Abstract
Clausena lansium, commonly known as wampee, is a subtropical fruit from the Rutaceae family characterized by its high nutrient content and numerous bioactive substances. This low-fat fruit is abundant in fiber, vitamins, minerals, and essential amino acids. Wampee has been found to contain several bioactive compounds, including essential oils, phenolic compounds, and alkaloids. These bioactive constituents provide numerous health-enhancing properties, such as antioxidant, neuroprotective, anticarcinogenic, anti-inflammatory, hepatoprotective, antidiabetic, and antimicrobial effects. The relationship between these compounds and their impacts on health has been explored in various studies. While the disease-prevention efficacy of C. lansium has been established, additional research is necessary to elucidate the precise mechanisms and metabolic pathways involved. This paper presents a comprehensive review of wampee, focusing on its bioactive compounds, the beneficial effects derived from its consumption, and the evidence supporting the development of wampee-based functional foods in future studies.
1. Introduction
Clausena lansium (Lour.) Skeel, commonly referred to as wampee, is a subtropical fruit belonging to the Rutaceae family, whose origins are traced back to China.1 It has been cultivated in China for more than 1500 years, and several varieties have been developed through selective breeding.2 The fruit is commercially grown in China, Vietnam, Thailand, Malaysia, the Philippines, and India due to its high-value status. Wampee typically exhibits an oval shape and measures approximately 2.0 cm in diameter. Its yellow peel encases 1–5 seeds, as shown in Figure 1. It generally ripens from May to August in different cultivation areas due to geographical and climatic differences.3 The edible part of the wampee includes the pulp and peel. Besides being consumed as fresh fruit, wampee also can be processed into long-shelf-life food products, such as jams, beverages, preserved fruits, and wines. Dried wampee in Thailand is popular with locals.4 Processing is a possible way to expand the wampee market.
Figure 1.

Wampee fruits on the tree.
In addition, wampee could be employed in medicine rather than just being consumed as a typical food. In the view of Asian folk medicine, such as traditional Chinese medicine (TCM), the leaves, stem, bark, root, peel, and seeds of wampees can be used to treat various diseases. The water extraction of wampee leaves and peel can be used to treat certain dermatological disorders, acute and chronic viral hepatitis, as well as asthma.5 The therapeutic properties of the wampee’s root, bark, and stem were effective in addressing bronchitis and malarial fever, while the pulp and seeds were purportedly beneficial in alleviating symptoms of cough, dyspepsia, and specific gastrointestinal disorders.6,7 The wampee is renowned not only for its fruit but also for its substantial medicinal properties. Clausenolide-1-ethyl ether, a limonoid extracted from wampee, has been scientifically demonstrated to exhibit anti-HIV activity. Another compound, clausine-D, also derived from the wampee plant, possesses an antiplatelet effect that can aid in preventing blood clot formation. Additional pharmacological benefits attributed to the wampee include antibacterial, antifungal, and antimalarial activities. The myriad of therapeutic potentials this plant offers thus emphasizes its significance in medical research.8 In previous decades, with the help of advanced research techniques, the chemical composition and nutrient profiles of the wampee were explored. Relevant research has demonstrated that wampee is a rich source of various nutrients and phytochemicals, such as polyphenols, minerals, vitamins, and flavonoids, which possess health-promoting properties. These beneficial effects include antioxidative, anti-inflammatory, and antimicrobial activity.9
Several researchers have reported the bioactive compounds isolated from wampee, but no comprehensive information about the chemical profiles and bioactivities of wampee has been reported. This review aims to summarize the chemical constituents and corresponding bioactivities of different parts of the wampee. This comprehensive review is helpful for the better development of wampee fruit and related products.
2. Chemical Compositions
2.1. Proximate Compositions
The nutritional value of wampee is primarily attributed to its pulp, which serves as the primary edible portion. Supplemental Table 1 demonstrates the major macronutrient composition of wampee determined in several types of research. Wampee is a kind of tropical fruit with a high water content, and water occupies around 78.93 to 84.00% of the fresh weight (FW) of wampee pulp. The ash content in the wampee ranges from 0.9 to 2.6 g/100 g of FW. In every 100 g of the consumable pulp of the wampee fruit, the composition comprises 0.61 to 3.78 g of protein, 0.10 to 0.28 g of fat, and 9.9 to 14.1 g of carbohydrates by fresh weight. The fiber content is up to 4.58 g/100 g of wampee, establishing it as an outstanding source of dietary fiber. Additionally, the appropriate ratio between soluble sugar (10.16 g/100 g on average) and total acid (1.55 g/100 g on average) balances the sweet and sour taste to wampee. The low fat and sugar contents in wampee result in a low energy level of around 47 kcal/100 g. The variance of constituent content in other research may be attributed to the growing conditions, geographic factors, and analytical methods.4,10,11
2.2. Vitamins and Minerals
Wampee could be a good supplement for vitamins and minerals in a daily diet. The most abundant vitamin in wampee is vitamin C (548 mg/kg), higher than other tropical fruits like lychee (364 mg/kg) and papaya (512 mg/kg).12,13 Other vitamins and precursors, including vitamin E (1.58 mg/kg), vitamin B1 (1.35 mg/kg), vitamin B2 (0.72 mg/kg), and niacin (0.33 mg/kg), as well as β-carotene (0.016 mg/kg), are also found in wampee. The wampee fruit also serves as a significant source of essential minerals. Each kilogram of wampee contains significant amounts of essential minerals such as 3500 mg potassium, 710 mg calcium, and 220 mg phosphorus, which are crucial for maintaining homeostasis in the body.11 The contents of vitamins and minerals for fresh wampee are summarized in Supplemental Table 1.
2.3. Protein and Amino Acid
The protein content of wampee is 6.20%, while abundant amino acids were found in wampee pulp.14 A total of 17 amino acids were isolated and quantified from wampee and summarized in Supplemental Table 2.14−16 Among the identified amino acids in wampee pulp, alanine was the most abundant, with a concentration of 10.50 mg/g, followed by asparagine (6.10 mg/g), glutamine (4.10 mg/g), serine (3.00 mg/g), lysine (2.30 mg/g), and leucine (2.00 mg/g).
Amino acid assessment indicates that wampee contains nine essential amino acids (EAA), including histidine, isoleucine, leucine, lysine, methionine, phenylalanine, threonine, valine, and arginine, which make up around 28% of the total amount of amino acids. Therefore, the consumption of wampee may contribute to the sufficient daily intake of essential amino acids. Amino acids play a crucial role in physiological activities and food taste development. Based on their taste properties in foods, amino acids could be classified into three groups: bitter, sweet, and umami. The amino acid assessment reveals that the sweet amino acids (Thr, Ser, Gly, and Ala) are the dominant group of amino acids accounting for nearly 41% of the total amino acids. Additionally, the umami amino acids (Asp and Glu) make up about 28% of the total amino acids in wampee. As a result, it is reasonable that the amino acids in wampee contribute to developing its sweet and umami taste profile.
2.4. Carbohydrates
The carbohydrate content of wampee is primarily made up of free sugars and polysaccharides. Fructose, glucose, and sucrose are the most dominant free sugars in wampee. Some research reported a slight difference in soluble sugar content among different species of wampee fruit.17 The total soluble sugar content could be up to 87.35 mg/g, consisting of fructose (40.14 mg/g), glucose (28.39 mg/g), and sucrose (18.81 mg/g). The median total soluble sugar content in wampee is 100 mg/g, including 50.6 mg/g of sucrose.18
Besides the free sugars, the polysaccharides occupy a considerable portion of carbohydrates in the wampee. Polysaccharide is a group of bioactive compounds with different health benefits, such as antioxidative and anti-inflammatory activities, etc. Additionally, it involves immunoregulation and cell activities, thus playing a critical role in health.19 Polysaccharides in wampee are mainly composed of pectin, cellulose, and hemicellulose. Various methodologies, such as microwave-assisted extraction and ethanol sedimentation, have obtained crude polysaccharide extracts from wampee.
The composition of monosaccharides in the wampee may exhibit variations, which can be attributed to the difference in extraction methodologies and the specific portions of the wampee fruit utilized. For example, the polysaccharides obtained from the seed and peel of wampee were composed of mannose, galacturonic acid, galactose, arabinose, and glucose with a ratio of 11.70:55.37:9.58:17.81:2.03 and 2.76:48.28:15.83:27.63:2.74, respectively.20,21
Furthermore, the difference in extraction method may cause the variation in monosaccharide composition, even from the same portion of the wampee. For example, galacturonic acid is one of the dominant monosaccharides in the pulp extract obtained through ethanol sedimentation, accounting for 62.16% of the total content. However, the ultrasonic microwave-assisted enzymatic method resulted in arabinose being the most abundant polysaccharide present in the pulp.22,23 The characteristics of polysaccharides extracted from a different portion of wampee have been summarized in Supplemental Table 3.
2.5. Volatile Compounds
Essential oils are natural bioactive products consisting of volatile compounds found in plants. The essential oils derived from wampee fruit exhibit potent health-promoting and pharmacological properties, including anti-inflammatory, antibacterial, and anticancer activities. These volatile components also contribute to the distinctive and appealing aroma of fruits. Thus, examining the chemical composition of essential oils and volatiles from wampee fruit is crucial, as they play a role in both its bioactivity and aroma. The volatile constituents of wampee essential oils can be classified into several major groups: terpenoids and their derivatives as well as alcohols and olefin compounds.
Various extraction methods could be applied to extract volatile compounds in plants, such as supercritical fluid extraction, hydrodistillation, steam distillation, and hydro-diffusion, etc.24 In addition, gas chromatography and mass spectrometry (GC-MS) are frequently utilized techniques to identify volatile compounds.
Volatile compounds were isolated and identified from fresh pulp, leaves, and seeds of wampee using GC-MS with a headspace sampler.25 It is reported that the volatile compounds from wampee pulp are mainly made up of monoterpenes (76.0%) and alcohols (17.5%). In the wampee leaves, 86% of the entire volatile fraction was characterized. The primary components among these constituents were sesquiterpenes, accounting for 28%, and monoterpenes, comprising 22%. The major volatile components in the seed of wampee were found to be sabinene, β-phellandrene, and 4-terpineol by using GC-MS analysis.26 Volatile oil was extracted from wampee pulp by supercritical fluid extraction.27 The main compositions in wampee essential oil were 4-terpineol (26.94%), followed by γ-terpinene (14.39%), β-phellandrene (8.24%), sabinene (5.58%), and cymene (5.01%). Among these substances, β-phellandrene is an attractive resource for its potential antifungal activities.26,28 There are some differences between the characterized volatile compositions in the studies, possibly due to the differences in temperature, pressure, and polarity of different extraction methods.29
Furthermore, the content of identical substances may exhibit considerable differences in various studies because of variations in climate, growing environment, and plucking. The volatile compositions in different parts of the wampee were also investigated, and it was found that monoterpene dominates the total volatile content.25 Sabinene was found to have the highest amount in all parts of the wampee: leaves (14.92%), pulp (50.64%), peel (69.07%), and seed (83.56%). The volatile compounds in the wampee were summarized in Table 1.
Table 1. Volatile and Phenolic Compounds of Wampeea.
| Volatile compounds of wampee | ||||
|---|---|---|---|---|
| % Relative
area |
||||
| Compound | Leaves | Pulp | Peel | Seed |
| Sabinene | 14.92 | 50.64 | 69.07 | 83.56 |
| β-Bisabolene | 9.88 | ND | ND | 0.15 |
| β-Caryophyllene | 7.72 | ND | ND | 0.55 |
| α-Zingiberene | 6.52 | ND | ND | 0.06 |
| 3-Cyclohexen-1-ol | ND | 15.17 | 0.28 | 0.51 |
| Cyclohexene | ND | 6.5 | 0.17 | 0.39 |
| 1,4-Cyclohexadiene | ND | 6.19 | 0.32 | ND |
| α-Phellandrere | 1.38 | 5.03 | 10.63 | 3.08 |
| α-Pinene | 1.99 | 2.08 | 9.41 | 4.26 |
| Myrcene | 1.1 | 1.7 | 3.15 | 2.94 |
| Acetic acid | 0.94 | 2.65 | 0.08 | 0.03 |
| Hexanal | 1.55 | 0.47 | 0.04 | ND |
| Isosativene | 0.38 | ND | 0.07 | 0.01 |
| Butanal | 8.61 | ND | ND | ND |
| Reference | Chokeprasert et al., 200725 | |||
| Phenolic
content of wampee (based on dry weight) | |||
|---|---|---|---|
| Portion | Phenolic compounds | Content (mg/100 g DW) | References |
| Leaves | Gallic acid | 224.7 | Chen et al., 201738 |
| (+)-Catechin | 762.3 | ||
| Vanillic acid | 609.1 | ||
| Caffeic acid | 1514.1 | ||
| Leaves | Syringic acid | 216.4 | |
| Epicatechin | 424.5 | ||
| p-Coumaric acid | 12836.2 | ||
| Ferulic acid | 5417.2 | ||
| Rutin | 9241.5 | ||
| Isoquercitrin | 2760.9 | ||
| Quercitrin | 2730.7 | ||
| Quercetin | 5641.3 | ||
| Peel | Proanthocyanidins | 1.88 | Chai et al., 201739 |
| Phenolic
content of wampee (based on fresh weight) | |||
|---|---|---|---|
| Portion | Phenolic compounds | Content (mg/100 g FW) | References |
| Leaves | Gallic acid | 90.3 | Kong et al., 201836 |
| Chlorogenic acid | 221.8 | ||
| Caffeic acid | 67.7 | ||
| Rutin | 1700.5 | ||
| Ferulic acid | 82.4 | ||
| Mature leaves | Quercetin | 2.87 | Chang, Lu et al., 201867 |
| Catechin | 1.91 | ||
| Feurlic acid | 2.94 | ||
| Chlorogenic acid | 1.39 | ||
| Vanillic acid | 1.66 | ||
| Pulp | Vanillic acid | 1.77 | Ye et al., 201932 |
| Ferulic acid | 2.43 | ||
| Rutin | 15.87 | ||
| Syringin | 15.85 | ||
| Catechin | 262.7 | ||
| Hesperetin | 1.63 | ||
| Leaves | p-Coumaric acid | 9.87 | Li et al., 201935 |
| 7-Hydroxycoumarin | 12.03 | ||
| Ferulic acid | 13.15 | ||
| Rutin | 98.94 | ||
| Pulp | Syringin | 9.8 | Chang et al., 202240 |
| Rutin | 656.8 | ||
| Benzoic acid | 350.1 | ||
| 2-Methoxycinnamic acid | 29.1 | ||
| Kaempferol | 20.7 | ||
| Hesperetin | 20.9 | ||
| Nobiletin | 39.1 | ||
| Tangeretin | 48 | ||
“ND”: Not detected.
2.6. Phenolic Compounds
Phenolic compounds are a massive group of phytochemicals, which are secondary metabolites synthesized through the shikimic acid and phenylpropanoid pathways in plants.30 The typical chemical structure of phenolic compounds comprises an aromatic ring with one or more hydroxyls groups. Phenolic compounds can be divided into several classes, including phenolic acids (e.g., hydroxycinnamic acid, hydroxybenzoic acid), flavonoids (e.g., flavonols, flavanols, and anthocyanidins), tannins (e.g., hydrolyzable tannins, condensed tannins), stilbenes, coumarins, and lignans.31
The phenolic composition and concentrations of wampee may exhibit considerable discrepancies across various studies owing to the diversity in numerous aspects, such as geographical distribution, sample preparation, analytical approaches, and statistical analysis. Consequently, it is essential to consider these variables when evaluating the phenolic compound profiles derived from different studies.
The studies identifying phenolic compounds primarily focused on the pulp, peel, and leaf portion of wampee. Ye et al.32 extracted phytochemicals from wampee pulp using ethanol as the solvent and measured the phenolic content by the Folin–Ciocalteu method. The total phenolic content in wampee pulp was reported to be up to 907.8 mg gallic acid equivalent (GAE)/100 g FW, while the total flavonoid content in wampee pulp was up to 536.6 mg catechin equivalent (CE)/100 g FW. In another study, Prasad et al.33 investigated the phenolic content in wampee peel using the Folin–Ciocalteu method and found that the total phenolic content in the ethanol extract fraction is 4.62 GAE mg/100 g dry weight (DW). The highest level of total phenolic content was observed in the ethyl acetate fraction, which was 33 mg GAE/100 g DW. In addition, Chang et al.34 found that as the wampee leaves grow there is an accumulation of total phenolic content.
At least 20 phenolic compounds have been isolated and identified from different parts of wampee, including hydroxybenzoic acid (gallic acid, vanillic acid, and benzoic acid), hydroxycinnamic acid (ferulic acid, 2-methoxycinnamic acid, chlorogenic acid, caffeic acid, and p-coumaric acid), flavonol (rutin, kaempferol, quercetin, and isoquercitrin), flavanone (hesperetin), flavone (nobiletin, tangerretin), flavanol (catechin), and phenolic glycosides (syringin).32,35−40 In addition, coumarin is a class of phenolic compounds widely existing in wampee. All of these identified compounds were summarized in Table 1.
2.7. Alkaloids
Alkaloids represent a significant class of plant-derived secondary metabolites exhibiting pharmacological activity, and their biodynamic activities may benefit human health.41 Alkaloids are abundant in different parts of the wampee and could be classified into several types.
2.7.1. Carbazole Alkaloid
Carbazole alkaloid is an important subgroup of alkaloids. The fundamental chemical composition of carbazole is characterized by a central pyrrole ring, which is coalesced with two benzene rings on either side.42 This class of compounds is considered as an anticancer agent because of their biological activities.43
Serving as a significant source of carbazole alkaloids, various compounds have been extracted and identified from multiple sections of the wampee plant. Ten carbazole alkaloids named claulansines A–J were extracted from the stem of the wampee. Among these alkaloids, 10 μM of claulansines A, F, H, I, and J effectively protected pheochromocytoma (PC12) cells from apoptosis.44 Furthermore, claulansine F was found to be able to promote neuritogenesis in PC12 cells by activating the ERK signaling pathway, thus exerting its neuroprotective activity.
Moreover, a new carbazole alkaloid, claulansine K, was first isolated from wampee peels and exhibited in vitro α-glucosidase inhibitory activity.45 One year later, Du et al.46 characterized two new carbazole alkaloid claulansine S and T in the stem of the wampee. This research group extracted another seven carbazole alkaloids named claulansines L–R from the wampee stem in the same year. They subsequently discovered that claulansines N, P, Q, and S demonstrated hepatoprotective properties in human HepG2 hepatoma cells.47 Subsequently, Liu et al.48 extensively studied the alkaloid profiles in wampee fruits and isolated 16 carbazole alkaloids, while six were first reported. The research group also evaluated the neuroprotective activities of these alkaloids toward the human neuroblastoma SH-SY5Y cell line, proposing that wampee could potentially serve as a preventative and therapeutic agent for Parkinson’s disease. Additionally, five new carbazole alkaloids were extracted from the stem of wampee, named claulansine A (Figure 2A), claulansine U, claulansine V, claulansine W, and (−)-(2′R)-claulamine A, respectively. These compounds presented potential protective effects on PC12 cells against serum deprivation and anticancer activities.49 Recently, a new carbazole alkaloid, called claulansine X, has been identified in the ethanol extract of the wampee stem, exhibiting a moderate neuroprotective influence on the PC12 cell line.50
Figure 2.
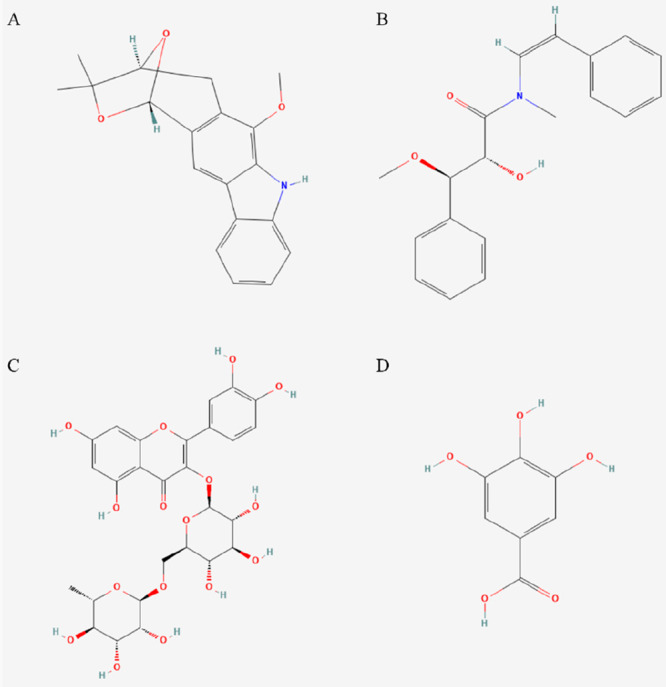
Structures of some important secondary metabolites: (A) claulansine A; (B) clausenalansamide C; (C) rutin; (D) gallic acid. The image was extracted from PubChem.
In short, more than 40 carbazole alkaloids have been isolated and identified from different parts of the wampee and are summarized in Table 2. They are an essential and characteristic feature of a wampee.
Table 2. Alkaloids in Wampee.
| Carbazole
alkaloid in wampee | |||||
|---|---|---|---|---|---|
| No. | Name | Molecular formula | Source | Bioactivities | References |
| 1 | 3-Formyl-6-methoxycarbazole | C14H11NO2 | Root | - | Li et al., 19916 |
| 2 | Methyl 6-methoxycarbazole-3-carboxylate | C15H13NO3 | - | ||
| 3 | 3-Formyl-1,6-dimethoxycarbazole | C15H13NO3 | - | ||
| 4 | Mafaicheenamines A | C19H19NO4 | Twigs | Anticancer | Maneerat & Laphookhieo, 201088 |
| 5 | Mafaicheenamine B | C19H21NO5 | |||
| 6 | Mafaicheenamines C | C19H19NO3 | |||
| 7 | Mafaicheenamines D | C19H18NO2 | Root | Anticancer | Maneerat et al., 201289 |
| 8 | Mafaicheenamines E | C19H18NO3 | |||
| 9 | Claulansine A | C19H19NO3 | Stem | Neuroprotective | Liu et al., 201244 |
| 10 | Claulansine B | C19H19NO4 | |||
| 11 | Claulansine C | C19H20NO5 | |||
| 12 | Claulansine D | C19H19NO5 | |||
| 13 | Claulansine E | C16H13NO3 | |||
| 14 | Claulansine F | C19H17NO3 | |||
| 15 | Claulansine G | C19H17NO2 | |||
| 16 | Claulansine H | C19H19NO4 | |||
| 17 | Claulansine I | C18H18NO2 | |||
| 18 | Claulansine J | C14H11NO4 | |||
| 19 | Claulansine K | C25H23NO8 | Peel | - | Deng et al., 201445 |
| 20 | 6-Methoxy-9H-carbazole-3-carboxylic acid | C14H11NO3 | Stem | Anticancer | Jiang et al., 201490 |
| 21 | Claulansine L | C25H23NO8 | Stem | Hepatoprotective | Du, Liu, Li, Ma et al., 20155 |
| 22 | Claulansine M | C18H15NO2 | |||
| 23 | Claulansine N | C14H12NO3 | |||
| 24 | Claulansine O | C15H14NO3 | |||
| 25 | Claulansine P | C21H23NO4 | |||
| 26 | Claulansine Q | C15H15NO | |||
| 27 | Claulansine R | C16H17NO2 | |||
| 28 | Claulansine S | C17H19NO | Stem | Hepatoprotective | Du, Liu, Li, Yang et al., 201546 |
| 29 | Claulansine T | C18H21NO2 | |||
| 30 | Claulansium A | C19H17NO4 | Branches | Anticancer | Peng et al., 201891 |
| 31 | Claulansium B | C19H21NO3 | |||
| 32 | Clausenalansine A | C18H16NO3 | Fruit | Neuroprotective | Liu, Guo, Liu et al., 201948 |
| 33 | Clausenalansine B | C14H14NO2 | |||
| 34 | Clausenalansine C | C15H12NO4 | |||
| 35 | Clausenalansine D | C15H14NO3 | |||
| 36 | Clausenalansine E | C14H14NO2 | |||
| 37 | Clausenalansine F | C15H16NO2 | |||
| 38 | Claulansine U | C19H17NO4 | Stem | Against serum deprivation injury | Sun et al., 202049 |
| 39 | Claulansine V | C19H15NO4 | Against serum deprivation injury | ||
| 40 | Claulansine W | C19H19NO3 | Anticancer | ||
| 41 | (−)-(2′R)-Claulamine A | C19H17NO3 | / | ||
| 42 | Claulansine X | C19H20NO4 | Stem | Neuroprotective | Sun et al., 202150 |
| Amine
alkaloid in wampee | |||||
|---|---|---|---|---|---|
| No. | Name | Molecular formula | Source | Bioactivities | References |
| 1 | Clausenamide | C18H19NO3 | Leaves | Hepatoprotective | Yang, Chen et al., 198792 |
| 2 | Neoclausenamide | C18H19NO3 | |||
| 3 | Dehydroccloclausenamide | C18H17NO2 | |||
| 4 | Cycloclausenamide | C18H17NO2 | Leaves | Hepatoprotective | Yang et al., 198893 |
| 5 | (E)-N-(4-Methoxyphenethyl)-2-methylbut-2-enamide | C14H19NO2 | Leaves | - | Zhao et al., 201194 |
| 6 | Lansiumamide B | C18H17NO | Leaves | Anti-inflammatory | Matsui et al., 201355 |
| 7 | Lansiumamide C | C17H18NO | |||
| 8 | Lansamide I | C18H18NO | |||
| 9 | SB-204900 | C18H17NO2 | |||
| 10 | Clausenalansamides C | C19H21NO3Na | Leaves | - | Shen et al., 201754 |
| 11 | Clausenalansamides D | C18H18ClNO2Na | |||
| 12 | Clausenalansamides E | C18H18ClNO2Na | |||
| 13 | Clausenalansamides F | C19H21SNO4Na | |||
| 14 | Clausenalansamides G | C23H29NO8Na | |||
| 15 | Lansiumamide A | C17H15NO | Seeds | - | Lin, 198956 |
| 16 | Lansiumamide B | C18H17NO | |||
| 17 | Lansiumamide C | C18H19NO | |||
| 18 | Lansumide I | C18H17NO | |||
| 19 | Clausenalansamide A | C18H19NO3 | Seeds | Nematicidal | Fan, Chen, Mei, Xu et al., 201895 |
| 20 | Clausenalansamide B | C18H19NO2 | |||
| 21 | 1′-Methoxyl-clausenalansamide B | C19H21NO3 | Seeds | Nematicidal | Fan, Chen, Mei, Kong et al., 201857 |
| 22 | 3-Dehydroxy-3-methoxyl-secoclausenamide | C18H21NO3 | |||
| 23 | 2′-Dehydroxy-2′-acetoxyl-clausenalansamide B | C20H21NO3Na | |||
| 24 | Neoclausenamide A | C20H21NO3Na | |||
| 25 | Neoclausenamide B | C27H25NO4Na | |||
| 26 | Acetylclausenamide | C20H19O3N | Stem | Neuroprotective | Sun et al., 202150 |
| 27 | Anhydroclausenamide | C18H18O2N | |||
| 28 | 1-Chloro-benzenepropanamide | C17H19O2NCl | |||
| 29 | Clauamide A | C16H17NO3 | Stem | Hepatoprotective | Du, Liu, Li, Yang et al., 201546 |
| 30 | (+)-(3S,4R,5S,6S)-Clauselansine C | C18H17NO2 | Stem | Neuroprotective | Liu, Du et al., 201762 |
Fan et al. recently reported that the pivotal regulatory enzymes, shikimate kinase and anthranilate synthase, upregulate carbazole alkaloid production in wampee through the carbazole alkaloid biosynthetic pathway.51 Alkaloids were discovered in different portions of the wampee, including the pulp, stem, branch, leaf, twig, root, and peel, and the stem contains the most abundant alkaloid profiles. Hence, the wampee stem can be considered an important source of carbazole alkaloids. Some of these carbazole alkaloids demonstrated antineoplastic, hepatoprotective, and neuroprotective properties in in vitro tests. In addition, Li et al. also suggested that carbazoles isolated from Clausena plants are a vital heterocyclic class of anticancer agents.52 However, further in-depth studies are needed to investigate the potential mechanisms of the bioactivities of these carbazole alkaloids to illustrate the health benefits of wampee.
2.7.2. Amine Alkaloid
Amide alkaloids are another important category of alkaloids abundant in various portions of the wampee. This group of compounds has attracted attention due to their excellent biological activities.
An amide alkaloid named clausenamide was first isolated and characterized from wampee leaves.53 In a later study, a greater variety of amide alkaloids were found in the leaves of wampee, such as clausenalansamides C (in Figure 2B) through G and clausenaline G,54 lansiumamide C, lansamide I, lansiumamide B, and SB-204900.55 Additionally, the wampee seed is another important source of amide alkaloids. Lin identified four cinnamamide derivatives in wampee seeds: lansiumamide A, lansumide-I, lansiumamide B, and lansiumamide C.56 Later, Fan, Chen, Mei, and Kong et al. isolated amide alkaloids from wampee seeds, including 1′-methoxyl-clausenalansamide B, 3-dehydroxy-3-methoxyl-secoclausenamide, 2′-dehydroxy-2′-acetoxyl-clausenalansamide B, neoclausenamide-A, clausenalansamide A, clausenalansamide B, and neoclausenamide-B, and confirmed their nematicidal activity.57 Among the amide alkaloids derived from wampee seeds, lansiumamide B exhibits the broadest biological activities. These include an antiobesity effect,58 larvicidal activity,59 antibacterial activity,60 and fungicidal activity.61
Furthermore, continued research into the bioactive compounds in the wampee plant revealed the discovery of new amide alkaloids in the stem of the wampee. Du et al. reported clauamide A, isolated from wampee seeds, has potential hepatoprotective capacity.46 Liu et al. extracted (+)-(3S,4R,5S,6S)-clauselansine C from wampee seeds and confirmed its neuroprotective activity.62 Recently, three more new amide alkaloids were found in wampee seeds, including acetylclausenamide, anhydroclausenamide, and 1-chloro-benzenepropanamide.50
At least 30 amide alkaloids were extracted and characterized from different portions of the wampee. The amide alkaloids isolated from wampee were summarized in Table 2. The biological activities of amide alkaloids have been extensively investigated, revealing an array of potential therapeutic applications such as neuroprotective, anti-inflammatory, nematicidal, hepatoprotective, antidepressant, and antinociceptive effects. Hence, analyzing the amide alkaloid composition of wampee could potentially offer novel perspectives on its utilization, including the development of health-enhancing products. Also, developing the byproducts of wampee, like seeds, peels, leaves, and twigs, is a sustainable strategy that promotes the valorization of low-value products in the food industry.
3. Health-Promoting Effects of Wampee
As mentioned in the previous section, wampee is a rich source of several bioactive compounds. The health-promoting effects of wampee reported in previous research include antioxidative, anti-inflammatory, hepatoprotective, antibacterial, and antineoplastic properties, etc. Most of the work was conducted using the in vitro model. However, these outcomes obtained from in vitro studies provide fundamental evidence for in-depth animal and clinical research.
3.1. Antioxidative Effects
Excessive production of reactive oxygen species (ROS) can result in an imbalanced redox reaction in tissues, leading to oxidative stress or even oxidative damage in the body.63 ROS are produced both endogenously, as metabolic byproducts stemming from cellular oxygen metabolism within the organism, and exogenously, originating from environmental factors including ultraviolet radiation, ionizing radiation, pollutants, heavy metals, and xenobiotics.64 The most common ROS include hydroxyl radicals, hydrogen peroxide, and superoxide anions. Excessive ROS may cause irreversible cellular damage by inducing DNA/RNA damage, protein oxidation, and lipid peroxidation of polyunsaturated fatty acids. Consequently, increased oxidative stress heightens the risk of chronic diseases such as cancer, neurodegenerative disorders, and diabetes.65 Hence, the dietary intake of antioxidants could be a feasible strategy to neutralize ROS and reduce the risk of related health problems.
To evaluate the antioxidative properties of wampee, a range of in vitro assays were used, including 2,2′-azinobis(3-ethylbenzothiazoline-6-sulfonate) (ABTS), oxygen radical absorbance capacity (ORAC), ferric reducing antioxidant power (FRAP), 2,2-diphenyl-1-picrylhydrazyl (DPPH), Trolox equivalent antioxidant capacity (TEAC), peroxide radical scavenging capacity (PSC), cellular antioxidant activity (CAA), self-oxidation of 1,2,3-phentriol, and lipid peroxidation assays.
The antioxidant capacity of wampee pulp can be primarily attributed to its rich phenolic compound content. Ye et al.32 examined the correlation between total phenolic content and antioxidant properties across five wampee pulp varieties. The total phenolic content was positively associated with both in vitro and in vivo antioxidant assays. The variety with the highest total phenolic content (907.8 mg of GAE/100 g) displayed the most potent antioxidant capacity in the ORAC, DPPH, and FRAP assays, with values of 11.03 mmol of Trolox equivalent/100 g, 208.1 mg ascorbic acid equivalent/100 g, and 279.7 mmol Fe2+/100 g, respectively. On the contrary, the variety with the lowest level of total phenolics (49.25 mg GAE/100 g) exhibited the weakest antioxidant capacity, with corresponding assay values of 1.08 mmol Trolox equivalent/100 g, 10.55 mg ascorbic acid equivalent/100 g, and 72.84 mmol Fe2+/100 g, respectively. Other research has similarly observed that wampee pulp with higher phenolic content displays enhanced free radical scavenging capabilities.34,40 In addition, Ye et al. analyzed the association between phenolic monomers and antioxidant properties, finding that in vitro antioxidant capacity was primarily attributable to phenolic monomers such as ferulic acid, catechin, and syringic acid, while hesperetin contributed most to the in vivo antioxidant capacity of wampee pulp.32 Moreover, Zeng et al. reported that the ethanol extract of wampee fruit inhibited the expression of NF-κB, thereby protecting PC-12 cells from oxidative stress.66
Wampee leaves exhibit remarkable antioxidant potential due to their rich phenolic composition. Chang et al. observed that wampee leaf buds with the highest total phenolic content (2046 mg GAE/100 g) yielded the highest ORAC value (415.8 μmol Trolox equivalent/g).67 As the leaves developed, the total phenolic content consistently decreased, causing an initial reduction in antioxidant potential, followed by a subsequent increase. Interestingly, the total flavonoid content displayed an upward trend during leaf development. This observation can be attributed to the fact that despite the decline in total phenolic content affecting the antioxidant potential of wampee leaves the simultaneous production of flavonoid compounds may compensate for and sustain their antioxidant capacity. Thus, flavonoids represent a significant subgroup of total phenolics that may considerably contribute to the antioxidant properties of wampee leaves.
Prasad et al. examined the antioxidative properties of wampee peel extracts using four solvents: ethanol, hexane, ethyl acetate, butanol, and water.33 The ethyl acetate extract demonstrated significant antioxidative potential, surpassing that of butylated hydroxytoluene (BHT) in in vitro tests. In the DPPH assay, antioxidative capacities were ranked as follows at a concentration of 50 μg/mL: ethyl acetate > BHT > water > ethanol > butanol > hexane. This outcome is likely due to the total phenolic content in the different fractions, with ethyl acetate, water, ethanol, butanol, and hexane fractions containing 330.0, 54.0, 46.2, 30.3, and 7.9 μg/g DW, respectively.33 A similar study also reported higher total phenolic content in the ethyl acetate fraction.1
Ethyl acetate is a polar solvent, whereas hexane is typically used to extract nonpolar substances, indicating that the phenolic compounds in the wampee peel may possess high polarity. Moreover, the total phenolic content in the water fraction was significantly lower than that in the ethyl acetate fraction, suggesting that most antioxidative compounds in the wampee peel were not water-soluble. Thus, an appropriate solvent with both polar and hydrophobic properties can enhance the efficacy of isolating the predominant antioxidant constituents from the wampee peel.
In addition to phenolic substances, other compounds, such as polysaccharides extracted from wampee, have been identified as potential antioxidants.20 The antioxidative capacity of acidic heteropolysaccharide extracted from wampee seed exhibited a concentration-dependent manner in both in vitro and in vivo tests. Polysaccharide extracts significantly reduced the malondialdehyde (MDA) level while increasing the SOD and glutathione (GSH) levels in high-fat diet-fed rats, demonstrating their potent ability to manage oxidative stress in vivo.21 However, different fractions of polysaccharide extracts might exhibit varying antioxidative properties. Peng et al.20 attributed the variation to the phenols attached to polysaccharides, while Wu et al.21 ascribed it to differences in uronic acid content and structural characteristics.
Overall, various portions of the wampee exhibit significant antioxidant properties. Most research has primarily focused on the in vitro antioxidant capacity of wampee. Nonetheless, it is crucial to acknowledge that outcomes derived from in vitro tests may not simply be extrapolated to in vivo circumstances. The chemical and molecular targets of in vitro tests, such as ABTS and DPPH, are sterically hindered stable radicals that do not accurately represent short-lived radicals in the body.68
In summary, phenolic compounds and polysaccharides in wampee are the primary constituents contributing to its antioxidative activity. Table 3 summarizes the antioxidant activity of wampee. Further comprehensive in vivo antioxidant tests are necessary to provide more evidence of the antioxidant properties of the wampee and determine the specific mechanisms.
Table 3. Antioxidative Activity of Wampeea.
| Portion | Peel | Pulp | Pulp |
|---|---|---|---|
| Bioactive compound | Phenolics | Phenolics | Phenolics |
| Solvent | Ethyl acetate | Acetone | Acetone |
| TPC | 330 ± 9.9 μg/g dry weight, expressed as gallic acid equivalents | 7.92 mg/g GAE FW | 9.07 mg/g GAE FW |
| TFC | 7.16 mg/g CE FW | 3.50 mg/g CE FW | |
| DPPH | 50 μg/mL (95% scavenging activity) | 76.72 mg AsA equiv/100 g FW | 208.1 mg AsA equiv/100 g FW |
| FRAP | 264.3 mmol Fe2+/100 g FW | 279.7 mmol Fe2+/100 g FW | |
| ABTS | |||
| Superoxide anion radical scavenging activity | 50 μg/mL (88% scavenging activity) | ||
| PSC | 34.7 μmol Vit. C equiv/100 g FW | 4927 μmol AsA equiv per 100 g FW | |
| ORAC | 5.44 mmol TE/100 g FW | 11.03 mmol TE/100 g FW | |
| Cell antioxiant in vivo | |||
| Major finding | Ethyl acetate extract of wampee peel exhibits superior antioxidative capacity, which is mainly related to phenolic content. | Antioxidant capacity of wampee fruit is related to the total phenolic and total flavonoid contents. | It was apparent that wampee fruits had higher phenolic and flavonoid contents resulting in better antioxidant activities in vitro and cellular. |
| Reference | Prasad et al., 20101 | Chang, Ye et al., 201834 | Ye et al., 201932 |
“TPC”: Total phenolic content; “TFC”: Total flavonoid content; “DPPH”: 1,1-diphenyl-2-picrylhydrazyl; “FRAP”: Ferric reducing antioxidant power; “ABTS”: 2,2′-Azinobis(3-ethylbenzthiazoline-6-sulfonate); “PSC”: Peroxide radical scavenging capacity; “ORAC”: Oxygen radical absorbance capacity.
3.2. Neuroprotection
Neurodegenerative diseases comprise a group of progressive disorders that impact neurons in the nervous system, leading to a wide array of symptoms, including cognitive dysfunction, impaired movement, memory loss, and, ultimately, death. Some of the most prevalent neurodegenerative diseases include Alzheimer’s disease (AD), Parkinson’s disease (PD), Huntington’s disease (HD), and amyotrophic lateral sclerosis (ALS).69 The pathogenesis of these diseases, however, remains incompletely understood. Presently, there is a burgeoning interest in exploring natural products for their potential to target the hypothesized pathogenesis of neurodegenerative diseases. Adrenal pheochromocytoma (PC12) cells and human neuroblastoma SH-SY5Y cells are two widely used cell lines for establishing neuron injury models and investigating neuroprotective compounds.70,71 Prior studies have demonstrated that numerous bioactive compounds derived from wampee exhibit promising potential in protecting neuronal cells and may offer new insights into innovative treatments for neurodegenerative diseases.
Alkaloids comprise a large family of compounds with potential neuroprotective activity isolated from wampee. (−)-Clausenamide was one of the first compounds discovered with potential neuroprotective activity derived from wampee. Hu et al. demonstrated that (−)-clausenamide (1–100 μM) significantly alleviates Aβ-induced neurotoxicity in differentiated PC12 cells in a dose-dependent manner, as indicated by cell viability assays.72 This compound inhibited the production of reactive oxygen species (ROS) and the activation of caspase-3, which serve as markers for oxidative stress and apoptosis, respectively. Additionally, (−)-clausenamide activates the Nrf2/HO-1 antioxidant pathways, which play a crucial role in regulating oxidative stress and inflammation. Consequently, (−)-clausenamide may have potential as a natural neuroprotective agent for preventing and treating neurodegenerative diseases such as Alzheimer’s disease. However, further research, including in vivo studies, is required to fully understand the mechanisms of action and potential therapeutic applications of this compound. Liu et al. developed a neuron injury model using a PC12 cell line and sodium nitroprusside (SNP) as an inducer.44 They discovered that four alkaloids extracted from the wampee stem significantly increased cell viability against SNP-induced neurotoxicity. In a subsequent study, Liu et al. investigated the neuroprotective effects of alkaloids derived from the wampee stem.62 They observed that 5 out of 14 alkaloids moderately alleviate neurotoxicity induced by okadaic acid in PC12 cells. A total of 13 alkaloids were identified, while 5 of them could moderately alleviate the neurotoxicity induced by okadaic acid in PC12 cells.
Liu, Guo, and Liu et al. discovered that 16 carbazole alkaloids extracted from wampee pulp demonstrated significant protective capabilities against 6-OHDA-induced apoptosis in human neuroblastoma SH-SY5Y cells, similar to the positive control, curcumin.48 Subsequently, Liu et al. isolated 12 geranylated carbazole alkaloids from the leaves and stems of the wampee, which exhibited similar protective activity with curcumin against 6-OHDA-induced neurotoxicity in SH-SY5Y cells.73 More recently, Sun et al. reported that two carbazole alkaloids isolated from wampee stems showed only moderate protective effects on PC12 cells against serum deprivation injury.49 Collectively, these studies underscore the neuroprotective potential of alkaloids derived from wampee, suggesting possible therapeutic applications for neurodegenerative diseases. However, as most of these investigations were preliminary, the mechanisms underlying the compounds’ neuroprotective effects remain unclear, necessitating further research.
In addition to alkaloids, phenolic compounds have also demonstrated potential as neuroprotective agents. Li et al. extracted a flavonoid, Bu-7, from wampee leaves, which significantly enhanced the viability of PC12 cells following rotenone-induced injury.74 Bu-7 inhibited the JNK and p38 signaling pathways via phosphorylation, consequently preventing rotenone-induced apoptosis in PC12 cells. Moreover, Bu-7 treatment attenuated mitochondrial membrane permeabilization and the collapse of the mitochondrial membrane potential (MMP), indicating the significant neuroprotective potential of Bu-7 and its possible application in treating Parkinson’s disease. Later, Zeng et al. investigated the activity of wampee fruit ethanol extract in a hydrogen-peroxide-induced PC12 cell model.66 The wampee fruit extracts significantly reversed cell apoptosis in PC12 cells in a dose-dependent manner, reduced intracellular ROS generation, and prevented MMP collapse. Furthermore, the extract treatment inhibited the NF-κB pathway and downregulated caspase-3 expression, suggesting that the intracellular antioxidative capacity of wampee contributes to its protective effects against neurodegenerative diseases.
Further investigation of the neuroprotective effects of wampee using in vivo models is necessary. Tongun and Phachonpai developed a memory impairment model in rats induced by chronic restraint stress.75 In the object recognition assessment, rats administered wampee peel extract (600 mg/kg) exhibited a higher discrimination index, while in the Morris water maze evaluation, they exhibited reduced escape latency and increased retention duration. The activity of the antioxidant enzyme in rat brain tissue, such as superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GSH-Px), was enhanced after wampee peel extract supplementation. The treatment group exhibited lower acetylcholinesterase (AChE) activity, indicating a higher essential neurotransmitter (ACh) level. Phachonpai and Tongun76 reported similar findings and suggested that phenolic compounds might be the primary bioactive substances responsible for the antidementia activity of the wampee. In another study, Phachonpai and Tongun77 induced focal cerebral ischemia in rats through middle cerebral artery occlusion (MCAO). Rats supplemented with the wampee peel extract displayed higher cognition test scores than the MCAO control group. Additionally, wampee peel extract treatment reduced inflammatory biomarkers and improved neuronal survival and cholinergic neurons in the hippocampal regions. The specific experiment design for the wampee neuroprotection effect was shown in Figure 3.
Figure 3.
Experimental design of wampee neuroprotection effect and test index.
Both the in vitro and in vivo studies presented above demonstrate the considerable potential of wampee for addressing neurological issues. Alkaloids and phenolic compounds appear to be the primary categories of bioactive substances in wampee that contribute to its neuroprotective properties. However, further research is needed to elucidate the precise mechanisms of action of these bioactive compounds. Table 4 provides a summary of the neuroprotective effects of wampee.
Table 4. Neuroprotective Effects of Wampee.
| No. | Sources | Model | Study design | Result | References |
|---|---|---|---|---|---|
| 1 | (−)-Clausenamide isolated from wampee leaves | Aβ 25–35-induced cell apoptosis in PC12 cells | Cell viability, Western blot, MMP, flow cytometry | (−)-Clausenamide (10 μM) reduces the collapse of MMP by 42.4% | Hu et al., 201072 |
| (−)-Clausenamide (10 μM) treatment increased the viability of PC12 to 90% from 70% | |||||
| (−)-Clausenamide inactivated the p38 MAPK pathway and inhibited the expression of P53 and caspase 3 | |||||
| 2 | Alkaloids isolated from wampee stem | SNP-induced toxicity in PC12 cells | Cell viability | The cell viability of negative control group was 76%, while the treatment of four alkaloids (10 μM) with neuroprotective potential restored the value to 91.0, 88.2, 84.8, and 83.7%, respectively. | Liu et al., 201244 |
| 3 | Alkaloids isolated from wampee stem | Okadaic acid-induced injury in PC12 cells | Cell viability | The cell viability of negative control group was 70.5%, while the treatment of five alkaloids (10 μM) with neuroprotective potential restored the value to 91.2, 89.7, 83.5, 83.4, and 83.3%, respectively. | Liu, Du et al., 201762 |
| 4 | Alkaloids isolated from wampee pulp | 6-Hydroxydopamine-induced cell apoptosis in human neuroblastoma SH-SY5Y cells | Cell viability | The EC50 value of 16 carbazole alkaloids ranged from 0.36 to 10.69 μM, while the EC50 value for curcumin (positive control) was 5.82 μM. | Liu, Guo, Liu et al., 201948 |
| 5 | Alkaloids isolated from wampee stem and leaves | 6-Hydroxydopamine-induced cell apoptosis in human neuroblastoma SH-SY5Y cells | Cell viability | The EC50 value of 12 geranylated carbazole alkaloids ranged from 0.48 to 12.36 μM, while the EC50 value for curcumin (positive control) was 6.03 μM. | Liu, Guo, Wang et al., 201973 |
| 6 | Alkaloids isolated from wampee stem | Serum deprivation injury in PC12 cells | Cell viability | The cell viabilities of negative control, positive control, and alkaloid treatment groups (10 μM) were 47.4, 83.8, 67.8, and 63.3%, respectively | Sun et al., 202049 |
| 7 | Flavonoid isoalted from wampee leaves | Rotenone-induced injury in PC12 cells | Cell viability, Western blot, flow cytometry, measurement of mitochondrial membrane potential | The cell viability of cells treated with rotenone was 69.2%, and this increased significantly by 14.4%, 3.1%, and 18.1% after treated with 0.1, 1, and 10 μM Bu-7, respectively., | Li et al., 201174 |
| Bu-7 attenuated the rotenone-induced mitochondrial potential reduction in cells. | |||||
| Bu-7 inhibited activation of the JNK and p38 signaling pathways and suppressed caspase 3 activity in the rotenone-treated cells. | |||||
| Bu-7 excerted its activities in a bell-shaped dose–response relationship. | |||||
| 8 | Ethanol extract of wampee pulp | H2O2-induced neurotoxicity in PC12 cells | Cell viability, Western blot, measurement of mitochondrial membrane potential | The cell viability of negative control group was 55%, but the wampee fruit extract (1, 10, 20 μM) increased this value to 64.12, 72.13, and 76.26%, respectively. | Zeng et al., 201466 |
| Wampee pulp extract downregulated caspase-3 and pnf-κB p65 and suppressed NF-κB p65 entry to the nucleus from the cytoplasm. | |||||
| 9 | Ethanol extract of wampee peel | Memory impairment induced by chronic restraint stress in rats | Object recognition test, Morris water maze test, ache activity lipid peroxidation, SOD activity determination | High dose of wampee peel extract (600 mg/kg) significantly increased the discrimination index of rats. The rats received wampee peel extract treatment displayed shorter time of escape of latency and longer retention time. | Tongun & Phachonpai, 202075 |
| Wampee peel extract decreased the lipid peroxidation index. It improved the antioxidant enzymes activities while inhibiting pain. | |||||
| 10 | Ethanol extract of wampee peel | Rats received permanent right middle cerebral artery occlusion | Morris water maze test, passive avoidance test, determination of cerebral infarct volume, Nissl staining and survival neuron densities counts, determination of the cholinergic neuron densities, measurement of lipid peroxidation | Medium dose of wampee peel extract treatment (400 mg/kg) significantly reduced the brian infract volume of rats; decreased the MDA level in the brain; and enhanced the neuronal survival and cholinergic neurons in hippocampal regions. | Phachonpai & Tongun, 2021b77 |
| 11 | Ethanol extract of wampee peel | Memory impairment induced by chronic restraint stress in rats | Object recognition test, Morris water maze test, superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GSH-Px), and ache activities and the malondialdehyde (MDA) determination | High dose of wampee peel extract treatment (600 mg/kg) increased the discrimination index of rats. The rats that received wampee peel extract treatment displayed shorter time of escape of latency and longer retention time. | Phachonpai & Tongun, 2021a76 |
| Wampee peel extract alleviated oxidative stress. It improved the antioxidant enzyme activities while inhibiting pain. |
3.3. Anti-Inflammation Effects
Inflammation is a multifaceted physiological response to tissue injury, infection, and other forms of cellular stress. Acute inflammation is an essential defense mechanism protecting the body from harmful stimuli. However, chronic inflammation contributes to the pathogenesis of numerous diseases such as arthritis, cardiovascular disease, and cancer. This persistent inflammatory state is characterized by the continuous activation of immune cells, tissues, and signaling pathways, leading to the production of pro-inflammatory cytokines, chemokines, and other mediators that may harm healthy tissues and exacerbate disease.78
Wampee contains various bioactive compounds known for their anti-inflammatory properties. Inflammation can be induced by carrageenin, leading to the formation of edema in rat paws. A 200 mg/kg methanolic extract of wampee leaves and indomethacin treatments reduced rat paw edema by 55.5% and 64.0% within four hours, respectively, suggesting the potential of wampee extract as an anti-inflammatory agent.7 Similarly, Huang et al. discovered that wampee leaf extract diminished TNF-α production by suppressing the TLR4/MYD88/TRAF6 signaling pathways in LPS-treated RAW 264.7 cells.79 Matsui et al. identified two cinnamamides, lansiumamide B and SB-204900, that inhibited histamine release and decreased IL-6, COX-2, and TNF-α formation, thus exhibiting anti-inflammatory activity in rat basophilic leukemia cells.55 The mechanism of the wampee anti-inflammation effect is shown in Figure 4. Wampee stem-derived alkaloids demonstrated anti-inflammatory potential comparable to curcumin in LPS-induced murine microglial BV2 cells.80 The alkaloid 3-formyl-6-methoxycarbazole, isolated from wampee roots, showed anti-inflammatory activity similar to dexamethasone, significantly suppressing TNF-α at a concentration of 50 μg/mL in LPS-induced monocyte cells.81 Shen et al. reported that alkaloids from the wampee stem not only inhibited TNF-α and NO production but also suppressed fMLP/CB-induced superoxide anion generation in LPS-induced RAW 264.7 cells.82 Likewise, coumarin and alkaloid compounds from wampee leaves, such as wampetin and imperatorin, effectively reduced superoxide anion generation in RAW 264.7 cells with IC50 values of 6.8 and 1.7 μM, respectively.54
Figure 4.
Mechanism of wampee anti-inflammation effect.
In summary, both in vivo and in vitro studies have demonstrated the potent anti-inflammatory properties of wampee. It has been posited that the notable anti-inflammatory compound present in the wampee could be attributed to alkaloids, which exhibit their effects by inhibiting inflammatory signaling cascades and reducing the production of reactive species. Table 5 summarizes the anti-inflammation effect of wampee.
Table 5. Health Benefit Effects of Wampee.
| Health effect | Sources | Cell model | Result | Reference |
|---|---|---|---|---|
| Anti-inflammation effect | Alkaloids isolated from wampee stem | Murine microglial BV2 cells | Carbazole alkaloids displayed considerable anti-inflammatory potential against LPS-stimulated NO production in cell model, with IC50 values of around 5 μM | Liu et al., 201580 |
| Alkaloids and coumarins isolated from wampee stem | Human neutrophils and RAW264.7 cell lines | Imperatorin, isoheraclenin. and osthol were the most potent inhibitors for fMLP/CB-induced superoxide anion generation with IC 50 values of 0.20, 0.81, and 0.0086 μM, respectively. | Shen et al., 201282 | |
| The extracted alkaloid, such as wampetin, decreases up to 98.9% NO of control which is higher than positive control (indomethacin, 30.9%). | ||||
| Wampetin also downregulated the expression of TNF-α. | ||||
| Alkaloids and coumarins isolated from wampee leaves | Human neutrophils | Imperatorin and wampetin exhibited potent inhibitory ability toward fMLP/CB-induced superoxide anion generation with IC50 values of 1.7 and 6.8 μM, respectively. | Shen et al., 201754 | |
| Anti-inflammation effect | Ethyl acetate extract of wampee leaves | RAW264.7 cell lines | Wampee extract inactivated TNF-α in a dose-dependent manner, through suppressing TLR4/MYD88/TRAF6 pathways, while up to 100 μg/mL wampee extract inhibited 80.6% TNF-α expression. | Huang et al., 201979 |
| Alkaloid isolated from wampee root | Monocyte cell line U937, human gingival fibroblast (HGF) cell line | 3-Formyl-6-methoxycarbazole (50 μg/mL) displayed comparable inhibitory ability to dexamethasone (positive control) in suppressing TNF-α. | Rodanant et al., 201581 | |
| Methanol extract of wampee stem | Rats fed with high-fat diet | 100 mg/kg wampee extract had comparable inflammatiory effect to indomethacin that significantly alleviated the rat paw edema induced by inflammation. | Adebajo et al., 20097 | |
| Hepatoprotective | Methanol extract of wampee stem | Rats fed with high-fat diet | The low dose (100 mg/kg) and high dose (200 mg/kg) could decrease sleep duration time 5.3% and 8.4%, respectively. The methanol extract increased AST, ALT and ALP activity in serum and decreased these enzymes’ activity in plasma. | Adebajo et al., 20097 |
| Leaves of wampee including eight compounds | CCl4-induced hepatotoxicity in mice | These eight compounds (250 mg/kg) could decrease the elevated SGPT of CCl4-intoxicated mice. The cyclo-clausenamide was the most potent one among these compounds. The inhibition rate reached 76.7% after the cyclo-clausenamide treatment. | Liu et al., 199684 | |
| Glucopyranoside isolated from wampee stem | HepG2 (human hepatocellular liver carcinomacell line) cells | The HepG2 cell survival rate was increased around 10% after the extracted compound (10 μM) treatment. | Liu et al., 201762 | |
| Antidiabetes | Polyphenol extract from wampee leaves | Streptozotocin-induced type 2 diabetic rats | Higher body weight. | Kong et al., 201836 |
| Lower food intake. | ||||
| Less excretion. | ||||
| Lower level of fasting blood glucose. | ||||
| Lower organ coefficient. | ||||
| Less liver damage and lipid accumulation. | ||||
| Methanol extract of wampee stem | Glucose-induced INS-1 cell line, rat | The rat treated with methanol extract of wampee stem (100 mg/kg) showed 15.8% lower level of glucose than control group. | Adebajo et al., 20097 | |
| The extract also increased the insulin secretion in both in vivo (38.5% higher than control) and in vitro (1.74 times over control) tests. | ||||
| Proanthocyanidins isolated from wampee pulp | Chemical reaction model | Proanthocyanidins isolated from wampee (0.26 μg/mL) suppressed the enzyme activity to 50%. | Yang et al. 202083 | |
| Antimicrobial | Essential oils extracted from pericarps | Candida strains | The essential oil extracted from chicken heart wampee which was rich in β-phellandrene and β-sesquiphellandrene had the most powerful effect. The inhibition zone diameter of C.glabrata was 23.1 mm after the essential oils (10 μL/disc) treatment. | He et al., 201987 |
| Essential oils and three main compounds extracted from seed | Candida strains | The 4-terpineol was the most potent compound among the essential oils and its main compound. The inhibition zone diameters of five Candida species was 26.7–35.6 mm after the 4-terpineol (10 μL/disc) treatment. | Ma et al., 202196 | |
| Three carbazole alkaloids extracted from twigs roots | Porphyromonas gingivalis | Inhibition zone diameters of Porphyromonas gingivalis was 19.06–20.94 mm after the lansine (50 μg/mL) treatment. The lansine was the most powerful compound among extracted carbazole alkaloids. | Rodanant et al., 201581 |
3.4. Hepatoprotection
In recent years, increasing interest has emerged in exploring the potential hepatoprotective activity of the wampee and its constituents. Du et al. isolated several novel carbazole alkaloids from the wampee stem and assessed their hepatoprotective activity against N-acetyl-p-aminophenol (APAP)-induced toxicity in HepG2 cells, a human hepatocellular carcinoma cell line.47 The study discovered that five of these alkaloids demonstrated hepatoprotective activity comparable to bicyclol, a drug with established hepatoprotective effects. Similarly, Liu et al. identified a monoterpenoid from the stem of the wampee that exhibited similar activity to bicyclol in protecting HepG2 cells from APAP-induced toxicity.83
The hepatoprotective properties of wampees have been investigated in animal models. Carbon tetrachloride (CCl4) is a widely used agent to induce liver injury in mice by generating the trichloromethyl-oxygen radical through hepatic cytochrome P450 activation, leading to lipid peroxidation. Liu et al. demonstrated that administering eight alkaloids isolated from wampee leaves at a dosage of 250 mg/kg significantly decreased serum alanine transaminase (ALT) levels in CCl4-intoxicated mice.84 Moreover, the two alkaloids protected against acetaminophen- and thioacetamide-induced liver damage. It is hypothesized that these alkaloids might exhibit hepatoprotective effects by inhibiting the interaction between CCl4 and hepatic cytochrome P450, consequently diminishing the production of subsequent free radicals.
Additionally, carbon tetrachloride (CCl4)-induced liver injury results in the rapid release of alanine transaminase (ALT), aspartate transaminase (AST), and alkaline phosphatase (ALP) into the bloodstream. Elevated serum levels of these enzymes were indicative of liver damage. Adebajo et al. discovered that administering a 200 mg/kg dosage of methanolic wampee leaf extract improved the activities of ALT, AST, and ALP in the livers of CCl4-intoxicated mice while simultaneously downregulating these enzymes’ activity in plasma.7 The mechanism of the wampee hepatoprotective effect is also illustrated in Figure 5. Consequently, these findings indicate that wampee extract could potentially mitigate hepatocellular damage induced by CCl4in vivo. Table 5 summarizes the hepatoprotective effect of wampee.
Figure 5.
Mechanism of the wampee hepatoprotective effect.
3.5. Antidiabetic Effects
Numerous extracts from wampee have demonstrated antidiabetic properties. Adebajo et al.7 discovered that wampee stem extract effectively reduced glucose levels in rats. This extract treatment increased insulin secretion in both in vivo and in vitro experiments. Additionally, the wampee peel extract (3.4 mL/kg/day) significantly mitigated the physiological abnormalities associated with diabetes, while decreasing swelling and lipid accumulation in rat organs. Furthermore, an 8-week oral administration of lansiumamide B (20 mg/kg), an alkaloid isolated from the seeds of wampee, significantly reduced the body weight of HFD-fed mice by diminishing the visceral and subcutaneous fat tissues. The serum cholesterol, high-density lipoprotein (HDL), and low-density lipoprotein (LDL) levels in high-fat diet fed mice were reduced by 30.4%, 22.1%, and 47.7%, respectively.58
Kong et al. investigated the impact of polyphenol extracts from the wampee leaf on streptozotocin (STZ)-induced type 2 diabetic rats.36 The extract exhibited potent antihyperlipidemic properties, which effectively reduced the concentrations of total cholesterol (TC), triglycerides (TG), low-density lipoprotein cholesterol (LDL-C), and high-density lipoprotein cholesterol (HDL-C) within a four-week duration. The test index was illustrated in Figure 6. The bioactive constituents in wampee peel extract, including rutin (in Figure 2C), gallic acid (in Figure 2D), ferulic acid, chlorogenic acid, and caffeic acid, were identified to exhibit potential antidiabetic properties.36
Figure 6.
Wampee extract was used for diabetes treatment.
α-Glucosidase is an enzyme responsible for breaking down complex carbohydrates into simple sugars in the small intestine. This process is crucial for the absorption of carbohydrates into the bloodstream. However, in individuals with diabetes mellitus, α-glucosidase activity can result in elevated blood sugar levels after a meal, a phenomenon termed postprandial hyperglycemia. Condensed tannins and proanthocyanidins isolated from wampee have demonstrated inhibitory effects against α-glucosidase through competitive inhibition and conformational alteration in a dose-dependent manner.85 Moreover, Shen et al. found that a high-fat diet altered gut microbiota composition in mice and increased the expression of inflammatory cytokines, such as tumor necrosis factor-α (TNF-α), while oral administration of wampee extract could reverse these changes.86 Consequently, wampee may attenuate hyperglycemia and hyperlipidemia by inhibiting α-glucosidase and positively modulating the abundance of gut microbiota associated with metabolic parameters, thereby exhibiting antidiabetic activity.
These findings suggest the potential of wampee in preventing diabetes and provide scientific evidence for developing wampee-based functional food. Table 5 summarized the antidiabetic activities of the wampee.
3.6. Antimicrobial Effects
Wampee has potential antimicrobial activity. Both He et al.87 and Ma et al.26 found that the essential oils (EOs) of Clausena lansium played an important role in inhibiting the growth of five Candida species. The EOs, extracted from chicken heart wampee (CHW) pericarp and abundant in β-phellandrene and β-sesquiphellandrene, showed the most significant antimicrobial efficacy. For example, He et al.87 reported a 23.1 mm inhibition zone diameter for β-phellandrene, while Ma et al.26 observed comparable outcomes. Following β-phellandrene treatment, the inhibition zone diameters for the five Candida species examined ranged from 15.6 mm to 22.1 mm. In addition to β-phellandrene, the principal constituents of the EOs, sabinene, and 4-terpineol, they also demonstrated inhibitory effects on the growth of the bacterial strain. The compound 4-terpineol demonstrated the most significant efficacy, with inhibition zone diameters ranging from 26.7 to 35.6 mm when tested against five distinct Candida species.26 The extracts from the twigs and roots of Clausena lansium also showed antimicrobial activity. The P. gingivalis growth was habited after three carbazole alkaloid treatments. Among the investigated carbazole alkaloids, lansine exhibited the most potent efficacy, as evidenced by its inhibition zone diameters ranging from 19.06 to 20.94 mm.81 Based on the current studies, different bioactive compounds isolated from wampee exhibited potential antimicrobial activity.
Nonetheless, no research has been conducted to investigate the precise mechanisms underlying the effects of these compounds. Therefore, further investigation is required to understand the antimicrobial effect of wampee. These compounds could be extracted from wampee and made into an antimicrobial drug for further application. Table 5 summarized the antimicrobial effects of wampee.
4. Conclusions
The wampee fruit has been the subject of numerous studies due to its abundant nutrient content. This comprehensive review thoroughly studies the health implications and chemical constituents of wampee. In summary, wampee is an excellent source of dietary fiber with low-fat and low-calorie content and is enriched with significant levels of vitamin C, potassium, calcium, and nine essential amino acids. It is a good source of low-calorie food. Numerous bioactive constituents in wampee contribute to an array of health-promoting effects, such as antioxidative properties and neuroprotection and anticancer, anti-inflammatory, hepatoprotective, antidiabetic, and antimicrobial activities. The beneficial promoting effect and its corresponding bioactive substances have been studied and identified from wampee. However, the specific mechanism of these substances has not been explained clearly. The metabolic pathways and toxicology research of the bioactive substances extracted from wampee still require further study.
Acknowledgments
We are grateful to all the members of the Food Science and Technology Program at BNU-HKBU United International College for their scientific assistance.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.3c02759.
Supplemental Tables 1–3 as mentioned in the text (PDF)
Author Contributions
# These authors contributed equally.
This study is supported by a research grant (project code: UICR0200007–23) from BNU-HKBU United International College.
The authors declare no competing financial interest.
Special Issue
Published as part of the ACS Omegavirtual special issue “Phytochemistry”.
Supplementary Material
References
- Prasad K. N.; Xie H.; Hao J.; Yang B.; Qiu S.; Wei X.; Chen F.; Jiang Y. Antioxidant and anticancer activities of 8-hydroxypsoralen isolated from wampee [Clausena lansium (Lour.) Skeels] peel. Food Chem. 2010, 118 (1), 62–66. 10.1016/j.foodchem.2009.04.073. [DOI] [Google Scholar]
- Zhao Z.; Gao A.; Huang J.; Luo R.; Yu K. Screening of sweet wampee [Clausena lansium (Lour.) Skeels] progenies in the early growth stage based on chloroplast genome analysis. Genet. Resour. Crop Evol. 2021, 68, 1747–1750. 10.1007/s10722-021-01134-3. [DOI] [Google Scholar]
- Rodrigues S.; de Brito E. S.; de Oliveira Silva E.. Wampee—Clausena lansium. In Exotic Fruits; Rodrigues S., de Oliveira Silva E., de Brito E. S., Eds.; Academic Press: 2018; pp 439–441. [Google Scholar]
- Lim T., Clausena lansium. In Edible Medicinal and Non-medicinal Plants; Springer, 2012; pp 871–883. [Google Scholar]
- Du Y.; Liu H.; Li C.; Ma J.; Zhang D.; Li L.; Sun H.; Bao X.; Zhang D. Bioactive carbazole alkaloids from the stems of Clausena lansium. Fitoterapia 2015, 103, 122–128. 10.1016/j.fitote.2015.03.018. [DOI] [PubMed] [Google Scholar]
- Wen-Shyong L.; McChesney J. D.; El-Feraly F. S. Carbazole alkaloids from Clausena lansium. Phytochemistry 1991, 30 (1), 343–346. 10.1016/0031-9422(91)84151-H. [DOI] [Google Scholar]
- Adebajo A.C.; Iwalewa E.O.; Obuotor E.M.; Ibikunle G.F.; Omisore N.O.; Adewunmi C.O.; Obaparusi O.O.; Klaes M.; Adetogun G.E.; Schmidt T.J.; Verspohl E.J. Pharmacological properties of the extract and some isolated compounds of Clausena lansium stem bark: anti-trichomonal, antidiabetic, anti-inflammatory, hepatoprotective and antioxidant effects. J. Ethnopharmacol. 2009, 122 (1), 10–19. 10.1016/j.jep.2008.11.015. [DOI] [PubMed] [Google Scholar]
- Ismail A. A.; Ahmad B. A.; Mohamed A.; RasedeeAbdullah; Siddig I. A.; Mohamed Y. I.; Zeenelabdin A. A review of traditional uses, phytochemical and pharmacological aspects of selected members of Clausena genus (Rutaceae). J. Med. Plants Res. 2012, 6 (38), 5107–5118. 10.5897/JMPR12.317. [DOI] [Google Scholar]
- Peng W.; Fu X.; Li Y.; Xiong Z.; Shi X.; Zhang F.; Huo G.; Li B. Phytochemical study of stem and leaf of Clausena lansium. Molecules 2019, 24 (17), 3124. 10.3390/molecules24173124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H.; Zhao Z.; Gao A.; Luo R. Quality evaluation of 23 species of Clausena lansium (Lour.) Skeels germplasm resources based on principal component analysis and cluster analysis. Chin J. Trop Crop. 2022, 7, 1357–1364. 10.3969/j.issn.1000-2561.2022.07.006. [DOI] [Google Scholar]
- Li S.; Chen W.; Xu Y.; Xiao G.; Wu J.; Chen Z. Nutrition composition of seedless wampee. Food Sci. Technol. 2005, 06, 96–98. [Google Scholar]
- Wall M. M. Ascorbic acid and mineral composition of longan (Dimocarpus longan), lychee (Litchi chinensis) and rambutan (Nephelium lappaceum) cultivars grown in Hawaii. J. Food Compost Anal. 2006, 19 (6), 655–663. 10.1016/j.jfca.2005.12.001. [DOI] [Google Scholar]
- Wall M. M. Ascorbic acid, vitamin A, and mineral composition of banana (Musa sp.) and papaya (Carica papaya) cultivars grown in Hawaii. J. Food Compost Anal. 2006, 19 (5), 434–445. 10.1016/j.jfca.2006.01.002. [DOI] [Google Scholar]
- Huang W.-Y.; Cai Y.-Z.; Corke H.; Sun M. Survey of antioxidant capacity and nutritional quality of selected edible and medicinal fruit plants in Hong Kong. J. Food Compost Anal. 2010, 23 (6), 510–517. 10.1016/j.jfca.2009.12.006. [DOI] [Google Scholar]
- Zhang Y.; Huang Y.; Huang J.; Li R.; Liu J. Study on amino acids from the fruit of Clausena lansium. J. Chin. Med. Mater. 2006, 29 (9), 921–924. 10.13863/j.issn1001-4454.2006.09.019. [DOI] [PubMed] [Google Scholar]
- Dai H.; Lai Z.; Huang B.; Li J.; Xiao W.; Wang X. Determination of amino acids in flesh of waxberry and wampee by RP-HPLC. J. Zhongkai Univ. Agric. Technol. 2008, 21 (003), 7–11. [Google Scholar]
- Sun D.; Lu X.; Liang J.; Hu Y.; Xie J. Fruit quality and constituent of sugars and organic acids in wampee cultivars. Chin. J. Trop. Crops. 2012, 33 (8), 1418–1421. [Google Scholar]
- Lu Y.; Lin Z.; Zeng Y.; Qiu J.; Pan Q. Diversity of wampee (Clausena Lansium (Lour.) Skeels) germplasms in China revealed based on fruit quality traits. Mol. Plant Breed. 2015, 13 (2), 317–330. [Google Scholar]
- Yang W.; Zhao P.; Li X.; Guo L.; Gao W. The potential roles of natural plant polysaccharides in inflammatory bowel disease: A review. Carbohydr. Polym. 2022, 277, 118821 10.1016/j.carbpol.2021.118821. [DOI] [PubMed] [Google Scholar]
- Peng J.; Bu Z.; Ren H.; He Q.; Yu Y.; Xu Y.; Wu J.; Cheng L.; Li L. Physicochemical, structural, and functional properties of wampee (Clausena lansium (Lour.) Skeels) fruit peel pectin extracted with different organic acids. Food Chem. 2022, 386, 132834 10.1016/j.foodchem.2022.132834. [DOI] [PubMed] [Google Scholar]
- Wu H.; Min T.; Li X.; Li L.; Lai F.; Tang Y.; Yang X. Physicochemical properties and antioxidant activities of acidic polysaccharides from wampee seeds. Int. J. Biol. Macromol. 2013, 59, 90–95. 10.1016/j.ijbiomac.2013.04.020. [DOI] [PubMed] [Google Scholar]
- Song C.; Huang F.; Liu L.; Zhou Q.; Zhang D.; Fang Q.; Lei H.; Niu H. Characterization and prebiotic properties of pectin polysaccharide from Clausena lansium (Lour.) Skeels fruit. Int. J. Biol. Macromol. 2022, 194, 412–421. 10.1016/j.ijbiomac.2021.11.083. [DOI] [PubMed] [Google Scholar]
- Wen P.; Pei Z.; Zhu T.; Yu Z.; Geng Y.; Chen C.; Xue C. Preparation technology optimization of soluble dietary fiber and its structure characterization and composition of monosaccharide from Clausena lansium sarcocarp. Sci. Technol. Food Ind. 2020, 41 (21), 29–36. 10.13386/j.issn1002-0306.2020020124. [DOI] [Google Scholar]
- Tongnuanchan P.; Benjakul S. Essential oils: Extraction, bioactivities, and their uses for food preservation. J. Food Sci. 2014, 79 (7), R1231–R1249. 10.1111/1750-3841.12492. [DOI] [PubMed] [Google Scholar]
- Chokeprasert P.; Charles A. L.; Sue K.-H.; Huang T.-C. Volatile components of the leaves, fruits and seeds of wampee [Clausena lansium (Lour.) Skeels]. J. Food Compost Anal. 2007, 20 (1), 52–56. 10.1016/j.jfca.2006.07.002. [DOI] [Google Scholar]
- Ma Y.; Wang Y.; Zhou X.; Yang H.; Zhang H.; Chen W.; Zhang H.; Zhang Y.; He X. The influence of the chemical composition of essential oils of Clausena lansium seeds on the growth of Candida strains. Sci. Rep. 2021, 11 (1), 1–10. 10.1038/s41598-021-99188-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y.; Zhang Y.; Huang J.; Li R.; Liu J. Study on chemical constituents of volatile oil and trace elements from fruits of Clausena lansium.Chin. J. Chin. Mater. Med. 2006, 31 (11), 898–900. [PubMed] [Google Scholar]
- Tampieri M. P.; Galuppi R.; Macchioni F.; Carelle M. S.; Falcioni L.; Cioni P. L.; Morelli I. The inhibition of Candida albicans by selected essential oils and their major components. Mycopathologia 2005, 159 (3), 339–345. 10.1007/s11046-003-4790-5. [DOI] [PubMed] [Google Scholar]
- Yousefi M.; Rahimi-Nasrabadi M.; Pourmortazavi S. M.; Wysokowski M.; Jesionowski T.; Ehrlich H.; Mirsadeghi S. Supercritical fluid extraction of essential oils. Trends Anal. Chem. 2019, 118, 182–193. 10.1016/j.trac.2019.05.038. [DOI] [Google Scholar]
- Laura A.; Moreno-Escamilla J. O.; Rodrigo-García J.; Alvarez-Parrilla E., Phenolic compounds. In Postharvest physiology and biochemistry of fruits and vegetables; Elsevier: 2019; pp 253–271. [Google Scholar]
- Saranraj P.; Behera S. S.; Ray R. C., Traditional foods from tropical root and tuber crops: Innovations and challenges. In Innovations in traditional foods; Elsevier: 2019; pp 159–191. [Google Scholar]
- Ye Y.; Chang X.; Brennan M. A.; Brennan C. S.; Guo X. Comparison of phytochemical profiles, cellular antioxidant and anti-proliferative activities in five varieties of wampee (Clausena lansium) fruits. Int. J. Food Sci. Technol. 2019, 54 (7), 2487–2493. 10.1111/ijfs.14205. [DOI] [Google Scholar]
- Prasad K. N.; Hao J.; Yi C.; Zhang D.; Qiu S.; Jiang Y.; Zhang M.; Chen F. Antioxidant and anticancer activities of wampee (Clausena lansium (Lour.) Skeels) peel. J. Biotechnol. Biomed. 2009, 612805 10.1155/2009/612805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang X.; Ye Y.; Pan J.; Lin Z.; Qiu J.; Guo X.; Lu Y. Comparative assessment of phytochemical profiles and antioxidant activities in selected five varieties of wampee (Clausena lansium) fruits. Int. J. Food Sci. Technol. 2018, 53 (12), 2680–2686. 10.1111/ijfs.13877. [DOI] [Google Scholar]
- Li Q.; Chang X.; Guo R.; Wang Q.; Guo X. Dynamic effects of fermentation on phytochemical composition and antioxidant properties of wampee (Clausena lansium (Lour.) Skeel) leaves. Food Sci. Nutr. 2019, 7 (1), 76–85. 10.1002/fsn3.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong F.; Su Z.; Guo X.; Zeng F.; Bi Y. Antidiabetic and lipid-lowering effects of the polyphenol extracts from the leaves of Clausena lansium (Lour.) Skeels on streptozotocin-induced type 2 diabetic rats. J. Food Sci. 2018, 83 (1), 212–220. 10.1111/1750-3841.14004. [DOI] [PubMed] [Google Scholar]
- Chang X.; Lu Y.; Lin Z.; Qiu J.; Guo X.; Pan J.; Mehmood A. A. Impact of leaf development stages on polyphenolics profile and antioxidant activity in Clausena lansium (Lour.) Skeels. Biomed Res. Int. 2018, 7093691 10.1155/2018/7093691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G.-L.; Zhang X.; Chen S.-G.; Han M.-D.; Gao Y.-Q. Antioxidant activities and contents of free, esterified and insoluble-bound phenolics in 14 subtropical fruit leaves collected from the south of China. J. Funct. Foods 2017, 30, 290–302. 10.1016/j.jff.2017.01.011. [DOI] [Google Scholar]
- Chai W.-M.; Lin M.-Z.; Feng H.-L.; Zou Z.-R.; Wang Y.-X. Proanthocyanidins purified from fruit pericarp of Clausena lansium (Lour.) Skeels as efficient tyrosinase inhibitors: Structure evaluation, inhibitory activity and molecular mechanism. Food Funct. 2017, 8 (3), 1043–1051. 10.1039/C6FO01320A. [DOI] [PubMed] [Google Scholar]
- Chang X.; Ye Y.; Pan J.; Lin Z.; Qiu J.; Peng C.; Guo X.; Lu Y. Comparative analysis of phytochemical profiles and antioxidant activities between sweet and sour wampee (Clausena lansium) Fruits. Foods 2022, 11 (9), 1230. 10.3390/foods11091230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debnath B.; Singh W. S.; Das M.; Goswami S.; Singh M. K.; Maiti D.; Manna K. Role of plant alkaloids on human health: A review of biological activities. Mater. Today Chem. 2018, 9, 56–72. 10.1016/j.mtchem.2018.05.001. [DOI] [Google Scholar]
- Yang J.-H.; Wang X.-Y.; Zhou Y.-P.; Lu R.; Chen C.-H.; Zhang M.-H.; Cheng Y.-Y.; Morris-Natschke S. L.; Lee K.-H.; Wang Y.-S. Carbazole alkaloids from Clausena anisum-olens: Isolation, characterization, and anti-HIV evaluation. Molecules 2020, 25 (1), 99. 10.3390/molecules25010099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaikh M. S.; Karpoormath R.; Thapliyal N.; Rane R. A.; Palkar M. B.; Faya A. M.; Patel H. M.; Alwan W. S.; Jain K.; Hampannavar G. A. Current perspective of natural alkaloid carbazole and its derivatives as antitumor agents. Anti-Cancer Agents Med. Chem. 2015, 15 (8), 1049–1065. 10.2174/1871520615666150113105405. [DOI] [PubMed] [Google Scholar]
- Liu H.; Li C.-J.; Yang J.-Z.; Ning N.; Si Y.-K.; Li L.; Chen N.-H.; Zhao Q.; Zhang D.-M. Carbazole alkaloids from the stems of Clausena lansium. J. Nat. Prod. 2012, 75 (4), 677–682. 10.1021/np200919a. [DOI] [PubMed] [Google Scholar]
- Deng H.; Mei W.; Wang H.; Guo Z.; Dong W.; Wang H.; Li S.; Dai H. Carbazole alkaloids from the peels of Clausena lansium. J. Asian Nat. Prod. Res. 2014, 16 (10), 1024–1028. 10.1080/10286020.2014.930442. [DOI] [PubMed] [Google Scholar]
- Du Y.; Liu H.; Li C.; Yang J.; Ma J.; Zhang D.; Sun H.; Zhang D. Carbazole and amide alkaloids from the stems of Clausena lansium. J. Asian Nat. Prod. Res. 2015, 17 (11), 1048–1053. 10.1080/10286020.2015.1052414. [DOI] [PubMed] [Google Scholar]
- Du Y.-Q.; Liu H.; Li C.-J.; Ma J.; Zhang D.; Li L.; Sun H.; Bao X.-Q.; Zhang D.-M. Bioactive carbazole alkaloids from the stems of Clausena lansium. Fitoterapia 2015, 103, 122–128. 10.1016/j.fitote.2015.03.018. [DOI] [PubMed] [Google Scholar]
- Liu Y.; Guo J.; Liu Y.; Hu S.; Yan G.; Qiang L.; Fu Y. Carbazole alkaloids with potential neuroprotective activities from the fruits of Clausena lansium. J. Agric. Food Chem. 2019, 67 (20), 5764–5771. 10.1021/acs.jafc.9b00961. [DOI] [PubMed] [Google Scholar]
- Sun X.; Ma J.; Li C.; Zang Y.; Huang J.; Wang X.; Chen N.; Chen X.; Zhang D. Carbazole alkaloids with bioactivities from the stems of Clausena lansium. Phytochem. Lett. 2020, 38, 28–32. 10.1016/j.phytol.2020.03.004. [DOI] [Google Scholar]
- Sun X.; Li C.; Ma J.; Zang Y.; Huang J.; Chen N.; Wang X.; Zhang D. New amide alkaloids and carbazole alkaloid from the stems of Clausena lansium. Fitoterapia 2021, 154, 104999 10.1016/j.fitote.2021.104999. [DOI] [PubMed] [Google Scholar]
- Fan Y.; Sahu S. K.; Yang T.; Mu W.; Wei J.; Cheng L.; Yang J.; Liu J.; Zhao Y.; Lisby M.; Liu H. The Clausena lansium (Wampee) genome reveal new insights into the carbazole alkaloids biosynthesis pathway. Genomics 2021, 113 (6), 3696–3704. 10.1016/j.ygeno.2021.09.007. [DOI] [PubMed] [Google Scholar]
- Li H.; Feng Z.; Wang Y.; Lin L. Anticancer carbazole alkaloids and coumarins from Clausena plants: A review. Chin. J. Nat. Med. 2017, 15 (12), 881–888. 10.1016/S1875-5364(18)30003-7. [DOI] [PubMed] [Google Scholar]
- Yang M.; Cao Y.; Li W.; Yang Y.; Chen Y.; Huang L. Isolation and structural elucidation of Clausenamide from the leaves of Clausena lansium (Lour.) Skeels. Acta Pharm. Sin. 1987, 22 (1), 33–40. [PubMed] [Google Scholar]
- Shen D.; Kuo P.; Huang S.; Hwang T.; Chan Y.; Shieh P.; Ngan N.; Thang T.; Wu T. Constituents from the leaves of Clausena lansium and their anti-inflammatory activity. J. Nat. Med. 2017, 71 (1), 96–104. 10.1007/s11418-016-1033-x. [DOI] [PubMed] [Google Scholar]
- Matsui T.; Ito C.; Furukawa H.; Okada T.; Itoigawa M. Lansiumamide B and SB-204900 isolated from Clausena lansium inhibit histamine and TNF-α release from RBL-2H3 cells. Inflamm. Res. 2013, 62 (3), 333–341. 10.1007/s00011-012-0586-8. [DOI] [PubMed] [Google Scholar]
- Lin J.-H. Cinnamamide derivatives from Clausena lansium. Phytochemistry 1989, 28 (2), 621–622. 10.1016/0031-9422(89)80063-9. [DOI] [Google Scholar]
- Fan Y.; Chen H.; Mei W.; Kong F.; Li F.; Chen P.; Cai C.; Huang M.; Dai H. Nematicidal amide alkaloids from the seeds of Clausena lansium. Fitoterapia 2018, 128, 20–25. 10.1016/j.fitote.2018.04.023. [DOI] [PubMed] [Google Scholar]
- Huang L.; Li D.; Xu Y.-S.; Feng Z.-L.; Meng F.-C.; Zhang Q.-W.; Gan L.-S.; Lin L.-G. Clausoxamine, an alkaloid possessing a 1, 3-oxazine-4-one ring from the seeds of Clausena lansium and the anti-obesity effect of lansiumamide B. RSC Adv. 2017, 7 (74), 46900–46905. 10.1039/C7RA09793J. [DOI] [Google Scholar]
- Han Y.; Li L.-c.; Hao W.-b.; Tang M.; Wan S.-q. Larvicidal activity of lansiumamide B from the seeds of Clausena lansium against Aedes albopictus (Diptera: Culicidae). Parasitol. Res. 2013, 112 (2), 511–516. 10.1007/s00436-012-3161-x. [DOI] [PubMed] [Google Scholar]
- Li L.; Feng X.; Tang M.; Hao W.; Han Y.; Zhang G.; Wan S. Antibacterial activity of Lansiumamide B to tobacco bacterial wilt (Ralstonia solanacearum). Microbiol. Res. 2014, 169 (7–8), 522–526. 10.1016/j.micres.2013.12.003. [DOI] [PubMed] [Google Scholar]
- Xu H.; Chen T.; Huang L.; Shen Q.; Lian Z.; Shi Y.; Ouyang M.-A.; Song L. Synthesis and fungicidal activity of lansiumamide A and B and their derivatives. Molecules 2018, 23 (7), 1499. 10.3390/molecules23071499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J.; Du Y.; Li C.; Li L.; Chen F.; Yang J.; Chen N.; Zhang D. Alkaloids from the stems of Clausena lansium and their neuroprotective activity. RSC Adv. 2017, 7 (56), 35417–35425. 10.1039/C7RA06753D. [DOI] [Google Scholar]
- Rotariu D.; Babes E. E.; Tit D. M.; Moisi M.; Bustea C.; Stoicescu M.; Radu A.-F.; Vesa C. M.; Behl T.; Bungau A. F.; Bungau S. G. Oxidative stress–Complex pathological issues concerning the hallmark of cardiovascular and metabolic disorders. Biomed. Pharmacother. 2022, 152, 113238 10.1016/j.biopha.2022.113238. [DOI] [PubMed] [Google Scholar]
- Pizzino G.; Irrera N.; Cucinotta M.; Pallio G.; Mannino F.; Arcoraci V.; Squadrito F.; Altavilla D.; Bitto A. Oxidative stress: harms and benefits for human health. Oxid. Med. Cell. Longev. 2017, 8416763 10.1155/2017/8416763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivas U. S.; Tan B. W.; Vellayappan B. A.; Jeyasekharan A. D. ROS and the DNA damage response in cancer. Redox Biol. 2019, 25, 101084 10.1016/j.redox.2018.101084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng X.; Zhou X.; Cui L.; Liu D.; Wu K.; Li W.; Huang R. The fruits of wampee inhibit H2O2-induced apoptosis in PC12 cells via the NF-κB pathway and regulation of cellular redox status. Molecules 2014, 19 (6), 7368–7387. 10.3390/molecules19067368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang X.; Lu Y.; Lin Z.; Qiu J.; Guo X.; Pan J.; Abbasi A. M. Impact of leaf development stages on polyphenolics profile and antioxidant activity in Clausena lansium (Lour.) Skeels. Biomed Res. Int. 2018, 7093691 10.1155/2018/7093691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaich K. M.; Tian X.; Xie J. Hurdles and pitfalls in measuring antioxidant efficacy: A critical evaluation of ABTS, DPPH, and ORAC assays. J. Funct. Foods 2015, 14, 111–125. 10.1016/j.jff.2015.01.043. [DOI] [Google Scholar]
- Maragakis N. J.; Rothstein J. D. Mechanisms of disease: Astrocytes in neurodegenerative disease. Nat. Clin. Pract. Neurol. 2006, 2 (12), 679–689. 10.1038/ncpneuro0355. [DOI] [PubMed] [Google Scholar]
- Xicoy H.; Wieringa B.; Martens G. J. The SH-SY5Y cell line in Parkinson’s disease research: A systematic review. Mol. Neurodegener. 2017, 12 (1), 1–11. 10.1186/s13024-017-0149-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiatrak B.; Kubis-Kubiak A.; Piwowar A.; Barg E. PC12 cell line: Cell types, coating of culture vessels, differentiation and other culture conditions. Cells 2020, 9 (4), 958. 10.3390/cells9040958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J.-F.; Chu S.-F.; Ning N.; Yuan Y.-H.; Xue W.; Chen N.-H.; Zhang J.-T. Protective effect of (−) Clausenamide against Aβ-induced neurotoxicity in differentiated PC12 cells. Neurosci. Lett. 2010, 483 (1), 78–82. 10.1016/j.neulet.2010.07.067. [DOI] [PubMed] [Google Scholar]
- Liu Y.; Guo J.; Wang X.; Liu Y.; Zhang W.; Wang T.; Qiang L.; Fu Y. Geranylated carbazole alkaloids with potential neuroprotective activities from the stems and leaves of Clausena lansium. Bioorg. Chem. 2019, 92, 103278 10.1016/j.bioorg.2019.103278. [DOI] [PubMed] [Google Scholar]
- Li B.; Yuan Y.; Hu J.; Zhao Q.; Zhang D.; Chen N. Protective effect of Bu-7, a flavonoid extracted from Clausena lansium, against rotenone injury in PC12 cells. Acta Pharmacol. Sin. 2011, 32 (11), 1321–1326. 10.1038/aps.2011.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tongun T.; Phachonpai W. Cognitive booster of wampee peel extract on chronic restraint stress-induced memory dysfunction in rats. J. Appl. Pharm. Sci. 2020, 10 (7), 019–026. 10.7324/JAPS.2020.10703. [DOI] [Google Scholar]
- Phachonpai W.; Tongun T. Cognition enhancing effects of Clausena lansium (Lour.) peel extract attenuate chronic restraint stress-induced memory deficit in rats. Heliyon 2021, 7 (5), e07003 10.1016/j.heliyon.2021.e07003. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Phachonpai W.; Tongun T. Neuroprotective and cognitive enhancing effects of Clausena lansium (Lour.) skeels peels extract in ischemic rat brains. J. Appl. Pharm. Sci. 2021, 11 (9), 001–008. 10.7324/JAPS.2021.110901. [DOI] [Google Scholar]
- Shin S. A.; Joo B. J.; Lee J. S.; Ryu G.; Han M.; Kim W. Y.; Park H. H.; Lee J. H.; Lee C. S. Phytochemicals as anti-inflammatory agents in animal models of prevalent inflammatory diseases. Molecules 2020, 25 (24), 5932. 10.3390/molecules25245932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang G.; Li J.; Li W.; Liu T.; Jiang G.; Wu T.; Zou Y.; Huang J.; Tao L.; Zhu Z.; et al. Suppression of inflammation by ethanol extract of Clausena lansium via modulation of TLR4/MYD88/TRAF6 signaling pathway in RAW 264.7 macrophages. Eur. J. Inflamm. 2019, 17, 2058739219841973 10.1177/2058739219841973. [DOI] [Google Scholar]
- Liu J.; Li C.-J.; Ni L.; Yang J.-Z.; Li L.; Zang C.-x.; Bao X.-Q.; Zhang D.; Zhang D.-M. Anti-inflammatory alkaloid glycoside and quinoline alkaloid derivates from the stems of Clausena lansium. RSC Adv. 2015, 5 (98), 80553–80560. 10.1039/C5RA14173G. [DOI] [Google Scholar]
- Rodanant P.; Surarit R.; Laphookhieo S.; Kuvatanasuchati J. In vitro evaluation of the antibacterial and anti-inflammation activities of Clausena lansium (Lour.) Skeels. Songklanakarin J. Sci. Technol. 2015, 37 (1), 43–48. [Google Scholar]
- Shen D.-Y.; Chao C.-H.; Chan H.-H.; Huang G.-J.; Hwang T.-L.; Lai C.-Y.; Lee K.-H.; Thang T. D.; Wu T.-S. Bioactive constituents of Clausena lansium and a method for discrimination of aldose enantiomers. Phytochemistry 2012, 82, 110–117. 10.1016/j.phytochem.2012.06.019. [DOI] [PubMed] [Google Scholar]
- Liu J.; Li C.-J.; Du Y.-Q.; Li L.; Sun H.; Chen N.-H.; Zhang D.-M. Bioactive compounds from the stems of Clausena lansium. Molecules 2017, 22 (12), 2226. 10.3390/molecules22122226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G. T.; Li W. X.; Chen Y. Y.; Wei H. L. Hepatoprotective action of nine constituents isolated from the leaves of Clausena lansium in mice. Drug Dev. Res. 1996, 39 (2), 174–178. . [DOI] [Google Scholar]
- Ou-Yang C.; Chai W.; Xu X.; Song S.; Wei Q.; Huang Q.; Zou Z. Inhibitory potential of proanthocyanidins from the fruit pulp of Clausena lansium (Lour.) Skeels against α-glucosidase and non-enzymatic glycation: Activity and mechanism. Process Biochem. 2020, 91, 364–373. 10.1016/j.procbio.2020.01.006. [DOI] [Google Scholar]
- Shen S.; Liao Q.; Huang L.; Li D.; Zhang Q.; Wang Y.; Lee S. M.-Y.; Lin L. Water soluble fraction from ethanolic extract of Clausena lansium seeds alleviates obesity and insulin resistance, and changes the composition of gut microbiota in high-fat diet-fed mice. J. Funct. Foods 2018, 47, 192–199. 10.1016/j.jff.2018.05.057. [DOI] [Google Scholar]
- He X.; Zhang L.; Chen J.; Sui J.; Yi G.; Wu J.; Ma Y. Correlation between chemical composition and antifungal activity of Clausena lansium essential oil against Candida spp. Molecules 2019, 24 (7), 1394. 10.3390/molecules24071394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maneerat W.; Laphookhieo S. Antitumoral alkaloids from Clausena lansium. Heterocycles 2010, 81 (5), 1261. 10.3987/COM-10-11924. [DOI] [Google Scholar]
- Maneerat W.; Ritthiwigrom T.; Cheenpracha S.; Laphookhieo S. Carbazole alkaloids and coumarins from Clausena lansium roots. Phytochem. Lett. 2012, 5 (1), 26–28. 10.1016/j.phytol.2011.08.013. [DOI] [Google Scholar]
- Jiang H.-Y.; Zhang W.-J.; You C.-X.; Yang K.; Fan L.; Feng J.-B.; Chen J.; Yang Y.-J.; Wang C.-F.; Deng Z.-W.; Yin H.-B.; Du S.-S. Two new cytotoxic constituents from the Clausena lansium (Lour.) Skeels. Phytochem. Lett. 2014, 9, 92–95. 10.1016/j.phytol.2014.04.016. [DOI] [Google Scholar]
- Peng W.; Zheng L.; Ji C.; Shi X.; Xiong Z.; Shangguan X. Carbazole alkaloids isolated from the branch and leaf extracts of Clausena lansium. Chin. J. Nat. Med. 2018, 16 (7), 509–512. 10.1016/S1875-5364(18)30087-6. [DOI] [PubMed] [Google Scholar]
- Ming-He Y.; Yan-Yong C.; Liang H. Studies on the chemical constituents of Clausena Lansium (Lour.) Skeels II: the isolation and structural elucidation of neoclausenamide and dehydrocycloclausenamide. Acta Chimica Sinica English Edition 1987, 5 (3), 267–272. 10.1002/cjoc.19870050312. [DOI] [Google Scholar]
- Ming-He Y.; Yan-Yong C.; Liang H. Three novel cyclic amides from Clausena lansium. Phytochemistry 1988, 27 (2), 445–450. 10.1016/0031-9422(88)83117-0. [DOI] [Google Scholar]
- Zhao Q.; Yang J.-Z.; Li C.-J.; Chen N.-H.; Zhang D.-M. A new megastigmane glucoside and a new amide alkaloid from the leaves of Clausena lansium (Lour.) Skeels. J. Asian Nat. Prod. Res. 2011, 13 (04), 361–366. 10.1080/10286020.2011.558841. [DOI] [PubMed] [Google Scholar]
- Fan Y.; Chen H.; Mei W.; Xu S.; Zhou L.; Huang M. Amide alkaloids from the seeds of Clausena lansium and their nematicidal activities. J. Trop. Subtrop. Bot. 2018, 26 (1), 85–91. [Google Scholar]
- Ma Y.; Wang Y.; Zhou X.; Yang H.; Zhang H.; Chen W.; Zhang H.; Zhang Y.; He X. The influence of the chemical composition of essential oils of Clausena lansium seeds on the growth of Candida strains. Sci. Rep. 2021, 11 (1), 1–10. 10.1038/s41598-021-99188-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.






