Abstract
Background:
Exposure to zoonotic diseases is a significant occupational risk in veterinary medicine. In this study, we characterized personal protective equipment use, injury frequency, and Bartonella seroreactivity in Washington State veterinary workers.
Methods:
Using a risk matrix developed to reflect occupational risk factors for exposure to Bartonella and multiple logistic regression, we explored determinants of risk for Bartonella seroreactivity.
Results:
Depending on the titer cutoff used, Bartonella seroreactivity was between 24.0% and 55.2%. No significant predictors of seroreactivity were found, although the relationship between high-risk status and increased seroreactivity for some Bartonella species approached significance. Serology for other zoonotic and vector borne pathogens did not identify consistent cross reactivity with Bartonella antibodies.
Conclusion:
The predictive power of the model was likely limited by the small sample size and high level of exposure to risk factors for most participants. Given the high proportion of veterinarians seroreactive to one or more of the three Bartonella spp. known to infect dogs and cats in the United States, as well as seroreactivity to other zoonoses, and the unclear relationship between occupational risk factors, seroreactivity, and disease expression, more research is needed in this area.
Keywords: Bartonella henselae, Bartonella vinsonii, Bartonella koehlerae, risk matrix, zoonotic disease epidemiology, veterinarian occupational health
Introduction
Veterinary workers regularly face occupational exposure to zoonotic pathogens. While zoonotic infections can be subclinical and self-limiting, they can also be severe or significantly impact quality of life. In a survey of Oregon Veterinary Medicine Association members, nearly half (47%) of respondents reported contracting a zoonotic disease during their career (Jackson and Villarroel, 2012). In a 2012 study of Canadian veterinarians, 17% reported that they were diagnosed with or treated for a zoonotic disease in the past 5 years (Epp and Waldner, 2012). A recent systematic review and meta-analysis of veterinary occupational hazards found a pooled proportion estimate of zoonotic disease infection of 17% among veterinarians with proportions up to 31%, 26%, and 24% for specific infections such as bartonellosis, Q fever, and viral infection, respectively (Adebowale et al., 2021).
Despite these risks, surveys have reported low use of personal protective equipment (PPE) and lack of comprehensive infection prevention and control planning in veterinary workplaces (Attard et al., 2012; Lipton et al., 2008; Murphy et al., 2010). According to a survey of over 2000 veterinarians in the United States, almost all (92–99%) engage in needle recapping; less than one-quarter reported using appropriate PPE when handling patients with dermatologic (17.9%), gastrointestinal (21.4%), respiratory (6.3%), or neurologic symptoms (16.5%); and just over half (55%) always wash their hands before eating or drinking at work (Wright et al., 2008). As a result, veterinary workers remain vulnerable to zoonotic diseases, although the magnitude of the risk remains poorly understood.
Bartonella is a zoonotic pathogen associated with occupational veterinary exposures (Lantos et al., 2014; Oliveira et al., 2010; Oteo et al., 2017). Bartonella is relevant to veterinary workers because of the presence of these bacteria in blood, tissues, and effusions in companion animals and livestock (Bradley et al., 2014; Chomel et al., 2006; Okaro et al., 2017). Bartonella is particularly common in domestic cats—studies have documented 30–40% seroreactivity for Bartonella henselae, the presence of several other Bartonella species, and up to 75% bacteremic prevalence (Álvarez-Fernández et al., 2018; Fabbi et al., 2004). Human Bartonella infection can cause a wide range of illness. B. henselae infections are typically characterized by self-limited, regional lymphadenopathy but can also cause severe disease, particularly in immunocompromised individuals (Mascarelli et al., 2011; Mosepele et al., 2012).
Because of the expanding number of Bartonella species, the spectrum of disease they can cause, and their presence in companion animals and livestock, Bartonella has been proposed as an underrecognized threat, particularly to veterinary workers (Breitschwerdt, 2015). Because bloodborne infection with Bartonella koehlerae and vinsonii based on culture, PCR amplification, and DNA sequencing has been implicated in rheumatic disease manifestations, neurologic symptoms, and musculoskeletal conditions, further research on these species is critical (Breitschwerdt et al., 2010a; Breitschwerdt et al., 2010b; Breitschwerdt et al., 2008; Maggi et al., 2012; Maggi et al., 2011; Mozayeni et al., 2018). We sought to address this gap in the literature by including these species in this study.
Sequential and lifetime studies are needed to correlate the occupational risk of zoonotic exposure to actual health outcomes. Many point-in-time surveys of zoonotic disease in veterinary workers rely on self-reported symptoms, making misclassification of infection likely. In the current study, we measured seroreactivity to B. henselae, B. koehlerae, and B. vinsonii subsp. berkhoffii in a sample of 97 veterinarians at a Washington Veterinary Medical Association conference. To assess for serological cross reactivity, we also tested for exposure to other zoonotic and vector borne pathogens. Using a risk matrix developed from a survey, we investigated occupational risk factors for exposure to Bartonella to explore determinants of risk for Bartonella seroreactivity.
Methods
This study is a cross-sectional convenience survey of veterinarians in the Pacific Northwest. A self-administered survey of veterinary practice characteristics, work practices, exposure to potentially infectious materials, injuries, and health outcomes was completed by 97 veterinary professionals at the 2019 Pacific Northwest Veterinary Conference. Blood samples were collected from each participant and analyzed by immunofluorescent antibody assay (IFA) for antibodies specific to B. henselae, B. koehlerae, B. vinsonii subsp. berkhoffii, Brucella abortus, Coxiella burnetii, Francisella tularensis, Anaplasma phagocytophilum, Ehrlichia chaffeensis, Rickettsia conorii, and Rickettsii typhi. This study was approved by UW human subjects research under STUDY00000042: GAZER.
Laboratory methods
B. vinsonii subsp. berkhoffii, B. henselae, and B. koehlerae antibodies were determined in the Intracellular Pathogens Research Laboratory (IPRL) at North Carolina State University (Raleigh, NC) using cell culture grown bacteria as antigens and following standard immunofluorescent antibody assay (IFA) techniques as previously described. A canine isolate of B. vinsonii subsp. berkhoffii genotype II (NCSU 95CO-08, Winnie), and feline isolates of B. henselae SA2 strain (NCSU 95FO-099, Missy) and B. koehlerae (NCSU 09FO-01, Trillium), were passed from agar plate grown cultures into Bartonella-permissive cell lines, that is, the DH82 (a canine monocytoid) cell line for strains B. henselae SA2 and B. koehlerae and Vero cells (a mammalian fibroblast cell line) for B. vinsonii subsp. berkhoffii genotype II, for IFA testing. For each antigen, heavily infected cell cultures were spotted onto 30-well Teflon-coated slides (Cell-Line/Thermo Scientific), air-dried, acetone-fixed, and stored frozen.
Fluorescein conjugated goat anti-human IgG (Cappel, ICN) was used to detect bacteria within cells using a fluorescent microscope (Carl Zeiss Microscopy, LLC, Thornwood, NY). Serum samples diluted in a phosphate-buffered saline (PBS) solution containing normal goat serum, Tween-20, and powdered nonfat dry milk to block nonspecific antigen-binding sites were first screened at dilutions of 1:16 to 1:64. All sera that were reactive at a reciprocal titer of 64 were further tested with two-fold dilutions to an endpoint titer. To avoid confusion with possible nonspecific binding found at low dilutions, cutoffs of 1:64 and 1:128 were selected as seroreactive titers.
A 1:64 IFA titer was considered the minimal seroreactive titer based upon testing sera of 32 healthy volunteers from a local medical school, in which there was no seroreactivity to B. koehlerae, no seroreactivity to B. vinsonii subsp. berkhoffii genotypes I and III, only one individual was B. henselae seroreactive (at an IFA titer of 1:64), but for reasons that remain unclear, nearly half of these individuals were seroreactive to B. vinsonii subsp. berkhoffii genotype II (Breitschwerdt et al., 2010).
Indirect fluorescent antibody testing to assess cross reactivity to Bartonella spp. antigens was performed using previously frozen, blinded serum samples at Fuller Laboratories, a research testing (FDA Registration No. 3004036192) and Medical Device Manufacturing Laboratory, licensed by the state of California (Fuller Laboratories, Fullerton, CA). All sera were screened by the company's Quality Control Supervisor at a 1:64 dilution in phosphate-buffered saline, incubated for 30 min, then washed, and incubated for 30 min with an affinity purified goat anti-human IgG (Fc-specific) with Alexa Fluor 488 conjugation (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA) for 30 min (all at 37°C). A senior investigator with 30 years of IFA reading experience reassessed seroreactivity at 1:64 and 1:256 endpoint dilution. A 1:64 dilution was considered a positive result for all five genera for assessing cross reactivity to Bartonella species.
Risk matrix
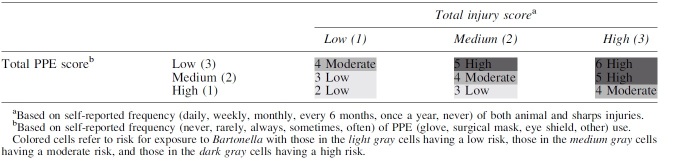
A risk matrix is a common semiquantitative tool in occupational health research and risk management, which considers the probability of exposure to a hazard and the likelihood of an adverse outcome, allowing assignment of individuals or tasks into ordered categories characterizing the risk of an adverse outcome (Arnetz et al., 2014; Jensen et al., 2022; Sieber et al., 1991). To consider the likelihood of exposure to Bartonella, we combined frequency of PPE use across tasks and reported animal-related injury collected on the survey, as these measures would most impact whether Bartonella has a port of entry into an individual.
Frequency use score for PPE was determined by assessing reported glove, surgical mask, eye shield, and other PPE use for nine tasks where exposure to blood, saliva, needle sticks, or animal bites and scratches could occur, on a 0–4 frequency scale (0 = never use, 4 = always use). Scores on tasks were summed to create a total PPE use score with a maximum of 144 points. High PPE use was defined as ≥37 points (average of >4 points per task, e.g., multiple pieces of PPE sometimes or one piece of PPE always), moderate PPE use as 19–36 points (average of >2 and ≤4 points per task, e.g., no more than multiple pieces of PPE rarely or one piece of PPE often), and low PPE use as 0–18 points (average of ≤2 points per task, e.g., no more than two pieces of PPE rarely or one piece of PPE sometimes).
Score for occupational injury was assigned by assessing frequency of animal injury and needle stick injury in the past year on a scale of 0–5 (0 = never, 5 = daily). Scores were summed for a total injury score with a maximum of 10 points. A low injury score was defined as ≤3 points (e.g., no more than monthly for one type of injury or every 6 months for two types), moderate injury score as 4–6 points (e.g., daily for one type of injury or monthly for two types), and high injury score as ≥7 points (e.g., at least weekly for one type of injury and monthly for the other).
To create an overall risk score, PPE use scores were reverse coded, with high use assigned a value of 1, moderate use assigned a value of 2, and low use assigned a value of 3. Low injury was assigned a value of 1, moderate injury a value of 2, and high injury was assigned a value of 3. The two categories (PPE use and injury) were summed to create the overall risk score, with the range of scores being 2–6. Low level of exposure risk was defined as 2–3 total points (high PPE use and low injury), moderate level as 4 total points, and high level as 5–6 points (low PPE use and high injury). The resultant risk matrix and variables are outlined in Appendix Tables A1–A3.
Logistic regression model
We generated a logistic regression model, including total risk, career length, cat ownership, and dog ownership, as possible predictors of overall Bartonella seroreactivity at the 1:128 cutoff [Logit(p) = β0 + β1Xrisklevel + β3Xcareerlength + β4Xpetcat + β4Xpetdog]. The same model was used for the outcomes B. henselae seroreactivity, B. koehlerae seroreactivity, and B. vinsonii subsp. berkhoffii seroreactivity. A chi-squared test was used to assess the relationship between Bartonella seroreactivity and general health rating.
Results
Participant characteristics
Most of the 97 participants were veterinarians, although veterinary technicians, practice managers, and students were also included (Table 1). The sample was mostly female and of white race/ethnicity. Categories for jobs and race were not mutually exclusive. Age ranged from 23 to 71 years (mean age = 48.2). The mean career length for participants was 22.3 years (Table 2). As reported in Tables 1 and 2, exposure to cats and dogs was high for the majority of the sample. Scratches were the most common occupational injury. Cats were the most common animal source of injury.
Table 1.
Demographic Characteristics
| Demographic characteristics | n (%) | Mean | SD |
|---|---|---|---|
| Age | 46.6 | 13.1 | |
| Gender | |||
| Female | 82 (85.4) | ||
| Male | 12 (12.5) | ||
| Other | 2 (2.1) | ||
| Race/ethnicitya | |||
| White | 82 (84.4) | ||
| Black | 0 | ||
| Hispanic | 2 (2.1) | ||
| Asian | 3 (3.1) | ||
| American Indian/Alaska Native | 0 | ||
| Native Hawaiian/Pacific Islander | 1 (1.0) | ||
| Other | 1 (1.0) | ||
| Pet ownershipa | |||
| Cat | 71 (74.0) | ||
| Dog | 72 (75) | ||
Participants could check more than one category; some participants did not answer.
SD, standard deviation.
Table 2.
Occupational Characteristics
| Occupational characteristics | n (%) | Mean | SD |
|---|---|---|---|
| Length of career (years) | 22.3 | 12.6 | |
| Job titlea | |||
| Owner | 27 (28.1) | ||
| Veterinarian | 76 (79.2) | ||
| Veterinary technician | 9 (9.4) | ||
| Veterinary assistant | 0 | ||
| Veterinary student | 1 (1.0) | ||
| Practice manager | 3 (3.1) | ||
| Otherb | 6 (6.3) | ||
| Practice typea | |||
| Small animal | 81 (84.4) | ||
| Large animal | 3 (3.1) | ||
| Mixed practice | 13 (13.5) | ||
| Otherb | 11 (11.5) | ||
| Occupational cat/dog exposurea | |||
| Cat | 94 (97.9) | ||
| Dog | 92 (95.8) | ||
| Most common injury typea | |||
| Bite | 8 (8.3) | ||
| Scratch | 70 (72.9) | ||
| Kick | 5 (5.2) | ||
| Needle stick | 16 (16.7) | ||
| Other | 6 (6.3) | ||
| Most common animal injury sourcea | |||
| Cat | 50 (52.1) | ||
| Dog | 29 (30.2) | ||
| Other | 16 (16.7) | ||
Participants could check more than one category.
Other jobs included technical services vet, state/army vet, department chair/instructor, public health vet; other practice types included exotic animals, zoos.
Most participants (93.8%) reported being in excellent or good health (Table 3). However, many participants also reported being diagnosed with a condition such as allergies, arthritis, or a chronic musculoskeletal disorder (Table 3). Only one participant reported diagnosis with bartonellosis.
Table 3.
Reported Health Status
| Self-reported health | n (%) |
|---|---|
| Health rating | |
| Excellent | 35 (36.5) |
| Good | 55 (57.3) |
| Fair | 6 (6.3) |
| Poor | 0 |
| Diagnosesa | |
| Allergies | 42 (43.8) |
| Chronic Musculoskeletal Disorder | 34 (35.4) |
| Arthritis | 27 (28.1) |
| Zoonotic infectionb | 18 (18.8) |
| Asthma | 16 (16.7) |
| Dermatitis | 12 (12.5) |
| Immunocompromising Disorder | 6 (6.3) |
| Other | 10 (10.4) |
Participants could check more than one category.
Zoonotic infections included ringworm, cryptosporidiosis, salmonellosis, psittacosis, roundworms, and bartonellosis.
Bartonella seroreactivity
At the 1:64 titer cutoff, 54.2% (53) of participants were seroreactive to at least one Bartonella species 32.3% (31) for B. henselae, 36.5% (35) for B. koehlerae, and 23.7% (23) for B. vinsonii subsp. berkhoffii. At the 1:128 cutoff, 24.0% of participants were seroreactive to at least one Bartonella species (11.5% for B. henselae, 15.6% for B. koehlerae, and 8.3% for B. vinsonii subsp. berkhoffii). Twenty-six (27.1%), 17 (17.7%), and 10 (10.4%) participants were reactive to 1, 2, or 3 Bartonella species, respectively. Forty-four (46.0%) participants were not seroreactive to any of the three Bartonella species at 1:16 and 1:32 screening dilutions.
Assessment of Bartonella cross-reactivity
Table 4 compares Bartonella spp. seropositivity to seropositivity to other zoonotic bacteria. For all but one pathogenic organism, there was no statistical association with Bartonella seropositivity. However, there was a statistically significant correlation between F. tularensis antibodies and Bartonella antibodies, but this was based on a low total number of F. tularensis-positive samples (N = 19/97). Of the 15 study participants with titers ranging from 1:256 to 1:1024 to 1 or more of the Bartonella spp. antigens, 7 were not seroreactive to any of the 6 other genera tested. Of the remaining eight of these Bartonella spp. seroreactors, three had antibodies only to A. phagocytophilum and two only to F. tularensis. Seroreactivity to individual and combinations of Bartonella spp. antigens is summarized in Table 5. The highest geometric mean titers were to B. henselae and B. koehlerae, respectively.
Table 4.
Serology Results for 97 Veterinary Workers Tested for Bartonella spp. Antibodies and for Antibodies to Other Zoonotic or Vector-Borne Infectious Diseases
| Total (≥1:64) seroreactive | Bartonella seroreactive (≥1:64) | Bartonella nonseroreactive (≤1:32) | p a | |
|---|---|---|---|---|
| Brucella abortus | 1 | 1 | 0 | 0.360 |
| Coxiella burnetii Phase I | 3 | 1 | 2 | 0.451 |
| Coxiella burnetii Phase II | 9 | 4 | 5 | 0.519 |
| Francisella tularensis | 19 | 15 | 4 | 0.018 |
| Anaplasma phagocytophilum | 22 | 16 | 6 | 0.053 |
| Ehrlichia chaffeensis | 9 | 5 | 4 | 0.954 |
| Rickettsia rickettsii | 7 | 5 | 2 | 0.354 |
| Rickettsia typhi | 4 | 3 | 1 | 0.849 |
Significant values are in bold.
Association evaluated by chi-squared test.
Table 5.
Total, Individual, and Combination Reactivity at the 1:64 Cutoff to Bartonella spp. Antigens
| Reactivity to antigen total (%) | GMT | Range | Reactivity to antigen only (%) | |
|---|---|---|---|---|
| Any Bartonella antigen | 53 (54.6) | — | — | — |
| Bartonella henselae | 14 (14.4) | 196.6 | 64–1024 | 6 (6.2) |
| Bartonella koehlerae | 9 (9.3) | 92.4 | 64–256 | 7 (7.2) |
| Bartonella vinsonii berkhoffii | 3 (3.1) | 64 | 64 | 0 (0) |
| Bh and Bk | 6 (6.2) | — | — | — |
| Bh and Bvb | 1 (1.0) | — | — | — |
| Bk and Bvb | 10 (10.3) | — | — | — |
| Bh, Bk, and Bvb | 10 (10.3) | — | — | — |
GMT, geometric mean titer.
Correlation of Bartonella seroreactivity and risk factors
No significant predictors of seroreactivity were found using the initial logistic regression model, likely because of the small sample size, lack of variability in risk factors, and limited sensitivity of Bartonella IFA (seronegative infection). In a model comparing high risk to low and moderate risk instead of all risk categories to each other, values approaching or achieving significance were found for the relationship between high risk and general Bartonella seroreactivity, as well as B. koehlerae seroreactivity (Table 6). A significant inverse relationship between career length and general Bartonella seroreactivity was observed in this high-risk model (odds ratio [OR] = 0.51 [0.27–0.98], p = 0.043). No significant relationship between Bartonella seroreactivity and general health rating was found.
Table 6.
Association Between High Total Risk Status and Bartonella Status
| Species | OR | 95% CI | p |
|---|---|---|---|
| All Bartonella | 2.95 | 0.99–8.79 | 0.052 |
| Bartonella henselae | 2.03 | 0.49–8.32 | 0.33 |
| Bartonella koehlerae | 3.35 | 1.01–11.18 | 0.049 |
| Bartonella vinsonii subsp. berkhoffii | 2.00 | 0.41–9.84 | 0.39 |
Significant values are in bold.
CI, confidence interval; OR, odds ratio.
Discussion
This study emphasizes that Bartonella exposure, as assessed by IFA testing of incompletely defined specificity, is likely frequent. Because few serosurveys of Bartonella in veterinary workers have been conducted, it is difficult to compare our findings to existing literature. However, our seroreactivity findings are comparable to a 2017 serosurvey of Bartonella in veterinary workers by Oteo et al. This serosurvey, like ours, was conducted at a conference of veterinary medicine professionals. At the 1:64 cutoff point, we found that 32.7% of participants were seroreactive to B. henselae and 36.5% were seroreactive to B. koehlerae, similar to Oteo's findings of 37.1% and 41.6%. Our results differed for B. vinsonii subsp. berkhoffii.
We found that 24.0% of participants were seroreactive to B. vinsonii subsp. berkhoffii, while Oteo found a higher percentage of 56.2. This may be due to Oteo's use of B. vinsonii subsp. berkhoffii genotype III, a genotype found more frequently in Europe, versus our use of B. vinsonii subsp. berkhoffii genotype II, the most prevalent subsp. in dogs and humans in the United States. At the 1:128 cutoff, we found similar seroreactivity to B. henselae (11.5% vs. 10.1%), higher seroreactivity to B. koehlerae (15.6% vs. 10.1%), and lower seroreactivity to B. vinsonii subsp. berkhoffii (8.3% vs. 12.4%), suggesting that B. koehlerae exposure may occur more frequently in the northwestern United States (Osikowicz et al., 2021).
In another study of veterinary workers, 42 of 97 (44%) veterinary personnel had detectable Bartonella antibodies with an IFA titer of 1:64 or greater to at least one Bartonella species antigen (Lantos et al., 2014). While seroprevalence was not reported by species, detection of Bartonella DNA using enrichment culture and molecular amplification showed that 56% of participants with detectable species Bartonella DNA were infected with B. henselae, 26% with B. vinsonii, and 22% with B. koehlerae, suggesting that exposure to Bartonella species varies by region.
Published findings indicate that seroreactivity to Bartonella may be higher in veterinarians than the general population, although seroreactivity can only be directly compared for B. henselae in most studies, due to the lack of human serosurveys of B. koehlerae and B. vinsonii subsp. berkhoffii. A serosurvey of healthy adults in Korea found a B. henselae prevalence of 15.0% (1:64 cutoff) and a serosurvey of children in Jordan found a prevalence of 11% (1:64 cutoff) (Al-Majali and Al-Qudah, 2004; Kwon et al., 2017). However, a serosurvey of healthy students in Germany found that 30% (1:64 cutoff) were seroreactive to B. henselae, similar to the 32.3% prevalence in our sample (Sander et al., 1998).
Further research is necessary for more definitive estimates of seroprevalence, particularly for different regions and study populations before a confident comparison can be made. However, seroprevalence appears to be highest in populations with extensive animal and arthropod exposures (Breitschwerdt et al., 2007).
Importantly, in our study, 27.8% of the individuals tested were seroreactive to only one Bartonella sp. antigen when assessed at the 1:128 titer, and 17.7% were only reactive to two of the IFA antigens. When dogs infected with B. koehlerae were experimentally infected with B. henselae or B. vinsonii subsp. berkhoffii, seroreactivity was only detected to B. koehlerae and the other infecting Bartonella sp. (Balakrishnan et al., 2013). Although it was not unusual for study participants to have had seroreactivity to one, two, or all three of the IFA antigens, we acknowledge that IFA cannot definitively determine the infecting species or if the individual has been exposed to more than one species, as a result of repeat exposures. Coinfection with more than one Bartonalla spp. has been documented in veterinary workers based upon PCR amplification and DNA sequence confirmation (Maggi et al., 2011).
Overall, relationships between hypothesized risk factors and seroreactivity were not significant in our original logistic regression model. However, there was a trend toward increasing seroreactivity with increasing risk level. This trend was more visible when comparing high exposure risk participants to all other participants and significant or near-significant relationships were found between high exposure risk level and general Bartonella seroreactivity (OR = 2.95, p = 0.052) as well as B. koehlerae seroreactivity (OR = 3.35, p < 0.05). Cat and dog ownership were not significant predictors of seroreactivity. Because veterinary workers have regular exposure to cats and dogs in both their training and career, this is not unexpected. Exposure to a small number of cats or dogs at home would not likely affect most veterinary workers' overall exposure.
The extent to which there is serological cross-reactivity between Bartonella and other known pathogens to which veterinary workers are exposed remains unclear. Interestingly, as the genus Brucella is phylogenetically most closely related to the genus Bartonella and therefore would most likely share immunogenic cross-reactive antigens, only one study participant was seroreative to B. abortus antigens. In contrast, Francisella, which is a distantly related genus, showed a statistical association with Bartonella spp. antibodies. Using a microagglutination assay and a 1:128 cutoff value, Telford and colleagues found landscapers working on Martha's Vineyard to be at occupational risk of Francisella exposure (Feldman et al., 2003). To our knowledge, there are no studies that specifically address Francisella spp. exposure risks in veterinary workers, but it is a recognized hazard in this occupational group, and our findings suggest the need for further exploration of this occupational risk.
Despite the statistical association, the low number of F. tularensis-positive samples means that cross-reactivity to this pathogen would not explain the high rate of Bartonella seropositivity in our study. Also, the lack of cross-reactivity in six sera with the highest Bartonella antibody titers obtained in this study to all other tested genera provides additional support for the specificity of Bartonella spp. IFA, when testing human sera.
Limitations to the study include the small sample size and homogeneity of risk factors in the sample, which likely limited our ability to identify significant risk factors for Bartonella seroreactivity. Using a more heterogenous sample and including practice type or frequency of occupational exposure to cats and dogs in future analyses could help clarify these relationships. Similarly, a larger sample size would allow us the degrees of freedom to investigate individual risk factors in a multiple logistic regression model instead of combining individual risk factors into a risk matrix, which could help to identify specific work practices that may relate to Bartonella seroreactivity.
This study supports previous studies that document veterinary occupational exposure to Bartonella species, particularly species with pets as reservoir hosts. As an emerging infectious disease, bartonellosis should be further explored and monitored in this population. While reducing illness from Bartonella is worthwhile in itself, Bartonella can also be used as an indicator of risk posed by other emerging infectious diseases and to evaluate the effectiveness of PPE use and injury prevention against blood- and saliva-borne zoonotic infections in general. In human medicine, research has shown that some of the main impediments to PPE are usability, availability, and clear communication, and that addressing these issues in hospital-based interventions can be effective in increasing compliance (Williams et al., 2019). Veterinary medicine faces similar impediments and interventions similar to those effective in human medicine could be considered for future implementation and evaluation (Robin et al., 2017).
Future research that includes multiple Bartonella species, uses serology in addition to self-report or PCR testing, and compares veterinary workers with a wide range of work practices and animal exposures will hopefully clarify the role of Bartonella in human and animal health, inform interventions that improve the safety of veterinary workplaces, and ultimately decrease the burden of Bartonella in this population.
Acknowledgments
The content is solely the responsibility of the authors and does not necessarily represent the official views of National Institute for Occupational Safety and Health (NIOSH). Serological testing was conducted by Julie Bradley, and Chance Liedig provided technical assistance (IPRL, Center for Comparative Medicine and Translational Research, College of Veterinary Medicine, North Carolina State University).
Appendix
Appendix Table A1.
Development of the Risk Matrix: Variables Combined into the Total Personal Protective Equipment Score
| Task | Glove use | Surgical mask use | Eye shield use | Other PPE use | PPE score |
|---|---|---|---|---|---|
| Cystocentesis | 0 = Never 1 = Rarely 2 = Sometimes 3 = Often 4 = Always |
0–4 | 0–4 | 0–4 | 0–16 |
| Drawing blood | |||||
| Prepping blood work | |||||
| Restraining patient | |||||
| Placing/removing IV | |||||
| Setting up/examining ear or skin cytology | |||||
| Cleaning surgical suites/instruments | |||||
| Performing dentistry | |||||
| Monitoring anesthetized patients | |||||
| Total PPE score | 0–144 |
PPE, personal protective equipment.
Appendix Table A2.
Variables Combined into the Total Injury Score
| Injury type | Daily | Weekly | Monthly | Every 6 months | Once a year | Never | Injury score |
|---|---|---|---|---|---|---|---|
| Animal injury | 5 | 4 | 3 | 2 | 1 | 0 | 0–5 |
| Sharps injury | |||||||
| Total injury score | 0–10 |
Appendix Table A3.
Risk Matrix Combining Frequency of Personal Protective Equipment Use and Injury to Determine Likelihood of Exposure to Bartonella Due to Job Practices
|
Authors' Contributions
N.T.: conceptualization, methodology, software, formal analysis, data curation, and writing—original draft. E.B.B.: resources, methodology, and writing—review and editing. L.F.: resources, testing, and review. M.B.: methodology and writing—review and editing. B.L.: methodology and writing—review and editing. P.R.: conceptualization, methodology, writing—review and editing, and supervision.
Author Disclosure Statement
The authors have no conflicts of interest to disclose.
Funding Information
Research reported in this thesis was supported by the National Institute for Occupational Safety and Health (NIOSH) under Federal Training Grant T42OH008433.
References
- Adebowale O, Fasanmi O, Awosile B, et al. . Systematic review and meta-analysis of veterinary-related occupational exposures to hazards. Open Vet Sci 2021;2:6–22; doi: 10.1515/ovs-2020-0104 [DOI] [Google Scholar]
- Al-Majali AM, Al-Qudah KM. Seroprevalence of Bartonella henselae and Bartonella quintana infections in children from Central and Northern Jordan. Saudi Med J 2004;25(11):1664–1669. [PubMed] [Google Scholar]
- Álvarez-Fernández A, Breitschwerdt EB, Solano-Gallego L. Bartonella infections in cats and dogs including zoonotic aspects. Parasites Vectors 2018;11(1):624; doi: 10.1186/s13071-018-3152-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnetz JE, Hamblin L, Ager J, et al. . Application and implementation of the hazard risk matrix to identify hospital workplaces at risk for violence. Am J Indus Med 2014;57(11):1276–1284; doi: 10.1002/ajim.22371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attard K, Burrows E, Kotiranta-Harris K, et al. . Veterinary infection control in Australia: Is there control? Aust Vet J 2012;90(11):438–441; doi: 10.1111/j.1751-0813.2012.00971.x [DOI] [PubMed] [Google Scholar]
- Balakrishnan N, Cherry NA, Linder KE, et al. . Experimental infection of dogs with Bartonella henselae and Bartonella vinsonii subsp. berkhoffii. Vet Immunol Immunopathol 2013;156(1):153–158; doi: 10.1016/j.vetimm.2013.09.007 [DOI] [PubMed] [Google Scholar]
- Bradley JM, Mascarelli PE, Trull CL, et al. . Bartonella henselae infections in an owner and two papillon dogs exposed to tropical rat mites (Ornithonyssus bacoti). Vector Borne Zoonotic Dis 2014;14(10):703–709; doi: 10.1089/vbz.2013.1492 [DOI] [PubMed] [Google Scholar]
- Breitschwerdt EB. Did Bartonella henselae contribute to the deaths of two veterinarians? Parasites Vectors 2015;8(1):317; doi: 10.1186/s13071-015-0920-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitschwerdt EB, Maggi RG, Duncan AW, et al. . Bartonella species in blood of immunocompetent persons with animal and arthropod contact. Emerg Infect Dis 2007;13(6):938–941; doi: 10.3201/eid1306.061337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitschwerdt EB, Maggi RG, Lantos PM, et al. . Bartonella vinsonii subsp. berkhoffii and Bartonella henselae bacteremia in a father and daughter with neurological disease. Parasites Vectors 2010a;3(1):29; doi: 10.1186/1756-3305-3-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitschwerdt EB, Maggi RG, Nicholson WL, et al. . Bartonella sp. bacteremia in patients with neurological and neurocognitive dysfunction. J Clin Microbiol 2008;46(9):2856–2861; doi: 10.1128/JCM.00832-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitschwerdt EB, Maggi RG, Robert Mozayeni B, et al. . PCR amplification of Bartonella koehlerae from human blood and enrichment blood cultures. Parasites Vectors 2010b;3(1):76; doi: 10.1186/1756-3305-3-76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomel BB, Boulouis H-J, Maruyama S, et al. . Bartonella spp. in pets and effect on human health. Emerg Infect Dis 2006;12(3):389–394; doi: 10.3201/eid1203.050931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epp T, Waldner C. Occupational health hazards in veterinary medicine: Zoonoses and other biological hazards. Can Vet J 2012;53(2):144–150. [PMC free article] [PubMed] [Google Scholar]
- Fabbi M, De Giuli L, Tranquillo M, et al. . Prevalence of Bartonella henselae in Italian stray cats: Evaluation of serology to assess the risk of transmission of bartonella to humans. J Clin Microbiol 2004;42(1):264–268; doi: 10.1128/jcm.42.1.264-268.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman KA, Stiles-Enos D, Julian K, et al. . Tularemia on martha's vineyard: seroprevalence and occupational risk. Emerg Infect Dis 2003;9(3):350–354; doi: 10.3201/eid0903.020462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson J, Villarroel A. A survey of the risk of zoonoses for veterinarians. Zoonoses Public Health 2012;59(3):193–201; doi: 10.1111/j.1863-2378.2011.01432.x [DOI] [PubMed] [Google Scholar]
- Jensen RC, Bird RL, Nichols BW. Risk assessment matrices for workplace hazards: Design for usability. Int J Environ Res Public Health 2022;19(5):2763; doi: 10.3390/ijerph19052763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon HY, Im JH, Lee SM, et al. . The seroprevalence of Bartonella henselae in healthy adults in Korea. Korean J Intern Med 2017;32(3):530–535; doi: 10.3904/kjim.2016.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lantos PM, Maggi RG, Ferguson B, et al. . Detection of Bartonella species in the blood of veterinarians and veterinary technicians: A newly recognized occupational hazard? Vector Borne Zoonotic Dis 2014;14(8):563–570; doi: 10.1089/vbz.2013.1512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton BA, Hopkins SG, Koehler JE, et al. . A survey of veterinarian involvement in zoonotic disease prevention practices. J Am Vet Med Assoc 2008;233(8):1242–1249; doi: 10.2460/javma.233.8.1242 [DOI] [PubMed] [Google Scholar]
- Maggi RG, Mascarelli PE, Pultorak EL, et al. . Bartonella spp. bacteremia in high-risk immunocompetent patients. Diagn Microbiol Infect Dis 2011;71(4):430–437; doi: 10.1016/j.diagmicrobio.2011.09.001 [DOI] [PubMed] [Google Scholar]
- Maggi RG, Mozayeni BR, Pultorak EL, et al. . Bartonella spp. bacteremia and rheumatic symptoms in patients from Lyme disease–endemic region. Emerg Infect Dis 2012;18(5):783–791; doi: 10.3201/eid1805.111366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascarelli PE, Iredell JR, Maggi RG, et al. . Bartonella species bacteremia in two patients with epithelioid hemangioendothelioma. J Clin Microbiol 2011;49(11):4006–4012; doi: 10.1128/JCM.05527-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosepele M, Mazo D, Cohn J. Bartonella infection in immunocompromised hosts: Immunology of vascular infection and vasoproliferation. Clin Dev Immunol 2012;2012:612809; doi: 10.1155/2012/612809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozayeni BR, Maggi RG, Bradley JM, et al. . Rheumatological presentation of Bartonella koehlerae and Bartonella henselae bacteremias. Medicine (Baltimore) 2018;97(17):e0465; doi: 10.1097/MD.0000000000010465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy CP, Reid-Smith RJ, Weese JS, et al. . Evaluation of specific infection control practices used by companion animal veterinarians in community veterinary practices in Southern Ontario. Zoonoses Public Health 2010;57(6):429–438; doi: 10.1111/j.1863-2378.2009.01244.x [DOI] [PubMed] [Google Scholar]
- Okaro U, Addisu A, Casanas B, et al. . Bartonella species, an emerging cause of blood-culture-negative endocarditis. Clin Microbiol Rev 2017;30(3):709–746; doi: 10.1128/CMR.00013-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira AM, Maggi RG, Woods CW, et al. . Suspected needle stick transmission of Bartonella vinsonii subspecies berkhoffii to a veterinarian. J Vet Intern Med 2010;24(5):1229–1232; doi: 10.1111/j.1939-1676.2010.0563.x [DOI] [PubMed] [Google Scholar]
- Osikowicz LM, Horiuchi K, Goodrich I, et al. . Exposure of domestic cats to three zoonotic Bartonella species in the United States. Pathogens 2021;10(3):354; doi: 10.3390/pathogens10030354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oteo JA, Maggi R, Portillo A, et al. . Prevalence of Bartonella spp. by culture, PCR and serology, in veterinary personnel from Spain. Parasites Vectors 2017;10(1):1–9; doi: 10.1186/s13071-017-2483-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robin C, Bettridge J, McMaster F. Zoonotic disease risk perceptions in the British veterinary profession. Prev Vet Med 2017;136:39–48; doi: 10.1016/j.prevetmed.2016.11.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sander A, Posselt M, Oberle K, et al. . Seroprevalence of antibodies to Bartonella henselae in patients with cat scratch disease and in healthy controls: Evaluation and comparison of two commercial serological tests. Clin Diagn Lab Immunol 1998;5(4):486–490; doi: 10.1128/CDLI.5.4.486-490.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieber WK Jr., Sundin DS, Frazier TM, et al. . Development, use, and availability of a job exposure matrix based on national occupational hazard survey data. Am J Indus Med 1991;20(2):163–174; doi: 10.1002/ajim.4700200204 [DOI] [PubMed] [Google Scholar]
- Williams VR, Leis JA, Trbovich P, et al. . Improving healthcare worker adherence to the use of transmission-based precautions through application of human factors design: A prospective multi-centre study. J Hosp Infect 2019;103(1):101–105; doi: 10.1016/j.jhin.2019.03.014 [DOI] [PubMed] [Google Scholar]
- Wright JG, Jung S, Holman RC, et al. . Infection control practices and zoonotic disease risks among veterinarians in the United States. J Am Vet Med Assoc 2008;232(12):1863–1872; doi: 10.2460/javma.232.12.1863 [DOI] [PubMed] [Google Scholar]


