurring in the inpatient or outpatient setting (primary or secondary diagnosis) after the first stroke/SE during the follow-up. MB-related medical costs were defined as the hospitalization costs associated with the first MB event plus all subsequent MB costs occurring in the inpatient or outpatient setting (primary or secondary diagnosis) after the first MB during the follow-up. Costs included all paid amounts, including Medicare payments, copayments, and deductibles incurred during the follow-up period. All-cause medical costs represent the sum of reimbursed costs for inpatient, outpatient (office, ER, and other outpatient costs), and other costs (durable medical equipment, skilled nursing facility, home health agency, and hospice costs); total health care costs represent the sum of medical and pharmacy costs. All cost outcomes were measured per patient per month (PPPM) and adjusted to 2014 U.S. dollars using the Consumer Price Index for medical care services.
Baseline Variables
Patient demographics (age, sex, and U.S. geographic region) and clinical characteristics (Charlson Comorbidity Index [CCI] score, CHADS2 score, CHA2DS2-VASc score, HAS-BLED score, comorbid conditions, and comedication use), as well as health care resource utilization, were assessed during the baseline period. The CHA2DS2-VASc stroke risk score was calculated using ICD-9-CM codes in the claims data as the summed total of the points determined for each diagnosis or characteristic and based on the CHADS2 score (congestive heart failure, hypertension, aged > 75 years, diabetes mellitus, prior stroke or transient ischemic attack or thromboembolism) plus vascular disease, aged 65-74 years, and sex.25 The HAS-BLED bleeding risk score was based on evidence of hypertension, abnormal kidney or liver function, stroke, bleeding, aged > 65 years, and drugs/alcohol abuse or dependence.26
Statistical Methods
All study variables were analyzed descriptively in each cohort, using apixaban as the reference. Means and standard deviations were reported for continuous variables, and student’s t-tests were used to detect differences. Percentages were reported for categorical variables, and chi-square tests were used to detect differences in these variables. A P value of 0.05 was used as the threshold for statistical significance.
Propensity score matching (PSM) was conducted to balance identified baseline demographics and clinical characteristics when comparing apixaban to dabigatran, rivaroxaban, or warfarin. Patients were matched 1:1 on the propensity scores generated by multivariable logistic regressions based on age, sex, geographic region, CCI score, CHA2DS2-VASc score, HAS-BLED score, prior bleed and stroke, comorbidities, baseline comedications, and baseline hospitalization. The covariates included in the PSM were determined based on clinical rationale. Nearest neighbor without replacement with a caliper of 0.01 was used to match the patients.27 The balance of covariates was checked based on standardized differences with a threshold of 10%.28
The incidence rates of hospitalization (all-cause, stroke-related, and MB-related) in the matched cohorts were calculated using the number of hospitalized patients divided by total person-years of exposure and multiplied by 100. Cox proportional hazards regression models were used to assess the likelihood of all-cause hospitalization, hospitalization due to stroke/SE, and hospitalization due to MB-related conditions in patients treated with other OACs relative to apixaban.27 Hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated for each outcome of interest.
Generalized linear models with log-link and a gamma distribution were used for the analysis of health care costs among the cohorts.29 Additionally, two-part models with bootstrapping were used in the analysis of MB- and stroke-related medical costs, given the high proportion of cost fields with 0 values. The marginal effect of costs, 95% CIs, and P values for each matched cohort were reported.
Sensitivity Analyses
Three sensitivity analyses were conducted. First, for the DOAC cohorts, standard-dose (dabigatran 150 mg, rivaroxaban 20 mg, and apixaban 5 mg) and low-dose (dabigatran 75 mg, rivaroxaban 10 mg/15 mg, and apixaban 2.5 mg) cohorts were created based on the index dosage. Each patient initiating warfarin was assigned to one of the 2 subgroups according to the dose of the matched DOAC patient (standard and low dose). The balance of baseline characteristics was tested in each subgroup; when imbalance was detected (standardized difference > 10%), the variable was included in the multivariate model. Risk of hospitalization (all-cause health care, stroke-related, and MB-related) was compared between the study cohorts, and the statistical significance of the interaction between treatments and subgroups was evaluated.
Second, patients were censored at 6 months to create a more balanced length of follow-up between the treatment groups. Third, only patients with ≥ 30 days of follow-up were evaluated to exclude patients with too short of a follow-up to develop any stroke/SE or MB events. The second and third analyses were to help address the more recent approval of apixaban relative to dabigatran and rivaroxaban.
Results
After applying the selection criteria, a total of 264,479 patients were identified: 125,257 warfarin, 20,979 dabigatran, 79,337 rivaroxaban, and 38,906 apixaban patients (Figure 1). Beforematching, patients prescribed warfarin were older and had poorer health status compared with apixaban patients, and apixaban patients were older with poorer health status compared with dabigatran and rivaroxaban patients (Appendix A, available in online article). After 1:1 PSM, 77,480 warfarin-apixaban, 41,580 dabigatran-apixaban, and 77,640 rivarox-aban-apixaban matched patients were included in the study (Table 1). Patients were followed for a median of 113 and 97 days for warfarin-apixaban cohorts, 106 and 98 days for dabigatran-apixaban cohorts, and 117 and 97 days for rivaroxaban-apixaban cohorts, respectively.
FIGURE 1.
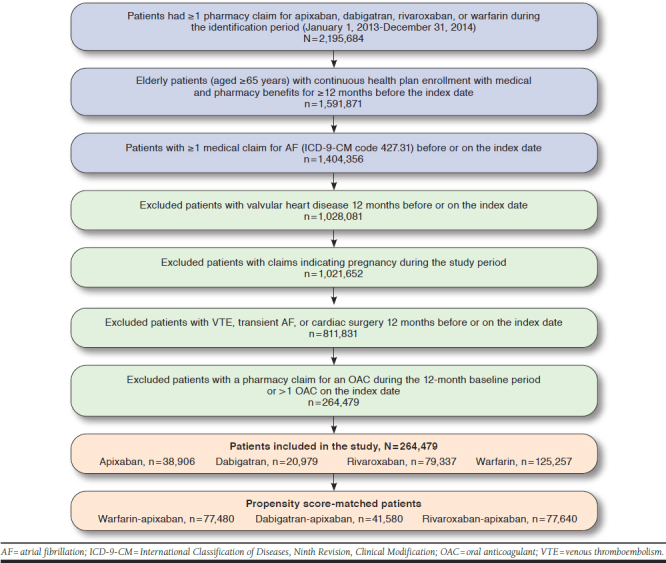
Patient Selection Criteria
TABLE 1.
PSM-Adjusted Baseline Characteristics and Outcomes
| Apixaban Cohort n = 38,740 | Warfarin Cohort n = 38,740 | Apixaban Cohort n = 20,790 | Dabigatran Cohort n = 20,790 | Apixaban Cohort n = 38,820 | Rivaroxaban Cohort n = 38,820 | |||||||
| n/mean | %/SD | n/mean | %/SD | n/mean | %/SD | n/mean | %/SD | n/mean | %/SD | n/mean | %/SD | |
| Age (years) | 78.3 | 7.4 | 78.2 | 7.3 | 77.2 | 7.2 | 77.1 | 7.0 | 78.3 | 7.4 | 78.3 | 7.2 |
| 65-74 | 13,578 | 35.0% | 13,888 | 35.8% | 8,529 | 41.0% | 8,435 | 40.6% | 13,644 | 35.1% | 13,596 | 35.0% |
| 75-84 | 16,407 | 42.4% | 16,179 | 41.8% | 8,707 | 41.9% | 8,796 | 42.3% | 16,454 | 42.4% | 16,523 | 42.6% |
| ≥ 85 | 8,755 | 22.6% | 8,673 | 22.4% | 3,554 | 17.1% | 3,559 | 17.1% | 8,722 | 22.5% | 8,701 | 22.4% |
| Gender | ||||||||||||
| Male | 18,365 | 47.4% | 18,179 | 46.9% | 10,417 | 50.1% | 10,503 | 50.5% | 18,414 | 47.4% | 18,334 | 47.2% |
| Female | 20,375 | 52.6% | 20,561 | 53.1% | 10,373 | 49.9% | 10,287 | 49.5% | 20,406 | 52.6% | 20,486 | 52.8% |
| U.S. geographic region | ||||||||||||
| Northeast | 7,107 | 18.3% | 7,191 | 18.6% | 4,130 | 19.9% | 4,183 | 20.1% | 7,098 | 18.3% | 7,052 | 18.2% |
| North Central | 8,114 | 20.9% | 7,987 | 20.6% | 4,805 | 23.1% | 4,827 | 23.2% | 8,110 | 20.9% | 8,167 | 21.0% |
| South | 17,227 | 44.5% | 17,367 | 44.8% | 7,997 | 38.5% | 7,980 | 38.4% | 17,318 | 44.6% | 17,307 | 44.6% |
| West | 6,266 | 16.2% | 6,164 | 15.9% | 3,848 | 18.5% | 3,790 | 18.2% | 6,268 | 16.1% | 6,245 | 16.1% |
| Other | 26 | 0.1% | 31 | 0.1% | 10 | 0.1% | 10 | 0.1% | 26 | 0.1% | 49 | 0.1% |
| Baseline comorbidity | ||||||||||||
| Baseline Charlson Comorbidity Index score | 2.7 | 2.6 | 2.8 | 2.6 | 2.5 | 2.4 | 2.5 | 2.4 | 2.7 | 2.5 | 2.7 | 2.5 |
| 0-1 | 15,365 | 39.7% | 15,094 | 39.0% | 9,127 | 43.9% | 8,955 | 43.1% | 15,451 | 39.8% | 15,293 | 39.4% |
| 2-3 | 11,618 | 30.0% | 11,639 | 30.0% | 6,175 | 29.7% | 6,373 | 30.7% | 11,640 | 30.0% | 11,865 | 30.6% |
| ≥ 4 | 11,757 | 30.3% | 12,007 | 31.0% | 5,488 | 26.4% | 5,462 | 26.2% | 11,729 | 30.2% | 11,662 | 30.0% |
| Baseline CHADS2 scorea | 2.7 | 1.4 | 2.7 | 1.4 | 2.6 | 1.4 | 2.6 | 1.4 | 2.7 | 1.4 | 2.7 | 1.4 |
| 0 = low risk | 1,453 | 3.8% | 1,474 | 3.8% | 1,024 | 4.9% | 986 | 4.7% | 1,455 | 3.7% | 1,450 | 3.7% |
| 1 = moderate risk | 6,548 | 16.9% | 6,286 | 16.2% | 3,881 | 18.7% | 3,867 | 18.6% | 6,592 | 17.0% | 6,577 | 16.9% |
| 2 = high risk | 11,279 | 29.1% | 11,163 | 28.8% | 6,298 | 30.3% | 6,251 | 30.1% | 11,318 | 29.2% | 11,263 | 29.0% |
| ≥ 2 = high risk | 19,460 | 50.2% | 19,817 | 51.2% | 9,587 | 46.1% | 9,686 | 46.6% | 19,455 | 50.1% | 19,530 | 50.3% |
| Baseline CHA2DS2-VASc scoreb | 4.6 | 1.7 | 4.6 | 1.7 | 4.3 | 1.7 | 4.4 | 1.7 | 4.5 | 1.7 | 4.6 | 1.7 |
| 0 = low risk | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% |
| 1 = moderate risk | 729 | 1.9% | 683 | 1.8% | 538 | 2.6% | 539 | 2.6% | 727 | 1.9% | 686 | 1.8% |
| 2 = high risk | 3,549 | 9.2% | 3,410 | 8.8% | 2,279 | 11.0% | 2,260 | 10.9% | 3,568 | 9.2% | 3,590 | 9.2% |
| ≥ 2 = high risk | 34,462 | 89.0% | 34,647 | 89.4% | 17,973 | 86.5% | 17,991 | 86.5% | 34,525 | 88.9% | 34,544 | 89.0% |
| Baseline HAS-BLED scorec | 3.2 | 1.2 | 3.3 | 1.2 | 3.1 | 1.2 | 3.1 | 1.2 | 3.2 | 1.2 | 3.2 | 1.2 |
| 0 = low risk | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% |
| 1-2 = moderate risk | 12,002 | 31.0% | 11,602 | 29.9% | 7,498 | 36.1% | 7,406 | 35.6% | 12,027 | 31.0% | 12,197 | 31.4% |
| ≥ 2 = high risk | 26,738 | 69.0% | 27,138 | 70.1% | 13,292 | 63.9% | 13,384 | 64.4% | 26,793 | 69.0% | 26,623 | 68.6% |
| Baseline prior bleed | 7,914 | 20.4% | 8,065 | 20.8% | 3,882 | 18.7% | 3,858 | 18.6% | 7,906 | 20.4% | 7,922 | 20.4% |
| Baseline prior stroke | 4,742 | 12.2% | 4,928 | 12.7% | 2,338 | 11.2% | 2,353 | 11.3% | 4,729 | 12.2% | 4,769 | 12.3% |
| Congestive heart failure | 11,277 | 29.1% | 11,545 | 29.8% | 5,656 | 27.2% | 5,688 | 27.4% | 11,246 | 29.0% | 11,234 | 28.9% |
| Diabetes | 13,850 | 35.8% | 14,007 | 36.2% | 7,557 | 36.3% | 7,572 | 36.4% | 13,855 | 35.7% | 13,935 | 35.9% |
| Hypertension | 34,117 | 88.1% | 34,357 | 88.7% | 17,970 | 86.4% | 18,004 | 86.6% | 34,195 | 88.1% | 34,173 | 88.0% |
| Renal disease | 9,158 | 23.6% | 9,344 | 24.1% | 3,906 | 18.8% | 3,893 | 18.7% | 9,107 | 23.5% | 9,086 | 23.4% |
| Myocardial infarction | 4,895 | 12.6% | 4,979 | 12.9% | 2,234 | 10.7% | 2,243 | 10.8% | 4,891 | 12.6% | 4,895 | 12.6% |
| Dyspepsia or stomach discomfort | 8,164 | 21.1% | 8,427 | 21.8% | 3,983 | 19.2% | 4,031 | 19.4% | 8,193 | 21.1% | 8,155 | 21.0% |
| Peripheral vascular disease | 21,370 | 55.2% | 21,683 | 56.0% | 10,713 | 51.5% | 10,692 | 51.4% | 21,432 | 55.2% | 21,387 | 55.1% |
| Transient ischemic attack | 3,032 | 7.8% | 3,117 | 8.0% | 1,528 | 7.3% | 1,513 | 7.3% | 3,032 | 7.8% | 3,100 | 8.0% |
| Coronary artery disease | 18,572 | 47.9% | 18,876 | 48.7% | 9,288 | 44.7% | 9,214 | 44.3% | 18,626 | 48.0% | 18,597 | 47.9% |
| Baseline medication use | ||||||||||||
| Angiotensin-converting enzyme inhibitor | 14,261 | 36.8% | 14,410 | 37.2% | 7,928 | 38.1% | 7,972 | 38.3% | 14,289 | 36.8% | 14,349 | 37.0% |
| Amiodarone | 3,248 | 8.4% | 3,193 | 8.2% | 1,599 | 7.7% | 1,631 | 7.8% | 3,303 | 8.5% | 3,253 | 8.4% |
| Angiotensin receptor | 10,467 | 27.0% | 10,538 | 27.2% | 5,227 | 25.1% | 5,248 | 25.2% | 10,550 | 27.2% | 10,552 | 27.2% |
| Baseline medication use | ||||||||||||
| Beta blockers | 21,834 | 56.4% | 21,927 | 56.6% | 11,100 | 53.4% | 11,175 | 53.8% | 21,899 | 56.4% | 22,071 | 56.9% |
| H2-receptor antagonist | 2,598 | 6.7% | 2,624 | 6.8% | 1,291 | 6.2% | 1,331 | 6.4% | 2,603 | 6.7% | 2,638 | 6.8% |
| Proton pump inhibitor | 12,475 | 32.2% | 12,646 | 32.6% | 6,126 | 29.5% | 6,137 | 29.5% | 12,524 | 32.3% | 12,473 | 32.1% |
| Antiplatelets | 7,030 | 18.1% | 7,127 | 18.4% | 3,119 | 15.0% | 3,094 | 14.9% | 7,087 | 18.3% | 7,016 | 18.1% |
| Statins | 23,692 | 61.2% | 23,916 | 61.7% | 12,049 | 58.0% | 12,036 | 57.9% | 23,761 | 61.2% | 23,755 | 61.2% |
| Index drug dosed | ||||||||||||
| Standard dose | 28,130 | 72.6% | 16,037 | 77.1% | 16,626 | 80.0% | 28,229 | 72.7% | 24,801 | 63.9% | ||
| Low dose | 10,610 | 27.4% | 4,753 | 22.9% | 4,164 | 20.0% | 10,591 | 27.3% | 14,019 | 36.1% | ||
| Follow-up time (days) | 144.6 | 133.3 | 181.1 | 173.6 | 145.4 | 133.2 | 185.2 | 183.9 | 144.7 | 133.3 | 182.3 | 176.5 |
| Median | 97 | 113 | 98 | 106 | 97 | 117 | ||||||
| Switch during follow-up | 1,461 | 3.8% | 2,553 | 6.6% | 771 | 3.7% | 2,318 | 11.1% | 1,460 | 3.8% | 2,346 | 6.0% |
aCHADS2: congestive heart failure, hypertension, aged ≥ 75 years, diabetes mellitus, prior stroke or transient ischemic attack, or venous thromboembolism.
bCHA2DS2-VASc: congestive heart failure, hypertension, aged ≥ 75 years, diabetes mellitus, prior stroke or transient ischemic attack or thromboembolism, vascular disease, aged 65-74 years, sex category.
cHAS-BLED: hypertension, abnormal renal and liver function, stroke, bleeding, labile international normalized ratios, elderly, drugs and alcohol.
dStandard dose: 5 mg twice a day apixaban, 150 mg twice a day dabigatran, 20 mg every day rivaroxaban; low dose: 2.5 mg twice a day apixaban, 75 mg twice a day dabigatran, 10 mg or 15 mg every day rivaroxaban.
PSM = propensity score matching; SD = standard deviation.
Baseline Characteristics
In the 3 postmatching cohorts, the mean age was around 78 years. The dabigatran-apixaban patients had the lowest mean CCI score (2.5), followed by rivaroxaban-apixaban (2.7) and warfarin-apixaban (2.8 and 2.7) patients. The CHA2DS2-VASc scores ranged from 4.3 to 4.6 across the cohorts. About 20% of all matched patients had baseline bleeding, and more than 10% had baseline stroke/SE (Table 1).
Hospitalization: All-Cause, Stroke/SE, and MB
Incidence of all-cause hospitalizations and hospitalizations related to MB and stroke/SE are shown in Figure 2.
FIGURE 2.
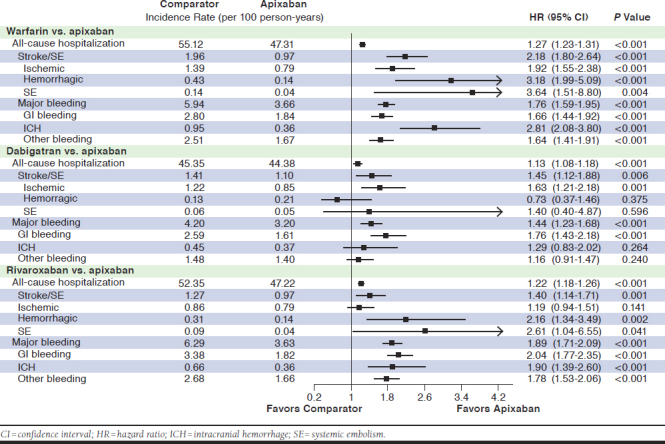
Hazard Ratios of All-Cause Hospitalization, Hospitalization Due to Stroke/SE, and Hospitalization Due to Major Bleeding for Propensity Score-Matched Patients
After PSM, OAC patients were significantly more likely to have an all-cause hospitalization compared with apixaban patients (warfarin: HR = 1.27, 95% CI = 1.23-1.31; dabigatran: HR = 1.13, 95% CI = 1.08-1.18; and rivaroxaban: HR = 1.22, 95% CI = 1.18-1.26).
Warfarin, dabigatran, and rivaroxaban treatment were each associated with a significantly higher likelihood of having a hospitalization due to stroke/SE compared with apixaban treatment (warfarin: HR = 2.18, 95% CI = 1.80-2.64; dabigatran: HR = 1.45, 95% CI = 1.12-1.88; and rivaroxaban: HR = 1.40, 95% CI = 1.14-1.71). They were also associated with a significantly higher risk of hospitalization due to MB-related conditions compared with apixaban treatment (warfarin: HR = 1.76, 95% CI = 1.59-1.95; dabigatran: HR = 1.44, 95% CI = 1.23-1.68; and rivaroxaban: HR = 1.89, 95% CI = 1.71-2.09).
Health Care Costs
Patients prescribed warfarin, dabigatran, and rivaroxaban had significantly higher all-cause total health care costs PPPM compared with apixaban patients (Table 2). Inpatient and outpatient costs were the main drivers for health care costs.
TABLE 2.
Adjusted Health Care Cost Comparisons
| PPPM Costsa | Apixaban Cohort (n = 38,470) | Warfarin Cohort (n = 38,470) | Apixaban Cohort (n = 20,790) | Dabigatran Cohort (n = 20,790) | Apixaban Cohort (n = 38,820) | Rivaroxaban Cohort (n = 38,820) | |||
|---|---|---|---|---|---|---|---|---|---|
| Marginal Effect ($) | Marginal Effect ($) | P Value | Marginal Effect ($) | Marginal Effect ($) | P Value | Marginal Effect ($) | Marginal Effect ($) | P Value | |
| All-cause ER/outpatient medical costs | 901 | 956 | < 0.001 | 895 | 873 | 0.010 | 902 | 932 | < 0.001 |
| All-cause hospitalization medical costs | 1,147 | 1,599 | < 0.001 | 1,080 | 1,307 | < 0.001 | 1,145 | 1,632 | < 0.001 |
| All-cause medical costsb | 2,402 | 3,207 | < 0.001 | 2,290 | 2,556 | < 0.001 | 2,399 | 3,178 | < 0.001 |
| Pharmacy costs | 781 | 370 | < 0.001 | 770 | 660 | < 0.001 | 781 | 701 | < 0.001 |
| All-cause health care costsb | 3,183 | 3,577 | < 0.001 | 3,060 | 3,217 | < 0.001 | 3,180 | 3,878 | < 0.001 |
aGeneralized linear models were used for the analysis of all-cause health care costs.
bAll-cause medical costs include all-cause ER/outpatient and hospitalization medical costs; all-cause health care costs include all-cause medical and pharmacy costs.
ER = emergency room; PPPM = per patient per month.
Warfarin, dabigatran, and rivaroxaban patients had significantly higher MB-related medical costs compared with apixaban patients (Figure 3). Warfarin patients had significantly higher stroke/SE-related medical costs compared with apixaban patients.
FIGURE 3.
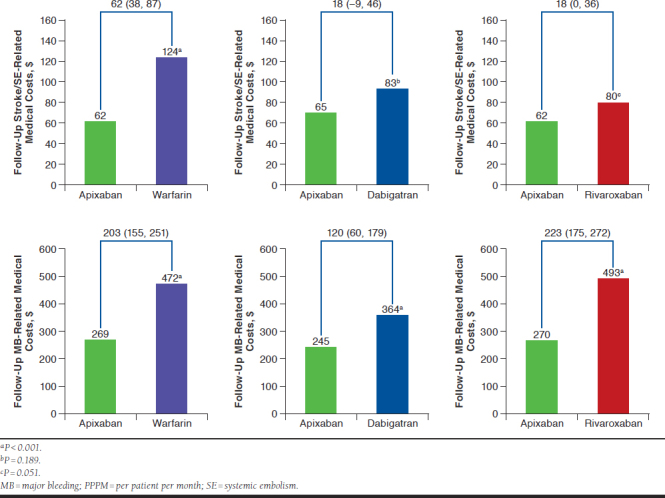
Comparisons of Stroke/SE-Related and MB-Related Medical Costs PPPM for Propensity Score-Matched Patients
Subgroup and Sensitivity Analyses Results
Results of the subgroup and sensitivity analyses were generally consistent with those of the main analysis (Appendix B, available in online article). Significant interactions were found for dose and all-cause, stroke/SE-related, and MB-related hospitalizations among apixaban and warfarin patients. Warfarin was associated with a higher risk of all-cause, stroke/SE-related, and MB-related hospitalizations compared with both standard-dose and low-dose apixaban, with a difference in magnitude. No other interactions were significant. The other sensitivity analyses were consistent with the main analysis.
Discussion
Using national Medicare data, we found that NVAF patients initiating warfarin, dabigatran, or rivaroxaban had a higher risk of all-cause, stroke/SE-related, and MB-related hospitalization compared with patients initiating apixaban. In addition, patients initiating warfarin, dabigatran, and rivaroxaban had significantly higher all-cause and MB-related health care costs compared with patients initiating apixaban.
The ARISTOTLE trial demonstrated a significantly lower risk of stroke/SE (HR = 0.79, 95% CI = 0.66-0.95, P = 0.01) and MB (HR = 0.69, 95% CI = 0.60-0.80, P < 0.001) for apixaban patients compared with warfarin patients, which is consistent with our results.7,30 In addition to clinical trials, a few observational studies comparing apixaban and warfarin have added real-world evidence in different patient populations.22,31-34 In a study of OptumLabs data by Yao et al. (2016), apixaban users had a 33% lower risk of stroke/SE and 55% lower risk of MB compared with warfarin.31 In a study of 4 pooled datasets by Li et al. (2017), apixaban demonstrated lower risks of stroke/SE (HR = 0.67, 95% CI = 0.59-0.76) and MB (HR = 0.60, 95% CI = 0.54-0.65) compared with warfarin.32
Although no head-to-head DOAC clinical trials are available, several real-world studies have compared the risks of stroke/SE and MB among dabigatran, rivaroxaban, and apixaban.33,35 In our analysis, apixaban had a lower risk of hospitalization due to stroke/SE and MB compared with the other DOACs. In a study of the MarketScan population by Lip et al. (2016), patients who initiated dabigatran had a numerically higher risk of MB, and those who initiated rivaroxaban had a significantly higher risk of MB compared with those who initiated apixaban.33 In Noseworthy et al. (2016), apixaban demonstrated a significantly lower risk of MB and a numerically lower risk of stroke/SE compared with dabigatran and rivaroxaban.35 However, we found in our study that dabigatran and rivaroxaban patients had a statistically significantly higher risk of both stroke/SE and MB than apixaban, which may be due to the larger sample size and hence increased power and different study populations.
The results of the sensitivity analyses showed consistent results with the primary analysis, which showed that standard-dose or low-dose apixaban was associated with a lower risk of all-cause, stroke/SE-related, and MB-related hospitalization compared with other OACs.
There are a few economic studies that have compared apixaban to warfarin, dabigatran, and rivaroxaban among NVAF patients. In studies using IMS PharMetrics Plus, Humana, and Optum claims databases, warfarin patients had significantly higher total all-cause health care costs, stroke/SE-related costs, and MB-related medical costs compared with apixaban.22,36-38 In Amin et al.’s (2013) observational claims database study, patients treated with apixaban versus warfarin had medical cost reductions of $493 for stroke, $752 for MB (excluding intracranial hemorrhage), and $1,245 for the combined outcome of both events.39 In claims studies comparing rivaroxaban and apixaban, rivaroxaban patients had higher all-cause hospitalization costs, all-cause health care costs, and MB-related medical costs compared with apixaban.22,36,37 Dabigatran patients were associated with similar stroke/SE- and MB-related medical costs and similar or higher all-cause health care costs compared with apixaban.22,36,37 In Deitelzweig et al.’s (2016) study comparing the all-cause hospitalization readmission costs of DOACs, rivaroxaban had significantly higher costs compared with apixaban (difference: $413; P = 0.003), and dabigatran had numerically higher costs versus apixaban ($142; P = 0.31).40 These studies are generally aligned with our findings on health care costs associated with apixaban relative to other oral anticoagulants.
Limitations
This study has several limitations. Given the nature of retrospective observational studies, only associations were assessed, and no causality can be concluded. This database contains information from the Medicare population and may not be generalizable to the entire U.S. population of NVAF patients. Additionally, administrative claims data are primarily collected for billing purposes rather than research, and the analysis is constrained by codes that may contain coding errors and missing data. In addition, the cause of stroke/SE and major bleeding is not available in the claims data. Moreover, unobserved confounders such as compliance, AF duration, and over-the-counter aspirin use may exist for which the analysis did not control. Nevertheless, we used PSM to balance observed demographics and clinical characteristics. The follow-up time was short, not uniform, and was not consistent with the clinical trials. Therefore, the sensitivity analysis with patients censored at 6 months was conducted to address the issue of imbalanced follow-up times. Sensitivity analysis results for MB and stroke/SE were consistent with those in the main analysis. Finally, the interpretation of stroke/SE-related outcomes should be carefully considered because of the low number of stroke/SE events.
Conclusions
This real-world observational study is one of the largest that has compared the risks of stroke/SE and MB and the associated health care costs between OACs in elderly NVAF patients.
In this study, apixaban was associated with significantly lower risks of all-cause, stroke/SE-related, and MB-related hospitalizations compared with warfarin, dabigatran, and rivaroxaban. Accordingly, apixaban showed significantly lower all-cause health care costs as well as MB-related medical costs. This study may assist clinicians in determining the appropriate OAC for OAC-naive elderly NVAF patients and could be informative to decision makers managing Medicare populations.
APPENDIX A. Pre-PSM Descriptive Baseline Characteristics and Outcomes
| Warfarin Cohort (N = 125,257) | Apixaban Cohort (N = 38,906) | Dabigatran Cohort (N = 20,979) | Rivaroxaban Cohort (N = 79,337) | |||||
| n/mean | %/SD | n/mean | %/SD | n/mean | %/SD | n/mean | %/SD | |
| Age (years) | 78.56 | 7.37 | 78.30 | 7.38 | 77.07 | 7.00 | 77.53 | 7.16 |
| 65-74 | 41,678 | 33.27% | 13,648 | 35.08% | 8,585 | 40.92% | 30,663 | 38.65% |
| 75-84 | 54,186 | 43.26% | 16,470 | 42.33% | 8,830 | 42.09% | 33,772 | 42.57% |
| ≥ 85 | 29,393 | 23.47% | 8,788 | 22.59% | 3,564 | 16.99% | 14,902 | 18.78% |
| Gender | ||||||||
| Male | 60,638 | 48.41% | 18,438 | 47.39% | 10,647 | 50.75% | 38,518 | 48.55% |
| Female | 64,619 | 51.59% | 20,468 | 52.61% | 10,332 | 49.25% | 40,819 | 51.45% |
| Geographic region | ||||||||
| Northeast | 24,944 | 19.91% | 7,110 | 18.27% | 4,239 | 20.21% | 14,298 | 18.02% |
| North Central | 38,533 | 30.76% | 8,115 | 20.86% | 4,882 | 23.27% | 18,387 | 23.18% |
| South | 40,386 | 32.24% | 17,383 | 44.68% | 7,987 | 38.07% | 32,912 | 41.48% |
| West | 21,241 | 16.96% | 6,272 | 16.12% | 3,837 | 18.29% | 13,592 | 17.13% |
| Other | 153 | 0.12% | 26 | 0.07% | 34 | 0.16% | 148 | 0.19% |
| Baseline comorbidity | ||||||||
| Charlson Comorbidity Index | 3.10 | 2.76 | 2.70 | 2.55 | 2.46 | 2.40 | 2.58 | 2.49 |
| CHADS2 scorea | 2.84 | 1.45 | 2.70 | 1.43 | 2.57 | 1.41 | 2.60 | 1.43 |
| CHA2DS2-VASc scoreb | 4.71 | 1.75 | 4.55 | 1.73 | 4.35 | 1.71 | 4.43 | 1.72 |
| HAS-BLED scorec | 3.29 | 1.30 | 3.23 | 1.24 | 3.07 | 1.21 | 3.17 | 1.23 |
| Baseline prior bleed | 31,052 | 24.79% | 7,921 | 20.36% | 3,878 | 18.49% | 16,932 | 21.34% |
| Baseline prior stroke | 19,297 | 15.41% | 4,746 | 12.20% | 2,377 | 11.33% | 9,021 | 11.37% |
| Congestive heart failure | 43,374 | 34.63% | 11,295 | 29.03% | 5,753 | 27.42% | 21,517 | 27.12% |
| Diabetes | 50,006 | 39.92% | 13,884 | 35.69% | 7,683 | 36.62% | 28,152 | 35.48% |
| Hypertension | 108,611 | 86.71% | 34,276 | 88.10% | 18,152 | 86.52% | 69,011 | 86.98% |
| Renal disease | 36,657 | 29.27% | 9,175 | 23.58% | 3,902 | 18.60% | 16,045 | 20.22% |
| Myocardial infarction | 18,638 | 14.88% | 4,906 | 12.61% | 2,253 | 10.74% | 9,400 | 11.85% |
| Dyspepsia or stomach discomfort | 26,535 | 21.18% | 8,215 | 21.11% | 4,049 | 19.30% | 16,489 | 20.78% |
| Peripheral vascular disease | 69,381 | 55.39% | 21,502 | 55.27% | 10,762 | 51.30% | 41,938 | 52.86% |
| Transient ischemic attack | 9,789 | 7.82% | 3,044 | 7.82% | 1,527 | 7.28% | 5,829 | 7.35% |
| Coronary artery disease | 59,249 | 47.30% | 18,698 | 48.06% | 9,276 | 44.22% | 35,963 | 45.33% |
| Follow-up time (days) | 179.31 | 173.59 | 144.66 | 133.32 | 185.02 | 183.82 | 178.76 | 174.67 |
| Median | 111 | 97 | 106 | 113 | ||||
| All-cause hospitalization incidence rate (per 100 person-years) | 60.72 | 47.25 | 45.27 | 53.31 | ||||
| Stroke/SE incidence rate (per 100 person-years) | 1.98 | 0.97 | 1.40 | 1.25 | ||||
| Ischemic stroke | 1.43 | 0.79 | 1.21 | 0.87 | ||||
| Hemorrhage stroke | 0.42 | 0.14 | 0.13 | 0.29 | ||||
| SE | 0.13 | 0.04 | 0.06 | 0.08 | ||||
| Major bleeding incidence rate (per 100 person-years) | 6.56 | 3.64 | 4.18 | 6.30 | ||||
| Gastrointestinal bleeding | 3.12 | 1.83 | 2.58 | 3.53 | ||||
| Intracranial hemorrhage | 0.99 | 0.36 | 0.44 | 0.61 | ||||
| Other bleeding | 2.80 | 1.67 | 1.48 | 2.65 | ||||
| Follow-up all-cause health care costs ($ PPPM) | ||||||||
| All-cause ER/outpatient medical costs | 1,826 | 8,058 | 1,145 | 5,333 | 1,301 | 6,081 | 1,645 | 7,310 |
| All-cause hospitalization medical costs | 1,024 | 2,571 | 901 | 1,893 | 874 | 1,893 | 966 | 2,920 |
| Pharmacy costs | 375 | 885 | 780 | 1,690 | 659 | 1,046 | 701 | 1,097 |
| All-cause health care costs | 3,987 | 9,904 | 3,179 | 6,733 | 3,210 | 7,310 | 3,941 | 9,030 |
aCHADS2: congestive heart failure, hypertension, aged ≥ 75 years, diabetes mellitus, prior stroke, transient ischemic attack, or venous thromboembolism.
bCHA2DS2-VASc: congestive heart failure, hypertension, aged ≥ 75 years, diabetes mellitus, prior stroke or transient ischemic attack or thromboembolism, vascular disease, aged 65-74 years, sex category.
cHAS-BLED: hypertension, abnormal renal and liver function, stroke, bleeding, labile international normalized ratios, elderly, drugs and alcohol.
ER = emergency room; PPPM = per patient per month; PSM = propensity score matching; SD = standard deviation; SE = systemic embolism.
APPENDIX B. Risk of Hospitalization in Sensitivity Analyses Among Propensity Score-Matched Patients
| Warfarin vs. Apixaban | Dabigatran vs. Apixaban | Rivaroxaban vs. Apixaban | ||||
|---|---|---|---|---|---|---|
| Dosing Form | n = 76,940 | P Valuea | n = 41,580 | P Valuea | n = 77,640 | P Valuea |
| All-cause hospitalization | ||||||
| Standard doseb | 1.33 (1.28-1.38) | < 0.001 | 1.13 (1.07-1.19) | 0.409 | 1.21 (1.17-1.26) | 0.230 |
| Low doseb | 1.09 (1.03-1.16) | 1.18 (1.08-1.28) | 1.17 (1.11-1.23) | |||
| Stroke/SE | ||||||
| Standard dose | 2.55 (1.99-3.26) | 0.039 | 1.50 (1.08-2.10) | 0.914 | 1.48 (1.13-1.94) | 0.408 |
| Low dose | 1.67 (1.22-2.29) | 1.46 (0.95-2.23) | 1.25 (0.92-1.69) | |||
| Major bleeding | ||||||
| Standard dose | 1.93 (1.70-2.19) | 0.003 | 1.44 (1.19-1.74) | 0.782 | 1.90 (1.67-2.17) | 0.678 |
| Low dose | 1.38 (1.16-1.65) | 1.51 (1.16-1.97) | 1.82 (1.55-2.13) | |||
| Censoring at 6 months | n = 76,940 | P Value | n = 41,580 | P Value | n = 77,640 | P Value |
| All-cause hospitalization | 1.28 (1.24-1.32) | < 0.001 | 1.14 (1.09-1.20) | < 0.001 | 1.24 (1.20-1.29) | < 0.001 |
| Stroke/SE | 2.22 (1.80-2.74) | < 0.001 | 1.43 (1.08-1.90) | 0.013 | 1.33 (1.05-1.67) | 0.017 |
| Major bleeding | 1.74 (1.56-1.95) | < 0.001 | 1.39 (1.18-1.65) | < 0.001 | 1.85 (1.65-2.06) | < 0.001 |
| At least 30-day follow up | n = 68,536 | P Value | n = 36,882 | P Value | n = 68,684 | P Value |
| All-cause hospitalization | 1.25 (1.21-1.29) | < 0.001 | 1.11 (1.06-1.16) | < 0.001 | 1.19 (1.15-1.23) | < 0.001 |
| Stroke/SE | 2.21 (1.81-2.70) | < 0.001 | 1.43 (1.08-1.88) | 0.001 | 1.43 (1.16-1.77) | < 0.001 |
| Major bleeding | 1.72 (1.55-1.91) | < 0.001 | 1.42 (1.21-1.66) | < 0.001 | 1.87 (1.69-2.07) | < 0.001 |
Note: In the sensitivity analysis of dosing forms, standard-dose and low-dose dabigatran and rivaroxaban were compared with apixaban patients with the same dose.
aP value is for interaction in the dosing form sensitivity analysis.
bStandard dose: 5 mg twice a day apixaban, 150 mg twice a day dabigatran, 20 mg every day rivaroxaban; low dose: 2.5 mg twice a day apixaban, 75 mg twice a day dabigatran, 10 mg or 15 mg every day rivaroxaban.
SE = systemic embolism.
REFERENCES
- 1.Shea JB, Sears SF. Cardiology patient pages. A patient’s guide to living with atrial fibrillation. Circulation. 2008;117(20):e340-43. [DOI] [PubMed] [Google Scholar]
- 2.January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation. 2014;130(23):2071-104. [DOI] [PubMed] [Google Scholar]
- 3.Rahman F, Kwan GF, Benjamin EJ. Global epidemiology of atrial fibrillation. Nat Rev Cardiol. 2014;11(11):639-54. [DOI] [PubMed] [Google Scholar]
- 4.Kim MH, Johnston SS, Chu BC, Dalal MR, Schulman KL. Estimation of total incremental health care costs in patients with atrial fibrillation in the United States. Circ Cardiovasc Qual Outcomes. 2011;4(3):313-20. [DOI] [PubMed] [Google Scholar]
- 5.Lee WC, Lamas GA, Balu S, Spalding J, Wang Q, Pashos CL. Direct treatment cost of atrial fibrillation in the elderly American population: a Medicare perspective. J Med Econ. 2008;11(2):281-98. [DOI] [PubMed] [Google Scholar]
- 6.Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med. 2007;146(12):857-67. [DOI] [PubMed] [Google Scholar]
- 7.Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365(11):981-92. [DOI] [PubMed] [Google Scholar]
- 8.Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361(12):1139-51. [DOI] [PubMed] [Google Scholar]
- 9.Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365(10):883-91. [DOI] [PubMed] [Google Scholar]
- 10.Giugliano RP, Ruff CT, Braunwald E, et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369(22):2093-104. [DOI] [PubMed] [Google Scholar]
- 11.Mani H, Lindhoff-Last E. New oral anticoagulants in patients with nonvalvular atrial fibrillation: a review of pharmacokinetics, safety, efficacy, quality of life, and cost effectiveness. Drug Des Devel Ther. 2014;8:789-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harrington AR, Armstrong EP, Nolan PE Jr, Malone DC.. Costeffectiveness of apixaban, dabigatran, rivaroxaban, and warfarin for stroke prevention in atrial fibrillation. Stroke. 2013;44(6):1676-81. [DOI] [PubMed] [Google Scholar]
- 13.Xie L, Vo L, Keshishian A, et al. Comparison of hospital length of stay and hospitalization costs among patients with non-valvular atrial fibrillation treated with apixaban or warfarin: an early view. J Med Econ. 2016;19(8):769-76. [DOI] [PubMed] [Google Scholar]
- 14.Farr AM, Jing Y, Johnston S, et al. Comparison of hospital length of stay between hospitalized non-valvular atrial fibrillation patients treated with either apixaban or warfarin. Hosp Pract. 2015;43(3):172-79. [DOI] [PubMed] [Google Scholar]
- 15.Centers for Medicare & Medicaid Services, Office of Enterprise Data & Analytics, CMS Chronic Conditions Data Warehouse. Total Medicare enrollment: total, original Medicare, and Medicare Advantage and other health plan enrollment, calendar years 2011-2016. Available at: https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/CMSProgramStatistics/2016/Downloads/MDCR_ENROLL_AB/2016_CPS_MDCR_ENROLL_AB_1.pdf. Accessed April 16, 2020.
- 16.Willke RJ, Mullins CD. “Ten commandments” for conducting comparative effectiveness research using “real-world data.” J Manag Care Pharm. 2011;17(9 Suppl A):S10-15. Available at: https://www.jmcp.org/doi/10.18553/jmcp.2011.17.s9-a.S10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berger ML, Mamdani M, Atkins D, Johnson ML. Good research practices for comparative effectiveness research: defining, reporting and interpreting nonrandomized studies of treatment effects using secondary data sources: the ISPOR Good Research Practices for Retrospective Database Analysis Task Force Report--Part I. Value Health. 2009;12(8):1044-52. [DOI] [PubMed] [Google Scholar]
- 18.Cox E, Martin BC, Van Staa T, Garbe E, Siebert U, Johnson ML. Good research practices for comparative effectiveness research: approaches to mitigate bias and confounding in the design of nonrandomized studies of treatment effects using secondary data sources: the ISPOR Good Research Practices for Retrospective Database Analysis Task Force Report—Part II. Value Health. 2009;12(8):1053-61. [DOI] [PubMed] [Google Scholar]
- 19.Johnson ML, Crown W, Martin BC, Dormuth CR, Siebert U. Good research practices for comparative effectiveness research: analytic methods to improve causal inference from nonrandomized studies of treatment effects using secondary data sources: the ISPOR Good Research Practices for Retrospective Database Analysis Task Force Report—Part III. Value Health. 2009;12(8):1062-73. [DOI] [PubMed] [Google Scholar]
- 20.Jensen PN, Johnson K, Floyd J, Heckbert SR, Carnahan R, Dublin S.. A systematic review of validated methods identifying atrial fibrillation using administrative data. Pharmacoepidemiol Drug Saf. 2012;21(Suppl 1):141-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Teutsch C, Huisman MV, Lip GYH, et al. Persistence with dabigatran therapy for stroke prevention in patients with non-valvular atrial fibrillation: the Gloria-AF Registry. Blood. 2016;128(22):2616.27697774 [Google Scholar]
- 22.Amin A, Keshishian A, Trocio J, et al. Risk of stroke/systemic embolism, major bleeding and associated costs in non-valvular atrial fibrillation patients who initiated apixaban, dabigatran or rivaroxaban compared with warfarin in the United States Medicare population. Curr Med Res Opin. 2017;33(9):1595-604. [DOI] [PubMed] [Google Scholar]
- 23.Thigpen JL, Dillon C, Forster KB, et al. Validity of international classification of disease codes to identify ischemic stroke and intracranial hemorrhage among individuals with associated diagnosis of atrial fibrillation. Circ Cardiovasc Qual Outcomes. 2015;8(1):8-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cunningham A, Stein CM, Chung CP, Daugherty JR, Smalley WE, Ray WA. An automated database case definition for serious bleeding related to oral anticoagulant use. Pharmacoepidemiol Drug Saf. 2011;20(6):560-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest. 2010;137(2):263-72. [DOI] [PubMed] [Google Scholar]
- 26.Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJ, Lip GY. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest. 2010;138(5):1093-100. [DOI] [PubMed] [Google Scholar]
- 27.Austin PC. The use of propensity score methods with survival or time-to-event outcomes: reporting measures of effect similar to those used in randomized experiments. Stat Med. 2014;33(7):1242-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. 2009;28(25):3083-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blough DK, Ramsey SD. Using generalized linear models to assess medical care costs. Health Serv Outcomes Res Method. 2000;1(2):185-202. [Google Scholar]
- 30.Hylek EM, Held C, Alexander JH, et al. Major bleeding in patients with atrial fibrillation receiving apixaban or warfarin: the ARISTOTLE trial (apixaban for reduction in stroke and other thromboembolic events in atrial fibrillation): predictors, characteristics, and clinical outcomes. J Am Coll Cardiol. 2014;63(20):2141-47. [DOI] [PubMed] [Google Scholar]
- 31.Yao X, Abraham NS, Sangaralingham LR, et al. Effectiveness and safety of dabigatran, rivaroxaban, and apixaban versus warfarin in nonvalvular atrial fibrillation. J Am Heart Assoc. 2016;5(6):e003725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li XS, Deitelzweig S, Keshishian A, et al. Effectiveness and safety of apixaban versus warfarin in non-valvular atrial fibrillation patients in “realworld” clinical practice. A propensity-matched analysis of 76,940 patients. Thromb Haemost. 2017;117(6):1072-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lip GY, Keshishian A, Kamble S, et al. Real-world comparison of major bleeding risk among non-valvular atrial fibrillation patients initiated on apixaban, dabigatran, rivaroxaban, or warfarin. Thromb Haemost. 2016;116(5):975-86. [DOI] [PubMed] [Google Scholar]
- 34.Adeboyeje G, Sylwestrzak G, White J, et al. Comparative effectiveness and safety of anticoagulant therapy with warfarin, dabigatran, apixaban, or rivaroxaban in patients with nonvalvular atrial fibrillation [abstract 2]. Circ Cardiovasc Qual Outcomes. 2016;9(Suppl 2):A2. [Google Scholar]
- 35.Noseworthy PA, Yao X, Abraham NS, Sangaralingham LR, McBane RD, Shah ND. Direct comparison of dabigatran, rivaroxaban, and apixaban for effectiveness and safety in nonvalvular atrial fibrillation. Chest. 2016;150(6):1302-12. [DOI] [PubMed] [Google Scholar]
- 36.Lin J, Trocio J, Gupta K, et al. Major bleeding risk and healthcare economic outcomes of non-valvular atrial fibrillation patients newly-initiated with oral anticoagulant therapy in the real-world setting. J Med Econ. 2017;20(9):952-61. [DOI] [PubMed] [Google Scholar]
- 37.Deitelzweig S, Luo X, Gupta K, et al. All-cause, stroke/systemic embolism-, and major bleeding-related health-care costs among elderly patients with nonvalvular atrial fibrillation treated with oral anticoagulants. Clin Appl Thromb Hemost. 2020;24(4):602-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Deitelzweig S, Luo X, Gupta Ket al. Effect of apixaban versus warfarin use on health care resource utilization and costs among elderly patients with nonvalvular atrial fibrillation. J Manag Care Spec Pharm. 2017;23(11):1191-201. Available at: https://www.jmcp.org/doi/10.18553/jmcp.2017.17060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Amin A, Stokes M, Wu N, et al. Estimated medical cost reductions associated with apixaban in real-world patients with non-valvular atrial fibrillation. J Med Econ. 2013;16(10):1193-202. [DOI] [PubMed] [Google Scholar]
- 40.Deitelzweig S, Bruno A, Trocio J, et al. An early evaluation of bleedingrelated hospital readmissions among hospitalized patients with nonvalvular atrial fibrillation treated with direct oral anticoagulants. Curr Med Res Opin. 2016;32(3):573-82. [DOI] [PubMed] [Google Scholar]


