Abstract
The most potent and broad HIV envelope (Env)-specific antibodies often when reverted to their inferred germline versions representing the naive B cell receptor, fail to bind Env, suggesting that the initial responding B cell population not only exclusively comprises a naive population, but also a pre-existing cross-reactive antigen-experienced B cell pool that expands following Env exposure. Previously we isolated gp120-reactive monoclonal antibodies (mAbs) from participants in HVTN 105, an HIV vaccine trial. Using deep sequencing, focused on immunoglobulin G (IgG), IgA, and IgM, VH-lineage tracking, we identified four of these mAb lineages in pre-immune peripheral blood. We also looked through the ∼7 month postvaccination bone marrow, and interestingly, several of these lineages that were found in prevaccination blood were still persistent in the postvaccination bone marrow, including the CD138+ long-lived plasma cell compartment. The majority of the pre-immune lineage members included IgM, however, IgG and IgA members were also prevalent and exhibited somatic hypermutation. These results suggest that vaccine-induced gp120-specific antibody lineages originate from both naive and cross-reactive memory B cells.
Keywords: HIV-1, pre-immune, B cell, antibody repertoire, envelope, gp120, naive, memory
Introduction
Despite increased access to antiretrovirals for treatment and prevention, HIV-1 is still a major health burden, with ∼1.5 million new infections and ∼680,000 HIV-related deaths globally in 2020.1 Thus, the development of a safe and an effective preventive HIV vaccine remains a global priority. HIV-1 Envelope (Env) glycoprotein is the primary target of the humoral response to HIV-1 infection and the antibody (Ab) response has been correlated with vaccine-mediated protection from HIV infection.2–7 Although Env is highly immunogenic and readily induces Abs, Ab-mediated protection from HIV appears to be conferred, if at all, by only a minor subset of Ab clones from the overall Env-specific immunoglobulin (Ig) repertoire. Approximately 10%–50% of people living with HIV (PLWH) within several years of infection develop Abs with tremendous breadth able to neutralize diverse isolates, although typically not their own contemporary isolates,8,9 indicating that such broadly neutralizing antibodies are capable of being induced, and that if recapitulated by vaccination would likely confer protection from infection.
Numerous HIV-1 broadly neutralizing monoclonal antibodies (bnmAbs) have been isolated from PLWH and are the most well characterized of HIV-1-specific mAbs, including extensive deep-sequencing analysis of their clonal lineages. These bnmAbs typically have extensive somatic hypermutation and common molecular features in the heavy- and light-chain variable regions. Some of these bnmAbs share very specific structural features such as gene usage and complementarity-determining region 3 (CDR3) lengths, particularly observed among those targeting the CD4 binding site, referred to as VRC01-class bnmAbs.10,11 Many of these bnmAbs when reverted to their presumed germline or naive sequence fail to bind HIV Env,12–14 raising the possibility that they may arise from naive B cells that responded initially to a different antigen, acquiring cross-reactivity with HIV Env sufficient to respond to HIV infection, and then subsequently developed increasing affinity to HIV Env as part of their extensive somatic hypermutation process in response to chronic viremia.
Recently, the development of “germline-targeting” immunogens has showed their potential for binding predicted germline versions of bnmAbs, activating transgenic B cells and generating Ab responses in B cell receptor (BCR) transgenic mouse models.15–22 Havenar-Daughton et al. have used the eOD-GT8 immunogen successfully to isolate genuine human naive B cells ex vivo, showing some promise for the “germline-targeting” vaccine development concept.15 Subsequently, the eOD-GT8 60mer immunogen is in the early stages of clinical testing to determine the potential of germline targeting approaches for stimulating the human B cell repertoire.23
Several HIV-1 vaccine efficacy trials have been completed so far although no vaccine has been licensed to date.24 RV144 is the only immunogen-based preventive HIV vaccine trial that has demonstrated moderate protection so far; however, this regimen did not induce a substantial neutralizing Ab response.25 More recently, two phase 2b antibody-mediated prevention trials with the VRC01 bnmAb infusion in high-risk populations have found that the VRC01 was able to block acquisition of only ∼30% of the circulating strains, however, it was 75% effective at preventing acquisition of VRC01-sensitive strains (in vitro IC80 < 1 μg/mL).26–28 Although VRC01 could not prevent the overall HIV-1 acquisition more effectively than placebo, these “proof-of-concept” studies indicated the potential of bnmAbs to prevent HIV infection. All these studies highlight the need to understand the mechanisms of bnAb formation to develop an effective HIV-1 vaccine.26,27
Several findings from PLWH support that one of the possible developmental pathways for HIV-1 Env antibodies involve polyreactive B cells that cross-react with self and/or microbial antigens, particularly those that target the gp41 component of Env.29–31 In addition, Williams et al. reported that following HIV vaccination, a gp41-reactive mAb lineage, which cross-reacts to gut residing Escherichia coli RNA polymerase, developed. They further suggested that such cross-reactive populations may result in an immunodominance that minimizes the development of other Env-specific B cell responses to vaccination.32 In general, HIV-1 Env vaccine containing both gp120 and gp41 primarily induces an intestinal microbiota-derived cross-reactive gp41-dominant response along with a low frequency of gp120 response,32 whereas immunization with gp120-only results in a comparatively higher frequency of gp120-specific memory B cell response than the response that is induced from gp140 or gp160 vaccination.33,34
Moreover, several lines of evidence indicate that the precursors of Env-specific mAb lineages can be identified in the naive B cell repertoire15 and some antibodies with bnAb activity can also arise from naive B cells.35
Previously we isolated 66 gp120 reactive mAbs36 from the peripheral blood B cells of HVTN 105 trial participants,37 a phase 1 trial testing AIDSVAX® B/E (gp120 protein) that was used in RV144, combined with a DNA immunogen. To determine the origin of these mAb producing B cells, we used deep sequencing-based VH lineage tracking and identified several of these mAb lineages in peripheral blood before vaccination (pre-immune). The majority of the pre-immune gp120-reactive lineages included IgM, however, IgG and IgA members were also predominant before vaccination. In addition, the majority of the pre-immune lineage members exhibited somatic hypermutation before vaccination, suggesting that the vaccine-induced gp120-specific Ab lineages originated from both naive B cells and cross-reactive memory B cells.
Materials and Methods
Study participants
Blood samples for this study were obtained from 21 participants at the University of Rochester, who participated in the HVTN 105 phase 1 randomized, blinded, multisite HIV vaccine clinical trial (ClinicalTrials.gov NCT02207920).37 All procedures used in this study were approved by the Research Subjects Review Board at the University of Rochester Medical Center, all participants provided written informed consent, and all research was performed in accordance with relevant guidelines/regulations. All participants were seronegative for HIV infection at the time of enrollment for the study. Participants received different combinations of AIDSVAX B/E [HIV Env gp120 of clade B (MN) and E (A244)], DNA-HIV-PT123 (three plasmids containing DNA of clade C 96ZM651 gag, clade C 96ZM651 gp140, and clade C CN54 pol-nef), and placebo administered intramuscularly at four time points over a period of 6 months (Fig. 1B).
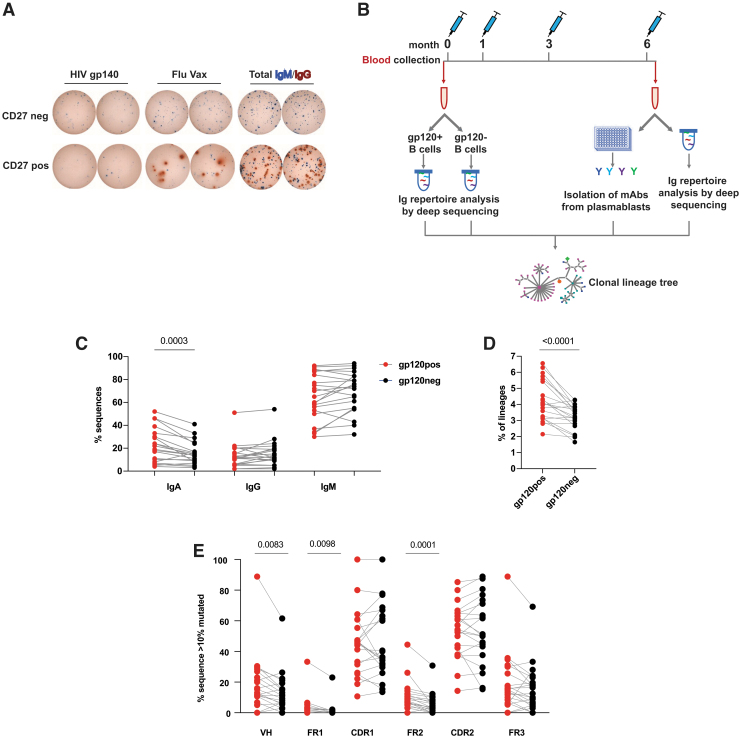
FIG. 1.
Characterization of pre-immune B cell repertoire in blood. (A) Representative ELISpot showing Env-reactive pre-immune CD27pos (memory) and CD27neg B cells isolated by magnetic bead separation in HIV-negative healthy subjects (blue, IgM; red, IgG). (B) Time line showing the collection of peripheral blood at baseline (month 0) and 7 days after final immunization (M6) from HVTN 105 participants (n = 21) who received AIDSVAX B/E Protein and DNA-HIV-PT123 plasmid immunizations intramuscularly at months 0, 1, 3, and 6. (C–E) Frequency of (C) IgA, IgG, and IgM, (D) lineages with long HCDR3 (≥22 amino acid), (E) expanded lineages with ≥10% average mutation from germline in gp120pos and gp120neg B cell fractions. Each symbol represents an individual subject. Env, envelope; Ig, immunoglobulin.
Peripheral blood mononuclear cells (PBMCs) were obtained before the vaccination and 7 days after the final vaccination. Bone marrow and tonsil biopsy samples were collected 7 months after the final vaccination.
HIV-specific antibody secreting cells ELISpot
The frequency of HIV-specific antibody secreting cells in total PBMCs was determined by ELISpot similar to as previously described.36,38,39 Briefly, sterile 96-well polyvinylidene fluoride membrane plates (MilliporeSigma, USA) were coated overnight at 4°C with 50 μL of 5 μg/mL of HIV-1 Env gp140 SF162.B (NIH AIDS Reagent Program) or 1 μg/mL of anti-human IgG or IgM (Jackson ImmunoResearch, West Grove, PA, USA) in PBS. Plates were blocked with RPMI1640 (Corning, VA, USA) media with 10% fetal bovine serum (Atlanta Biologicals, GA, USA) for 2 h at 37°C. Then PBMCs were added in a final volume of 200 μL per well in triplicate. The plates were incubated for ∼40 h at 37°C in 5% CO2 and then washed with PBS containing 0.1% Tween 20.
Bound antibodies were detected with 50 μL of 1 μg/mL of alkaline phosphatase-conjugated anti-human IgA and horseradish peroxidase-conjugated anti-human IgG (diluted in PBS containing 0.1% Tween 20 and 1% BSA) Ab (Jackson ImmunoResearch) for 2 h at 37°C in 5% CO2 and then developed with VECTOR Blue, Alkaline Phosphatase Substrate Kit III (Vector Laboratories, Burlingame, CA). The spots per well were counted using the CTL immunospot reader (Cellular Technologies Ltd., Shaker Heights, OH, USA).
VH next-generation sequencing
For the Ig VH sequencing library preparation, PBMCs were collected before the start of the vaccination trial (at M0). gp120+ve and gp120-ve B cell fractions were isolated by magnetic selection using biotinylated HIV Env protein gp120 MN.B (ARP-12570, NIH AIDS Reagent Program) and A244.AE (ARP-12569, NIH AIDS Reagent Program). These proteins were selected on the basis of previous reports that showed that the truncated gp120 protein with the first 11 amino acid deletion without the addition of HSV-1 gD tag is sufficient to trigger the immunological response in human.40 PBMCs were also collected at multiple time points (M1 = 7 days post 2nd vaccination, M3 = 7 days post 3rd vaccination, M6 = 7 days post 4th vaccination) over the course of vaccination. The bone marrow (CD138+ fraction, CD138− fraction, or total) and peripheral blood B cells were collected ∼7 months after the final vaccination (M13).
The Ig heavy-chain libraries were prepared as described in Piepenbrink et al., 202141 with modifications. Briefly, total RNA was isolated using the RNeasy Mini Kit (Qiagen, Germany) and treated with DNase I (Turbo DNA-free Kit; Invitrogen, Lithuania). Approximately 1/9 part of this RNA was used for cDNA synthesis in a 20 μL reaction with the qScript cDNA Synthesis Kit (Quantabio, MA, USA). Two rounds of PCRs were carried out to generate the libraries with a cocktail of VH1–6 forward primers and IgA, IgG, and IgM reverse primers36 with the modifications adapted for the Illumina Nextera approach. Following PCR, amplicons were analyzed in 1% agarose gel and bands corresponding to ∼600 bp were purified with the QiAquick Gel Extraction Kit (Qiagen, USA). All the reactions were carried out in triplicates and pooled together during the gel purification.
The individual libraries were further purified using the ProNex size-selective purification system (Promega, WI, USA) to select products between the 500 and 700 bp range. Final products were submitted to the University of Alabama at Birmingham Genomics Core Laboratory, where quality control and quantification were performed using qRT-PCR. Finally, the libraries were pooled together in equimolar ratio and sequenced on an Illumina MiSeq system (Illumina, Inc., CA, USA) using 2 × 300 bp paired-end kits (Illumina MiSeq Reagent Kit v3, 600-cycle; Illumina Inc.).
Sequence analysis of the repertoire
Sequence analysis was performed using an in-house custom analysis pipeline described previously.36,42,43 Briefly, all sequences were aligned with www.imgt.org/HighV-QUEST44 following quality filtering and paired-read joining. Categorization of sequences as naive or non-naive was done to minimize ambiguity, and the potential of false calls of naive sequences within the non-naive bin given mutations has been previously reported in naive BCR analysis mutation calling as a result of sequencing error and allelic variability,45–50 with naive defined as expressing IgM and <1% nt mutation from IMGT assigned germline allele, and non-naive defined as >10% nt mutation. To normalize intersample variations, 130 K sequences were randomly chosen from each library and then analyzed for this study. All clonal lineage assignments were based on identical VH and JH region calls, identical HCDR3 length, and ≥85% HCDR3 nucleotide sequence similarity.
Expanded clones were determined (Supplementary Fig. S1) by plotting size-ranked lineages (% partitions of total sequences) stacked on along the y-axis, and the normalized size per lineage (% of total sequences) plotted on the x-axis. The “knee” of the resulting curve indicates the lineages above which are considered “expanded.” This knee is determined by drawing a line from the top of the curve to the plot origin, and then identifying the longest perpendicular line to the clonality curve.
For targeted resolution of pre-immune lineage members of gp120-specific mAb-based lineages and the subsequent lineage tree construction, mAb sequences were parsed against the libraries, and sequences having the same V and J gene usage, HCDR3 length, and ≥80% HCDR3 similarities to the mAb sequence were identified and underwent further filtering using Partis, a multihidden Markov model-based method,51 to confirm the clonal relationship of lineage members. For lineage tree construction, within a lineage, instances of only a single identical nucleotide sequence were removed, with the exception of sequences that match any M0 or M13 or inferred node sequence. The resulting sequences were analyzed using PHYLIP's dnapars tool (version 3.69s).52 The output file was then parsed using in-house custom scripts, collapsing any duplicate sequences into an individual node, and was visualized using Cytoscape.53
mAb generation
Mature mAbs were cloned out from plasmablasts isolated 7 days after the final vaccination as previously described in Basu et al.36 Pre-immune heavy chain representatives were selected on the basis of highest amino acid similarity from the respective germlines. Predicted germline versions were designed with germline V, J, and pre-immune CDR3 sequences for the heavy chains and mature CDR3 for the light chains. These V-regions were synthesized from gBlocks (IDT) and then used for the production of recombinant mAbs as full human IgG1 as described previously.36,54,55 For the pre-immune version mAbs, pre-immune heavy chains were paired with mature light chains, and for the germline version mAbs, germline heavy chains were paired with germline light chains. After 8 days of transfection, mAbs were purified from the culture supernatant using Magna Protein A beads (Promega).
Enzyme-linked immunosorbent assay
The reactivity profiles of mAbs against HIV Env proteins were detected by ELISA. Briefly, ELISA plates (Nunc MaxiSorp; Thermo Fisher Scientific, NY, USA) were coated overnight at 4°C with 50 μL of 0.5 μg/mL of HIV Env gp120 (MN.B or A244.AE, NIH AIDS Reagent Program) in PBS and blocked with 3% BSA in PBS for 30 min at room temperature. Plasma samples were tested at 1:2,500 dilution and mAbs were tested at 10-fold dilutions (100, 10, 1, and 0.1 μg/mL). Fifty microliters of the sample (diluted in PBS containing 0.05% Tween 20 or PBST) was added per well in triplicate and incubated for 1 h. The reaction was detected using peroxidase-conjugated anti-human IgG (Jackson ImmunoResearch), diluted 1:2,000 in PBST. Mean optical density values of triplicate test samples were divided by control (PBST) and represented as relative units (RU).
Surface plasmon resonance
Surface plasmon resonance (SPR) experiments were performed on a Biacore T200 with a CM5 sensor and human IgG capture kit (Cytiva) at 25°C using HEPES buffered saline (HBS) with 0.005% P20 as the running buffer. Anti-human IgG was captured using the protocol recommended by Cytiva over all four flow paths. Between 535 and 580 RU of each Ab was captured using a contact time of 60 s and a 10 μL/min flow rate in an HBS running buffer containing 3 mM EDTA and 0.005% P20. MN.B gp120 (AIDS Reagent Repository) was used as the analyte diluted between 1.6 and 200 nM. gp120 MN.B was injected over flow paths 1, 2, 3, and 4 with a contact time of 120 s, flow rate of 30 μL/min, and a long dissociation of 1,200 s.
Flow path 1 was used as reference, while antibodies were bound in flow paths 2, 3, or 4. 3 M magnesium chloride was injected over all four flow paths for regeneration using a contact time of 30 s and flow rate of 30 μL/min. 1:1 Langmuir model was used to determine the kinetics of each Ab. Because of the low level of binding of gp120 (or the saturation at low gp120 MN.B concentrations), steady-state kinetics were attempted for both pre-immune 1098B8 and germline 1098B8 by reducing the captured Ab to ∼120 RU and increasing the contact time of analyte to 300 s. The pre-immune 1098B8 mAb did not fit well to 1:1 Langmuir kinetics and the lower binding could only be estimated.
Statistical analysis
Statistical analysis of VH deep sequencing data was done with GraphPad Prism v7.0 or MATLAB, using the unpaired Mann–Whitney test or Wilcoxon matched-pairs singed rank test, as appropriate.
Results
Identification of HIV-1 Env-specific B cells in pre-immune blood
We have previously observed the presence of HIV-1 Env gp140 reactive B cells in healthy participants both within the peripheral blood CD27− naive B cell and CD27+ memory B cell compartments (Fig. 1A), suggesting the presence of pre-existing non-naive HIV-1-Env reactive B cell populations. To characterize this pre-immune HIV-1 Env reactive B cell compartment further, we collected peripheral blood samples from 21 healthy participants of the HVTN 105 HIV-1 vaccine clinical trial before and after immunization (Fig. 1B). The participants received four vaccinations over a period of 6 months with different combinations of AIDSVAX B/E bivalent gp120 protein, which includes gp120 MN.B and gp120 A244.AE proteins, and DNA-HIV-PT123, consisting of plasmids expressing 96ZM651.C gp140, ZM96.C gag, and CN54.C pol-nef.37
Human gut microbiota reactive antibodies are known to recognize gp41 subunit of the HIV-1 Env.29,37,56–58 Therefore, we decided to isolate peripheral blood pre-immune HIV-1-Env-specific (gp120pos) B cells using magnetic bead-based enrichment with gp120 MN.B and A244.AE proteins that was a part of HVTN 105 vaccine to maximize isolation of gp120-reactive B cells. The remaining fraction of B cells (gp120neg) was used for comparison.
Characterization of pre-immune B cell repertoire in blood
To understand the molecular features of HIV-1 Env-specific pre-immune BCR repertoire, we analyzed characteristics of the heavy chain variable region by deep sequencing of these gp120pos and gp120neg peripheral blood B cells from all 21 participants. Comparing the isotype distribution (Fig. 1C), we found IgM was the predominant isotype in both the gp120pos and gp120neg repertoires overall, as expected, since naive IgM+ B cells compose the majority of the peripheral B cell compartment in healthy individuals. Non-IgM isotypes (i.e., IgG or IgA) were also substantially present in gp120pos (ranging from 3.8% to 52% for IgA and from 1.5% to 50.9% for IgG) as well as in gp120neg (ranging from 2.7% to 41.5% for IgA and from 1.9% to 53.7% for IgG) in pre-immune blood.
However, on the individual participant basis, higher frequencies of IgM (in 15/21; 71% of the participants) and IgG (in 12/21; 57% of the participants) were found in gp120neg compared with gp120pos. In contrast, the gp120pos fraction was significantly enriched (p = .0003) for IgA with a higher IgA proportion in 86% of the participants (18/21) compared with their gp120neg population.
The CDR3 is the most variable region of the Ab molecule and a major determinant for antigen recognition and binding. Among various known characteristics, the presence of long HCDR3, as defined as ≥22 aa, is a common feature for some well-characterized bnmAbs against HIV-1, and several Env-reactive mAbs isolated from HIV vaccine trials also possess this feature.36,59 We searched for this trait in the pre-immune peripheral blood B cells and found significant (p < .0001) enrichment of lineages with long HCDR3 in the gp120pos over gp120neg fraction in 17 of the 21 (∼81%) participants (Fig. 1D).
Next, we wanted to assess the presence of somatically mutated Abs in the pre-immune repertoire as an indication of antigen experience. Since the predominant population in the peripheral blood of healthy is expected to be naive B cells, which lack somatic hypermutation, BCR sequences with >10% nt mutations compared with germline were analyzed to focus on the non-naive population only. There was no significant difference in the overall VH mutation level between the gp120pos and gp120neg non-naive B cell fractions (not shown). Assessment of expanded clonal lineages, those that predominate the repertoires (Supplementary Fig. S1), revealed that there was a significantly (p = .0083) higher frequency of mutated (>10% nt) VH sequences in gp120pos (Fig. 1E). This increased VH mutation in gp120pos compared with gp120neg was most evident in framework region 1 (FR1) (p = .0098) and FR2 (p = .0001). Together, these results indicate that the peripheral blood pre-immune gp120pos compartment includes IgM, IgG, and IgA B cells, with proportionally more IgA, clones with longer HCDR3, and increased somatic hypermutation in FR1 and FR2.
Expansion of HIV-1 Env-specific pre-immune B cells following HIV Env vaccination
The pre-immune HIV Env-reactive B cell fraction is assumed to not have had any prior exposure to HIV Env and is anticipated to have a certain degree of representation in the resulting HIV Env vaccine-induced B cell repertoire. Previously, for the HIV Env vaccine phase 1 trial HVTN 105, BCR repertoires from peripheral blood 7 days after the final vaccination (M6), expected to be dominated by vaccine-induced IgG plasmablasts, were sequenced.36 We sought to understand the degree of overlap between the gp120pos prevaccination and the vaccine-induced peripheral blood BCR repertoire. Nine out of 11 participants for whom M6 samples were analyzed had postvaccination lineages that could be traced back to the HIV-1 Env-specific pre-immune B cell compartment. However, as expected, due to the large size and diversity of the repertoire and limitation of sequencing depth, only a few lineages were shared between the pre- and post-vaccination samples.
To understand whether these shared pre-immune lineages originated from the naive compartment or not, pre-immune sequences having ≤1% nucleotide mutations from germline in the variable gene were considered derived from naive B cells, and those with >10% nucleotide mutations from germline in the variable gene were considered derived from non-naive B cells (Supplementary Table S1). These criteria were based on previous observations of somatic hypermutation in naive BCR repertoire analysis to account for sequencing errors and allelic miscalls43–48 and to minimize the potential of false identification of a naive B cell within the non-naive bin. Although a similar number of shared lineages between M6 and pre-immune gp120pos were found in the naive and the non-naive compartments (Fig. 2A), the shared lineages between M6 and gp120pos pre-immune comprised a significantly (p = .0117) higher proportion (highest 2.4%) of the non-naive compartment of the gp120pos pre-immune compared with the proportion of the gp120pos pre-immune naive compartment that was shared with M6 (highest 0.20%) (Fig. 2B).
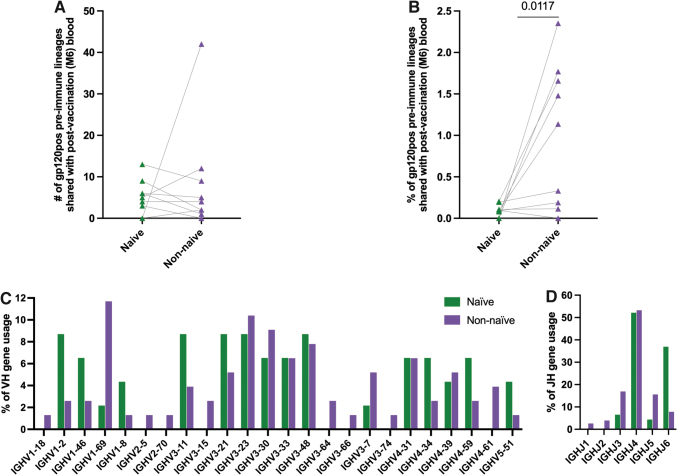
FIG. 2.
Expansion of HIV-1 Env-specific pre-immune B cells following HIV Env vaccination. gp120pos pre-immune B cells that shared lineages with M6 peripheral blood that was obtained 7 days after final immunization were split into naive (≤1% nucleotide mutations from germline in the variable gene) and non-naive (>10% nucleotide mutations from germline in the variable gene) compartments and characterized. Each symbol represents an individual subject. (A) Number of lineages, (B) percentage of lineages, (C) VH and (D) JH gene usage.
Among the lineages that are shared between pre-immune and postvaccination (M6), there was a higher frequency of VH1–2, VH1–8, and VH5–51 lineages in the naive than in non-naive pre-immune lineage members (Fig. 2C). Contrastingly, the frequency of VH1–69 lineages was higher in non-naive (11.7%) than in naive (2.2%) pre-immune members. Among the JH gene usage, increased JH6 usage in the naive than in the non-naive population was most evident (Fig. 2D). Together, these results suggest that the non-naive gp120pos pre-immune compartment is preferentially responsive to this HIV vaccine than the naive gp120pos pre-immune compartment. These results further suggest that the VH1–2, VH1–8, and VH5–51 gp120pos lineages may preferentially arise from the naive pre-immune compartment, whereas the VH1–69 gp120 lineages may preferentially arise from the non-naive pre-immune compartment following HIV Env vaccine.
Mixed origins of vaccine-induced gp120-specific pre-immune repertoire
Observing shared lineage members in the pre- and postvaccination peripheral blood samples, we next sought to definitively characterize the dynamics of the gp120 pre-immune lineages that respond to vaccination. Previously we isolated 66 gp120-specific mAbs from HVTN 105 participants after their last vaccination.36 We sought to determine whether the precursor B cells for these mAbs were present in the peripheral blood pre-immune B cell compartment and subsequently participated in the Env-reactive B cell response to the vaccination. We parsed our mAbs against the pre-immune deep-sequencing database using the criteria of identical VH and JH, HCDR3 length, ≥80% HCDR3 nucleotide sequence similarity, and clonal assignment based on the Partis multihidden Markov model program,51 consistent with previous deep-sequencing clonal lineage analyses60–62 to identify the pre-immune clonal lineage members, and subsequently identified four lineages from three of the study participants (Table 1; Supplementary Fig. S2). No distinct binding or molecular features such as V or J family gene usage distinguished these mAbs from the other mAbs that were not found in the pre-immune repertoire (not shown).
Table 1.
Summary of Vaccine-Induced gp120-Reactive Monoclonal Antibody Lineages Identified in Pre-immune Peripheral Blood
| Subject | mAb (M6) |
Pre-immune (M0) |
Other samples containing lineage members | Total no. of seqs. in cluster associated with the mAb | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | V gene | J gene | VH mutation %, AA | Isotype | No. of members in blood | Avg. VH mutation %, nt | Avg. VH mutation %, AA | Avg. HCDR3 nt similarity % | Avg. HCDR3 AA similarity % | Isotype | |||
| 056 | 1131A5 | VH1–46 | JH6 | 8.2 | IgG1 | 9 | 3 (0.3–7.4) | 5.2 (0–12.2) | 83.2 (82–87.2) | 73.5 (61.5–77) | IgM(9) | M1, M6 | 1,447 |
| 1131D12 | VH1–46 | JH6 | 18.2 | IgG1 | 23 | 10.8 (3.2–13.4) | 18.5 (7.3–24.4) | 95.8 (92–96.8) | 98.3 (90.5–100) | IgA(19) IgM(4) | M1, M3, BM, tonsil | 1,056 | |
| 211 | 1095A8 | VH3–74 | JH4 | 14.3 | IgA1 | 301 | 9 (3.6–19.5) | 15.7 (6.5–36.6) | 96.8 (80.4–100) | 93.5 (58.8–100) | IgA(190) IgG(9) IgM(38) Undefined(64) |
M1, M3, M6 | 1,740 |
| 097 | 1098B8 | VH1–2 | JH5 | 6.1 | IgG1 | 6 | 9.2 (9–10.1) | 16.5 (16.3–17.4) | 80.5 | 83.3 | IgG(6) | M6, M13, BM | 43,942 |
M1 = 7 dp 2nd vaccination and 1 m.p.f.v; M3 = 7 dp 3rd vaccination and 3 m.p.f.v; M6 = 7 dp 4th and final vaccination, 6 m.p.f.v; M13 = 13 m.p.f.v (7 months postfinal vaccination); BM (7 months postfinal vaccination); tonsil (7 months postfinal vaccination).
BM, bone marrow; dp, days post; mAb, monoclonal antibody; Ig, immunoglobulin; m.p.f.v, month post 1st vaccination.
Three of these four mAbs utilize VH1, which we previously reported to be dominant in the vaccine-induced lineages in HVTN 105.36 Pre-immune members of 2/4 lineages (1131D12, 1095A8) were found to be IgM and IgA and/or IgG, with a minimum of 6% VH amino acid mutation from germline, indicating they developed from a non-naive pre-immune origin. The 1098B8 lineage members were only found as IgG in pre-immune, also indicating development from a non-naive pre-immune origin. The 1131D12 lineage members were found as IgA and somatically mutated IgM in pre-immune indicating the development from a non-naive pre-immune origin. Members of the 1131D12 lineage were also found in tonsil 7 months after the final vaccination, all as IgA, indicating the mucosal presence of this lineage. Among these three non-naive pre-immune origin lineages, substantial VH somatic hypermutation (6%–37% AA) in the pre-immune lineage members was evident.
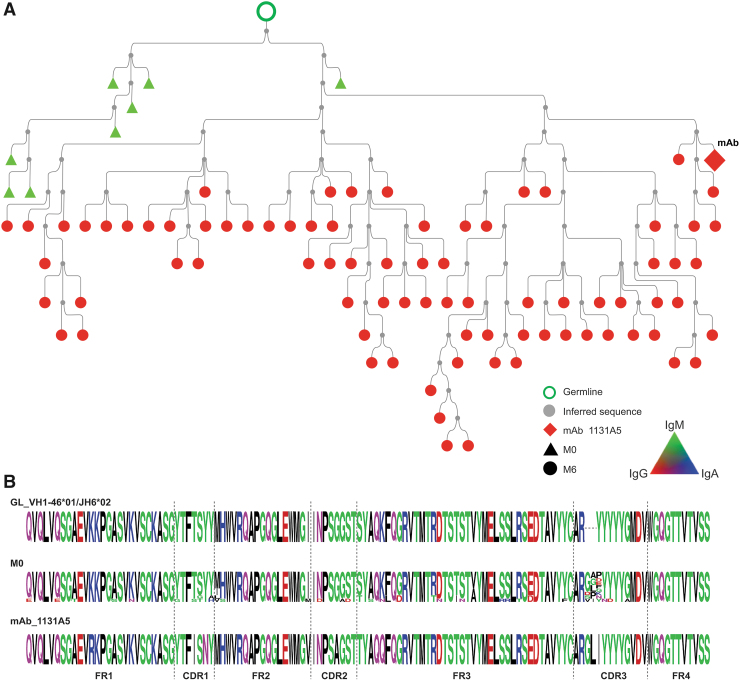
The 1131A5 lineage was found as only IgM in pre-immune, including lineage members in their germline configuration, lacking any VH amino acid mutation, suggesting that the 1131A5 lineage developed, at least in part, from a naive pre-immune origin.
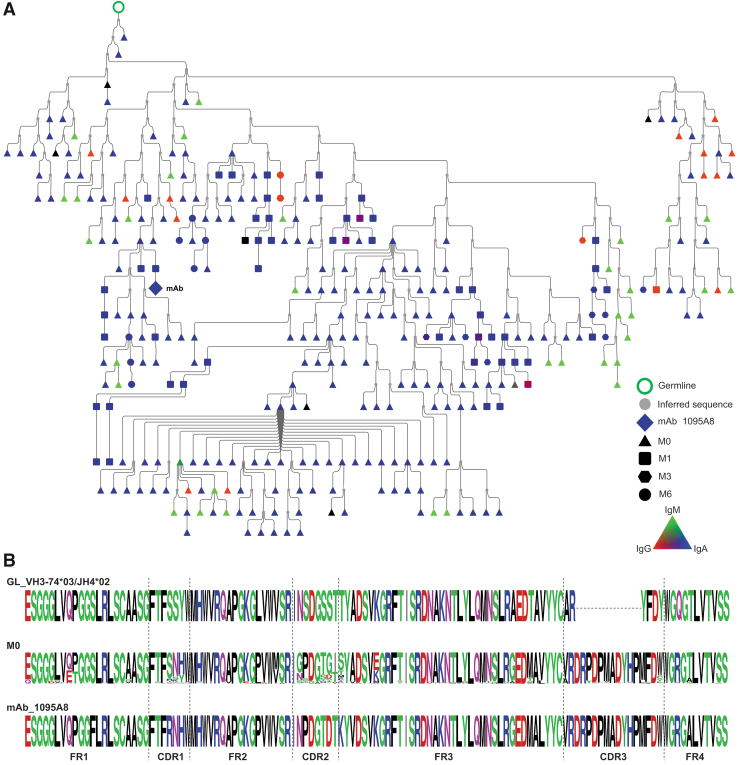
We selected three representative lineages to investigate further and constructed lineage trees from the clonally related sequences. The 1131A5 lineage (Fig. 3) had the lowest somatic mutation (0.3%–7.4% nt) observed in pre-immune members (M0), which consisted of only IgM and was considerably mutated when isolated from postvaccination blood as an IgG mAb. This lineage is likely indicative of a naive B cell responding to HIV gp120 proceeding through affinity maturation and class switching. The 1095A8 lineage (Fig. 4) includes pre-immune members that are highly somatically mutated (6.5%–36.6% AA) and IgA, IgG, and IgM. The lineage spans multiple time points following the vaccination course, with IgA dominance in pre-immune and throughout.
FIG. 3.
HIV-1 Env reactive 1131A5 lineage in pre-immune blood. (A) Phylogenic analysis and alignments of 11131A5 lineage. Lineage members were defined as same heavy-chain V and J gene usage, HCDR3 length, and >80% HCDR3 similarity to the mAb sequence, and clustered based on Partis analysis. Individual nodes indicate identical nucleotide sequences (n = 1–6). Black nodes indicate undefined isotype, symbol shape indicates time point. (B) Alignments depict germline, pre-immune lineage members, and M6 mAb-derived sequences. mAb, monoclonal antibody.
FIG. 4.
HIV-1 Env reactive 1095A8 lineage in pre-immune blood. (A) Phylogenic analysis and alignments of 1095A8 lineage. Lineage members were defined as same heavy-chain V and J gene usage, HCDR3 length, and >80% HCDR3 similarity to the mAb sequence, and clustered based on Partis analysis. Individual nodes indicate identical nucleotide sequences (n = 1–37). Black nodes indicate undefined isotype, symbol shape indicates time point. Nodes with sequences from multiple time points are in black borders. (B) Alignments depict germline, pre-immune lineage members, and M6 mAb sequences.
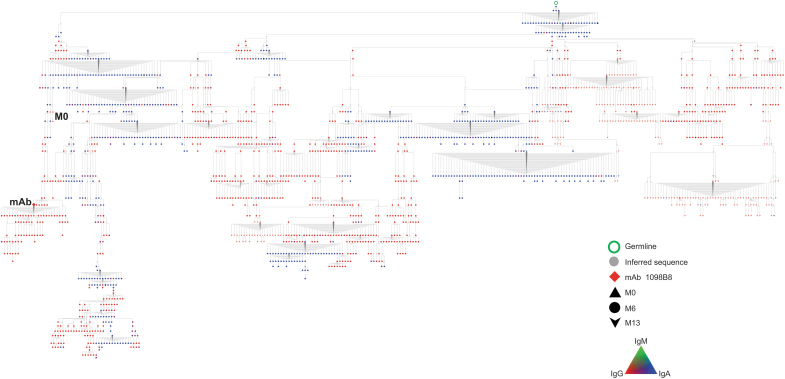
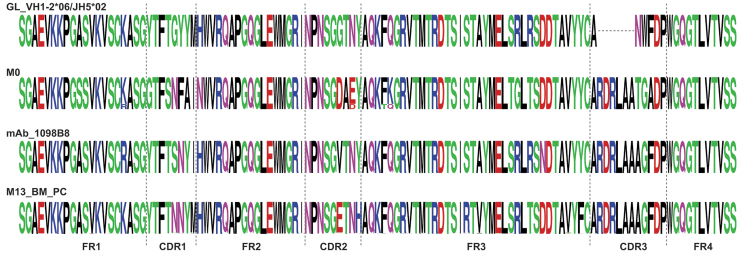
The 1098B8 pre-immune lineage members (Fig. 5) were identified as IgG and had substantial mutation from germline (16.3%–17.4% AA) (Fig. 6).
FIG. 5.
HIV-1 Env reactive 1098B8 lineage in pre-immune blood. Phylogenic analysis of 1098B8 lineage. Lineage members were defined as same heavy-chain V and J gene usage, HCDR3 length, and >80% HCDR3 similarity to the mAb sequence, and clustered based on Partis analysis. M13 sequences were obtained from peripheral blood, total BM, and CD138+ BM PC. Individual nodes indicate identical nucleotide sequences (n = 1–653). Black nodes indicate undefined isotype, symbol shape indicates time point. BM, bone marrow; PC, plasma cells.
FIG. 6.
Sequence alignment of 1098B8 lineage members. Alignments depict germline, pre-immune lineage members, M6 mAb, and M13 BM PC-derived sequences for 1098B8 lineage.
Lineage members with additional somatic mutations were also present as CD138+ long-lived plasma cells in the bone marrow 7 months after final vaccination and also in peripheral blood memory B cells. This suggests that the lineage represents a non-naive B cell responding to HIV gp120 and resulting in additional clonal expansion and somatic hypermutation.
gp120 binding by pre-immune antibodies
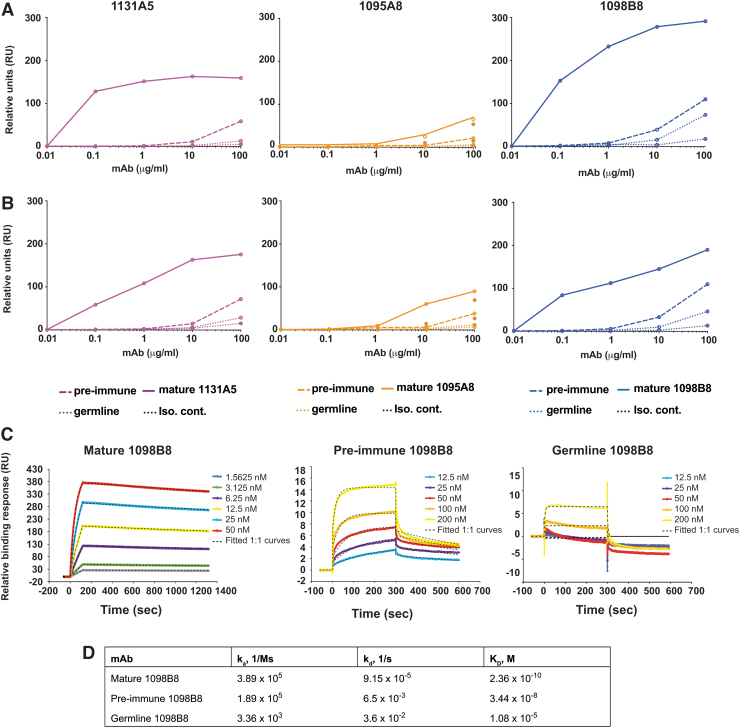
We tested the gp120 binding ability of the pre-immune versions of these lineages (Fig. 7). The representatives of each pre-immune lineage and the germline versions were synthesized and complemented with the mature or the germline light-chain versions. The pre-immune mAbs showed lower reactivity than the postvaccination mature Abs against HIV-1 Env of the vaccine component strains MN.B [AIDSVAX B/E] (Fig. 7A) and A244.AE [AIDSVAX B/E] (Fig. 7B), but higher than their predicted germline versions. We further tested one of these lineages for the binding affinity against HIV-1 Env MN.B gp120 by SPR. Mature 1098B8 mAb bound gp120 with a dissociation constant (KD) of 236 pM, demonstrating high affinity (Fig. 7C, D). As expected, the pre-immune mAb showed lower but appreciable binding (KD = 34.4 nM). The germline mAb showed a general lack of binding to gp120, and therefore, the kinetic constant could not be similarly determined.
FIG. 7.
gp120 binding by pre-immune antibodies. (A, B) Reactivity of germline and pre- and post-vaccination versions of representative mAbs to (A) MN.B and (B) A244.AE gp120 determined by ELISA (n = 3 replicates per dilution). A HIV-irrelevant hIgG1 was used as an isotype control. Each line represents the mean of an individual mAb. (C) Binding profile of germline and pre- and post-vaccination versions of 1098B8 lineage to MN.B gp120 determined by surface plasmon resonance analysis and (D) their dissociation constants (KD).
Together, these results suggest that 1098B8 developed from a non-naive pre-immune origin as a result of an initial naive B cell, which had minimal gp120 reactivity, responding to a non-HIV Env antigen and undergoing somatic hypermutation and acquiring HIV Env cross-reactivity, and subsequently responding to the HIV Env vaccine and undergoing HIV Env-directed affinity maturation.
Discussion
While HIV Env is immunogenic and its delivery in numerous forms such as plasmid DNA, protein, and viral vector readily induces Env-specific Ab and B cells, only a minority subset of the responding repertoire is typically able to target the most consequential epitopes or have the potential for conferring protection. As HIV vaccine development progresses, a major emphasis has been placed in trying to focus the humoral vaccine response to highly conserved epitopes and elicit clonal lineages that preferentially target these epitopes. To aid in such nuanced HIV vaccine development efforts, we sought to characterize the state and features of the peripheral blood gp120-specific B cell repertoire before immunization, and the features of those pre-immune B cell clonal lineages that ultimately respond to the vaccine. We determined that in humans a major proportion of the vaccine-induced gp120-specific BCR repertoire originates from non-naive B cells present before immunization.
Most of the pre-immune gp120-specific repertoire consisted of IgM (∼60%), however, IgA and IgG comprised ∼25% and ∼15%, respectively. Although a significant level of Ab diversity has been observed in the pre-immune gp120-specific repertoire, it cannot be ruled out that the use of trimeric gp120 instead of monomeric gp120 protein for isolating B cells could have a higher potential to bind to the rare Ab expressing or lower affinity IgM precursor B cells.63,64 Another factor to consider is that glycans found on HIV-1 Env and other pathogens are also present on host molecules,65,66 suggesting that the pre-immune gp120 reactive B cell population may include cross-reactive but weakly glycol-specific B cells. In addition, the absence of particular glycans (e.g., presence of glycan holes) on the antigens has shown to improve HIV Env-specific immune responses.67–69 However, several bnAbs, which recognize the natural heavily glycosylated epitopes on HIV-Env protein, have been well-characterized.70,71
Half of the gp120-specific mAb lineages we were able to track back to pre-immune included pre-immune IgA clonal members. While the functional relevance and consequences of the IgA compartment as a major origin of gp120-vaccine-induced responses are to be determined, it is consistent with past findings during early HIV infection and exposure that an IgA response to predominantly gp41 but also gp12072–74 is observed particularly in mucosa.75,76 IgA antibodies and B cells are major contributors to mucosal protection, and although we did not look in-depth at the mucosal BCR repertoire and only following immunization, IgA members of the 1131D12 lineage were evident in tonsil after vaccination, and IgA pre-immune members were present in peripheral blood, which may suggest a mucosal association with the origin of this lineage.
The systemic and mucosal IgA BCR repertoire is strongly influenced by B1 B cells,77,78 which are an unconventional B cell population that responds quickly against mucosal pathogens through innate-like recognition via IgM, but also that can further develop into highly antigen-specific IgG and IgA B cells77,79,80 indeed, about half of the mucosal IgA has a B1 origin.77,78 Future studies that directly evaluate pre-immune mucosal B cells or mucosal immunization may provide additional insight into the development of protective IgA responses to HIV vaccines. Together this suggests that further assessment of the contribution of mucosal IgA and B1 B cells to the pre-immune gp120 compartment is warranted.
An enrichment of long HCDR3 and increased somatic hypermutation in FR1 and FR2 was evident in the pre-immune gp120 compartment, suggesting intrinsic germline and acquired molecular features that determine recognition of gp120. This is consistent with the observation that long HCDR3 is frequently found in responses to HIV infection and vaccination, particularly with Abs targeting gp120.59,81 In addition, there have been reports of acquired FR1 and FR2 motifs that may be conserved among public clonotypes recognizing gp120.82–85
VH1–2 and VH1–46 usage among the gp120 repertoire is particularly notable, given their use among VRC01-class antibodies that target the CD4 binding site of gp120 and have a potent and broad neutralizing activity. Although VH1–2 and VH1–46 were not overrepresented in the gp120 pre-immune compartment, they were overrepresented among the pre-immune lineages of naive origin that responded to the immunization. In contrast, VH1–69, which is known to be frequent in antiviral responses,86 and VH3–23, which is the most common VH gene used in the human Ig repertoire,45,87 were overrepresented in those vaccine responding pre-immune lineages of non-naive origin. It is anticipated that tuning vaccines to elicit VH-specific/class-type responses, such as being done with the “germline-targeting” approaches, may be more consistent and reproducible among vaccinees when targeting the naive pre-immune compartment, and subsequently more uniform, as being dependent on just germline representations.
However, induction of VH-specific/class-type responses that are overrepresented in the non-naive pre-immune compartment (e.g., VH1–69) would likely be more inconsistent and highly variable among vaccinees, and heavily dependent on exposure history to the cross-reactive non-HIV antigens that stimulated the initial naive B cell and the acquisition of cross-reactivity to gp120. Such a process would likely be difficult to reverse engineer into a vaccination strategy, and it remains unclear, given the likely redundant pathways to generate bnAbs against the same epitope,88,89 whether pre-immune non-naive and naive lineages can equally develop into bnAbs. Due to the infrequent nature of gp120-specificity in the pre-immune repertoire and limitations of deep sequencing approaches, highly quantitative tracking and characterization of the lineages that respond or do not respond to immunization are difficult but should be pursued further given the fundamental heterogeneity we have defined here.
As mature light chains were used to reconstruct the pre-immune version mAbs, the contribution of the light-chain somatic hypermutation in the affinity maturation could not be resolved in this study. We observed that the non-naive pre-immune VH (e.g., 1098B8, 1095A8) conferred greater gp120 binding activity than the germline VH. This is consistent with previous demonstration that many HIV bnAbs, including 3BNC60, PGT145, CAP256-VRC26, NIH45–46, b12, and 2G12 when reverted to germline, have little to no recognition of unmodified gp120.12,14,84,90–92 This supports the possibility that at least a substantial proportion of the non-naive pre-immune gp120-specific compartment acquired affinity for gp120 through the affinity maturation process against another antigen. Through this process, these clonal lineages and their memory B cells would have likely expanded beyond naive frequencies, which could have serious implications for immunodominance, and provide rationale for the substantial representation of non-naive origin lineages in the postvaccine gp120-specific repertoire.
A number of studies have shown that the pre-existing immunity significantly affects the Ab responses in the setting of infections/vaccinations that include influenza,93,94 SARS-CoV-2,95,96 Zika,97 and yellow fever.98 Several gp120 reactive antibodies have demonstrated cross-reactivity with bacterial antigens,32,99,100 but the sources and dynamics of these cross-reactive antigens remain to be fully resolved. If these initiating antigens or microbes could be identified, they may be highly beneficial to use in a prepriming approach to establish the non-naive pre-immune gp120 compartment, readied to respond to gp120 immunogens.
The current study was not able to resolve these initiating antigens, nor the precise gp120 epitopes recognized by the described mAbs or their dependence on glycosylated residues, and is a major emphasis of ongoing efforts. Because mature HCDR3 forms as a result of the combined influence of random V-D-J recombination, N additions, and somatic mutation, precisely determining the germline HCDR3 is not feasible, and the contribution of individual germline HCDR3 residues in conferring gp120 reactivity with the lineages we have identified should be examined.
In RV144, the decreased risk of infection observed in vaccinated participants was strongly correlated with the gp120-specific Ab response, but protection did not appear to be long-lived, highlighting that durability is a critical element of effective HIV vaccine. We have previously demonstrated that the HVTN 105 vaccine regimen induces long-lived bone marrow plasma cells,36 the major source of systemic plasma Ab, and here we observe gp120-specific mAb lineage members persisting in the bone marrow for both lineages that arise from naive (1131A5) and non-naive (1098B8, 1131D12, 1095A8) origins. Although we cannot exclude some pre-immune seeding of the bone marrow with these non-naive origin lineage members, this suggests that origin of the gp120 humoral response, whether naive or non-naive, does not grossly hinder the ability to establish long-lived gp120-specific plasma cells.
It remains to be determined to what extent pre-immune gp120 reactive plasma antibodies may occur and their relationship with bone marrow plasma cells and influence to the humoral response to HIV vaccination and infection.
In conclusion, we have demonstrated that the pool of B cells that respond to initial immunization with HIV Env includes a substantial proportion of antigen-experienced non-naive B cells. Further defining the mechanisms by which the pre-immune Env-reactive B cell compartment develops and responds to vaccination will likely be necessary for developing broadly efficacious HIV vaccines and may provide insights for other viral vaccines seeking to induce precise Ab classes or germlines.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation. The Ab and repertoire RNA sequences are available from the database of Genotypes and Phenotypes (dbGaP) (https://www.ncbi.nlm.nih.gov/gap/) accession number phs002027.v2.p1.
Supplementary Material
Acknowledgments
We are grateful for the assistance of the Rochester Victory Alliance and its staff. We are grateful for the assistance of the University of Rochester Flow Cytometry Core Facility, and the University of Alabama at Birmingham Genomics Core Laboratory and Multidisciplinary Molecular Interaction Core. We are most grateful for the participation of the HVTN 105 study volunteers, and thank the HVTN protocol team for their support of this project. We are very appreciative of the assistance provided by the HIV Vaccine Trials Network in enabling and coordinating this project. A version of this article has been published on preprint server BioRxiv https://www.biorxiv.org/content/10.1101/2021.09.01.458551v1
Authors' Contributions
M.B., A.F.R., J.L., L.-X.M, C.A.B., M.C.K., and J.J.K. conceived and designed the study. J.L. and L.-X.M. provided critical samples. M.B. and M.S.P. performed the experiments. M.B., M.S.P., C.F., A.F.R., and J.J.K. performed the data curation and analysis. M.B. and J.J.K. drafted and edited the article.
Author Disclosure Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding Information
This research was partially funded by the National Institutes of Health [5R21AI116285, 5R01AI117787, 5R01DE027245 to J.J.K., UM1AI069511 to M.C.K.], the University of Rochester Center for AIDS Research P30AI078498 (NIH/NIAID), the University of Alabama at Birmingham Center for AIDS Research P30AI027767 (NIH/NIAID), and the University of Alabama at Birmingham HIV Clinical Trials Unit UM1AI069452 (NIH/NIAID).
Supplementary Material
References
- 1. WHO. WHO HIV/AIDS Data and Statistics. 2019. Available from: https://www.who.int/hiv/data/en [Last accessed: June 30, 2022].
- 2. Haynes BF, Gilbert PB, McElrath MJ, et al. Immune-correlates analysis of an HIV-1 vaccine efficacy trial. N Engl J Med 2012;366(14):1275–1286; doi: 10.1056/NEJMoa1113425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chung AW, Kumar MP, Arnold KB, et al. Dissecting polyclonal vaccine-induced humoral immunity against HIV using systems serology. Cell 2015;163(4):988–998; doi: 10.1016/j.cell.2015.10.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zolla-Pazner S, deCamp A, Gilbert PB, et al. Vaccine-induced IgG antibodies to V1V2 regions of multiple HIV-1 subtypes correlate with decreased risk of HIV-1 infection. PLoS One 2014;9(2):e87572; doi: 10.1371/journal.pone.0087572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Karasavvas N, Billings E, Rao M, et al. The Thai Phase III HIV Type 1 Vaccine trial (RV144) regimen induces antibodies that target conserved regions within the V2 loop of gp120. AIDS Res Hum Retroviruses 2012;28(11):1444–1457; doi: 10.1089/aid.2012.0103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zolla-Pazner S, deCamp AC, Cardozo T, et al. Analysis of V2 antibody responses induced in vaccinees in the ALVAC/AIDSVAX HIV-1 vaccine efficacy trial. PLoS One 2013;8(1):e53629; doi: 10.1371/journal.pone.0053629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yates NL, Liao HX, Fong Y, et al. Vaccine-induced Env V1-V2 IgG3 correlates with lower HIV-1 infection risk and declines soon after vaccination. Sci Transl Med 2014;6(228):228ra39; doi: 10.1126/scitranslmed.3007730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bonsignori M, Liao HX, Gao F, et al. Antibody-virus co-evolution in HIV infection: Paths for HIV vaccine development. Immunol Rev 2017;275(1):145–160; doi: 10.1111/imr.12509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hraber P, Seaman MS, Bailer RT, et al. Prevalence of broadly neutralizing antibody responses during chronic HIV-1 infection. AIDS 2014;28(2):163–169; doi: 10.1097/QAD.0000000000000106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. West AP Jr, Diskin R, Nussenzweig MC, et al. Structural basis for germ-line gene usage of a potent class of antibodies targeting the CD4-binding site of HIV-1 gp120. Proc Natl Acad Sci U S A 2012;109(30):E2083–E2090; doi: 10.1073/pnas.1208984109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wu X, Zhang Z, Schramm CA, et al. Maturation and diversity of the VRC01-antibody lineage over 15 years of chronic HIV-1 infection. Cell 2015;161(3):470–485; doi: 10.1016/j.cell.2015.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hoot S, McGuire AT, Cohen KW, et al. Recombinant HIV envelope proteins fail to engage germline versions of anti-CD4bs bNAbs. PLoS Pathog 2013;9(1):e1003106; doi: 10.1371/journal.ppat.1003106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ahlers JD. All eyes on the next generation of HIV vaccines: Strategies for inducing a broadly neutralizing antibody response. Discov Med 2014;17(94):187–199. [PubMed] [Google Scholar]
- 14. Xiao X, Chen W, Feng Y, et al. Germline-like predecessors of broadly neutralizing antibodies lack measurable binding to HIV-1 envelope glycoproteins: Implications for evasion of immune responses and design of vaccine immunogens. Biochem Biophys Res Commun 2009;390(3):404–409; doi: 10.1016/j.bbrc.2009.09.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Havenar-Daughton C, Sarkar A, Kulp DW, et al. The human naive B cell repertoire contains distinct subclasses for a germline-targeting HIV-1 vaccine immunogen. Sci Transl Med 2018;10(448); doi: 10.1126/scitranslmed.aat0381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jardine JG, Ota T, Sok D, et al. HIV-1 VACCINES. Priming a broadly neutralizing antibody response to HIV-1 using a germline-targeting immunogen. Science 2015;349(6244):156–161; doi: 10.1126/science.aac5894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jardine J, Julien JP, Menis S, et al. Rational HIV immunogen design to target specific germline B cell receptors. Science 2013;340(6133):711–716; doi: 10.1126/science.1234150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Escolano A, Steichen JM, Dosenovic P, et al. Sequential immunization elicits broadly neutralizing anti-HIV-1 antibodies in Ig knockin mice. Cell 2016;166(6):1445–1458, e12; doi: 10.1016/j.cell.2016.07.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Briney B, Sok D, Jardine JG, et al. Tailored immunogens direct affinity maturation toward HIV neutralizing antibodies. Cell 2016;166(6):1459–1470, e11; doi: 10.1016/j.cell.2016.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Abbott RK, Lee JH, Menis S, et al. Precursor frequency and affinity determine B cell competitive fitness in germinal centers, tested with germline-targeting HIV vaccine immunogens. Immunity 2018;48(1):133–146, e6; doi: 10.1016/j.immuni.2017.11.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Huang D, Abbott RK, Havenar-Daughton C, et al. B cells expressing authentic naive human VRC01-class BCRs can be recruited to germinal centers and affinity mature in multiple independent mouse models. Proc Natl Acad Sci U S A 2020;117(37):22920–22931; doi: 10.1073/pnas.2004489117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lin YR, Parks KR, Weidle C, et al. HIV-1 VRC01 germline-targeting immunogens select distinct epitope-specific B cell receptors. Immunity 2020;53(4):840–851, e6; doi: 10.1016/j.immuni.2020.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. ClinicalTrials.gov. A phase I trial to evaluate the safety and immunogenicity of eOD-GT8 60mer vaccine, adjuvanted. 2021. Available from: https://clinicaltrials.gov/ct2/show/NCT03547245 [Last accessed: June 30, 2022].
- 24. Ng'uni T, Chasara C, Ndhlovu ZM. Major scientific hurdles in HIV vaccine development: Historical perspective and future directions. Front Immunol 2020;11:590780; doi: 10.3389/fimmu.2020.590780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, et al. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med 2009;361(23):2209–2220; doi: 10.1056/NEJMoa0908492 [DOI] [PubMed] [Google Scholar]
- 26. Edupuganti S, Mgodi N, Karuna ST, et al. Feasibility and successful enrollment in a proof-of-concept HIV prevention trial of VRC01, a broadly neutralizing HIV-1 monoclonal antibody. J Acquir Immune Defic Syndr 2021;87:671–679; doi: 10.1097/QAI.0000000000002639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mgodi NM, Takuva S, Edupuganti S, et al. A phase 2b study to evaluate the safety and efficacy of VRC01 broadly neutralizing monoclonal antibody in reducing acquisition of HIV-1 infection in women in sub-Saharan Africa: Baseline findings. J Acquir Immune Defic Syndr 2021;87:680–687; doi: 10.1097/QAI.0000000000002649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Corey L, Gilbert PB, Juraska M, et al. Two randomized trials of neutralizing antibodies to prevent HIV-1 acquisition. N Engl J Med 2021;384(11):1003–1014; doi: 10.1056/NEJMoa2031738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Planchais C, Kok A, Kanyavuz A, et al. HIV-1 envelope recognition by polyreactive and cross-reactive intestinal B cells. Cell Rep 2019;27(2):572–585, e7; doi: 10.1016/j.celrep.2019.03.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Trama AM, Moody MA, Alam SM, et al. HIV-1 envelope gp41 antibodies can originate from terminal ileum B cells that share cross-reactivity with commensal bacteria. Cell Host Microbe 2014;16(2):215–226; doi: 10.1016/j.chom.2014.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mouquet H, Nussenzweig MC. Polyreactive antibodies in adaptive immune responses to viruses. Cell Mol Life Sci 2012;69(9):1435–1445; doi: 10.1007/s00018-011-0872-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Williams WB, Liao HX, Moody MA, et al. HIV-1 VACCINES. Diversion of HIV-1 vaccine-induced immunity by gp41-microbiota cross-reactive antibodies. Science 2015;349(6249):aab1253; doi: 10.1126/science.aab1253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Moody MA, Yates NL, Amos JD, et al. HIV-1 gp120 vaccine induces affinity maturation in both new and persistent antibody clonal lineages. J Virol 2012;86(14):7496–7507; doi: 10.1128/JVI.00426-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wiehe K, Easterhoff D, Luo K, et al. Antibody light-chain-restricted recognition of the site of immune pressure in the RV144 HIV-1 vaccine trial is phylogenetically conserved. Immunity 2014;41(6):909–918; doi: 10.1016/j.immuni.2014.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Krebs SJ, Kwon YD, Schramm CA, et al. Longitudinal analysis reveals early development of three MPER-directed neutralizing antibody lineages from an HIV-1-infected individual. Immunity 2019;50(3):677–691, e13; doi: 10.1016/j.immuni.2019.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Basu M, Piepenbrink MS, Francois C, et al. Persistence of HIV-1 Env-specific plasmablast lineages in plasma cells after vaccination in humans. Cell Rep Med 2020;1(2); doi: 10.1016/j.xcrm.2020.100015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rouphael NG, Morgan C, Li SS, et al. DNA priming and gp120 boosting induces HIV-specific antibodies in a randomized clinical trial. J Clin Invest 2019;129(11):4769–4785; doi: 10.1172/JCI128699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kobie JJ, Alcena DC, Zheng B, et al. 9G4 autoreactivity is increased in HIV-infected patients and correlates with HIV broadly neutralizing serum activity. PLoS One 2012;7(4):e35356; doi: 10.1371/journal.pone.0035356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kobie JJ, Zheng B, Bryk P, et al. Decreased influenza-specific B cell responses in rheumatoid arthritis patients treated with anti-tumor necrosis factor. Arthritis Res Ther 2011;13(6):R209; doi: 10.1186/ar3542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Alam SM, Liao HX, Tomaras GD, et al. Antigenicity and immunogenicity of RV144 vaccine AIDSVAX clade E envelope immunogen is enhanced by a gp120N-terminal deletion. J Virol 2013;87(3):1554–1568; doi: 10.1128/JVI.00718-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Piepenbrink MS, Park JG, Oladunni FS, et al. Therapeutic activity of an inhaled potent SARS-CoV-2 neutralizing human monoclonal antibody in hamsters. Cell Rep Med 2021;2(3):100218; doi: 10.1016/j.xcrm.2021.100218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Nogales A, Piepenbrink MS, Wang J, et al. A highly potent and broadly neutralizing H1 influenza-specific human monoclonal antibody. Sci Rep 2018;8(1):4374; doi: 10.1038/s41598-018-22307-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tipton CM, Fucile CF, Darce J, et al. Diversity, cellular origin and autoreactivity of antibody-secreting cell population expansions in acute systemic lupus erythematosus. Nat Immunol 2015;16(7):755–765; doi: 10.1038/ni.3175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Aouinti S, Giudicelli V, Duroux P, et al. IMGT/StatClonotype for pairwise evaluation and visualization of NGS IG and TR IMGT clonotype (AA) diversity or expression from IMGT/HighV-QUEST. Front Immunol 2016;7(339); doi: 10.3389/fimmu.2016.00339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wu YC, Kipling D, Dunn-Walters DK. The relationship between CD27 negative and positive B cell populations in human peripheral blood. Front Immunol 2011;2:81; doi: 10.3389/fimmu.2011.00081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ghraichy M, Galson JD, Kovaltsuk A, et al. Maturation of the human immunoglobulin heavy chain repertoire with age. Front Immunol 2020;11:1734; doi: 10.3389/fimmu.2020.01734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ohlin M, Scheepers C, Corcoran M, et al. Inferred allelic variants of immunoglobulin receptor genes: A system for their evaluation, documentation, and naming. Front Immunol 2019;10:435; doi: 10.3389/fimmu.2019.00435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ghraichy M, von Niederhausern V, Kovaltsuk A, et al. Different B cell subpopulations show distinct patterns in their IgH repertoire metrics. Elife 2021;10: doi: 10.7554/eLife.73111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Claireaux M, Caniels TG, de Gast M, et al. A public antibody class recognizes a novel S2 epitope exposed on open conformations of SARS-CoV-2 spike. bioRxiv 2021; doi: 10.1101/2021.12.01.470767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hazanov L, Mehr R, Wu Y-CB, et al. Lineage tree analysis of high throughput immunoglobulin sequencing clarifies B cell maturation pathways. International Workshop on Artificial Immune Systems (AIS), Taormina, Italy; 2015; pp. 1–6; doi: 10.1109/AISW.2015.7469231. [DOI] [Google Scholar]
- 51. Ralph DK, Matsen FA. Likelihood-based inference of B cell clonal families. PLoS Comput Biol 2016;12(10):e1005086; doi: 10.1371/journal.pcbi.1005086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Felsenstein J. PHYLIP (Phylogeny Inference Package) version 3.6. Department of Genome Sciences, University of Washington, Seattle, WA, USA; 2005. [Google Scholar]
- 53. Shannon P, Markiel A, Ozier O, et al. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res 2003;13(11):2498–2504; doi: 10.1101/gr.1239303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kobie JJ, Zheng B, Piepenbrink MS, et al. Functional and molecular characteristics of novel and conserved cross-clade HIV envelope specific human monoclonal antibodies. Monoclon Antib Immunodiagn Immunother 2015;34(2):65–72; doi: 10.1089/mab.2014.0064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Tiller T, Meffre E, Yurasov S, et al. Efficient generation of monoclonal antibodies from single human B cells by single cell RT-PCR and expression vector cloning. J Immunol Methods 2008;329(1–2):112–124; doi: 10.1016/j.jim.2007.09.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Williams WB, Han Q, Haynes BF. Cross-reactivity of HIV vaccine responses and the microbiome. Curr Opin HIV AIDS 2018;13(1):9–14; doi: 10.1097/COH.0000000000000423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Zolla-Pazner S, Edlefsen PT, Rolland M, et al. Vaccine-induced human antibodies specific for the third variable region of HIV-1 gp120 impose immune pressure on infecting viruses. EBioMedicine 2014;1(1):37–45; doi: 10.1016/j.ebiom.2014.10.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Cram JA, Fiore-Gartland AJ, Srinivasan S, et al. Human gut microbiota is associated with HIV-reactive immunoglobulin at baseline and following HIV vaccination. PLoS One 2019;14(12):e0225622; doi: 10.1371/journal.pone.0225622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Easterhoff D, Moody MA, Fera D, et al. Boosting of HIV envelope CD4 binding site antibodies with long variable heavy third complementarity determining region in the randomized double blind RV305 HIV-1 vaccine trial. PLoS Pathog 2017;13(2):e1006182; doi: 10.1371/journal.ppat.1006182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Soto C, Bombardi RG, Branchizio A, et al. High frequency of shared clonotypes in human B cell receptor repertoires. Nature 2019;566(7744):398–402; doi: 10.1038/s41586-019-0934-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Setliff I, McDonnell WJ, Raju N, et al. Multi-donor longitudinal antibody repertoire sequencing reveals the existence of public antibody clonotypes in HIV-1 infection. Cell Host Microbe 2018;23(6):845–854, e6; doi: 10.1016/j.chom.2018.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Davis CW, Jackson KJL, McCausland MM, et al. Influenza vaccine-induced human bone marrow plasma cells decline within a year after vaccination. Science 2020;370(6513):237–241; doi: 10.1126/science.aaz8432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Kovacs JM, Nkolola JP, Peng H, et al. HIV-1 envelope trimer elicits more potent neutralizing antibody responses than monomeric gp120. Proc Natl Acad Sci U S A 2012;109(30):12111–12116; doi: 10.1073/pnas.1204533109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Heydarchi B, Center RJ, Bebbington J, et al. Trimeric gp120-specific bovine monoclonal antibodies require cysteine and aromatic residues in CDRH3 for high affinity binding to HIV Env. MAbs 2017;9(3):550–566; doi: 10.1080/19420862.2016.1270491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Williams WB, Meyerhoff RR, Edwards RJ, et al. Fab-dimerized glycan-reactive antibodies are a structural category of natural antibodies. Cell 2021;184(11):2955–2972, e25; doi: 10.1016/j.cell.2021.04.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Yu JS, Ma BJ, Scearce RM, et al. Anti-Ebola MAb 17A3 reacts with bovine and human alpha-2-macroglobulin proteins. J Virol Methods 2010;168(1–2):248–250; doi: 10.1016/j.jviromet.2010.04.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Zhu C, Dukhovlinova E, Council O, et al. Rationally designed carbohydrate-occluded epitopes elicit HIV-1 Env-specific antibodies. Nat Commun 2019;10(1):948; doi: 10.1038/s41467-019-08876-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Wagh K, Hahn BH, Korber B. Hitting the sweet spot: Exploiting HIV-1 glycan shield for induction of broadly neutralizing antibodies. Curr Opin HIV AIDS 2020;15(5):267–274; doi: 10.1097/COH.0000000000000639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Schorcht A, Cottrell CA, Pugach P, et al. The glycan hole area of HIV-1 envelope trimers contributes prominently to the induction of autologous neutralization. J Virol 2022;96(1):e0155221; doi: 10.1128/JVI.01552-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Griffith SA, McCoy LE. To bnAb or not to bnAb: Defining broadly neutralising antibodies against HIV-1. Front Immunol 2021;12:708227; doi: 10.3389/fimmu.2021.708227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Haji-Ghassemi O, Blackler RJ, Martin Young N, et al. Antibody recognition of carbohydrate epitopesdagger. Glycobiology 2015;25(9):920–952; doi: 10.1093/glycob/cwv037 [DOI] [PubMed] [Google Scholar]
- 72. Ruiz MJ, Ghiglione Y, Falivene J, et al. Env-specific IgA from viremic HIV-infected subjects compromises antibody-dependent cellular cytotoxicity. J Virol 2016;90(2):670–681; doi: 10.1128/JVI.02363-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Amos JD, Himes JE, Armand L, et al. Rapid development of gp120-focused neutralizing B cell responses during acute simian immunodeficiency virus infection of African green monkeys. J Virol 2015;89(18):9485–9498; doi: 10.1128/JVI.01564-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Yates NL, Stacey AR, Nolen TL, et al. HIV-1 gp41 envelope IgA is frequently elicited after transmission but has an initial short response half-life. Mucosal Immunol 2013;6(4):692–703; doi: 10.1038/mi.2012.107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Seaton KE, Ballweber L, Lan A, et al. HIV-1 specific IgA detected in vaginal secretions of HIV uninfected women participating in a microbicide trial in Southern Africa are primarily directed toward gp120 and gp140 specificities. PLoS One 2014;9(7):e101863; doi: 10.1371/journal.pone.0101863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Granados-Gonzalez V, Claret J, Berlier W, et al. Opposite immune reactivity of serum IgG and secretory IgA to conformational recombinant proteins mimicking V1/V2 domains of three different HIV type 1 subtypes depending on glycosylation. AIDS Res Hum Retroviruses 2008;24(2):289–299; doi: 10.1089/aid.2007.0187 [DOI] [PubMed] [Google Scholar]
- 77. Kroese FG, Butcher EC, Stall AM, et al. Many of the IgA producing plasma cells in murine gut are derived from self-replenishing precursors in the peritoneal cavity. Int Immunol 1989;1(1):75–84; doi: 10.1093/intimm/1.1.75 [DOI] [PubMed] [Google Scholar]
- 78. Budeus B, Kibler A, Brauser M, et al. Human cord blood B cells differ from the adult counterpart by conserved Ig repertoires and accelerated response dynamics. J Immunol 2021;206:2839–2851; doi: 10.4049/jimmunol.2100113 [DOI] [PubMed] [Google Scholar]
- 79. Shikina T, Hiroi T, Iwatani K, et al. IgA class switch occurs in the organized nasopharynx- and gut-associated lymphoid tissue, but not in the diffuse lamina propria of airways and gut. J Immunol 2004;172(10):6259–6264; doi: 10.4049/jimmunol.172.10.6259 [DOI] [PubMed] [Google Scholar]
- 80. Beagley KW, Murray AM, McGhee JR, et al. Peritoneal cavity CD5 (Bla) B cells: Cytokine induced IgA secretion and homing to intestinal lamina propria in SCID mice. Immunol Cell Biol 1995;73(5):425–432; doi: 10.1038/icb.1995.66 [DOI] [PubMed] [Google Scholar]
- 81. Wu X, Kong XP. Antigenic landscape of the HIV-1 envelope and new immunological concepts defined by HIV-1 broadly neutralizing antibodies. Curr Opin Immunol 2016;42:56–64; doi: 10.1016/j.coi.2016.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Karray S, Juompan L, Maroun RC, et al. Structural basis of the gp120 superantigen-binding site on human immunoglobulins. J Immunol 1998;161(12):6681–6688. [PubMed] [Google Scholar]
- 83. Henderson R, Watts BE, Ergin HN, et al. Selection of immunoglobulin elbow region mutations impacts interdomain conformational flexibility in HIV-1 broadly neutralizing antibodies. Nat Commun 2019;10(1):654; doi: 10.1038/s41467-019-08415-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Scheid JF, Mouquet H, Ueberheide B, et al. Sequence and structural convergence of broad and potent HIV antibodies that mimic CD4 binding. Science 2011;333(6049):1633–1637; doi: 10.1126/science.1207227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Zhou JO, Zaidi HA, Ton T, et al. The effects of framework mutations at the variable domain interface on antibody affinity maturation in an HIV-1 broadly neutralizing antibody lineage. Front Immunol 2020;11:1529; doi: 10.3389/fimmu.2020.01529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Chen F, Tzarum N, Wilson IA, et al. VH1-69 antiviral broadly neutralizing antibodies: Genetics, structures, and relevance to rational vaccine design. Curr Opin Virol 2019;34:149–159; doi: 10.1016/j.coviro.2019.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Kraj P, Rao SP, Glas AM, et al. The human heavy chain Ig V region gene repertoire is biased at all stages of B cell ontogeny, including early pre-B cells. J Immunol 1997;158(12):5824–5832. [PubMed] [Google Scholar]
- 88. Pappas L, Foglierini M, Piccoli L, et al. Rapid development of broadly influenza neutralizing antibodies through redundant mutations. Nature 2014;516(7531):418–422; doi: 10.1038/nature13764 [DOI] [PubMed] [Google Scholar]
- 89. Lorin V, Fernandez I, Masse-Ranson G, et al. Epitope convergence of broadly HIV-1 neutralizing IgA and IgG antibody lineages in a viremic controller. J Exp Med 2022;219(3); doi: 10.1084/jem.20212045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Andrabi R, Voss JE, Liang CH, et al. Identification of common features in prototype broadly neutralizing antibodies to HIV envelope V2 apex to facilitate vaccine design. Immunity 2015;43(5):959–973; doi: 10.1016/j.immuni.2015.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Gorman J, Soto C, Yang MM, et al. Structures of HIV-1 Env V1V2 with broadly neutralizing antibodies reveal commonalities that enable vaccine design. Nat Struct Mol Biol 2016;23(1):81–90; doi: 10.1038/nsmb.3144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Xiao X, Chen W, Feng Y, et al. Maturation pathways of cross-reactive HIV-1 neutralizing antibodies. Viruses 2009;1(3):802–817; doi: 10.3390/v1030802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Angeletti D, Gibbs JS, Angel M, et al. Defining B cell immunodominance to viruses. Nat Immunol 2017;18(4):456–463; doi: 10.1038/ni.3680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Li GM, Chiu C, Wrammert J, et al. Pandemic H1N1 influenza vaccine induces a recall response in humans that favors broadly cross-reactive memory B cells. Proc Natl Acad Sci U S A 2012;109(23):9047–9052; doi: 10.1073/pnas.1118979109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Song G, He WT, Callaghan S, et al. Cross-reactive serum and memory B cell responses to spike protein in SARS-CoV-2 and endemic coronavirus infection. bioRxiv 2020; doi: 10.1101/2020.09.22.308965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Ng KW, Faulkner N, Cornish GH, et al. Preexisting and de novo humoral immunity to SARS-CoV-2 in humans. Science 2020;370(6522):1339–1343; doi: 10.1126/science.abe1107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Rogers TF, Goodwin EC, Briney B, et al. Zika virus activates de novo and cross-reactive memory B cell responses in dengue-experienced donors. Sci Immunol 2017;2(14); doi: 10.1126/sciimmunol.aan6809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Chan KR, Wang X, Saron WAA, et al. Cross-reactive antibodies enhance live attenuated virus infection for increased immunogenicity. Nat Microbiol 2016;1(12):16164; doi: 10.1038/nmicrobiol.2016.164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Jeffries TL Jr, Sacha CR, Pollara J, et al. The function and affinity maturation of HIV-1 gp120-specific monoclonal antibodies derived from colostral B cells. Mucosal Immunol 2016;9(2):414–427; doi: 10.1038/mi.2015.70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Han Q, Williams WB, Saunders KO, et al. HIV DNA-adenovirus multiclade envelope vaccine induces gp41 antibody immunodominance in rhesus macaques. J Virol 2017;91(21); doi: 10.1128/JVI.00923-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation. The Ab and repertoire RNA sequences are available from the database of Genotypes and Phenotypes (dbGaP) (https://www.ncbi.nlm.nih.gov/gap/) accession number phs002027.v2.p1.