Abstract
Purpose:
To evaluate the pharmacokinetic profiles of the ocular hypotensive agent QLS-101, a novel ATP-sensitive potassium channel opening prodrug, and its active moiety levcromakalim, following topical ophthalmic and intravenous dosing of normotensive rabbits and dogs.
Methods:
Dutch belted rabbits (n = 85) and beagle dogs (n = 32) were dosed with QLS-101 (0.16–3.2 mg/eye/dose) or formulation buffer for 28 days. Pharmacokinetic profiles of QLS-101 and levcromakalim were evaluated in ocular tissues and blood by LC-MS/MS. Tolerability was assessed by clinical and ophthalmic examinations. Maximum systemic tolerated dose was evaluated in beagle dogs (n = 2) following intravenous bolus administrations of QLS-101 (0.05 to 5 mg/kg).
Results:
Plasma analysis following topical dosing of QLS-101 (0.8–3.2 mg/eye/dose) for 28 days indicated an elimination half-life (T1/2) of 5.50–8.82 h and a corresponding time (Tmax) range of 2–12 h in rabbits, and a T1/2 of 3.32–6.18 h with a Tmax range of 1–2 h in dogs. Maximum tissue concentration (Cmax) values ranged from 54.8–540 (day 1) to 50.5–777 ng/mL (day 28) in rabbits, and 36.5–166 (day 1) to 47.0–147 ng/mL (day 28) in dogs. Levcromakalim plasma T1/2 and Tmax were similar to QLS-101, while Cmax was consistently lower. Topical ophthalmic delivery of QLS-101 was well tolerated in both species, with sporadic mild ocular hyperemia noted in the group treated with the highest concentration (3.2 mg/eye/dose). Following topical ophthalmic dosing, QLS-101 and levcromakalim were found primarily in the cornea, sclera, and conjunctiva. Maximum tolerated dose was determined to be 3 mg/kg.
Conclusions:
QLS-101 was converted to its active moiety levcromakalim and showed characteristic absorption, distribution, and safety profiles of a well-tolerated prodrug.
Keywords: ATP-sensitive potassium channel, glaucoma, intraocular pressure, prodrug, levcromakalim, CKLP1
Introduction
Glaucoma, a progressive neurodegenerative disease of the eye, affects over 80 million people worldwide and is a leading cause of irreversible blindness.1,2 Elevated intraocular pressure (IOP) is the most prevalent risk factor for the disease, and currently all treatments are designed to lower IOP to slow disease progression and associated vision loss.3–6
Physiologically, IOP is maintained by the balance between production of aqueous humor by the ciliary epithelial cells and its egress from the anterior chamber through the trabecular and uveoscleral outflow pathways. Resistance to aqueous humor outflow is the main cause of elevated IOP. Therefore, agents that either reduce the rate of aqueous humor production or facilitate outflow of aqueous humor from the anterior chamber are commonly used by clinicians to lower IOP.
Many of these IOP lowering therapeutics may be associated with adverse side effects and tachyphylaxis.7 Due to variable efficacy and drug resistance, many patients require multiple drugs that affect both aqueous formation and outflow to lower IOP and slow disease progression.8 Unfortunately, nearly 50% of patients are noncompliant to their medical therapies due in part to the use of multiple medications, variance in dosing regimens, and adverse side effects, thus impacting successful outcomes.9,10 As a result, there is a significant need for identification of novel ocular hypotensive therapeutics that will effectively lower IOP with minimal side effect profiles and work through a novel mode of action.
Over the past decade, a new class of IOP-lowering therapeutics known as ATP-sensitive potassium channel openers has been identified and characterized.11–16 The ability of ATP-sensitive potassium channel openers such as levcromakalim to lower IOP has been described in human anterior segments, and normotensive and ocular hypertensive animal models.12–16 Because of its limited aqueous solubility, a water-soluble prodrug of levcromakalim called cromakalim prodrug 1 (CKLP1) was developed and shown to have similar efficacy to its active moiety.13,15–18 Similar to other ATP-sensitive potassium channel openers, CKLP1 was shown to significantly lower IOP in multiple normotensive (C57BL/6J mice, Dutch belted rabbits, hound dogs, African green monkeys) and ocular hypertensive animal models (TGF-β2 overexpressing mice, steroid-induced C57BL/6J mice, and DBA/2J mice).15–18
Following conversion of CKLP1 to its active moiety levcromakalim by endogenous phosphatases, the drug was found to reduce episcleral venous pressure, a unique mode of action compared with currently available ocular hypotensive agents.16,18,19 Due to this unique mechanism, CKLP1 was shown to work in an additive manner when used in combination with existing ocular hypotensive agents such as latanoprost, timolol, and a Rho kinase inhibitor.18
Recently, Qlaris Bio, Inc., has developed a free acid form of CKLP1 which, when fully dissolved at equimolar doses in phosphate-buffered saline (PBS), is indistinguishable from CKLP1. We have previously demonstrated in preclinical models that, similar to CKLP1, QLS-101 is hydrolyzed to form the active moiety levcromakalim and significantly lowers IOP with once-daily dosing.19 In the current study, we evaluated the pharmacokinetic profiles of QLS-101 and its active moiety levcromakalim, following in vivo treatments at various doses. The treatment range was selected based on previously published reports with CKLP1 from our laboratory that evaluated the effect of these doses on IOP, tolerability, and pharmacokinetics.12–19 We also evaluated the ocular and systemic safety and tolerability of QLS-101 following topical ophthalmic and intravenous dosing in normotensive Dutch belted rabbits and beagle dogs.
Methods
Reagents
QLS-101 was synthesized as a phosphate ester prodrug by Aptuit (Oxford) Ltd, an Evotec Company (Abingdon, Oxfordshire, UK) as previously reported.13,19 Formulations were prepared by dissolving QLS-101 in PBS and adjusting the pH to 6.5 with sodium hydroxide. The sodium chloride content was determined to control the osmolality (290 ± 25 mOsm/kg) of the prodrug product. Formulations of QLS-101 were prepared as isotonic solutions for 0.4%, 2.0%, and 4.0% QLS-101. For the 8.0% solution, the formulation resulted in a slightly hypertonic solution (440–465 mOsm/kg). Deuterated QLS-101 (QLS-101-d6) and the active moiety levcromakalim (QLS-100-d6) were also manufactured by Aptuit for use as stable isotope internal standards for liquid chromatography with tandem mass spectrometry (LC-MS/MS) analysis. Analysis was performed using qualified methods at NorthEast BioAnalytical Laboratories LLC (Hamden, CT).
Animal care
All animal experiments were preapproved by the Institutional Animal Care and Use Committee of the facilities where they were performed and adhered to the recommendations in the Guide for the Care and use of Laboratory Animals of the National Institutes of Health, and the tenets of the ARVO Statement for the Use of Animals in Vision Research. Animals were acclimated to the study environment for 7–15 days. Male and female Dutch belted, pigmented rabbits >6 months of age were procured from either Covance (n = 53) or Envigo Global Services, Inc. (Denver, CO; n = 32). Male and female beagle dogs (5–7 months old) were obtained from Marshall BioResources (North Rose, NY). Beagle dogs receiving topical ophthalmic dosing (n = 32) and intravenous dosing to assess effects on blood pressure (n = 4) were housed in groups ≤3 animals of the same sex and dosing group, while beagle dogs used to determine maximum tolerated dose (MTD; n = 2) were housed individually. All animals were housed in a climate-controlled room with 12-h light–12-h dark cycles and unlimited access to food and water.
Topical ophthalmic treatment with QLS-101
Dutch belted rabbits and beagle dogs were treated topically once daily in both eyes for 28 consecutive days with 40 μL of either QLS-101 at doses of 0.16, 0.8, 1.6, or 3.2 mg/eye/dose (0.4%, 2.0%, 4.0%, or 8.0%) or formulation buffer (without the drug). In both species, 2 animals of each sex from the formulation buffer and 3.2 mg/eye/dose QLS-101-treated groups were placed after 28 days of treatment into a 14-day no-treatment/recovery group.
Intravenous treatment with QLS-101
To quantify effects of QLS-101 on blood pressure and heart rate, various doses of QLS-101 were administered by intravenous injection to the cephalic vein of 4 male beagle dogs (∼2 years of age) previously implanted with DSI PhysioTel® Digital L21 transmitters (Data Sciences International, St. Paul, MN) in a modified lead II configuration. Each animal received a single dose of formulation buffer and 3 doses of QLS-101 (0.075, 0.5, and 1.5 mg/kg) using a 4 × 4 Latin-Square crossover paradigm, with each animal receiving a different dose from the others at each time point. Doses were administered 7 days apart. Cardiovascular parameters, including heart rate, body temperature, and systemic arterial blood pressure were monitored for 26 h before the study, and then for 2 h before and 24 h after administration of each dose, recording at a 1-min logging rate.
To quantify MTD and plasma pharmacokinetics following systemic administration, ascending doses of QLS-101 were administered to 1 male and 1 female beagle dog by single intravenous injections into the cephalic vein. Doses were administered with a dose escalation design, with a minimum observation period of 48 h between each injection (0.05 mg/kg dosed on day 1; 0.5 mg/kg dosed on day 3; 1.5 mg/kg dosed on day 8; 3 mg/kg dosed on day 10; and 5 mg/kg dosed on day 14). For pharmacokinetic analysis, plasma was isolated from blood samples collected from each animal before dosing and at 1, 3, 6, 8, and 24 h following each dose.
Clinical and ocular examinations
In all studies, animals were observed twice daily for morbidity and mortality. Food consumption and body weights were determined each day just before dosing. Overall health was monitored through assessment of heart rate, body temperature, and general animal behavior. Cage-side observations were performed daily, with particular attention paid to both eyes. A veterinary ophthalmologist performed complete ocular examinations using a slit lamp biomicroscope and indirect ophthalmoscope to evaluate ocular surface morphology and anterior segment inflammation on all animals. The Hackett and McDonald ocular grading system was used for scoring. In animals dosed topically, gross ocular examinations, including the modified Draize scale, occurred once before the study, at 4 h postdose on days 3, 7, 14, and 28, and at the end of the 14-day recovery period before necropsy.
Collection of blood and tissues for pharmacokinetic and pathology analysis
For plasma pharmacokinetic analysis, blood samples were drawn before dosing and at 1, 2, 4, 8, 12, and 24 h postdose on days 1 and 28, and in recovery animals just before sacrifice. Following sacrifice, all animals were subjected to a complete necropsy examination, where eyes, extraocular tissues, and peripheral organs were isolated and examined both at the macroscopic and microscopic levels (Supplementary Table S1). A board-certified veterinary pathologist performed histopathologic evaluation.
Blood clinical pathology analysis
Before study initiation, on day 27 before sacrifice, and postrecovery in applicable animals, blood was collected from Dutch belted rabbits and beagle dogs treated with topical ophthalmic administration with QLS-101 (0.8, 1.6, and 3.2 mg/eye/dose) or formulation buffer through jugular venipuncture. Whole blood was analyzed for hematology parameters using an ADVIA 120 Hematology System (Siemens, Malvern, PA). Plasma was analyzed for coagulation parameters with a STA Compact Stago Analyzer (Diagnostica Stago, Parsippany, NJ). Serum was analyzed for clinical chemistry parameters using a COBAS 6000 machine (Roche Diagnostics, Indianapolis, IN).
Collection of ocular tissues for drug distribution analysis
To determine ocular drug distribution, both eyes of Dutch belted rabbits (n = 24) were treated with 40 μL eyedrops containing QLS-101 (0.16 or 0.8 mg/eye/dose) once daily for 28 consecutive days. Animals were sacrificed either on day 6, day 17, or on day 28 before dosing, and then 2, 4, and 23-h postdose (n = 2 animals/per group). These interim sacrifices reduced the number of animals from n = 24 per dose on day 5 to n = 16 per dose on day 16 and n = 8 per dose on day 27. Data were collected from these 3 days and compared to day 1 levels to evaluate pharmacokinetic parameters and drug accumulation following short-term, intermediate, and long-term dosing. Following animal sacrifice, the right eye from each animal was removed and dissected while frozen. Samples of cornea, lens, iris, ciliary body, retina, choroid, vitreous humor, sclera, optic nerve, bulbar conjunctiva, orbital fat, and lacrimal gland were isolated from each animal. Tissues were shipped on dry ice for analysis by LC-MS/MS (NorthEast BioAnalytical Laboratories, LLC).
Quantification of QLS-101 and levcromakalim
LC-MS/MS was used to detect QLS-101 and levcromakalim in rabbit ocular tissues as well as plasma from both Dutch belted rabbits and beagle dogs. The lower limit of quantitation (LLOQ) for QLS-101 and levcromakalim was calculated to be 1.999 and 0.499 ng/mL, respectively. For each analysis, stable isotope (deuterated) internal standards QLS-101-d6 and QLS-100-d6 (levcromakalim) were dissolved in acetonitrile and added to plasma samples along with appropriate quality controls and freshly prepared calibrators containing known quantities of QLS-101 and levcromakalim.
Frozen ocular tissue samples were thawed and homogenized in 1 × PBS (4 mL/g of tissue, yields dilution factor = 5) using a Qiagen TissueLyzer LT bead shaker homogenizer with stainless steel beads (5 mm diameter; QIAGEN, Germantown, MD). Tissues were homogenized for 5 min at 5 oscillations per second. Liquid chromatographic separation was performed using a gradient of acetonitrile and 10 mM ammonium formate on a Kinetex 5 μm EVO C18 50 × 2.1 mm analytical column with a Security Guard Gemini C18 3.2 mm internal diameter guard column (Phenomenex, Torrance, CA).
Detection was accomplished by mass spectrometry with a Sciex API 5000 operated in positive ion electrospray mode using multiple reaction monitoring (MRM) optimized for detection of the test analytes and internal standards. The MRM parent and product ions for the compounds and stable isotope internal standards were monitored at m/z 367 > 86, 287 > 86, 373 > 92, and 293 > 92 for QLS-101, levcromakalim, QLS-101-d6, and QLS-100-d6, respectively. For all methods, the intraday and interday bias and precision criteria were ±20% for the LLOQ, and ±15% for tested levels above the LLOQ relative to the expected nominal concentrations for QLS-101 and levcromakalim in each test system.
Statistical analysis
Values are expressed as mean ± standard error of mean wherever applicable. All statistical tests were conducted at the 5% significance level. Mean values within the same group or animal were compared using Student's paired t-test at 5% confidence levels as appropriate. Tissue pharmacokinetic parameters such as the maximum tissue concentration (Cmax) and the corresponding time (Tmax), the concentration–time curve (AUC), and elimination half-life (T1/2) were estimated using noncompartmental rank-sum analysis with the linear trapezoidal rule [Phoenix WinNonlin (v8.0); Certara Corporation, Princeton, NJ].
Results
Pharmacokinetic assessment of QLS-101 in Dutch belted rabbits following topical ophthalmic treatment
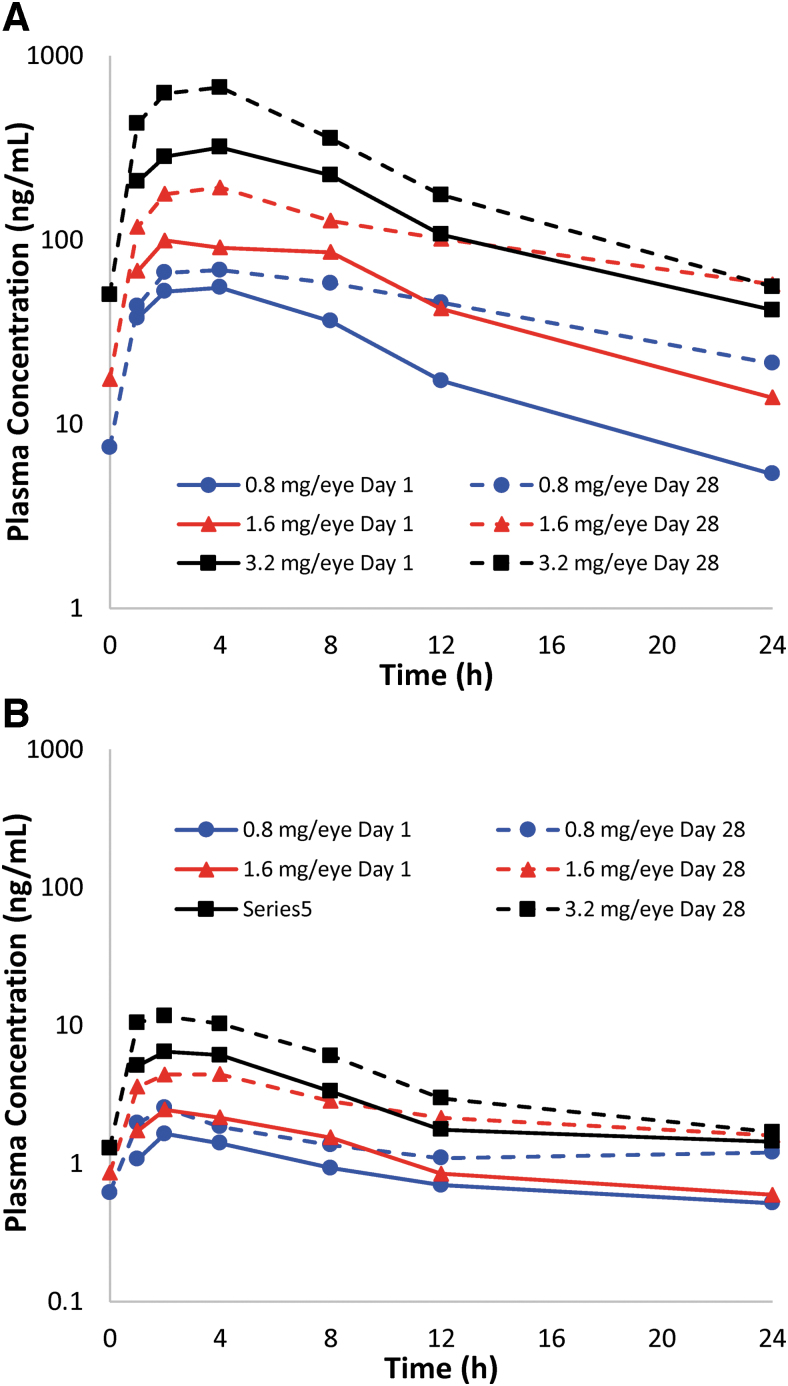
QLS-101 was administered topically (40 μL drops) to both eyes of male and female Dutch belted rabbits once daily at doses of 0 mg/eye/dose (formulation buffer; n = 10), 0.8 mg/eye/dose (n = 6), 1.6 mg/eye/dose (n = 6), or 3.2 mg/eye/dose (n = 10) for 28 consecutive days. To assess systemic pharmacokinetics of QLS-101, plasma was isolated from blood that was collected at various time points over 24 h postdose from all animals on days 1 and 28 following treatment (Fig. 1A). On day 1, QLS-101 Tmax range across all doses was calculated to be 1–8 h in males, and 2–24 h in females. Similarly, Tmax for QLS-101 on day 28 ranged from 2 to 12 h in both males and females (Table 1). Plasma Cmax and AUC0–24h values on days 1 and 28 increased in a dose-dependent, but not dose-proportional manner. The individual T1/2 ranged from 2.83 to 8.48 h on day 1 and from 5.33 to 8.82 h on day 28 across the different doses.
FIG. 1.
Mean plasma concentration versus time profiles of QLS-101 and levcromakalim in Dutch belted rabbits. (A) Plasma concentrations of QLS-101 following once-daily topical ocular administration of 3 concentrations of QLS-101 for 28 consecutive days. (B) Plasma concentrations of levcromakalim following once-daily topical ocular administration of 3 concentrations of QLS-101 for 28 consecutive days. Presence of levcromakalim in plasma confirms conversion of QLS-101 to levcromakalim. Solid lines represent day 1 and dashed lines represent day 28 data.
Table 1.
Plasma Pharmacokinetics in Dutch Belted Rabbits Following Topical Ophthalmic Administration with QLS-101
| QLS-101 | |||||||
|---|---|---|---|---|---|---|---|
| Day | Gender | Dose (mg/eye/dose) | Tmax (h) | Cmax (ng/mL) | tlast (h) | AUC0–24h (h × ng/mL) | T1/2 (h) |
| 1 | Male | 0.8 | 4 (1–8) | 69.60 ± 70.20 | 24 | 657 ± 474 | 5.19 ± ID |
| 1.6 | 2 (2–8) | 130 ± 156 | 24 | 1,290 ± 1,240 | 2.83 ± ID | ||
| 3.2 | 4 (2–8) | 540 ± 365 | 24 | 5,070 ± 2,520 | 3.82 ± 1.21 | ||
| Female | 0.8 | 2 (2–2) | 54.80 ± 40.60 | 24 | 535 ± 233 | 6.71 ± ID | |
| 1.6 | 8 (2–8) | 99.20 ± 49.30 | 24 | 1,220 ± 588 | NC | ||
| 3.2 | 8 (2–24) | 161 ± 65.60 | 24 | 2,120 ± 701 | 8.48 ± ID | ||
| 28 | Male | 0.8 | 12 (2–12) | 50.50 ± 2.44 | 24 | 882 ± 57.10 | NC |
| 1.6 | 4 (4–4) | 178 ± 146 | 24 | 2,230 ± 1,300 | 6.12 ± ID | ||
| 3.2 | 4 (2–8) | 777 ± 421 | 24 | 7,200 ± 2,680 | 5.50 ± 1.73 | ||
| Female | 0.8 | 4 (2–8) | 101 ± 66.40 | 24 | 1,280 ± 631 | 7.52 ± ID | |
| 1.6 | 4 (4–12) | 218 ± 148 | 24 | 3,050 ± 1,490 | 8.82 ± ID | ||
| 3.2 | 4 (2–8) | 630 ± 514 | 24 | 5,930 ± 3,460 | 5.33 ± ID | ||
| Levcromakalim | |||||||
|---|---|---|---|---|---|---|---|
| Day | Gender | Dose (%) (mg/eye/dose) | Tmax (h) | Cmax (ng/mL) | tlast (h) | AUC0–24h (h × ng/mL) | T1/2 (h) |
| 1 |
Male |
0.8 |
4 (2–4) |
1.55 ± 0.29 |
12 (8–24) |
14.70 ± 7.52 |
NC |
| |
|
1.6 |
4 (2–4) |
2.57 ± 2.09 |
12 (12–24) |
21.90 ± 12.00 |
NC |
| 3.2 |
2 (1–4) |
9.95 ± 4.92 |
24 (12–24) |
86.10 ± 25.80 |
5.20 ± 2.30 |
||
| Female |
0.8 |
2 (1–2) |
1.93 ± 0.34 |
8 (8–8) |
9.60 ± 1.14 |
NC |
|
| |
1.6 |
4 (2–24) |
2.17 ± 1.34 |
24 (24–24) |
25.10 ± 14.80 |
7.41 ± ID |
|
| 3.2 |
2 (1–24) |
4.23 ± 1.90 |
24 (12–24) |
42.20 ± 17.90 |
3.22 ± ID |
||
| 28 |
Male |
0.8 |
2 (2–2) |
2.31 ± 0.57 |
24 (24–24) |
34.70 ± 13.80 |
NC |
| |
1.6 |
4 (2–4) |
5.31 ± 2.51 |
24 (24–24) |
63.10 ± 20.30 |
7.92 ± ID |
|
| 3.2 |
2 (1–8) |
15.20 ± 5.70 |
24 (24–24) |
136 ± 21.00 |
7.55 ± 2.38 |
||
| Female |
0.8 |
2 (1–2) |
2.78 ± 0.95 |
24 (24–24) |
28.00 ± 9.37 |
NC |
|
| 1.6 |
2 (1–4) |
5.15 ± 3.52 |
24 (24–24) |
60.40 ± 31.70 |
NC |
||
| 3.2 | 4 (1–8) | 11.10 ± 4.83 | 24 (24–24) | 98.10 ± 36.00 | 6.58 ± ID | ||
AUC, concentration–time curve; Cmax, maximum tissue concentration; ID, insufficient data; NC, not calculable; T1/2, elimination half-life; Tmax, corresponding time.
Levcromakalim, the active moiety of QLS-101, was also identified in all blood samples across all doses and time points but at lower levels than QLS-101 (Fig. 1B and Table 1). Mean exposure to levcromakalim represented between 1.76% (day 1) and 4.57% (day 28) of mean QLS-101 Cmax, and 1.65% (day 1) and 3.93% (day 28) of mean QLS-101 AUC0–24h. Similar to QLS-101, plasma levels of levcromakalim increased proportionally to the QLS-101 treatment dose. The plasma Tmax of levcromakalim was observed between 1 and 4 h in males and between 1 and 24 h in females on day 1; and between 1 and 8 h in both sexes on day 28. When estimated, the individual T1/2 ranged from 3.22 to 7.92 h, and was not dependent on sex, dose, or time, similar to QLS-101 parameters (Fig. 1B).
Two male and 2 female rabbits from the 3.2 mg/eye/dose QLS-101 and formulation buffer-treated groups were allowed to recover for 14 days at the conclusion of the 28-day treatment period. Plasma samples collected from these animals at the end of the recovery period exhibited undetectable levels of QLS-101 (LLOQ = 1.999 ng/mL). Levcromakalim was quantifiable only in female recovery animals, but not males, at 14 days postdose (LLOQ = 0.499 ng/mL).
Ocular distribution of QLS-101 in Dutch belted rabbits following topical ophthalmic treatment
To determine ocular distribution of QLS-101 and its active moiety, levcromakalim, female Dutch belted rabbits (n = 24 per group) were treated once daily to both eyes with either 0.16 mg/eye/dose or 0.8 mg/eye/dose QLS-101 for 28 consecutive days. While QLS-101 was detectable in most ocular tissues sampled, higher concentrations were noted in tissues isolated from animals administered the 0.8 mg/eye/dose, specifically in the cornea, sclera, conjunctiva, and lacrimal gland (Table 2). Lesser concentrations were identified in the lens and orbital fat. Interestingly, lowest levels of QLS-101 were found in vitreous humor while aqueous humor contained levels below quantitation.
Table 2.
Ocular Tissue Pharmacokinetic Parameters for QLS-101 in Dutch Belted Rabbits Following Topical Ophthalmic Administration
| |
Day 6 |
Day 17 |
Day 28 |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Dose solution (mg/eye/dose) | Tissue | Cmax (ng/g) | AUC0–23h (h × ng/g) | Tmax (h) | Cmax (ng/g) | Tmax (h) | Cmax (ng/g) | AUC0–23h (h × ng/g) | Tmax (h) |
| 0.16 | Cornea | 1,510 | 24,900 | 4 | 885 | 2 | 82 | 1,310 | 4 |
| 0.16 | Aqueous Humor | BLQ | BLQ | BLQ | BLQ | BLQ | 6.29 | 66.1 | 4 |
| 0.16 | Conjunctiva | 161 | 1,980 | BLQ | 116 | 2 | 640 | 8,920 | 23 |
| 0.16 | Sclera | 3,570 | 50,000 | 4 | 580 | 4 | 58.5 | 1,010 | 4 |
| 0.16 | Lacrimal gland | 128 | 2,790 | 23 | 126 | 4 | 79.3 | 958 | 4 |
| 0.16 | Iris/CB | 117 | 1,320 | 2 | 28.9 | 2 | BLQ | BLQ | BLQ |
| 0.16 | Lens | 5.91 | 11.8 | 2 | BLQ | BLQ | BLQ | BLQ | BLQ |
| 0.16 | Vitreous | 45.4 | 498 | 4 | 4.5 | 4 | 9.85 | 9.85 | BLQ |
| 0.16 | Fat | 46.1 | 596 | 4 | 41.4 | 2 | 81.9 | 1,510 | 4 |
| 0.16 | Retina | 448 | 5,410 | 4 | 75.7 | 4 | BLQ | BLQ | BLQ |
| 0.16 | Optic nerve | 255 | 3,650 | 4 | 48.3 | 4 | BLQ | BLQ | BLQ |
| 0.8 | Cornea | 6,680 | 104,000 | 4 | 2,440 | 4 | 416 | 4,330 | 2 |
| 0.8 | Aqueous humor | 142 | 1,770 | 4 | 32.9 | 2 | 29.4 | 315 | 2 |
| 0.8 | Conjunctiva | 1,590 | 14,300 | 2 | 506 | BLQ | 2,390 | 31,200 | 4 |
| 0.8 | Sclera | 3,330 | 37,600 | 2 | 857 | 4 | 237 | 3,850 | 4 |
| 0.8 | Lacrimal gland | 219 | 3,720 | 4 | 437 | 4 | 219 | 2,850 | 2 |
| 0.8 | Iris/CB | 581 | 3,920 | 2 | 142 | 4 | 23.1 | 199 | 2 |
| 0.8 | Lens | 101 | 248 | 2 | BLQ | BLQ | BLQ | BLQ | BLQ |
| 0.8 | Vitreous | 10.4 | 109 | 4 | BLQ | BLQ | BLQ | BLQ | BLQ |
| 0.8 | Fat | 168 | 1,200 | 2 | 90.5 | 2 | 259 | 2,630 | 2 |
| 0.8 | Retina | 104 | 2,090 | 23 | 96.8 | 2 | 28.8 | 254 | 2 |
| 0.8 | Optic nerve | 165 | 910 | 2 | 47.9 | 2 | 19.1 | 38.3 | 2 |
BLQ, below limit of quantification; CB, ciliary body.
Pharmacokinetic parameters were not estimated for levcromakalim, as multiple time points had concentrations that were below the LLOQ. However, levcromakalim was detectable in some anterior segment tissues following ocular administration of QLS-101. In 0.16 mg/eye/dose QLS-101-treated animals, levcromakalim was sporadically detected in the cornea, conjunctiva, sclera, and lacrimal gland. In 0.8 mg/eye/dose QLS-101-treated animals, levcromakalim was primarily detected in the cornea (3.51–59.3 ng/g of tissue), conjunctiva (3.03–26.4 ng/g), lacrimal gland (4.28–13.4 ng/g), and iris/ciliary body (1.41–2.02 ng/g). Levcromakalim was only detected in aqueous humor on day 28 at low concentrations.
Tolerability of QLS-101 in Dutch belted rabbits following topical ophthalmic treatment
Throughout the study, no change in food consumption was observed, and animal body weight increased similarly to formulation buffer and untreated controls (Supplementary Table S2). Clinical parameters for blood chemistry values were all within normal ranges (Table 3), as were blood differential and coagulation measurements (Supplementary Table S2). At the conclusion of the 28-day topical ophthalmic treatment period, no QLS-101-related adverse systemic effects were identified, and no unscheduled deaths were noted. Animals from the 3.2 mg/eye/dose QLS-101-treated group that were allowed to recover for 14 days also showed no clinical findings.
Table 3.
Blood Chemistry of Dutch Belted Rabbits Following 21 Days of Topical Ophthalmic Administration of QLS-101
| Males |
|||||
|---|---|---|---|---|---|
| Vehicle | QLS-101 (0.8 mg/eye/dose) | QLS-101 (1.6 mg/dose/eye) | QLS-101 (3.2 mg/eye/dose) | Historical control range | |
| Aspartate aminotransferase (U/L) | 21.20 ± 3.00 | 22.70 ± 12.40 | 17.00 ± 0.00 | 18.40 ± 2.80 | 15.10–67.60 |
| Alanine aminotransferase (U/L) | 28.80 ± 7.90 | 40.00 ± 8.50 | 23.00 ± 5.30 | 29.40 ± 8.60 | 16.60–53.70 |
| Alkaline phosphatase (U/L) | 88.60 ± 13.50 | 106.70 ± 27.70 | 93.70 ± 3.50 | 106.00 ± 24.80 | 43.70–155.10 |
| Total bilirubin (mg/dL) | 0.04 ± 0.02 | 0.03 ± 0.03 | 0.02 ± 0.02 | 0.03 ± 0.02 | 0.00–0.05 |
| Urea nitrogen (mg/dL) | 19.00 ± 2.30 | 16.70 ± 1.20 | 19.00 ± 2.60 | 17.60 ± 2.70 | 14.10–23.80 |
| Creatinine (mg/dL) | 0.80 ± 0.07 | 0.83 ± 0.06 | 0.87 ± 0.15 | 0.84 ± 0.05 | 0.70–1.05 |
| Creatinine kinase (U/L) | 687.00 ± 237.60 | 475.70 ± 86.50 | 436.00 ± 144.50 | 488.00 ± 116.80 | 428.50–29,866.30 |
| Glucose (mg/dL) | 120.60 ± 2.40 | 129.70 ± 8.50 | 118.70 ± 7.40 | 126.20 ± 11.70 | 118.00–148.50 |
| Cholesterol (mg/dL) | 22.60 ± 1.70 | 21.30 ± 2.30 | 29.30 ± 8.50 | 25.40 ± 5.50 | 21.00–47.10 |
| Triglycerides (mg/dL) | 35.40 ± 8.30 | 36.00 ± 5.20 | 34.00 ± 7.20 | 40.80 ± 13.90 | 23.70–51.90 |
| Total protein (g/dL) | 5.70 ± 0.10 | 5.73 ± 0.06 | 5.90 ± 0.10 | 5.72 ± 0.19 | 5.46–6.20 |
| Albumin/globulin ratio (G) | 3.52 ± 0.34 | 3.47 ± 0.12 | 3.10 ± 0.20 | 3.40 ± 0.25 | 2.81–3.79 |
| Calcium (mg/dL) | 13.44 ± 0.28 | 13.67 ± 0.21 | 13.50 ± 0.26 | 13.70 ± 0.54 | 11.89–14.08 |
| Phosphorus (mg/dL) | 5.44 ± 0.11 | 5.13 ± 0.46 | 5.67 ± 0.15 | 5.44 ± 0.40 | 4.70–6.35 |
| Sodium (mmol/L) | 138.60 ± 1.10 | 139.30 ± 1.20 | 140.30 ± 0.60 | 139.80 ± 0.80 | 137.60–144.00 |
| Potassium (mmol/L) | 4.40 ± 0.22 | 4.30 ± 0.26 | 4.37 ± 0.25 | 4.40 ± 0.39 | 4.16–5.66 |
| Chloride (mmol/L) | 103.20 ± 1.50 | 102.00 ± 1.70 | 103.30 ± 0.60 | 102.40 ± 1.90 | 99.00–105.00 |
| |
Females |
||||
|---|---|---|---|---|---|
| Vehicle | QLS-101 (0.8 mg/eye/dose) | QLS-101 (1.6 mg/eye/dose) | QLS-101 (3.2 mg/eye/dose) | Historical control range | |
| Aspartate aminotransferase (U/L) |
21.60 ± 6.50 |
19.30 ± 2.50 |
16.00 ± 4.40 |
17.40 ± 3.10 |
13.00–49.10 |
| Alanine aminotransferase (U/L) |
34.40 ± 9.30 |
29.00 ± 7.00 |
20.70 ± 7.20 |
25.00 ± 5.80 |
19.00–52.00 |
| Alkaline phosphatase (U/L) |
109.20 ± 19.00 |
90.00 ± 10.40 |
86.70 ± 24.80 |
80.00 ± 11.40 |
65.10–162.30 |
| Total bilirubin (mg/dL) |
0.02 ± 0.02 |
0.04 ± 0.01 |
0.03 ± 0.03 |
0.02 ± 0.02 |
0.00–0.06 |
| Urea nitrogen (mg/dL) |
25.40 ± 3.8 0 |
25.70 ± 2.10 |
22.30 ± 3.20 |
23.40 ± 4.30 |
13.10–28.80 |
| Creatinine (mg/dL) |
0.88 ± 0.08 |
1.03 ± 0.12 |
0.93 ± 0.06 |
0.90 ± 0.07 |
0.70–1.10 |
| Creatinine kinase (U/L) |
515.60 ± 70.40 |
417.00 ± 100.30 |
452.00 ± 200.10 |
470.00 ± 109.60 |
371.50–28,004.60 |
| Glucose (mg/dL) |
117.00 ± 8.20 |
122.70 ± 11.20 |
122.70 ± 9.90 |
121.20 ± 12.3 |
109.20–142.00 |
| Cholesterol (mg/dL) |
35.20 ± 2.70 |
40.70 ± 2.90 |
38.00 ± 3.50 |
38.80 ± 9.30 |
31.00–60.80 |
| Triglycerides (mg/dL) |
33.40 ± 3.30 |
38.70 ± 8.10 |
35.00 ± 1.00 |
35.80 ± 9.00 |
24.10–45.90 |
| Total protein (g/dL) |
5.70 ± 0.37 |
5.90 ± 0.20 |
5.80 ± 0.35 |
5.68 ± 0.35 |
5.20–6.60 |
| Albumin/globulin ratio (G) |
3.26 ± 0.18 |
3.10 ± 0.20 |
3.17 ± 0.55 |
3.34 ± 0.44 |
2.40–3.98 |
| Calcium (mg/dL) |
13.32 ± 0.40 |
13.50 ± 0.26 |
13.40 ± 0.20 |
13.50 ± 0.43 |
12.41–13.90 |
| Phosphorus (mg/dL) |
5.00 ± 0.22 |
5.17 ± 0.25 |
4.70 ± 0.26 |
5.08 ± 0.23 |
4.50–5.99 |
| Sodium (mmol/L) |
138.20 ± 1.10 |
139.70 ± 1.20 |
138.00 ± 2.00 |
138.20 ± 0.80 |
136.00–144.00 |
| Potassium (mmol/L) |
4.46 ± 0.26 |
4.10 ± 0.10 |
4.10 ± 0.10 |
4.36 ± 0.17 |
3.90–4.90 |
| Chloride (mmol/L) | 102.40 ± 1.80 | 102.30 ± 2.10 | 101.70 ± 1.50 | 101.20 ± 2.30 | 99.0 0–105.00 |
Ocular examination showed that topical ophthalmic application of QLS-101 was well tolerated across the various doses (0.8, 1.6, and 3.2 mg/eye/dose). In only a few eyes, mild redness was noted, including 2 eyes treated with formulation buffer (Table 4). Redness was more prevalent at the 3.2 mg/eye/dose concentration, but in most cases did not persist between time points with the exception of 1 female in the 0.8 mg/eye/dose-treated group. Additionally, mild swelling was reported on day 14 in 1 eye of 1 animal that received 1.6 mg/eye/dose QLS-101, but it resolved by day 21. Superficial corneal opacities described as very mild were reported on day 28 in 2 of 12 eyes treated with 1.6 mg/eye/dose, and in 3 of 20 eyes treated with 3.2 mg/eye/dose of QLS-101, but these were deemed clinically insignificant by the examiners. Based on the absence of any significant adverse events, the no-observed-adverse-effect level (NOAEL) for dosing both eyes was determined to be 3.2 mg/eye once daily.
Table 4.
Summary of Observed Eye Redness During Gross Ocular Examination in Dutch Belted Rabbits Dosed Once Daily by Topical Ophthalmic Administration with Various QLS-101 Concentrations for 28 Days
| Treatment | Eye | Day |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| −7 |
3 |
7 |
14 |
21 |
28 |
29 |
42 |
||
| Number of eyes (severity level)a | |||||||||
| Formulation buffer | OD | — | — | — | — | — | 1 (2) | — | — |
| OS | — | — | — | — | — | 1 (2) | — | — | |
| QLS-101 (0.8 mg/eye/dose) | OD | — | — | — | 1 (2) | — | 2 (2) | — | N/A |
| OS | — | — | — | — | — | 3 (2) | 1 (2) | N/A | |
| QLS-101 (1.6 mg/eye/dose) | OD | — | — | — | 1 (2) | — | 3 (2) | — | N/A |
| OS | — | — | — | 1 (1) | — | — | — | N/A | |
| QLS-101 (3.2 mg/eye/dose) | OD | — | 1 (2) | 1 (2) | 2 (2) | — | 3 (2) | — | — |
| OS | — | 1 (2) | — | — | — | 6 (2) | — | — | |
Values represent the number of animals identified with ocular redness. Number in parenthesis represents the magnitude of observed redness (1 = very mild; 2 = mild). No other relevant findings were observed during gross ocular examination.
N/A, not available; OD, right eye; OS, left eye.
Pharmacokinetics of QLS-101 in beagle dogs following topical ophthalmic treatment
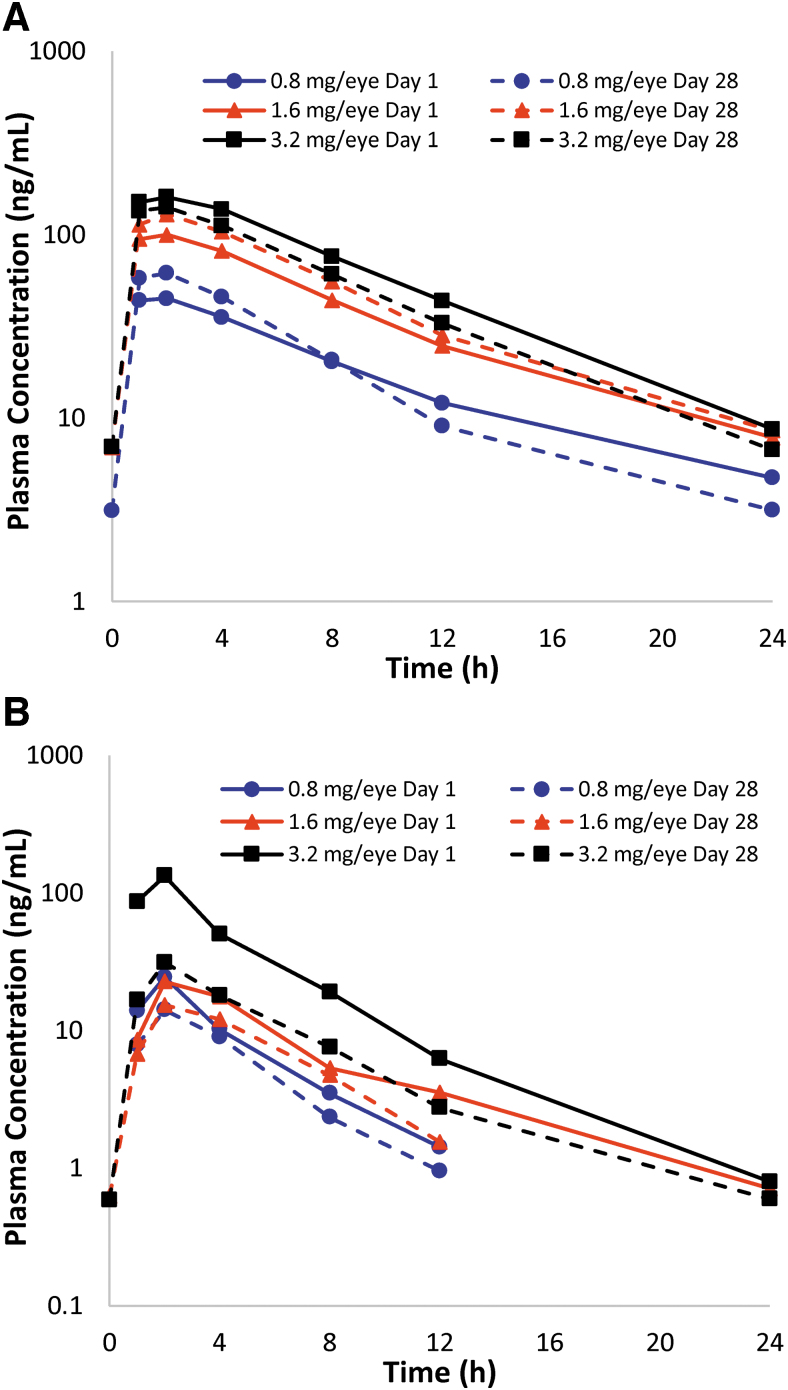
To evaluate pharmacokinetic parameters following topical ophthalmic administration in dogs, formulation buffer or QLS-101 at concentrations of 0.8, 1.6, or 3.2 mg/eye/dose were administered once daily for 28 consecutive days to both eyes (n = 3 of each sex for each concentration). Pharmacokinetic analyses of QLS-101 concentrations showed that Tmax occurred between 1 and 2 h in all dose groups in both male and female animals (Table 5). The mean T1/2 of QLS-101 ranged between 3.69 and 4.82 h in males and 4.12–6.18 h in females on day 1, and 3.32–5.08 h in males and 3.68–5.15 h in females on day 28 (Fig. 2A shows combined time vs. concentration curve). In male dogs, Cmax values increased in a dose-dependent manner from day 1 (36.5–166 ng/mL) to day 28 (47.0–147.0 ng/mL) but were not dose proportional. Cmax in females showed a dose-dependent trend on day 1 (range 55.5–156.0 ng/mL), but by day 28, Cmax values [80.2 ng/mL (0.8 mg/eye/dose), 201.0 ng/mL (1.6 mg/eye/dose), 139 ng/mL (3.2 mg/eye/dose)] were not dose dependent (Table 5).
Table 5.
Plasma Pharmacokinetic Parameters for QLS-101 and Levcromakalim in Beagle Dogs Dosed Topically with QLS-101 Eyedrops for 28 Days
| QLS-101 | |||||||
|---|---|---|---|---|---|---|---|
| Day | Gender | Dose (mg/eye/dose) | Tmax (h) | Cmax (ng/mL) | tlast (h) | AUC0–24h (h × ng/mL) | T1/2 (h) |
| 1 | Male | 0.8 | 1 (1–2) | 36.5 ± 17.2 | 12 (12–12) | 231 ± 108 | 3.69 ± ID |
| 1.6 | 1 (1–2) | 73.7 ± 50.5 | 12 (12–24) | 520 ± 274 | 4.26 ± ID | ||
| 3.2 | 2 (2–2) | 166 ± 32.0 | 24 (24–24) | 1,520 ± 115 | 4.82 ± 0.872 | ||
| Female | 0.8 | 2 (2–2) | 55.5 ± 26.5 | 12 (12–24) | 496 ± 371 | 4.12 ± ID | |
| 1.6 | 2 (2–2) | 129 ± 39.7 | 24 (24–24) | 1,220 ± 207 | 6.18 ± 1.37 | ||
| 3.2 | 2 (1–2) | 156 ± 79.0 | 24 (12–24) | 1,480 ± 832 | 4.92 ± 0.558 | ||
| 28 | Male | 0.8 | 1 (1–2) | 47.0 ± 7.44 | 12 (12–12) | 272 ± 24.1 | 3.44 ± 0.252 |
| 1.6 | 1 (1–2) | 57.3 ± 36.3 | 12 (12–12) | 381 ± 180 | 3.32 ± ID | ||
| 3.2 | 2 (2–2) | 147 ± 37.9 | 24 (24–24) | 1,260 ± 205 | 5.08 ± 1.23 | ||
| Female | 0.8 | 2 (1–2) | 80.2 ± 14.0 | 12 (12–24) | 545 ± 81.9 | 3.68 ± 1.17 | |
| 1.6 | 2 (2–2) | 201 ± 105 | 24 (24–24) | 1,750 ± 890 | 4.95 ± 0.654 | ||
| 3.2 | 2 (1–2) | 139 ± 66.0 | 24 (24–24) | 1,200 ± 462 | 5.15 ± 1.06 | ||
| Levcromakalim | |||||||
|---|---|---|---|---|---|---|---|
| Day | Gender | Dose (mg/eye/dose) | Tmax (h) | Cmax (ng/mL) | tlast (h) | AUC0–24h (h × ng/mL) | T1/2 (h) |
| 1 |
Male |
0.8 |
2 (1–2) |
25.3 ± 5.05 |
12 (8–12) |
99.8 ± 4.28 |
3.64 ± ID |
| |
|
1.6 |
4 (2–4) |
24.4 ± 15.0 |
12 (12–12) |
133 ± 51.0 |
3.24 ± ID |
| 3.2 |
2 (2–2) |
76.0 ± 26.9 |
24 (12–24) |
361 ± 105 |
3.44 ± 0.10 |
||
| Female |
0.8 |
2 (2–2) |
25.1 ± 6.32 |
12 (12–12) |
92.7 ± 8.00 |
2.65 ± 1.05 |
|
| |
1.6 |
2 (2–4) |
23.2 ± 6.76 |
12 (12–24) |
125 ± 58.0 |
3.61 ± ID |
|
| 3.2 |
2 (1–2) |
72.2 ± 42.5 |
12 (12–24) |
294 ± 180 |
2.73 ± 0.42 |
||
| 28 |
Male |
0.8 |
2 (2–4) |
10.6 ± 1.79 |
12 (8–12) |
49.1 ± 7.85 |
2.35 ± ID |
| |
1.6 |
2 (2–8) |
15.5 ± 12.7 |
12 (12–12) |
70.7 ± 35.5 |
3.12 ± ID |
|
| 3.2 |
2 (1–4) |
31.0 ± 13.0 |
12 (12–24) |
166 ± 67.1 |
3.38 ± 1.15 |
||
| Female |
0.8 |
2 (2–2) |
18.4 ± 3.55 |
12 (8–12) |
80.1 ± 4.94 |
2.51 ± ID |
|
| 1.6 |
2 (2–4) |
19.9 ± 2.80 |
12 (12–12) |
105 ± 6.57 |
2.06 ± ID |
||
| 3.2 | 2 (2–4) | 32.9 ± 22.6 | 24 (12–24) | 154 ± 76.5 | 4.90 ± 2.44 | ||
FIG. 2.
Mean plasma concentration versus time profiles of QLS-101 and levcromakalim in Beagle dogs. (A) Plasma concentrations of QLS-101 following once-daily topical ocular administration of 3 concentrations of QLS-101 for 28 consecutive days. (B) Plasma concentrations of levcromakalim following once-daily topical ocular administration of 3 concentrations of QLS-101 for 28 consecutive days. Presence of levcromakalim in plasma confirms conversation of QLS-101 to levcromakalim. Solid lines represent day 1 and dashed lines represent day 28 data.
Similar to what was observed in rabbits, mean exposure to QLS-101 in beagle dogs exceeded that of levcromakalim across all doses and time points (Table 5). The Tmax of levcromakalim on day 1 was observed between 1 and 4 h and day 28 between 1 and 8 h in both sexes. In both male and female dogs, peak plasma concentrations of levcromakalim were found to increase in a dose-dependent, but not dose-proportional manner. Mean T1/2 was between 2.65–3.64 h on day 1 and 2.06–4.90 on day 28 (Fig. 2B).
To assess animals following cessation of treatment, 2 male and 2 female dogs from the 3.2 mg/eye/dose QLS-101 and formulation buffer-treated groups were monitored for 14 days posttreatment. At the completion of the recovery period, blood was collected from each animal for final analysis of QLS-101 and levcromakalim. Plasma levels of both QLS-101 and levcromakalim were found to be below quantifiable levels in all animals.
Tolerability of QLS-101 in beagle dogs following topical ophthalmic treatment
After 28 days of bilateral once-daily dosing, topical ophthalmic treatment with QLS-101 was well tolerated. Food consumption and body weights were found to be within normal ranges, as were blood differential and coagulation parameters (Supplementary Table S3). All treated animals regardless of the QLS-101 dose showed blood chemistry values within the normal range (Table 6). Similarly, no clinical findings were observed during the 14-day recovery period.
Table 6.
Blood Chemistry of Beagle Dogs Following 27 Days of Topical Ophthalmic Administration of QLS-101
| Males | |||||
|---|---|---|---|---|---|
| Vehicle | QLS-101 (0.8 mg/eye/dose) | QLS-101 (1.6 mg/dose/eye) | QLS-101 (3.2 mg/eye/dose) | Historical control range | |
| Aspartate aminotransferase (U/L) | 25.2 ± 5.8 | 26.0 ± 2.6 | 26.7 ± 3.5 | 28.4 ± 5.6 | 22.0–46.4 |
| Alanine aminotransferase (U/L) | 28.0 ± 5.3 | 28.0 ± 1.7 | 27.3 ± 4.6 | 28.2 ± 5.1 | 21.0–91.7 |
| Alkaline phosphatase (U/L) | 98.0 ± 27.8 | 107.0 ± 48.2 | 89.0 ± 9.2 | 81.2 ± 16.0 | 46.6–531.7 |
| Total bilirubin (mg/dL) | 0.028 ± 0.018 | 0.013 ± 0.023 | 0.013 ± 0.023 | 0.044 ± 0.026 | 0.000–0.100 |
| Urea nitrogen (mg/dL) | 16.0 ± 1.6 | 13.7 ± 2.3 | 15.7 ± 2.9 | 17.4 ± 4.9 | 10.0–24.0 |
| Creatinine (mg/dL) | 0.60 ± 0.00 | 0.60 ± 0.00 | 0.57 ± 0.06 | 0.58 ± 0.08 | 0.30–0.90 |
| Creatinine kinase (U/L) | 197.0 ± 50.6 | 224.7 ± 44.7 | 179.0 ± 22.3 | 188.4 ± 76.7 | 138.8–634.9 |
| Glucose (mg/dL) | 99.2 ± 3.3 | 105.3 ± 3.2 | 98.0 ± 7.8 | 101.2 ± 3.6 | 82.2–117.0 |
| Cholesterol (mg/dL) | 146.6 ± 16.8 | 137.3 ± 13.4 | 158.0 ± 9.8 | 120.6 ± 14.4 | 110.0–201.7 |
| Triglycerides (mg/dL) | 34.4 ± 6.3 | 35.0 ± 5.2 | 34.7 ± 6.4 | 27.2 ± 6.8 | 18.0–104.0 |
| Total protein (g/dL) | 5.12 ± 0.19 | 5.13 ± 0.23 | 4.77 ± 0.12 | 4.88 ± 0.18 | 5.00–6.60 |
| Albumin/globulin ratio (G) | 1.68 ± 0.11 | 1.80 ± 0.10 | 1.97 ± 0.12 | 1.70 ± 0.17 | 1.20–2.34 |
| Calcium (mg/dL) | 10.24 ± 0.26 | 10.37 ± 0.15 | 10.17 ± 0.25 | 9.98 ± 0.25 | 9.70–10.97 |
| Phosphorus (mg/dL) | 5.60 ± 0.69 | 5.60 ± 0.61 | 5.43 ± 0.15 | 5.20 ± 0.34 | 4.26–6.80 |
| Sodium (mmol/L) | 146.2 ± 1.1 | 145.0 ± 1.0 | 145.3 ± 1.2 | 144.8 ± 0.4 | 142.6–149.0 |
| Potassium (mmol/L) | 4.56 ± 0.30 | 4.43 ± 0.06 | 4.50 ± 0.26 | 4.48 ± 0.19 | 4.10–5.20 |
| Chloride (mmol/L) | 110.4 ± 1.8 | 108.3 ± 1.5 | 110.0 ± 0.0 | 110.2 ± 0.8 | 103.0–114.0 |
| Females | |||||
|---|---|---|---|---|---|
| Vehicle | QLS-101 (0.8 mg/eye/dose) | QLS-101 (1.6 mg/dose/eye) | QLS-101 (3.2 mg/eye/dose) | Historical control range | |
| Aspartate aminotransferase (U/L) |
25.4 ± 1.1 |
34.7 ± 10.7 |
30.0 ± 7.0 |
30.4 ± 6.8 |
22.0–45.0 |
| Alanine aminotransferase (U/L) |
25.6 ± 7.2 |
40.7 ± 20.2 |
26.3 ± 2.5 |
34.0 ± 6.8 |
18.0–42.0 |
| Alkaline phosphatase (U/L) |
89.2 ± 24.9 |
78.3 ± 4.5 |
72.7 ± 11.7 |
93.8 ± 23.3 |
52.0–155.0 |
| Total bilirubin (mg/dL) |
0.048 ± 0.008 |
0.033 ± 0.006 |
0.067 ± 0.012 |
0.048 ± 0.008 |
0.000–0.110 |
| Urea nitrogen (mg/dL) |
16.2 ± 2.8 |
15.0 ± 1.0 |
14.3 ± 1.5 |
14.4 ± 2.3 |
11.0–22.0 |
| Creatinine (mg/dL) |
0.60 ± 0.00 |
0.53 ± 0.06 |
0.60 ± 0.00 |
0.54 ± 0.05 |
0.50–0.80 |
| Creatinine kinase (U/L) |
217.2 ± 52.6 |
407.0 ± 283.7 |
216.0 ± 87.5 |
318.4 ± 207.4 |
135.6–402.2 |
| Glucose (mg/dL) |
97.4 ± 5.1 |
98.0 ± 6.6 |
94.7 ± 5.0 |
93.0 ± 4.5 |
77.0–112.0 |
| Cholesterol (mg/dL) |
146.0 ± 13.2 |
126.0 ± 9.2 |
128.7 ± 6.8 |
115.2 ± 24.8 |
110.0–204.0 |
| Triglycerides (mg/dL) |
37.0 ± 7.1 |
24.0 ± 3.6 |
35.3 ± 5.5 |
24.2 ± 4.8 |
19.0–55.0 |
| Total protein (g/dL) |
5.16 ± 0.17 |
3.30 ± 0.10 |
3.53 ± 0.12 |
3.48 ± 0.08 |
4.90–6.10 |
| Albumin/globulin ratio (G) |
2.28 ± 0.15 |
1.97 ± 0.12 |
2.17 ± 0.38 |
2.24 ± 0.09 |
1.40–2.60 |
| Calcium (mg/dL) |
10.64 ± 0.17 |
10.37 ± 0.12 |
10.50 ± 0.20 |
10.50 ± 0.17 |
9.70–11.00 |
| Phosphorus (mg/dL) |
5.48 ± 0.33 |
5.03 ± 0.35 |
5.43 ± 0.29 |
5.28 ± 0.33 |
4.30–6.00 |
| Sodium (mmol/L) |
147.0 ± 0.7 |
145.3 ± 0.6 |
146.0 ± 1.0 |
145.4 ± 1.7 |
141.0–149.0 |
| Potassium (mmol/L) |
4.58 ± 0.13 |
4.43 ± 0.23 |
4.67 ± 0.23 |
4.54 ± 0.11 |
4.00–4.90 |
| Chloride (mmol/L) | 109.0 ± 0.7 | 109.7 ± 0.6 | 109.0 ± 1.7 | 109.8 ± 1.1 | 106.0–113.0 |
QLS-101 was found to be systemically well tolerated in all treated groups (0.8, 1.6, and 3.2 mg/eye/dose). Some incidental nonadverse changes were observed in the thymus that included decreased organ weight, small size, and decreased lymphoid cellularity. These findings were present in both sexes across all groups, including controls. Additionally, a nonadverse decrease in red blood cell mass parameters, including a 0.80–0.89 × baseline mean in red blood cell count, hemoglobin, and hematocrit in both sexes treated with 3.2 mg/eye/dose QLS-101 were noted, although all values remained within a normal range (Supplementary Table S3). These findings were fully reversed in females and partially reversed in males (0.91–0.93 × baseline mean) following the 14-day recovery period in animals treated with 3.2 mg/eye/dose QLS-101. Based on the overall low severity levels and lack of noteworthy adverse effects, the NOAEL in this study was considered to be 3.2 mg/eye/dose.
Similar to results seen in Dutch belted rabbits, some mild eye redness was noted clinically in all animals, irrespective of dosing or treatment schedule (Table 7). Treatment with QLS-101 at the 3.2 mg/eye/dose resulted in a higher incidence of moderate redness of the eyes (Table 7). Minor conjunctival hyperemia was also noted across all groups with highest incidence in the 3.2 mg/eye/dose QLS-101-treated group. These results were reversible, intermittent, and deemed nonadverse.
Table 7.
Summary of Observed Eye Redness During Gross Ocular Examination in Beagle Dogs Following Once-Daily Topical Ophthalmic Dosing with 3 Concentrations of QLS-101 for 28 Days
| Treatment | Eye | Day |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| −4/−3 |
3 |
7 |
14 |
21 |
28 |
29 |
42 |
||
| Number of eyes (severity level)a | |||||||||
| Formulation buffer | OD | 3 (2) | 4 (2) | 2 (2) | 5 (2) | 1 (3), 4 (2) | 4 (2) | 1 (2) | — |
| OS | 1 (2) | 4 (2) | 1 (2) | 4 (2) | 1 (3), 5 (2) | 4 (2) | 3 (2) | — | |
| QLS-101 (0.8 mg/eye/dose) | OD | — | 1 (2) | 1 (2) | 2 (2) | 3 (2) | — | — | N/A |
| OS | — | 1 (2) | 1 (2) | 2 (2) | — | — | N/A | ||
| QLS-101 (1.6 mg/eye/dose) | OD | — | 2 (2) | 2 (2) | 4 (2) | 1 (2) | 3 (2) | N/A | |
| OS | — | 1 (2) | 2 (2) | 3 (2) | 1 (2) | 5 (2) | 1 (2) | N/A | |
| QLS-101 (3.2 mg/eye/dose) | OD | — | 5 (2) | 2 (3), 4 (2) | 1 (3), 9 (2) | 2 (3), 6 (2) | 1 (3), 6 (2) | 5 (2) | — |
| OS | — | 6 (2) | 2 (3), 4 (2) | 1 (3), 9 (2) | 2 (3), 7 (2) | 1 (3), 7 (2) | 5 (2) | — | |
Values represent the number of animals identified with ocular redness. Number in parenthesis represents the magnitude of observed redness (1 = very mild; 2 = mild; 3 = moderate). No other relevant findings such as eye swelling, or eye discharge were observed during gross ocular examination.
A small number of uncommon ocular changes were also observed on histopathology. This included increased mitosis in the corneal epithelium in 1 male in the 1.6 mg/eye/dose treatment group and 1 female in the 3.2 mg/eye/dose treatment group. This was reversible as the female dog was part of the 14-day recovery group, and no mitosis was noted by the end of the recovery period. Lastly, 2 of the 3 females in the 3.2 mg/eye/dose treatment group exhibited lacrimal gland acinar atrophy that was determined to be unrelated to the current treatment regimen.
Cardiovascular outcomes following intravenous administration of QLS-101 in beagle dogs
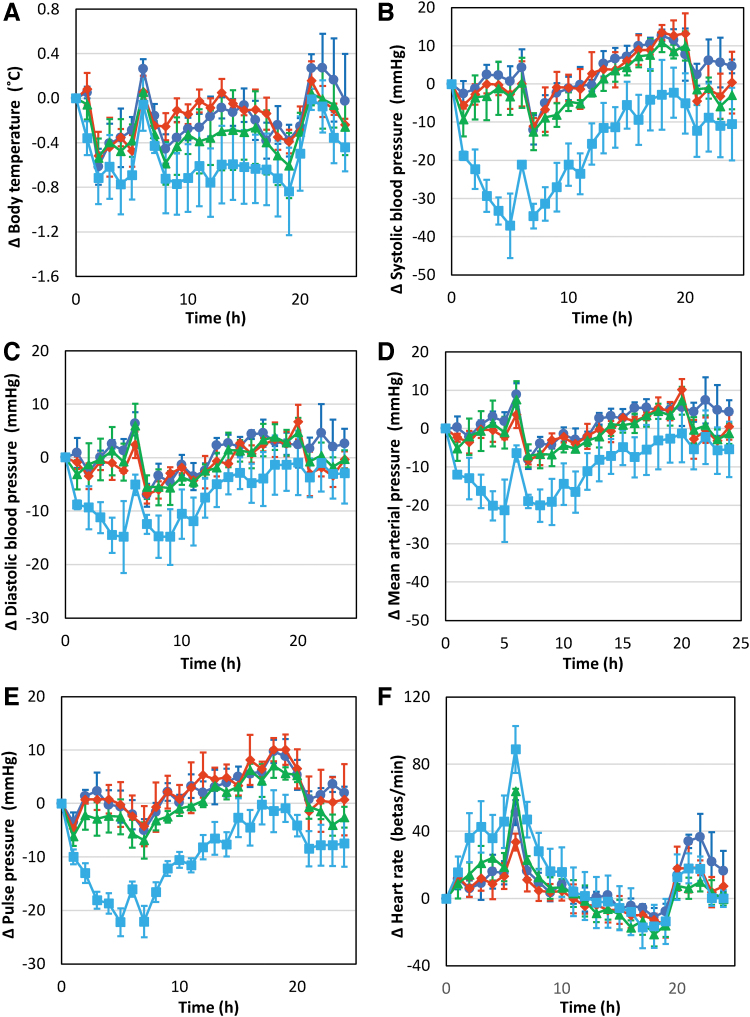
To determine the effect of QLS-101 on blood pressure, formulation buffer and multiple doses (0.075, 0.5, and 1.5 mg/kg) of QLS-101 were administered to male dogs (n = 4) as single bolus injections every 7 days in a 4 × 4 Latin-Square crossover design. Treatments resulted in no mortality, no changes in food consumption or body weight, and no QLS-101-related clinical signs. There was a slight decrease in body temperature that averaged 0.38°C relative to baseline adjusted controls (38.05°C predose compared with 38.32°C) in animals administered the 1.5 mg/kg dose (Fig. 3A). However, these changes did not reach statistical significance and were attributed to the wide range of baseline body temperatures.
FIG. 3.
Cardiovascular outcomes in beagle dogs after intravenous administration of QLS-101. Effect of single intravenous doses of QLS-101 at doses of 0 (dark blue), 0.075 (red), 0.5 (green), and 1.5 mg/kg (light blue) on (A) body temperature, (B) systolic blood pressure, (C) diastolic blood pressure, (D) mean arterial pressure, (E) mean pulse pressure, and (F) mean heart rate. Data are expressed as the mean change from predose baseline ± standard error of mean.
At intravenous injected concentrations of 0.075 and 0.5 mg/kg of QLS-101, no changes were noted in systolic, diastolic, mean arterial, and pulse pressure when compared with formulation buffer. In contrast, intravenous injection with 1.5 mg/kg showed transient decreases in systolic, diastolic, mean arterial, and pulse pressures within 10 h (Fig. 3B–E). Between 1 and 10 h postdose, the average systolic pressure decreased 14 mmHg (9%), with a maximal decrease of 25 mmHg (16%) from 4 to 5 h postdose (Fig. 3B). During the same period, average decreases in diastolic and mean arterial pressures were 4 mmHg (5%, Fig. 3C) and 6 mmHg (5%, Fig. 3D), respectively, relative to concurrent control absolute values. Decreases in diastolic pressure resulted in an overall decrease in pulse pressure of 10 mmHg (14%, Fig. 3E), relative to concurrent control absolute values.
Increases in heart rate were noted in 1 out of 4 animals at the 0.5 mg/kg dose, and in all 4 animals following administration of the 1.5 mg/kg dose, between 1 and 10 h after administration. At the 0.5 mg/kg dose, increased heart rate in the affected animal was noted between 1 and 7 h postdose and averaged 42 beats per minute (+44%) relative to its predose baseline. At 1.5 mg/kg, all animals exhibited statistically significant heart rate increases between 1 and 10 h postdose that averaged 25 beats per minute (+29%), relative to baseline adjusted controls (Fig. 3F). The maximal increase in heart rate was noted from 4 to 7 h postdose and averaged 33 beats per minute (+34%). These results are well recognized characteristics of lowering blood pressure.
Maximum tolerable dose and pharmacokinetics of QLS-101 following intravenous administration in beagle dogs
To calculate systemic MTD, escalating doses (0.05, 0.5, 1.5, 3, and 5 mg/kg/day) of QLS-101 were administered as intravenous injections to 1 male and 1 female beagle allowing at least 48 h between ascending doses. Treatments resulted in no mortality, and no QLS-101-related effects on food consumption or body weight were noted in either animal. Clinical signs that occurred as a result of QLS-101 treatment included observations of red discoloration of the pinnae (≥0.5 mg/kg doses) and gums (≥3 mg/kg) in both dogs, and the left forelimb of the female dog (≥0.5 mg/kg doses) (Table 8). At the 5 mg/kg dose, both dogs showed increased heart rate and felt warm suggesting a potential increase in body temperature. Additionally, the female dog exhibited nonedematous partly closed eyes following administration of the 5 mg/kg dose. Based on these observations, the MTD for intravenous administration of QLS-101 was determined to be 3 mg/kg.
Table 8.
Summary of Clinical Findings in Beagle Dogs Dosed Intravenously with QLS-101
| Finding | 0.05 mg/kg |
0.5 mg/kg |
1.5 mg/kg |
3 mg/kg |
5 mg/kg |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| M | F | M | F | M | F | M | F | M | F | |
| Pinna redness | X | — | X | — | X | X | X | X | X | X |
| Limb redness | — | — | — | X | — | X | X | X | — | — |
| Gingiva redness | — | — | — | — | — | X | X | X | X | X |
| Generalized redness | — | — | — | — | — | — | X | X | X | X |
| Abnormally increased heart rate | — | — | — | — | — | — | — | — | X | X |
| Warm to touch | — | — | — | — | — | — | — | — | X | X |
| Partly closed eyes | — | — | — | — | — | — | — | — | X | — |
X represents positive response. — Represents no observed indication.
F, female; M, male.
Pharmacokinetic parameters calculated in animals receiving escalating doses of QLS-101 by intravenous injection showed that QLS-101 exposure, as determined by Cmax and AUC, was dose proportional in both dogs (Table 9). Cmax ranged from 245 (0.05 mg/kg) to 22,400 ng/mL (5 mg/kg) in the male and 356 (0.05 mg/kg) to 29,300 ng/mL (5 mg/kg) in the female. AUC0–24h ranged from 1,120 (0.05 mg/kg) to 159,000 h × ng/mL (5 mg/kg) in the male and 2,100 (0.05 mg/kg) to 311,000 h × ng/mL (5 mg/kg) in the female. For levcromakalim, exposure in both dogs appeared to be proportional to the QLS-101 dose administered; however, no pharmacokinetic parameters were calculated in the female dog on day 1 as only 2 of the 6 time points yielded quantifiable levels. While the female dog consistently demonstrated higher plasma levels of both QLS-101 and levcromakalim after dosing, sex differences in AUC0–24h values following each dose were <2-fold. There were no obvious differences in levcromakalim pharmacokinetic parameters between the male and female dogs.
Table 9.
Pharmacokinetic Parameters for QLS-101 and Levcromakalim in Beagle Dogs Following Intravenous Dosing with QLS-101
| Pharmacokinetic Parameters | 0.05 mg/kg |
0.5 mg/kg |
1.5 mg/kg |
3 mg/kg |
5 mg/kg |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| M | F | M | F | M | F | M | F | M | F | |
| QLS-101 | ||||||||||
| Cmax (ng/mL) | 245 | 356 | 2,600 | 2,990 | 7,690 | 9,050 | 14,400 | 18,400 | 22,400 | 29,300 |
| AUC0–24h (ng·h/mL) | 1,120 | 2,100 | 14,300 | 20,900 | 43,200 | 61,200 | 85,100 | 128,000 | 159,000 | 311,000 |
| Tmax (h) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| T1/2 (h) | 2.23 | 3.26 | 2.84 | 4.11 | 2.91 | 4.68 | 6.22 | 2.94 | 3.47 | 5.98 |
| Levcromakalim | ||||||||||
| Cmax (ng/mL) | 0.826 | NR | 7.3 | 5.13 | 18 | 15.6 | 42.7 | 28.3 | 65.8 | 82.7 |
| AUC0–24h (ng·h/mL) | 5.04 | NR | 45.5 | 81.3 | 183 | 241 | 476 | 386 | 741 | 1,040 |
| Tmax (h) | 1 | NR | 1 | 3 | 1 | 3 | 1 | 6 | 1 | 3 |
| T1/2 (h) | 9.66 | NR | 6.89 | 10.3 | 5.27 | 7.58 | 6.48 | 5.07 | 7.67 | 7.82 |
NR, not reported.
Discussion
The current study was designed to evaluate pharmacokinetic and tolerability parameters using good laboratory practice for manufactured QLS-101 (0.8–3.2 mg/eye/dose) in 2 FDA-accepted animal species to enable an Investigational New Drug (IND) application by Qlaris Bio, Inc. Following topical ophthalmic or intravenous administration, QLS-101 was converted to its active moiety levcromakalim in ocular tissues and blood. Analysis of ocular and systemic side effects, including cardiovascular parameters, peripheral organ histology, and clinical chemistry did not reveal any significant findings, indicating excellent drug tolerability at topical ophthalmic doses up to 3.2 mg/eye (3.2 mg/kg for a 2 kg rabbit, 0.64 mg/kg for a 10 kg dog). Intravenous administration predictably resulted in higher plasma concentrations of both QLS-101 and levcromakalim compared with topical ophthalmic dosing but was similarly well tolerated at doses up to 3 mg/kg.
Observed adverse events following systemic exposure were dose dependent and considered to be minor and transient. Altogether, the results describe a relevant pharmacokinetic profile and provide confidence that QLS-101 at clinical topical ophthalmic doses of up to 3.2 mg/eye per day should show good tolerability and safety when used in human clinical trials.
To develop QLS-101 as a viable topical ophthalmic therapeutic option to lower IOP in patients with primary open-angle glaucoma or ocular hypertension, QLS-101 must first penetrate the cornea to access the anterior chamber, where aqueous humor will facilitate circulation before egress by the outflow pathways. Based on our previous studies,19 we also surmised that the endogenous phosphatases would convert (at least partially) QLS-101 to its active moiety levcromakalim. Therefore, we first sought to understand the pharmacokinetics of QLS-101 and its active moiety within their primary site of action. In this study, we observed QLS-101 and levcromakalim in the cornea, iris/ciliary body, and sclera of Dutch belted rabbits following topical ophthalmic applications suggesting QLS-101 was actively being converted to levcromakalim. The pharmacokinetic parameters generated in the various ocular tissues suggests that QLS-101 is converted to levcromakalim in the eye but does not validate whether that tissue was responsible for the conversion. Therefore, we refrained from commenting on the metabolic profile of QLS-101 and only assert that following treatment, the drug was converted to its active moiety levcromakalim. However, because minimal levcromakalim was found in the aqueous humor, it suggests that conversion of QLS-101 to levcromakalim occurs in the ophthalmic tissues by tissue-bound phosphatases. This is consistent with in vitro studies that suggest multiple ocular tissues convert QLS-101 to levcromakalim.19
Systemic exposure to ocular therapeutics is often low but expected, as aqueous humor drains into the systemic venous circulation by way of the episcleral veins.21 Therefore, it was important to assess the pharmacokinetics of QLS-101 and levcromakalim in plasma following ocular topical application. The maximum concentrations of QLS-101 and levcromakalim increase in rabbit plasma by 1.37–3.91 × and 1.44–2.62 × , respectively, after 28 days of QLS-101 dosing compared with day 1. The exception to this was the Cmax of QLS-101 in males treated with 0.8 mg/eye/dose, which decreased on average by 0.73 × , but this could be attributed to the large, measured variability on day 1. Similar increases in the AUC0–24h of QLS-101 (1.34–2.80 × increase) and levcromakalim (1.58–2.92 × ) were noted between days 1 and 28 in all rabbits. In contrast, in beagle dogs the average Cmax of QLS-101 moderately increased between days 1 and 28 in all animals treated with 0.8 mg/eye/dose (1.29–1.45 × ), and in females treated with 1.6 mg/eye/dose (1.56 × ) but decreased in 1.6 mg/eye/dose-treated males (0.78 × ) and all 3.2 mg/eye/dose-treated animals (0.89 × ). Similar patterns were observed in the AUC0–24h values.
In all rabbits, regardless of QLS-101 dose, levcromakalim Cmax and AUC0–24h decreased between days 1 and 28. This species difference may be the result of the extensive retrobulbar plexus present in rabbits, which allows for medicines delivered by eyedrops to directly enter the bloodstream,15 a physiological feature that is not as prominent in dogs.22 It may also be related to the difference in animal sizes, as blood volume is roughly 15–35 mL/kg lower in rabbits.23,24 Increases in Cmax values do indicate a possible accumulation of the drug with continuous treatment, a phenomenon also noted in CKLP1-treated Dutch belted rabbits.15 Whether additional drug present in the system can result in better efficacy in IOP reduction is an interesting question and will require additional studies. However, the current study with QLS-101 as well as previous pharmacokinetic reports with CKLP1 show that even after long-term treatment of up to 90 days, there was little to no contraindicative side effects with the drug and a lowered IOP was maintained throughout the treatment period.15,17
All current treatments for glaucoma have side effects, which can limit patient compliance. It is noteworthy to mention that ophthalmic treatment with QLS-101 resulted in minimal hyperemia. In Dutch belted rabbits, treatment with QLS-101 only resulted in mild transient hyperemia in a small proportion of animals. While more instances of hyperemia were recorded in beagle dogs as the QLS-101 dose increased, it is important to note that animals treated with only the formulation buffer also exhibited mild-to-moderate hyperemia, suggesting that this redness may be species specific for topical ophthalmic administrations rather than drug related. The importance of the extremely low hyperemia rate in animals treated with therapeutic doses (currently up to 0.8 mg/eye/dose) of QLS-101 is underscored by evidence that hyperemia was attributed in the Glaucoma Adherence and Persistency Study (GAPS) as the primary cause of stopping or switching medications in 63% of patients who cited adverse events.20
The active moiety of prodrug QLS-101, levcromakalim, was developed by Beecham Pharmaceuticals in the 1980s as an oral treatment for systemic hypertension, and the systemic vasodilatory properties of levcromakalim and other ATP-sensitive potassium channel openers have been previously reported.17,25–27 Therefore, it was necessary to evaluate the effects of QLS-101 on various cardiovascular parameters. Using intravenous injection, changes in blood pressure were only noted in animals receiving the highest dose (1.5 mg/kg), and in only 1 animal following the 0.5 mg/kg dose. In previous studies in humans with levcromakalim, single oral doses of 0.5–1.5 mg were considered to be well tolerated, while also eliciting marked decreases in blood pressure in patients with hypertension.25 The plasma Cmax in these patients increased in a dose-dependent manner and ranged from 5 to 20 ng/mL, comparable to what was seen in our animal study following intravenous dosing.
We speculate that clinical doses of QLS-101, which will be administered by eyedrops will not elicit a reduction in systemic blood pressure in humans, considering that the drug concentration will be lower. Additionally, what does get into the systemic circulation will be diluted due to a greater total average blood volume in humans compared with dogs. QLS-101 also converts to levcromakalim slowly in human tissues further reducing the circulating levels of levcromakalim.19
Overall, the safety profile of QLS-101 is encouraging, particularly because the results from clinical chemistry panels indicate that QLS-101, even at concentrations as high as 3.2 mg/eye/dose, does not elicit any change in critical blood chemistry parameters. Similarly, hematology and coagulation results after 28 days of dosing indicate that while some changes were noted in red blood cell counts particularly in dogs given 3.2 mg/eye/dose QLS-101, all findings remained within the “normal” range. Also, no microscopic changes were noted in either the spleen or the bone marrow of these animals, and therefore these findings were considered to be nonadverse. It should be noted that the safety profile discussed herein are contingent on the limited doses used to elucidate the pharmacokinetic properties of QLS-101. The current data does not indicate or establish any meaningful concentrations for human clinical studies but provides a range of doses that are well tolerated in large animals such as the rabbit and dog.
The adverse events observed following topical ophthalmic or intravenous dosing could be the result of levcromakalim's target-mediated vasodilation. The most common adverse event observed when QLS-101 was administered topically to both eyes was dose-related increases in conjunctival hyperemia or overall ocular redness/congestion. Upon ocular examination, this observed redness was not the result of inflammation in either species. In the MTD study, systemic side effects included redness of the pinnae, limbs, and gingiva (consistent with peripheral vasodilation), which initially presented following administration of the 1.5 mg/kg dose, and continued at higher doses, along with a mildly increased heart rate. Similarly, heart rate increased in a dose-dependent manner in dogs administered intravenous doses of 1.5 mg/kg QLS-101. These observations were transient and therefore considered nonadverse but are also consistent with the known vasodilatory action of levcromakalim. However, given that these events were only observed in the highest dose ranges and were also sporadic and transient, we consider QLS-101 at topical ophthalmic doses up to 3.2 mg/eye to be safe and well tolerated in animals, which allows for a minimum of 4-fold margin of safety based upon current QLS-101 doses being tested in the clinic.
In summary, this study provides the pharmacokinetic parameters to help understand the absorption, distribution, and elimination characteristics of QLS-101 and its active moiety levcromakalim. It also demonstrates that topical ophthalmic-administered QLS-101 is converted to its active moiety levcromakalim in normotensive large animal models, and that daily topical ophthalmic doses up to 3.2 mg/eye are well tolerated. Along with its increased aqueous solubility and ease of formulation for comfortable ocular administrations, these studies help establish QLS-101 as a prime candidate for first-in-human clinical trials for the treatment of elevated IOP in glaucoma and related syndromes.
Supplementary Material
Authors' Contributions
U.R.C.: writing (lead); review and editing (equal); conceptualization (supporting); methodology (equal); investigation (equal); resources (equal); and formal analysis (equal). C.L.P-S.: writing (lead); review and editing (equal); conceptualization (supporting); methodology (equal); investigation (equal); resources (equal); and formal analysis (equal). R.S.: review and editing (equal); conceptualization (supporting); methodology (supporting); investigation (equal); resources (equal); and formal analysis (equal). H.K.S.: review and editing (equal); conceptualization (supporting); methodology (supporting); investigation (supporting); resources (equal); and formal analysis (equal).
B.R.: review and editing (equal); conceptualization (supporting); methodology (supporting); investigation (supporting); resources (equal); and formal analysis (equal). R.C.: review and editing (equal); conceptualization (supporting); methodology (supporting); investigation (supporting); resources (equal); and formal analysis (equal). P.I.D.: review and editing (equal); conceptualization (supporting); methodology (supporting); investigation (supporting); resources (supporting); and formal analysis (equal). T.H.: review and editing (equal); conceptualization (lead); methodology (equal); investigation (equal); resources (equal); and formal analysis (equal). B.M.W.: review and editing (equal); conceptualization (lead); methodology (equal); investigation (equal); resources (equal); and formal analysis (equal).
M.P.F.: review and editing (equal); conceptualization (lead); methodology (equal); investigation (equal); resources (equal); and formal analysis (equal).
Author Disclosure Statement
C.L.P-S., H.K.S., R.C., T.H., and B.M.W. are employees of Qlaris Bio, Inc. M.P.F., P.I.D., T.H., and B.M.W. have patents associated with QLS-101. M.P.F. and P.I.D. have licensed products associated with Qlaris Bio, Inc. M.P.F., R.S., and B.R. are consultants for Qlaris Bio, Inc. U.R.C. has no conflicts of interest.
Funding Information
This study was funded by NIH grant EY21727 (M.P.F.), Mayo Clinic Department of Ophthalmology grant (U.R.C.), and Mayo Foundation (M.P.F.).
Supplementary Material
References
- 1. Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol 2006;90:262–267; doi: 10.1136/bjo.2005.081224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tham YC, Li X, Wong TY, et al. . Global prevalence of glaucoma and projections of glaucoma burden through 2040: A systematic review and meta-analysis. Ophthalmology 2014;121:2081–2090; doi: 10.1016/j.ophtha.2014.05.013 [DOI] [PubMed] [Google Scholar]
- 3. Comparison of glaucomatous progression between untreated patients with normal-tension glaucoma and patients with therapeutically reduced intraocular pressures. Collaborative Normal-Tension Glaucoma Study Group. Am J Ophthalmol. 1998;126:487–497; doi: 10.1016/s0002-9394(98)00223-2 [DOI] [PubMed] [Google Scholar]
- 4. The Advanced Glaucoma Intervention Study (AGIS): 7. The relationship between control of intraocular pressure and visual field deterioration. The AGIS Investigators. Am J Ophthalmol 2000;130:429–440; doi: 10.1016/s0002-9394(00)00538-9 [DOI] [PubMed] [Google Scholar]
- 5. Heijl A, Leske MC, Bengtsson B, et al. . Reduction of intraocular pressure and glaucoma progression: Results from the Early Manifest Glaucoma Trial. Arch Ophthalmol 2002;120:1268–1279; doi: 10.1001/archopht.120.10.1268 [DOI] [PubMed] [Google Scholar]
- 6. Kass MA, Heuer DK, Higginbotham EJ, et al. . The Ocular Hypertension Treatment Study: A randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol 2002;120:701–713; discussion 829–730; doi: 10.1001/archopht.120.6.701 [DOI] [PubMed] [Google Scholar]
- 7. King AJ, Fernie G, Azuara-Blanco A, et al. . Treatment of Advanced Glaucoma Study: A multicentre randomised controlled trial comparing primary medical treatment with primary trabeculectomy for people with newly diagnosed advanced glaucoma-study protocol. Br J Ophthalmol 2018;102:922–928; doi: 10.1136/bjophthalmol-2017-310902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mehta AA, Kanu LN, Sood-Mendiratta S, et al. . Experience with netarsudil 0.02% and latanoprostene bunod 0.024% as adjunctive therapy for glaucoma. Eur J Ophthalmol 2022;32:322–326; doi: 10.1177/1120672121998913 [DOI] [PubMed] [Google Scholar]
- 9. Rajurkar K, Dubey S, Gupta PP, et al. . Compliance to topical anti-glaucoma medications among patients at a tertiary hospital in North India. J Curr Ophthalmol 2018;30:125–129; doi: 10.1016/j.joco.2017.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Newman-Casey PA, Robin AL, Blachley T, et al. . The most common barriers to glaucoma medication adherence: A cross-sectional survey. Ophthalmology 2015;122:1308–1316; doi: 10.1016/j.ophtha.2015.03.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chowdhury UR, Holman BH, Fautsch MP. ATP-sensitive potassium (K(ATP)) channel openers diazoxide and nicorandil lower intraocular pressure in vivo. Invest Ophthalmol Vis Sci 2013;54:4892–4899; doi: 10.1167/iovs.13-11872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Roy Chowdhury U, Bahler CK, Holman BH, et al. . Ocular hypotensive effects of the ATP-sensitive potassium channel opener cromakalim in human and murine experimental model systems. PLoS One 2015;10:e0141783; doi: 10.1371/journal.pone.0141783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Roy Chowdhury U, Viker KB, Stoltz KL, et al. . Analogs of the ATP-sensitive potassium (KATP) channel opener cromakalim with in vivo ocular hypotensive activity. J Med Chem 2016;59:6221–6231; doi: 10.1021/acs.jmedchem.6b00406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Roy Chowdhury U, Dosa PI, Fautsch MP. ATP sensitive potassium channel openers: A new class of ocular hypotensive agents. Exp Eye Res 2017;158:85–93; doi: 10.1016/j.exer.2016.04.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Roy Chowdhury U, Kudgus RA, Rinkoski TA, et al. . Pharmacological and pharmacokinetic profile of the novel ocular hypotensive prodrug CKLP1 in Dutch-belted pigmented rabbits. PLoS One 2020;15:e0231841; doi: 10.1371/journal.pone.0231841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Roy Chowdhury U, Millar JC, Holman BH, et al. . Effect of ATP-sensitive potassium channel openers on intraocular pressure in ocular hypertensive animal models. Invest Ophthalmol Vis Sci 2022;63:15; doi: 10.1167/iovs.63.2.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Roy Chowdhury U, Kudgus RA, Holman BH, et al. . Pharmacological profile and ocular hypotensive effects of cromakalim prodrug 1, a novel ATP-sensitive potassium channel opener, in normotensive dogs and nonhuman primates. J Ocul Pharmacol Ther 2021;37:251–260; doi: 10.1089/jop.2020.0137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Roy Chowdhury U, Rinkoski TA, Bahler CK, et al. . Effect of cromakalim prodrug 1 (CKLP1) on aqueous humor dynamics and feasibility of combination therapy with existing ocular hypotensive agents. Invest Ophthalmol Vis Sci 2017;58:5731–5742; doi: 10.1167/iovs.17-22538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pervan-Steel CL, Roy Chowdhury U, Sookdeo HK, et al. . Ocular hypotensive properties and biochemical profile of QLS-101, a novel ATP-sensitive potassium (KATP) channel opening prodrug. Invest Ophthalmol Vis Sci 2022;63:26; doi: 10.1167/iovs.63.4.26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Friedman DS, Hahn SR, Gelb L, et al. . Doctor-patient communication, health-related beliefs, and adherence in glaucoma results from the Glaucoma Adherence and Persistency Study. Ophthalmology 2008;115:1320–1327; doi: 10.1016/j.ophtha.2007.11.023 [DOI] [PubMed] [Google Scholar]
- 21. Huang AS, Francis BA, Weinreb RN. Structural and functional imaging of aqueous humour outflow: A review. Clin Exp Ophthalmol 2018;46:158–168; doi: 10.1111/ceo.13064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Williams D. Rabbit and rodent ophthalmology. Eur J Companion Animal Prac 2007;17:242–252. [Google Scholar]
- 23. Jutkowitz LA, Rozanski EA, Moreau JA, et al. . Massive transfusion in dogs: 15 Cases (1997–2001). J Am Vet Med Assoc 2002;220:1664–1669; doi: 10.2460/javma.2002.220.1664 [DOI] [PubMed] [Google Scholar]
- 24. Melillo A. Rabbit clinical pathology. J Exot Pet Med 2007;16:135–145; doi: 10.1053/j.jepm.2007.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hamilton TC, Beerahee A, Moen JS, et al. . Levcromakalim. Cardiovasc Drug Rev 1993;11:199–222; doi: 10.1111/j.1527-3466.1993.tb00276.x [DOI] [Google Scholar]
- 26. Suzuki S, Yano K, Kusano S, et al. . Antihypertensive effect of levcromakalim in patients with essential hypertension. Study by 24-h ambulatory blood pressure monitoring. Arzneimittelforschung 1995;45:859–864. [PubMed] [Google Scholar]
- 27. Tinker A, Aziz Q, Li Y, et al. . ATP-sensitive potassium channels and their physiological and pathophysiological roles. Compr Physiol 2018;8:1463–1511; doi: 10.1002/cphy.c170048 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.