Abstract
BACKGROUND
Pneumonia and bloodstream infections (BSI) due to extensively drug-resistant (XDR) Acinetobacter baumannii, XDR Pseudomonas aeruginosa, and carbapenem-resistant Enterobacterales (CRE) are associated with high mortality rates, and therapeutic options remain limited. This trial assessed whether combination therapy with colistin and meropenem was superior to colistin monotherapy for the treatment of these infections.
METHODS
The OVERCOME (Colistin Monotherapy versus Combination Therapy) trial was an international, randomized, double-blind, placebo-controlled trial. We randomly assigned participants to receive colistin (5 mg/kg once followed by 1.67 mg/kg every 8 hours) in combination with either meropenem (1000 mg every 8 hours) or matching placebo for the treatment of pneumonia and/or BSI caused by XDR A. baumannii, XDR P. aeruginosa, or CRE. The primary outcome was 28-day mortality, and secondary outcomes included clinical failure and microbiologic cure.
RESULTS
Between 2012 and 2020, a total of 464 participants were randomly assigned to treatment, and 423 eligible patients comprised the modified intention-to-treat population. A. baumannii was the predominant trial pathogen (78%) and pneumonia the most common index infection (70%). Most patients were in the intensive care unit at the time of enrollment (69%). There was no difference in mortality (43 vs. 37%; P=0.17), clinical failure (65 vs. 58%; difference, 6.8 percentage points; 95% confidence interval [CI], −3.1 to 16.6), microbiologic cure (65 vs. 60%; difference, 4.8 percentage points; 95% CI, −5.6 to 15.2), or adverse events (acute kidney injury, 52 vs. 49% [P=0.55]; hypersensitivity reaction, 1 vs. 3% [P=0.22]; and neurotoxicity, 5 vs. 2% [P=0.29]) between patients receiving monotherapy and combination therapy, respectively.
CONCLUSIONS
Combination therapy with colistin and meropenem was not superior to colistin monotherapy for the treatment of pneumonia or BSI caused by these pathogens. (Funded by the National Institute of Allergy and Infectious Diseases, Division of Microbiology and Infectious Diseases protocol 10-0065; ClinicalTrials.gov number, NCT01597973.)
Introduction
Pneumonia and bloodstream infections (BSI) due to extensively drug-resistant (XDR) Acinetobacter baumannii, XDR Pseudomonas aeruginosa, and carbapenem-resistant Enterobacterales (CRE) are associated with devastating outcomes.1-4 The U.S. Centers for Disease Control and Prevention recognizes these pathogens as serious or urgent threats to human health,5 and the World Health Organization highlights that they are of the highest priority for research and development of new therapeutic agents, given the paucity of treatment options.6
Despite the renaissance of novel agents in the past decade for the treatment of carbapenem-resistant gram-negative pathogens, unmet needs remain. Novel agents are not universally available and not active against all carbapenem-resistant pathogens, and, following their adoption in clinical practice, resistance to these agents has proliferated.7 Colistin therefore remains an essential and frequently used agent for the management of infections due to XDR gramnegative bacilli in the United States8 and worldwide.
Concerns exist regarding colistin’s safety and efficacy as monotherapy.9 Due to colistin’s potent in vitro synergy when combined with carbapenems, many experts recommend that colistin be combined with a carbapenem for the treatment of infections caused by carbapenem-resistant gram-negative bacilli, despite the presence of in vitro carbapenem resistance and a lack of robust clinical evidence supporting this strategy. Importantly, there are significant risks associated with widespread carbapenem use, including further resistance development and an increase in adverse events, including Clostridioides difficile–associated disease.10 The OVERCOME (Colistin Monotherapy versus Combination Therapy) trial, a randomized, double-blind, placebo-controlled trial assessing outcomes in carbapenem-resistant gram-negative bacilli, was designed to assess whether combination therapy with colistin and a carbapenem is superior to colistin monotherapy.
Methods
TRIAL OVERVIEW
The OVERCOME trial was a randomized, double-blind, placebo-controlled trial conducted at hospitals in the United States, Thailand, Taiwan, Israel, Greece, Italy, and Bulgaria from October 2012 to August 2020 (ClinicalTrials.gov number, NCT01597973). The institutional review board at each site approved the protocol, and all participants, or their authorized representatives, provided written informed consent. The trial protocol, provided with the full text of this article at evidence.nejm.org, was designed by the principal investigator (K.S.K.) and approved by the National Institutes of Health and the U.S. Food and Drug Administration. The trial was performed in accordance with the principles of the Declaration of Helsinki.11 The data were obtained by the site investigators and coordinators (provided in the Supplementary Appendix, available with the full text of this article at evidence.nejm.org), and analysis was performed by the statistical team (G.W.D., L.S., and K.L.) in collaboration with the first and last authors on the publication (K.S.K. and J.M.P.). The authors assume responsibility for the accuracy of the data and analyses and the trial results presented. The initial draft of the manuscript was prepared by J.M.P. and K.S.K. and reviewed and edited by all coauthors, and all authors agreed to publish the manuscript.
PATIENTS AND TRIAL PROCEDURES
Patients were eligible for enrollment if they were 18 years of age or older and had pneumonia and/or BSI caused by XDR A. baumannii, XDR P. aeruginosa, or CRE, with in vitro susceptibility to colistin (colistin minimum inhibitory concentration [MIC] ≤2 mg/l)12 (Table 1). Patients were ineligible if they received 72 hours or more of polymyxin treatment within 96 hours of enrollment or if they had a life expectancy of 24 hours or less. The trial protocol lists the full inclusion and exclusion criteria (Section 5.2), as well as detailed definitions of trial pathogens (Section 5.2.1), trial infections (BSI and pneumonia, Section 5.2.3), and microbiologic methods (Section 7.2.3).
Table 1.
Study Definitions.*
| Term | Definition |
|---|---|
| XDR Acinetobacter baumannii | A. baumannii resistant to all but one or two classes of antibiotics. For the purposes of this study, XDR A. baumannii was defined as an A. baumannii not susceptible to one or more group 2 carbapenems and ampicillin/sulbactam, in addition to representatives of ≥3 classes of antimicrobial agents (including broad-spectrum penicillins, third/fourth-generation cephalosporins, aminoglycosides, fluoroquinolones, trimethoprim-sulfamethoxazole, minocycline, and tigecycline) |
| XDR Pseudomonas aeruginosa | P. aeruginosa that exhibits in vitro nonsusceptibility to all tested carbapenems and to all other traditional antipseudomonal β-lactams tested (e.g., cefepime, ceftazidime, piperacillin/tazobactam) |
| Carbapenem-resistant Enterobacterales |
Escherichia coli, Klebsiella spp., or Enterobacter spp., which were either
|
| Pneumonia | A patient with a positive culture with a study pathogen from a respiratory specimen (e.g., sputum, bronchoalveolar lavage, pleural effusion) at the time of study enrollment and either
|
| Bloodstream infection |
|
Detailed definitions of trial pathogens and infection types are provided in the trial protocol (Sections 5.2.1 and 5.2.3, respectively) available at evidence.nejm.org. MIC denotes minimum inhibitory concentration; and XDR, extensively drug-resistant.
Patients were enrolled in the trial based on carbapenem and colistin susceptibility test results performed at the participating site. Confirmatory susceptibility testing by broth microdilution (BMD) for all available isolates was performed either at trial sites or the central laboratory (details are provided in the trial protocol, Sections 7.2.1 and 7.2.2). If isolates were found to be colistin susceptible at the trial site by a method other than BMD and the patient received on-trial treatment but the index pathogen was subsequently found to be colistin resistant by BMD at the central laboratory, the patient was removed from the modified intention-to-treat (mITT) analysis (described in the following section) but was still assessed in the all-treated population.
RANDOMIZATION, TRIAL TREATMENTS, AND POPULATIONS
All participants received intravenous colistin (loading dose of 5 mg/kg followed by 1.67 mg/kg every 8 hours). Participants were randomly assigned to receive an intravenous carbapenem or a matching normal saline placebo. Imipenem (500 mg every 6 hours as a 30-minute infusion) was used initially, as the National Institutes of Health requirements allowed only for the use of generic medications, and four patients were randomly assigned to this treatment. Once meropenem became generically available, the protocol was amended, and in November 2013, imipenem was switched to meropenem (1000 mg every 8 hours as a 30-minute infusion) because of meropenem’s ease of administration and improved safety profile. Trial medications were renally dose adjusted by trial personnel as detailed in the trial protocol (Sections 6.2 and 6.3). The duration of trial drug therapy was 7 to 14 days and determined by the treating physician. If it was determined that treatment was needed beyond 14 days, therapy was continued off-trial at the discretion of the treating physician, and the participant was categorized as a clinical failure.
Eight separate balanced block randomization sequences were used for each participating hospital. Strata were defined according to infection type, organism confirmation status (confirmed or presumptive), and, for patients with pneumonia only, severity of illness (measured by using the Acute Physiology and Chronic Health Evaluation II [APACHE II] score,13 with patients stratified according to scores <25 vs. ≥25). APACHE II has a range of scores from 0 to 71, and an APACHE II score of 25 is associated with a predicted in-hospital mortality of 55%. No additional stratification was made for the pneumonia group, but patients with BSI were further stratified according to primary (the BSI is not secondary to other sources) versus secondary (the BSI is secondary to infection at a distal site) bacteremia.14 Varying block sizes were used within each stratum. The eight randomization sequences for each site were generated by using nQuery Advisor version 4.0, GraphPad Software. A binder with the eight sequences was used by each hospital’s unblinded research pharmacist.
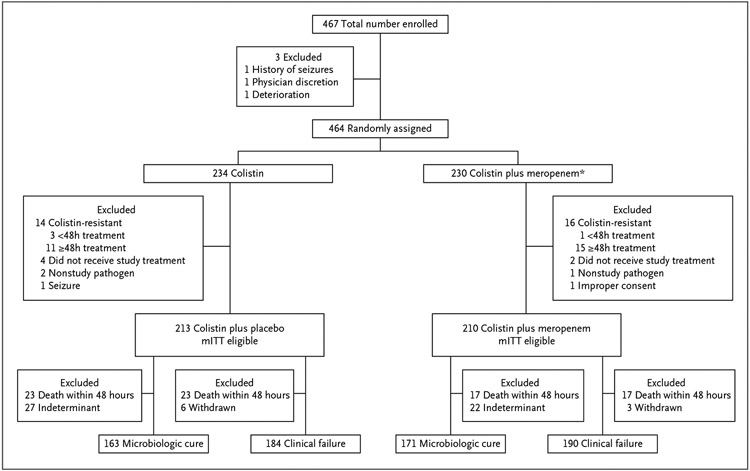
The ITT population consisted of all randomly assigned patients. The mITT population included all randomly assigned patients with a trial pathogen who received at least one dose of trial medication and whose trial pathogens exhibited colistin susceptibility according to BMD (Fig. 1). The all-treated population included all patients in the mITT population as well as patients initially randomly assigned to a trial arm who received more than 48 hours of a trial drug but were subsequently excluded from the mITT population due to identification of a colistin-resistant trial pathogen according to central laboratory BMD testing. For patients with BSI, blood cultures were obtained daily until they were negative for 2 consecutive days. For patients with pneumonia, respiratory cultures were obtained daily (when feasible) until they were negative for 2 consecutive days.
Figure 1. Consolidated Standards of Reporting Trials Flow Diagram for Enrollment in the OVERCOME Trial.
*Includes four patients who received colistin in combination with imipenem. mITT denotes modified intention-to-treat; and OVERCOME, Colistin Monotherapy versus Combination Therapy.
An independent data and safety monitoring committee was established to regularly review data and make recommendations regarding continuation of the trial. A clinical research organization was used to ensure the integrity of data, trial protocol process, and trial medication accountability.
OUTCOMES
The primary outcome was all-cause 28-day mortality in the mITT population. Secondary efficacy outcomes, including clinical failure and microbiologic cure, were evaluated in patients who survived more than 48 hours after enrollment. Patients who were withdrawn from the study before completing trial therapy for any reason other than meeting clinical failure criteria were deemed indeterminant for clinical failure and were subsequently excluded from this end point (Fig. 1). Clinical failure was a composite end point defined as meeting any of the following: death either while on trial therapy or within 7 days following completion; receipt of rescue therapy for the trial pathogen within 7 days of completion of trial treatment; removal from the trial due to an adverse event considered related to trial treatment; bacteremia more than 5 days after initiation of trial treatment for patients with BSI; or failure to improve or worsening of oxygenation by the end of trial treatment in patients with pneumonia. For patients with pneumonia who were mechanically ventilated at baseline, improvement of oxygenation was defined as any of the following occurring by the end of trial therapy: removal from mechanical ventilation, an increase in the ratio of arterial oxygen partial pressure to the fraction of inspired oxygen (PaO2/FIO2) by 100 mm Hg, or an increase in PaO2/FIO2 to a value 300 mm Hg or higher. For patients who remained on mechanical ventilation at the end of trial treatment without a recorded PaO2/FIO2 ratio on that date, recorded ratios from ±2 days from end of therapy were used to assess oxygenation status at the end of therapy, with preference given to the value closest to the end of therapy. If no values were available from within this time window, patients were indeterminant for this outcome. For patients with pneumonia who were not on mechanical ventilation at baseline, worsening was defined as requiring mechanical ventilation at the end of trial treatment.
Microbiologic cure was only assessed for patients in whom repeat specimens were obtained and was defined as eradication of pathogen from the infection site (i.e., negative cultures) by the end of treatment. For trial patients with pneumonia who completed a full course of trial therapy but did not have a documented negative respiratory culture, microbiologic outcomes were determined by the date of their last recorded positive respiratory sample according to the following criteria. If the patient’s last respiratory culture was positive and was collected 5 or more days after trial enrollment, they were considered a microbiologic failure. If the patient’s last respiratory culture was positive and obtained 4 days or less after enrollment, the patient was classified as indeterminant for microbiologic cure, as no inference could be made with respect to eradication at the end of therapy. Furthermore, if a patient was removed from the trial early due to death or treatment failure before documented eradication of the pathogen from the respiratory sample, they were categorized as a microbiologic failure.
Safety outcomes evaluated included decreases in renal function, as well as neurologic and hypersensitivity reactions. Acute kidney injury (AKI) was defined according to the RIFLE (Risk, Injury, Failure, Loss of kidney function, and End-stage kidney disease) criteria.15 All patients who received a trial drug for 48 hours or more with a baseline creatinine clearance of 30 ml/min or higher were eligible for AKI assessment, regardless of whether they remained in the mITT population. Details of the case definition of other adverse events16 are provided in the Supplemental Appendix.
STATISTICAL ANALYSIS
The target sample size, a total of at least 422 patients with known mortality status, was selected to give 80% power to detect a 28% relative reduction in mortality with combination therapy, assuming 50% mortality in the monotherapy arm. One interim analysis with a P value of 0.003 required for stopping was planned when one half (n=211) of the target sample size was enrolled. Stopping for futility was planned if the conditional power under the alternative dropped below 5%. The primary mortality analysis used a chi-square test, and associated analyses included Kaplan–Meier survival curve estimation and log-rank testing. Cox regression was used for secondary survival analysis. The t tests or Wilcoxon rank-sum tests were used to assess other continuous or ordinal variables as appropriate.
For secondary outcomes, no prespecified multiple comparison adjustment was included in the statistical analysis plan, and thus no significance tests are presented for those outcomes. Instead, only the estimated percentage-point differences ([colistin plus placebo] – [colistin plus meropenem]) and the associated 95% confidence intervals (CIs) are presented. The widths of those CIs have not been adjusted for multiplicity. Thus, the CIs should not be used to reject or not reject treatment effect hypotheses. No data were missing for the primary outcome or for the secondary outcomes beyond what has been previously described. For clinical failure, patients who were indeterminant in terms of the improvement in oxygenation criteria were assessed based on the other components of the composite outcome. Those who met another component of clinical failure were considered clinical failures, whereas those who did not meet any other failure criteria were considered not to be failures. Patients who were indeterminant for the microbiologic cure of pneumonia outcome were excluded from the analysis of this outcome.
Results
DESCRIPTION OF THE COHORT
A total of 467 patients were enrolled, 464 were randomly assigned to the trial, and 423 met inclusion criteria and comprised the mITT population (Fig. 1). Twenty-six patients who were randomly assigned to a trial arm were treated for 48 hours or more based on non-BMD colistin susceptibility testing performed at the site that was later determined to be colistin resistant when tested via BMD by the central laboratory or at the site. These patients were subsequently removed from the mITT group. Results between treatment arms, including these patients (i.e., the all-treated population; n=449), are provided in Table S2. Other reasons for removal of randomly assigned patients from the mITT are detailed in the Supplementary Appendix, and described in Fig. 1.
The mITT population included 213 patients who received colistin in combination with placebo and 210 who received colistin in combination with a carbapenem (n=206 meropenem and n=4 imipenem [hereafter referred to as colistin plus meropenem]). Baseline characteristics of patients in the two treatment arms are listed in Table 2 and Table S1. The mean age of the cohort was 68.0–16.0 years; 158 (37%) were female, 216 (51%) were White, and 173 (41%) were Asian. The most frequent comorbidities were hypertension (n=262 [62%]), diabetes (n=167 [39%]), and chronic lung disease (n=163 [39%]). Most patients were critically ill, with 291 (69%) in an intensive care unit at the time of enrollment. The trial population was highly reflective of the population most affected by these infections globally (Table S4).
Table 2.
Baseline Characteristics.*
| Characteristic | Colistin plus Placebo (n=213) |
Colistin plus Meropenem (n=210) |
|---|---|---|
| Demographics | ||
| Age — yr | 67.6±16.6 | 68.5±15.5 |
| Race — no. (%) | ||
| White | 108 (51) | 108 (51) |
| Asian | 89 (42) | 84 (40) |
| Other | 16 (8) | 18 (9) |
| Female — no. (%) | 83 (39) | 75 (36) |
| Region — no. (%) | ||
| Israel | 81 (38) | 85 (40) |
| Thailand | 64 (30) | 59 (28) |
| Taiwan | 26 (12) | 26 (12) |
| United States | 21 (10) | 22 (10) |
| European Union | 21 (10) | 18 (9) |
| Select comorbid conditions — no. (%) | ||
| Diabetes mellitus | 82 (39) | 85 (40) |
| Chronic lung disease | 84 (39) | 79 (38) |
| Chronic renal disease | 64 (30) | 71 (34) |
| Malignancy | 49 (23) | 50 (24) |
| Charlson Comorbidity Index score — median (IQR)† | 5 (4–7) | 5 (4–7) |
| Key patient characteristics at time of infection onset | ||
| Serum creatinine — mg/dl | 1.64±1.40 | 1.50±1.24 |
| Intensive care unit residence — no. (%) | 148 (69) | 143 (68) |
| Mechanical ventilation — no. (%) | 132 (62) | 120 (57) |
| APACHE II score — median (IQR)‡ | 22 (17–26) | 21 (17–26) |
| Vasopressor use — no. (%) | 30 (14) | 30 (14) |
| Infection characteristics — no. (%) | ||
| Infection type | ||
| Pneumonia | 152 (71) | 146 (70) |
| With secondary bacteremia | 10 (5) | 13 (6) |
| Bloodstream infection | 61 (29) | 64 (30) |
| Infecting organism | ||
| Acinetobacter baumannii∫ | 165 (77) | 164 (78) |
| Enterobacterales | 34 (16) | 35 (17) |
| Pseudomonas aeruginosa | 23 (11) | 20 (10) |
Plus–minus values are means±SD. APACHE II denotes Acute Physiology and Chronic Health Evaluation II; and IQR, interquartile range.
Charlson Comorbidity Index scores range from 0 to 41, with higher scores representing a lower estimated 10-year survival rate. A score of 5 represents an estimated 10-year survival rate of 21%.
APACHE II scores range from 0 to 71; a score of 20 to 24 has an estimated mortality of 40%, whereas an APACHE II score of 25 is associated with a predicted in-hospital mortality of 55%. APACHE II was only assessed in those with pneumonia in the intensive care unit at the time of enrollment.
This included two non–extensively drug-resistant A. baumannii isolates susceptible to ampicillin/sulbactam, in which the patient had a penicillin allergy contraindicating ampicillin/sulbactam.
Pneumonia was the most common type of index infection (n=298 [70%]), which included 23 patients with a concomitant BSI (Table 2 and Table S1). The remaining 125 patients were enrolled with a BSI. The most common causative pathogen was A. baumannii (n=329 [78%]) followed by CRE (n=69 [16%]) and P. aeruginosa (n=43 [10%]). Seventeen patients (4%) in the cohort had multiple trial pathogens as the cause of their index infection, and 93 (22%) were coinfected with a nontrial pathogen. The median time from onset of index infection to administration of appropriate therapy (i.e., therapy with in vitro activity against the trial pathogen) was 3 days (interquartile range, 2 to 4 days). Before enrollment, 290 patients (69%) received a median of 1 day of treatment with a drug active against the trial pathogen. When a patient received a nontrial drug active against the trial pathogen before enrollment, the drug in almost all cases was colistin or polymyxin B (n=288 [99%]), and 52 (18%) patients who received an active, nontrial drug before enrollment received colistin in combination with a carbapenem for a median duration of 1 day before enrollment (Table S1).
TWENTY-EIGHT–DAY MORTALITY
There was no significant difference in 28-day mortality between the colistin monotherapy and colistin plus meropenem combination therapy trial groups (43 vs. 37%; P=0.17) (Table 3), and there was no difference in time to mortality (Fig. 2). Similarly, there was no difference in mortality between treatment arms in any infection type or pathogen subgroup. When assessing 28-day mortality according to time to appropriate therapy, or when excluding patients who received pre-enrollment colistin plus carbapenem combination therapy or who were coinfected with nontrial pathogens, there remained no difference between treatment arms (Table S2).
Table 3.
Twenty-Eight–Day Mortality.*
| Cause of Mortality | Colistin plus Placebo | Colistin plus Meropenem | Difference (95% CI)† | P Value |
|---|---|---|---|---|
| Overall | 92/213 (43) | 77/210 (37) | 6.5 (−2.8 to 15.8) | 0.17 |
| Pneumonia | 69/152 (45) | 59/146 (40) | 5.0 (−6.2 to 16.2) | |
| BSI | 23/61 (38) | 18/64 (28) | 9.6 (−6.8 to 26.0) | |
| Acinetobacter baumannii | 76/165 (46) | 69/164 (42) | 4.0 (−6.7 to 14.7) | |
| CRE | 11/34 (32) | 6/35 (17) | 15.2 (−4.9 to 35.3) | |
| Pseudomonas aeruginosa | 10/23 (43) | 5/20 (25) | 18.5 (−9.3 to 46.2) |
Data are n/N (%), where n is the number of patients who died and N represents the total number of patients in the subgroup. BSI denotes bloodstream infection; CI, confidence interval; and CRE, carbapenem-resistant Enterobacterales.
Differences and 95% CIs are listed as differences in percentage points of ([colistin plus placebo] – [colistin plus meropenem]). The widths of the CIs have not been adjusted for multiplicity. Thus, the CIs should not be used to reject or not reject treatment effect hypotheses.
Figure 2.
Kaplan–Meier Survival Curves for Trial Patients Treated with Combination Therapy (Colistin plus Meropenem) and Monotherapy (Colistin plus Placebo).
CLINICAL FAILURE AND MICROBIOLOGIC CURE
A total of 374 patients were eligible for analysis of the clinical failure end point (Fig. 1). There was no difference in clinical failure between the monotherapy and combination therapy trial groups (65 vs. 58%; difference, 6.8 percentage points; 95% CI, −3.1 to 16.6), nor were there any differences between treatment arms in any infection type or pathogen subgroup (Table 4 and Table S3). In analyses accounting for the impact of time to appropriate therapy or pre-enrollment receipt of colistin plus carbapenem combination therapy on clinical failure, there remained no difference in clinical failure rate between treatment arms.
Table 4.
Secondary Outcomes.*
| Outcome | Colistin plus Placebo | Colistin plus Meropenem | Difference (95% CI)† or P Value |
|---|---|---|---|
| Clinical failure‡ | |||
| Overall | 119/184 (65) | 110/190 (58) | 6.8 (−3.1 to 16.6) |
| Death by 7 days’ posttreatment | 52/184 (28) | 39/190 (21) | 7.7 (−0.9 to 16.4) |
| Need for rescue therapy | 44/184 (24) | 42/190 (22) | 1.8 (−6.7 to 10.3) |
| Discontinue due to adverse event | 12/184 (7) | 21/190 (11) | -4.5 (−10.2 to 1.2) |
| BSI >5 days∫ | 4/59 (7) | 2/67 (3) | 3.8 (−3.8 to 11.4) |
| Oxygenation failure¶ | 72/123 (59) | 53/117 (46) | 12.9 (0.3–25.4) |
| Pneumonia | 96/132 (73) | 87/134 (65) | 7.8 (−3.3 to 18.9) |
| Bloodstream infection | 23/52 (44) | 23/56 (41) | 3.2 (−15.5 to 21.8) |
| Acinetobacter baumannii | 95/140 (68) | 88/146 (60) | 7.6 (−3.5 to 18.7) |
| CRE | 18/32 (56) | 16/33 (48) | 7.8 (−16.4 to 32.0) |
| Pseudomonas aeruginosa | 12/20 (60) | 11/19 (58) | 2.1 (−28.8 to 33.0) |
| Microbiologic cure∥ | |||
| Overall | 106/163 (65) | 103/171 (60) | 4.8 (−5.6 to 15.2) |
| Pneumonia | 59/114 (52) | 48/115 (42) | 10.0 (−2.8 to 22.9) |
| BSI | 47/49 (96) | 55/56 (98) | -2.3 (−8.8 to 4.2) |
| A. baumannii | 76/121 (63) | 74/130 (57) | 4.9 (−7.2 to 17.0) |
| CRE | 23/29 (79) | 24/32 (75) | 4.3 (−16.7 to 25.3) |
| P. aeruginosa | 9/19 (47) | 6/17 (35) | 12.1 (−19.9 to 44.0) |
| Adverse events of interest | |||
| Acute kidney injury** | 88/170 (52) | 85/174 (49) | 0.55 |
| Risk | 36/169 (21) | 30/174 (17) | |
| Injury | 31/169 (18) | 29/174 (17) | |
| Failure | 21/169 (12) | 26/174 (15) | |
| Hypersensitivity reaction†† | 3/229 (1) | 7/227 (3) | 0.22 |
| Neurotoxicity†† | 11/229 (5) | 5/227 (2) | 0.29 |
| Seizures†† | 3/229 (1) | 3/227 (1) | 1.00 |
Data are n/N (%), where n is the number of patients meeting the outcome and N is the total number of patients eligible. BSI denotes bloodstream infection; CI, confidence interval; and CRE, carbapenem-resistant Enterobacterales.
Differences and 95% CIs are listed as differences in percentage points of ([colistin plus placebo] – [colistin plus meropenem]). The widths of the CIs have not been adjusted for multiplicity. Thus, the CIs should not be used to reject or not reject treatment effect hypotheses.
Clinical failure was only assessed in patients who survived >48 hours from randomization and was a composite end point of death, the need for rescue therapy, discontinuation for an adverse event, persistent bacteremia, and failure to improve oxygenation. For the overall clinical failure outcome listed, the n/N (%) is listed for meeting the overall end point as well as each component of the composite. A patient could fail for more than one reason, and, therefore, the sum of the components of the end point is greater than that of the overall end point.
BSI is defined as bacteremia >5 days following initiation of study treatment — limited to patients with bacteremia.
Oxygenation failure is defined as failure to improve or worsening of oxygenation by the end of study treatment in patients with pneumonia. For patients with pneumonia who were mechanically ventilated at baseline, improvement of oxygenation was defined as any of the following occurring by the end of study therapy: removal from mechanical ventilation, an increase in the ratio of arterial oxygen partial pressure to fraction of inspired oxygen by 100 mm Hg, or an increase in the ratio of arterial oxygen partial pressure to fraction of inspired oxygen to a value ≥300. For patients with pneumonia who were not on mechanical ventilation at baseline, worsening was defined as requiring mechanical ventilation at the end of study treatment — limited to patients with pneumonia. A total of 26 patients (n = 9 colistin plus placebo and n = 17 colistin plus meropenem) were indeterminant for this variable.
Microbiologic cure was only assessed in patients from whom repeat specimens were obtained.
Acute kidney injury was limited to patients with baseline creatinine clearance ≥30 ml/min who received ≥48 hours of treatment.
Patients who were properly consented and received ≥1 dose of study medication were assessed for these adverse events.
A total of 334 patients were eligible for analysis of the microbiologic end point (Fig. 1). Microbiologic cure was shown in 106 (65%) of 163 patients receiving monotherapy compared with 103 (60%) of 171 receiving combination therapy (difference, 4.8 percentage points; 95% CI, −5.6 to 15.2). There were no differences in microbiologic cure between treatment arms in terms of infection type or infecting pathogen (Table 4).
SAFETY
A similar number of patients receiving monotherapy and combination therapy were removed from the trial due to adverse events (12 of 213 [6%] vs. 21 of 210 [10%], respectively; P=0.094). Most of these events (70%) were AKI. Overall, AKI rates were similar between patients receiving monotherapy versus those receiving combination therapy (88 of 170 [52%] vs. 85 of 174 [49%]; P=0.55). Neurotoxicity (4%) and hypersensitivity reactions (1%) were infrequent and occurred at similar rates in both treatment arms (Table 4).
Discussion
In this randomized, double-blind, placebo-controlled trial, there was no difference between colistin plus meropenem combination therapy and colistin monotherapy with respect to the primary outcome of 28-day mortality or the secondary outcomes of clinical failure and microbiologic cure in patients with pneumonia or BSI caused by XDR gram-negative pathogens or CRE. This lack of benefit with colistin plus meropenem combination therapy is consistent with the findings from a recent open-label trial, AIDA (the European Union’s Multicenter Open-label Randomised Controlled Trial to Compare Colistin Alone Versus Colistin plus Meropenem), that compared these same treatment regimens.17 As with OVERCOME, AIDA consisted predominantly of A. baumannii infections (77%), with 88% of patients having either pneumonia or BSI. Other randomized controlled trials comparing colistin monotherapy versus combination therapy with colistin and either rifampin18,19 or fosfomycin20 for invasive A. baumannii infections have also failed to show benefit with combination therapy. In totality, the evidence suggests that colistin-based combination therapy is no more effective than colistin monotherapy for carbapenem-resistant A. baumannii.
Intriguingly, although CRE and P. aeruginosa accounted for less than 25% of trial pathogens in both OVERCOME and AIDA,17 there was consistency in both studies with regard to trends in impact of combination therapy on mortality. Both trials reported numerical reductions in 28-day mortality with combination therapy compared with colistin alone for CRE (32 vs. 17% and 35 vs. 21% in OVERCOME and AIDA, respectively) and P. aeruginosa (43 vs. 25% and 31 vs. 25%) but were underpowered to assess significant differences in these subgroups. Further study of colistin plus meropenem combination therapy against CRE and P. aeruginosa may be warranted.
The current trial also highlights safety concerns with high-dose colistin therapy. Similar to results of other randomized controlled studies using comparable colistin dosing strategies,17,21-23 treatment was associated with a high rate of AKI, which occurred in roughly one half of enrolled patients. In two thirds of AKI instances, there was at least a doubling of serum creatinine. These results highlight the importance of novel antimicrobial agents with activity against resistant gram-negative pathogens, which are less nephrotoxic.
The strengths of the current trial are in the design. OVERCOME was a randomized, double-blind trial for the treatment of XDR gram-negative pathogens. This trial was conducted at 21 enrollment sites in seven countries and used a clinical research organization to ensure adherence to a complex and challenging protocol. Given the significant complexities in patient population and biases that would be expected in a less rigorously conducted trial, these strengths provide confidence in the accuracy of the findings.
There are limitations that warrant consideration. First, as is true in any clinical trial of pneumonia in critically ill patients, diagnosing pneumonia and determining the influence of the trial pathogen on outcomes are challenging. Therefore, even though OVERCOME used strict inclusion criteria, it is possible that patients were included who did not have pneumonia, which would bias this study toward the null. Second, although our primary outcome of 28-day mortality is considered the gold standard end point for the treatment of severe infections, in complex trial populations such as the one in OVERCOME, mortality can be influenced by many variables unrelated to infection and treatment. Third, there are other outcomes of interest pertaining to the role of colistin and meropenem combination therapy that were not reported in this article, including the development of antimicrobial resistance. Analyses of the OVERCOME data in terms of the impact of combination therapy on the emergence of colistin resistance are ongoing.
Finally, the dosage of meropenem in OVERCOME (1000 mg every 8 hours as a 30-minute infusion) is lower than that recommended by recent guidance documents for resistant gram-negative organisms, and some evidence supports that a dosage of 2000 mg every 8 hours as a 3-hour infusion may improve outcomes for CRE when isolates have MICs to meropenem 8 mg/l or less.24,25 However, it is important to note that when the OVERCOME trial was initiated, these data did not yet exist, and the meropenem dose was selected based on providing synergy with colistin, rather than providing activity against low-level carbapenem-resistant isolates. Furthermore, it is unlikely that meropenem dosing affected the results of OVERCOME for two main reasons. First, the AIDA trial used the more aggressive meropenem dosing, and, similar to OVERCOME, outcomes were not improved in the combination therapy arm.16 Second, very few isolates in OVERCOME (<10%) (Table S1) had meropenem MIC values of 4 to 8 mg/l, in which a more aggressive meropenem dosing strategy might have affected outcomes. For these reasons, we think that the lower dosing of meropenem used in OVERCOME is unlikely to have contributed to the failure of combination therapy in improving patient outcomes.
Conclusions
The receipt of colistin plus meropenem combination therapy did not affect 28-day mortality, clinical failure, or microbiologic cure compared with colistin monotherapy. Furthermore, the high rates of mortality and clinical failure in infections caused by A. baumannii seen in both treatment arms in this trial highlight the urgent need for non-polymyxin treatment alternatives for invasive infections caused by this pathogen. Future trials of the colistin plus meropenem combination may be warranted for CRE and P. aeruginosa, given the numerical trends toward decreased mortality in both OVERCOME and AIDA. However, alternative treatment strategies, including the use of novel agents where active and available, should be considered, as numerous publications suggest improved efficacy and safety with novel β-lactam/β-lactamase inhibitor combinations compared with colistin-based regimens for infections caused by these pathogens.6,26,27
Supplementary Material
Footnotes
Disclosures
Supported by the National Institute of Allergy and Infectious Diseases (Division of Microbiology and Infectious Diseases protocol 10-0065).
Author disclosures and other supplementary materials are available at evidence.nejm.org.
References
- 1.Santoro A, Franceschini E, Meschiari M, et al. Epidemiology and risk factors associated with mortality in consecutive patients with bacterial bloodstream infection: impact of MDR and XDR bacteria. Open Forum Infect Dis 2020;7:ofaa461. DOI: 10.1093/ofid/ofaa461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fitzpatrick MA, Suda KJ, Poggensee L, et al. Epidemiology and clinical outcomes associated with extensively drug-resistant (XDR) Acinetobacter in US Veterans’ Affairs (VA) medical centers. Infect Control Hosp Epidemiol 2021;42:305–310. DOI: 10.1017/ice.2020.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Duin D, Arias CA, Komarow L, et al. Molecular and clinical epidemiology of carbapenem-resistant Enterobacterales in the USA (CRACKLE-2): a prospective cohort study. Lancet Infect Dis 2020;20:731–741. DOI: 10.1016/S1473-3099(19)30755-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis [published correction appears in Lancet 2022;400:1102]. Lancet 2022;399:629–655. DOI: 10.1016/S0140-6736(21)02724-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. Antibiotic Resistance Threats in the United States. December 2019. U.S. Department of Health and Human Services; (www.cdc.gov/drugresistance/pdf/threats-report/2019-ar-threats-report-508.pdf). [Google Scholar]
- 6.van Duin D, Lok JJ, Earley M, et al. Colistin versus ceftazidime-avibactam in the treatment of infections due to carbapenem-resistant Enterobacteriaceae. Clin Infect Dis 2018;66:163–171. DOI: 10.1093/cid/cix783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ho S, Nguyen L, Trinh T, MacDougall C. Recognizing and overcoming resistance to new beta-lactam/beta-lactamase inhibitor combinations. Curr Infect Dis Rep 2019;21:39. DOI: 10.1007/s11908-019-0690-9. [DOI] [PubMed] [Google Scholar]
- 8.Clancy CJ, Potoski BA, Buehrle D, Nguyen MH. Estimating the treatment of carbapenem-resistant Enterobacteriaceae infections in the United States using antibiotic prescription data. Open Forum Infect Dis 2019;6:ofz344. DOI: 10.1093/ofid/ofz344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsuji BT, Pogue JM, Zavascki AP, et al. International consensus guidelines for the optimal use of the polymyxins: endorsed by the American College of Clinical Pharmacy (ACCP), European Society of Clinical Microbiology and Infectious Diseases (ESCMID), Infectious Diseases Society of America (IDSA), International Society for Anti-infective Pharmacology (ISAP), Society of Critical Care Medicine (SCCM), and Society of Infectious Diseases Pharmacists (SIDP). Pharmacotherapy 2019;39:10–39. DOI: 10.1002/phar.2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schechner V, Fallach N, Braun T, Temkin E, Carmeli Y. Antibiotic exposure and the risk of hospital-acquired diarrhoea and Clostridioides difficile infection: a cohort study. J Antimicrob Chemother 2021;76: 2182–2185. DOI: 10.1093/jac/dkab151. [DOI] [PubMed] [Google Scholar]
- 11.World Medical Association. WMA Declaration of Helsinki — Ethical Principles for Medical Research Involving Human Subjects. September 5, 2022. (https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involying-human-subjects/). [Google Scholar]
- 12.European Committee on Antimicrobial Susceptibility Testing. Clinical breakpoints — breakpoints and guidance. January 1, 2022. (https://www.eucast.org/clinical_breakpoints/). [Google Scholar]
- 13.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med 1985;13:818–829. DOI: 10.1097/00003246-198510000-00009. [DOI] [PubMed] [Google Scholar]
- 14.National Healthcare Safety Network. Bloodstream Infection Event (Central-Line–Associated Bloodstream Infection and Non–Central-Line–Associated Bloodstream Infection). January 2022. (https://www.cdc.gov/nhsn/pdfs/pscmanual/4psc_clabscurrent.pdf). [Google Scholar]
- 15.Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P. Acute renal failure — definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care 2004;8:R204–R212. DOI: 10.1186/cc2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.U.S. Department of Health and Human Services. Common Terminology Criteria for Adverse Events (CTCAE). May 28, 2009. (https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf). [Google Scholar]
- 17.Paul M, Daikos GL, Durante-Mangoni E, et al. Colistin alone versus colistin plus meropenem for treatment of severe infections caused by carbapenem-resistant Gram-negative bacteria: an open-label, randomised controlled trial. Lancet Infect Dis 2018;18:391–400. DOI: 10.1016/S1473-3099(18)30099-9. [DOI] [PubMed] [Google Scholar]
- 18.Durante-Mangoni E, Signoriello G, Andini R, et al. Colistin and rifampicin compared with colistin alone for the treatment of serious infections due to extensively drug-resistant Acinetobacter baumannii: a multicenter, randomized clinical trial. Clin Infect Dis 2013;57:349–358. DOI: 10.1093/cid/cit253. [DOI] [PubMed] [Google Scholar]
- 19.Aydemir H, Akduman D, Piskin N, et al. Colistin vs. the combination of colistin and rifampicin for the treatment of carbapenem-resistant Acinetobacter baumannii ventilator-associated pneumonia. Epidemiol Infect 2013;141:1214–1222. DOI: 10.1017/S095026881200194X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sirijatuphat R, Thamlikitkul V. Preliminary study of colistin versus colistin plus fosfomycin for treatment of carbapenem-resistant Acinetobacter baumannii infections. Antimicrob Agents Chemother 2014;58:5598–5601. DOI: 10.1128/AAC.02435-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brown ML, Motsch J, Kaye KS, et al. Evaluation of renal safety between imipenem/relebactam and colistin plus imipenem in patients with imipenem-nonsusceptible bacterial infections in the randomized, phase 3 RESTORE-IMI 1 Study. Open Forum Infect Dis 2020;7:ofaa054. DOI: 10.1093/ofid/ofaa054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wunderink RG, Giamarellos-Bourboulis EJ, Rahav G, et al. Effect and safety of meropenem-vaborbactam versus best-available therapy in patients with carbapenem-resistant Enterobacteriaceae infections: the TANGO II randomized clinical trial. Infect Dis Ther 2018;7:439–455. DOI: 10.1007/s40121-018-0214-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McKinnell JA, Dwyer JP, Talbot GH, et al. Plazomicin for infections caused by carbapenem-resistant Enterobacteriaceae. N Engl J Med 2019;380:791–793. DOI: 10.1056/NEJMc1807634. [DOI] [PubMed] [Google Scholar]
- 24.Paul M, Carrara E, Retamar P, et al. European Society of Clinical Microbiology and Infectious Diseases (ESCMID) guidelines for the treatment of infections caused by multidrug-resistant Gram-negative bacilli (endorsed by European Society of Intensive Care Medicine). Clin Microbiol Infect 2022;28:521–547. DOI: 10.1016/j.cmi.2021.11.025. [DOI] [PubMed] [Google Scholar]
- 25.Tamma PD, Aitken SL, Bonomo RA, Mathers AJ, van Duin D, Clancy CJ. Infectious Diseases Society of America 2022 guidance on the treatment of extended-spectrum β-lactamase producing Enterobacterales (ESBL-E), carbapenem-resistant Enterobacterales (CRE), and Pseudomonas aeruginosa with difficult-to-treat resistance (DTR-P. aeruginosa). Clin Infect Dis 2022;75:187–212. DOI: 10.1093/cid/ciac268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pogue JM, Kaye KS, Veve MP, et al. Ceftolozane/tazobactam vs polymyxin or aminoglycoside-based regimens for the treatment of drug-resistant Pseudomonas aeruginosa. Clin Infect Dis 2020;71:304–310. DOI: 10.1093/cid/ciz816. [DOI] [PubMed] [Google Scholar]
- 27.Shields RK, Nguyen MH, Chen L, et al. Ceftazidime-avibactam is superior to other treatment regimens against carbapenem-resistant Klebsiella pneumoniae bacteremia. Antimicrob Agents Chemother 2017;61:e00883–17. DOI: 10.1128/AAC.00883-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.